Abstract
Aims
Local therapy including surgery or radiotherapy has been reported for the treatment of non-small cell lung cancer (NSCLC) with synchronous solitary metastasis, while studies with other local ablative treatment are rare. Here, we summarized our single-center experience of microwave ablation (MWA) for both primary and metastatic lesions in NSCLC patients with synchronous solitary extracranial metastases.
Patients and methods
We retrospectively screened our institute database from January 2014 to Jun 2019. NSCLC patients with synchronous extracranial solitary metastasis with primary and metastatic lesions that were treated with MWA were identified and analyzed.
Results
Of the 1472 stage IV NSCLC patients found, 38 were diagnosed with synchronous extracranial solitary metastasis and 29 of them received MWA for primary and metastatic lesions. The most common distant metastases were contralateral lung metastases (14 cases), followed by bone (6), liver (4), adrenal gland (3) and pleura metastases (1). Median OS and PFS was 21.5 and 12.5 months, respectively. Patients with N0 had significantly longer PFS (median 18.5 vs. 8.0 months) and OS (median 42.7 vs. 19.0 months). In addition, systemic therapy was showed to be a prognostic factor for better PFS (12.9 vs. 7.5 months). Clinical pathological factors including age, histology, T stage, PS score, and metastasis locations are not significantly associated with survival.
Conclusions
MWA may serve as an alternative treatment for NSCLCs with synchronous solitary extracranial metastases.
Keywords: Non-small cell lung cancer, Synchronous solitary metastases, Microwave ablation, Thermal ablation
Introduction
Clinically, only a small portion of non-small cell lung cancers (NSCLCs) are diagnosed at an early stage, while over 60% patients present with locally advanced or metastatic disease (stage III or IV) at initial diagnosis, at which point surgical resection may not be considered (Siegel et al. 2019; Travis et al. 2015). Despite the progress in anticancer therapy including targeted agents or newly developed checkpoint inhibitor, prognosis for metastatic NSCLC remains to be improved.
Stage IV NSCLC is a heterogeneous disease. Multiple preclinical and clinical data increasingly supported the concept that patients with oligometastases present a distinct population from widely spread metastatic disease (Hellman and Weichselbaum 1995; Wong et al. 2016; Lussier et al. 2011; Weichselbaum and Hellman 2011). Based on the 8th edition of the TNM classification of lung cancer, the M1a category includes cases of pleural or pericardial effusions, contralateral or bilateral lung/pleural nodules, or a combination of these parameters. Solitary metastasis in a single distant organ has been newly classified as M1b, while multiple lesions in single or multiple organs should be reclassified in a new M1c category. Both M1a and M1b have been classified in IVa stage while widespread metastasis is categorized as IVb disease, suggesting that patients presenting with synchronous solitary metastasis may present a distinct population. For these patients, life expectancy and prognosis may be better and aggressive local ablative therapy can be beneficial. Several studies suggested that selected patients who present with synchronous solitary metastases may have long-term survival if receiving surgical resection or radiotherapy for both primary and metastatic lesions (Tonnies et al. 2014; Salah et al. 2012; Ambrogi et al. 2001; Toffart et al. 2018).
In previously published literatures, local therapy for patients with solitary metastases mainly consists of surgery, chemoradiotherapy or multi-modality treatment, while studies concerning other local ablative treatment have been rarely reported. Image-guided thermal ablation techniques including radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation have been widely applied for NSCLC with oligometastatic disease, especially for those that are not surgical candidates (Vogl et al. 2017; Baere et al. 2016; Ni et al. 2019; Solbiati 2018; Ye et al. 2018; Hertzanu and Ye 2019; Wei et al. 2019). Thermal ablation showed good safety, efficacy, and local disease control while preserving lung parenchyma. Studies have showed advantages of MWA in contrast to other thermal ablation techniques in large tumors, in locations adjacent to vessels and in fully perfused areas (Palussiere et al. 2017). Our recently published results indicated that local MWA consolidative therapy with concurrent TKIs resulted in better local control and survival than TKIs monotherapy in EGFR-mutant advanced NSCLCs with extracranial oligometastases (Ni et al. 2020). The present report was initiated to summarize our single-center experience of MWA to primary and metastatic lesions for NSCLC patients with synchronous solitary extracranial metastases.
Methods
Patients
This is a retrospective single-center study. We analyzed the database of patients who underwent NSCLC and were treated with MWA between January 2014 and Jun 2019 at the Shandong Provincial Hospital affiliated to Shandong First Medical University. Of those, 29 NSCLC patients with synchronous extracranial solitary metastasis who received MWA for both primary and metastatic lesions were retrospectively identified. The study was approved by the ethics committee of Shandong Provincial Hospital affiliated to Shandong First Medical University and written informed consent to use the clinical data for research was obtained from each participant before the medical intervention started. Patients were evaluated with physical examination, systemic examination of either contrast-enhanced computed tomography (CT) of the chest and abdomen, bone scans, or positron emission tomography/computed tomography (PET–CT), brain CT or magnetic resonance imaging (MRI). Patients who met the following criteria were enrolled: (1) pathologically or cytologically confirmed NSCLC; (2) stage IV disease according to the 7th edition of the American Joint Committee on Cancer staging system, with synchronous solitary metastatic disease; (3) primary NSCLC and metastases technically completely microwave ablated; (4) no further metastatic lesions were detected (solitary metastasis); (5) ≥ 18 years old, and Eastern Cooperative Oncology Group (ECOG) performance status (PS) score ≤ 2.
The exclusion criteria included: (1) patients who did not undergo a systemic examination prior to enrolling in this study; (2) diagnosed with synchronous brain metastasis at baseline; (3) newly diagnosed distant metastasis within 1 month after ablation therapy. Patients’ selection steps are shown in Fig. 1.
Fig. 1.
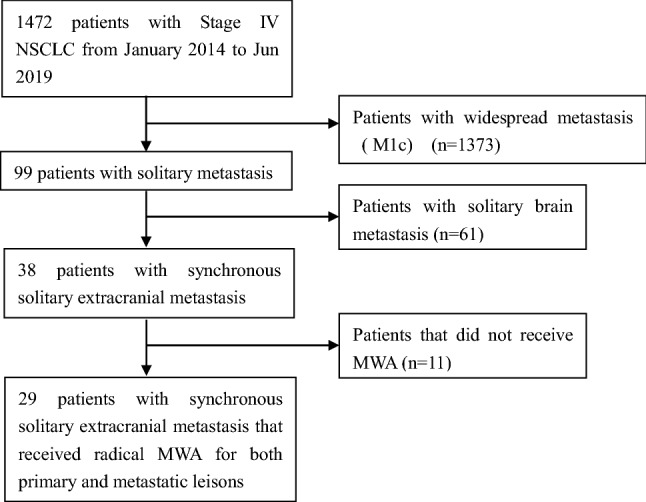
Flow chart of patients’ treatments
Treatment procedures
All patients had been discussed in a multidisciplinary tumor board, where a consensus was reached that MWA treatment to all known sites of disease was clinically reasonable and feasible. To limit potential bias, including selection bias, patients were included based on the ‘intention-to-treat’ decision of the multidisciplinary tumor board. Detailed ablation procedures were as we previously described. Generally, needle biopsy and MWA of primary tumors was performed first and the metastasis was then ablated. The interval between primary tumor and metastasis treatment allowed the administration of induction chemotherapy, adjuvant chemotherapy or TKIs and was less than 1 months in all cases. One month after ablation, contrast-enhanced computed tomography (CT) and/or magnetic resonance (MR) imaging was performed to determine the local response (complete or incomplete ablation) based on criteria as we previously described (Ye et al. 2018). Patients received contrast-enhanced CT or MR scan every 1–2 months after ablation to assess their response to treatment and to identify adverse events.
Data processing and statistical analysis
All patients were analyzed for baseline characteristics, tumor and treatment characteristics, ablation completeness, ablation complications and 30-day mortality. Primary endpoints were overall survival (OS) and progression-free survival (PFS). OS was calculated from the first day of ablation treatment until the last date of follow-up or death. PFS was calculated from first day of treatment until any relapse, local or distant, or death (whichever occurred first). Kaplan–Meier curve and log-rank test were used for survival analyses. Cox proportional hazards model was used to calculate the hazard ratio (HR) and corresponding 95% confidence intervals (CI). P values were two-sided and considered significant if < 0.05. Statistical analysis was performed using SPSS for Windows Version 17.0 (IBM, Chicago, IL).
Results
Patient characteristics
Based on the inclusion and exclusion criteria, 29 NSCLC patients with synchronous solitary extracranial metastases who received MWA for both primary and metastatic lesions were included in the present study (patients’ characteristics shown in Table 1). Adenocarcinoma was the predominant histologic type (18, 62.1%). All adenocarcinomas were detected for EGFR gene mutations and TKIs were admitted as first-line therapy whenever applicable. In all patients, 24 (82.8%) received post-ablation systemic therapy, of which 6 patients received EGFR-TKIs.
Table 1.
Baseline patient characteristics
| Variables | Numbers (%) |
|---|---|
| Age | |
| Median (IQR) | 68 (58–78) |
| Sex | |
| Male | 9 (31.0) |
| Female | 20 (69.0) |
| ECOG performance status | |
| 0–1 | 21 (72.4) |
| 2 | 8 (27.6%) |
| Histology | |
| Adenocarcinoma | 15 (51.7) |
| Squamous cell carcinoma | 12 (41.4) |
| NSCLC, undefined | 2 (6.9) |
| T stage | |
| T1 | 14 (48.3) |
| T2 | 13 (44.8) |
| T3 | 2 (6.9) |
| Nodal status | |
| N0 | 10 (34.5) |
| N1 | 10 (34.5) |
| N2/N3 | 9 (31.0) |
| Metastasis location | |
| Contralateral lung | 14 (48.3) |
| Bone | 6 (20.7) |
| Liver | 4 (13.8) |
| Adrenal gland | 3 (10.3) |
| Pleura | 1 (3.4) |
The clinical N involvement was N0 in 10 patients (34.5%), N1 in 10 patients (34.5%), N2 in 6 patients (21%) and N3 in 3 patients (10%). The most common distant metastases were contralateral lung metastases (14 cases), followed by bone (6), liver (4), adrenal gland (3) and pleura metastases (1). The site of the bone metastasis was rib in two patients, vertebra in four patients.
Outcomes and prognostic factors
Median OS of all patients was 21.5 months (IQR 12.5–25.1 months) and PFS was 12.5 months (IQR 7.0–16.0 months) (Fig. 2). Patients with N0 had significantly longer PFS (median 18.5 vs. 8.0 months, HR 0.27, 95% CI 0.11–0.65, P < 0.01) (Fig. 3a) and OS (median 42.7 vs. 19.0 months, HR 0.20, 95% CI 0.06–0.66, P < 0.01) (Fig. 3b). In addition, systemic therapy was showed to be a prognostic factor for better PFS (12.9 vs. 7.5 months, HR 0.18, 95% CI 0.03–0.95, P = 0.04) (Fig. 4a), and nearly retained significance for OS (24.5 vs. 18.2 months, HR 0.14, 95% CI 0.02–1.01, P = 0.052) (Fig. 4b). Multivariable Cox regression analysis confirmed that negative nodal involvement was associated with better PFS and OS. Other clinical pathological factors including age, histology, T stage, PS score, and metastasis locations are not significantly associated with patients’ survival (Table 2).
Fig. 2.
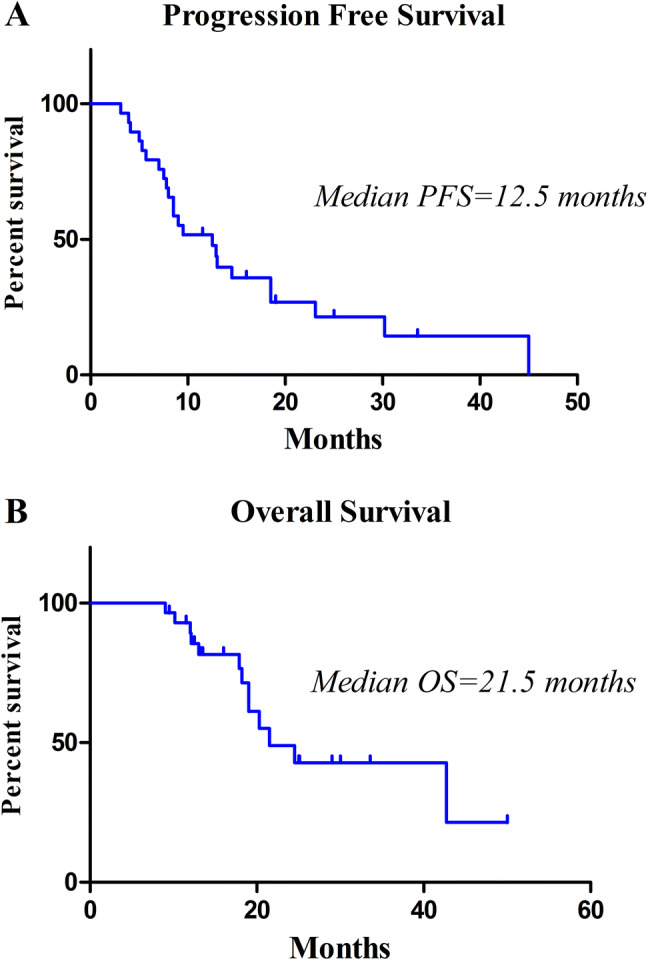
Progression-free survival (a) and overall survival (b) of all patients
Fig. 3.
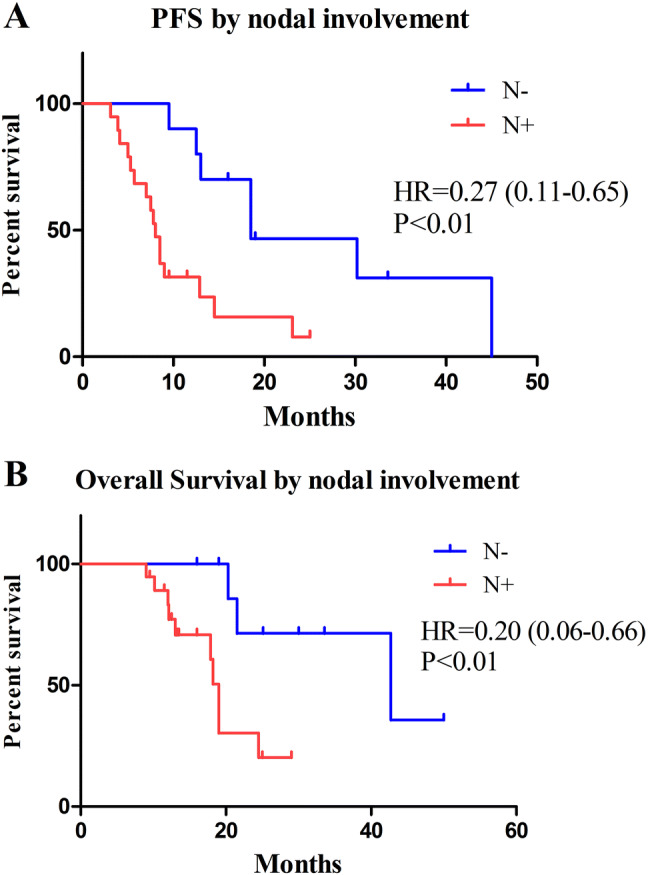
Kaplan–Meier analysis of PFS (a) and OS (b) by nodal involvement. Patients with N0 had significantly longer PFS (median 18.5 vs. 8.0 months, HR 0.27, 95% CI 0.11–0.65, P < 0.01) and OS (median 42.7 vs. 19.0 months, HR 0.20, 95% CI 0.06–0.66, P < 0.01)
Fig. 4.

Kaplan–Meier analysis of PFS (a) and OS (b) by systemic treatment. Patients with systemic treatment have better PFS (12.9 vs. 7.5 months, HR 0.18, 95% CI 0.03–0.95, P = 0.04) and relatively long OS (24.5 vs. 18.2 months, HR 0.14, 95% CI 0.02–1.01, P = 0.052)
Table 2.
Results of Cox regression analyses
| Variables | Multivariable Cox regression analysis for PFS and OS | |||||
|---|---|---|---|---|---|---|
| PFS | OS | |||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Gender (male vs. female) | 1.87 | 0.39–4.37 | 0.37 | 2.58 | 1.78–3.55 | 0.76 |
| Age (< 65 vs. ≥ 65) | 0.89 | 0.80–0.99 | 0.45 | 0.99 | 0.84–1.20 | 0.98 |
| ECOG score (0–1 vs. 2) | 0.91 | 0.31–2.65 | 0.86 | 0.39 | 0.11–1.36 | 0.14 |
| Histology (adenocarcinoma vs. others) | 2.25 | 0.76–6.70 | 0.15 | 0.94 | 0.27–3.2 | 0.92 |
| T stage (T1 vs. T2/T3) | 1.59 | 0.67–3.76 | 0.29 | 2.32 | 0.85–6.36 | 0.10 |
| Nodal status (N2/N3 vs. N0/N1) | 3.57 | 1.89–6.73 | < 0.01 | 3.36 | 1.51–7.45 | < 0.01 |
| Metastasis location (lung vs. other sites) | 2.54 | 0.64–4.58 | 0.53 | 1.65 | 0.67–3.47 | 0.34 |
Treatment complications
Generally, most MWA complications were mild and well tolerated. There was no perioperative mortality during ablation procedures or within 30 days after MWA. Twenty patients (69%) suffered mild or moderate pain after ablation, but no severe post-ablation pain occurred. Post-ablation pneumothorax image findings were observed in 10 (23.1%) lung ablation sessions, while 4 (40%) of them received chest tube drainage. Self-limited, post-ablation syndrome including fever (below 38.5 °C), fatigue, general malaise, nausea, and vomiting, etc., occurred in 11/58 (19%) of ablation sessions. Hypertensive crises occurred in one adrenal gland MWA procedure (33.3%) and were managed by anesthesiology with phentolamine and sodium nitroprusside.
Discussion
NSCLC patients with distant metastases were classified as having stage IV disease and a poor prognosis. However, there is growing evidence supporting the benefits of aggressive local therapy to primary and metastatic lesions for patients with synchronous oligometastasis. More restricted than the definition of oligometastasis, we enrolled patients with synchronous solitary extracranial metastasis. The present study was conducted to describe criteria for selecting patients who would benefit from MWA treatment to primary tumor and synchronous metastases.
Several retrospective series concerning surgery for synchronous solitary metastasis reported a median survival ranging between 13.5 and 41 months (Tonnies et al. 2014; Collaud et al. 2012; Mordant et al. 2012; Hanagiri et al. 2012). In a recently published study with 1592 advanced stage NSCLC patients, 109 (7%) had a synchronous solitary metastasis (M1 disease), of which 90 (83%) received local therapy including surgery or radiotherapy of curative intent at the primary and metastatic sites. Local therapy for both sites was associated with long-term survival (Toffart et al. 2018). However, most series reporting on patients with synchronous solitary metastases have focused on brain metastases. Moreover, local therapy applied in these studies mainly included surgical resection or radiotherapy.
The most common synchronous distant metastatic sites in NSCLC patients are the brain, bone, liver and adrenal gland (Quint et al. 1996). Brain was reported as the most frequent (60%) solitary metastatic site (Mordant et al. 2012; Downey et al. 2002; Luketich et al. 1995; Nieder et al. 2019), while studies concerning solitary extracranial metastasis are scarce. Luketich et al. initially identified 14 NSCLC patients who received local treatment of their primary lesions followed by solitary extracranial metastases resection. An overall 10-year survival of 86% was observed in these patients (Luketich et al. 1995). Ambrogi et al. conducted a small sample retrospective study and enrolled nine NSCLC patients with synchronous solitary extracranial metastasis: adrenal gland (n = 5), cutaneous (n = 2), axillary lymph node (n = 1) and kidney (n = 1). Resection of both primary tumor and solitary metastases was associated with a 5-year survival rate of 55.6% (Ambrogi et al. 2001). In a systemic review of 51 articles including 62 cases with solitary metastasis (other than adrenal gland or brain) who received combined primary cancer treatment and metastasis resection, the 5-year survival rate reached 50% for the entire cohort (Salah et al. 2012). The long-term survival of patients who underwent simultaneous resection or radiotherapy of primary and metastatic tumors stimulated our interest in the role of MWA therapy for this situation, especially for those who are not amenable for surgical resection. MWA has several advantages compared to RFA, achieving higher temperatures, larger ablation zones, less intraprocedural pain, as well as reduced susceptibility to the heat sink effect (Pusceddu et al. 2019). Our previous published results indicated that MWA as local consolidative therapy leads to better disease control and survival in EGFR-mutant advanced NSCLC patients with extracranial oligometastasis (Ni et al. 2020). In the present study, all patients received MWA for their primary and metastatic lesions and had a median PFS of 12.5 months and OS of 21.5 months. This encouraging result is consistent and comparable with previously series in surgery or radiotherapy, which expands the clinical practice of MWA for stage IV NSCLCs.
According to our results, clinical nodal involvement (N0 versus N+) was a prognostic factor of survival. Indeed, patients with clinical N0 stage had a median survival of 42.7 months compared to 19 months for lymph node positive patients (P < 0.01). These findings were in accordance with multiple studies on surgical or radiation treatment (Salah et al. 2012; Ambrogi et al. 2001; Collaud et al. 2012; Downey et al. 2002). The second factor of good prognosis is related to the addition of systemic chemotherapy/TKIs. Multiple retrospective studies commonly supported that prognosis might be ameliorated by systemic therapy (Hanagiri et al. 2012; Griffioen et al. 2013; Congedo et al. 2012). As local MWA cannot be applied for hilar or mediastinal lymph node metastases, combination with thoracic radiotherapy or aggressive systemic therapy should be considered in these patients subgroup.
Our study still has some limitations. First, this is a single-center retrospective study with small sample size. Second, patients with brain metastasis were excluded in this study. Therefore, further investigation of combined primary pulmonary MWA and brain radiotherapy for NSCLCs with solitary brain metastasis may be interesting and necessary. Moreover, without a control group with solitary metastasis who did not receive aggressive MWA therapy, it is difficult to ascertain whether MWA actually improves survival.
In conclusion, aggressive MWA therapy of primary and metastatic lesions in selected NSCLC patients with synchronous extracranial solitary metastasis can result in long-term survival. Intra-thoracic positive nodal involvement appears as a significant clinical factor in predicting survival and patients with additional systemic therapy have better survival than those without. Prospective clinical trials, ideally randomized, should evaluate the role of MWA therapy in NSCLCs with synchronous extracranial solitary metastases.
Acknowledgements
This study has received funding by National Natural Science Foundation of China (81502610).
Compliance with ethical standards
Conflict of interest
The authors report no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ambrogi V, Tonini G, Mineo TC (2001) Prolonged survival after extracranial metastasectomy from synchronous resectable lung cancer. Ann Surg Oncol 8(8):663–666 [DOI] [PubMed] [Google Scholar]
- Collaud S, Stahel R, Inci I, Hillinger S, Schneiter D, Kestenholz P et al (2012) Survival of patients treated surgically for synchronous single-organ metastatic NSCLC and advanced pathologic TN stage. Lung Cancer 78(3):234–238 [DOI] [PubMed] [Google Scholar]
- Congedo MT, Cesario A, Lococo F, De Waure C, Apolone G, Meacci E et al (2012) Surgery for oligometastatic non-small cell lung cancer: long-term results from a single center experience. J Thorac Cardiovasc Surg 144(2):444–452 [DOI] [PubMed] [Google Scholar]
- de Baere T, Tselikas L, Catena V, Buy X, Deschamps F, Palussiere J (2016) Percutaneous thermal ablation of primary lung cancer. Diagn Interv Imaging 97(10):1019–1024 [DOI] [PubMed] [Google Scholar]
- Downey RJ, Ng KK, Kris MG, Bains MS, Miller VA, Heelan R et al (2002) A phase II trial of chemotherapy and surgery for non-small cell lung cancer patients with a synchronous solitary metastasis. Lung Cancer 38(2):193–197 [DOI] [PubMed] [Google Scholar]
- Griffioen GH, Toguri D, Dahele M, Warner A, de Haan PF, Rodrigues GB et al (2013) Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): patient outcomes and prognostic factors. Lung Cancer 82(1):95–102 [DOI] [PubMed] [Google Scholar]
- Hanagiri T, Takenaka M, Oka S, Shigematsu Y, Nagata Y, Shimokawa H et al (2012) Results of a surgical resection for patients with stage IV non-small-cell lung cancer. Clin Lung Cancer 13(3):220–224 [DOI] [PubMed] [Google Scholar]
- Hellman S, Weichselbaum RR (1995) Oligometastases. J Clin Oncol 13(1):8–10 [DOI] [PubMed] [Google Scholar]
- Hertzanu Y, Ye X (2019) Computed tomography-guided percutaneous microwave ablation: a new weapon to treat ground-glass opacity-lung adenocarcinoma. J Cancer Res Ther 15(2):265–266 [DOI] [PubMed] [Google Scholar]
- Luketich JD, Martini N, Ginsberg RJ, Rigberg D, Burt ME (1995) Successful treatment of solitary extracranial metastases from non-small cell lung cancer. Ann Thorac Surg 60(6):1609–1611 [DOI] [PubMed] [Google Scholar]
- Lussier YA, Xing HR, Salama JK, Khodarev NN, Huang Y, Zhang Q et al (2011) MicroRNA expression characterizes oligometastasis(es). PLoS ONE 6(12):e28650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordant P, Arame A, De Dominicis F, Pricopi C, Foucault C, Dujon A et al (2012) Which metastasis management allows long-term survival of synchronous solitary M1b non-small cell lung cancer? Eur J Cardiothorac Surg 41(3):617–622 [DOI] [PubMed] [Google Scholar]
- Ni Y, Liu B, Ye X, Fan W, Bi J, Yang X et al (2019) Local thermal ablation with continuous EGFR tyrosine kinase inhibitors for EGFR-mutant non-small cell lung cancers that developed extra-central nervous system (CNS) oligoprogressive disease. Cardiovasc Interv Radiol 42(5):693–699 [DOI] [PubMed] [Google Scholar]
- Ni Y, Ye X, Yang X, Huang G, Li W, Wang J et al (2020) Microwave ablation as local consolidative therapy for patients with extracranial oligometastatic EGFR-mutant non-small cell lung cancer without progression after first-line EGFR-TKIs treatment. J Cancer Res Clin Oncol 146(1):197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieder C, Haukland E, Mannsaker B, Pawinski AR, Yobuta R, Dalhaug A (2019) Presence of brain metastases at initial diagnosis of cancer: patient characteristics and outcome. Cureus 11(2):e4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palussiere J, Catena V, Buy X (2017) Percutaneous thermal ablation of lung tumors—radiofrequency, microwave and cryotherapy: where are we going? Diagn Interv Imaging 98(9):619–625 [DOI] [PubMed] [Google Scholar]
- Pusceddu C, Melis L, Sotgia B, Guerzoni D, Porcu A, Fancellu A (2019) Usefulness of percutaneous microwave ablation for large non-small cell lung cancer: a preliminary report. Oncol Lett 18(1):659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint LE, Tummala S, Brisson LJ, Francis IR, Krupnick AS, Kazerooni EA et al (1996) Distribution of distant metastases from newly diagnosed non-small cell lung cancer. Ann Thorac Surg 62(1):246–250 [DOI] [PubMed] [Google Scholar]
- Salah S, Tanvetyanon T, Abbasi S (2012) Metastasectomy for extra-cranial extra-adrenal non-small cell lung cancer solitary metastases: systematic review and analysis of reported cases. Lung Cancer 75(1):9–14 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34 [DOI] [PubMed] [Google Scholar]
- Solbiati LA (2018) A valuable guideline for thermal ablation of primary and metastatic lung tumors. J Cancer Res Ther 14(4):725–726 [DOI] [PubMed] [Google Scholar]
- Toffart AC, Duruisseaux M, Brichon PY, Pirvu A, Villa J, Selek L et al (2018) Operation and chemotherapy: prognostic factors for lung cancer with one synchronous metastasis. Ann Thorac Surg 105(3):957–965 [DOI] [PubMed] [Google Scholar]
- Tonnies M, Pfannschmidt J, Bauer TT, Kollmeier J, Tonnies S, Kaiser D (2014) Metastasectomy for synchronous solitary non-small cell lung cancer metastases. Ann Thorac Surg 98(1):249–256 [DOI] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB et al (2015) The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 10(9):1243–1260 [DOI] [PubMed] [Google Scholar]
- Vogl TJ, Nour-Eldin NA, Albrecht MH, Kaltenbach B, Hohenforst-Schmidt W, Lin H et al (2017) Thermal ablation of lung tumors: focus on microwave ablation. Rofo 189(9):828–843 [DOI] [PubMed] [Google Scholar]
- Wei Z, Yang X, Ye X, Huang G, Li W, Han X et al (2019) Camrelizumab combined with microwave ablation improves the objective response rate in advanced non-small cell lung cancer. J Cancer Res Ther 15(7):1629–1634 [DOI] [PubMed] [Google Scholar]
- Weichselbaum RR, Hellman S (2011) Oligometastases revisited. Nat Rev Clin Oncol 8(6):378–382 [DOI] [PubMed] [Google Scholar]
- Wong AC, Watson SP, Pitroda SP, Son CH, Das LC, Stack ME et al (2016) Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer 122(14):2242–2250 [DOI] [PubMed] [Google Scholar]
- Ye X, Fan W, Wang H, Wang J, Wang Z, Gu S et al (2018) Expert consensus workshop report: Guidelines for thermal ablation of primary and metastatic lung tumors (2018 edition). J Cancer Res Ther 14(4):730–744 [DOI] [PubMed] [Google Scholar]


