Abstract
Purpose
Retinoids have proved to be effective for hematologic malignancies treatment but till nowadays, their use as single agent for the solid tumor’s management is still controversial. All-trans retinoic acid (ATRA), the main active metabolite of vitamin A, exerts non-genomic interactions with different members of the protein kinase C (PKC) family, recognized modulators of different tumor progression pathways. To determine whether a group of patients could become benefited employing a retinoid therapy, in this study we have evaluated whether PKCα expression (a poor prognosis marker in breast cancer) could sensitizes mammary cells to ATRA treatment.
Methods
PKCα overexpression was achieved by stable transfection and confirmed by western blot. Transfected PKC functionality was determined by nuclear translocation-induction and confocal microscopy. In vitro proliferation was evaluated by cell counting and cell cycle distribution was analyzed by flow cytometry. In vivo studies were performed to evaluate orthotopic tumor growth and experimental lung colonization. Retinoic acid response elements (RARE) and AP1 sites-dependent activity was studied by gene reporter assays and retinoic acid receptors (RARs) were measured by RT-qPCR.
Results
Our findings suggest that high PKCα levels improve the differentiation response to ATRA in a RAR signaling-dependent manner. Moreover, RARβ expression appears to be critical to induce ATRA sensitization, throughout AP1 trans-repression.
Conclusion
Here we propose that retinoids could lead a highly personalized anticancer treatment, bringing benefits to patients with aggressive breast tumors resulting from high PKCα expression but, an adequate expression of the RARβ receptor is required to ensure the effect on this process.
Electronic supplementary material
The online version of this article (10.1007/s00432-020-03368-7) contains supplementary material, which is available to authorized users.
Keywords: PKCα, ATRA, Proliferation, Tumor growth, Metastasis dissemination
Introduction
Breast cancer is the most common malignancy in women, with about 2.1 million new cases and more than 600 thousand deaths worldwide in 2018 (Bray et al. 2018). Despite improvements in early diagnosis, surgical and adjuvant therapies, the mortality rate remains high. Therefore, it is imperative to continue searching novel approaches for breast cancer prevention, detection and treatment.
Retinoids are known for their antiproliferative, differentiative, immunomodulatory, and apoptosis-inducing effects (Gentilini et al. 2017; Gudas 1994). In this regard, all-trans retinoic acid (ATRA), the main active metabolite of Vitamin A, is nowadays used in clinical treatment of acute promyelocytic leukemia (APL), changing the clinical course of this disease (Tallman and Altman 2009). After the success in APL, both ATRA and synthetic retinoids were employed in clinical settings for the treatment of different solid malignancies, with controversial results (Decensi et al. 2009; Zanardi et al. 2006). Nevertheless, some phase II clinical trials are being evaluated nowadays (Dobrotkova et al. 2018; Garattini et al. 2014).
ATRA elicits genomic and non-genomic actions. Genomic effects occur through two kind of nuclear receptors: retinoic acid receptors (RARs-α, -β, and -γ) and retinoid X receptors (RXRs-α, -β, and -γ). Transcription activation occurs in a ligand-dependent manner and includes the formation of RAR/RXR heterodimers that bind to retinoic acid response elements (RAREs) located in the promoter regions of target genes (Tang and Gudas 2011).
Non-genomic effects remain poorly understood, however they have been reported in multiple studies (Al Tanoury et al. 2013), which include: effects on neuron spine formation (Chen and Napoli 2008), rapid activation of extracellular signal regulated kinase 1/2 (Gupta et al. 2008; Wu et al. 2013) and modulation of protein kinase C activity (Ochoa et al. 2003).
The protein kinase C (PKC) is a family of phospholipid-dependent serine/threonine kinases that influences a wide variety physiological process including proliferation, differentiation and apoptosis, through the phosphorylation of target proteins (Dekker and Parker 1994; Griner and Kazanietz 2007; Newton 2018; Nishizuka 1995). PKC isozymes have been classified into three groups on basis of their structural similarities and cofactor requirements: classical, novel and atypical (Garg et al. 2014; Newton 1995).
PKCα, classical PKC isozyme, has been long recognized as a key regulator of multiple aspects of tumor growth, including proliferation, survival, differentiation and motility. In many instances, a correlation between elevated PKCα expression/activation and tumor aggressiveness has been reported (Cameron et al. 2008; Haughian et al. 2009; Kong et al. 2005). Unfortunately, the clinical success of PKCα modulators is modest so far, mainly due the highly tissue-specific functions, as well as the fact that this kinase may act as tumor promoter or a tumor suppressor depending on the tissue context (Urtreger et al. 2012).
As mentioned above, the non-genomic effect of ATRA includes the interaction with different members of the PKC family. In this sense, several works found that ATRA not only induces cell arrest, but also activates the expression of different PKCs which in turn increase RARE transcriptional activity (Cho et al. 1997; Kambhampati et al. 2003). In line with this result, we have demonstrated that the pharmacological inhibition of PKCα and PKCδ activity in mammary cancer cells, prevented the activation RARs by ATRA (Berardi et al. 2015). The complex interaction between the retinoid system and the PKC family lies in the fact that others authors have described contradictroy findings. Delmotte et al. showed that PKCα and PKCγ can phosphorylate RARα on Ser157 reducing its ability to form an active heterodimer with RXR, afecting the expression of proteins involved in differentiation and/or cell growth inhibition (Delmotte et al. 1999). Other authors have proposed that ATRA can act as PKCα ligand, competing with the lipid activator, thus acting as a PKC inhibitor (Radominska-Pandya et al. 2000).
Within the context of the retinoids/PKC interaction, in this work we have evaluated whether PKCα overexpression sensitizes human tumor-derived mammary cells to ATRA treatment. Moreover, the underlying mechanism involved in the lack of sensitization was also analyzed, reinforcing the importance of the RARβ receptor in this process.
In sum, here we propose that ATRA may become an alternative to interfere with mammary tumors progression, particularly in a high PKCα expression context. Additionally, an adequate expression of the retinoid system components is required, to ensure the effect on this process.
Materials and methods
Reagents and antibodies
Media for cell culture and G418 were from Gibco BRL Laboratories (Rockville, MD). Fetal bovine serum (FBS) was from GBO (Buenos Aires, Argentina). Lipofectamine 2000 was purchased from Thermo Fisher Scientific (Waltham, MA). Monoclonal anti-PKCα was purchased from BD Biosciences (San Diego, CA). Acrylamide, horseradish peroxidase conjugated anti-mouse antibodies, ATRA and phorbol 12-myristate 13-acetate (PMA) were obtained from Sigma (St. Louis, MO). Hybond-P membranes and chemiluminescence reagents (ECL) were from GE Healthcare Bio-Sciences (Little Chalfont, UK). Other reagents for polyacrylamide gel electrophoresis were obtained from Bio-Rad (Richmond, CA).
Cell lines and culture conditions
The human breast cancer cell line T47D, positive for estrogen and progesterone expression and the triple negative MDA-MB231 cell line, were obtained from ATCC. Cells routinely maintained in Dulbecco’s modified Eagle’s medium (DMEM/F12) supplemented with 10% FBS and gentamicin (80 μg/ml) in a humidified atmosphere with 5% CO2 in the air. The murine mammary HER2 positive cell line LM3 (Urtreger et al. 1997) was cultured in minimum essential medium (MEM) with the same supplement and conditions mentioned above. Serial passages were conducted treating the monolayers with 0.25% trypsin (Invitrogen, Carlsbad, CA) and 0.02% EDTA in Ca2+ free and Mg2+ free phosphate-buffered saline (PBS) twice a week.
Expression vectors, transfection and selection
T47D and MDA-MB231 cells were stably transfected with 5 μg of pGFPN1-PKCα, a mammalian expression vector encoding for PKCα, using Lipofectamine 2000 (obtaining the T47D-PKCα and MDA-PKCα sub-lines, respectively). Both cell lines, transfected with the empty vector were used as control (T47D-Vector and MDA-Vector). Forty-eight hours after transfection, cells were selected by adding G418 to the culture medium (500 μg/ml). After selection, G418-resistant clones corresponding to each transfectant were pooled to avoid clonal variations. Same procedure was performed using the murine cell line LM3 to generate LM3-PKCα and develop the in vivo assays. In this case, we employ pMTH-PKCα, a mammalian expression vector encoding for murine PKCα isoform. LM3 cells, transfected with the empty vector, were used as control. Transfection assays were carried out in at least two independent experiments and cells were maintained in culture for 10–12 passages and then discarded. For transient expression of RARβ isotype in MDA-MB231 cells, the pcDNA3.1-RARβ expression vector was used, employing the transfection conditions described above. Same vector, without RARβ insert was used as control.
Western blot
Semiconfluent monolayers were washed twice with ice-cold PBS and then lysed with 1% Triton X-100 in PBS by scrapping with a Teflon scrapper. Samples were denatured by boiling in sample buffer with 5% β-mercaptoethanol and run in 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Fifty μg of protein was loaded in each lane and gels were blotted to Hybond-P membranes. Membranes were blocked by incubation during 1 h with PBS containing 5% skim-milk plus 0.1% Tween-20. Then membranes were incubated with the first antibody overnight at 4 °C, and finally with a secondary antibody coupled to horseradish peroxidase. Detection was performed by chemiluminescence. Bands were digitalized with a Photo/Analyst Express System (Fotodyne Inc. Hartland, WI) and signal intensity was quantified with Gel-Pro Analyzer software.
Evaluation of transfected PKC functionality
Semiconfluent monolayers of transfected T47D cells grown on glass coverslides were treated with PMA (50 nM) up to 5 min and then fixed with 10% formaldehyde in PBS for 15 min. Since the expression vector results in a PKCα-GFP fusion protein, PKCα localization was analyzed by confocal microscope employing an LSM 510 Meta confocal Zeiss microscope and processed with ImageJ software. The appearance of fluorescent mark on the cell membrane is indicative of an efficient expression of a phorbol ester responsive PKCα.
Proliferation assays
Proliferative potential was determined by assessing transfected T47D and MDA-MB231 cell number during the exponential growth phase of unsynchronized monolayer cultures. Briefly, 1 × 105 cells were seeded onto 35 mm Petri dishes in DMEM/F12 supplemented with 10% FBS, 80 μg/ml gentamicin and 250 μg/ml G418 (n = 3). At different times after seeding, cells were washed twice with PBS, trypsinized, counted using a hemocytometer and trypan blue exclusion and population doubling time was determined.
Analysis of cell cycle distribution by flow cytometry
T47D and MDA-MB231 transfectants were treated with ATRA (1 µM) or vehicle for 72 h. Then cells were detached and fixed with 70% ice cold ethanol. After staining with propidium iodide (100 mg/ml), samples were examined for DNA content by flow cytometry using an Epics Elite ESP coulter cytometer (Beckman coulter, Fullerton, CA).
Gene reporter assay
MCF7 and T47D transfectants were seeded onto 48-well plates (1 × 105/well). Twenty-four hours later, cells were transfected with RARE (PTK-RARE-Luc) or AP1 (PTK-TRE-Luc) reporter plasmids together with pTK-Renilla vector as control, using Lipofectamine 2000 according to manufacturer instructions. Lipofectamine reagent, DNA ratio was 3:1. Firefly and renilla luciferase activities were determined 48 h later employing the dual luciferase assay kit (Promega, Madison, WI) and measuring luminescence with a Wallac Micro-Beta scintillation counter (PerkinElmer Life Sciences Richmond, CA). Firefly luciferase reporter activity was normalized to the renilla luciferase activity.
Quantitative real-time PCR (qRT-PCR)
Total RNA was prepared using Tri Reagent (Merck, Darmstadt, Germany). cDNA was prepared with the iScript cDNA synthesis kit and amplified by real-time PCR using a CFX96 Real-Time PCR detection systems kit (Bio-Rad Richmond, CA) and SYBR green PCR master mix (Applied Biosystems, Carlsbad, CA). PCR products were obtained using the primers indicated in table S1, which correspond to mouse RAR α, β, γ and GAPDH (used as housekeeping gene). Relative changes in gene expression were calculated with the 2-∆∆CT or 2-∆RAR∆CT method (Livak and Schmittgen 2001).
Animals
For in vivo assays, randomized inbred female BALB/c mice, 2–4 months old (20–25 g), obtained from the Animal Care Division of the Institute of Oncology “A. H. Roffo” were employed. Animals were kept five per cage, under automatic 12 h light/12 h darkness schedule, and provided with sterile pellets and tap water ad libitum. For housing, rectangular polycarbonate cages (20 × 29 × 14 cm) with irradiated pine wood (15 KGy) as bed were employed. All animal studies were conducted in accordance with the standards of animal care as outlined in the NIH and ARRIVE Guidelines for the Care and Use of Laboratory Animals. Besides, protocols have the approval of the Committee for the Use and Care of laboratory Animals (CICUAL) of the Institute of Oncology “A. H. Roffo”, University of Buenos Aires.
Animals were euthanized by CO2 inhalation at the experimental endpoint. We established the human endpoint when mice met one of the following signs: Loss of > 20% of the initial weight, lethargy, bristling coat and/or hemorrhagic diarrhea.
Orthotopic tumor growth
Mice were orthotopically inoculated into the fat pad of the 4th mammary gland with 2 × 105 of LM3 transfectants. The order of injection was randomized to eliminate any difference that may bias the outcome. When tumors became detectable, 15 days after cell inoculation, each mouse received a subcutaneous silastic pellet containing ATRA (10 mg) or an empty pellet as control. Mice were monitored daily and tumor diameters were measured with a sliding caliper twice a week. Tumor volume was calculated using the following formula: V = ¾(π × L × 2 W), where L is the longest and W is the shortest diameter. Forty days after tumor inoculation, mice were sacrificed and necropsied. Tumors were fixed in 10% formalin, embedded in paraffin and 5 μm sections were mounted on glass slides and stained with hematoxylin and eosin for histopathological studies.
Lung colonization assay
To study the effect of ATRA on lung colonization, an experimental metastasis assay was performed as previously described (Grossoni et al. 2009). Five days before cell inoculation each mouse received a subcutaneous silastic pellet containing ATRA (10 mg) or an empty pellet as control. Then 2 × 105 cells were injected into the tail vein using a 27-gauge needle. Mice were monitored daily and sacrificed 21 days later. Lungs were removed, fixed in Bouin’s solution and the number of superficial lung nodules was counted under a dissecting microscope.
Statistical analysis
All assays were performed in triplicate, and independent experiments repeated at least twice. Statistical differences between groups were calculated by applying ANOVA, Student’s t test or Krustal-Wallis tests, as indicated in each case. A p value < 0.05 was considered significant.
Results
Overexpression of PKCα in human cell lines
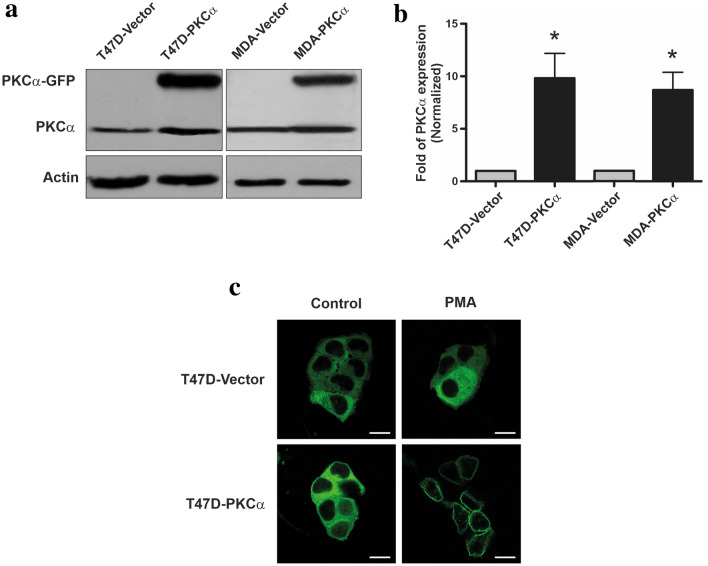
To investigate whether high PKCα expression levels sensitize human tumor-derived cell lines to ATRA treatment; a stable transfection approach was employed. T47D and MDA-MB231 cells were transfected with the pGFPN1 expression vector alone (as a control) or containing the complete sequence of the human PKCα isoform. Western blot analysis revealed the presence of a specific band corresponding to PKCα-GFP in PKCα-transfected cells but not in vector transfected ones (Fig. 1a). Considering the endogenous expression, we achieved a ~ tenfold increase in PKCα expression relative to control transfectants in both cell lines (Fig. 1b). The overexpression of PKCα did not alter the expression of other PKC isozymes, present in T47D and MDA-MB231 cells (data not shown). All cell lines obtained after separated transfection experiments showed similar expression levels and biological properties described below.
Fig. 1.
Overexpression of PKCα in T47D and MDA-MB231 cells. a Whole cell lysates prepared from T47D and MDA-MB231 cells transfected with pGFPN1-PKCα or pGFPN1 (vector alone) were resolved on 10% SDS-PAGE and blotted with an anti-PKCα antibody (50 μg protein/lane). b Densitometric analysis of PKC overexpression. Results were expressed in fold of PKCα expression relative to vector-transfected cells expression. Each data point represents the mean ± S.D. three independent experiments. *p < 0.05 versus the respective vector-transfected cell line (ANOVA test). c T47D cells transfected with pGFPN1-PKCα or pGFPN1 vector alone were incubated with PMA (50 nM) for 10 min, fixed and PKCα-GFP localization was analyzed by confocal microscope (scale bar: 50 μm). Results are representative of three independent experiments
Since the expression vector results in a PKCα-GFP fusion protein, we could confirm by confocal microscopy that high percentage of cells (> 85%) overexpress this kinase (Fig. 1c). Moreover, the treatment of T47D-PKCα cells with phorbol 12-myristate 13-acetate (PMA) induced the translocation of the transfected PKC from the cytosol to the membrane in less than 3 min after activation (Fig. 1c). Similar results were observed in MDA-PKCα cells (data not shown). These results suggested that PKCα ectopically expressed is phorbol ester responsive and functionally active.
ATRA induces grow inhibition and cell cycle arrest in T47D-PKCα cells
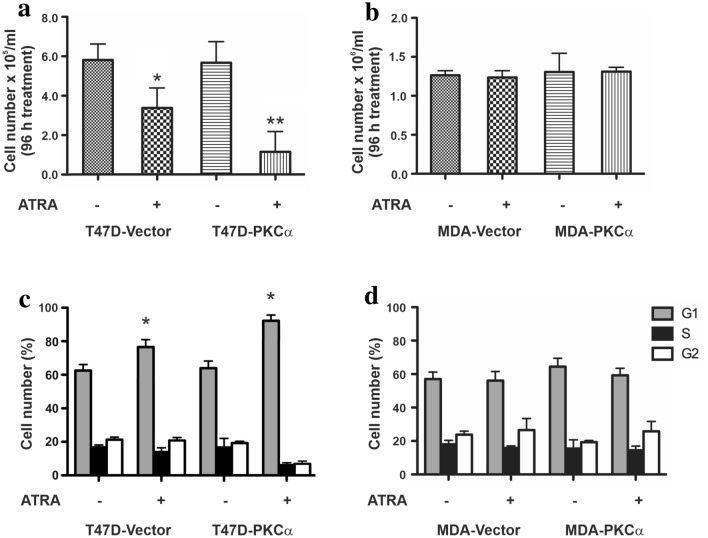
To determine whether PKCα overexpression alters the response to ATRA, we analyzed the proliferative capacity of T47D and MDA-MB231 transfectants after 96 h treatment. T47D cells, showed an important increase in population doubling time (PDT) from 18.5 ± 0.6 h to 24.3 ± 0.7 h (p < 0.05), after ATRA treatment. These differences were further increased, from 19.1 ± 0.5 h to 27.4 ± 0.5 h, upon PKCα overexpression (p < 0.05). Although, the overexpressed PKCα did not modulate the proliferative potential of T47D cells itself (Fig. 2a), it clearly enhances ATRA cytostatic effect showing a significant decrease in cultures cell content (Fig. 2a) and an increase in the percentage of cells in the G1 phase of the cell cycle (Fig. 2c). On the other hand, ATRA did not alter MDA-MB231 proliferative potential, even when these cells overexpress PKCα (PDT: 18.6 ± 0.8 and 19.2 ± 0.4 h, respectively). No changes in cell number (Fig. 2b) or cell cycle arrest could be detected after ATRA treatment (Fig. 2d).
Fig. 2.
Effect on in vitro proliferation and cell cycle progression of T47D and MDA-MB231 transfectants. a, b Cell number was assessed 96 h after the treatment with ATRA (1 µM). Each data point represents the mean ± S.D. of triplicate determinations. *p < 0.05 versus untreated T47D-Vector cells, **p < 0.01 versus untreated T47D-PKCα cells (ANOVA test). At least three independent experiments were performed with similar results. c, d T47D and MDA-MB231 cells overexpressing or not PKCα were treated with ATRA (1 µM) for 48 h and then stained with propidium iodide to analyze cycle distribution by flow cytometry. Each data point represents the mean ± S.D. of triplicate determinations. *p < 0.05 versus the respective untreated transfectant (ANOVA test). At least three independent experiments were performed with similar results
PKCα overexpression induces a highly aggressive tumor phenotype, although ATRA-sensitive
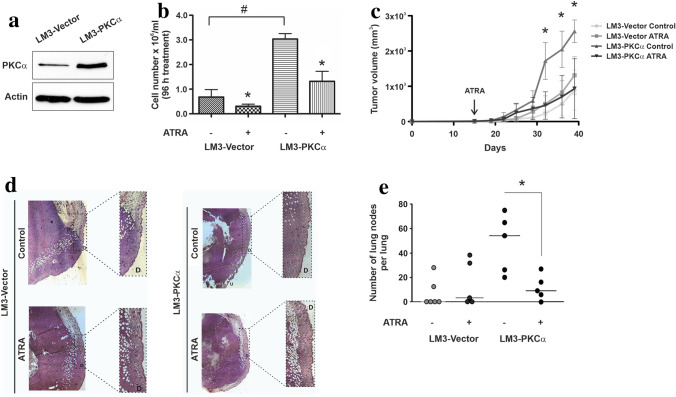
Employing the LM3 mammary tumor model, BALB/c syngeneic, we studied the existence of an in vivo correlation with the results obtained in vitro. This murine cell line was also transfected with a PKCα encoding vector, generating the LM3-PKCα cell line which displays a similar increase in PKCα expression (~ eightfold) described for the human mammary cell lines (Fig. 3a). In this murine model, PKCα overexpression per se significantly increased the in vitro proliferative potential as already described for other cell models (Haughian et al. 2009; Kong et al. 2005; Wu et al. 2013). Interestingly, the proliferative capacity was highly reduced upon ATRA treatment (Fig. 3b).
Fig. 3.
Effect of PKCα overexpression on tumor growth and lung colonization in a murine experimental model. a Whole cell lysates prepared from LM3-Vector or LM3-PKCα cells were resolved on 10% SDS-PAGE and blotted with anti-PKCα antibodies (50 μg/lane). Actin expression level was used as protein loading control. A representative experiment is shown. b Cell number was assessed 96 h after ATRA (1 µM) treatment. Each data point represents the mean ± S.D. of triplicate determinations, *p < 0.05 versus the respective untreated LM3 transfectant, #p < 0.05 versus untreated LM3-Vector cells (ANOVA test). At least three independent experiments were performed with similar results. c LM3-PKCα and LM3-Vector transfected cells were harvested from subconfluent monolayers and orthotopically inoculated into the fat pad of BALB/c mice. Fifteen days later, animals receive a silastic pellet containing ATRA (10 mg) or an empty pellet as control. Tumor diameters were measured twice a week and used to calculate tumor volume. Each data point represents the mean ± S.D. (n = 6), *p < 0.05 versus ATRA-treated LM3-PKCα cells (ANOVA test). Two independent experiments were performed with similar results. d Effect of ATRA on histopathological features of LM3-PKCα and LM3-Vector tumors. Tumors were fixed in 10% formalin and slides were stained with hematoxylin and eosin (D: Normal dermis, U: Ulcerated dermis). e LM3 cells, overexpressing or not PKCα, were injected in the lateral tail vein of mice carrying a subcutaneous silastic pellet containing ATRA (10 mg) or an empty pellet as control. Each data point represents the number of lung nodules per animal. The figure shows the results of one experiment representative of three independent assays. *p < 0.05 versus untreated LM3-PKCα cells (Kruskall-Wallis test)
On the other hand, in vivo tumor progression showed that LM3 cells overexpressing PKCα are markedly affected by ATRA treatment unlike tumors derived from the inoculation of LM3 cells transfected with the empty vector (Fig. 3c). Furthermore, the overexpression of PKCα itself induces the development in vivo of highly proliferative tumors that were histopathological aggressive, even ulcerating the dermis (Fig. 3d). On the contrary, upon ATRA treatment LM3-PKCα derived tumors showed a reduced necrotic center and were unable to invade dermis and epidermis layers (Fig. 3d). No histological differences were found in tumors generated from LM3-Vector transfected inoculation, treated or not with ATRA.
Next we have evaluated the effect of ATRA on experimental tumor cell dissemination. For this purpose, transfected LM3 cells were injected in the lateral tail vein of BALB/c mice, and 21 days later mice were sacrificed, and lung nodules were recorded. Interestingly, ATRA treatment significantly reduced the number of pulmonary nodules only in PKCα overexpressing cells, as compared to the control group (Fig. 3e). No significant changes in lung nodes number could be detected in LM3-Vector cells treated or not with ATRA.
ATRA treatment modulates RARE and AP-1 sites in a differential manner due to PKCα expression
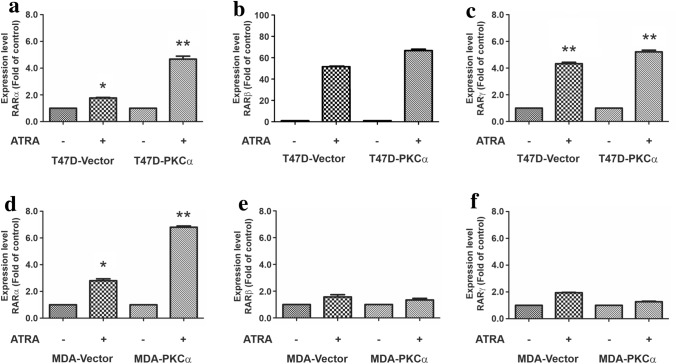
The previous results showed an inhibitory effect of ATRA treatment on in vitro and in vivo proliferation in a high PKCα-expression environment. Conversely, MDA-MB231 cells proved to be ATRA insensitive, either overexpressing or not PKCα. These differences led us to evaluate the existence of a differential ability of retinoic receptors to activate RARE promoter sequences in response to ATRA treatment. Through a gene reporter assay, we could observe that T47D responds to ATRA showing an increase in RARE-dependent luciferase activity. This increase was even higher when cells overexpress PKCα (Fig. 4a).
Fig. 4.
Evaluation of RARE an AP-1-dependent activity through reporter gene assays. a, b T47D and MDA-MB231 cells overexpressing or not PKCα were transfected with the TK luciferase plasmid containing the retinoic acid response element. Cells were co-transfected with an expression vector encoding renilla luciferase in a 1:10 ratio. After transfection cells were treated with ATRA (1 µM) and luciferase/renilla ratio was determined. Each data point represents the mean ± S.D. of triplicate determinations. **p < 0.01 versus the respective untreated transfectant, #p < 0.05 versus ATRA-treated T47D-Vector cells (ANOVA test). Three independent experiments were performed with similar results. c, d T47D and MDA-MB231 overexpressing or not PKCα were transfected with TK luciferase plasmid containing AP-1 sites. Cells were co-transfected with expression plasmids encoding renilla in a 1:10 ratio. After transfection cells were treated or not with ATRA (1 µM) and luciferase/renilla ratio was determined. Each data point represents the mean ± S.D. of triplicate determinations. *p < 0.05 and **p < 0.01 versus the respective untreated T47D transfectant (ANOVA test). Three independent experiments were performed with similar results
Surprisingly, in MDA-MB 231 cells, ATRA also increased luciferase activity indicating a significant activation of retinoic acid receptors (Fig. 4b). In this case, overexpression of PKCα did not increase basal retinoic acid receptors activity.
Since it has been reported that some of the effects induced by retinoids are not only consequence of a RARE activity increase, we decided to focus on AP-1 trans-repression also, through a gene reporter assay. Upon ATRA treatment, an important decrease in AP-1-dependent luciferase activity could be detected in T47D cells (Fig. 4c). Again, as already observed in RARE luciferase activity, AP-1 modulation was higher when cells overexpress PKCα. On the other hand, in MDA-MB231-treated cells, no AP-1 trans-repression could be observed, even when the cells overexpress PKCα (Fig. 4d).
RARα expression levels are highly increased by ATRA when PKCα is overexpressed
To get insight the differences observed between the cell lines in the biological response to ATRA, next we study through qPCR, the expression levels of the different retinoid receptors under basal conditions and after ATRA treatment. In both cell lines, RARα expression level increases upon ATRA treatment (Fig. 5). Moreover, PKCα overexpression significantly increases RARα levels after ATRA treatment in both cell lines (Fig. 5a–d). On the other hand, RARβ and RARγ also respond to ATRA treatment with increased expression in T47D cells (Fig. 5b, c). Both receptors were almost undetectable in constitutive conditions and a highly expressed after the treatment. In MDA-MB231 cells, no increase in RARβ or RARγ levels could be detected in response to ATRA treatment neither in vector cells nor in PKCα overexpressing cells (Fig. 5e, f).
Fig. 5.
Modulation of RAR isotypes expression in T47D and MDA-MB231 trasnfectants. T47D and MDA-MB231 cells transfected with PKCα or the empty vector as control were treated or not with ATRA (1 µM) and the different RAR isotypes expression was analyzed by qPCR (RARα panels a and d, RARβ panels b–e and RARγ panels c–f). *p < 0.05 and **p < 0.01 versus the respective untreated transfectant (ANOVA test). Three independent experiments were performed with similar results
Rescue of AP-1 sites trans-repression by RARβ re-expression impairs MDA-MB231 cells proliferation
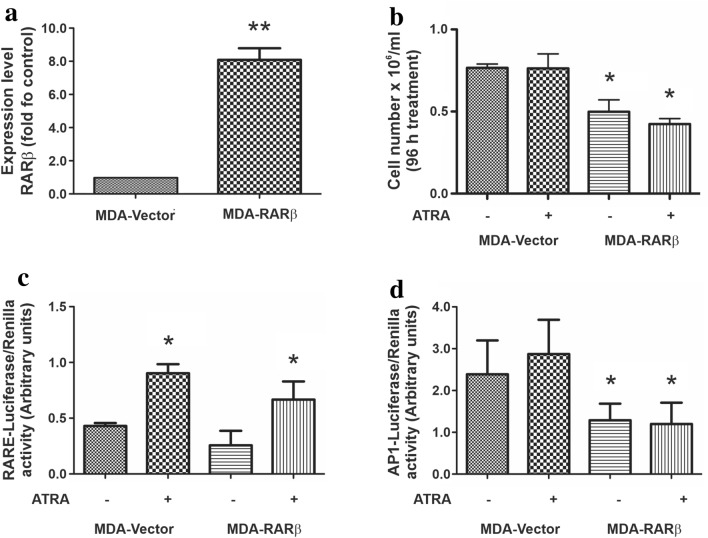
Given that in MDA-MB231 cells RARs were incapable of trans-repress AP-1 sites upon ATRA treatment, next we investigated whether RARβ could restore this ability. Although both RARβ and RARγ levels are practically undetectable in this cell line, we chose to express RARβ since this retinoid receptor has been described as the main responsible of trans-repressing activities (Lin et al. 2000). As shown in Fig. 6a, we could induce an important increase in RARβ expression upon transfection. Next, we perform a new proliferation assay but, in this case, treating MDA-RARβ and MDA-Vector cells with ATRA for 96 h. Cells re-expressing RARβ showed a reduced proliferation rate even when they were not exposed to ATRA (Fig. 6b).
Fig. 6.
Role of RARβ on MDA-MB231 response to ATRA treatment. a MDA-MB231 cells were transfected with RARβ or with the empty vector as control. RARβ expression levels were evaluated by RT-qPCR. **p < 0.01 versus vector transfected cells (Student’s t test). b Cell number was assessed 96 h after the treatment with ATRA (1 µM). Each data point represents the mean ± S.D. of triplicate determinations, *p < 0.05 versus MDA-Vector cells that received the same treatment (ANOVA test). At least three independent experiments were performed with similar results. c MDA-MB231 expressing or not RARβ were transfected with the TK luciferase plasmid containing the retinoic acid response element. Cells were co-transfected with an expression vector encoding renilla luciferase in a 1:10 ratio. After transfection cells were treated with ATRA (1 µM) and luciferase/renilla ratio was determined. Each data point represents the mean ± S.D. of triplicate determinations. *p < 0.05 versus the respective untreated transfectant (ANOVA test). Three independent experiments were performed with similar results. d MDA-MB231 expressing or not RARβ were transfected with the TK luciferase plasmid containing AP1 sites. Cells were co-transfected with expression plasmids encoding renilla in a 1:10 ratio. After transfection, cells were treated with ATRA (1 µM) and luciferase/renilla ratio was determined. Each data point represents the mean ± S.D. of triplicate determinations. *p < 0.05 versus MDA-Vector cells that received the same treatment (ANOVA test). Three independent experiments were performed with similar results
To study whether the effects on cell proliferation could involve RARβ activity, we performed the gene reporter assays employing the RARβ transfectants. As already observed in MDA-MB231 wild-type cells, RARE-luciferase activity increased when cells were treated with ATRA and no further modulations could be detected in response to RARβ overexpression (Fig. 6c). On the other hand, a significant reduction in AP-1 luciferase activity could be detected in MDA-MB231 cells expressing RARβ, whether treated or not with ATRA (Fig. 6d), indicating that RARβ expression could be essential to restore ATRA sensitivity.
Discussion
Since ATRA has been approved for the management for chronic myeloid leukemia (Castaigne et al. 1990) there have been great efforts to achieve a retinoid-based treatment for solid tumors. The fact that breast cancer is a complex disease with a wide variety of subgroups with different histopathological and genetic characteristics, results in diverse responses to retinoids treatment and this is probably the reason for their clinical failure. Only few reports evidence a positive impact of retinoids in the metastatic dissemination of solid tumors (Simon et al. 2002; Zanardi et al. 2006).
In this work, we analyzed the implication of two different molecules in the response to ATRA. On one hand PKCα, a classical PKC isoform whose interaction with the retinoid system was described by us as well as other authors (Berardi et al. 2015; Cho et al. 1997; Delmotte et al. 1999; Kambhampati et al. 2003; Radominska-Pandya et al. 2000) and on the other hand, the β isotype of the retinoid acid receptor whose expression has proved to be crucial for the antiproliferative effect of retinoids. The importance of RARβ in tumor progression can be inferred since this receptor is silenced in several types of cancer (Gao et al. 2013; Picard et al. 1999; Wang et al. 2003; Widschwendter et al. 2000; Youssef et al. 2004).
Human breast carcinomas usually present a differential expression of PKC isoforms (Ali et al. 2009). Although in breast cancer PKCα is considered a poor prognosis marker due to its association with a reduced disease-free survival and relapse, neither T47D-PKCα cells nor MDA-MB231-PKCα cells presented higher aggressiveness in vitro than their respective control counterparts. However, a clear increase in the malignant potential could be evidenced employing the murine cell model.
In vitro, T47D cells responded to ATRA treatment diminishing its proliferation rate. Nevertheless, PKCα overexpression sensitized these cells to ATRA treatment, inducing a marked inhibition of cell growth, possibly through the cell cycle arrestment. This result clearly confirms the evidences postulated by us as well as other authors of the existence of a crosstalk between PKC and retinoid pathways (Berardi et al. 2015; Cho et al. 1997; Delmotte et al. 1999; Kambhampati et al. 2003; Radominska-Pandya et al. 2000; Wu et al. 2017).
We have previously demonstrated that retinoid treatment increases PKCα expression (Berardi et al. 2015) and an exhaustive analysis revealed that the PKCα gene promoter indeed contains RARE sites (Desai et al. 1999). Despite the modulation of PKCα gene expression, Talmage and coworkers have demonstrated that ATRA interacts with PKCα through its calcium binding domain and this unconventional interaction leads the inhibition of cell proliferative processes (Tighe and Talmage 2004).
Regarding growth inhibition, MDA-MB231 is a well-known ATRA-unresponsive cell model. The rationale behind overexpressing PKCα was to evaluate whether these cells could become retinoid sensitive since this PKC isoform is capable of activating RARα (Berardi et al. 2015). Upon translocate to the nucleus, RARα participates in RARβ and γ expression, so the lack of some of these components makes all retinoid system fail. Although, our results indicate that PKCα is not enough to guarantee this sensitivity we could establish a pattern of biological features, which include the trans-repression of AP-1 sites by RARβ, to predict a positive response to the retinoid differentiating therapy. In this sense, the re-expression of RARβ allowed the rescue of the retinoid system that led to reduce cell proliferation.
To confirm whether PKCα participates in the activation of retinoic acid receptors a reporter gene assays was performed. We found that in T47D cells, high PKCα levels favor the expression of genes with RARE sequences in their promoters and this effect was even more pronounced after ATRA stimulation. This correlates with the enhanced proliferation sensitivity to ATRA observed in this cell line. However, we found that MDA-MB231 cells can also activate the expression from RARE sequences when they are exposed to retinoids. Although this result is unexpected, since this is the first step in the response to ATRA, it would be indicating that in these cells RARs are active and functional.
Another well-known transcriptional consequence of retinoid receptors activation is their repressive activity on AP-1 sites (Dedieu and Lefebvre 2006). AP-1 is a transcription factor composed of multiple Jun (c-Jun, JunB, and JunD) and Fos (c-Fos, FosB, Fra1, and Fra2) members, implicated in the regulation of various physiological and pathological cellular processes including proliferation, differentiation, growth, apoptosis, cell migration, and transformation (Shaulian 2010). Tonetti and collaborators reported that PKCα overexpression leads to a higher AP-1 activity in T47D cells (Tonetti et al. 2000). In coincidence, here we report a higher AP-1 activity in T47D-PKCα cells, but the inhibition achieved in response to ATRA was higher in these cells as compared to vector ones. On the other hand, in MDA-MB231 no AP-1 inhibition could be detected, even when the express high PKCα levels.
The RARβ receptor has been described as the main responsible of AP-1 inhibition (Karamouzis et al. 2004; Lin et al. 2000; Yang et al. 1997), thus we next analyzed the expression of all RAR components in both cell models.
As expected, T47D cells express α, β, and γ RAR isotypes and their levels were increased after ATRA treatment. Moreover, when T47D-PKCα cells were exposed to the retinoid, RARα significantly increased its expression as compared to T47D-Vector. The increase in this RAR could be responsible for both the higher RARE luciferase activity and, as this receptor is involved in cell differentiation, for the cell cycle arrest observed. MDA-MB231 cells also responded to ATRA increasing RARα expression, especially when cells overexpress PKCα, but RARβ and γ levels were extremely low and no modulation could be detected after the treatment (Stefanska et al. 2010). The absence of these receptor allows AP-1 sites to remain functional despite retinoid treatment. In this sense, it is important to note that the RARβ isotype is highly implicated in the retinoid-induced antiproliferative/differentiating phenotype (Flamini et al. 2014). To corroborate the impact of the RARβ isotype on biological features that lead to a differentiated status, we have analyzed the effect of its re-expression in MDA-MB231 cells. Under this condition, both proliferation and AP-1 activity were diminished, proving the fundamental function of this receptor in retinoids-mediated antiproliferative effect. The clear response, even in the absence of ATRA treatment, is consistent the constitutive expression of RARβ, achieved through the transfection procedure.
To look for an in vivo correlation that summarizes our in vitro findings, we performed studies employing the LM3 experimental model, syngeneic in BALB/c mice. While ATRA induced a modest effect on vector transfected LM3 cells, tumor growth was significantly impaired in a PKCα overexpressed context. Moreover, the invasion of adjacent tissues was also reduced under this condition. Even though in the murine model PKCα overexpression increases the malignant potential, this kinase highly favors ATRA response both in vivo and in vitro, as observed in T47D cells. Thus, retinoid therapy would be more effective in a high PKCα levels context. However, RARβ expression is another important player to be considered since it plays a very important role in the response to retinoids, the trans-repression of AP-1 sites.
In conclusion, our results support the idea of using retinoids as a highly personalized treatment bringing benefits to patients with aggressive breast tumors, resulting from the expression of PKCα. Nevertheless, significant levels of the RARβ receptor are also required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Dr. Elisa Bal de Kier Joffé for her constant support and useful discussions.
Abbreviations
- ATRA
All-trans retinoic acid
- RA
Retinoic Acid
- RAR
Retinoic acid receptor
- RARE
Retinoic acid response elements
- PKC
Protein Kinase C
- DAG
Diacylglycerol
- FBS
Fetal bovine serum
- PBS
Phosphate-buffered saline
- PAGE
Polyacrylamide gel electrophoresis
- SDS
Sodium dodecyl sulfate
- PMA
Phorbol 12-myristate 13-acetate
Author contributions
MIDB and DEB carried out all experiments, prepared figures and drafted the manuscript. SMC, DID and MGP participated in data analysis and interpretation of results AJU and LBT conceived the study and participated in data analysis. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the Instituto Nacional del Cancer and CONICET (PIP No. 11220150100320 CO).
Data availability
All the data generated during this study are included in this article.
Compliance with ethical standards
Conflict of interest
The authors read and approved the manuscript and declare that they have no competing interests.
Ethics approval and consent to participate
All animal studies were conducted in accordance with the standards of animal care as outlined in the NIH and ARRIVE Guidelines for the Care and Use of Laboratory Animals. Protocols have the approval of the Committee for the Use and Care of laboratory Animals (CICUAL) of the Institute of Oncology “A. H. Roffo”, University of Buenos Aires.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
María Inés Díaz Bessone and Damián Emilio Berardi made equal contributions to the manuscript.
Laura Beatriz Todaro and Alejandro Jorge Urtreger share senior authorship.
References
- Al Tanoury Z, Piskunov A, Rochette-Egly C (2013) Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res 54:1761–1775. 10.1194/jlr.R030833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Al-Sukhun S, El-Rayes BF, Sarkar FH, Heilbrun LK, Philip PA (2009) Protein kinases C isozymes are differentially expressed in human breast carcinomas. Life Sci 84:766–771. 10.1016/j.lfs.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi DE, Bessone MI, Motter A, de Kier B, Joffe ED, Urtreger AJ, Todaro LB (2015) Involvement of protein kinase C alpha and delta activities on the induction of the retinoic acid system in mammary cancer cells. Mol Carcinog 54:1110–1121. 10.1002/mc.22181 [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA. Cancer J Clin 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Cameron AJ, Procyk KJ, Leitges M, Parker PJ (2008) PKC alpha protein but not kinase activity is critical for glioma cell proliferation and survival. Int J Cancer 123:769–779. 10.1002/ijc.23560 [DOI] [PubMed] [Google Scholar]
- Castaigne S, Chomienne C, Daniel MT, Ballerini P, Berger R, Fenaux P, Degos L (1990) All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I Clin Results Blood 76:1704–1709 [PubMed] [Google Scholar]
- Chen N, Napoli JL (2008) All-trans-retinoic acid stimulates translation and induces spine formation in hippocampal neurons through a membrane-associated RARalpha. FASEB J 22:236–245. 10.1096/fj.07-8739com [DOI] [PubMed] [Google Scholar]
- Cho Y, Tighe AP, Talmage DA (1997) Retinoic acid induced growth arrest of human breast carcinoma cells requires protein kinase C alpha expression and activity. J Cell Physiol 172:306–313. 10.1002/(SICI)1097-4652(199709)172:3<306:AID-JCP4>3.0.CO;2-S [DOI] [PubMed] [Google Scholar]
- Decensi A et al (2009) Randomized double-blind 2 x 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol 27:3749–3756. 10.1200/JCO.2008.19.3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedieu S, Lefebvre P (2006) Retinoids interfere with the AP1 signalling pathway in human breast cancer cells. Cell Signal 18:889–898. 10.1016/j.cellsig.2005.08.001 [DOI] [PubMed] [Google Scholar]
- Dekker LV, Parker PJ (1994) Protein kinase C–a question of specificity. Trends Biochem Sci 19:73–77. 10.1016/0968-0004(94)90038-8 [DOI] [PubMed] [Google Scholar]
- Delmotte MH, Tahayato A, Formstecher P, Lefebvre P (1999) Serine 157, a retinoic acid receptor alpha residue phosphorylated by protein kinase C in vitro, is involved in RXR.RARalpha heterodimerization and transcriptional activity. J Biol Chem 274:38225–38231. 10.1074/jbc.274.53.38225 [DOI] [PubMed] [Google Scholar]
- Desai DS, Hirai S, Karnes WE Jr, Niles RM, Ohno S (1999) Cloning and characterization of the murine PKC alpha promoter: identification of a retinoic acid response element. Biochem Biophys Res Commun 263:28–34. 10.1006/bbrc.1999.1307 [DOI] [PubMed] [Google Scholar]
- Dobrotkova V, Chlapek P, Mazanek P, Sterba J, Veselska R (2018) Traffic lights for retinoids in oncology: molecular markers of retinoid resistance and sensitivity and their use in the management of cancer differentiation therapy. BMC Cancer 18:1059. 10.1186/s12885-018-4966-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamini MI, Gauna GV, Sottile ML, Nadin BS, Sanchez AM, Vargas-Roig LM (2014) Retinoic acid reduces migration of human breast cancer cells: role of retinoic acid receptor beta. J Cell Mol Med 18:1113–1123. 10.1111/jcmm.12256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T et al (2013) The association of retinoic acid receptor beta2(RARbeta2) methylation status and prostate cancer risk: a systematic review and meta-analysis. PLoS ONE 8:e62950. 10.1371/journal.pone.0062950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garattini E et al (2014) Retinoids and breast cancer: from basic studies to the clinic and back again. Cancer Treat Rev 40:739–749. 10.1016/j.ctrv.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Garg R, Benedetti LG, Abera MB, Wang H, Abba M, Kazanietz MG (2014) Protein kinase C and cancer: what we know and what we do not. Oncogene 33:5225–5237. 10.1038/onc.2013.524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilini LD et al (2017) Stable and high expression of Galectin-8 tightly controls metastatic progression of prostate cancer. Oncotarget 8:44654–44668. 10.18632/oncotarget.17963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griner EM, Kazanietz MG (2007) Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer 7:281–294. 10.1038/nrc2110 [DOI] [PubMed] [Google Scholar]
- Grossoni VC, Todaro LB, Kazanietz MG, de Kier Joffe ED, Urtreger AJ (2009) Opposite effects of protein kinase C beta1 (PKCbeta1) and PKCepsilon in the metastatic potential of a breast cancer murine model. Breast Cancer Res Treat 118:469–480. 10.1007/s10549-008-0299-4 [DOI] [PubMed] [Google Scholar]
- Gudas LJ (1994) Retinoids and vertebrate development. J Biol Chem 269:15399–15402 [PubMed] [Google Scholar]
- Gupta P et al (2008) Retinoic acid-stimulated sequential phosphorylation, PML recruitment, and SUMOylation of nuclear receptor TR2 to suppress Oct4 expression. Proc Natl Acad Sci USA 105:11424–11429. 10.1073/pnas.0710561105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughian JM, Reno EM, Thorne AM, Bradford AP (2009) Protein kinase C alpha-dependent signaling mediates endometrial cancer cell growth and tumorigenesis. Int J Cancer 125:2556–2564. 10.1002/ijc.24633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambhampati S et al (2003) Activation of protein kinase C delta by all-trans-retinoic acid. J Biol Chem 278:32544–32551. 10.1074/jbc.M301523200 [DOI] [PubMed] [Google Scholar]
- Karamouzis MV, Sotiropoulou-Bonikou G, Vandoros G, Varakis I, Papavassiliou AG (2004) Differential expression of retinoic acid receptor beta (RARbeta) and the AP-1 transcription factor in normal, premalignant and malignant human laryngeal tissues. Eur J Cancer 40:761–773. 10.1016/j.ejca.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Kong C et al (2005) Role of protein kinase C-alpha in superficial bladder carcinoma recurrence. Urology 65:1228–1232. 10.1016/j.urology.2005.01.007 [DOI] [PubMed] [Google Scholar]
- Lin F, Xiao D, Kolluri SK, Zhang X (2000) Unique anti-activator protein-1 activity of retinoic acid receptor beta. Cancer Res 60:3271–3280 [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method Methods 25:402–408. 10.1006/meth.2001.1262(1262 S1046-2023(01)91262-9 [pii]) [DOI] [PubMed] [Google Scholar]
- Newton AC (1995) Protein kinase C: structure, function, and regulation. J Biol Chem 270:28495–28498. 10.1074/jbc.270.48.28495 [DOI] [PubMed] [Google Scholar]
- Newton AC (2018) Protein kinase C: perfectly balanced. Crit Rev Biochem Mol Biol 53:208–230. 10.1080/10409238.2018.1442408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y (1995) Protein kinase C and lipid signaling for sustained cellular responses. FASEB J 9:484–496 [PubMed] [Google Scholar]
- Ochoa WF, Torrecillas A, Fita I, Verdaguer N, Corbalan-Garcia S, Gomez-Fernandez JC (2003) Retinoic acid binds to the C2-domain of protein kinase C(alpha). Biochemistry 42:8774–8779. 10.1021/bi034713g [DOI] [PubMed] [Google Scholar]
- Picard E et al (1999) Expression of retinoid receptor genes and proteins in non-small-cell lung cancer. J Natl Cancer Inst 91:1059–1066. 10.1093/jnci/91.12.1059 [DOI] [PubMed] [Google Scholar]
- Radominska-Pandya A, Chen G, Czernik PJ, Little JM, Samokyszyn VM, Carter CA, Nowak G (2000) Direct interaction of all-trans-retinoic acid with protein kinase C (PKC) Implications for PKC signaling and cancer therapy. J Biol Chem 275:22324–22330. 10.1074/jbc.M907722199 [DOI] [PubMed] [Google Scholar]
- Shaulian E (2010) AP-1–The Jun proteins: oncogenes or tumor suppressors in disguise? Cell Signal 22:894–899. 10.1016/j.cellsig.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Simon D et al (2002) Clinical impact of retinoids in redifferentiation therapy of advanced thyroid cancer: final results of a pilot study. Eur J Nucl Med Mol Imaging 29:775–782. 10.1007/s00259-001-0737-6 [DOI] [PubMed] [Google Scholar]
- Stefanska B, Rudnicka K, Bednarek A, Fabianowska-Majewska K (2010) Hypomethylation and induction of retinoic acid receptor beta 2 by concurrent action of adenosine analogues and natural compounds in breast cancer cells. Eur J Pharmacol 638:47–53. 10.1016/j.ejphar.2010.04.032 [DOI] [PubMed] [Google Scholar]
- Tallman MS, Altman JK (2009) How I treat acute promyelocytic leukemia. Blood 114:5126–5135. 10.1182/blood-2009-07-216457 [DOI] [PubMed] [Google Scholar]
- Tang XH, Gudas LJ (2011) Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol 6:345–364. 10.1146/annurev-pathol-011110-130303 [DOI] [PubMed] [Google Scholar]
- Tighe AP, Talmage DA (2004) Retinoids arrest breast cancer cell proliferation: retinoic acid selectively reduces the duration of receptor tyrosine kinase signaling. Exp Cell Res 301:147–157. 10.1016/j.yexcr.2004.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti DA, Chisamore MJ, Grdina W, Schurz H, Jordan VC (2000) Stable transfection of protein kinase C alpha cDNA in hormone-dependent breast cancer cell lines. Br J Cancer 83:782–791. 10.1054/bjoc.2000.1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urtreger A, Ladeda V, Puricelli L, Rivelli A, Vidal M, Delustig E, Joffe E (1997) Modulation of fibronectin expression and proteolytic activity associated with the invasive and metastatic phenotype in two new murine mammary tumor cell lines. Int J Oncol 11:489–496. 10.3892/ijo.11.3.489 [DOI] [PubMed] [Google Scholar]
- Urtreger AJ, Kazanietz MG, de Kier B, Joffe ED (2012) Contribution of individual PKC isoforms to breast cancer progression. IUBMB Life 64:18–26. 10.1002/iub.574 [DOI] [PubMed] [Google Scholar]
- Wang Y et al (2003) Hypermethylation-associated inactivation of retinoic acid receptor beta in human esophageal squamous cell carcinoma. Clin Cancer Res 9:5257–5263 [PubMed] [Google Scholar]
- Widschwendter M et al (2000) Methylation and silencing of the retinoic acid receptor-beta2 gene in breast cancer. J Natl Cancer Inst 92:826–832. 10.1093/jnci/92.10.826 [DOI] [PubMed] [Google Scholar]
- Wu B, Zhou H, Hu L, Mu Y, Wu Y (2013) Involvement of PKCalpha activation in TF/VIIa/PAR2-induced proliferation, migration, and survival of colon cancer cell SW620. Tumour Biol 34:837–846. 10.1007/s13277-012-0614-x [DOI] [PubMed] [Google Scholar]
- Wu MJ, Kim MR, Chen YS, Yang JY, Chang CJ (2017) Retinoic acid directs breast cancer cell state changes through regulation of TET2-PKCzeta pathway. Oncogene 36:3193–3206. 10.1038/onc.2016.467 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Yang L, Kim HT, Munoz-Medellin D, Reddy P, Brown PH (1997) Induction of retinoid resistance in breast cancer cells by overexpression of cJun. Cancer Res 57:4652–4661 [PubMed] [Google Scholar]
- Youssef EM et al (2004) Hypermethylation of the retinoic acid receptor-beta(2) gene in head and neck carcinogenesis. Clin Cancer Res 10:1733–1742. 10.1158/1078-0432.ccr-0989-3 [DOI] [PubMed] [Google Scholar]
- Zanardi S, Serrano D, Argusti A, Barile M, Puntoni M, Decensi A (2006) Clinical trials with retinoids for breast cancer chemoprevention. Endocr Relat Cancer 13:51–68. 10.1677/erc.1.00938 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated during this study are included in this article.