Abstract
Purpose
Presently, liver cancer is still one of the malignant tumors with high mortality. As far as the treatment of liver cancer is concerned, the most effective method is still liver transplantation. But every year, many liver cancer patients die from the lack of a proper liver transplant, or from waiting for a liver transplant. Therefore, it is very important to find new and effective treatment for patients with liver cancer.
Methods
Herein, the cell model and the orthotropic liver tumor mice model have been performed to verify the results of our treatment. We found that the in situ synthesized gold nanocluster–PTEN (GNC–PTEN) complexes can effectively target and realize the fluorescence imaging of the liver tumor.
Results
GNC–PTEN complexes could inhibit the proliferation, invasion, and metastasis of liver cancer cells. And the results also showed that GNC–PTEN complexes could be well targeted liver tumor at 6 h and the liver tumor in mice group treated with GNC–PTEN complexes almost disappeared.
Conclusion
This is a simply and effectively method to realize liver cancer imaging and inhibition. This may raise the possibility for the accurate image/diagnosis and simultaneously efficient treatment of liver cancer in the relevant clinic application.
Electronic supplementary material
The online version of this article (10.1007/s00432-020-03163-4) contains supplementary material, which is available to authorized users.
Keywords: Liver cancer, The orthotropic liver tumor model, In situ, Synthesized, surgical orthotropic implantation
Introduction
The World Cancer report recently released by the World Health Organization pointed out that the number of new liver cancer cases and deaths in China accounted for more than half of the new cases and deaths worldwide (Mcguire 2016). The relevant death rate of liver cancer is also high, and the mortality rate is second only to lung cancer in 2012. At present, liver transplantation is the main treatment for liver cancer. The 1-year and 5-year survival rates of liver transplantation patients exceed 85% and 70%, respectively (Mazzaferro et al. 2008). However, there are two main disadvantages of liver transplantation: liver transplantation has caused great damage to donors, so far 19 donors have died all over the world. On the other hand, the normal liver for liver transplantation is extremely scarce, and many patients die while waiting for the donor. Therefore, the discoveries of more effective treatments make it particularly important for more liver cancer patients while waiting for donors.
Therefore, it is an urgent technical problem to find a new and effective method for the treatment of liver cancer. The development of gene therapy (Gan et al. 2018; Huang et al. 2018a, b) and nanotechnology (Abed et al. 2019; Surnar et al. 2018; Xu et al. 2019) makes us more and more hopeful to overcome this difficult problem. Phosphatase and tensin homologue (PTEN) is a tumor suppressor gene and can inhibit the occurrence and development of liver cancer (Chai et al. 2017; Xin et al. 2018; Zheng et al. 2018). And the expression of PTEN was negatively correlated with the clinical grade of liver cancer. Higher the expression of PTEN, lower the clinic grade of liver cancer (Yidi et al. 2019). Many experiments have also shown that overexpression of PTEN can inhibit the invasion and metastasis of liver cancer, in vitro and in vivo (Feng et al. 2019; Jiang et al. 2019). In the previous study (Wang et al. 2019), we found that the in situ synthesized gold nanoclusters could inhibit the development of tumor by inhibiting the expression of oncogene AKT and PI3K and mTOR molecules in the PI3K signal pathway, but did not affect the expression of tumor suppression gene–PTEN. Therefore, we speculate that if PTEN can be added to the tumor inhibition process at the same time, then there will be a better inhibitory effect on the PI3K signal pathway, and, thus, will better inhibit the occurrence and development of cancer. Combined with PTEN, there have been found that a fluorescent GNC–PTEN complexes’ self-assembled in situ can be utilized to inhibition the proliferation of subcutaneous tumors (Wang et al. 2020). In this contribution, we observed that this complex could also well target on liver cancer. At the same time, this complex has a low risk of stranding in normal liver tissue and targets liver tumors directly, which is different from other delivery drug carriers (Sadauskas et al. 2007). This liver cancer treatment will provide the right type of treatment to liver tumors with minimal systemic toxicity. Therefore, this could be act as an effective method for the treatment of liver cancer by directly delivering PTEN to the tumor site.
In our recent studies, we have committed to making use of the characteristics of tumor microenvironment, such as low pH, and relatively high GSH and NADP + reductive substances, to synthesize a variety of metal nanoclusters in situ. Not only can the multimodal imaging of the tumors be realized (Du et al. 2017; Sana et al. 2018; Wang et al. 2013; Zhao et al. 2016), but also the effective treatment of the tumors can be realized through the nanoclusters’ photothermal therapy. In this contribution, using PTEN DNA and the gold nanocluster precursor, we have found a simple way to accurately perform GNC-PTEN complexes in subcutaneous tumors and obtain a well therapeutic result. Herein, we found that this in situ synthesis of GNC-PTEN complexes can not only effectively prevent the proliferation, invasion, and metastasis of liver cancer cells in vitro, but also can better treat orthotropic liver tumors in vivo. And the effect of treatment is better than that of subcutaneous tumor. At the same time, using the fluorescence characteristics of gold nanocluster, we can also readily achieve liver tumor imaging. It can be used to monitor the tumor in real time and improve the success rate of resection of liver cancer. We hope that this simple and effective method for the theranostics of liver cancer in situ can be further applied to clinical practice in the future.
Materials and methods
In situ biosynthetic GNC–DNA complexes
Initially, relevant cancer cells were incubated under different conditions. When the cell density reached 80%, it was digested with trypsin and placed in a 15 ml sterile centrifuge tube for 800 rpm for 3 min. After that, the supernatant will be emptied. 3 ml of the cell complete medium were added and centrifuged. Repeat this step for three times. Then, 3 ml medium was added and mixed evenly with the cell precipitate. Afterwards, transfer the cell suspension into the culture bottle (25 cm2 square torticollis breathable cap cell culture bottle, CORNING). Add HAuCl4 solution (pH = 7.2) (aladdin; CAS: 16903-35-8) to culture bottle and ensure the final concentration of HAuCl4 solution to 0.68 ng/µl. Gently shake counterclockwise and mix it well with the medium. Place the bottle in the cell incubator. A few minutes later, after waiting for the cell to settle, add PTEN DNA solution to the medium (final concentration: 2 ng/μl). At least 8 h incubation later, the cells can be used to do other experimental measurements. Here, we used HepG2 and SMMC-7721 cancer cell lines, respectively, in this study.
Cell culture
All cells were purchased from ATCC. Both HepG2 and SMMC-7721 were cultured with 10% fetal bovine serum (FBS, ZATA, America), penicillin (100 U/ml, #P3032, Sigma Chemicals, St Louis, MO, USA), and streptomycin (100 g/ml, Sigma Chemicals), respectively, but the medium was PRMI1640 and DMEM. In all experiments relevant cells were cultured in a humidified incubator with 5% CO2 at 37 °C.
Wound healing assay
When the density of cell is about to reached 95% in 6-well culture board, scratch the bottom of the plate with the pipet tip. Then, at 0 h, 24 h, 48 h, the scratch spacing at the same position was recorded. Three visual fields were randomly selected for each group, and the data were counted with Image Pro Plus 6.0.
Matrigel invasion assay
This experiment was conducted in accordance with the instructions for the transwell cell culture insert (8 μm pore size, BD Bioscience, New Jersey, USA). Initially, put the transwell insert into 24-well plates. Tile 20 μl 10% Matrigel inside the insert. A total of 2 × 105 cells in 200 μl of serum-free medium were added to the top of transwell insert. The bottom is the culture medium within 20% FBS. Then, 24-well plates were incubated for 36–48 h at cell incubator, and then, the bottom cells in the transwell insert were fixed with methanol, stained with hematoxylin, and observed under an inverted microscope. The same group of transwell cell culture inserts should be fixed and stained at the same time. The number of invasive cells were counted by ImageJ. Five fields were randomly selected for each experiment and averaged counted.
MTT cytotoxicity assay
200 μl complete medium which contained about 5000 cells was placed in 96-well plates. After 4–6 h of culture, 80 μl PTEN solution was added in every cell-cultured hole. Then, different concentrations (pH = 7.2) of HAuCl4 solution were added. After 24 h, complete the experiment, according to the MTT instruction. Finally, the absorbance was measured at 490 nm wavelength.
Cell proliferation analysis
1000 cells, incubated under different conditions, were inoculated into per hole. After cultured in cell incubator for 24 h, five holes were randomly selected every day. After incubated with 20 μl MTT for 4 h, the culture medium was sucked out. And then, 200 μl DMSO was added per hole. The absorbance at 490 nm wavelength was measured. After 6 days, the absorbance curve was drawn.
The orthotropic tumor model
Liver orthotropic cancer model were established in Balb/c nude mice to mimic the natural cancer microenvironment by surgical orthotropic implantation (SOI). Briefly, HepG2 tumors in the exponential growth phase, grown subcutaneously in nude mice, were resected aseptically and removed it into DMEM medium. Measured by ruler, 1 cm3 of tumor tissue was sutured into the liver of the blank nude mice. Fifteen days after the tumor suture operation, the mice were randomly divided into four different groups: PBS; free PTEN; GNCs; GNC-PTEN complexes (eight mice per group). Different kinds of reagents were injected intravenously: HAuCl4 solution (0.3 mmol) and PTEN (40 µg), only HAuCl4 solution (0.3 mmol), only PTEN (40 µg PTEN), and PBS (equal volume with HAuCl4 solution). After the first injection, one mouse was randomly selected from each group and the fluorescence intensity was observed at different time points within 24 h. After 24 h, the mice were euthanized and imaged for ex vivo organ imaging. All mice were killed on day 38, their main organs were taken, and the liver was weighed and imaged. At the same time, the tumor length (L) and width (W) were measured, and calculated the volume according to V = l × w2/2. All applicable institutional and/or national guidelines for the care and use of animals were followed. And we declare that all experiments on animal were conducted in accordance with the 1964 Helsinki Declaration. All experiments involving mice were approved by the National Institute of Biological Science and Animal Care Research Advisory Committee of Southeast University, and the experiments were conducted by following the guidelines of the Animal Research Ethics Board of Southeast University.
Results and discussion
GNC-PTEN complexes can effectively inhibit the development of hepatoma cells in vitro
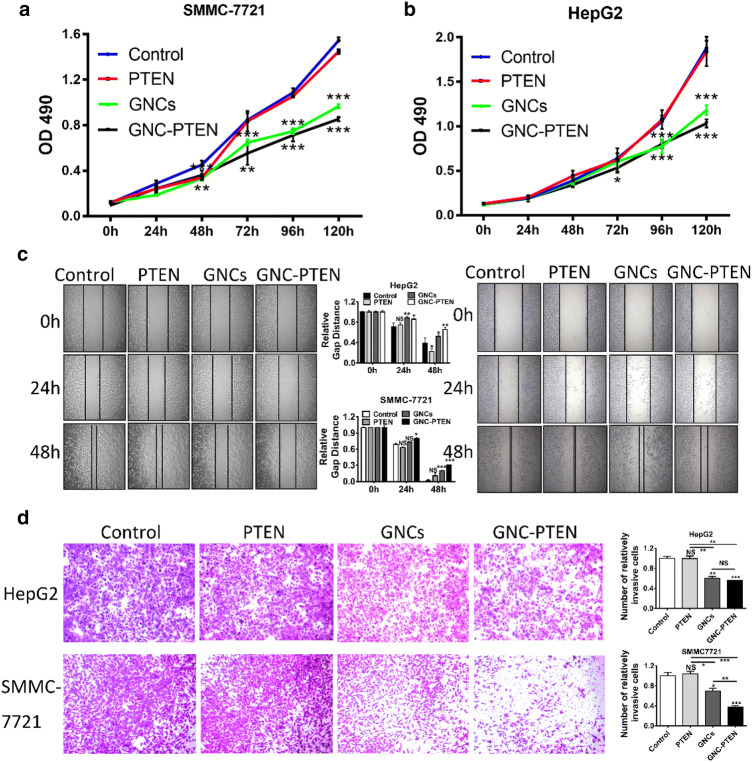
First, using MTT cytotoxicity experiments, we ensure that cell proliferation is not affected (Fig. S1). Accordingly, the optimum concentration of GNCs formed with PTEN was found. And then, we carried out the MTT cell proliferation assays (Chen et al. 2018; Gerlier and Thomasset 1986) (Fig. 1a, b), which is a classic experiment to detect cell proliferation ability. The hepatoma cells, HepG2 and SMMC-7721, in the same growth state were divided into four groups on an average, respectively. The different groups of cells were added with gold nanocluster precursor and PTEN gene solution at the same time, only PTEN gene solution, only the gold nanocluster precursor, and equal volume of saline, respectively. After 24 h, MTT cell proliferation experiments were carried out to record the proliferation states of cells within 120 h. Therefore, based on the final results, it can be seen that the GNC–PTEN complexes can effectively inhibit the proliferation of the hepatoma cells. Then, after the cells were incubated under the same conditions, we did scratch healing (Menon et al. 2009; Vega et al. 2014) (Fig. 1c) and transwell experiments (Brackenbury and Djamgoz 2007; Cabreara et al. 2003) (Fig. 1d). The scratch healing results showed that at 48 h, the scratch distance of the group containing GNC-PTEN complexes was significantly larger than that of the other three groups. This suggests that the complexes could inhibit the migration of hepatoma cells. And the results of transwell invasion experiments showed that under the same experimental conditions, the number of cells at the bottom of the inserts with the GNC-PTEN complex-containing group was significantly less than that in the other three groups. This proved that the complexes could inhibit the invasion of hepatoma cells. Above all, the results of these cell experiments suggest that the GNC–PTEN complexes can inhibit the growth and development of hepatoma cells in vitro.
Fig. 1.
GNC–PTEN complexes can effectively inhibit the proliferation, migration, and invasion of hepatoma cells in vitro. a,b MTT proliferation assay of HepG2 cells and SMMC-7721 cells after incubating with different reagents, respectively. The blue line, red line, green line, and black line represent the negative control group, PTEN group (PTEN, 2 ng/µl), in situ synthetic GNCs (HAuCl4, 0.68 ng/µl), and in situ GNC–PTEN complex group (PTEN, 2 ng/µl; HAuCl4, 0.68 ng/µl), respectively. c The left and right sides are the representative results of scratch healing experiment of HepG2 cells and SMMC-7721 cells after incubating with different reagents, respectively. The statistical results are in the middle. At each point in time, three fields of photographs were randomly selected, and the picture was 100×. d. Transwell invasion experiments of HepG2 and SMMC-7721 after incubating with different reagents, respectively. The representative results (100×) are shown in the graph. The statistical results of c and d were completed by imageJ. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; NS no significant statistical significance
In situ biosynthetic GNC–PTEN complexes for cancer bio-imaging in orthotropic tumor treatment model
Tumor-bearing mouse model is one of the most classical models in animal experiments. At present, there are two main tumor-bearing mice models: one is subcutaneous tumor (Wan et al. 2018; Woroniecka et al. 2018) and the other is orthotropic tumor model (Tian et al. 2018; Zhang et al. 2019). The orthotropic tumor, because of its tumor growth in situ, is more reliable to be able to simulate organ tumor in vivo, so it has been favored by many researchers. However, the orthotropic tumor model has some technical difficulty compared to the subcutaneous tumor. The construction of orthotropic tumor mainly consists of two methods: one is to inject a suitable number of cells in situ (Jiang and Ren 2008; Lei et al. 2017) and the other is to suture tumors of a certain size in situ through by surgical orthotropic implantation (SOI) (Okada et al. 2018; Thalheimer et al. 2009). Although in situ injection of cells is faster, there is no guarantee that the same number of cells actually attached to an organ-forming tumor, in the experiments. Therefore, the results of the experiments conducted by this method may have greatly intergroup error. Hence, to minimize the differences within the group, we used the surgical suture method to construct an orthotropic liver tumor model (Fig. 2a).
Fig. 2.
Bioimaging of tumor in vivo. a The steps of constructing an orthotropic mice model of hepatocellular carcinoma. Briefly, resected aseptically the subcutaneous tumor in nude mice, grown by injecting HepG2 cells of the exponential growth phase. Then, removed it into DMEM medium. 1 mm3 size tumor was sutured into the liver of another mouse by SOI. The mice were randomly divided into four groups with eight mice in each group. b The figures of fluorescence dynamic biological distribution in vivo, after intravenous injection of different preparations. The top–down preparations are Control group, the PTEN group, the GNC–PTEN complexes’ group, and the GNCs’ group. The fluorescence intensity of the control group was standardized to one. c Quantitative analysis of the relative fluorescence light intensity of liver
In previous studies, we found that GNC–PTEN complexes could target subcutaneous tumor bio-imaging, so we guessed whether it is possible to target liver cancer. Herein, after intravenous injection of different reagents, one mouse in each group was selected to observe the targeted image results, with the help of the animal fluorescence imaging system in vivo. The fluorescence intensity of animals within 24 h was recorded (Fig. 2b, c). It can be observed that the fluorescent GNC–PTEN complexes aggregate in the liver tumor and the fluorescence intensity reaches its peak at 6 h. And after 6 h, the fluorescence intensity of the tumor site was significantly higher than that of the group containing only GNCs. At the 24th hour, the mice were euthanized and the main organs were taken out to observe the fluorescence intensity. In the experimental group containing the GNC–PTEN complexes, we found that only the tumor had fluorescence ex vivo, which further confirmed the well targeting of the complexes to the liver tumor (Fig. 3).
Fig. 3.
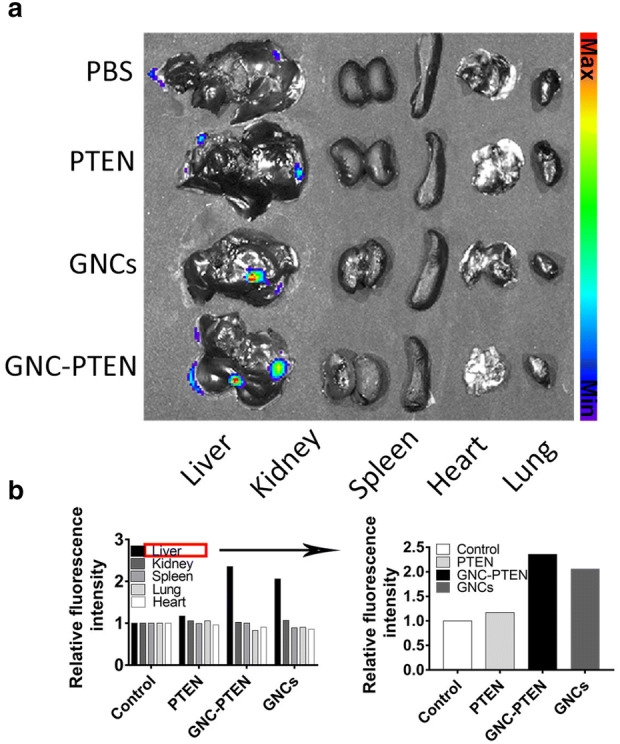
Bioimaging of different organs ex vivo imaging. a At the 24th hour, the mice were euthanized and the main organs (tumor-bearing liver, kidney, spleen, lung, and heart) were taken out for ex vivo imaging. The red circle represents the tumor above the liver. b Fluorescence intensities of individual organs from mice treated with different groups. The fluorescence intensity of the control group was standardized to one
In situ biosynthetic GNC–PTEN complexes for cancer inhibition in orthotropic tumor treatment model
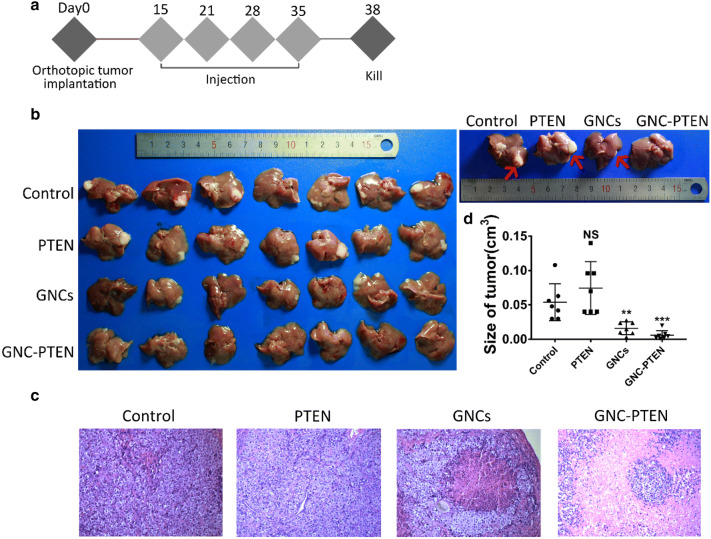
Since the GNC–PTEN complexes was found to inhibit the development of liver tumor cells in vitro, we speculated whether the complexes could inhibit the growth of tumor cells in vivo. And we continued to observe the growth status of the remaining seven mice in each group. According to the treatment model shown in Fig. 4a, four groups of mice were intravenously injected with different reagents on days 15, 21, 28, and 35, namely, HAuCl4 solution (3 mmol) and PTEN (60 μg), only HAuCl4 solution (3 mmol), only PTEN solution (60 μg), and only equal volume of saline solution. The mice were euthanized on the 38th day and the size of the tumor was observed (Fig. 4b). It can be seen that the liver tumor within the in situ synthetized GNC–PTEN complexes was significantly decreased, and some of the tumors even disappeared, compared with the control group and the PTEN group. These results were consistent with the haematoxylin and eosin-stained slices of the four representative liver results. The area of tumor tissue within GNC–PTEN complexes was obviously smaller than that of the other three groups (Fig. 4c). The statistical results of the specific tumor size are shown in Fig. 4d. No significant change of mouse liver weights was observed for the mice during the experimental period (Fig. S2), indicating that there was no apparent liver toxicity caused by in situ synthetized GNC–PTEN complexes. And the results of haematoxylin and eosin-stain (Fig. S3) showed that there was no significant difference between the main organs of the experimental group and the control group, which means that the experimental group did not cause pathological damage to the organs.
Fig. 4.
In situ biosynthetic GNC–PTEN complexes for cancer inhibition in orthotropic tumor treatment model. a Scheme of in situ synthesis of GNC–PTEN complexes treatments. Two weeks after liver tumor transplantation, mice received various formulations via tail veil injections for four times, and killed on day 38. b Ex vivo images of all orthotropic tumors in livers on day 38. Representative images of livers are on the right, and tumors were pointed out by red arrow. c Haematoxylin and eosin-stained sections of liver tumor (100×). d. Tumor size measured on day 38. **p < 0.01, ***p < 0.001
These results prove that our hypothesis is correct; that is, the in situ synthetized GNC–PTEN complexes can effectively inhibit the progression of liver tumors and even eliminate it. This is consistent with the results of hepatoma cell experiments. The experimental results show that GNC–PTEN complexes has a good targeting and therapeutic effect on liver tumors, and can be used in the comprehensive treatment and diagnosis of liver tumors. Especially in surgical resection of liver tumor, the size and location of the tumor can be monitored in real time, which has a good clinical application prospect.
Conclusions
In summary, in this contribution, we have established the cell model and the orthotropic liver tumor mice model to exploit the possibility to in situ biosynthesize gold nanocluster–PTEN (GNC–PTEN) complexes for effectively targeting and bio-imaging of the liver tumor in vivo. Our observations demonstrate that the self-assembly fluorescent GNC–PTEN complexes can precisely target on liver tumors, which will be not only beneficial to the accurate bio-imaging of liver tumors, but also efficiently inhibit the development of liver tumors. These suggested that the biosynthesized complexes could well integrate cancer diagnosis and treatment. Using the natural low pH value and the strong reductive microenvironment of the tumors, this complex can be readily synthesized in situ directly in the tumor tissue, and would not affect the normal organs. This simple and effective method not only requires a fewer material types and low toxicity, but also can greatly reduce the cost of medical care. It is hopeful that the in situ synthetized GNC–PTEN complex model in cancer cells/tissues can be promisingly utilized in clinics in the future to treat patients with liver cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (91753106, 21675023, and 81325011), Primary Research & Development Plan of Jiangsu Province (BE2019716), and the National Key Research and Development Program of China (2017YFA0205300).
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maonan Wang and Lishan Wang are common first authors.
Contributor Information
Jiahua Zhou, Email: zhoujh@seu.edu.cn.
Xuemei Wang, Email: xuewang@seu.edu.cn.
References
- Abed Z, Beik J, Laurent S et al (2019) Iron oxide–gold core–shell nano-theranostic for magnetically targeted photothermal therapy under magnetic resonance imaging guidance. J Cancer Res Clin Oncol 145:1213–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Djamgoz M (2007) Nerve growth factor enhances voltage-gated Na+ channel activity and Transwell migration in Mat-LyLu rat prostate cancer cell line. J Cell Physiol 210:602–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreara R, Tu Z, Firpi RJ et al (2003) An immunomodulatory role for CD4+ CD25+ T lymphocytes in hepatitis C infection. Gastroenterology 124:A710 [DOI] [PubMed] [Google Scholar]
- Chai Y, Xiaoyu L, Haiyan W (2017) Correlation between expression levels of PTEN and p53 genes and the clinical features of HBsAg-positive liver cancer. J BUON 22:942 [PubMed] [Google Scholar]
- Chen JF, Wu P, Xia R et al (2018) STAT3-induced lncRNA HAGLROS overexpression contributes to the malignant progression of gastric cancer cells via mTOR signal-mediated inhibition of autophagy. Mol Cancer 17:6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du T, Zhao C, Ur Rehman F et al (2017) Rapid and multimodal in vivo bioimaging of cancer cells through in situ biosynthesis of Zn&Fe nanoclusters. Nano Res 10:2626–2632 [Google Scholar]
- Feng J, Dang Y, Zhang W et al (2019) PTEN arginine methylation by PRMT6 suppresses PI3K–AKT signaling and modulates pre-mRNA splicing. Proc Natl Acad Sci 116:6868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan H, Chen L, Sui X et al (2018) Enhanced delivery of sorafenib with anti-GPC3 antibody-conjugated TPGS-b-PCL/Pluronic P123 polymeric nanoparticles for targeted therapy of hepatocellular carcinoma. Mater Sci Eng 91:395–403 [DOI] [PubMed] [Google Scholar]
- Gerlier D, Thomasset N (1986) Use of MTT colorimetric assay to measure cell activation. J Immunol Methods 9:457–463 [DOI] [PubMed] [Google Scholar]
- Huang C, Zheng J, Ma D et al (2018a) Hypoxia-triggered gene therapy: a new drug delivery system to utilize photodynamic-induced hypoxia for synergistic cancer therapy. J Mater Chem B 6:6424–6430 [DOI] [PubMed] [Google Scholar]
- Huang K-W, Lai Y-T, Chern G-J et al (2018b) Galactose derivative-modified nanoparticles for efficient siRNA delivery to hepatocellular carcinoma. Biomacromolecules 19:2330–2339 [DOI] [PubMed] [Google Scholar]
- Jiang HF, Ren J (2008) Inoculation of murine bone marrow mesenchymal stem cells induces tumor necrosis in mouse with orthotopic hepatocellular carcinoma. Beijing Da Xue Xue Bao Yi Xue Ban 40:453–458 [PubMed] [Google Scholar]
- Jiang Z, Zhou Q, Ge C et al (2019) Rpn10 promotes tumor progression by regulating hypoxia-inducible factor 1 alpha through the PTEN/Akt signaling pathway in hepatocellular carcinoma. Cancer Lett 10:1–11 [DOI] [PubMed] [Google Scholar]
- Lei Y, Tang L, Xie Y (2017) Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat Commun 8:15130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaferro V, Chun YS, Poon RTP et al (2008) Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol 15:1001–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcguire S (2016) World cancer report 2014. Geneva, Switzerland: World Health Organization, International agency for research on cancer, WHO Press, 2015. Adv Nutr 7:418–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon MB, Ronkina N, Schwermann J et al (2009) Fluorescence-based quantitative scratch wound healing assay demonstrating the role of MAPKAPK-2/3 in fibroblast migration. Cytoskeleton 66:1041–1047 [DOI] [PubMed] [Google Scholar]
- Okada S, Vaeteewoottacharn K, Kariya R (2018) Establishment of a patient-derived Tumor Xenograft model and application for precision cancer medicine. Chem Pharm Bull 66:225–230 [DOI] [PubMed] [Google Scholar]
- Sadauskas E, Wallin H, Stoltenberg M et al (2007) Kupffer cells are central in the removal of nanoparticles from the organism. Part Fibre Toxicol 4(1):10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sana S, Ur RF, Tianyu Du et al (2018) Real-time multimodal bioimaging of cancer cells and exosomes through biosynthesized iridium and iron nanoclusters. ACS Appl Mater Interfaces 10:26056–26063 [DOI] [PubMed] [Google Scholar]
- Surnar B, Basu U, Banik B et al (2018) Nanotechnology-mediated crossing of two impermeable membranes to modulate the stars of the neurovascular unit for neuroprotection. Proc Natl Acad Sci 115:E12333–E12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalheimer A, Otto C, Bueter M et al (2009) Tumor cell dissemination in a human colon cancer animal model: orthotopic implantation or intraportal injection? Eur Surg Res 42:195–200 [DOI] [PubMed] [Google Scholar]
- Tian Yu, Gujie Mi, Qian C et al (2018) Acid-induced activated cell-penetrating peptide-modified cholesterol-conjugated polyoxyethylene sorbitol oleate mixed micelles for pH-triggered drug release and efficient brain tumor targeting based on a charge reversal mechanism. ACS Appl Mater Interfaces 10:43411–43428 [DOI] [PubMed] [Google Scholar]
- Vega JM, Grande AM, van der Zwaag S et al (2014) On the role of free carboxylic groups and cluster conformation on the surface scratch healing behaviour of ionomers. Eur Polym J 57:121–126 [Google Scholar]
- Wan H, Yue J, Zhu S et al (2018) A bright organic NIR-II nanofluorophore for three-dimensional imaging into biological tissues. Nat Commun 9:1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang G, Li Q et al (2013) In vivo self-bio-imaging of tumors through in situ biosynthesized fluorescent gold nanoclusters. Sci Rep 3:1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yu Z, Feng H et al (2019) In situ biosynthesized gold nanoclusters inhibiting cancer development via the PI3K–AKT signaling pathway. J Mater Chem B 7:5336–5344 [DOI] [PubMed] [Google Scholar]
- Wang M, Chen Y, Cai W et al (2020) In situ self-assembling Au-DNA complexes for targeted cancer bioimaging and inhibition. Proc Natl Acad Sci 117:308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woroniecka K, Chongsathidkiet P, Rhodin KE et al (2018) T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res 24:1846–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Mengying W, Qiuyu M (2018) Long noncoding RNA HULC accelerates liver cancer by inhibiting PTEN via autophagy cooperation to miR15a. Mol Cancer 17:94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Cheng X, Tan L et al (2019) Microwave responsive nanoplatform via P-selectin mediated drug delivery for treatment of hepatocellular carcinoma with distant metastasis. Nano Lett 19:2914–2927 [DOI] [PubMed] [Google Scholar]
- Yidi H, Meizhu C, Aili W et al (2019) STAT3-induced upregulation of lncRNA CASC11 promotes the cell migration, invasion and epithelial-mesenchymal transition in hepatocellular carcinoma by epigenetically silencing PTEN and activating PI3K/AKT signaling pathway. Biochem Biophys Res Commun 508:472–479 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cai M, Bao C et al (2019) Endoscopic Cerenkov luminescence imaging and image-guided tumor resection on hepatocellular carcinoma-bearing mouse models. Nanomedicine 17:62–70 [DOI] [PubMed] [Google Scholar]
- Zhao C, Du T, Rehman FU et al (2016) Biosynthesized gold nanoclusters and iron complexes as scaffolds for multimodal cancer bioimaging. Small 12:6255–6265 [DOI] [PubMed] [Google Scholar]
- Zheng Q, Lin Z, Xu J et al (2018) Long noncoding RNA MEG3 suppresses liver cancer cells growth through inhibiting β-catenin by activating PKM2 and inactivating PTEN. Cell Death Dis 9:253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.