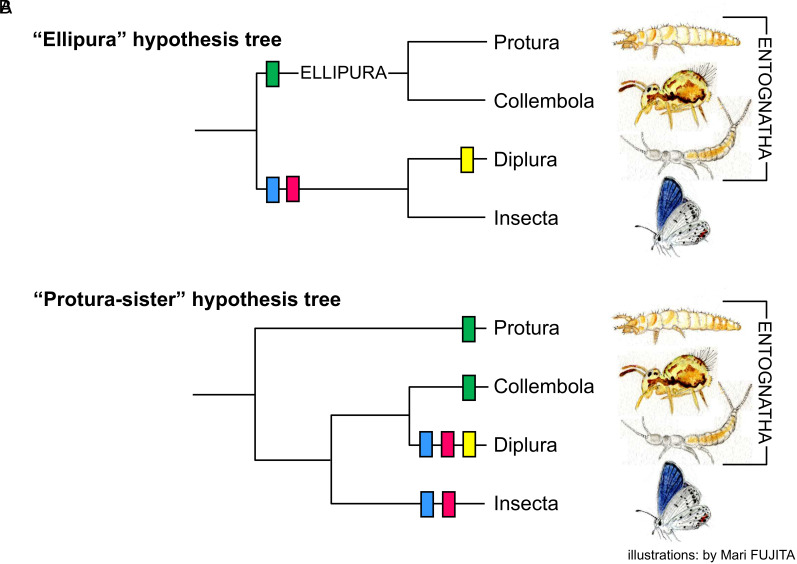
Du et al. (1) addressed the longstanding issue of the phylogenetic relationships of the early lineages of Hexapoda, i.e., Protura, Collembola, Diplura, and Insecta (=Ectognatha). They obtained new transcriptome sequences for one proturan and two dipluran species and analyzed them, along with genomic and transcriptomic data for 42 other hexapods and 9 crustaceans from NCBI. Using various evolutionary models, among the phylogenetic trees constructed, they chose the one formulated as {Protura + [(Collembola + Diplura) + Insecta]} (“Protura-sister” hypothesis) and determined Protura as the earliest diverging hexapod lineage.
Du et al. used embryologic data for verification but failed to pursue crucial/subsequent information. For example, they argued that Collembola and Diplura share a similar mode of blastokinesis (2) to tacitly suggest their affinity. However, subsequent studies proved that Protura also exhibit blastokinesis of this type (3, 4); hence, the characteristic is common among Protura, Collembola, and Diplura. They discriminated Protura from other hexapods by noting that its embryonic membrane can differentiate into the body wall (4); however, a recent study determined that the collembolan embryonic membrane possesses this ability as well (5). In any case, this shared feature, being an ancestral trait, cannot contribute to phylogenetic discussion. Therefore, what is significant is not the presence of this ability in proturan and collembolan embryonic membranes, but rather the loss of this ability in the embryonic membranes of Diplura and Insecta, as well as the acquisition of a second embryonic membrane, the “amnion,” in these two lineages. Each of these two features is regarded as the shared derived or synapomorphic features of Diplura and Insecta; therefore, the sister group relationship of them strongly suggested (2–7). At this point, four early hexapod lineages have only been established based on trichotomy. However, data on entognathy formation indicate that the entognathy in Protura and Collembola formed through a synapomorphic “ellipuran type,” whereas that in Diplura formed through an autapomorphic “dipluran type” (2, 3, 5, 6). Thus, the hexapod basal branchings can be reconstructed as [Ellipura (=Protura + Collembola) + (Diplura + Insecta)], thereby recovering the “Ellipura” hypothesis (Fig. 1A). The Protura-sister hypothesis requires three times the parallel acquisition for the embryological features concerned (Fig. 1B).
Fig. 1.
Mapping of embryological features on the Ellipura hypothesis tree (A) and Protura-sister hypothesis tree (B). In the Ellipura hypothesis tree (A), the embryological features are parsimoniously mapped on the lineages, as the apomorphic or derived feature defining each lineage, but in the Protura-sister hypothesis, several parallel acquisitions of the features need to be assumed. Protura, Collembola, and Diplura possess a specialized mouthpart named entognathy, in which the jaws are covered by the gena, collectively called the “Entognatha.” Blue: loss of the embryonic membrane’s ability to differentiate into the body wall, pink: acquisition of the amnion, green: formation of the ellipuran-type entognathy, and yellow: formation of the dipluran-type entognathy.
Du et al. also cited morphological information to support their Protura-sister hypothesis. They highlighted the special features of sperm in Protura; however, these only emphasize the monophyly of this group because they are autapomorphic features of Protura (8). They further cited the segmented antenna as a common feature of Collembola and Diplura, but this feature also does not substantiate phylogenetic argument since it is symplesiomorphic to these orders (9).
Thus, we identified several notable issues with the argument of Du et al. Azar (10) wrote a commentary praising Du et al., but he made similar errors; in his Fig. 1C, a morphological feature plotted on the Collembola-Diplura lineage as “morphological evidence” is the symplesiomorphic “segmented antenna.” Additionally, the five colorfully lined squares on the Protura lineage, presented as morphological evidence, merely indicate the monophyletic status of Protura.
Acknowledgments
Author contributions
R.M., M.F., S.T., Y.I., K.S., and M.M. wrote the paper.
Competing interests
The authors declare no competing interest.
References
- 1.Du S., et al. , Revisiting the four Hexapoda classes: Protura as the sister group to all other hexapods. Proc. Natl. Acad. Sci. U.S.A. 121, e2408775121 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikeda Y., Machida R., Embryogenesis of the dipluran Lepidocampa weberi Oudemans (Hexapoda, Diplura, Campodeidae): External morphology. J. Morphol. 237, 101–115 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Fukui M., Machida R., Embryonic development of Baculentulus densus (Imadaté): An outline (Hexapoda: Protura, Acerentomidae). Proc. Arthropod. Embryol. Soc. Jpn. 41, 21–28 (2006). [Google Scholar]
- 4.Machida R., Evidence from embryology for reconstructing the relationships of hexapod basal clades. Arthropod Syst. Phylog. 64, 95–104 (2006). [Google Scholar]
- 5.Tomizuka S., Machida R., Embryonic development of a collembolan, Tomocerus cuspidatus Börner, 1909: With special reference to the development and developmental potential of serosa (Hexapoda: Collembola, Tomoceridae). Arthropod Struct. Dev. 44, 157–272 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Sekiya K., Machida R., Embryonic development of Occasjapyx japonicus (Enderlein): Notable features (Hexapoda: Diplura, Dicellurata). Proc. Arthropod. Embryol. Soc. Jpn. 44, 13–18 (2009). [Google Scholar]
- 7.Masumoto M., Machida R., Development of embryonic membranes in the silverfish Lepisma saccharina Linnaeus (Insecta: Zygentoma, Lepismatidae). Tissue Cell 38, 159–169 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Dallai R., Gottardo M., Beutel R. G., Structure and evolution of insect sperm: New interpretations in the age of phylogenomics. Annu. Rev. Entomol. 61, 1–23 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Matsuda R., Morphology and Evolution of the Insect Head (Memoir of the American Entomological Institute, 1965), No. 4. [Google Scholar]
- 10.Azar D., Commentary: Robust resolved phylogeny of hexapods’ four classes. Proc. Natl. Acad. Sci. U.S.A. 121, e2418732121 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]