Abstract
Background
Adjuvant chemotherapy could improve the prognosis of stage II–III non-small cell lung cancer (NSCLC). However, its influences on stage IB were controversial. The purpose of this study was to investigate whether patients with stage IB NSCLC could benefit from adjuvant chemotherapy.
Methods
Stage IB NSCLC in 2010–2015 was selected from the surveillance, epidemiology, and end result database. Chi square test was used to compare the clinical characteristics of patients with different adjuvant chemotherapy status. Kaplan–Meier survival curves were plotted by the log-rank test. Cox proportional hazard regression was used to perform multivariate analysis on overall survival (OS), and the life table method was employed to calculate 1-, 3-, and 5-year survival rates.
Results
A total of 2915 patients were included in this study, and the number of patients with visceral pleural invasion (VPI) was 1096 (37.6%), of which 145 (13.2%) received adjuvant chemotherapy. There was no statistical difference in OS among the total population with or without chemotherapy (p = 0.295), nor in patients with VPI (p = 0.216). In patients with VPI, the 1-, 3-, 5-year survival curves of patients who are receiving adjuvant chemotherapy showed an upward trend compared with patients who did not. Additionally, female, high differentiated, adenocarcinoma, and tumor size ≤ 3 cm were also independent prognostic factors for improving the prognosis of patients with VPI.
Conclusion
In our study, stage IB NSCLC did not benefit from adjuvant chemotherapy, even in patients with VPI. However, the significance of adjuvant chemotherapy in patients with VPI is still worth further exploration.
Electronic supplementary material
The online version of this article (10.1007/s00432-020-03276-w) contains supplementary material, which is available to authorized users.
Keywords: NSCLC, Visceral pleural invasion, Adjuvant chemotherapy
Introduction
Although great progress has been achieved in the treatment of lung cancer, it is still the malignant tumor with the highest mortality and morbidity in the world (Bray et al. 2018). American Cancer Society estimates that 228,150 new lung cancer patients and 142,670 lung cancer deaths will occur in 2019 (Siegel et al. 2019). Adjuvant therapies including postoperative adjuvant chemotherapy and chemoradiotherapy (Zou et al. 2010) are commonly used in patients with lung cancer after surgery. In recent years, more and more studies find that adjuvant chemotherapy can improve the prognosis of patients with stage II–III non-small cell lung cancer (NSCLC) (Park et al. 2018; Salazar et al. 2016; Taylor et al. 2004; Holmes 1994). However, whether NSCLC with early stage, especially stage IB can benefit from adjuvant chemotherapy is still controversial (Burdett et al. 2015; Butts et al. 2010; Hung et al. 2016; Roselli et al. 2006; Zhang et al. 2018).
Visceral pleural invasion (VPI) was used to describe the characteristics of tumors in the tumor, node, metastasis (TNM) classification of the International Union Against Cancer (UICC) staging system in the mid-1970s (Yoshida et al. 2009). He Huang et al. (2015) found that stage I NSCLC patients with visceral pleural metastasis had a higher risk of death (HR = 1.427, p = 0.000) and recurrence (HR = 1.6, p = 0.000). Another study also found that lung cancer with VPI (PL1: 63.6%, PL2: 54.1%) had significantly lower 5-year overall survival (OS) rate than patients without VPI (PL0: 75.9%) (Adachi et al. 2015). Thus, VPI was a vital risk factor for the prognosis of patients with lung cancer. So far, few researches payed attention to whether stage IB NSCLC with VPI can benefit from adjuvant chemotherapy (Park et al. 2018).
The surveillance, epidemiology, and end results (SEER) database is one of the largest public cancer databases in the world, which was established by the National Cancer Institute of the United States, and accounts for about 30% of the U.S. population (Park et al. 2012). The SEER database is an important resource for understanding tumor characteristics and treatment in different groups. Therefore, the purpose of this study is to explore whether adjuvant chemotherapy can improve the survival of patients with IB NSCLC, and further illustrate the role of adjuvant chemotherapy in patients with VPI. Through the analysis of the SEER database, we hoped to provide valuable information to facilitate further implementation of adjuvant chemotherapy in stage IB NSCLC with VPI in clinical practice.
Materials and methods
Selection of patients
Patients with stage IB NSCLC experienced lobectomy from 2010 to 2015 were selected by SEER*Stat software version 8.3.5. Patients with complete clinical data were included, and we excluded the patients whose age, race, differentiation, and survival time were unknown. According to the 8th edition of TNM staging, T2a was defined as tumor size ≤ 4 cm, so patients with tumors > 4 cm were removed. Patients underwent radiotherapy were not included in this study.
Variable classification
Age of diagnosis, gender, race, grade, laterality, tumor size, histologic type, and adjuvant chemotherapy status were obtained from the database. Age was divided into 20–59, 60–74, and ≥ 75 years old (Stein 2010). Tumor size was categorized into ≤ 3 and 3–4 cm. According to the pathological classification of lung cancer mentioned by Thakur et al. (2018), patients were divided into adenocarcinoma (8140, 8250–8253, 8255, 8260, 8323, 8480–8481, 8550, 8560, 8570, and 8574), squamous cell carcinoma (8070–8073 and 8083) and other types of NSCLC. We used codes 00, 10, 20 in CS site-specific factor 2 to get VPI status PL0 (no evidence of VPI and tumor does not completely traverse the elastic layer), PL1 (invasion beyond the visceral elastic pleura, but limited to the pulmonary pleura and tumor extends through the elastic layer), and PL2 (invasion to the surface of the pulmonary pleura and tumor extends to the surface of the visceral pleura). PL1 and PL2 indicated the presence of VPI, while PL0 represents the absence of VPI.
Statistical analysis
The distribution differences of age, gender, race, grade, laterality, tumor size, and histologic type were compared by the Pearson Chi square test. The Kaplan–Meier method was used to estimate OS. Variables related with OS (p < 0.05) in the univariable analysis or with important clinical value were included into a multivariable analysis. The 1-, 3-, and 5-year survival rates were calculated by the life table method. For all analyses, p value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics 25 (IBM, NY). Because the patient data in the SEER database have been identified, this study dispensed with signing informed consent forms.
Result
Population characteristics
Finally, 2915 patients from the SEER database were eligible to enter the cohort, in which 1096 patients were diagnosed with VPI, accounting for about 37.6% in the cohort. The filtering flowchart was shown in Fig. 1. There was no significant difference in gender (p = 0.355), race (p = 0.756), histology (p = 0.079), tumor size (p = 0.381), and laterality (p = 0.389) between patients treat with or without adjuvant chemotherapy. Patients receiving adjuvant chemotherapy were more likely to be younger (33.0% vs. 19.2%, p < 0.0001), diagnosed with VPI+ (44.8% vs. 36.7%, p = 0.005) and lower differentiated (51.9% vs. 31.5%, p < 0.0001) compared with patients who did not receive chemotherapy. Detailed clinicopathological features were summarized in Table 1.
Fig. 1.
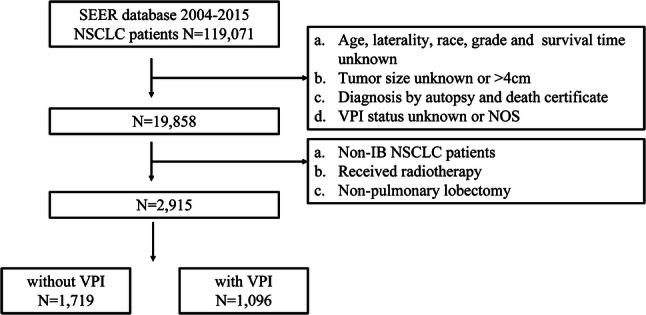
Flowchart of patient selection
Table 1.
Clinical characteristics of patients
| Variables | Adjuvant chemotherapy | p value | |||
|---|---|---|---|---|---|
| Yes | No/unknown | ||||
| N | % | N | % | ||
| Age | < 0.0001 | ||||
| 20–59 | 107 | 33.0 | 499 | 19.2 | |
| 60–74 | 191 | 59.0 | 1393 | 53.8 | |
| ≥ 75 | 26 | 8.0 | 699 | 27.0 | |
| Gender | 0.355 | ||||
| Male | 145 | 44.8 | 1230 | 47.5 | |
| Female | 179 | 55.2 | 1361 | 52.5 | |
| Race | 0.756 | ||||
| White | 269 | 83.0 | 2110 | 81.4 | |
| Black | 26 | 8.0 | 237 | 9.1 | |
| Others | 29 | 9.0 | 244 | 9.4 | |
| Gradea | < 0.0001 | ||||
| I–II | 156 | 48.1 | 1776 | 68.5 | |
| III–IV | 168 | 51.9 | 815 | 31.5 | |
| Histology | 0.079 | ||||
| Adenocarcinoma | 230 | 71.0 | 1697 | 65.5 | |
| Squamous cell carcinoma | 63 | 19.4 | 650 | 25.1 | |
| Others | 31 | 9.6 | 244 | 9.4 | |
| Tumor size | 0.381 | ||||
| 0–3 cm | 120 | 37.0 | 1025 | 39.6 | |
| 3.1–4 cm | 204 | 63.0 | 1566 | 60.4 | |
| Laterality | 0.389 | ||||
| Left | 121 | 37.3 | 1032 | 39.8 | |
| Right | 203 | 62.7 | 1559 | 60.2 | |
| VPI status | 0.005 | ||||
| VPI− | 179 | 55.2 | 1640 | 63.3 | |
| VPI+ | 145 | 44.8 | 951 | 36.7 | |
aI—well differentiated, II—moderate differentiated, III—poorly differentiated, IV—undifferentiated
In patients diagnosed with VPI, there was no significant difference in gender (p = 0.091), race (p = 0.439), and laterality (p = 0.425) between patients treated with or without adjuvant chemotherapy. Patients receiving adjuvant chemotherapy were more likely to be younger (33.1% vs. 17.5%, p < 0.0001), adenocarcinoma (82.1% vs. 76.2%, p = 0.041), large tumors (33.8% vs. 24.5%, p = 0.017), and lower differentiated (42.8% vs. 32.0%, p = 0.010) compared with patients who did not receive chemotherapy. Detailed clinicopathological features were summarized in Table 2.
Table 2.
Clinical characteristics of VPI+ patients
| Variables | Adjuvant chemotherapy | p value | |||
|---|---|---|---|---|---|
| Yes | No/unknown | ||||
| N | % | N | % | ||
| Age | < 0.0001 | ||||
| 20–59 | 48 | 33.1 | 166 | 17.5 | |
| 60–74 | 86 | 59.3 | 527 | 55.4 | |
| ≥ 75 | 11 | 7.6 | 258 | 27.1 | |
| Gender | 0.091 | ||||
| Male | 55 | 37.9 | 432 | 45.4 | |
| Female | 90 | 62.1 | 519 | 54.6 | |
| Race | 0.439 | ||||
| White | 115 | 79.3 | 754 | 79.3 | |
| Black | 11 | 7.6 | 97 | 10.2 | |
| Others | 19 | 13.1 | 100 | 10.5 | |
| Gradea | 0.010 | ||||
| I–II | 83 | 57.2 | 647 | 68.0 | |
| III–IV | 62 | 42.8 | 304 | 32.0 | |
| Histology | 0.041 | ||||
| Adenocarcinoma | 119 | 82.1 | 725 | 76.2 | |
| Squamous cell carcinoma | 15 | 10.3 | 175 | 18.4 | |
| Others | 11 | 7.6 | 51 | 5.4 | |
| Tumor size | 0.017 | ||||
| 0–3 cm | 96 | 66.2 | 718 | 75.5 | |
| 3.1–4 cm | 49 | 33.8 | 233 | 24.5 | |
| Laterality | 0.425 | ||||
| Left | 52 | 35.9 | 374 | 39.3 | |
| Right | 93 | 64.1 | 577 | 60.7 | |
aI—well differentiated, II—moderate differentiated, III—poorly differentiated, IV—undifferentiated
Survival analysis
Unadjusted survival curves were used to compare the OS of patients who are receiving adjuvant chemotherapy with those who did not in different cohorts (Fig. 2). There was no statistical difference in OS between the total NSCLC population with or without chemotherapy (p = 0.057), nor in VPI status (p = 0.467) (Table 3). Furthermore, patients were divided into two groups based on the status of adjuvant chemotherapy. Survival rates of 1-, 3-, and 5-year in different subgroups were calculated, in which the effect of adjuvant chemotherapy on the prognosis of stage IB NSCLC could be demonstrated more intuitively. The 1-year survival rate of female (93%) and squamous cell carcinoma (73%) in the chemotherapy group was lower than that in patients without chemotherapy (94 and 87%), and later, with the extension of follow-up time, the 5-year survival rate (80 and 66%) was higher than in patients with chemotherapy (71 and 51%). The survival rate of patients between 20 and 59 was not improved even if they received chemotherapy. In other subgroups, the 1-, 3-, and 5-year survival rates between patients who received chemotherapy or not were gradually increasing. For example, in male patients, the 1-, 3-, and 5-year survival rates were 4, 12 and 19%, respectively. Specific difference values of variables were shown in Table 4. We also use this method to compare survival rates in patients with VPI. The 1-year survival rate of squamous cell carcinoma (73%) in the chemotherapy group was lower than that in patients without chemotherapy (87%). In other subgroups, the 1-, 3-, and 5-year survival rates between patients who received chemotherapy or not were gradually increasing. Specific difference values of variables were shown in Table 5.
Fig. 2.
Overall survival curve of adjuvant chemotherapy and non-adjuvant chemotherapy in different cohorts. a Comparison of overall survival rate between adjuvant chemotherapy and non-adjuvant chemotherapy in patients with stage IB NSCLC; b comparison of overall survival rate between adjuvant chemotherapy and non-adjuvant chemotherapy in stage IB NSCLC patients with VPI; c comparison of overall survival rate between adjuvant chemotherapy and non-adjuvant chemotherapy in stage IB NSCLC patients without VPI
Table 3.
Univariate and multivariate analysis of the patients selected
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| p value | HR | 95% CI | p value | |
| Age | < 0.0001 | < 0.0001 | ||
| 20–59 | Ref. | |||
| 60–74 | 1.542 | 1.226–1.938 | ||
| ≥ 75 | 2.732 | 2.146–3.479 | ||
| Gender | < 0.0001 | < 0.0001 | ||
| Male | Ref. | |||
| Female | 0.736 | 0.634–0.853 | ||
| Race | 0.139 | NA | ||
| White | ||||
| Black | ||||
| Others | ||||
| Gradea | < 0.0001 | < 0.0001 | ||
| I–II | Ref. | |||
| III–IV | 1.415 | 1.215–1.648 | ||
| Histology | < 0.0001 | 0.140 | ||
| Adenocarcinoma | Ref. | |||
| Squamous cell carcinoma | 1.179 | 0.996–1.395 | ||
| Others NSCLC | 0.989 | 0.751–1.303 | ||
| Tumor size | 0.009 | 0.021 | ||
| 0–3 cm | Ref. | |||
| 3.1–4 cm | 1.245 | 1.034–1.499 | ||
| Laterality | 0.557 | NA | ||
| Left | ||||
| Right | ||||
| Adjuvant chemotherapy | 0.057 | 0.233 | ||
| Yes | Ref. | |||
| No/unknown | 1.168 | 0.905–1.507 | ||
| VPI status | 0.467 | 0.013 | ||
| VPI− | Ref. | |||
| VPI+ | 1.261 | 1.050–1.514 | ||
aI—well differentiated, II—moderate differentiated, III—poorly differentiated, IV—undifferentiated
Table 4.
Difference values of 1-, 3- and 5-year OS rate in patients with adjuvant chemotherapy and without adjuvant chemotherapy
| Variables | Adjuvant chemotherapy | Difference (%) | |
|---|---|---|---|
| Yes (%) | No/unknown (%) | ||
| Gender | |||
| Male | |||
| 1 year | 93 | 89 | 4 |
| 3 year | 84 | 72 | 12 |
| 5 year | 79 | 60 | 19 |
| Female | |||
| 1 year | 93 | 94 | − 1 |
| 3 year | 84 | 82 | 2 |
| 5 year | 80 | 71 | 9 |
| Age | |||
| 20–59 | |||
| 1 year | 92 | 96 | − 4 |
| 3 year | 84 | 88 | − 4 |
| 5 year | 75 | 79 | − 4 |
| 60–74 | |||
| 1 year | 92 | 92 | 0 |
| 3 year | 79 | 80 | − 1 |
| 5 year | 74 | 69 | 5 |
| ≥75 | |||
| 1 year | 96 | 85 | 11 |
| 3 year | 77 | 68 | 9 |
| 5 year | 66 | 50 | 16 |
| Gradea | |||
| I–II | |||
| 1 year | 95 | 94 | 1 |
| 3 year | 86 | 82 | 4 |
| 5 year | 81 | 69 | 12 |
| III–IV | |||
| 1 year | 90 | 88 | 2 |
| 3 year | 81 | 69 | 12 |
| 5 year | 78 | 59 | 18 |
| Histology | |||
| Adenocarcinoma | |||
| 1 year | 96 | 93 | 3 |
| 3 year | 85 | 81 | 4 |
| 5 year | 80 | 69 | 11 |
| Squamous cell carcinoma | |||
| 1 year | 73 | 87 | − 14 |
| 3 year | 66 | 66 | 0 |
| 5 year | 66 | 51 | 15 |
| Others | |||
| 1 year | 91 | 90 | 1 |
| 3 year | 91 | 71 | 20 |
| 5 year | 91 | 71 | 20 |
| Tumor size | |||
| 0–3 cm | |||
| 1 year | 95 | 94 | 2 |
| 3 year | 88 | 79 | 9 |
| 5 year | 85 | 68 | 17 |
| 3.1–4 cm | |||
| 1 year | 90 | 88 | 2 |
| 3 year | 75 | 73 | 2 |
| 5 year | 69 | 59 | 10 |
aI—well differentiated, II—moderate differentiated, III—poorly differentiated, IV—undifferentiated
Table 5.
Difference values of 1-, 3- and 5-year OS rate in VPI+ patients with adjuvant chemotherapy and without adjuvant chemotherapy
| Variables | Adjuvant chemotherapy | Difference (%) | |
|---|---|---|---|
| Yes (%) | No/unknown (%) | ||
| Gender | |||
| Male | |||
| 1 year | 93 | 89 | 4 |
| 3 year | 84 | 70 | 14 |
| 5 year | 79 | 58 | 21 |
| Female | |||
| 1 year | 93 | 93 | 0 |
| 3 year | 82 | 82 | 0 |
| 5 year | 80 | 71 | 9 |
| Age | |||
| 20–59 | |||
| 1 year | 96 | 96 | 0 |
| 3 year | 91 | 88 | 3 |
| 5 year | 87 | 81 | 6 |
| 60–74 | |||
| 1 year | 92 | 92 | 0 |
| 3 year | 79 | 76 | 3 |
| 5 year | 76 | 68 | 8 |
| ≥ 75 | |||
| 1 year | 91 | 86 | 5 |
| 3 year | 82 | 70 | 12 |
| 5 year | 82 | 45 | 37 |
| Gradea | |||
| I–II | |||
| 1 year | 95 | 93 | 2 |
| 3 year | 86 | 80 | 6 |
| 5 year | 81 | 67 | 14 |
| III–IV | |||
| 1 year | 90 | 86 | 4 |
| 3 year | 78 | 69 | 9 |
| 5 year | 78 | 59 | 19 |
| Histology | |||
| Adenocarcinoma | |||
| 1 year | 96 | 92 | 4 |
| 3 year | 84 | 80 | 4 |
| 5 year | 80 | 68 | 12 |
| Squamous cell carcinoma | |||
| 1 year | 73 | 87 | − 14 |
| 3 year | 73 | 64 | 9 |
| 5 year | 73 | 51 | 22 |
| Others | |||
| 1 year | 91 | 90 | 1 |
| 3 year | 91 | 71 | 20 |
| 5 year | 91 | 71 | 20 |
| Tumor size | |||
| 0–3 cm | |||
| 1 year | 95 | 92 | 3 |
| 3 year | 88 | 78 | 10 |
| 5 year | 86 | 67 | 19 |
| 3.1–4 cm | |||
| 1 year | 90 | 87 | 3 |
| 3 year | 72 | 72 | 0 |
| 5 year | 69 | 59 | 10 |
Prognosis factors of patients
We found that there was a statistical difference between patients diagnosed with and without VPI in the COX multivariate analysis of the total population (Table 3). In details, patients with VPI have a worse prognosis (HR: 1.261, 95% CI 1.050–1.514, p = 0.013). However, there was no significant statistical difference between patients receiving adjuvant chemotherapy and not receiving adjuvant chemotherapy (p = 0.233).
Then we analyzed the prognostic factors in patients with VPI. The association of variables including age, gender, race, grade, histology, tumor size, laterality, and adjuvant chemotherapy status with survival was conducted by univariate regression analysis. Significantly, difference was found in specific variables except race (p = 0.070) and liberality (p = 0.181).
Multivariate analysis showed that age, gender, grade, histological grade were independent prognostic factors associated with OS in stage IB NSCLC patients with VPI (Table 6). In details, with the increase of the patient’s age, the patient’s prognosis is getting worse and worse (age 60–74, HR: 1.872, 95% CI 1.259–2.783; age ≥ 75, HR: 3.011, 95% CI 1.976–4.588, p < 0.0001), female had a better OS than male (HR: 0.683, 95% CI 0.537–0.869, p = 0.002), and a worse prognosis was found in low differentiated (HR: 1.404, 95% CI 1.096–1.800, p = 0.007), squamous cell carcinoma (HR: 1.454, 95% CI 1.101–1.920, p = 0.025). There was no statistical difference in OS between the total population with or without chemotherapy (p = 0.216).
Table 6.
Univariate and multivariate analysis of the VPI+ patients
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| p value | HR | 95% CI | p value | |
| Age | < 0.0001 | < 0.0001 | ||
| 20–59 | Ref. | |||
| 60–74 | 1.872 | 1.259–2.783 | ||
| ≥ 75 | 3.011 | 1.976–4.588 | ||
| Gender | 0.001 | 0.002 | ||
| Male | Ref. | |||
| Female | 0.683 | 0.537–0.869 | ||
| Race | 0.070 | NA | ||
| White | ||||
| Black | ||||
| Others | ||||
| Gradea | 0.003 | 0.007 | ||
| I–II | Ref. | |||
| III–IV | 1.404 | 1.096–1.800 | ||
| Histology | 0.012 | 0.025 | ||
| Adenocarcinoma | Ref. | |||
| Squamous cell carcinoma | 1.454 | 1.101–1.920 | ||
| Others NSCLC | 0.928 | 0.532–1.617 | ||
| Tumor size | 0.023 | 0.106 | ||
| 0–3 cm | Ref. | |||
| 3.1–4 cm | 1.237 | 0.956–1.602 | ||
| Laterality | 0.181 | NA | ||
| Left | ||||
| Right | ||||
| Adjuvant chemotherapy | 0.029 | 0.216 | ||
| Yes | Ref. | |||
| No/unknown | 1.294 | 0.860–1.946 | ||
aI—well differentiated, II—moderate differentiated, III—poorly differentiated, IV—undifferentiated
We removed the age factor and re-analyzed the data (Supplementary Table 1). Multivariate analysis showed that gender, grade, histological grad, tumor size, and adjuvant chemotherapy status were independent prognostic factors associated with OS in stage IB NSCLC patients with VPI. In details, female had a better OS than male (HR: 0.714, p = 0.006), and a worse prognosis was found in low differentiated (HR: 1.393, 95% CI 1.087–1.784, p = 0.009), squamous cell carcinoma (HR: 1.596, 95% CI 1.212–2.101, p = 0.001), large tumors (HR: 1.313, 95% CI 1.015–1.699, p = 0.038), and patients without adjuvant chemotherapy (HR: 1.548, 95% CI 1.035–2.313, p = 0.033).
Discussion
The role of adjuvant chemotherapy in early stage lung cancer patients was uncertain. One study (Jang et al. 2017) demonstrated that the 5-year survival rate between 208 patients who receiving adjuvant chemotherapy and 110 patients who did not exhibited a statistical difference (79% vs. 92%). However, another research (Park et al. 2018) divided 89 patients with stage IB NSCLC into two groups based on the status of receiving adjuvant chemotherapy, and found that adjuvant chemotherapy did not improve disease-free survival and OS, even in high-risk patients, such as patients with VPI.
The incidence of VPI in early stage NSCLC is about 25% (Lakha et al. 2014), and most current studies consider that VPI is an risk factor affecting the prognosis of patients with early stage NSCLC (Qian et al. 2019; Shimizu et al. 2005; Shin et al. 2019; Wang et al. 2011; Oven Ustaalioglu et al. 2013; Maeda et al. 2011), but only a few studies do not (Hung et al. 2010; Nitadori et al. 2013; Travis et al. 2008). In 2004, Shimizu et al. (2004) reviewed the clinicopathological features and prognosis of 1653 patients with T1–T3 NSCLC who underwent surgery, and they found that patients with PL1 or PL2 tumors ≤ 3 cm had the same 5-year survival rate, which were significantly lower than patients with the same size of PL0 tumors. The survival rates of patients with PL1 or PL2 tumors > 3 m and T3 cancer patients were essentially the same. According to the results of the study, they suggested that tumors ≤ 3 cm had VPI and should be classified as T2, and tumors > 3 cm should be upgraded to T3 state. In the seventh of the TNM classification for lung cancer, tumors with VPI were classified as T2a, even if they were ≤ 3 cm (Takizawa et al. 2018). In 2014, Sameer Lakha et al. (2014) suggested that early stage lung cancer with pleural metastasis or tumor size of 3–5 and 5–7 cm should be reclassified to stages IIA (instead of stage IB) and IIB (instead of stage IIA), respectively, and adjuvant chemotherapy was recommended. A meta-analysis (Jiang et al. 2015) of 13 articles related to VPI found that VPI was a significant adverse prognostic factor in patients with node-negative NSCLC, regardless of tumor size, and patients with stage IB NSCLC and large tumor size with VPI might be considered for adjuvant chemotherapy after surgical resection. In addition to the above studies, Kang et al. (2003), Chang et al. (2012) also recommended that patients with VPI should receive adjuvant chemotherapy. However, one research (Park et al. 2018) insists that VPI is a high risk factor for prognosis, but it is not a decisive factor for adjuvant chemotherapy. In our study, adjuvant chemotherapy is not a prognostic factor for stage IB NSCLC patients with VPI. Therefore, our conclusion is consistent with the last article (Park et al. 2018). VPI is not the determining factor of adjuvant chemotherapy, although it is one of the high risk factors for prognosis. If patients with VPI really progress to stage II, whether they could benefit from adjuvant chemotherapy requires further research. Therefore, it is particularly important to find an appropriate treatment for VPI, which may affect the prognosis of 25% of patients with early NSCLC.
Our study found that there was no statistical difference in prognosis between patients with VPI who received chemotherapy and those who did not receive adjuvant chemotherapy. In the total study population, adjuvant chemotherapy did not affect the prognosis. Therefore, our study suggested that patients with VPI in stage IB were not the beneficiaries of adjuvant chemotherapy. Our conclusions were consistent with those of two others similar studies (Park et al. 2018; Zhang et al. 2016). However, our sample size is larger and the conclusion is relatively more reliable. In the above two studies, 27 and 22 VPI patients received adjuvant chemotherapy, respectively, while there were 145 patients in our study. Our study also proved that age, gender, pathological subtypes, and tumor size were independent factors affecting the prognosis of postoperative NSCLC patients with VPI in stage IB, which were consistent with previous studies (Yang et al. 2017). We further found that there was a statistical difference in OS between receiving and not receiving chemotherapy in stage IB NSCLC with VPI, when age factors were removed. By comparing the differences between receiving adjuvant chemotherapy group and not receiving adjuvant chemotherapy group in survival rates for 1-, 3- and 5-years, we found that these differences tended to expand as the follow-up time lengthened. Although the results were only a descriptive statistic, it could still provide guidance for clinical research on the role of adjuvant chemotherapy.
Our study still has some limitations. First, this was a retrospective study rather than a prospective randomized study. Second, previous studies (Okamoto et al. 2018; Okuda et al. 2018; Urushiyama et al. 2018) have found that there were differences in the efficacy of different chemotherapy regimens, and we do not know the specific chemotherapy regimens, so we cannot compare the detailed chemotherapy regimens. Because the classification of chemotherapy in the SEER database is YES and NO/unknown, it is not possible to determine the number of unknown and whether this group of people would have an impact on the results. Prospective studies are needed to verify our conclusions. Third, according to the degree of visceral pleura metastasis, it can be further divided into PL0, PL1, PL2, and PL3. One study (Tanju et al. 2019) found that there was no difference in prognosis between PL0 and PL1, but there was a difference between PL1 and PL2. While another study (Seok et al. 2017) found that there was no survival difference between PL1 and PL2. Therefore, we do not know the effect of adjuvant chemotherapy on pleural invasion of different degrees of metastasis, especially PL1 and PL2. Finally, our study design was based on the SEER database, so the conclusions might only apply to patients in the United States.
In conclusion, this was the first large-scale database study to explore whether patients with stage IB NSCLC with VPI could benefit from adjuvant chemotherapy.
In our study, stage IB NSCLC did not benefit from adjuvant chemotherapy, even in patients with VPI. However, the significance of adjuvant chemotherapy in patients with VPI is still worth further exploration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
JX, XZ, SH, WDP, BX, YL, SJZ, QL, and CL developed the study. JX, XZ, and SH are contributors in writing the manuscript. WDP, BX, and YL analyzed the patient data. SJZ, QL, and CL completed the final review of the manuscript. All authors discussed the results and approved the final manuscript.
Funding
This work was supported by a Grant from the International Science and Technology Cooperation Project of the Changzhou Science and Technology Bureau (number CZ20140016).
Compliance with ethical standards
Conflict of interest
The authors declare they have no conflict of interest
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jun Xie, Xian Zhang and Song Hu contributed equally to this work and are co-first authors.
Contributor Information
Qing Li, Email: liqblk@163.com.
Chong Li, Email: zeyou06@163.com.
References
- Adachi H et al (2015) Influence of visceral pleural invasion on survival in completely resected non-small-cell lung cancer. Eur J Cardiothorac Surg 48(5):691–697 (discussion 697) [DOI] [PubMed] [Google Scholar]
- Bray F et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424 [DOI] [PubMed] [Google Scholar]
- Burdett S et al (2015) Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev 3:CD011430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts CA et al (2010) Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 28(1):29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YL et al (2012) The significance of visceral pleural surface invasion in 321 cases of non-small cell lung cancers with pleural retraction. Ann Surg Oncol 19(9):3057–3064 [DOI] [PubMed] [Google Scholar]
- Holmes EC (1994) Surgical adjuvant therapy for stage II and stage III adenocarcinoma and large cell undifferentiated carcinoma. Chest 106(6 Suppl):293S–296S [DOI] [PubMed] [Google Scholar]
- Huang H et al (2015) Visceral pleural invasion remains a size-independent prognostic factor in stage I non-small cell lung cancer. Ann Thorac Surg 99(4):1130–1139 [DOI] [PubMed] [Google Scholar]
- Hung JJ et al (2010) Prognostic factors in pathological stage IB nonsmall cell lung cancer greater than 3 cm. Eur Respir J 36(6):1355–1361 [DOI] [PubMed] [Google Scholar]
- Hung JJ et al (2016) Adjuvant chemotherapy improves the probability of freedom from recurrence in patients with resected stage IB lung adenocarcinoma. Ann Thorac Surg 101(4):1346–1353 [DOI] [PubMed] [Google Scholar]
- Jang HJ et al (2017) Effect of adjuvant chemotherapy after complete resection for pathologic stage IB lung adenocarcinoma in high-risk patients as defined by a new recurrence risk scoring model. Cancer Res Treat 49(4):898–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L et al (2015) The impact of visceral pleural invasion in node-negative non-small cell lung cancer: a systematic review and meta-analysis. Chest 148(4):903–911 [DOI] [PubMed] [Google Scholar]
- Kang JH, Kim KD, Chung KY (2003) Prognostic value of visceral pleura invasion in non-small cell lung cancer. Eur J Cardiothorac Surg 23(6):865–869 [DOI] [PubMed] [Google Scholar]
- Lakha S et al (2014) Prognostic significance of visceral pleural involvement in early-stage lung cancer. Chest 146(6):1619–1626 [DOI] [PubMed] [Google Scholar]
- Maeda R et al (2011) Poor prognostic factors in patients with stage IB non-small cell lung cancer according to the seventh edition TNM classification. Chest 139(4):855–861 [DOI] [PubMed] [Google Scholar]
- Nitadori JI et al (2013) Visceral pleural invasion does not affect recurrence or overall survival among patients with lung adenocarcinoma </= 2 cm: a proposal to reclassify T1 lung adenocarcinoma. Chest 144(5):1622–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T et al (2018) A phase II randomized trial of adjuvant chemotherapy with S-1 versus S-1 plus cisplatin for completely resected pathological stage II/IIIA non-small cell lung cancer. Lung Cancer 124:255–259 [DOI] [PubMed] [Google Scholar]
- Okuda K et al (2018) S-1 vs. paclitaxel plus carboplatin as adjuvant chemotherapy for completely resected stage II/IIIA non-small-cell lung cancer. Mol Clin Oncol 8(1):73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oven Ustaalioglu BB et al (2013) Prognostic factors for lymph node negative stage I and IIA non-small cell lung cancer: multicenter experiences. Asian Pac J Cancer Prev 14(11):6287–6292 [DOI] [PubMed] [Google Scholar]
- Park HS et al (2012) Overview of the surveillance, epidemiology, and end results database: evolution, data variables, and quality assurance. Curr Probl Cancer 36(4):183–190 [DOI] [PubMed] [Google Scholar]
- Park BJ et al (2018a) Temporal and regional distribution of initial recurrence site in completely resected N1-stage II lung adenocarcinoma: the effect of postoperative adjuvant chemotherapy. Lung Cancer 117:7–13 [DOI] [PubMed] [Google Scholar]
- Park HJ et al (2018b) Efficacy of adjuvant chemotherapy for completely resected stage IB non-small cell lung cancer: a retrospective study. J Thorac Dis 10(4):2279–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J et al (2019) Adjuvant chemotherapy candidates in stage I lung adenocarcinomas following complete lobectomy. Ann Surg Oncol 26(8):2392–2400 [DOI] [PubMed] [Google Scholar]
- Roselli M et al (2006) Postsurgical chemotherapy in stage IB nonsmall cell lung cancer: long-term survival in a randomized study. Int J Cancer 119(4):955–960 [DOI] [PubMed] [Google Scholar]
- Salazar MC et al (2016) Adjuvant chemotherapy for T3 non-small cell lung cancer with additional tumor nodules in the same lobe. J Thorac Oncol 11(7):1090–1100 [DOI] [PubMed] [Google Scholar]
- Seok Y, Jeong JY, Lee E (2017) Extent of visceral pleural invasion and the prognosis of surgically resected node-negative non-small cell lung cancer. Thorac Cancer 8(3):197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K et al (2004) Visceral pleural invasion classification in non-small cell lung cancer: a proposal on the basis of outcome assessment. J Thorac Cardiovasc Surg 127(6):1574–1578 [DOI] [PubMed] [Google Scholar]
- Shimizu K et al (2005) Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg 130(1):160–165 [DOI] [PubMed] [Google Scholar]
- Shin JW et al (2019) Prognostic factors in stage IIB non-small cell lung cancer according to the 8th edition of TNM staging system. Korean J Thorac Cardiovasc Surg 52(3):131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34 [DOI] [PubMed] [Google Scholar]
- Stein CF et al (2010) Global burden of disease 2000: version 2, methods and results. Global program on evidence for health policy, 2010, Discussion Paper 50
- Takizawa H et al (2018) Autofluorescence for the diagnosis of visceral pleural invasion in non-small-cell lung cancer. Eur J Cardiothorac Surg 53(5):987–992 [DOI] [PubMed] [Google Scholar]
- Tanju S et al (2019) Level of pleural invasion effects on prognosis in lung cancer. Tumori 105(2):155–160 [DOI] [PubMed] [Google Scholar]
- Taylor NA et al (2004) Equivalent outcome of patients with clinical Stage IIIA non-small-cell lung cancer treated with concurrent chemoradiation compared with induction chemotherapy followed by surgical resection. Int J Radiat Oncol Biol Phys 58(1):204–212 [DOI] [PubMed] [Google Scholar]
- Thakur MK et al (2018) Risk of second lung cancer in patients with previously treated lung cancer: analysis of surveillance, epidemiology, and end results (SEER) data. J Thorac Oncol 13(1):46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis WD et al (2008) Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol 3(12):1384–1390 [DOI] [PubMed] [Google Scholar]
- Urushiyama H et al (2018) Oral fluorouracil vs vinorelbine plus cisplatin as adjuvant chemotherapy for stage II–III. A non-small cell lung cancer: propensity score-matched and instrumental variable analyses. Cancer Med 7(10):4863–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WL, Shen-Tu Y, Wang ZQ (2011) Prognostic factors for survival of stage IB upper lobe non-small cell lung cancer patients: a retrospective study in Shanghai, China. Chin J Cancer Res 23(4):265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X et al (2017) Prognostic value of visceral pleural invasion in non-small cell lung cancer: a propensity score matching study based on the SEER registry. J Surg Oncol 116(3):398–406 [DOI] [PubMed] [Google Scholar]
- Yoshida J et al (2009) Visceral pleura invasion impact on non-small cell lung cancer patient survival: its implications for the forthcoming TNM staging based on a large-scale nation-wide database. J Thorac Oncol 4(8):959–963 [DOI] [PubMed] [Google Scholar]
- Zhang H et al (2016) The predictive and prognostic values of factors associated with visceral pleural involvement in resected lung adenocarcinomas. Onco Targets Ther 9:2337–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T et al (2018) Meta-analysis of adjuvant chemotherapy versus surgery alone in T2aN0 stage IB non-small cell lung cancer. J Cancer Res Ther 14(1):139–144 [DOI] [PubMed] [Google Scholar]
- Zou B et al (2010) A multicenter retrospective analysis of survival outcome following postoperative chemoradiotherapy in non-small-cell lung cancer patients with N2 nodal disease. Int J Radiat Oncol Biol Phys 77(2):321–328 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



