Abstract
Dictyostelium amoebae accomplish a starvation-induced developmental process by aggregating into a mound and forming a single fruiting body with terminally differentiated spores and stalk cells. culB was identified as the gene disrupted in a developmental mutant with an aberrant prestalk cell differentiation phenotype. The culB gene product appears to be a homolog of the cullin family of proteins that are known to be involved in ubiquitin-mediated protein degradation. The culB mutants form supernumerary prestalk tips atop each developing mound that result in the formation of multiple small fruiting bodies. The prestalk-specific gene ecmA is expressed precociously in culB mutants, suggesting that prestalk cell differentiation occurs earlier than normal. In addition, when culB mutant cells are mixed with wild-type cells, they display a cell-autonomous propensity to form stalk cells. Thus, CulB appears to ensure that the proper number of prestalk cells differentiate at the appropriate time in development. Activation of cyclic AMP-dependent protein kinase (PKA) by disruption of the regulatory subunit gene (pkaR) or by overexpression of the catalytic subunit gene (pkaC) enhances the prestalk/stalk cell differentiation phenotype of the culB mutant. For example, culB− pkaR− cells form stalk cells without obvious multicellular morphogenesis and are more sensitive to the prestalk O (pstO) cell inducer DIF-1. The sensitized condition of PKA activation reveals that CulB may govern prestalk cell differentiation in Dictyostelium, in part by controlling the sensitivity of cells to DIF-1, possibly by regulating the levels of one or more proteins that are rate limiting for prestalk differentiation.
Development in multicellular organisms is precisely regulated in time and space such that morphogenesis and cell differentiation are coordinated. Dictyostelium development is characterized by the chemotactic aggregation of starved cells and the subsequent formation of a multicellular fruiting body composed of the differentiated spores and stalk cells. Two major cell types, prestalk and prespore, initially differentiate within the aggregate in a spatially independent manner. The prestalk cells then sort to the presumptive tip of the aggregate, and subsequently form the tip of an elongated finger-like structure (72, 75, 76). When this finger falls over to form a migrating slug, most of the prestalk cells remain in the anterior region. During terminal-cell differentiation and fruiting body formation, the slug rears up to form a “second finger” and then flattens into a structure resembling a Mexican hat. The culmination of development begins with the formation of a stalk tube that is initiated in the center of this structure by a subpopulation of prestalk cells. The rest of the prestalk cells in the tip migrate into the stalk tube and differentiate into stalk cells. As the stalk forms, it lifts the prespore cells aloft while the prespore cells actively move up it before differentiating into spores within the nascent sorus (64). There is substantial genetic and biochemical evidence for signaling between prestalk and prespore cells and for the coordinated movement of prestalk and prespore cells that is required for spatially and temporally regulated terminal differentiation (see, e.g., references 5, 11, 26, 30, 31, and 56). Recent work has focused on the molecular nature of these signaling events and how they are regulated (13, 15, 21).
One of the key control points for coordinating morphogenesis and cell differentiation in Dictyostelium involves the activation of cyclic AMP (cAMP)-dependent protein kinase (PKA) (20, 42). PKA is involved in a wide range of developmental processes in Dictyostelium, as has been found in metazoan species. It translates signals from the outside of cells, such as neurotransmitters, peptide transmitters, and growth hormones, into specific phosphorylation of downstream target proteins (69). PKA also plays a critical role in learning and memory in Drosophila and mice (2, 16, 59). In Dictyostelium, PKA regulates aggregation, prespore and prestalk cell differentiation, morphogenesis, and terminal cell differentiation (reviewed in reference 41). PKA activity is controlled through regulation of the intracellular cAMP concentration. Increased cAMP concentrations activate PKA by binding to its regulatory subunit(s) and releasing an active catalytic subunit(s). Although PKA is essential for Dictyostelium development, it is believed that multiple signaling pathways cooperate with PKA to regulate the precise timing of developmental events (5–7, 10, 13, 24, 31, 33, 40, 43, 45, 46, 50, 53, 55, 63, 65).
A number of experiments have shown that increased PKA activity allows Dictyostelium cells to develop rapidly, differentiate precociously, or even bypass critical regulatory events throughout development (1, 34, 58, 70, 71). We sought to identify developmental regulators that were more or less independent of PKA control. To do this, we performed a genetic screen for modifiers of the developmental phenotype of a mutant with constitutively active PKA (pkaR−, a regulatory-subunit null mutant). Since activation of PKA allows cells to bypass many of the regulatory events controlled by cAMP during early development, we expected to identify genes that function independently or “downstream” of PKA-promoted regulatory events. One mutant from the screen resulted from a null mutation in culB, a gene that encodes a protein with a high degree of similarity to the cullins.
Cullins from an evolutionarily conserved family of proteins that were first recognized in Caenorhabditis elegans (37). They are involved in a cascade of reactions in which ubiquitin is transferred from a ubiquitin-activating enzyme, E1, to a ubiquitin-conjugating enzyme, E2, and then to a lysine residue in the target protein through an E3-ubiquitin ligase (18, 49, 79, 80). The E3 complex acts as a bridge between the specific target and the appropriate E2, thereby providing specificity to the ubiquitin transfer reaction (54). Multiple rounds of ubiquitination of the initial conjugate lead to polyubiquitination of the target protein, which is subsequently recognized and degraded by the 26S proteosome. Cullins are subunits of some E3 ubiquitin-ligase complexes, such as the anaphase-promoting complex and SCF complex (19, 60, 79, 80). The anaphase-promoting complex is critical for regulating anaphase progression during the cell cycle, while SCF complexes mediate the degradation of a wide array of regulatory proteins in yeast and in mammals, such as the cyclin-dependent kinase inhibitors, transcription factors, and DNA replication initiation proteins (18, 77). Cullin function is fairly well understood in the SCF ubiquitin-ligase complex (reviewed in reference 18). In the SCF complex, the cullin protein Cdc53 interacts with the Skp1 protein. Skp1, in turn, interacts with an F-box protein that confers target specificity. The SCF is brought together with the ubiquitin conjugase E2 through the interaction of Cdc53 with E2.
Recently, ubiquitin-mediated protein degradation has been implicated in the regulation of cell differentiation and morphogenesis in Dicytostelium. Cells that are mutant in NosA, a homolog of deubiquitinating enzymes, arrest at the tight-aggregate stage (51). Disruption of Ubc1, a ubiquitin-conjugating enzyme, leads to arrest at the aggregate stage (14). A null mutation in the F-box protein, Mekkα, or overexpression of its F-box domain and WD repeats causes abnormal cell type proportioning (12). Finally, the cullin homolog CulA appears to regulate PKA activity by controlling the level of RegA, a cAMP-specific phosphodiesterase (46). Here, we describe the role of CulB in Dictyostelium development and present evidence that CulB regulates prestalk cell differentiation and morphogenesis.
MATERIALS AND METHODS
Cell culture, transformation and development.
Dictyostelium cells were grown in HL-5 liquid medium or on SM nutrient agar in association with bacteria (61). Ax4 was used as the wild-type strain in these studies. pkaR− cells were generated from Ax4 cells as described previously and used as the parental strain for large-scale mutagenesis (71). Restriction enzyme-mediated integration mutagenesis using DpnII, blasticidin selection, and linearized pBsr1 vector was performed as previously described (3, 38). Approximately 80,000 independent mutants were screened for developmental morphologies that differed from the parental strain. A full description of this screen will be described elsewhere (B. Wang and A. Kuspa, unpublished data). Selected strains were isolated, and their pBSR1 insertions and flanking genomic regions were cloned by plasmid rescue. Recapitulation of the original insertion events by homologous recombination and selection for blasticidin resistance was performed as described previously (3, 39). DNA transformations were performed by electroporation (44) or by calcium phosphate precipitation and glycerol shock (48). For development, cells were washed in phosphate buffer (50 mM KH2PO4, 50 mM Na2HPO4 [pH 6.1]) and spread onto Millipore filters or onto 1% nonnutrient agar plates (61).
cDNA cloning and plasmid construction.
Standard DNA and RNA manipulations were carried out as described previously (52). A cDNA library in the Lambda ACT2 vector (constructed by Sijie Lu) was used to isolate a full-length culB cDNA. The 1.6-kb XbaI genomic fragment of culB, which encodes the N terminus of CulB, was used as a probe to screen the library. Nine positive clones were obtained from 2.5 × 105 phage. The phage were converted into plasmids as described previously (47). The phage with the longest insert, p8, was thus converted into plasmid pcul8B. The analysis of the pcul8B sequence and the genomic sequence of culB confirmed that pcul8B contained the predicted full-length culB cDNA. The cDNA insert is 2.5 kb long and is predicted to encode a 772-amino-acid CulB protein. A CulB expression vector was generated by inserting the culB cDNA into the pDXA-HY vector, in frame with the upstream actin15 promoter and protein tag sequence (44). Plasmid pCulBsr was constructed by circularizing the culB XbaI genomic fragment by ligation with T4 ligase and inserting BamHI-linearized pBsr1 plasmid (a gift from W. F. Loomis) into the BamHI site within the fragment. To generate culB insertion mutations, pCulBsr was linearized with XbaI and introduced into cells by electroporation.
Molecular analyses.
Total RNA was prepared from cells developing on Millipore filters. Cells were collected at various times during development, washed, and frozen on dry ice. RNA was purified with TRIZol reagent (Invitrogen) as specified by the manufacturer. For Northern analyses, 10 μg of RNA was loaded into each lane and size fractionated on 1% agarose gels containing 2.2 M formaldehyde. Gel transfer and membrane hybridization to 32P-labeled DNA probes were performed by standard procedures (52). RNase protection assays were carried out as specified by the manufacturer of the assay (Ambion RPA II). A 360-bp fragment covering the insertion site of culB was generated by PCR using primers 5′GAAGGTGGTTTAGCTCCAG3′ and 5′TAATACGACTCACTATAGGGAGGGACCTAACTTCTCTTCC3′. The latter primer includes a T7 promotor at its 5′ end for use in synthesizing the antisense RNA probe used in the RNase protection assays. Southern analyses of genomic DNA were carried out with 32P-labeled DNA probes as described previously (52).
Spore assay.
After 48 h of development on filters, the cells were collected into 20 mM potassium phosphate buffer (pH 6.2) and treated with the nonionic detergent NP-40 (0.4%) for 10 min at room temperature. After the detergent treatment, the cells were washed with potassium phosphate buffer twice and disaggregated by trituration with an 18-gauge needle. Ellipsoid and refractile spores were counted by phase-contrast microscopy and plated clonally on SM agar plates with bacteria. The number of resulting colonies was used as an estimate of the number of viable spores in each sample, and this was used to calculate the total number of spores produced from 5 × 107 input cells. Under these conditions, wild-type cells (Ax4) produce 67% ± 8% detergent-resistant spores and 82% ± 5% of these produce viable colonies on bacterial growth plates. At least three independent determinations were carried out for each strain and are reported as the mean ± standard error of the mean.
Submerged-monolayer assay.
The submerged-monolayer assay was modified from an assay described previously (29). For stalk cell induction, vegetative cells were washed once with KK2 buffer (16.5 mM KH2PO4, 3.8 mM K2HPO4 [pH 6.2]) and three times with stalk buffer (10 mM morpholineethanesulfonic acid [MES], 2 mM NaCl, 10 mM KCl, 1 mM CaCl2, 200 μg of penicillin-streptomycin per ml [pH 6.2]). The cells were then plated into 24-well tissue culture plates at a density of 104 cells/cm2 and incubated in the presence or absence of differentiation-inducing factor (DIF). After a 48-h incubation, the buffer was removed without disturbing the cells. A Calcofluor solution (0.01%) (25) was added to the wells for 5 min. The Calcofluor solution was then removed, and the cells were observed immediately by microscopy. Only the cells that were vacuolated and stained by Calcofluor were counted as stalk cells (28). Cells were observed with a 32× objective lens, and the number of fluorescent vacuolated cells and the total number of cells were counted. At least 300 cells were counted for each assay, and the percentage of stalk cells formed was calculated by dividing the number of fluorescent vacuolated cells by the total number of cells. For spore cell induction, pkaR− cells and culB− pkaR− cells were washed once with KK2 buffer and three times with spore buffer (10 mM MES, 20 mM NaCl, 20 mM KCl, 1 mM CaCl2, 1 mM MgCl2) and then incubated in spore buffer supplemented with 5 mM cAMP (34).
Assays for DIF and PKA.
The DIF bioassay was based on the ability of DIF to induce stalk cell differentiation in isolated pkaR− cells in submerged monolayers. Briefly, the monolayer assay to induce stalk cell formation was performed as described above. The supernatants from the different conditions were collected after 48 h of incubation and added to at least three different wells for each assay. Fresh pkaR− cells were diluted 40-fold into the wells to a density of 104 cell/cm2. The number of stalk cells was determined after 48 h of incubation. The percentage of stalk cells was calculated as described above. PKA activity measurements were carried out using the SignaTECT PKA assay system (Promega). Cell extracts containing 10 μg of protein were prepared as specified by the manufacturer and were used in reactions with 5 mM cAMP and in the presence or absence of 10 μM PKA-specific inhibitor PKI, which inhibits the Dictyostelium enzyme (42). PKA activity is defined as the amount of Kemptide substrate phosphorylated (nanomoles per minute per milligram of protein) in the absence of PKI minus the amount phosphorylated in the presence of PKI.
RESULTS
Identification of CulB, a putative cullin homolog.
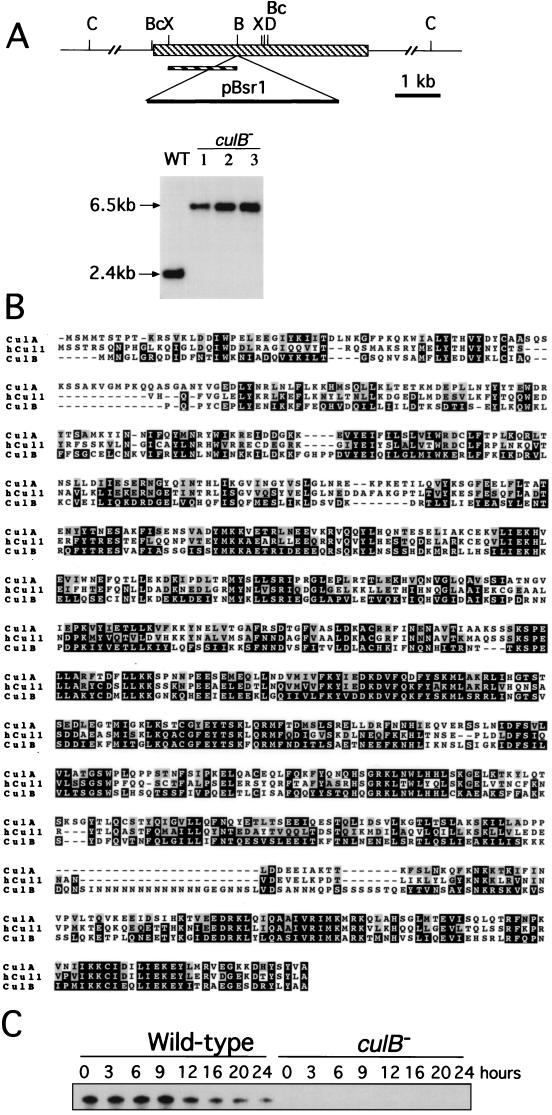
In an attempt to identify developmental genes that function relatively independently of PKA control, we carried out a screen for genetic modifiers of a mutant with constitutively active PKA (see Materials and Methods). From this screen, we isolated culB, a gene that is predicted to encode a cullin homolog. Disruption of the culB gene results in cells that form smaller fruiting bodies. By cloning, mapping, and sequencing the disrupted culB locus, we found that the mutation resulted from a plasmid insertion in the middle of the culB gene and the loss of the 3′ half of the gene along with 8 kb of flanking genomic DNA (data not shown). To test whether the disruption of the culB gene or the missing genomic DNA caused the mutant phenotype, we constructed a new targeting vector, pCulBsr, and introduced it into wild-type cells. The mutant phenotype was recapitulated in the homologous recombinants that acquired a simple insertion in culB, as confirmed by Southern analysis (Fig. 1A).
FIG. 1.
Characterization of culB. (A) culB genomic locus. pBsr1 is a 4.1-kb plasmid with the selectable Bsr cassette that was inserted into the BamHI site of the culB gene (hatched rectangle). Genomic DNA from wild-type, culB− (clones 1 and 2) or culB− pkaR− (clone 3) cells was digested with BclI, separated by agarose gel electrophoresis, blotted, and probed with the XbaI-BamHI genomic DNA fragment (hatched bar) in a standard Southern analysis. Restriction enzyme sites: C, ClaI; X, XbaI: B, BamHI; Bc, BclI; D, DpnII. (B) Comparison of the predicted amino acid sequences of CulB, CulA, and human Cul1 (hCul1). The amino acid identities between CulB (AF144717), CulA (AF020287), and human Cul1 (U58087) are in black, and amino acid similarities are in highlighted in gray. (C) Expression of culB. RNA was extracted from cells at the indicated times after the initiation of development. RNase protection assays carried out on 10 μg of RNA from each sample revealed a single RNA species.
The isolation and analysis of genomic and cDNA clones of culB revealed a gene that is predicted to encode a 772-amino-acid protein that has 37% identity to the human Cul-1 protein and 40% identity to the Dictyostelium CulA protein (Fig. 1B). All of the mutant phenotypes associated with the culB mutation that are described below could be rescued when the full-length culB cDNA was expressed in culB− cells under the control of the actin 15 promoter, confirming that the phenotypes were caused by the loss of CulB function (data not shown). Expression of the culB mRNA could not be detected by Northern analyses of wild-type cells. However, using an RNase protection assay, we found that culB was expressed in vegetative cells, and its mRNA level remained constant throughout the first 9 h of development but decreased gradually to the end of the development (Fig. 1C). In culB− cells, the mRNA could not be detected, even by RNase protection assays, confirming that it is a null mutant.
culB− mutants display aberrant prestalk cell differentiation.
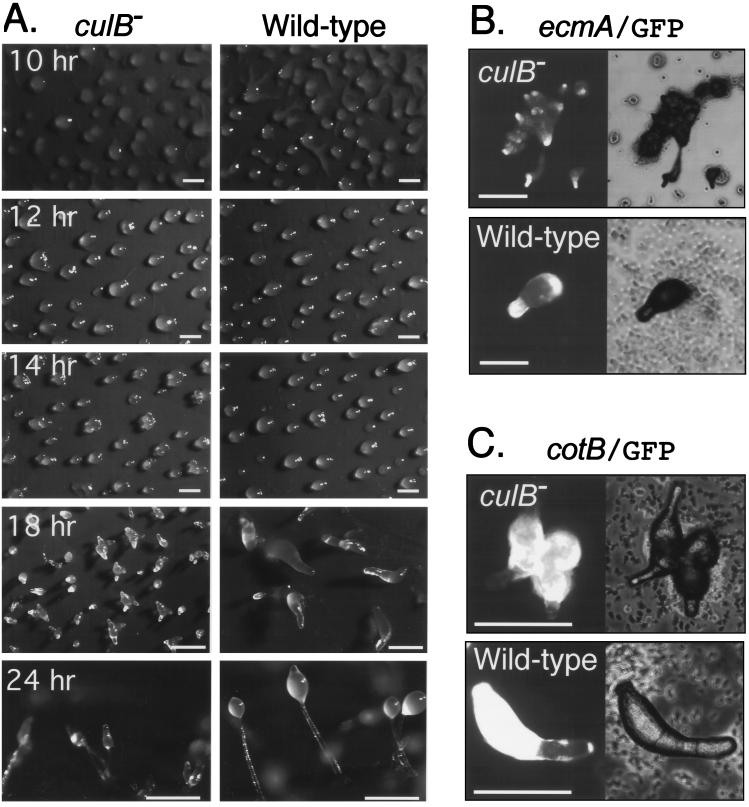
culB− cells aggregated on filters in a manner similar to wild-type cells, but multiple tips formed on most of the aggregates, each of which later elongated into a finger structure. These fingers then continued to develop and finally culminated to form small fruiting bodies (Fig. 2A). The culB− cells produced 17% ± 2.8% of the wild-type number of viable spores. The formation of aggregates with supernumerary tips suggested that prestalk cell differentiation was abnormal in the culB− mutant. Therefore we examined the sorting and terminal differentiation of the major cell types in culB− cells by marking them with green fluorescent protein (GFP). Using ecmA/GFP as a prestalk marker, and cotB/GFP as a prespore marker, we found that prestalk cells and prespore cells sorted appropriately during the development of culB− cells. Within the aggregates of culB− mutants, although they were multitipped, ecmA-positive cells were clearly located in the tips, just as in the wild type (Fig. 2B). In fruiting bodies, ecmA-positive cells composed the stalk cell compartments including the upper cup, lower cup, stalk, and basal disk (data not shown). The cotB/GFP-marked prespore cells remained in the lower half of the aggregate and finger structure and eventually formed spores within the spore head of the fruiting bodies (Fig. 2C).
FIG. 2.
Abnormal prestalk cell differentiation in culB− cells. (A) Cells were deposited on filters and allowed to develop, and photographs were taken at the indicated times. Bars, 0.5 mm. (B and C) Cells transformed with the reporter plasmids ecmA/GFP, a prestalk marker (B), or cotB/GFP, a prespore marker (C), were photographed after 18 h of development on filters.
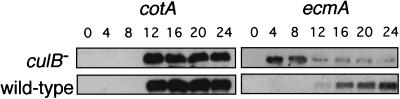
Examination of the temporal pattern of cell-type-specific gene expression revealed that ecmA was expressed precociously in culB− cells (Fig. 3). EcmA mRNA accumulated to detectable levels in culB− cells after 4 h of development, 8 h earlier than it could be detected in wild-type cells. This suggests that the disruption of culB results in precocious prestalk cell differentiation. The expression pattern of the prespore gene cotA in culB− cells was similar to that in wild-type cells, indicating that prespore cell differentiation is not affected in culB− cells (Fig. 3).
FIG. 3.
Cell-type-specific gene expression in culB− mutants. The expression of cotA (a prespore gene) and ecmA (a prestalk gene) was examined by extracting RNA from cells at the indicated times of development and analyzing them on Northern blots. For each gene probe, the blots were hybridized in the same chamber, and representatives of three independent experiments are shown.
The propensity of culB− mutants to form stalk cells is cell autonomous.
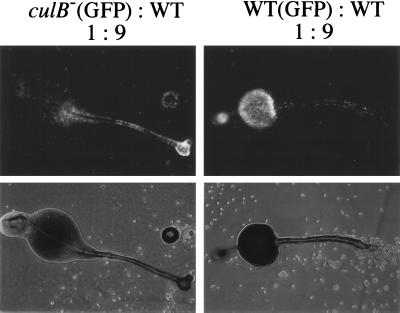
To test whether culB− cells have a cell autonomous propensity to form stalk cells, we assessed the differentiation of culB− cells in culB−/wild-type chimeras. culB− cells carrying an act15/GFP construct, which marks all cells, were mixed with unmarked cells in various combinations. In the context of an excess of wild-type cells (9:1 ratio), the culB− cells were found distributed in the stalk, basal disk, upper cup, and lower cup of the fruiting body, suggesting that they have a tendency to form stalk cells (Fig. 4). GFP-marked wild-type cells were distributed in both the stalk region and the spore region when mixed with unmarked wild-type cells (Fig. 4). In control experiments, GFP-marked culB− cells were found distributed throughout the fruiting body when mixed with unmarked culB− cells (1:9 ratio), whereas marked wild-type cells were detected mainly in the spore population when mixed with an excess of culB− cells (data not shown). These experiments indicate that the culB− mutant has a cell-autonomous propensity to form stalk cells placed in the environment of developing wild-type cells.
FIG. 4.
culB− cells have a propensity to form stalk cells in culB−/wild-type chimeras. Cells marked with an actin/GFP reporter plasmid were mixed with unmarked wild-type cells in a 1:9 ratio and allowed to develop into fruiting bodies. The actin/GFP-marked wild-type cells (WT) used as a control were mixed with unmarked wild-type cells.
culB− cells are defective in slug formation and migration.
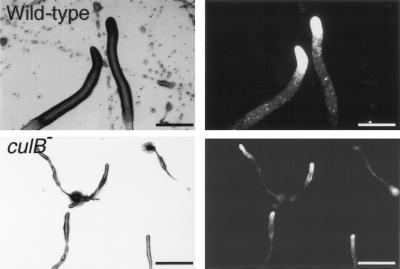
Prestalk cells within the tips of migrating slugs are thought to control slug phototaxis and thermotaxis (reviewed in references 22 and 23). As further evidence that prestalk cell differentiation is abnormal in culB mutants, we found that culB− cells were defective in slug formation and migration. When deposited on water agar plates and exposed to unidirectional light, culB− cells had a propensity to form aggregates and culminate rather than to form slugs. When slugs did form, they were smaller than wild-type slugs and not as mobile (Fig. 5). Wild-type slugs migrated about 1 to 2 cm in 24 h, while the culB− slugs all migrated less than 0.5 mm in 24 h.
FIG. 5.
The culB− mutant is defective in slug formation and migration. Cells were deposited on nonnutrient agar plates and kept in a dark chamber with unidirectional light for 24 h. Both wild-type and culB− mutant slugs, marked with ecmA/GFP, were visualized by bright-field (left) and fluorescence (right) microscopy. Bars, 0.25 mm.
Stalk cell differentiation is uncoupled from morphogenesis in culB− pkaR− cells.
It has been shown that PKA is essential for prestalk cell differentiation (reviewed in reference 41). It also has been shown that CulA is required for the degradation of RegA, a cAMP phosphodiesterase that negatively regulates PKA activity, and that the culA− mutant phenotype can be rescued by activating PKA (46). To explore the possible functional relationship between CulB and PKA, we examined double mutants and found that the activation of PKA in culB− cells exacerbated their phenotype. PKA was activated in culB− cells by either overexpressing the catalytic subunit gene (pkaC) (by using an actin promoter to drive the expression of pkaC) or inactivating the regulatory subunit gene (pkaR). The culB− [act15/pkaC] cells and the culB− pkaR− cells displayed similar phenotypes in all of our assays, and so only the analyses of the culB− pkaR− cells are described below.
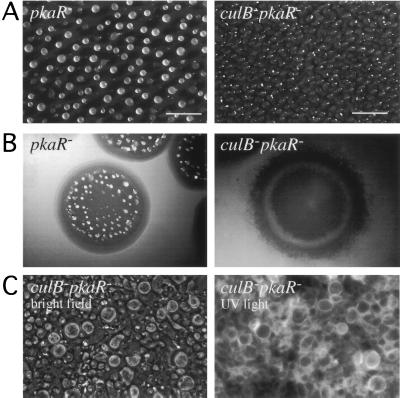
During development on filters, the culB− pkaR− cells accumulated into amorphous structures resembling the early stages of aggregation, but they did not form distinct aggregates (Fig. 6A). No spores were detected from cells deposited on filters for up to 48 h (see below). When plated with bacteria to allow growth and development, culB− pkaR− cells failed to aggregate (Fig. 6B). The colony surface remained flat for more than 5 days after the bacteria had been consumed. A circle of accumulated cells often appeared along the edge of the colonies, but they usually disappeared after the colonies expanded further. The control pkaR− cells aggregated and formed spherical structures within the colony, as expected (Fig. 6B). Although the culB− pkaR− cells did not aggregate after growth on bacteria, they did appear to differentiate since ecmA/GFP-positive cells were detectable in the colonies (data not shown). In addition, when cells were scraped from culB− pkaR− colonies and observed microscopically, vacuolated stalk-like cells were apparent. Calcofluor staining of these cells revealed cellulose-containing cell walls that are indicative of stalk cells (Fig. 6C). Other aggregation-deficient strains that we tested, such as PkaC-null or CRAC-null cells, do not produce cells that stain with Calcofluor (unpublished observations). However, no refractile ellipsoid spores were detected among culB− pkaR− cells, suggesting that the spore cell differentiation did not occur. We directly tested for viable spores after 48 h of development on filters. Some round, nonrefractile cells survived detergent treatment (∼0.05% of input cells), but fewer than 1 in 105 of the cells that were plated retained viability, as evidenced by colony formation on growth plates. These results suggest that the activation of PKA in the absence of CulB causes stalk cell differentiation without multicellular morphogenesis and without appreciable sporulation.
FIG. 6.
The culB− pkaR− cells differentiate into stalk cells without obvious morphogenesis. (A) Phenotype of pkaR and culB− pkaR− cells after 24 h of development on filters. (B) Colonies of pkaR and culB− pkaR− cells photographed after 5 days. (C) culB− pkaR− cells scraped from the colony surface after 5 days and stained with Calcofluor. Bars, 1 mm.
Prestalk differentiation is precocious and prespore differentiation is absent in culB− pkaR− cells.
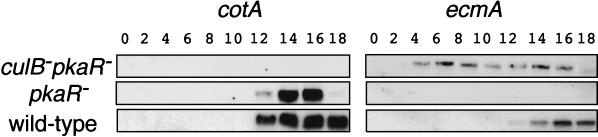
To examine the progression of cell differentiation during culB− pkaR− cell development, we studied the expression of cell-type-specific genes by Northern analysis. The prestalk ecmA gene was expressed in wild-type cells 12 h after starvation, as expected, when aggregates had formed. In culB− pkaR− cells, however, ecmA expression could be detected as early as 4 h after starvation, as in culB− cells. In the pkaR− control cells, ecmA expression could not be detected, and so compared to pkaR− cells, ecmA expression was also elevated in culB− pkaR− cells (Fig. 7). Our inability to detect ecmA expression by Northern analysis in pkaR− cells is reproducible, but it does not indicate that ecmA expression is absent in these cells. When the pkaR− cells carrying an ecmA/GFP reporter construct were examined after 36 h of development, about 5 to 10% of the cells were found to be GFP-positive stalk cells, indicating that the ecmA promoter is active over the course of development (data not shown). The prespore-specific gene cotA was expressed in wild-type cells and pkaR− cells normally after 12 h of starvation (Fig. 7). However, cotA was not expressed in culB− pkaR− cells. This is consistent with the observation that culB− pkaR− cells do not form spores and suggests that prespore cell differentiation does not occur in these cells.
FIG. 7.
Cell-type-specific gene expression in culB− pkaR− cells. Expression of the prestalk ecmA gene and the prespore cotA gene was examined by extracting RNA from cells at the indicated times of development (in hours) and analyzing 10 μg of RNA from each sample on Northern blots. For each gene probe, the blots were hybridized in the same chamber, and representatives of three independent experiments are shown.
culB− pkaR− cells are hypersensitive to DIF-1.
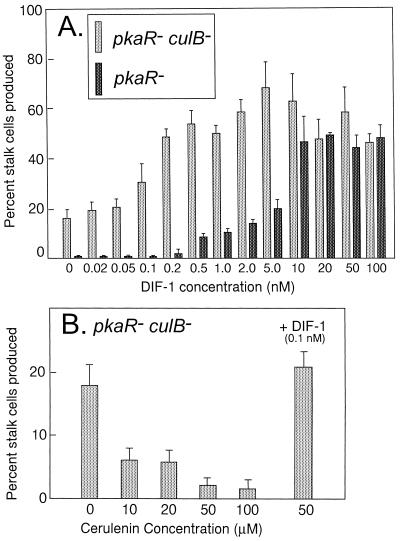
By incubation with cAMP and DIF-1 at low cell density, wild-type cells differentiate into cells with the characteristics of stalk cells in that they express prestalk- and stalk-specific genes, become vacuolated, produce cellulose, and lose viability (68). The addition of cAMP is thought to bring the cells to a DIF-responsive state, after which time they can be induced to form these stalk cells by treatment with DIF-1 (9). When pkaR− cells were incubated under submerged-culture conditions, they formed stalk cells after the addition of >0.2 nM DIF alone, without added cAMP (Fig. 8). These cells displayed a nonlinear response to DIF-1 concentrations between 0.5 and 10 nM and a maximal response to 10 to 100 nM DIF-1, where about half of the cells differentiated. These results with pkaR− cells are consistent with earlier findings (34, 63). However, about 17% of the culB− pkaR− cells formed stalk cells without added DIF-1 or cAMP (Fig. 8). Compared to pkaR− cells, a 5-fold-lower concentration of DIF-1 (0.1 nM) was required to elicit any response from the culB− pkaR− cells in this assay and about 50-fold less DIF-1 (0.2 versus 10 nM) induced a similar maximum percentage of stalk cell formation. The response of culB− pkaR− cells in this assay suggests that they are more sensitive to exogenous DIF-1 than are the control pkaR− cells. In similar DIF-1 assays, wild-type and culB− cells both required cAMP and DIF-1 for the formation of stalk cells and their response to DIF-1 was similar to that of pkaR− cells (data not shown).
FIG. 8.
culB− pkaR− mutants display an increased sensitivity to exogenous DIF in submerged culture. (A) culB− pkaR− cells and pkaR− cells were incubated at low density in submerged culture for 48 h. The percentage of vacuolated stalk cells was determined at different DIF concentrations. The mean and standard error of triplicate determinations are shown. (B) An inhibitor of DIF biosynthesis, cerulenin, inhibits the stalk-like cell differentiation of culB− pkaR− cells in submerged culture. culB− pkaR− cells were incubated for 48 h under the same conditions as in panel A in the presence of cerulenin.
If increased cellular synthesis of DIF-1 could account for the response of culB− pkaR− cells in the stalk cell induction assay, measurable DIF-1 should be detectable in the submerged culture media, assuming that excess DIF-1 is not completely dissolved in the cell membranes. We tested this by assaying for DIF-1 by its ability to convert pkaR− cells into stalk cells. Supernatants harvested from culB− pkaR− cells did not induce pkaR− cells to form stalk cells significantly more than did the control supernatant (the percentages of pkaR− test cells producing stalk-like cells were 1.5% ± 0.6% and 2.3% ± 0.5% for pkaR− and culB− pkaR− supernatant, respectively, in the absence of DIF after 48 h and 46% ± 2.0% and 43% ± 1.4% for pkaR− and culB− pkaR− supernatant, respectively, in the presence of 100 nM DIF after 48 h). This indicates that culB− pkaR− cells do not secrete large amounts of DIF-1 under these conditions (detection limit, 0.2 nM; Fig. 8A). It is generally accepted that the low-cell-density conditions of this assay prevent cell-cell communication during stalk cell formation since wild-type cells do require exogenous DIF-1 in order to differentiate. Any mutant with altered sensitivity to DIF-1 might challenge this assumption. For example, if 0.2 nM DIF-1 is normally achieved in this assay by mutant or wild-type cells, it would not have been detected in the assay described above, but it might influence stalk cell formation by culB− pkaR− cells. Therefore, we also tested whether culB− pkaR− cells required any DIF-1 that they secrete during the assay to induce their own differentiation. We incubated culB− pkaR− cells in submerged culture monolayers for 48 h and replaced the incubation buffer with fresh buffer every 5 h to remove any soluble DIF-1. About 15% of the culB− pkaR− cells still formed stalk cells. These results, taken together, suggest that secreted DIF-1 cannot account for the stalk cell differentiation of culB− pkaR− cells in the submerged-culture assay.
The assays above for secreted DIF-1 left open the possibility that endogenous DIF-1 that is retained by each cell supplies the DIF-1 needed for culB− pkaR− cells to differentiate into stalk cells. We tested whether the stalk cell differentiation of culB− pkaR− cells was DIF-1 dependent by examining their response to the polyketide synthase inhibitor cerulenin. Cerulenin has been shown to inhibit DIF-1 biosynthesis with a 50% inhibitory concentration of 1 to 2 μM and with maximal inhibition at concentrations of 30 to 100 μM (35). In the presence of 10 μM cerulenin, the formation of stalk cells by culB− pkaR− cells decreased about threefold and ≥50 μM cerulenin reduced the formation of stalk cells to levels roughly equivalent to that observed in the parental pkaR− cells without added DIF-1 (Fig. 8). The addition of DIF-1 together with cerulenin restores normal levels of stalk cell differentiation to the culB− pkaR− cells, demonstrating that the inhibition of stalk cell formation by cerulenin is not due to general cellular toxicity. These results suggest that endogenous DIF-1 synthesis is required for the differentiation of culB− pkaR− cells into stalk cells under these conditions.
DISCUSSION
We isolated and characterized the culB gene and found that it is involved in the regulation of prestalk cell differentiation and morphogenesis. Our results suggest that prestalk cell differentiation is actively suppressed until the proper time during development so that the optimal proportion of stalk and spore cells results. Given the similarity of CulB to known cullin proteins, it may act in a ubiquitin-mediated protein degradation pathway that mediates this suppression, perhaps by limiting the level of a protein, or set of proteins, that promote stalk cell differentiation. There may also be mechanisms to suppress prespore differentiation since prespore cells can be induced to sporulate prematurely by PKA activation (34), but CulB does not appear to be directly involved since prespore cell differentiation is relatively unaffected in the culB− mutant.
The differentiation of most prestalk cells occurs as the aggregate forms, and they are initially intermingled with prespore cells and “undifferentiated” cells, but they can be recognized by their expression of the ecmA or ecmB genes (reviewed in reference 73). Over the next several hours, many of the prestalk cells sort to the apical tip of the aggregate, and it is thought that once there, they coordinate further morphogenetic movements and differentiation events. The precocious expression of ecmA, which marks all prestalk cell subtypes to some extent, indicates that precocious prestalk differentiation is occurring during the development of culB mutants. It is possible that the abnormal morphogenesis of both the culB− and culB− pkaR− mutants is caused by this inappropriate prestalk cell differentiation. However, it is not clear whether the defects are due to alterations in the signaling capacity of the prematurely appearing prestalk cells or to an accelerated rate of the appearance of one cell type (prestalk) relative to the other (prespore). The fact that these mutants do not appear to inhibit the development of wild-type cells in chimeras, combined with the fact that they differentiate into all cell types when developing on their own, is suggestive of a cell-autonomous proportioning defect.
The cell type-proportioning defect observed in culB mutants is likely to be the cause of the observed morphological defects during development. The relative sizes of the prestalk and prespore regions of the aggregate and slug are thought to be controlled dynamically (reviewed in reference 36). The multiple tips that form in culB mutant aggregates “organize” the construction of multiple small fruiting bodies from a single aggregate. The formation of multiple distinct prestalk tips could result from the formation of excess prestalk cells that are less sensitive to the normal mechanisms that control prestalk/prespore ratios or that maintain prestalk cells as a single distinct tissue. However, it is difficult to imagine how the precocious appearance of prestalk cells causes the block in aggregation observed in culB− pkaR− cells. The aggregation deficiency of the culB− pkaR− cells cannot be simply due to the immobility of stalk cells, since mature stalk cells are only observed well after the time cells would normally aggregate. Moreover, the motility of culB− pkaR− cells appears to be relatively normal since they participate in aggregation when they are mixed with wild-type cells (unpublished observations). The culB− cells expressed ecmA precociously, 4 h after development, but the morphological defects (e.g., multiple tips) appeared only after aggregation, when, we presume, PKA becomes most active. We expect that in culB− pkaR− cells PKA is active from the start of development, and this could explain why the defects manifest themselves earlier. Similar early phenotypes result when PkaC is expressed in culB− cells, again suggesting that active PKA causes the premature prestalk/stalk cell differentiation that we observe. These results imply that PkaC and CulB act in opposition to each other to control prestalk/stalk cell differentiation and morphogenesis: PkaC promotes prestalk cell differentiation, while CulB suppresses it. However, this relationship is not a simple one since PKA activation appears to inhibit some aspects of prestalk cell differentiation, as evidenced by the severe reduction in ecmA expression in pkaR− cells (Fig. 7).
DIF-1 is one of the signals involved in prestalk differentiation in Dictyostelium (reviewed in reference 36). It induces prestalk gene expression and stalk cell formation among individual amoebae in vitro but is not required for these events in vivo (17, 57, 66, 67, 68, 74). Genetic ablation of DIF-1 production has demonstrated the requirement for DIF-1 in the differentiation of the pstO subclass of prestalk cells but not other prestalk cell types (66). Although the correct proportioning of prespore cells and prestalk cell subtypes probably results from the interplay of a number of signaling systems, DIF-1 appears to be a component of the signaling system that controls part of prestalk cell subspecialization. Under submerged low-cell-density conditions, DIF-1 induces stalk cell formation after cells have been incubated with cAMP (9). It is thought that PKA activation promotes DIF-1 induction of stalk cells since pkaR− cells can be induced to form stalk cells by exogenous DIF-1 alone, without the cAMP preincubation (63). The culB− pkaR− cells can differentiate into stalk cells without added cAMP or DIF-1, suggesting a cell-intrinsic propensity of these cells to differentiate into stalk cells. However, in vitro stalk cell differentiation in these cells still appears to depend on DIF-1, since cerulenin inhibits their differentiation. Since culB− pkaR− cells do not appear to overproduce DIF-1 and since these mutants are more sensitive to added DIF-1 than are pkaR− cells, the stalk cell formation of culB− pkaR− cells in submerged culture appears to be mediated by normal levels of endogenous DIF synthesis.
It is well known that growing cells are predisposed to become one cell type or the other during development (e.g., references 27 and 78). A number of physiological factors have been shown to bias cell differentiation, such as cell cycle-dependent differences in cytosolic calcium (8). For instance, vegetative cells grown with or without glucose preferentially differentiate as spores and stalk cells, respectively. Recent evidence suggests that these intrinsic biases are mediated by altered sensitivity to DIF-1 (67). It has been shown recently that a small amount of DIF-1 is produced early in development, about 5 h before tipped aggregates are formed (35). Our data are consistent with the notion that culB− pkaR− cells display a precocious prestalk cell differentiation due to an increased sensitivity to DIF-1 during early aggregation. To the extent that the submerged-culture bioassay accurately reflects differentiation responses in vivo, subnanomolar levels of DIF-1 could mediate the precocious prestalk cell differentiation that we observed. The genetic inference from these experiments is that CulB is normally required to delay prestalk cell differentiation by regulating, in part, the cellular response to DIF-1. Abnormal expression of any component of the DIF-1 response pathway might render cells more sensitive to DIF-1. A DIF-1-binding protein has been identified, and its activity peaks during aggregation when the pstO-prespore cell divergence occurs (32). It is tempting to speculate that this DIF-1-binding protein, or some other effector of the DIF response, is subject to regulation by CulB.
The ubiquitinylation of proteins through the SCF complex appears to play a major role in regulating development in Dictyostelium. Null mutations in genes that encode ubiquitin E2 conjugase, Ubc1, and an F-box protein, Mekkα, result in abnormal development (12, 14). Overexpression in wild-type cells of the part of MEKKα that contains the F-box motif and WD40 repeats results in a phenotype that is very similar to that of culB− mutants, a smaller fruiting body with extended spore head (12). The Mekkα-null cells also display a propensity to differentiate into stalk cells when mixed with wild-type cells (12). Thus, one attractive idea is that Mekkα and CulB are components of the same SCF complex that regulates cell differentiation in Dictyostelium. A Dictyostelium Skp1 homolog has been characterized, but null mutants have resisted isolation, suggesting that it is essential (62; C. West, personal communication). In the future, it will be important to establish whether Mekkα, CulB, and Skp1 function as a complex and to define possible target proteins for such a complex.
CulB is 40% identical to another putative cullin in Dictyostelium, CulA. The culA mutants form aggregates but fail to produce prespore or prestalk cells efficiently and eventually form large aggregates with multiple protrusions and many vacuolated stalk-like cells (46). Since CulA appears to control the stability of RegA, a cAMP-specific phosphodiesterase, it has been proposed that the culA mutant phenotype is caused by reduced intracellular cAMP levels and thus by reduced PKA activity. This is consistent with the observation that culA mutants are rescued by overexpressing PkaC (46). Curiously, culA mutants can be rescued by expressing CulA specifically in prestalk cells, which suggests that the lack of prespore cells in CulA-null cells is an indirect effect of the lack of prestalk cells. Thus, CulA, like CulB, appears to impinge more directly on prestalk cell differentiation at the cellular level. Finally, the absence of CulA in prestalk cells has effects roughly opposite to the absence of CulB. The simplest interpretation of these data is that CulA and CulB regulate the destruction of proteins that have opposing effects on prestalk cell differentiation. Regulating the relative activities of CulA- and CulB-mediated events would provide another level of control for coordinating cell differentiation and morphogenesis in Dictyostelium.
Acknowledgments
This work was supported by a grant to A.K. from the National Institutes of Health (GM52359).
We thank Negin Iranfar and Bill Loomis for sequencing culB genomic DNA and cDNA, and we thank Sijie Lu for many helpful discussions. We also thank Rick Firtel and Chris West for communicating results prior to publication.
REFERENCES
- 1.Abe, K., and K. Yanagisawa. 1983. A new class of rapid developing mutants in Dictyostelium discoideum: implications for cyclic AMP metabolism and cell differentiation. Dev. Biol. 95: 200–210. [DOI] [PubMed] [Google Scholar]
- 2.Abel, T., P. V. Nguyen, M. Barad, T. A. Deuel, E. R. Kandel, and R. Bourtchouladze. 1997. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88: 615–626. [DOI] [PubMed] [Google Scholar]
- 3.Adachi, H., T. Hasebe, K. Yoshinaga, T. Ohta, and K. Sutoh. 1994. Isolation of Dictyostelium discoideum cytokinesis mutants by restriction enzyme-mediated integration of the blasticidin S resistance marker. Biochem. Biophys. Res. Commun. 205: 1808–1814. [DOI] [PubMed] [Google Scholar]
- 4.Anjard, C., S. Pinaud, R. R. Kay, and C. D. Reymond. 1992. Overexpression of DdPK2 protein kinase causes rapid development and affects the intracellular cAMP pathway of Dictyostelium discoideum. Development 115: 785–790. [DOI] [PubMed] [Google Scholar]
- 5.Anjard, C., C. Zeng, W. F. Loomis, and W. Nellen. 1998. Signal transduction pathways leading to spore differentiation in Dictyostelium discoideum. Dev. Biol. 193: 146–155. [DOI] [PubMed] [Google Scholar]
- 6.Aubry, L., M. Maeda, R. Insall, P. N. Devreotes, and R. A. Firtel. 1997. The Dictyostelium mitogen-activated protein kinase ERK2 is regulated by ras and cAMP-dependent protein kinase (PKA) and mediates PKA function. J. Biol. Chem. 272: 3883–3886. [DOI] [PubMed] [Google Scholar]
- 7.Aubry, L., and R. A. Firtel. 1999. Integration of signaling networks that regulate Dictyostelium differentiation. Annu. Rev. Cell Dev. Biol. 15: 469–517. [DOI] [PubMed] [Google Scholar]
- 8.Azhar, M., P. K. Kennady, G. Pande, M. Espiritu, W. Holloman, D. Brazill, R. H. Gomer, and V. Nanjundiah. 2001. Cell cycle phase, cellular Ca2+ and development in Dictyostelium discoideum. Int. J. Dev. Biol. 45: 405–414. [PubMed] [Google Scholar]
- 9.Berks, M., and R. R. Kay. 1988. Cyclic AMP is an inhibitor of stalk cell differentiation in Dictyostelium discoideum. Dev. Biol. 126: 108–114. [DOI] [PubMed] [Google Scholar]
- 10.Chang, W. T., P. A. Thomason, J. D. Gross, and P. C. Newell. 1998. Evidence that the RdeA protein is a component of a multistep phosphorelay modulating rate of development in Dictyostelium. EMBO J. 17: 2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, T. L., W. A. Wolf, and R. L. Chisholm. 1998. Cell-type-specific rescue of myosin function during Dictyostelium development defines two distinct cell movements required for culmination. Development 125: 3895–3903. [DOI] [PubMed] [Google Scholar]
- 12.Chung, C. Y., T. B. Reddy, K. Zhou, and R. A. Firtel. 1998. A novel, putative MEK kinase controls developmental timing and spatial patterning in Dictyostelium and is regulated by ubiquitin-mediated protein degradation. Genes Dev. 12: 3564–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung, C. Y., S. Funamoto, and R. A. Firtel. 2001. Signaling pathways controlling cell polarity and chemotaxis. Trends Biochem. Sci. 26(9): 557–566. [DOI] [PubMed] [Google Scholar]
- 14.Clark, A., A. Nomura, S. Mohanty, and R. A. Firtel. 1997. A ubiquitin-conjugating enzyme is essential for developmental transitions in Dictyostelium. Mol. Biol. Cell 8: 1989–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dormann, D., J. Y. Kim, P. N. Devreotes, and C. J. Weijer. 2001. cAMP receptor affinity controls wave dynamics, geometry and morphogenesis in Dictyostelium. J. Cell Sci. 114: 2513–2523. [DOI] [PubMed] [Google Scholar]
- 16.Drain, P., E. Folkers, and W. G. Quinn. 1991. cAMP-dependent protein kinase and the disruption of learning in transgenic flies. Neuron 6: 71–82. [DOI] [PubMed] [Google Scholar]
- 17.Early, V. E., and J. G. Williams. 1988. A Dictyostelium prespore-specific gene is transcriptionally repressed by DIF in vitro. Development 103: 519–524. [DOI] [PubMed] [Google Scholar]
- 18.Elledge, S. J., and J. W. Harper. 1998. The role of protein stability in the cell cycle and cancer. Biochim Biophys. Acta 1377: M61–M70. [DOI] [PubMed] [Google Scholar]
- 19.Feldman, R. M., C. C. Correll, K. B. Kaplan, and R. J. Deshaies. 1997. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell 91: 221–230. [DOI] [PubMed] [Google Scholar]
- 20.Firtel, R. A. 1995. Integration of signaling information in controlling cell- fate decisions in Dictyostelium. Genes Dev. 9: 1427–1444. [DOI] [PubMed] [Google Scholar]
- 21.Firtel, R. A., and R. Meili. 2000. Dictyostelium: a model for regulated cell movement during morphogenesis. Curr. Opin. Genet. Dev. 10: 421–427. [DOI] [PubMed] [Google Scholar]
- 22.Fisher, P. R. 1997. Genetics of phototaxis in a model eukaryote, Dictyostelium discoideum. Bioessays 19: 397–407. [DOI] [PubMed] [Google Scholar]
- 23.Fisher, P. R. 1997. Directional movement of migrating slugs, p. 437–452. In Y. Maeda, K. Inouye, and I. Takeuchil (ed.), Dictyostelium—a model system for cell and developmental biology. Universal Academy Press, Tokyo, Japan.
- 24.Gaskins, C., A. M. Clark, L. Aubry, J. E. Segall, and R. A. Firtel. 1996. The Dictyostelium MAP kinase ERK2 regulates multiple, independent developmental pathways. Genes Dev. 10: 118–128. [DOI] [PubMed] [Google Scholar]
- 25.Giloh, H., and J. W. Sedat. 1982. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science 217: 1252–1255. [DOI] [PubMed] [Google Scholar]
- 26.Ginsburg, G. T., and A. R. Kimmel. 1997. Autonomous and nonautonomous regulation of axis formation by antagonistic signaling via 7-span cAMP receptors and GSK3 in Dictyostelium. Genes Dev. 11: 2112–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomer, R. H., and R. A. Firtel. 1987. Cell-autonomous determination of cell-type choice in Dictyostelium development by cell-cycle phase. Science 237: 758–762. [DOI] [PubMed] [Google Scholar]
- 28.Harrington, B. J., and K. B. Raper. 1968. Use of a fluorescent brightener to demonstrate cellulose in the cellular slime mold. J. Appl. Microbiol. 16: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harwood, A. J., S. E. Plyte, J. Woodgett, H. Strutt, and R. R. Kay. 1995. Glycogen synthase kinase 3 regulates cell fate in Dictyostelium. Cell 80: 139–148. [DOI] [PubMed] [Google Scholar]
- 30.Hopper, N. A., C. Anjard, C. D. Reymond, and J. G. Williams. 1993. Induction of terminal differentiation of Dictyostelium by cAMP-dependent protein kinase and opposing effects of intracellular and extracellular cAMP on stalk cell differentiation. Development 119: 147–154. [DOI] [PubMed] [Google Scholar]
- 31.Hopper, N. A., A. J. Harwood, S. Bouzid, M. Veron, and J. G. Williams. 1993. Activation of the prespore and spore cell pathway of Dictyostelium differentiation by cAMP-dependent protein kinase and evidence for its upstream regulation by ammonia. EMBO J. 12: 2459–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Insall, R., and R. R. Kay. 1990. A specific DIF binding protein in Dictyostelium. EMBO. J. 9: 3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawata, T., A. Shevchenko, M. Fukuzawa, K. A. Jermyn, N. F. Totty, N. V. Zhukovskaya, A. E. Sterling, M. Mann, and J. G. Williams. 1997. SH2 signaling in a lower eukaryote: a STAT protein that regulates stalk cell differentiation in Dictyostelium. Cell 89: 909–916. [DOI] [PubMed] [Google Scholar]
- 34.Kay, R. R. 1989. Evidence that elevated intracellular cyclic AMP triggers spore maturation in Dictyostelium. Development 105: 753–759. [Google Scholar]
- 35.Kay, R. R. 1998. The biosynthesis of Differentiation-Inducing Factor, a chlorinated signal molecule regulating Dictyostelium development. J. Biol. Chem. 273: 2669–2675. [DOI] [PubMed] [Google Scholar]
- 36.Kay, R. R., P. Flatman, and C. R. L. Thompson. 1999. DIF signalling and cell fate. Semin. Cell Dev. Biol. 10: 577–585. [DOI] [PubMed] [Google Scholar]
- 37.Kipreos, E. T., L. E. Lander, J. P. Wing, W. W. He, and E. M. Hedgecock. 1996. cul-1 is required for cell cycle exit in C. elegans and identifies a novel gene family. Cell 85: 829–839. [DOI] [PubMed] [Google Scholar]
- 38.Kuspa, A., and W. F. Loomis. 1992. Tagging developmental genes in Dictyostelium by restriction enzyme-mediated integration of plasmid DNA. Proc. Natl. Acad. Sci. USA 89: 8803–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuspa, A., and W. F. Loomis. 1994. Transformation of Dictyostelium—gene disruptions, insertional mutagenesis, and promoter traps. Methods Mol. Genet. 3: 3–21. [Google Scholar]
- 40.Loomis, W. F. 1996. Genetic networks that regulate development in Dictyostelium cells. Microbiol. Rev. 60: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loomis, W. F. 1998. Role of PKA in the timing of developmental events in Dictyostelium cells. Microbiol. Mol. Biol. Rev. 62: 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mann, S. K. O., W. M. Yonemoto, S. S. Taylor, and R. A. Firtel. 1992. DdPK3, which plays essential roles during Dictyostelium development, encodes the catalytic subunit of cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89: 10701–10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mann, S. K. O., J. M. Brown, C. Briscoe, C. Parent, G. Pitt, P. N. Devreotes, and R. A. Firtel. 1997. Role of cAMP-dependent protein kinase in controlling aggregation and postaggregative development in Dictyostelium. Dev. Biol. 183: 208–221. [DOI] [PubMed] [Google Scholar]
- 44.Manstein, D. J., H. P. Schuster, P. Morandini, and D. M. Hunt. 1995. Cloning vectors for the production of proteins in Dictyostelium discoideum. Gene 162: 129–134. [DOI] [PubMed] [Google Scholar]
- 45.Mohanty, S., K. A. Jermyn, A. Early, T. Kawata, L. Aubry, A. Ceccarelli, P. Schaap, J. G. Williams, and R. A. Firtel. 1999. Evidence that the Dictyostelium Dd-STATa protein is a repressor that regulates commitment to stalk cell differentiation and is also required for efficient chemotaxis. Development 126: 3391–3405. [DOI] [PubMed] [Google Scholar]
- 46.Mohanty, S., S. Lee, N. Yadava, M. J. Dealy, R. S. Johnson, and R. A. Firtel. 2001. Regulated protein degradation controls PKA function and cell-type differentiation in Dictyostelium. Genes Dev. 15: 1435–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulligan, J. T., and S. J. Elledge. 1994. The construction and use of cDNA libraries for genetic selections, p. 65–81. In J. R. Johnston (ed.), Molecular genetics of Yeast: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 48.Nellen, W., and R. A. Firtel. 1985. High-copy-number transformants and co-transformation in Dictyostelium. Gene 39: 155–163. [DOI] [PubMed] [Google Scholar]
- 49.Patton, E. E., A. R. Willems, D. Sa, L. Kuras, D. Thomas, K. L. Craig, and M. Tyers. 1998. Cdc53 is a scaffold protein for multiple Cdc34/Skp1/F-box protein complexes that regulate cell division and methionine biosynthesis in yeast. Genes Dev. 12: 692–705. (Erratum, 12:3144.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pitt, G. S., N. Milona, J. Borleis, K. C. Lin, R. R. Reed, and P. N. Devreotes. 1992. Structurally distinct and stage-specific adenylyl cyclase genes play different roles in Dictyostelium development. Cell 69: 305–315. [DOI] [PubMed] [Google Scholar]
- 51.Pukatzki, S., N. Tordilla, J. Franke, and R. H. Kessin. 1998. A novel component involved in ubiquitination is required for development of Dictyostelium discoideum. J. Biol. Chem. 273: 24131–24138. [DOI] [PubMed] [Google Scholar]
- 52.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 53.Saxe, C. L., III, Y. M. Yu, C. Jones, A. Bauman, and C. Haynes. 1996. The cAMP receptor subtype cAR2 is restricted to a subset of prestalk cells during Dictyostelium development and displays unexpected DIF-1 responsiveness. Dev. Biol. 174: 202–213. [DOI] [PubMed] [Google Scholar]
- 54.Scheffner, M., U. Nuber, and J. M. Huibregtse. 1995. Protein ubiquitination involving an E1–E2-E3 enzyme ubiquitin thioester cascade. Nature 373: 81–83. [DOI] [PubMed] [Google Scholar]
- 55.Shaulsky, G., D. Fuller, and W. F. Loomis. 1998. A cAMP-phosphodiesterase controls PKA-dependent differentiation. Development 125: 691–699. [DOI] [PubMed] [Google Scholar]
- 56.Shaulsky, G., A. Kuspa, and W. F. Loomis. 1995. A multidrug resistance transporter serine protease gene is required for prestalk specialization in Dictyostelium. Genes Dev. 9: 1111–1122. [DOI] [PubMed] [Google Scholar]
- 57.Shaulsky, G., and W. F. Loomis. 1996. Initial cell type divergence in Dictyostelium is independent of DIF-1. Dev. Biol. 174: 214–220. [DOI] [PubMed] [Google Scholar]
- 58.Simon, M. N., O. Pelegrini, M. Veron, and R. R. Kay. 1992. Mutation of protein kinase-A causes heterochronic development of Dictyostelium. Nature 356: 171–172. [DOI] [PubMed] [Google Scholar]
- 59.Skoulakis, E. M., D. Kalderon, and R. L. Davis. 1993. Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning and memory. Neuron 11: 197–208. [DOI] [PubMed] [Google Scholar]
- 60.Skowyra, D., K. L. Craig, M. Tyers, S. J. Elledge, and J. W. Harper. 1997. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91: 209–219. [DOI] [PubMed] [Google Scholar]
- 61.Sussman, M. 1987. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 28: 9–29. [DOI] [PubMed] [Google Scholar]
- 62.Tengumnuay, P., H. R. Morris, A. Dell, M. Panico, T. Paxton, and C. M. West. 1998. The cytoplasmic F-box binding protein SKP1 contains a novel pentasaccharide linked to hydroxyproline in Dictyostelium. J. Biol. Chem. 273: 18242–18249. [DOI] [PubMed] [Google Scholar]
- 63.Thomason, P. A., D. Traynor, G. Cavet, W. T. Chang, A. J. Harwood, and R. R. Kay. 1998. An intersection of the cAMP/PKA and two-component signal transduction systems in Dictyostelium. EMBO J. 17: 2838–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomason, P., D. Traynor, and R. R. Kay. 1999. Taking the plunge-terminal differentiation in Dictyostelium. Trends Genet. 15: 15–19. [DOI] [PubMed] [Google Scholar]
- 65.Thomason, P. A., D. Traynor, J. B. Stock, and R. R. Kay. 1999. The RdeA-RegA system: a eukaryotic phospho-relay controlling cAMP breakdown. J. Biol. Chem. 274: 27379–27384. [DOI] [PubMed] [Google Scholar]
- 66.Thompson, C. R., and R. R. Kay. 2000. The role of DIF-1 signaling in Dictyostelium development. Mol. Cell 6: 1509–1514. [DOI] [PubMed] [Google Scholar]
- 67.Thompson, C. R. L., and R. R. Kay. 2000. Cell-fate choice in Dictyostelium: intrinsic biases modulate sensitivity to DIF signaling. Dev. Biol. 227: 56–64. [DOI] [PubMed] [Google Scholar]
- 68.Town, C. D., J. D. Gross, and R. R. Kay. 1976. Cell differentiation without morphogenesis in Dictyostelium discoideum. Nature 262: 717–719. [DOI] [PubMed] [Google Scholar]
- 69.Walsh, D. A., and S. M. Van Patten. 1994. Multiple pathway signal transduction by the cAMP-dependent protein kinase. FASEB J. 8: 1227–1236. [DOI] [PubMed] [Google Scholar]
- 70.Wang, B., and A. Kuspa. 1997. Dictyostelium development in the absence of cAMP. Science 277: 251–254. [DOI] [PubMed] [Google Scholar]
- 71.Wang, B., G. Shaulsky, and A. Kuspa. 1999. Multiple developmental roles for CRAC, a cytosolic regulator of adenylyl cyclase. Dev. Biol. 208: 1–13. [DOI] [PubMed] [Google Scholar]
- 72.Williams, J. G. 1991. Regulation of cellular differentiation during Dictyostelium morphogenesis. Curr. Opin. Genet. Dev. 1: 358–362. [DOI] [PubMed] [Google Scholar]
- 73.Williams, J. G. 1997. Prestalk and stalk cell heterogeneity in Dictyostelium, p. 293–304. In Y. Maeda, K. Inouye, and I. Takeuchi (ed.), Dictyostelium—a model system for cell and developmental biology. Universal Academy Press, Inc., Tokyo, Japan.
- 74.Williams, J. G., A. Ceccarelli, S. McRobbie, H. Mahbubani, R. R. Kay, A. Farly, M. Berks, and K. A. Jermyn. 1987. Direct induction of Dictyostelium prestalk gene expression by D1F provides evidence that D1F is a morphogen. Cell 49: 185–192. [DOI] [PubMed] [Google Scholar]
- 75.Williams, J. G., K. T. Duffy, D. P. Lane, S. J. Mcrobbie, A. J. Harwood, D. Traynor, R. R. Kay, and K. A. Jermyn. 1989. Origins of the prestalk-prespore pattern in Dictyostelium development. Cell 59: 1157–1163. [DOI] [PubMed] [Google Scholar]
- 76.Williams, J. G., and K. A. Jermyn. 1991. Cell sorting and positional differentiation during Dictyostelium morphogenesis, p. 261–272. In J. Gerhart (ed.), Cell-cell interactions in early development. Wiley-Liss, New York, N.Y.
- 77.Winston, J. T., P. Strack, P. Beer-Romero, C. Y. Chu, S. J. Elledge, and J. W. Harper. 1999. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 13: 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wood, S. A., R. R. Ammann, D. A. Brock, L. Li, T. Spann, and R. H. Gomer. 1996. RtoA links initial cell type choice to the cell cycle in Dictyostelium. Development 122: 3677–3685. [DOI] [PubMed] [Google Scholar]
- 79.Yu, H., J. M. Peters, R. W. King, A. M. Page, P. Hieter, and M. W. Kirschner. 1998. Identification of a cullin homology region in a subunit of the anaphase- promoting complex. Science 279: 1219–1222. [DOI] [PubMed] [Google Scholar]
- 80.Zachariae, W., A. Shevchenko, P. D. Andrews, R. Ciosk, M. Galova, M. J. Stark, M. Mann, and K. Nasmyth. 1998. Mass spectrometric analysis of the anaphase-promoting complex from yeast: identification of a subunit related to cullins. Science. 279: 1216–1219. [DOI] [PubMed] [Google Scholar]