Abstract
Purpose
Dissociated response (DR, reduction at baseline or increase < 20% in target lesions compared with nadir in the presence of new lesions) was observed in 20–34% of patients treated with immune checkpoint inhibitors (ICIs). DRs were defined as progression disease (PD) per response evaluation criteria in solid tumors (RECIST v1.1), while evaluation criteria related to immunotherapy incorporated the new lesions into the total tumor burden or conducted further evaluation after 4–8 weeks rather than declaring PD immediately. The main objective of this study is to compare survival between people who continuing initial ICIs treatment and those who switched to other anticancer therapy at the time of DR.
Patients and methods
235 patients with advanced lung cancer (LC) treated with ICIs were evaluated. Propensity score matching (PSM) was used to minimize potential confounding factors. Post-DR OS, target lesion changes were evaluated.
Results
52 patients had been estimated as DRs. After PSM, the continuing ICIs treatment Post-DR cohort still had a significantly longer median post-DR OS than discontinuing ICIs treatment Post-DR cohort, 10.63 months (95% CI 6.27–NA) versus 4.33 months (95% CI 1.77–NA), respectively (p = 0.016).
Conclusion
Within the limitations of this single-center retrospective analysis, clinically stable patients who were judged by clinicians to be eligible for continuing ICIs treatment post-DR derived apparent OS benefit than discontinuing counterpart.
Keywords: Lung cancer, Dissociated response, Immunotherapy, Overall survival
Introduction
Cancer immunotherapy using ICIs has emerged as an effective treatment option for advanced LC (Borghaei et al. 2015; Chatterjee et al. 2016; Garon et al. 2015; Gettinger et al. 2016; Herbst et al. 2016; Horn et al. 2018; Kanda et al. 2016; Rizvi et al. 2015). As clinical trials with ICIs were ongoing, it was noted that dissociated patterns of responses (reduction at baseline or increase < 20% in target lesions compared with nadir in presence of the new lesions) were seen (Balar and Weber 2017; Hodi et al. 2018; Hodi et al. 2016; Wolchok et al. 2009; Queirolo and Spagnolo 2017; Tazdait et al. 2018). DRs were observed in about 20–34% of patients treated with ICIs (Hodi et al. 2018). The appearance of new lesions in the course of treatment is assessed as progression disease (PD) per traditional RECIST v1.1, while the evaluation criteria related to immunotherapy, such as immune-related response criteria (irRC), immune-related RECIST (irRECIST), iRECIST, and Immune-Modified RECIST (imRECIST) incorporated the new lesions into the total tumor burden or conducted further evaluation after 4–8 weeks rather than declaring PD immediately(Bohnsack et al. 2014; Hodi et al. 2018; Wolchok et al. 2009; Seymour et al. 2017). This vital difference may result in an opposite option on continuing ICIs following the initial radiographic DR. Although prior researches introduced irRC, irRECIST, iRECIST and imRECIST based on several observational studies and revealed that patients continuing ICIs treatment post-DR experienced an objective response (relative to baseline), no further potential post-DR OS benefit was clearly described (Bohnsack et al. 2014; Hodi et al. 2018; Wolchok et al. 2009; Seymour et al. 2017). There is a need to better understand the potential OS benefit of continuing ICIs treatment post-DR. The main objective of this study is to compare survival between people who continuing initial ICIs treatment and those who switched to other anticancer therapy at the time of DR.
Materials and methods
Study design
In this single-center retrospective study, data were collected from all patients with advanced LC treated with ICIs from January 1, 2016, to June 1, 2019, in West China Hospital. Include criteria: developed new lesions but responded to initial ICIs treatment (reduction at baseline or increase < 20% in target lesions compared with nadir), and clinically considered new lesions as metastasis. Exclude criteria: patients with a concomitant second cancer, or without measurable lesions at baseline were excluded. Discontinuation unrelated to PD or DR was also excluded. The baseline CT had to be performed within 6 weeks before initiating ICIs treatment. Follow-up radiographic analyses based on scans were performed mostly every 4–6 weeks according to clinical routine. The target lesions were defined per RECIST v1.1. Radiographic changes in target lesions, new lesions, and OS were measured to characterize the efficacy of patients continuing ICIs treatment post-DR. For context, OS outcomes in the population of patients who switched to other anticancer therapy at the time of DR were also evaluated.
Patterns of responses
The definition of complete response (CR), partial response (PR) and stable disease (SD) was exactly the same in irRC, irRECIST, iRECIST and imRECIST and RECIST v1.1. Dissociated response was defined as reduction at baseline or increase < 20% in target lesions compared with nadir in the presence of new lesions. RECIST v1.1 defined PD without new lesions was defined as increase ≥ 20% in target lesions compared with nadir and without new lesions developed.
Statistical analyses
Descriptive statistics were presented as median and the absolute number with proportions (%) for continuous and categorical variables, respectively. Continuous variables were compared with the Mann–Whitney U or independent-sample t test, as appropriate. Categorical variables were compared with the χ2 test or Fisher’s exact test. Overall survival curves were estimated with the Kaplan–Meier method and compared by the log-rank test. To minimize confounding factors, a 1:1 nearest neighbor with a caliper of 0.2 PSM was performed, which allowed retrospective studies to be designed similar to randomized controlled trial (Austin 2009). A two-tailed p < 0.05 was considered statistically significant. Statistical analyses were conducted using R 3.6.1 software. Missing data were replaced with the mean value.
Results
Patient characteristics
Among the 273 consecutive LC patients treated with ICIs, 235 were eligible. 38 patients were excluded, 22 for the absence of confirmatory CT, 5 for non-measurable lesions, 9 for discontinuation unrelated to DR and progression (eight for economic issues and one for stroke) and 2 progression of concomitant primary cancer (one prostate cancer and one colorectal cancer). There were 10 (4.26%) patients discontinued ICIs because of AEs (four for interstitial pneumonia, one for rash, one for thrombocytopenia, one for abnormal liver function, one for musculoskeletal pain, one for infection along with atrial fibrillation, one for interstitial pneumonia along with hypothyroidism, diabetes). On the basis of tumor burden change, all of these patients had been estimated as non-progressive disease (CR + PR + SD) per RECIST v1.1. No DRs discontinued ICIs because of AEs. To data cutoff (December 10, 2019), with a median follow-up of 17 months, 52 (22.13%) patients had experienced dissociated response, consistent with published data (Hodi et al. 2018). Of the 52 DRs with median 12.15 months (range 0.83–45.70 months) of post-DR observation, 26 died and 26 were censored. Median time to DR was 3.39 months (range 0.77–8.53 months). New lesions of 52 DRs were observed in both visceral organs and lymph nodes, mainly presented in pulmonary (21, 40.38%), lymph nodes (9, 17.31%), brain (8, 15.38%), subcutaneous nodules (5, 9.61%), and bone (4, 7.69%). 19 (36.54%) DRs received ICIs treatment as first-line therapy, 19 (36.54%) combined with chemotherapy, and 50 (96.15%) were PD-1 treated. Tumor burden changes of 52 DRs were recorded from the time of first ICIs dose to last CT scan (Fig. 1).
Fig. 1.
Spider plot of total tumor burden changes in DRs with measurable new lesion from the baseline (measurable new lesions were incorporated into the total tumor burden).
Survival comparison of the continuing ICIs treatment post-DR cohort and the discontinuing ICIs treatment post-DR cohort after PSM
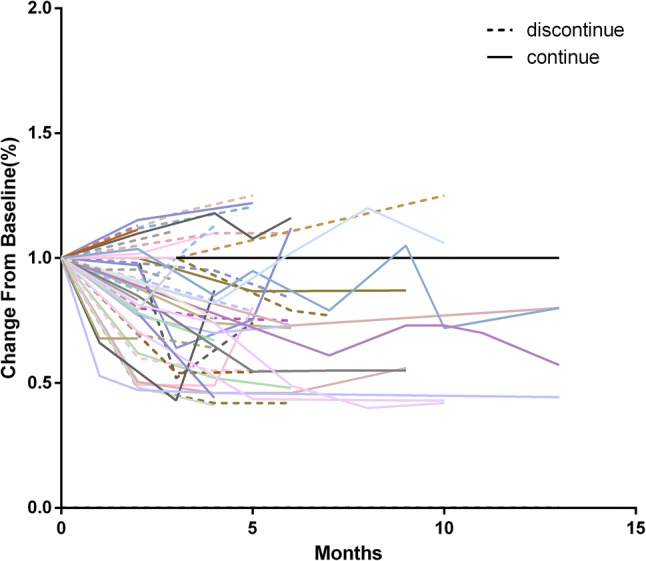
In all 52 DRs, the decision of continuing ICIs post-DR was mainly due to the clinical benefits observed by the clinicians based on the assessments of clinical improvements and tolerance. Among the 52 patients who had DR, 32 (61.54%) continued ICIs, 20 (38.46%) instead received subsequent anti-tumor therapy. Among 32 patients who continued ICIs post-DR, majority of the DRs (30) received the initial ICIs treatment, only two patients received ICIs different from the initial ones: one from Nivolumab to Pembrolizumab; one from Pembrolizumab combined with chemotherapy to Pembrolizumab monotherapy. Tumor burden changes of the 32 patients continuing ICIs post-DR were recorded from the time of DR (Fig. 2). To minimize potential confounding factors, PSM was performed based on age, sex, ICIs types, combined chemotherapy, PD-L1 expression, stage, number of prior therapy, histology, time to DR, tumor burden change, combined ICIs. Following PSM, two matched cohorts of 34 patients were created (Table 1).
Fig. 2.

ICIs antitumor activity post-DR (measurable new lesions were incorporated into the total tumor burden). a Waterfall plot of patients continuing ICIs post-DR, measuring the maximum reduction in the sum of longest diameters of the total tumor burden from DR, which was reset to zero at the appearance of new lesions; b spider plot of patients continuing ICIs post-DR
Table 1.
Characteristics of DR patients at baseline before and after PSM
| Characteristics n (%) | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| Discontinuing ICIs post-DR N = 20 | Continuing ICIs post-DR N = 32 | p value | Discontinuing ICIs post-DR N = 17 | Continuing ICIs post-DR N = 17 | p value | |
| Age [mean (SD)] | 56.80 (10.08) | 57.09 (11.85) | 0.927 | 57.76 (9.093) | 57.88 (12.12) | 0.975 |
| Sex | ||||||
| Male | 17 (85) | 24 (75) | 0.382 | 15 (88.2) | 13 (76.5) | 0.656 |
| Female | 3 (15) | 8 (25) | 2 (11.8) | 4 (23.5) | ||
| Histology | ||||||
| Adenocarcinoma | 12 (60.0) | 19 (59.4) | 0.293 | 11 (64.7) | 10 (58.8) | 0.724 |
| Squamous-cell carcinoma | 6 (30.0) | 11 (34.3) | 6 (35.3) | 7 (41.2) | ||
| Other | 2 (10.0) | 2 (6.3) | 0 (0.00) | 0 (0.00) | ||
| Treatment | ||||||
| PD-1 | 18 (90.0) | 32 (100.0) | 0.279 | 17 (100.0) | 17 (100.0) | – |
| PD-L1 | 2 (10.0) | 0 (0.0) | 0 (0.00) | 0 (0.00) | ||
| Combined chemotherapy | ||||||
| Yes | 10 (50.0) | 9 (28.1) | 0.111 | 8 (47.1) | 8 (47.1) | 1.000 |
| No | 10 (50.0) | 23 (71.9) | 9 (52.9) | 9 (52.9) | ||
| Number of prior therapy | ||||||
| 0 | 4 (20) | 15 (46.9) | 0.050 | 3 (17.6) | 5 (29.4) | 0.688 |
| ≥ 1 | 16 (80) | 17 (53.1) | 14 (82.4) | 12 (70.6) | ||
| PD-L1 expression [mean (SD)] | 32.10 (22.55) | 32.12 (27.37) | 0.997 | 34.00 (23.141) | 34.88 (27.973) | 0.921 |
| Stage | ||||||
| III | 3 (15) | 3 (9.4) | 0.664 | 2 (11.8) | 2 (11.8) | 1.000 |
| IV | 17 (85.0) | 29 (90.6) | 15 (88.2) | 15 (88.2) | ||
| Time to DR (months) | 3.03 (2.06) | 3.34 (2.24) | 0.625 | 3.22 (2.16) | 3.65 (2.62) | 0.604 |
| Tumor burden change | 0.91 (0.22) | 0.81 (0.19) | 0.091 | 0.917 (0.21) | 0.87 (0.18) | 0.496 |
| Combined immunotherapy | ||||||
| Yes | 1 (5.0) | 0 (0.00) | 0.385 | 0 (0.00) | 0 (0.00) | – |
| No | 19 (95.0) | 32 (100.0) | 17 (100.0) | 17 (100.0) | ||
| Dosage of ICIs | ||||||
| Standard* | 20 (100.0) | 32 (100.0) | – | 17 (100.0) | 17 (100.0) | – |
| Nonstandard | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
SD standard deviation, PD-1 programmed cell death protein 1, PD-L1 programmed cell death 1 ligand 1, Tumor burden change from the first dose of ICIs to DR; Standard* conventional-dose according to instructions or large scale clinical trials: nivolumab 3 mg/kg q2w; pembrolizumab 2 mg/kg q3w; atezulimab 1200 mg q3w; nivolumab combined with ipilimumab 3 mg/kg q2w for nivolumab, 1 mg/kg q6w for ipilimumab; pembrolizumab combined with chemotherapy 200 mg q3w for pembrolizumab; nivolumab combined with chemotherapy 360 mg q3w for nivolumab; camrelizumab 200 mg q2w; tislelizumab 200 mg q3w
Post-DR OS was defined from the time of first dose of ICIs post-DR to death. After PSM, baseline characteristics were relatively comparable, the continuing ICIs post-DR cohort still had a significantly longer median post-DR OS than discontinuing ICIs post-DR cohort, 10.63 months (95% CI 6.27–NA) versus 4.33 months (95% CI 1.77–NA), (p = 0.016) (Fig. 3).
Fig. 3.
Kaplan–Meier overall survival estimates after PSM. The continuing ICIs post-DR cohort (n = 17) still had a significantly longer median post-DR OS than discontinuing ICIs post-DR cohort (n = 17), 10.63 months (95% CI 6.27–NA) versus 4.33 months (95% CI 1.77–NA), respectively (p = 0.016)
To verify the reliability of the analysis using PSM, the multivariate analysis was performed using a Cox proportional hazards model. Variables with p value < 0.2 (Switch, Combined chemotherapy, Number of Prior Therapy, Tumor burden change) in univariate analyses were included in a multivariate model as possible confounders or effect modifiers. Backward-stepwise elimination was used to generate a minimum adequate model and excluded variables (p > 0.05) were retested in the minimum model. The result showed that continuing ICIs post-DR is an independent predictor for survival of DR patients [p = 0.013, HR = 2.718, 95% CI (1.235–5.982)], consistent with the former analysis using PSM.
Survival comparison of RECIST v1.1 defined PD without new lesions and DRs; CR + PR + SD and DRs
In the 235 patients assessed by central review, 84 (35.74%) had non-progressive disease (CR + PR + SD) and 151 (64.26%) had PD per RECIST v1.1. Among 151 RECIST v1.1 defined PDs, 84 were defined as PD without new lesions per RECIST v1.1.
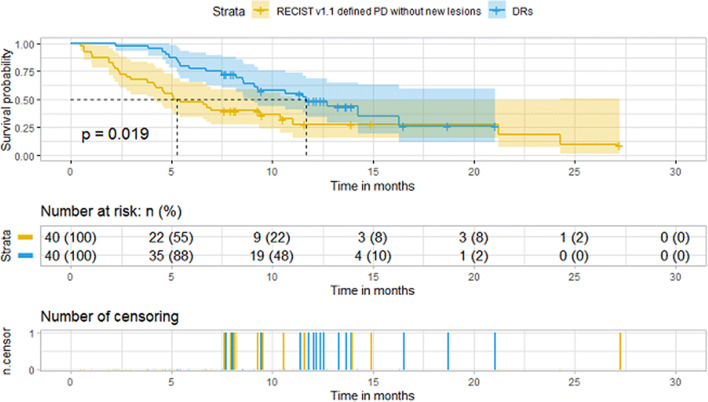
To compare the survival, PSM was utilized to create matched cohorts (DR match CR + PR + SD; DR match RECIST v1.1 defined PD without new lesions) based on age, sex, histology, ICIs types, combined chemotherapy, PD-L1 expression, stage, number of prior therapy, histology, combined ICIs, dose of ICIs. After 1:1 PSM, there was no significant difference in demographic and baseline characteristics between corresponding arms (Tables 2, 3). OS was defined from the time of the first dose of ICIs to death. We found that DRs had a significantly longer median OS than RECIST v1.1 defined PD without new lesions, 11.70 months (95% CI 9.10–NA) versus 5.25 months (95% CI 4.13–11.00), respectively (p = 0.019) (Fig. 4). The median OS was shorter in the DRs than in the CR + PR + SD group, 12.7 months (95% CI 9.27–NA) versus 23.1 (95% CI 14.5–NA), respectively (p = 0.047) (Fig. 5).
Table 2.
Characteristics of DRs and RECIST v1.1 defined PD without new lesions before and after PSM
| Characteristics n (%) | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| RECIST v1.1 defined PD without new lesions N = 84 | DRs N = 52 | p value | RECIST v1.1 defined PD without new lesions N = 40 | DRs N = 40 | p value | |
| Age [mean (SD)] | 57.80 (10.11) | 56.98 (11.10) | 0.660 | 57.38 (10.93) | 57.80 (10.77) | 0.816 |
| Sex | ||||||
| Male | 61 (72.6) | 41 (78.8) | 0.415 | 31 (77.5) | 31 (77.5) | 1.000 |
| Female | 23 (27.4) | 11 (21.2) | 9 (22.5) | 9 (22.5) | ||
| Histology | ||||||
| Adenocarcinoma | 38 (45.2) | 31 (59.6) | 0.257 | 20 (50.0) | 23 (57.5) | 0.598 |
| Squamous-cell carcinoma | 36 (42.9) | 17 (32.7) | 13 (32.5) | 13 (32.5) | ||
| Other | 10 (11.9) | 4 (7.7) | 7 (17.5) | 4 (10.0) | ||
| Treatment | ||||||
| PD-1 | 81 (96.4) | 50 (96.2) | 1.000 | 38 (95.0) | 38 (95.0) | 1.000 |
| PD-L1 | 3 (3.6) | 2 (3.8) | 2 (5.00) | 2 (5.00) | ||
| Combined chemotherapy | ||||||
| Yes | 21 (25.0) | 19 (36.5) | 0.151 | 13 (32.5) | 17 (42.5) | 0.356 |
| No | 63 (75.0) | 33 (63.5) | 27 (67.5) | 23 (57.5) | ||
| Number of prior therapy | ||||||
| 0 | 7 (8.3) | 19 (36.5) | 0.000 | 7 (17.5) | 7 (17.5) | 1.000 |
| ≥ 1 | 77 (91.7) | 33 (63.5) | 33 (82.5) | 33 (82.5) | ||
| PD-L1 expression [mean (SD)] | 26.86 (24.98) | 32.12 (25.39) | 0.238 | 32.93 (27.48) | 32.55 (26.06) | 0.950 |
| Stage | ||||||
| III | 8 (9.5) | 6 (11.5) | 0.707 | 5 (12.5) | 5 (12.5) | 1.000 |
| IV | 76 (90.5) | 46 (88.5) | 35 (87.5) | 35 (87.5) | ||
| Combined ICIs | ||||||
| Yes | 2 (2.4) | 1 (1.9) | 1.000 | 2 (5.0) | 1 (2.5) | 1.000 |
| No | 82 (97.6) | 51 (98.1) | 38 (95.0) | 39 (97.5) | ||
| Dosage of ICIs | ||||||
| Standard | 77 (91.7) | 52 (100) | 0.044 | 40 (100) | 40 (100) | – |
| Nonstandard | 7 (8.3) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
Table 3.
Characteristics of DRs and RECIST v1.1 defined CR + PR + SD before and after PSM
| Characteristics n (%) | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| RECIST v1.1 defined CR + PR + SD N = 84 | DRs N = 52 | p value | RECIST v1.1 defined CR + PR + SD N = 40 | DRs N = 40 | p value | |
| Age [mean (SD)] | 58.82 (9.44) | 56.98 (11.10) | 0.304 | 56.83 (10.21) | 57.63 (10.99) | 0.737 |
| Sex | ||||||
| Male | 66 (78.6) | 41 (78.8) | 0.970 | 32 (80.0) | 31 (77.5) | 0.785 |
| Female | 18 (21.4) | 11 (21.2) | 8 (20.0) | 9 (22.5) | ||
| Histology | ||||||
| Adenocarcinoma | 45 (53.6) | 31 (59.6) | 0.324 | 23 (57.5) | 22 (55.0) | 0.866 |
| Squamous-cell carcinoma | 25 (29.8) | 17 (32.7) | 12 (30.0) | 14 (35.0) | ||
| Other | 14 (16.7) | 4 (7.7) | 5 (12.5) | 4 (10.0) | ||
| Treatment | ||||||
| PD-1 | 76 (90.5) | 50 (96.2) | 0.317 | 40 (100.0) | 38 (95.0) | 0.494 |
| PD-L1 | 8 (9.5) | 2 (3.8) | 0 (0.00) | 2 (5.0) | ||
| Combined chemotherapy | ||||||
| Yes | 24 (28.6) | 19 (36.5) | 0.332 | 13 (32.5) | 17 (42.5) | 0.356 |
| No | 60 (71.4) | 33 (63.5) | 27 (67.5) | 23 (57.5) | ||
| Number of prior therapy | ||||||
| 0 | 48 (57.1) | 19 (36.5) | 0.020 | 14 (35.0) | 16 (40.0) | 0.644 |
| ≥ 1 | 36 (42.9) | 33 (63.5) | 26 (65.0) | 24 (60.0) | ||
| PD-L1 expression [mean (SD)] | 43.44 (25.30) | 32.12 (25.39) | 0.012 | 35.90 (21.37) | 35.93 (27.07) | 0.996 |
| Stage | ||||||
| III | 17 (20.2) | 6 (11.5) | 0.188 | 8 (20.0) | 5 (12.5) | 0.363 |
| IV | 67 (79.8) | 46 (88.5) | 32 (80.0) | 35 (87.5) | ||
| Combined immunotherapy | ||||||
| Yes | 1 (5.0) | 0 (0.00) | 1.000 | 0 (0.00) | 0 (0.00) | 1.000 |
| No | 19 (95.0) | 32 (100.0) | 17 (100.0) | 17 (100.0) | ||
| Dosage of ICIs | ||||||
| Standard | 80 (95.2) | 49 (94.2) | 1.000 | 37 (92.5) | 38 (95.0) | 1.000 |
| Nonstandard | 4 (4.8) | 3 (5.8) | 3 (7.5) | 2 (5) | ||
Fig. 4.
Kaplan–Meier overall survival estimates after PSM. DRs (n = 40) had a significantly longer median OS than RECIST v1.1 defined PD without new lesions (n = 40), 11.70 months (95% CI 9.10–NA) versus 5.25 months (95% CI 4.13–11.00), respectively (p = 0.019)
Fig. 5.
Kaplan–Meier overall survival estimates after PSM. The median OS was shorter in the DRs (n = 40) than in the CR + PR + SD group (n = 40), 12.7 months (95% CI 9.27–NA) versus 23.1 (95% CI 14.5–NA), respectively (p = 0.047)
Discussion
Our study indicated that the continuing ICIs post-DR group had a significantly better survival than the discontinuing counterpart. With the widespread use of ICIs, DRs were seen, which may be difficult to discern from established, radiographically undetectable lesions of baseline, as the latter may expand due to T-cell infiltration (Wolchok et al. 2009). Experience with ICIs has shown that the efficacy of ICIs is often not fully reflected in RECIST v1.1 (Hoos et al. 2015; Chmielowski 2018; Ferté and Marabelle 2017; Liang et al. 2020; Hodi et al. 2018; Hodi et al. 2016; Hodi et al. 2014; Wolchok et al. 2009; Nishino et al. 2013; Nishino et al. 2017; Queirolo and Spagnolo 2017; Seymour et al. 2017; Tazdait et al. 2018). To capture the DRs and other unconventional patterns of antitumor activity associated with ICIs and provide a comprehensive efficacy assessment of ICIs, irRC, irRECIST, iRECIST and imRECIST were developed (Chmielowski 2018; Bohnsack et al. 2014; Ferté and Marabelle 2017; Liang et al. 2020; Hodi et al. 2018; Hodi et al. 2016; Wolchok et al. 2009; Nishino 2016; Nishino et al. 2013; Nishino et al. 2014; Seymour et al. 2017). One of the key principles of these criteria was to allow patients who otherwise would have to discontinue ICIs therapy to continue, despite the appearance of new lesions or other atypical patterns. While as the RECIST working group recommended, these guidelines were created to provide a consistent framework for the management of data collected in ICIs clinical trials to test and validate RECIST v1.1 and suggest modifications if necessary, and not intended to guide clinical practice. Treatment decisions rest with the patient and their health-care team (Seymour et al. 2017). Thus, oncologists need to decide whether to continue ICIs treatment at the time of DR.
Result of some prior retrospective studies supported the notion that initial ICIs treatment should be continued at the time of DR. The study which developed irRC analyzed the data of advanced melanoma patients treated with ipilimumab and found that DRs who continued ipilimumab treatment at the time of DR subsequently experienced an objective response (relative to baseline). This finding was accordant with some of our results. Hence ICIs treatment was encouraged to be continued as tumors may begin to shrink or stabilize post-DR. While no further potential OS benefit of continuing initial ICIs at the time of DR was clearly described (Wolchok et al. 2009).
Some retrospective studies have shown that continuing initial ICIs treatment beyond RECIST v1.1 defined PD (DRs were included) appeared to have better survival than the discontinued counterpart. To date, analyses of post-PD effects of atezolizumab in metastatic urothelial carcinoma (mUC) and non-small cell lung cancer (NSCLC), nivolumab in renal cell carcinoma (RCC), melanoma and NSCLC, and anti-PD-1 agents in metastatic NSCLC and melanoma have been reported (Necchi et al. 2017; Escudier et al. 2017; Ricciuti et al. 2019; Kazandjian et al. 2017; Gandara et al. 2018; Long et al. 2017; Liang et al. 2020; Beaver et al. 2018; George et al. 2016; Tazdait et al. 2018). While few researches have carried out the subgroup analysis of the potential OS benefit of continuing ICIs post-DR. To the best of our knowledge, the present study is the first to conduct a survival comparison between continuing ICIs and discontinuing ICIs post-DR. The confounding bias was effectively controlled by PSM and the accuracy of these results was increased. In real world clinical practice, physicians can decide to continue ICIs when patients with good performance status experienced clinical symptoms improve. Patients with more aggressive disease were unintentionally separated from ones with more indolent disease by this approach, and it may introduce a bias. Given this, we hypothesized that clinically stable patients who were judged by clinicians to be eligible for continuing ICIs treatment at the time of DR derived apparent post-DR OS benefit than discontinued counterpart. The assumption is that patients benefit from ICIs treatment post-DR, but encourage DRs to continue initial ICIs treatment unintentionally prevents those who do not benefit from the present ICIs therapy from switching to the next-line anti-cancer therapy.
Our results also showed that DRs had better survival than RECIST v1.1 defined PD without new lesions, consistent with previous research that atypical response (DR and pseudoprogression) had longer median OS (Hodi et al. 2016, 2014; Tazdait et al. 2018). Notably, an observation which developed the imRECIST criteria, a modification to irRECIST, examined data from atezolizumab treated patients with NSCLC, mUC, RCC, and melanoma. Subgroup analysis revealed that patients who developed new lesions without target lesion progression had a similar or shorter OS compared with RECIST v1.1 defined PD without new lesions. These differences in result may be attributed to different ICI types and tumor types, as different response patterns might occur with the different agents across tumor types.
Limitations of this analysis include the retrospective assessment of response by central review, availability of clinical data for patients who continue ICIs treatment post-DR. In addition, small sample size and single-center limit the generalizability.
Within the limitations of this single-center retrospective analysis, clinically stable patients who were judged by clinicians to be eligible for continuing ICIs treatment post-DR derived apparent OS benefit than their discontinued counterpart. On the basis of these findings, larger-scale analyses of continuing ICIs treatment post-DR are warranted to quantify the OS benefit and prognostic differences of DRs in outcome estimations, and in addition, the safety profile needs to be explored.
Acknowledgements
The authors would like to thank all the clinical staff in the Department of thoracic oncology, West China Hospital, Sichuan University, who provided great help to this study.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by HZ, YS, WX, JH, LZ, JS, HW, YW and JZ. The first draft of the manuscript was written by HZ, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
None.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Sichuan University approved this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Austin PC (2009) Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28:3083–3107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balar AV, Weber JS (2017) PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother: CII 66:551–564. 10.1007/s00262-017-1954-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JA et al (2018) Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US food and drug administration pooled analysis. Lancet Oncol 19:229–239. 10.1016/s1470-2045(17)30846-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack O, Hoos A, Ludajic K (1070padaptation) 1070padaptation of the immune related response criteria: irrecist. Ann Oncol 25:iv369–iv369. 10.1093/annonc/mdu342.23 [Google Scholar]
- Borghaei H et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M et al (2016) Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol: Off J Eur Soc Med Oncol 27:1291–1298. 10.1093/annonc/mdw174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielowski B (2018) How should we assess benefit in patients receiving checkpoint inhibitor therapy? J Clin Oncol: Off J Am Soc Clin Oncol 36:835–836. 10.1200/jco.2017.76.9885 [DOI] [PubMed] [Google Scholar]
- Escudier B et al (2017) Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in checkMate 025. Eur Urol 72:368–376. 10.1016/j.eururo.2017.03.037 [DOI] [PubMed] [Google Scholar]
- Ferté C, Marabelle A (2017) iRECIST: a clarification of tumour response assessment in the immunotherapy era. Eur J Cancer 77:165–167. 10.1016/j.ejca.2017.02.015(Oxford, England: 1990) [DOI] [PubMed] [Google Scholar]
- Gandara DR et al (2018) Atezolizumab treatment beyond progression in advanced NSCLC: results from the randomized phase III OAK study. J Thorac Oncol 13:1906–1918. 10.1016/j.jtho.2018.08.2027 [DOI] [PubMed] [Google Scholar]
- Garon EB et al (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018–2028. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- George S et al (2016) Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: a subgroup analysis of a randomized clinical trial. JAMA Oncol 2:1179–1186. 10.1001/jamaoncol.2016.0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettinger S et al (2016) Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol: Off J Am Soc Clin Oncol 34:2980–2987. 10.1200/jco.2016.66.9929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London, England) 387:1540–1550. 10.1016/s0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- Hodi FS et al (2014) Patterns of response in patients with advanced melanoma treated with Pembrolizumab (MK-3475) and evaluation of immune-related response criteria (irRC). J Immunother Cancer 2:P103–P103. 10.1186/2051-1426-2-S3-P103 [Google Scholar]
- Hodi FS et al (2016) Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol: Off J Am Soc Clin Oncol 34:1510–1517. 10.1200/jco.2015.64.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS et al (2018) Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol: Off J Am Soc Clin Oncol 36:850–858. 10.1200/jco.2017.75.1644 [DOI] [PubMed] [Google Scholar]
- Hoos A, Wolchok JD, Humphrey RW, Hodi FS (2015) CCR 20th anniversary commentary: immune-related response criteria-capturing clinical activity in immuno-oncology. Clin Cancer Res: Off J Am Assoc Cancer Res 21:4989–4991. 10.1158/1078-0432.Ccr-14-3128 [DOI] [PubMed] [Google Scholar]
- Horn L et al (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379:2220–2229. 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- Kanda S et al (2016) Safety and efficacy of nivolumab and standard chemotherapy drug combination in patients with advanced non-small-cell lung cancer: a four arms phase Ib study. Ann Oncol: Off J Eur Soc Med Oncol 27:2242–2250. 10.1093/annonc/mdw416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazandjian D, Keegan P, Suzman DL, Pazdur R, Blumenthal GM (2017) Characterization of outcomes in patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1.1-defined disease progression in clinical trials. Semin oncology 44:3–7. 10.1053/j.seminoncol.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Liang H, Xu Y, Chen M, Zhong W, Wang M, Zhao J (2020) Patterns of response in metastatic NSCLC during PD-1 or PD-L1 inhibitor therapy: comparison of the RECIST 11 and iRECIST criteria. Thorac Cancer. 10.1111/1759-7714.13367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GV et al (2017) Nivolumab for patients with advanced melanoma treated beyond progression: analysis of 2 phase 3 clinical trials. JAMA Oncol 3:1511–1519. 10.1001/jamaoncol.2017.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necchi A et al (2017) Atezolizumab in platinum-treated locally advanced or metastatic urothelial carcinoma: post-progression outcomes from the phase II IMvigor210 study. Ann Oncol: Off J Eur Soc Med Oncol 28:3044–3050. 10.1093/annonc/mdx518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino M (2016) Immune-related response evaluations during immune-checkpoint inhibitor therapy: establishing a “common language” for the new arena of cancer treatment. J Immunother Cancer 4:30. 10.1186/s40425-016-0134-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS (2013) Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res: Off J Am Assoc Cancer Res 19:3936–3943. 10.1158/1078-0432.Ccr-13-0895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino M, Gargano M, Suda M, Ramaiya NH, Hodi FS (2014) Optimizing immune-related tumor response assessment: does reducing the number of lesions impact response assessment in melanoma patients treated with ipilimumab? J Immunother Cancer 2:17. 10.1186/2051-1426-2-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino M et al (2017) Immune-related tumor response dynamics in melanoma patients treated with pembrolizumab: identifying markers for clinical outcome and treatment decisions. Clin Cancer Res: Off J Am Assoc Cancer Res 23:4671–4679. 10.1158/1078-0432.Ccr-17-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queirolo P, Spagnolo F (2017) Atypical responses in patients with advanced melanoma, lung cancer, renal-cell carcinoma and other solid tumors treated with anti-PD-1 drugs: a systematic review. Cancer Treat Rev 59:71–78. 10.1016/j.ctrv.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Ricciuti B et al (2019) Safety and efficacy of nivolumab in patients with advanced non-small-cell lung cancer treated beyond progression. Clin Lung Cancer 20:178–185.e172. 10.1016/j.cllc.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Rizvi NA et al (2015) Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 16:257–265. 10.1016/s1470-2045(15)70054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour L et al (2017) iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18:e143–e152. 10.1016/s1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazdait M et al (2018) Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer 88:38–47. 10.1016/j.ejca.2017.10.017 [DOI] [PubMed] [Google Scholar]
- Wolchok JD et al (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res: Off J Am Assoc Cancer Res 15:7412–7420. 10.1158/1078-0432.Ccr-09-1624 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.