Abstract
Purpose
Glioblastoma multiforme (GBM) is a poorly curable disease due to its profound chemoresistance. Despite recent advances in surgery, radiotherapy and chemotherapy, the efficient treatment of GBMs is still a clinical challenge. Beside others, AT101, the R-(−) enantiomer of gossypol, and demethoxycurcumin (DMC), a curcumin-related demethoxy compound derived from Curcuma longa, were considered as possible alternative drugs for GBM therapy.
Methods
Using different human primary GBM cell cultures in a long-term stimulation in vitro model, the cytotoxic and anti-proliferative effects of single and combined treatment with 5 µM AT101 and 5 µM or 10 µM DMC were investigated. Furthermore, western blots on pAkt and pp44/42 as well as JC-1 staining and real-time RT-PCR were performed to understand the influence of the treatment at the molecular and gene level.
Results
Due to enhanced anti-proliferative effects, we showed that combined therapy with both drugs was superior to a single treatment with AT101 or DMC. Here, by determination of the combination index, a synergism of the combined drugs was detectable. Phosphorylation and thereby activation of the kinases p44/42 and Akt, which are involved in proliferation and survival processes, were inhibited, the mitochondrial membrane potential of the GBM cells was altered, and genes involved in dormancy-associated processes were regulated by the combined treatment strategy.
Conclusion
Combined treatment with different drugs might be an option to efficiently overcome chemoresistance of GBM cells in a long-term treatment strategy.
Keywords: Glioblastoma, AT101, Demethoxycurcumin, Proliferation, Cytotoxicity
Introduction
Glioblastomas (Glioblastoma multiforme, GBM) are highly malignant primary brain tumors (Louis et al. 2016), which are characterized by a profound chemoresistance. As a consequence, despite recent advances in surgery, radiotherapy and chemotherapy, only 5.6% of GBM patients survive more than 5 years after diagnosis (Ostrom et al. 2018; Stupp et al. 2005). One reason for this fatal prognosis is that GBM cells acquire and develop several mechanisms to escape from or adapt to conventional treatment regimen. Those are either based on molecular differences of distinct cells gained during gliomagenesis, the molecular adaption on certain therapeutics or environmental stimuli, or the close communication of tumor cells with their microenvironment. Thus, new, more efficient and alternative treatment strategies must be developed.
Beside others, possible alternative drugs attracting interest for GBM therapy are AT101, the R-(−)-enantiomer of the natural occurring cottonseed-derived polyphenol gossypol (Lu et al. 2017; Opydo-Chanek et al. 2017), and demethoxycurcumin (DMC), a yellow and slightly acidic curcumin-related demethoxy compound derived from Curcuma longa (Huang et al. 2012; Luthra et al. 2009).
Both AT101 and DMC seemed to be able to mediate anti-tumorigenic activities (alone or in combination with other drugs) in several tumor entities including GBMs (Adamski et al. 2017, 2018; Chen et al. 2017; Jarzabek et al. 2014; Keshmiri-Neghab et al. 2014; Kumar et al. 2018; Lal et al. 2018; Luthra et al. 2009; Wang et al. 2015). For example, AT101 triggered autophagic, mitophagic, or apoptotic cell death (Antonietti et al. 2017; Meyer et al. 2018; Voss et al. 2010; Warnsmann et al. 2018), which was (partly) accompanied by the induction of a G0/G1 cell cycle arrest (Guo et al. 2014). A combined gossypol/temozolomide (TMZ) treatment resulted in the inhibition of tumor-associated angiogenesis, invasion and proliferation, as well as in an enhanced cytotoxicity in GBMs (Adamski et al. 2017, 2018; Jarzabek et al. 2014), and gossypol significantly reduced clonogenic growth of radiation-affected GBM cells (Keshmiri-Neghab et al. 2014). DMC showed different kinds of action including, e.g., anti-oxidative, anti-inflammatory, and anti-tumorigenic activities (Huang et al. 2012; Luthra et al. 2009; Sandur et al. 2007; Somparn et al. 2007). DMC combined with TMZ inhibited the growth of human glioma xenografts in mice (Shi and Sun 2015) and suppressed the growth of human GBM cells by inducing a cell cycle arrest (Luthra et al. 2009; Huang et al. 2012).
However and contrary to these results, other studies showed that the anti-tumorigenic effects of both AT101 and DMC seemed to be limited. In detail, gossypol had only a low (but nevertheless measurable) response rate in a group of heavily pretreated, poor-prognosis patients with recurrent gliomas (Bushunow et al. 1999). AT101 single treatment was in comparison to TMZ less effective in killing GBM cells (Adamski et al. 2018), and DMC had only a mild regression effect on tumor growth compared with the control group in an orthotopic GBM xenograft model (Shi and Sun 2017).
Both natural products mediated their functional activities (alone or in combination with other drugs) e.g., by inducing a mitochondrial dysfunction (Meyer et al. 2018; Huang et al. 2012; Warnsmann et al. 2018), by interfering with the phosphatidylinositol 3-kinase-Akt pathway (Guo et al. 2014; Luthra et al. 2009), or by interacting with/regulating of pro-survival or dormancy-associated proteins (Adamski et al. 2017; Antonietti et al. 2017; Luthra et al. 2009; Opydo-Chanek et al. 2017). Further, a 18 kDa translocator protein, previously known as the peripheral-type benzodiazepine receptor localized in the mitochondrial outer membrane and identified as a key player in apoptotic signaling and cancer development, is known to have binding sites for both curcumin and gossypol (Li et al. 2013).
Thus, both DMC and AT101 seem to mediate their effects by an interaction with (partly) similar intracellular pathways; however, their anti-tumorigenic potential regarding GBM growth inhibition and cell death induction seems to be promising but limited/controversial. Hence, we hypothesized whether a combined treatment with AT101 and DMC may be more effective for GBM therapy. Accordingly, we stimulated human primary GBM cells in a long-term in vitro model with AT101 plus different concentrations of DMC, determined amounts of dead cells and still remaining growth rates in relation to respective controls, calculated the combination index of this combined treatment strategy, analyzed activated intracellular signal cascades, and visualized the affected mitochondrial membrane potential as well as expression of different genes known to be involved in therapeutic escape mechanisms of GBMs.
Materials and methods
Cultivation of human primary GBM cells
GBM samples were surgically obtained at the Department of Neurosurgery (Kiel, Germany) in accordance with the Helsinki Declaration of 1964 and its later amendments and with approval of the ethics committee of the University of Kiel, Germany, after written informed consent of donors (file references: D471/15 and D524/17). Tumors were diagnosed and classified according to WHO criteria by a pathologist. Cultured human primary GBM cells were generated by dissociation of tumor materials as described (Hattermann et al. 2010) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA or PANBiotech GmbH, Aidenbach, Germany), 1% penicillin–streptomycin (10,000 U/ml; Thermo Fisher Scientific, Waltham, MA, USA), and 2 mM additional l-glutamine (Thermo Fisher Scientific). Different GBM cells were checked for purity by immunostaining with cell type-specific markers and for the absence of Mycoplasma contamination as described (Adamski et al. 2017).
Cytotoxicity assay and determination of proliferation
For stimulation, human primary GBM cells (PC1-3) were seeded with 10% confluency in six-well culture dishes (30,000 cells for all three primary cultures, PC1-3, respectively). Subsequently, cells were stimulated with either 5 μM AT101 [stock dissolved at 100 mM in dimethyl sulfoxide (DMSO); Tocris, Bristol, UK], 5 or 10 μM DMC [(stock dissolved at 100 mM in DMSO); Sigma-Aldrich, St. Louis, MO, USA] as well as a combinations of 5 or 10 µM DMC plus 5 µM AT101 in DMEM without phenol-red (Life Technologies) supplemented with 10% FBS, 1% penicillin–streptomycin, and 2 mM additional l-glutamine for up to 6 days. Controls were stimulated with an equal volume of DMSO [0.01% (v/v)]. The cytotoxic effects of DMC, AT101, and DMSO treatment on human primary GBM cells were investigated with the CytoTox-FluorTM Cytotoxicity Assay (Promega, Madison, WI, USA) according to the manufacturer’s instruction. Briefly, supernatants of different AT101 and DMC stimulated and control cells were collected at days 3 and 6 of stimulation, mixed with the bis-AAF-R110 substrate, and measured in a fluorescence microplate reader (GENios, TECAN, Zürich, Switzerland) at 485/535 nm. The exact numbers of dead cells were determined according to a prepared standard of digitonin-lysed (82.5 μg/ml; Merck Millipore, Burlington, MA, USA) cell dilutions of each PC. Furthermore, the percentages (%) of dead cells in relation to the total amount of measured cells were calculated as described in Eqs. (1) and (2) after 3 and 6 days, respectively, of stimulation.
| 1 |
| 2 |
Moreover, the n-fold amount of (%) dead cells compared to the internal stimulation control DMSO was calculated for day 3 or 6, respectively. The cell survival was determined by counting viable cells at day 0, 3, and 6 of stimulation. Cell viabilities were calculated as n-fold numbers of live cells compared to the internal stimulation control DMSO for day 3 or 6, respectively. n = 8 (PC1) and n = 4 (PC2-3) for independent experiments.
Combination index
To analyze the influence of different drug combinations and their synergy quantification over the influence of their single effect, first, the fraction affected (FA) for each drug or drug combination was determined (see Eq. 3), by building the difference of the viability between the DMSO control (set as 1) and the individual stimulation (set as n-fold of the control).
| 3 |
Next, the Chou–Talalay method was used, with the CompuSyn software (https://www.combosyn.com) (Chou 2006). This analysis determines if the effect of a combined treatment is antagonistic [combination index (CI) > 1], additive (CI ~ 1), or synergistic (CI < 1).
Western blot
Human primary GBM cells (8.5 × 105, PC1) were stimulated for up to 6 days as described above in two independent experiments. Cells were harvested, and 3 μg protein per sample was used for western blotting experiments as described before (Hattermann et al. 2010). The primary antibodies were anti-phospho-p44/42 (1:500, #9101, rabbit; Cell Signaling, Danvers, MA, USA) and anti-phospho-Akt (1:250, #4060, rabbit; Cell Signaling) in 5% (w/v) bovine serum albumin (BSA)/Tris-buffered saline with 0.1% Tween (TBS-T); the secondary antibody was donkey anti-rabbit IgG-HRP (1:25,000, A16035; Invitrogen) in 2% (w/v) casein/TBS-T. Equal protein loading was confirmed by stripping and incubating the membranes with anti-glycerinaldehyde 3-phosphate dehydrogenase (GAPDH; 1:200; sc-47724; mouse, Santa Cruz Biotechnology, Dallas, TX, USA) in 2% (w/v) casein/TBS-T; the secondary antibody was donkey anti-mouse IgG-HRP (1:30,000, sc-2096; Santa Cruz Biotechnology) in 2% (w/v) casein/TBS-T as described before (Hattermann et al. 2010). The densities of kinase signals were measured using Image J software, signals of phosphorylated kinases were normalized to GAPDH, and ratios were plotted for each stimulation, respectively.
Visualization of mitochondrial membrane potential
Human primary GBM cells (3.0 × 105) were stimulated for up to 6 days as described above. After 6 days of stimulation, 20.0 × 105 GBM cells were reseeded, and the mitochondrial membrane potential was visualized with JC-1 (stock dissolved at 7.7 mM in DMSO; Invitrogen) according to the manufacturer’s instructions. Briefly, PC1 cells were incubated (37 °C, 5% CO2) with 2 µM JC-1 in DMEM without phenol-red (Life Technologies) for 30 min for mitochondrial staining. Next, cells were washed twice with phosphate-buffered saline (PBS), fixed with 2% paraformaldehyde, and microscoped at 488/529 nm (monomer form) and 488/595 (J-aggregate form). Exemplary pictures, taken with equal exposure times and settings, are shown. The green/red ratio was determined by Image J software and plotted for each stimulation, respectively (n = 3 independent experiments for PC1 with n = 4 analyzed pictures, respectively).
Reverse transcription and quantitative real-time PCR (qRT-PCR)
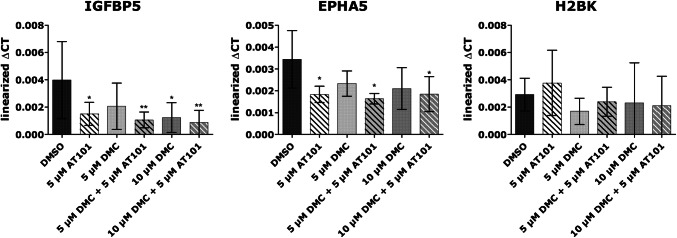
Human primary GBM cells (3.0 × 105, PC1) were stimulated for up to 6 days as described above in five independent experiments. The RNA of cells was isolated with the TRIzol® reagent (Invitrogen) according to the manufacturer’s instructions. DNase digestion, cDNA synthesis, and qRT-PCR were performed as described before (Hattermann et al. 2010) using TaqMan primer probes (Applied Biosystems, Waltham, MA, USA): ephrin type-A receptor 5 (EphA5) (Hs00300724_m1), glycerinaldehyde 3-phosphate dehydrogenase (GAPDH) (Hs99999905_m1), histone cluster 1 H2B family member K (H2BK) (Hs00955067_g1), insulin-like growth factor-binding protein 5 (IGFBP5) (Hs00181213_m1). Cycles of threshold (CT) were determined, and ∆CT values of each sample were calculated as CTgene of interest – CTGAPDH. Linearized ∆CT values are depicted in Fig. 4. The induction of gene expression upon stimulation was displayed as n-fold expression changes = 2∆CT control−∆CT stimulus.
Fig. 4.
Expression of IGFB5, EPHA5, and H2BK in PC 1 after 6 days of stimulation. Cells were either treated with 5 µM AT101, 5 or 10 µM DMC as well as the combination of 5 or 10 µM DMC plus 5 µM AT101 for up to 6 days and analyzed by qRT-PCR regarding the mRNA expression of IGFBP5, EphA5 and H2BK. Linearized ΔCT values were depicted of n = 5 independent experiments. A repeated one-way ANOVA followed by a Bonferroni’s multiple comparison test, comparing each column to all the other columns, of the linearized ΔCT was performed to check significant differences. Only significances between the different stimulations and DMSO, and between the combined treatment to their respective single treatments are depicted (*p < 0.05, **p < 0.01, ***p < 0.001)
Statistical analysis
Data was analyzed with the GraphPad Prism 5 Software (GraphPad Software, La Jolla, CA, USA). To check for statistical significance, a two-way ANOVA followed by a Bonferroni’s multiple comparison test, comparing each column to all the other columns was employed for n-fold viability and n-fold percentage of dead cells, whereas a repeated one-way ANOVA followed by Bonferroni’s multiple comparison test, comparing each column to all the other columns, was employed for the JC-1 staining and qRT-PCR. In general, the data are presented as mean ± standard deviation (SD). Asterisks indicate statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001. Only significances between the different stimulations and DMSO, and between the combined treatment to their respective single treatments are depicted.
Results
Combined treatment of AT101 and DMC was more effective in growth inhibition of GBM cells, whilst cell death was only moderately influenced
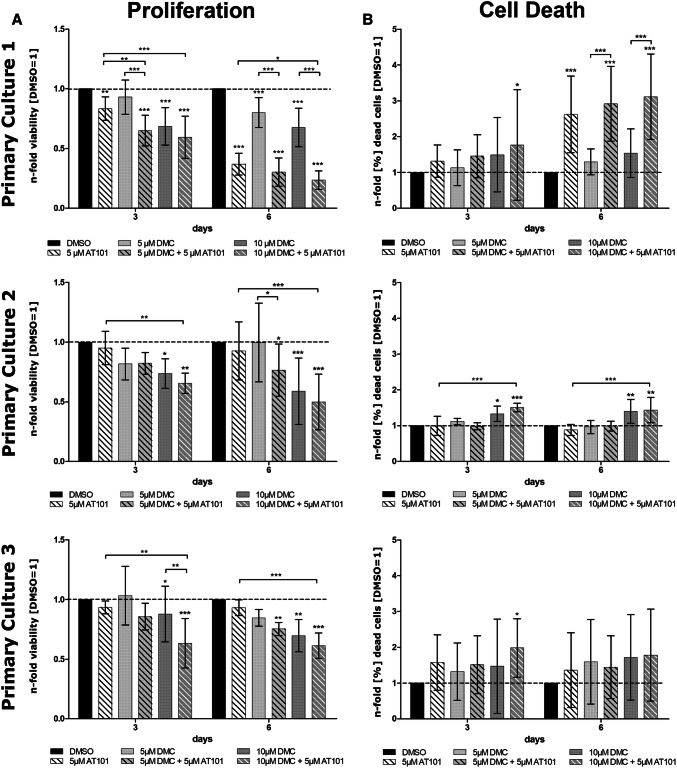
To investigate whether the combined treatment of AT101 and DMC was superior in relation to AT101 or DMC single treatment, respectively, three different human primary GBM cultures were treated with AT101 combined with two different DMC concentrations in a long-term stimulation in vitro model for up to 6 days, and n-fold cell viabilities as well as amounts of dead cells were determined in relation to controls and single treated samples (Fig. 1).
Fig. 1.
Effect of single and combined treatment with AT101 and DMC on n-fold viability and n-fold amount of (%) dead cells in three different human primary GBM cell cultures. Cells were either treated with 5 µM AT101, 5 or 10 µM DMC as well as the combination of 5 or 10 µM DMC plus 5 µM AT101 for up to 6 days. a Cell viability was counted at day 0, 3 and 6, and numbers of vital cells were depicted as n-fold numbers of live cells compared to the internal stimulation control (DMSO) for days 3 and 6, respectively. b Cytotoxicity assay was performed at days 3 and 6 and dead cells were compared in relation to live cells of the respective day and stimulation (% were calculated) and shown as n-fold amount of (%) dead cells compared to the internal stimulation control DMSO for day 3 or 6, respectively. a + bn = 8 independent experiments (PC1), n = 4 independent experiments (PC2, 3). A two-way ANOVA followed by a Bonferroni’s multiple comparison test, comparing each column to all the other columns, of the shown data was performed to check significant differences. Only significances between the different stimulations and DMSO, and between the combined treatment to their respective single treatments are depicted (*p < 0.05, **p < 0.01, ***p < 0.001)
Especially the combined treatment with 5 µM AT101 and 10 µM DMC yielded the best, statistically significant anti-proliferative effects in all primary GBM cultures tested after 3 and 6 days of treatment, respectively (Fig. 1a). If the combined treatment was performed with lower DMC concentrations (5 µM AT101 plus 5 µM DMC), the influence on n-fold cell viabilities was more heterogeneous. Whereas primary culture 1 (PC1) responded well, PC2 was nearly not affected, and PC3 to significant extents only after 6 days of treatment. When looking at the impact of AT101 or DMC single treatment, respectively, the effects were compared to the combined treatment less effective. However, AT101 showed a good anti-proliferative response in PC1, and 10 µM DMC was superior compared to 5 µM DMC regarding the inhibition of n-fold cell viabilities of all primary cultures especially after 6 days of treatment (Fig. 1a). Interestingly, the enhanced anti-proliferative effect of the combined AT101 and DMC treatment seemed to be regulated by either a more pronounced AT101 or DMC effect, respectively. In detail, in PC1 the AT101-mediated reduction of the n-fold cell viability was statistically significantly more efficient after addition of DMC after 3 and 6 days, respectively (pointing to a synergistic DMC effect), and the DMC-mediated anti-proliferative effect was statistically more efficient after addition of AT101 especially after 6 days of treatment (pointing to a synergistic AT101 effect). In both PC2 and PC3, these synergistic effects were also detectable; however, the most consistent one was the synergistic DMC effect regarding the 5 µM AT101 plus 10 µM DMC treatment compared to AT101 single treatment after 3 and 6 days, respectively. Indeed, after calculation of the combination index of the growth-inhibitory effects of the combined 5 µM AT101 plus 10 µM DMC treatment (6 days) using the CompuSyn software, a clear synergism of drugs was detectable for all investigated primary GBM cultures with the most powerful one in PC1 (Fig. 2).
Fig. 2.
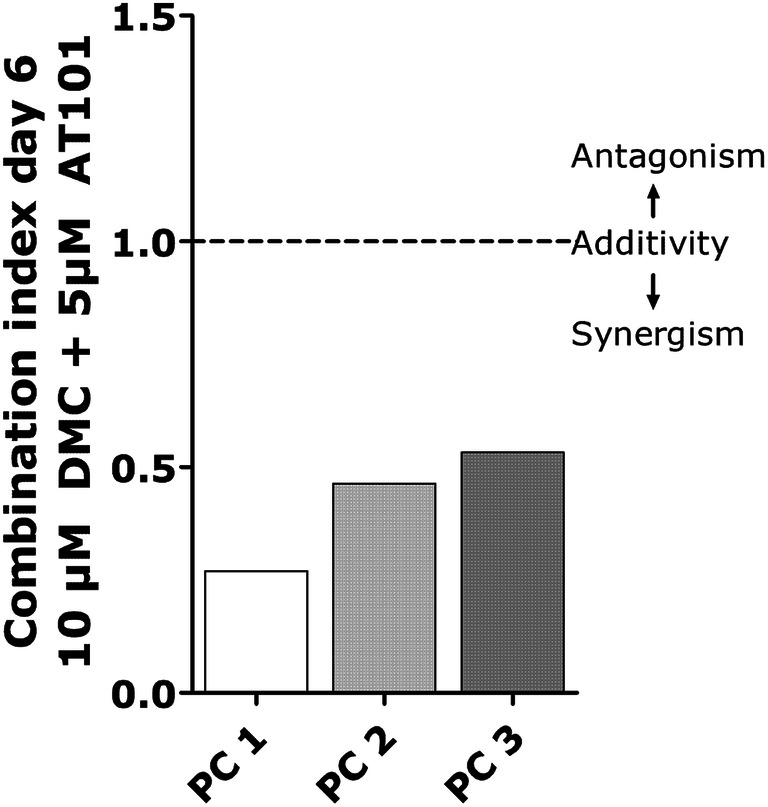
Combination index. Cell viability after 10 µM DMC and 5 µM AT101 treatment was analyzed in terms of the combined effect of different drugs by the Chou–Talalay method-combination index using the CompuSyn software
When looking at the drug-induced cell death [determined as n-fold (%) dead cells in relation to control samples; Fig. 1b], the measured drug effects were not as prominent and consistent as the influence on n-fold viabilities. Whereas in both PC1 and 2 the combined 5 µM AT101 plus 10 µM DMC treatment promoted cell death after 3 and 6 days of treatment, respectively, in PC3 no convincing effects were detectable. Single treatment strategies yielded only slight effects in PC1 regarding AT101 stimulation, as well as in PC2 regarding 10 µM DMC stimulation. Additionally, whereas in PC1 the DMC plus AT101-mediated induction of cell death was statistically more efficient compared to DMC single treatment after 6 days, in PC2, the AT101 plus DMC-mediated induction of cell death was slightly (but statistically significant) more efficient compared to AT101 single treatment after 3 and 6 days, respectively (Fig. 1b).
Combined treatment of AT101 and DMC influenced activated signal cascades, mitochondrial membrane potentials, as well as gene expression in GBM cells
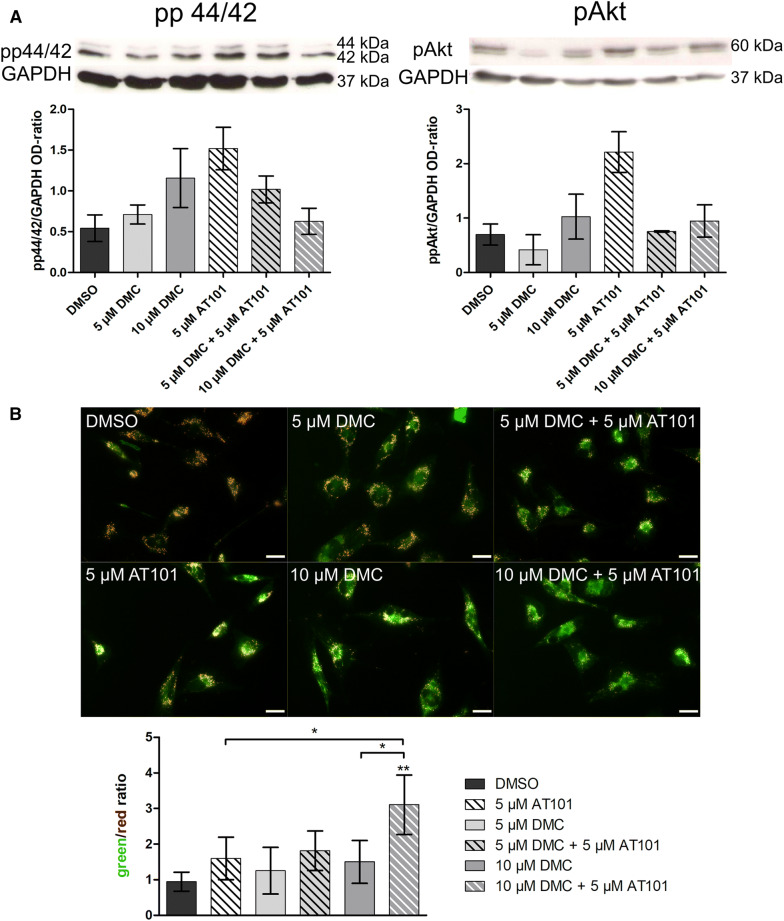
To evaluate whether the combined treatment influenced intracellular responses of treated GBM cells in our long-term stimulation in vitro model, firstly, we analyzed the activation/phosphorylation of the extracellular signal-regulated kinase (ERK) 1/2 (p44/42), and of Akt, which is also known as protein kinase B (Fig. 3a). These types of kinases are well known to be involved in critical pathways for human cancers, e.g., cell survival, proliferation, dissemination, nutrient metabolism, and resistance to drug therapy (Burotto et al. 2014; Rai et al. 2019). Whereas the single treatment with AT101 clearly favored the phosphorylation of both p44/42 and Akt, DMC single treatment only slightly influenced especially pp44/42 signals. Interestingly, in particular the combined 10 µM DMC plus 5 µM AT101 treatment distinctly reduced the AT101-mediated phosphorylation/activation of p44/42 and Akt to control levels as determined by densitometric analysis of signals of phosphorylated kinases normalized to glycerinaldehyde 3-phosphate dehydrogenase (GAPDH) (Fig. 3a).
Fig. 3.
Western blot on pp44/42 and pAkt (a) and visualization of mitochondrial membrane potential with JC-1 (b) in PC1 after 6 days of stimulation. Cells were either treated with 5 µM AT101, 5 or 10 µM DMC as well as the combination of 5 or 10 µM DMC plus 5 µM AT101 for up to 6 days. a pp44/42 and pAkt signals were analyzed by western blot and normalized to GAPDH. Exemplary data shown for PC1, n = 2. b JC-1 staining was performed on PC1, microscoped, and photographed with equal exposure times; scale bar equals 20 µm. The green/red ratio was determined using Image J. Exemplary data shown, n = 3 independent experiments. A repeated one-way ANOVA followed by a Bonferroni’s multiple comparison test, comparing each column to all the other columns, of the green/red ratio was performed to check significant differences. Only significances between the different stimulations and DMSO, and between the combined treatment to their respective single treatments are depicted (*p < 0.05, **p < 0.01, ***p < 0.001)
Next, we visualized the influence of the combined treatment on the membrane potential of mitochondria of long-term-treated GBM cells (Fig. 3b). Here, JC1 is able to incorporate into the mitochondrial membrane and to form aggregates due to the physiological membrane potential of mitochondria. This aggregation process changes the fluorescence properties of JC-1 leading to a shift from green to orange fluorescence. Whereas intact living cells stained with JC-1 are characterized by a pronounced orange fluorescence of mitochondria, cell death results in a breakdown of the mitochondrial membrane potential and a subsequent decrease of the orange fluorescence combined with a slight increase of the green one (Perry et al. 2011).
As depicted in Fig. 3b, the green/red JC1 ratio determined after 10 µM DMC plus 5 µM AT101 treatment was clearly greater than those measured in controls and single treated GBM samples (exemplary pictures are shown). Further, a statistical significance could be observed in comparison to single AT101 as well as to single 10 µM DMC treatment, respectively, pointing to a synergistic effect of the combined treatment once again.
Finally, we determined the influence of the combined AT101 plus DMC treatment on the mRNA expression of different genes in our long-term stimulation in vitro model. Since it is known that different treatment strategies could affect genes, which are involved in so-called dormancy-associated processes (Adamski et al. 2017), we focused on the expression of glioma-specific dormancy-associated genes like insulin-like growth factor-binding protein 5 (IGFBP5), ephrin type-A receptor 5 (EphA5), and histone cluster 1 H2B family member K (H2BK) (Almog et al. 2009; Satchi-Fainaro et al. 2012). Here, cancer dormancy is a widely described phenomenon of malignant tumors addressing a protected state which may occur, e.g., after an apparently successful therapeutic intervention (Adamski et al. 2017; Almog 2010), and IGFBP5, EphA5, as well as H2BK are upregulated in dormant GBM cells during treatment promoting cell survival (Almog et al. 2009; Satchi-Fainaro et al. 2012). Interestingly, in relation to control samples, IGFBP5 and EphA5 were downregulated especially in AT101 single or AT101 plus DMC combined treated GBM samples (Fig. 4), whereas H2BK expression was not affected.
Summarized, we showed that a combined therapy with both drugs was superior to the single treatment with AT101 or DMC especially due to an enhanced anti-proliferative effect in our long-term stimulation in vitro model using human primary GBM cells. By determination of the combination index, a synergism of the combined drugs was detectable. Further, the phosphorylation and thereby activation of the kinases p44/42 and Akt, which are involved in proliferation and survival processes, were inhibited, the mitochondrial membrane potential was altered, and genes involved in glioma dormancy-associated processes were inhibited by the combined treatment strategy.
Discussion
Since the efficient treatment of highly malignant, heterogeneously composed, and chemoresistant GBMs is still a clinical challenge, further therapeutic advances must be developed to extend the survival times of patients after diagnosis of this disease. Besides the widely used standard therapy, consisting of a concomitant radiation therapy with temozolomide (TMZ) followed by maintenance with TMZ (Stupp et al. 2005), several alternative drugs and treatment strategies are currently under consideration. In contrast to TMZ, the therapeutic benefit of which depends on its ability to alkylate/methylate DNA most frequently at the N-7 or O-6 positions of guanine residues, DMC and AT101 are characterized by different, multi-faceted kinds of action resulting in, e.g., anti-inflammatory, anti-oxidative, or anti-tumorigenic properties.
The different individual cellular responses of DMC or AT101-treated cells, respectively, were intensively investigated by different groups. In detail, Lal et al. (2018) showed that DMC was able to trigger apoptosis through caspase-8 and 9 activation and to inhibit Akt/NF-κB survival signaling in human glioma U87MG cells. This effect resulted after DMC-mediated induction of reactive oxygen species (ROS) via inhibition of the mitochondrial superoxide dismutase (MnSOD) favored by the establishment of π–π interactions with Tyr34 and Trp161 in the active site of MnSOD (Kumar et al. 2018; Lal et al. 2018). Further, DMC-induced ROS disrupted the mitochondrial membrane potential to trigger cytosolic release of cytochrome c and cleavage of caspase-9 (Kumar et al. 2018), and ROS led to a reduced expression and ubiquitination as well as to a proteasome degradation of CDC25C and cyclin B1 in U87MG glioma cells. As a consequence, DMC treatment suppressed the viability of U87MG glioma cells causing a G2/M arrest at 24 h and increased a sub-G1 apoptotic fraction at 48 h (Lal et al. 2018). Additionally, Huang et al. (2012) demonstrated that DMC exhibited a potent antineoplastic effect in GBM8401 glioma cells through loss of mitochondrial membrane potential, ultimately leading to apoptosis. Interestingly, Chen et al. (2017) showed that DMC-induced apoptosis and G0/G1 cell cycle were increased in glioma stem cells after suppressing the drug transporter ABCG2. ABCG2 suppression also increased the levels of ROS resulting in an increased cytochrome C release and caspase-3 activity. Concerning AT101, Warnsmann et al. (2018) mentioned that a treatment with gossypol selectively increased the hydrogen peroxidase levels and impaired mitochondrial respiration in GBM cells. In accordance with this, Voss et al. (2010) stated that AT101 enhanced cytochrome c release in glioma cells, and that a knockdown of mTOR sensitized glioma cells to autophagy, mitochondrial dysfunction, and cell death induced by AT101. Further, Meyer et al. (2018) showed that AT101 induced an early mitochondrial dysfunction to trigger mitophagic cell death in glioma cells.
Whereas in our study, AT101 or DMC single treatment was also able to trigger (partly) anti-proliferative effects and to (slightly) induce cell death as well, the combined AT101 plus DMC treatment was clearly more efficient. In accordance with this, in our long-term stimulation in vitro model using primary GBM cells, the single treatment with AT101 or DMC resulted in a disruption of the mitochondrial membrane potential, too. However, this effect was more enhanced by the combined AT101 plus DMC treatment strategy. Concerning the effects on signal kinases, only 5 µM DMC exhibited a slightly inhibitory effect on Akt phosphorylation, whereas both DMC and especially AT101 favored the phosphorylation and thereby activation of p44/42. However, the combined AT101 plus DMC treatment efficiently reduced the amounts of pp44/42 and pAkt to control values. Interestingly, Guo et al. (2014) showed that gossypol acetic acid mediated the induction of H2AX via activation of the phosphatidylinositol 3-kinase (PI3K) family. Since PI3K phosphorylates and activates Akt, these results are in accordance with those shown by our group.
As mentioned before, both AT101 and DMC are able to induce a cell cycle arrest (Guo et al. 2014; Luthra et al. 2009). However, a (reversible) growth arrest can also occur after an apparently successful therapeutic intervention (Adamski et al. 2017; Almog 2010). This so-called cellular dormancy is defined as a state in which either solitary or small groups of cells enter quiescence driven by intrinsic or extrinsic factors (Almog 2010). Expression analysis between dormant and fast-growing phenotypes of GBM cells revealed that a specific gene set is upregulated in dormant GBMs, including e.g., EphA5, thrombospondin, angiomotin, IGFBP5, and H2BK (Almog et al. 2009; Satchi-Fainaro et al. 2012). Now we were able to show that in relation to control samples, IGFBP5 and EphA5 were downregulated especially in AT101 single or AT101 plus DMC combined treated GBM samples pointing to the hypothesis that AT101 alone or in combination with DMC was able to regulate dormancy-associated gene expression. Interestingly, upon TMZ-induced dormancy in vitro IGFBP5, EphA5, as well as H2BK were upregulated (Adamski et al. 2017). However, a combined treatment with TMZ and AT101 diminished the amounts of surviving dormant GBM cells (Adamski et al. 2017).
Summarized, we showed that the combined treatment with AT101 plus DMC yielded enhanced anti-proliferative effects in a long-term stimulation GBM in vitro model mediated by the potentiated inhibition of pro-survival signal kinases, a more increased disruption of the mitochondrial membrane potential, and a decreased expression of dormancy-associated genes.
Acknowledgements
We thank Fereshteh Ebrahim and Brigitte Rehmke for expert technical assistance.
Abbreviations
- BSA
Bovine serum albumin
- CI
Combination index
- DMC
Demethoxycurcumin
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
Dimethylsulfoxide
- EphA5
Ephrin type-A receptor 5
- FBS
Fetal bovine serum
- GAPDH
Glycerinaldehyde 3-phosphate dehydrogenase
- GBM
Glioblastoma multiforme
- H2BK
Histone cluster 1 H2B family member K
- IGFBP5
Insulin-like growth factor-binding protein 5
- OD
Optical density
- PBS
Phosphate-buffered saline
- PC
Primary culture
- qRT-PCR
Quantitative real-time polymerase chain reaction
- TBS-T
Tris-buffered saline with 0.1% Tween
- TMZ
Temozolomide
Author contributions
JHF, CK, and CS conceived and designed the study; MM, VA, CS, and JHF performed the experiments and analyzed the data; CK and MS contributed materials and data and assisted in data analysis; JHF and MM wrote the paper; and all authors revised the manuscript.
Funding
This work was funded by the German Research Foundation (DFG) as part of the Research Training Group “Materials4Brain” (RTG2154; P8).
Compliance with ethical standards
Conflict of interest
Author Moiken Mehner declares that she has no conflict of interest. Author Carolin Kubelt declares that she has no conflict of interest. Author Vivian Adamski declares that she has no conflict of interest. Author Christina Schmitt declares that she has no conflict of interest. Author Michael Synowitz declares that he has no conflict of interest. Author Janka Held-Feindt declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human tumor samples were in accordance with the ethical standards of the institutional committee (ethics committee of the University of Kiel, Germany; file references: D471/15 and D524/17) and with the 1964 Helsinki Declaration and its later amendments. Written informed consent was obtained from all individual donors included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adamski V, Hempelmann A, Flüh C, Lucius R, Synowitz M, Hattermann K, Held-Feindt J (2017) Dormant glioblastoma cells acquire stem cell characteristics and are differentially affected by Temozolomide and AT101 treatment. Oncotarget 8:108064–108078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamski V, Schmitt C, Ceynowa F, Adelung R, Lucius R, Synowitz M, Hattermann K, Held-Feindt J (2018) Effects of sequentially applied single and combined temozolomide, hydroxychloroquine and AT101 treatment in a long-term stimulation glioblastoma in vitro model. J Cancer Res Clin Oncol 144:1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almog N (2010) Molecular mechanism underlying tumor dormancy. Cancer Lett 294:139–146 [DOI] [PubMed] [Google Scholar]
- Almog N, Ma L, Raychowdhury R, Schwager C, Erber R, Short S, Hlatky L, Vajkoczy P, Huber PE, Folkman J, Abdollahi A (2009) Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res 69:836–844 [DOI] [PubMed] [Google Scholar]
- Antonietti P, Linder B, Hehlgans S, Mildenberger IC, Burger MC, Fulda S, Steinbach JP, Gessler F, Rödel F, Mittelbronn M, Kögel D (2017) Interference with the HSF1/HSP70/BAG3 pathway primes glioma cells to matrix detachment and BH3 mimetic-induced apoptosis. Mol Cancer Ther 16:156–168 [DOI] [PubMed] [Google Scholar]
- Burotto M, Chiou VL, Lee J-M, Kohn EC (2014) The MAPK pathway across different malignancies: a new perspective. Cancer 120:3446–3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushunow P, Reidenberg MM, Wasenko J, Winfiled J, Lorenzo B, Lemke S, Himpler B, Corona R, Coyle T (1999) Gossypol treatment of recurrent adult malignant gliomas. J Neurooncol 43:79–86 [DOI] [PubMed] [Google Scholar]
- Chen L, Shi L, Wang W, Zhou Y (2017) ABCG2 downregulation in glioma stem cells enhances the therapeutic efficacy of demethoxycurcumin. Oncotarget 8:43237–43247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC (2006) Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58:621–681 [DOI] [PubMed] [Google Scholar]
- Guo Z, Zhao J, Song L, Ma JX, Wang CJ, Pei SY, Jiang C, Li SB (2014) Induction of H2AX phosphorylation in tumor cells by gossypol acetic acid is mediated by phosphatidylinositol 3-kinase (PI3K) family. Cancer Cell Int 14:e141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattermann K, Held-Feindt J, Lucius R, Sebens Müerköster S, Penfold MET, Schall TJ, Mentlein R (2010) The chemokine receptor CXCR7 is highly expressed in human glioma cells and mediates anti-apoptotic effects. Cancer Res 70:3299–3308 [DOI] [PubMed] [Google Scholar]
- Huang TY, Hsu CW, Chang WC, Wang MY, Wu JF, Hsu YC (2012) Demethoxycurcumin retards cell growth and induces apoptosis in human brain malignant glioma GBM 8401 cells. Evid Based Complement Altern Med 2012:396573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarzabek A, Amberger-Murphy V, Callanan JJ, Gao C, Zagozdzon AM, Shiels L, Wang J, Ligon KL, Rich BE, Dicker P, Gallagher WM, Prehn JHM, Byrne AT (2014) Interrogation of gossypol therapy in glioblastoma implementing cell line and patient-derived tumour models. Br J Cancer 111:2275–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshmiri-Neghab H, Goliaei B, Nikoofar A (2014) Gossypol enhances radiation-induced autophagy in glioblastoma multiforme. Gen Physiol Biophys 33:433–442 [DOI] [PubMed] [Google Scholar]
- Kumar R, Lal N, Nemaysh V, Luthra PM (2018) Demethoxycurcumin mediated targeting of MnSOD leading to activation of apoptotic pathway and inhibition of Akt/NF-κB survival signalling in human glioma U87 MG cells. Toxicol Appl Pharmacol 345:75–93 [DOI] [PubMed] [Google Scholar]
- Lal N, Nemaysh V, Luthra PM (2018) Proteasome mediated degradation of CDC25C and Cyclin B1 in demethoxycurcumin treated human glioma U87 MG cells to trigger G2/M cell cycle arrest. Toxicol Appl Pharmacol 356:76–89 [DOI] [PubMed] [Google Scholar]
- Li F, Xia Y, Meiler J, Ferguson-Miller S (2013) Characterization and modeling of the oligomeric state and ligand binding behavior of purified translocator protein 18 kDa from Rhodobacter sphaeroides. Biochemistry 52:5884–5899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820 [DOI] [PubMed] [Google Scholar]
- Lu Y, Li J, Dong C-E, Huang J, Zhou H-B, Wang W (2017) Recent advances in gossypol derivatives and analogs: a chemistry and biology view. Future Med Chem 9:1243–1275 [DOI] [PubMed] [Google Scholar]
- Luthra PM, Kumar R, Prakash A (2009) Demethoxycurcumin induces Bcl-2 mediated G2/M arrest and apoptosis in human glioma U87 cells. Biochem Biophys Res Commun 384:420–425 [DOI] [PubMed] [Google Scholar]
- Meyer N, Zielke S, Michaelis JB, Linder B, Warnsmann V, Rakel S, Osiewacz HD, Fulda S, Mittelbronn M, Münch C, Behrends C, Kögel D (2018) AT101 induces early mitochondrial dysfunction and HMOX1 (heme oxygenase 1) to trigger mitophagic cell death in glioma cells. Autophagy 14:1693–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opydo-Chanek M, Gonzola O, Marzo I (2017) Multifaceted anticancer activity of BH3 mimetics: current evidence and future prospects. Biochem Pharmacol 136:12–23 [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS (2018) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol 20:iv1–iv86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA (2011) Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques 50:98–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai SN, Dilnashin H, Birla H, Singh SS, Zahra W, Rathore AS, Singh BK, Singh SP (2019) The role of PI3K/Akt and ERK in neurodegenerative disorders. Neurotox Res 35:775–795 [DOI] [PubMed] [Google Scholar]
- Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB (2007) Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 8:1765–1773 [DOI] [PubMed] [Google Scholar]
- Satchi-Fainaro R, Ferber S, Segal E, Ma L, Dixit N, Ijaz A, Hlatky L, Abdollahi A, Almog N (2012) Prospective identification of glioblastoma cells generating dormant tumors. PLoS ONE 7:e44395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Sun G (2015) Low-dose DMC significantly enhances the effect of TMZ on glioma cells by targeting multiple signaling pathways both in vivo and in vitro. Neuromolecular Med 17:431–442 [DOI] [PubMed] [Google Scholar]
- Shi L, Sun G (2018) DMC is not better than TMZ on intracranial anti-glioma effects. J Cell Biochem 119:6057–6064 [DOI] [PubMed] [Google Scholar]
- Somparn P, Phisalaphong C, Nakornchai S, Unchern S, Morales NP (2007) Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol Pharm Bull 30:74–78 [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research, and Treatment of Cancer Brain Tumor, and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996 [DOI] [PubMed] [Google Scholar]
- Voss V, Senft C, Lang V, Ronellenfitsch MW, Steinbach JP, Seifert V, Kögel D (2010) The pan-Bcl-2 inhibitor (−)gossypol triggers autophagic cell death in malignant gliomas. Mol Cancer Res 8:1002–1016 [DOI] [PubMed] [Google Scholar]
- Wang J, Peng Y, Liu Y, Yang J, Huang M, Tan W (2015) AT101 inhibits hedgehog pathway activity and cancer growth. Cancer Chemother Pharmacol 76:461–469 [DOI] [PubMed] [Google Scholar]
- Warnsmann V, Meyer N, Hamann A, Kögel D, Osiewacz HD (2018) A novel role of the mitochondrial permeability transition pore in (−)gossypol-induced mitochondrial dysfunction. Mech Ageing Dev 170:45–58 [DOI] [PubMed] [Google Scholar]