Abstract
Purpose
Oral cavity squamous cell carcinoma (OCC) can spread to the neck without apparent lymphadenopathy. Pretreatment detection or prediction of occult metastasis might contribute to proper management of clinically node-negative (cN0) OCC. We examined the role of tumour quantitative 18-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/computed tomography (CT) measurements for predicting OCC occult metastasis and survival.
Methods
This study included 130 cN0 OCC patients who underwent 18F-FDG PET/CT scanning and subsequent curative surgery and neck dissection. Maximum, peak, and mean standardized uptake value (SUVmax, SUVpeak, and SUVmean), metabolic tumour volume (MTV), and total lesion glycolysis (TLG) were measured on pretreatment 18F-FDG PET/CT. Binary logistic regression was used to identify factors predicting occult cervical metastasis. Univariate and multivariate Cox proportional hazard regression were used to find factors associated with overall survival (OS).
Results
Pathological cervical metastasis (pN +) was found in 29 (22.3%) patients. Age, tumour differentiation, lymphovascular invasion, and T classification were significantly associated with pN + (all P < 0.05). After adjustment for these factors, MTV and TLG independently predicted pN + (P < 0.05). Invasion depth, lymphovascular invasion, T and N classifications, and overall TNM stage were significantly associated with OS. After adjustment for these factors, SUVmax and TLG independently predicted OS (all P < 0.05). Patients with TLG > 9.3 g had a 5.7-fold increased risk of overall mortality.
Conclusions
Tumour 18F-FDG PET/CT parameters might predict occult metastasis and survival in cN0 OCC patients.
Electronic supplementary material
The online version of this article (10.1007/s00432-020-03313-8) contains supplementary material, which is available to authorized users.
Keywords: Oral cavity squamous cell carcinoma, 18F-FDG PET/CT, Functional parameters, Occult metastasis, Survival
Introduction
Oral cavity squamous cell carcinoma (OCC) can spread to the neck via lymphatic drainage, without apparent lymph node (LN) disease (Farmer et al. 2015). Subclinical neck metastasis in patients with clinically LN-negative (cN0) OCC based on histological examination of elective dissection samples is as high as 34% (Shah et al. 1990; Farmer et al. 2015). Regional metastasis of OCC is associated with poor survival; metastatic LN burden decreases overall survival (OS) by nearly 50% (Ho et al. 2017). Subclinical neck LN metastasis is also a poor prognostic factor in cN0 OCC patients (Mucke et al. 2014). Therefore, elective rather than therapeutic neck dissection has been recommended for initial cN0 OCC that developed into clinically positive neck LN disease (cN +) (D'Cruz et al. 2015; Dik et al. 2016; Joo et al. 2019; Joo and Koo 2019). However, elective dissection can lead to surgical morbidities that might be avoided in up to 70% of cN0 OCC patients (Shah et al. 1990). Alternatively, sentinel LN biopsy is used to stage and treat subclinical neck disease in cN0 OCC (Schilling et al. 2015, 2017). Proper detection or prediction of occult cervical metastasis might be a rational approach for avoiding unnecessary neck surgery or sentinel LN biopsy.
Fluorine 18-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/computed tomography (CT) is used to assess the initial stage and treatment responses and to make posttreatment selections and prognostic prediction (Evangelista et al. 2014; Manca et al. 2016; Chong et al. 2017). The prognostic role of 18F-FDG PET/CT functional parameters in predicting treatment response and outcome and survival in head and neck cancer has been reported (Pak et al. 2014; Wang et al. 2019). Maximum standardized uptake value (SUVmax) measured on pretreatment 18F-FDG PET/CT scans predicts survival outcomes in OCC patients (Liao et al. 2009). Volumetric PET parameters, e.g. metabolic tumour volume (MTV) and total lesion glycolysis (TLG), have emerged as potentially better predictors of treatment responses (Pak et al. 2014; Wang et al. 2019).
Occult LN metastasis can be predicted by metabolic 18F-FDG PET parameters in several human solid tumours without apparent LN disease (Kim et al. 2014; Moon et al. 2014). However, tumour 18F-FDG uptake has been rarely used to predict occult metastasis in cN0 OCC patients. High MTV has been correlated with increased probability of occult metastasis of cN0 OCC (Chung et al. 2009). OCC-related clinical and pathological parameters, e.g. invasion depth and tumour differentiation, have been used to predict occult metastasis (Pentenero et al. 2005; Goerkem et al. 2010). 18F-FDG PET/CT functional parameters might be useful to predict occult metastasis of cN0 OCC. Therefore, we examined the usability of tumour quantitative 18F-FDG PET/CT analysis for predicting cervical metastasis and survival in cN0 OCC patients.
Materials and methods
Patients
This study prospectively enrolled consecutive OCC patients who underwent pretreatment 18F-FDG PET/CT and curative surgery between September 2010 and December 2016. Inclusion criteria were (1) patients aged 18 years or more with previously untreated cN0 OCC; (2) those who underwent 18F-FDG PET/CT within 3 weeks before treatment; (3) those who underwent curative surgery and neck dissection with or without postoperative adjuvant radiotherapy or chemoradiotherapy. cN0 was determined based on physical examination, imaging studies, and fine-needle aspiration biopsy before treatment. Cervical LNs suspected of having metastasis on images were preoperatively subjected to high-resolution ultrasonography-guided fine-needle aspiration. Tumours were included regardless of size, extent, and invasion if presenting cN0. Exclusion criteria were (1) patients for whom the volume of interest (VOI) of the primary tumour was difficult to define; (2) those with recurrent disease at presentation; (3) those with a history of neck surgery or irradiation for other cancers; (4) follow-up loss within 2 years after surgery. Eventually, 130 patients were included in this study. Tumours were staged according to the American Joint Committee on Cancer (AJCC) staging system (Edge et al. 2010). This study was approved by the Asan Medical Center Institutional Review Board and informed consent was obtained from each patient.
All patients underwent complete tumour extirpation and neck dissection. Elective neck dissection was performed at ipsilateral levels I–III or I–IV on the affected side. Bilateral neck dissection was performed in patients with median or bilateral tumours. Patients with adverse pathological features, e.g. positive resection margin, nodal positivity, perineural invasion, and lymphovascular invasion 'National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers' (2018), underwent postoperative radiotherapy or platinum-based concurrent chemoradiotherapy. After treatment completion, the patients were followed up every 1–3 months in the first year, every 2–4 months in the second year, every 6 months in the third to fifth years, and annually thereafter (Denaro et al. 2016). Recurrent or new lesions were checked with specific imaging techniques and biopsies and were treated with salvage surgery or palliative treatments.
Imaging analysis
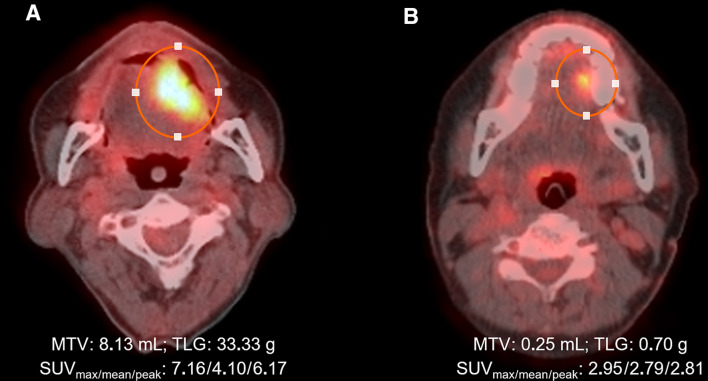
Patients underwent 18F-FDG PET/CT using a Biograph Sensation 16/TruePoint 40 system (Siemens, Knoxville, TN, USA) or a Discovery STE 8/690/710 system (GE Healthcare, Milwaukee, WI, USA). The patients fasted for at least 6 h to ensure serum glucose of 63–135 mg/dL prior to intravenous injection of 18F-FDG (370–640 MBq) followed by PET scanning. Image reconstruction and harmonization of SUVs from multiple PET systems were performed using standard methods (Boellaard et al. 2010; Lasnon et al. 2013). 18F-FDG-PET activity in VOIs drawn over primary tumours visible on PET images was determined by calculating the SUV as decay-corrected activity in the tissue divided by injected activity per lean body mass of the patient (Fig. 1). Intensity values were automatically converted to SUVs, and SUVmax, SUVpeak, SUVmean, and MTV were measured. For MTV measurement, primary tumour margins were delineated using an SUV 2.5 isocontour (Ryu et al. 2014). TLG was calculated as MTV × SUVmean.
Fig. 1.
Quantitative measurement of 18F-FDG PET functional parameters. VOI (circled) was set to include OCC with metabolic activity and the software automatically calculated the SUVmax, SUVmean, SUVpeak and MTV. Patient A (59-year-old men) with a 3 × 1.5 × 1.0 cm tumour in the tongue had single 5-mm-positive lymph node in left level Ib and died of disease at 42 months after curative surgery, whereas patient B (66-year-old women) with a 2.4 × 1.3 × 0.9 cm tumour in the tongue had no positive lymph nodes in the neck and has been disease-free for 56 months after surgery
Statistical analysis
The primary end point was pathological cervical LN metastasis (pN +). OS and disease-free survival (DFS) were secondary end points. Binary logistic regression was used to determine factors associated with pN + . Multivariate binary logistic regression was used to identify independent predictive factors using all variables with P < 0.1 in univariate analyses. Optimal cutoff values of PET/CT parameters were determined based on area under the receiver operating characteristics (ROC) curve (AUC) estimates of pN + , OS, and DFS. Univariate and multivariate analyses using a Cox proportional hazards model were conducted to identify relationships between clinicopathological factors or imaging parameters and OS and DFS. 18F–FDG PET/CT parameters were adjusted for variables with P < 0.05 in univariate analyses. Variables with multi-collinearity were separately fit. Survival curves were generated using the Kaplan–Meier method and survival data were compared using the log-rank test. The estimated odds ratio (OR), hazard ratio (HR) and 95% confidence interval (CI) were calculated. Two-sided P < 0.05 was considered significant. Statistical analyses were conducted using SPSS® version 24.0 (IBM, Armonk, NY, USA).
Results
Patient characteristics
This study included 84 (64.6%) male and 46 (35.4) female patients with a median age of 52 years (20–84 years). Patient characteristics of pN0 compared with pN + patients are summarized in Table 1. Tumour sites were the tongue (n = 107, 82.3%), mouth floor (n = 10, 7.7%), hard palate (n = 4, 3.1%), buccal region and gingiva (n = 3 each, 2.3%), lip (n = 2, 1.5%), and retromolar trigone (n = 1, 0.8%). Median tumour size and invasion depth were 1.8 cm (interquartile range 0.3–6.8 cm) and 0.6 cm (0.1–2.8 cm), respectively. Well, moderate, and poor differentiation was found in 77 (59.2%), 44 (33.8%), and 9 (6.9%) patients, respectively. One hundred and twenty-two patients (93.8%) were in early T1 or T2 classification. Twenty-nine (22.3%) patients had positive LNs with a median largest diameter of 0.4 cm (0.1–1.3 cm). The resection margin was grossly tumour free in all patients, but microscopically positive in five (3.8%) patients. Forty-six patients (35.4%) received postoperative radiotherapy or chemoradiotherapy. At follow-up at 46 months (range 4–100), any-site recurrence occurred in 27 (20.8%) patients, and at the last follow-up, 110 (84.6%) patients were disease free, 3 (2.3%) had recurrent disease, 16 (12.3%) died of the disease, and 1 (0.8%) died of other cause.
Table 1.
Patient characteristics (n = 130)
| Variable | Total, n (%) | pN0, n (%) | pN + , n (%) | Pa |
|---|---|---|---|---|
| No. of patients | 130 (100) | 101 (77.7) | 29 (22.3) | |
| Sex, female | 46 (35.4) | 36 (35.6) | 10 (34.5) | 0.908 |
| Age (years), median (range) | 52 (20–84) | 51 (20–82) | 60 (21–84) | 0.088 |
| > 60 | 41 (31.5) | 27 (26.7) | 14 (48.3) | 0.028* |
| Tumour site, tongue | 107 (82.3) | 81 (80.2) | 26 (89.7) | 0.239 |
| Tumour size, cm | ||||
| ≤ 2 | 82 (63.1) | 67 (55.3) | 15 (51.7) | 0.077 |
| 2.1–4 | 43 (33.1) | 32 (31.7) | 11 (37.9) | |
| > 4 | 5 (3.8) | 2 (2.0) | 3 (10.3) | |
| Histological differentiation | ||||
| Well | 77 (59.2) | 67 (66.3) | 10 (34.5) | 0.007* |
| Moderate | 44 (33.8) | 29 (28.7) | 15 (51.7) | |
| Poor | 9 (6.9) | 5 (5.0) | 4 (13.8) | |
| Tumour invasion depth, mm | ||||
| ≤ 5 | 56 (43.1) | 44 (43.6) | 12 (41.4) | 0.730 |
| 5.1–10 | 45 (34.6) | 36 (35.6) | 9 (31.0) | |
| > 10 | 29 (22.3) | 21 (20.8) | 8 (27.6) | |
| Lymphovascular invasion | 20 (15.4) | 12 (11.9) | 8 (27.6) | 0.039* |
| T classification | ||||
| T1–T2 | 122 (93.8) | 97 (96.0) | 25 (86.2) | 0.052* |
| T3–T4 | 8 (6.2) | 4 (4.0) | 4 (13.8) | |
| Treatment | ||||
| Surgery | 84 (64.6) | 78 (77.2) | 6 (20.7) | < 0.001* |
| Surgery + RT or CRT | 46 (35.4) | 23 (22.8) | 23 (79.3) | |
| Follow-up | ||||
| Median (range), months | 46 (4–100) | 47 (4–100) | 40 (8–95) | 0.308 |
| Any-site recurrence | 27 (20.8) | 17 (16.8) | 10 (34.5) | 0.039* |
| Last status, DOD or DOC | 17 (13.1) | 8 (7.9) | 9 (31.0) | 0.001* |
| Estimated mean survival, months | 87.8 | 92.4 | 69.9 | 0.002* |
CRT chemoradiotherapy; DOC died of other cause; DOD died of disease; pN0/pN + pathological lymph node negativity/positivity; RT radiotherapy; TNM tumour–node–metastasis staging according to American Joint Committee on Cancer (AJCC, 7th ed.)
aχ2 test except for age and median follow-up (t test), *P < 0.05
Predictive ability of 18F-FDG PET/CT parameters for occult cervical metastasis
18F-FDG PET parameters were quantitatively measured: the median (interquartile range) SUVmax, SUVmean, SUVpeak, MTV, and TLG were 4.1 (3.0–6.0), 3.3 (2.6–5.1), 3.1 (2.8–3.5), 2.6 (0.6–6.5) mL, and 8.0 (1.8–24.7) g, respectively. ROC curve analysis was used to identify predictors of pN + . Optimal cutoff values for pN + were 5.3 for SUVmax, 5.1 for SUVpeak, 3.5 for SUVmean, 6.6 mL for MTV, and 24.0 g for TLG. Binary logistic regression analyses showed that age (> 60 years), histological differentiation, lymphovascular invasion, and T classification were significantly associated with pN + (all P < 0.05) (Supplementary Table S1). Among 18F-FDG PET parameters at the defined cutoff values, MTV and TLG were associated with pN + (all P < 0.05). Multivariate regression showed that age and histological differentiation were independent predictors of pN + . After adjustment for age, histological differentiation, lymphovascular invasion, and T classification, MTV and TLG remained associated with pN + (P < 0.05) (Table 2). MTV > 6.6 mL or TLG > 24.0 g was associated with > 2.6-fold increased risk of cervical metastasis. AUCs of 18F-FDG PET parameters for pN + were 0.629 (95% CI 0.516–0.742; P = 0.032) for MTV and 0.626 (95% CI 0.512–0.740; P = 0.037) for TLG, and 0.600 (95% CI 0.495–0.721; P = 0.098) for tumour size. Sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of the 18F-FDG PET/CT parameters in association with pathological neck positivity are presented in Supplementary Table S2. Representative images for patients with low and high 18F-FDG PET values are shown in Fig. 1.
Table 2.
Univariate and multivariate analyses of PET parameters associated with pN +
| 18F–FDG PET/CT parameters | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| ORa | 95% CI | Pa | ORa | 95% CI | Pa | |
| SUVmax > 5.3 | 2.32 | 0.99–5.40 | 0.052 | 1.28 | 0.43–3.27 | 0.544 |
| SUVpeak > 5.1 | 2.19 | 0.90–5.33 | 0.082 | 1.14 | 0.96–3.30 | 0.541 |
| SUVmean > 3.5 | 2.39 | 1.00–5.73 | 0.050 | 1.38 | 0.84–3.21 | 0.240 |
| MTV > 6.6 mL | 2.86 | 1.18–6.94 | 0.020* | 2.72 | 1.07–7.78 | 0.037* |
| TLG > 24.0 g | 2.69 | 1.11–6.49 | 0.028* | 2.61 | 1.04–6.55 | 0.044* |
CI confidence interval; 18F-FDG 18F-fluorodeoxyglucose; MTV metabolic tumour volume; OR odds ratio; PET/CT positron emission tomography/computed tomography; pN + pathological neck–lymph–node positivity; SUV standardized uptake value; TLG total lesion glycolysis
aBinary logistic regression analyses; multivariate analyses were adjusted for age, histological differentiation, lymphovascular invasion, and T classification, *P < 0.05 (see Supplementary Table S1)
Relationship between 18F-FDG PET/CT parameters and post-reatment survival
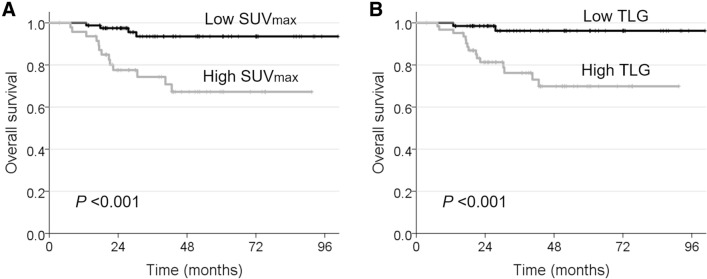
Optimal cutoff values of 18F-FDG PET parameters for OS and DFS determined by ROC curve analysis were 5.0 for SUVmax, 3.5 for SUVpeak, 3.1 for SUVmean, 2.9 mL for MTV, and 9.3 g for TLG. Univariate analyses showed that invasion depth (> 10 mm), lymphovascular invasion, T (T3–T4) and N (pN1–2) classification, overall TNM stage (III–IV) and postoperative radiotherapy or chemoradiotherapy were associated with poor OS (all P < 0.05) (Supplementary Table S3). Invasion depth (> 10 mm), lymphovascular invasion, T classification, overall TNM stage, and postoperative RT or CRT were also associated with poor DFS (all P < 0.05). High values of all 18F-FDG PET parameters were associated with poor OS and DFS (all P < 0.05). After adjustment for depth of tumour invasion, lymphovascular invasion, and T and N classifications for OS, and tumour invasion depth, lymphovascular invasion, and T classification for DFS, SUVmax and TLG remained associated with OS (P < 0.05) (Table 3). No 18F-FDG PET parameter was independently predictive of DFS (P > 0.1). Patients with TLG > 9.3 g had a 5.7-fold increased risk of overall mortality. Figure 2 shows Kaplan–Meier curves estimating OS for low and high values of SUVmax and TLG (log-rank test, P < 0.001).
Table 3.
Multivariate analyses of PET parameters associated with survival
| 18F–FDG PET/CT parameters | Overall survival | Disease-free survival | ||||
|---|---|---|---|---|---|---|
| HRa | 95% CI | Pa | HRa | 95% CI | Pa | |
| SUVmax > 5.0 | 3.44 | 1.01–11.71 | 0.048* | 1.53 | 0.65–3.61 | 0.336 |
| SUVpeak > 3.5 | 3.59 | 0.98–16.78 | 0.051 | 1.47 | 0.60–3.62 | 0.405 |
| SUVmean > 3.1 | 2.41 | 0.98–13.96 | 0.054 | 1.80 | 0.75–4.34 | 0.188 |
| MTV > 2.9 mL | 3.16 | 0.82–12.25 | 0.096 | 1.13 | 0.47–2.75 | 0.784 |
| TLG > 9.3 g | 5.74 | 1.20–27.44 | 0.029* | 1.54 | 1.62–3.79 | 0.352 |
CI confidence interval; 18F-FDG 18F-fluorodeoxyglucose; HR hazard ratio; MTV metabolic tumour volume; PET/CT positron emission tomography/computed tomography; SUV standardized uptake value; TLG total lesion glycolysis
aMultivariate Cox proportional hazard regression analyses were adjusted for invasion depth, lymphovascular invasion, and T and N classifications for overall survival, invasion depth, lymphovascular invasion, and T classification for disease-free survival, *P < 0.05 (see Supplementary Table S2)
Fig. 2.
Kaplan–Meier curves showing OS according to cutoff values of SUVmax (5.0, a) and TLG (9.3 g, b) calculated from pretreatment 18F-FDG PET/CT scans. Log-rank test, P < 0.001
Discussion
The present study showed the usability of quantitative 18F-FDG PET/CT measurements in predicting OCC cervical metastasis and survival. MTV and TLG were independent predictive factors for occult metastasis; MTV > 6.6 mL or TLG > 24.0 g was associated with a > 2.6-fold increased risk of occult metastasis. In addition, SUVmax > 5.0 and TLG > 9.3 g were independent predictive factors for OS, with TLG > 9.3 indicating a 5.7-fold increased risk of overall mortality. Thus, quantitative 18F-FDG PET/CT measurements might guide clinicians to establish proper surgical planning and prognostic prediction.
After adjustment for clinical and pathological factors, only MTV and TLG remained independent predictive factors for occult metastasis. Similarly, a previous study examining correlations between functional 18F-FDG PET parameters and occult metastasis in 43 cN0 OCC patients (Chung et al. 2009) revealed that only MTV (> 6.0 mL) was significantly associated with occult metastasis. SUVmax (> 6), lymphovascular invasion, and T classification did not correlate with occult metastasis, and TLG was not examined in the previous study. Different values of tumour SUVs, MTV, and TLG have been reported for predicting occult metastasis in other human solid tumours. SUVmax, SUVmean, MTV, and TLG were significantly associated with occult LN metastasis of cN0 oesophageal cancer, but only SUVmax was an independent predictive factor (Moon et al. 2014). SUVmax, MTV, and TLG were associated with occult metastasis of squamous cell lung cancer, and SUVmax and MTV remained significant predictive factors after adjustment for clinicopathological factors (Kim et al. 2014).
Several clinicopathological tumour characteristics are used to predict occult metastasis. Tumour thickness and invasion depth are reliable parameters for predicting occult metastasis in cN0 T1–T2 OCC, with cutoff values of 2–10 mm (Fukano et al. 1997; Pentenero et al. 2005; Hegde et al. 2017). However, the predictive abilities of tumour thickness and depth are not consistently supported; tumour lymphovascular invasion and poor differentiation might rather predict occult metastasis (Goerkem et al. 2010). Tumour invasion mode and satellite distance might also predict occult metastasis of OCC (Yang et al. 2011). In the present study, histological differentiation, not invasion depth, was significantly associated with occult metastasis. This discrepancy might result from different inclusion criteria. Clinicopathological factors might be used in combination with 18F-FDG parameters to increase the predictive power. Combination of clinical T classification and SUVmax increased the diagnostic value of each parameter predicting occult metastasis in cN0 oesophageal cancer (Moon et al. 2014). In the present study, combining parameters did not significantly increase the predictive power. Nonetheless, growing evidence indicates that metabolic 18F-FDG PET parameters might predict occult LN metastasis of cN0 tumours, including OCC (Chung et al. 2009; Kim et al. 2014; Moon et al. 2014).
We examined the prognostic values of SUV and volumetric measurements from pretreatment 18F-FDG PET/CT. SUV is a semi-quantitative measurement of the tissue glucose metabolic rate and a high value in a tumour may be associated with poor survival outcome. Increased 18F-FDG SUV or glucose transporter (Glut)-1 or Glut-3 overexpression is an independent predictor of poor survival in cN0 and cN + OCC patients (Kunkel et al. 2003; Tian et al. 2004; Hofele et al. 2009). MTV and TLG potentially are better predictors of treatment outcome and survival (Abgral et al. 2014; Pak et al. 2014). Pretreatment MTV and TLG have prognostic value in addition to clinical AJCC stage (Dibble et al. 2012). Tumour TLG was an independent factor of OS and DFS in 126 OCC patients (Abd El-Hafez et al. 2013). Preoperative MTV and TLG showed value in stratifying the likelihood of OS and DFS in 162 OCC patients (Ryu et al. 2014). The prognostic roles of SUVs, MTV, and TLG have been also examined in cN0 OCC. MTV was an independent predictor of survival in 57 cN0 OCC patients (Lee et al. 2012). In our study, SUVmax and TLG were predictive of OS, but not DFS, after adjustment for clinicopathological factors. Differences in prognostic values of PET parameters among studies might result from different inclusion criteria and cutoff values.
The present study included all tumour classes (T1–T4) and subsites in the oral cavity, which might also have affected the results. T3–T4 tumours are more likely to be removed by elective neck dissection. However, pN0 was found in half of the cN0 T3–T4 tumours in our study, indicating neck dissection might be unnecessary and rational application is recommended. We used a standardized surgical protocol, including neck dissection, which provided accurate pathological information of tumours and LNs. In addition, the considerable false-negative rates of the 18F-FDG PET/CT parameters might obscure their sole primary use for prediction of pathological neck positivity and determination of neck dissection procedure in clinical practice. The use of multiple PET/CT scanners might have affected the SUV analyses; however, SUVs from the different PET scanners were harmonized (Boellaard et al. 2010; Lasnon et al. 2013). Large-scale prospective trials are needed to validate our results and to confirm the clinical significance of quantitative 18F-FDG PET/CT parameters.
In conclusion, the present study suggests that tumour 18F-FDG PET/CT functional parameters, including SUVs, MTV, and TLG, might predict occult metastasis and survival in patients with cN0 OCC, with TLG being an independent predictor. Improved prediction may promote appropriate surgical planning and posttreatment risk stratification in cN0 OCC patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This study was supported by the National Research Foundation of Korea (NRF) grant, funded by the Ministry of Science and ICT (MSIT), the Government of Korea (No. 2019R1A2C2002259).
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research board and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent from all individual participants was obtained.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd El-Hafez YG, Moustafa HM, Khalil HF, Liao CT, Yen TC (2013) Total lesion glycolysis: a possible new prognostic parameter in oral cavity squamous cell carcinoma. Oral Oncol 49(3):261–268. 10.1016/j.oraloncology.2012.09.005 [DOI] [PubMed] [Google Scholar]
- Abgral R, Keromnes N, Robin P, Le Roux PY, Bourhis D, Palard X, Rousset J, Valette G, Marianowski R, Salaun PY (2014) Prognostic value of volumetric parameters measured by 18F-FDG PET/CT in patients with head and neck squamous cell carcinoma. Eur J Nucl Med Mol Imaging 41(4):659–667. 10.1007/s00259-013-2618-1 [DOI] [PubMed] [Google Scholar]
- Boellaard R, O'Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, Oyen WJ, Kotzerke J, Hoekstra OS, Pruim J, Marsden PK, Tatsch K, Hoekstra CJ, Visser EP, Arends B, Verzijlbergen FJ, Zijlstra JM, Comans EF, Lammertsma AA, Paans AM, Willemsen AT, Beyer T, Bockisch A, Schaefer-Prokop C, Delbeke D, Baum RP, Chiti A, Krause BJ (2010) FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging 37(1):181–200. 10.1007/s00259-009-1297-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong A, Ha JM, Han YH, Kong E, Choi Y, Hong KH, Park JH, Kim SH, Park JM (2017) Preoperative lymph node staging by FDG PET/CT with contrast enhancement for thyroid cancer: a multicenter study and comparison with neck CT. Clin Exp Otorhinolaryngol 10(1):121–128. 10.21053/ceo.2015.01424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Jeong HS, Son YI, So YK, Park GY, Choi JY, Hyun SH, Kim HJ, Ko YH, Baek CH (2009) Metabolic tumor volumes by [18F]-fluorodeoxyglucose PET/CT correlate with occult metastasis in oral squamous cell carcinoma of the tongue. Ann Surg Oncol 16(11):3111–3117. 10.1245/s10434-009-0621-3 [DOI] [PubMed] [Google Scholar]
- D'Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, Agarwal JP, Pantvaidya G, Chaukar D, Deshmukh A, Kane S, Arya S, Ghosh-Laskar S, Chaturvedi P, Pai P, Nair S, Nair D, Badwe R (2015) Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med 373(6):521–529. 10.1056/NEJMoa1506007 [DOI] [PubMed] [Google Scholar]
- Denaro N, Merlano MC, Russi EG (2016) Follow-up in head and neck cancer: do more does it mean do better? A systematic review and our proposal based on our experience. Clin Exp Otorhinolaryngol 9(4):287–297. 10.21053/ceo.2015.00976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibble EH, Alvarez AC, Truong MT, Mercier G, Cook EF, Subramaniam RM (2012) 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med 53(5):709–715. 10.2967/jnumed.111.099531 [DOI] [PubMed] [Google Scholar]
- Dik EA, Willems SM, Ipenburg NA, Rosenberg AJ, Van Cann EM, van Es RJ (2016) Watchful waiting of the neck in early stage oral cancer is unfavourable for patients with occult nodal disease. Int J Oral Maxillofac Surg 45(8):945–950. 10.1016/j.ijom.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (2010) Oral cavity cancer in AJCC cancer staging manual. Springer_Verlag, New York, pp 29–40 [Google Scholar]
- Evangelista L, Cervino AR, Chondrogiannis S, Marzola MC, Maffione AM, Colletti PM, Muzzio PC, Rubello D (2014) Comparison between anatomical cross-sectional imaging and 18F-FDG PET/CT in the staging, restaging, treatment response, and long-term surveillance of squamous cell head and neck cancer: a systematic literature overview. Nucl Med Commun 35(2):123–134. 10.1097/mnm.0000000000000022 [DOI] [PubMed] [Google Scholar]
- Farmer RW, McCall L, Civantos FJ, Myers JN, Yarbrough WG, Murphy B, O'Leary M, Zitsch R, Siegel BA (2015) Lymphatic drainage patterns in oral squamous cell carcinoma: findings of the ACOSOG Z0360 (Alliance) study. Otolaryngol Head Neck Surg 152(4):673–677. 10.1177/0194599815572585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukano H, Matsuura H, Hasegawa Y, Nakamura S (1997) Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck 19(3):205–210 [DOI] [PubMed] [Google Scholar]
- Goerkem M, Braun J, Stoeckli SJ (2010) Evaluation of clinical and histomorphological parameters as potential predictors of occult metastases in sentinel lymph nodes of early squamous cell carcinoma of the oral cavity. Ann Surg Oncol 17(2):527–535. 10.1245/s10434-009-0755-3 [DOI] [PubMed] [Google Scholar]
- Hegde P, Roy S, Shetty T, Prasad BR, Shetty U (2017) Tumor infiltration depth as a prognostic parameter for nodal metastasis in oral squamous cell carcinoma. Int J Appl Basic Med Res 7(4):252–257. 10.4103/ijabmr.IJABMR_66_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AS, Kim S, Tighiouart M, Gudino C, Mita A, Scher KS, Laury A, Prasad R, Shiao SL, Van Eyk JE, Zumsteg ZS (2017) Metastatic lymph node burden and survival in oral cavity cancer. J Clin Oncol 35(31):3601–3609. 10.1200/jco.2016.71.1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofele C, Freier K, Thiele OC, Haberkorn U, Buchmann I (2009) High 2-[18F]fluoro-2-deoxy-d-glucose (18FDG) uptake measured by positron emission tomography is associated with reduced overall survival in patients with oral squamous cell carcinoma. Oral Oncol 45(11):963–967. 10.1016/j.oraloncology.2009.06.008 [DOI] [PubMed] [Google Scholar]
- Joo YH, Koo BS (2019) Evolving strategy for surgical management of oral cancer: present and future. Clin Exp Otorhinolaryngol 12(2):101–102. 10.21053/ceo.2019.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo YH, Cho JK, Koo BS, Kwon M, Kwon SK, Kwon SY, Kim MS, Kim JK, Kim H, Nam I, Roh JL, Park YM, Park IS, Park JJ, Shin SC, Ahn SH, Won S, Ryu CH, Yoon TM, Lee G, Lee DY, Lee MC, Lee JK, Lee JC, Lim JY, Chang JW, Jang JY, Chung MK, Jung YS, Cho JG, Choi YS, Choi JS, Lee GH, Chung PS (2019) Guidelines for the surgical management of oral cancer: Korean Society of Thyroid-Head and Neck Surgery. Clin Exp Otorhinolaryngol 12(2):107–144. 10.21053/ceo.2018.01816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Song BI, Hong CM, Jeong SY, Lee SW, Lee J, Ahn BC (2014) Metabolic parameters using (1)(8)F-FDG PET/CT correlate with occult lymph node metastasis in squamous cell lung carcinoma. Eur J Nucl Med Mol Imaging 41(11):2051–2057. 10.1007/s00259-014-2831-6 [DOI] [PubMed] [Google Scholar]
- Kunkel M, Reichert TE, Benz P, Lehr HA, Jeong JH, Wieand S, Bartenstein P, Wagner W, Whiteside TL (2003) Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer 97(4):1015–1024. 10.1002/cncr.11159 [DOI] [PubMed] [Google Scholar]
- Lasnon C, Desmonts C, Quak E, Gervais R, Do P, Dubos-Arvis C, Aide N (2013) Harmonizing SUVs in multicentre trials when using different generation PET systems: prospective validation in non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging 40(7):985–996. 10.1007/s00259-013-2391-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Choi JY, Lee HJ, Baek CH, Son YI, Hyun SH, Moon SH, Kim BT (2012) Prognostic value of volume-based (18)F-fluorodeoxyglucose PET/CT parameters in patients with clinically node-negative oral tongue squamous cell carcinoma. Korean J Radiol 13(6):752–759. 10.3348/kjr.2012.13.6.752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CT, Chang JT, Wang HM, Ng SH, Hsueh C, Lee LY, Lin CH, Chen IH, Huang SF, Cheng AJ, See LC, Yen TC (2009) Pretreatment primary tumor SUVmax measured by FDG-PET and pathologic tumor depth predict for poor outcomes in patients with oral cavity squamous cell carcinoma and pathologically positive lymph nodes. Int J Radiat Oncol Biol Phys 73(3):764–771. 10.1016/j.ijrobp.2008.05.004 [DOI] [PubMed] [Google Scholar]
- Manca G, Vanzi E, Rubello D, Giammarile F, Grassetto G, Wong KK, Perkins AC, Colletti PM, Volterrani D (2016) (18)F-FDG PET/CT quantification in head and neck squamous cell cancer: principles, technical issues and clinical applications. Eur J Nucl Med Mol Imaging 43(7):1360–1375. 10.1007/s00259-015-3294-0 [DOI] [PubMed] [Google Scholar]
- Moon SH, Kim HS, Hyun SH, Choi YS, Zo JI, Shim YM, Lee KH, Kim BT, Choi JY (2014) Prediction of occult lymph node metastasis by metabolic parameters in patients with clinically N0 esophageal squamous cell carcinoma. J Nucl Med 55(5):743–748. 10.2967/jnumed.113.130716 [DOI] [PubMed] [Google Scholar]
- Mucke T, Mitchell DA, Wagenpfeil S, Ritschl LM, Wolff KD, Kanatas A (2014) Incidence and outcome for patients with occult lymph node involvement in T1 and T2 oral squamous cell carcinoma: a prospective study. BMC Cancer 14:346. 10.1186/1471-2407-14-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (NCCN) (2018) NCCN Clinical Practice Guidelines in Oncology: head and neck cancers. NCCN, Fort Washington [Google Scholar]
- Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, Kim EE, Lee DS (2014) Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med 55(6):884–890. 10.2967/jnumed.113.133801 [DOI] [PubMed] [Google Scholar]
- Pentenero M, Gandolfo S, Carrozzo M (2005) Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: a review of the literature. Head Neck 27(12):1080–1091. 10.1002/hed.20275 [DOI] [PubMed] [Google Scholar]
- Ryu IS, Kim JS, Roh JL, Cho KJ, Choi SH, Nam SY, Kim SY (2014) Prognostic significance of preoperative metabolic tumour volume and total lesion glycolysis measured by (18)F-FDG PET/CT in squamous cell carcinoma of the oral cavity. Eur J Nucl Med Mol Imaging 41(3):452–461. 10.1007/s00259-013-2571-z [DOI] [PubMed] [Google Scholar]
- Schilling C, Stoeckli SJ, Haerle SK, Broglie MA, Huber GF, Sorensen JA, Bakholdt V, Krogdahl A, von Buchwald C, Bilde A, Sebbesen LR, Odell E, Gurney B, O'Doherty M, de Bree R, Bloemena E, Flach GB, Villarreal PM, Fresno Forcelledo MF, Junquera Gutierrez LM, Amezaga JA, Barbier L, Santamaria-Zuazua J, Moreira A, Jacome M, Vigili MG, Rahimi S, Tartaglione G, Lawson G, Nollevaux MC, Grandi C, Donner D, Bragantini E, Dequanter D, Lothaire P, Poli T, Silini EM, Sesenna E, Dolivet G, Mastronicola R, Leroux A, Sassoon I, Sloan P, McGurk M (2015) Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur J Cancer 51(18):2777–2784. 10.1016/j.ejca.2015.08.023 [DOI] [PubMed] [Google Scholar]
- Schilling C, Shaw R, Schache A, McMahon J, Chegini S, Kerawala C, McGurk M (2017) Sentinel lymph node biopsy for oral squamous cell carcinoma. Where are we now? Br J Oral Maxillofac Surg 55(8):757–762. 10.1016/j.bjoms.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Shah JP, Candela FC, Poddar AK (1990) The patterns of cervical lymph node metastases from squamous carcinoma of the oral cavity. Cancer 66(1):109–113 [DOI] [PubMed] [Google Scholar]
- Tian M, Zhang H, Nakasone Y, Mogi K, Endo K (2004) Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study. Eur J Nucl Med Mol Imaging 31(1):5–12. 10.1007/s00259-003-1316-9 [DOI] [PubMed] [Google Scholar]
- Wang L, Bai J, Duan P (2019) Prognostic value of 18F-FDG PET/CT functional parameters in patients with head and neck cancer: a meta-analysis. Nucl Med Commun 40(4):361–369. 10.1097/mnm.0000000000000974 [DOI] [PubMed] [Google Scholar]
- Yang TL, Lou PJ, Chang YL, Wu CT, Wang CP, Ko JY (2011) Tumor satellite in predicting occult nodal metastasis of tongue cancer. Otolaryngol Head Neck Surg 145(4):599–605. 10.1177/0194599811411635 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.