Abstract
Purpose
Bone metastasis is the result of complex crosstalk between tumor cells and bone marrow cells. Bone marrow adipocytes (BMAs) are the most abundant cell type in adult bone marrow. Therefore, we explore the effects of BMAs on bone metastasis in lung cancer.
Methods
RNA-seq was used to compare the mRNA expression level of bone metastatic SBC5 cells and non-bone metastatic SBC3 cells. Rosiglitazone-induced marrow adiposity and intra-femoral injection of SBC5 cells were used to demonstrate the relationship between BMAs and SBC5 cells in vivo. Co-culture system, gene co-expression, gene ontology (GO) enrichment analysis and protein–protein interaction (PPI) network were used to explore the potential mechanism.
Results
BMAs specially enhance the invasion of bone metastatic SBC5 instead of non-bone metastatic SBC3 in vitro. SBC5 instead of SBC3 promoted osteoblast and osteoclast differentiation as well as de-differentiation of mature BMAs. Rosiglitazone-induced marrow adiposity significantly enhanced osteolytic lesion induced by SBC5 in vivo. RNA-seq revealed that compared with SBC3, S100A9 and S100A8 genes were the most prominent genes up-regulated in SBC5 cells. High expression of S100A8/9 in SBC5 could be responsible for the crosstalk between lung cancer cells and BMAs. More importantly, interleukin 6 receptor (IL6R), which is adjacent to S100A8/A9 in 1q21.3, was significantly up-regulated by BMAs in vitro. S100A8/A9 (1 μg/ml) could obviously enhance the osteoblastic differentiation and inhibit adipogenic differentiation, whereas TLR4 inhibitor TAK242 (10 μmol/l) significantly attenuated this effect.
Conclusions
Our study suggested that bone marrow adipocyte may communicate with lung cancer cells via 1q21.3 (S100A8/A9-IL6R)-TLR4 pathway to promote osteolytic bone destruction. 1q21.3 (S100A8/A9-IL6R) is a potential target for the treatment of lung cancer bone metastasis.
Electronic supplementary material
The online version of this article (10.1007/s00432-020-03277-9) contains supplementary material, which is available to authorized users.
Keywords: Bone metastasis, 1q21.3, Bone marrow adipocyte, IL6, Lung cancer
Background
Bone metastasis frequently occurred in patients with prostate cancer (65–90%), breast cancer (65–75%) and lung cancer (17–64%) (D'Oronzo et al. 2019). Most published studies involving bone metastasis have focused on breast cancer and prostate cancer, likely as a result of their higher incident of bone metastasis. However, lung cancer patients with bone metastasis indicate poorer prognosis (Brunetti et al. 2020). The survival time of breast cancer and prostate cancer with bone metastasis is roughly 24–36 months (Niikura et al. 2011) and 24 months (Gandaglia et al. 2015), respectively. In contrast, the median survival is about 5 months for lung cancer patients with bone metastasis (Riihimaki et al. 2014). The pathogenesis of lung cancer progression is different from breast cancer and prostate cancer. Thus, the mechanism of bone metastasis in lung cancer may be quite different from breast cancer and prostate cancer. Therefore, it is important to explore the mechanism of bone metastasis of lung cancer to improve the prognosis of lung cancer patients.
Clinical diagnosis and classification of bone metastasis are traditionally based on the radiographic appearance, which can be characterized by osteolytic, osteoblastic or mixed lesion. However, in some tumors, despite massive tumor cells infiltrate in the intertrabecular space, trabecular bone could stay intact. This condition is defined as intertrabecular metastasis, which is rarely diagnosed in clinical practice, owing to the insensitivity of radiographs and bone scans (Yamaguchi 2001). More importantly, the highest frequency of intertrabecular metastasis occurred in small cell lung cancer (SCLC) patients (Yamaguchi et al. 1996). We speculated that different crosstalk between lung cancer cells and bone marrow cells could determine the type of bone destruction.
The “seed and soil” hypothesis suggests that bone marrow provides a suitable “soil” for the “seed” (tumor cells) (Fidler et al. 2008). Among the diverse cells in the bone marrow niche, BMAs constituted about 70% of bone marrow volume in adult (Shafat et al. 2017) and are the most abundant cells in adult bone marrow (Gimble et al. 1996). However, the functions of BMAs have been neglected and underestimated for a long time. Most previous studies did not consider the potential roles of BMAs in bone metastasis. Hematological tumor cells and BMAs co-existed within the same bone marrow microenvironment. Thus, some earlier studies tried to investigate the relationship between the hematological tumor cells and BMAs. Most studies suggested that BMAs released free fatty acid (FFA) to support the survival of hematological tumor cells, such as the acute myeloid leukemia (Shafat et al. 2017; Tabe et al. 2017). Besides, adipocytes including, but not restricted to BMAs, were reported to promote tumor progression in acute lymphoblastic leukemia (Behan et al. 2009; Ehsanipour et al. 2013; Sheng et al. 2016) and multiple myeloma (Bullwinkle et al. 2016; Trotter et al. 2016). Until now, only one study has explored the roles of BMAs in solid tumor bone metastasis. Herroon and colleague demonstrated that BMAs enhanced tumor cells growth depended on the up-regulation of fatty acid-binding protein 4 (FABP4) in prostate cancer cells (Herroon et al. 2013). FABP4 has been traditionally regarded as FFA transporter in the cytoplasm. Similarly, this study also revealed about BMA's role as an energy provider. The endocrine and paracrine functions of BMAs in bone metastasis have not been reported in previous studies. What is more, there are no studies exploring the relationship between BMAs and lung cancer cells.
In summary, current knowledge about the roles of BMAs in bone metastasis is limited. In our study, we used two well established human SCLC cell lines. One is non-bone metastatic cell SBC3, another is bone metastatic cell SBC5. Our article demonstrated the reciprocal relationship between SBC5 (instead of SBC3) and BMAs in vitro and in vivo.
Methods
Cell lines and cell culture
The human SCLC cell lines SBC-5 and SBC-3 were established as previously reported (Miki et al. 2000). SBC3 and SBC5 cells were obtained from Okayama University. OP9 cells were obtained from American Type Culture Collection (ATCC). SBC3 and SBC5 were cultured in RPMI-1640 (Hyclone) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM l-glutamine (Gibco). OP9 cells were grown in ɑ-MEM medium containing 20% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin and 2 mM l-glutamine. All cell lines were maintained at 37 °C, 5% CO2, 95% humidity.
Animal studies
B-NSG female mice (8–11 weeks old) were purchased from Biocytogen (Beijing, China). BABL/c female mice (4–6 weeks old) were purchased from Chengdu Dashuo Experimental Animal Center (Chengdu, Sichuan, China). The mice were housed in specific pathogen-free conditions (22 °C, 12/12 h light/dark cycle) and given free access to water and diet. Rosiglitazone maleate (Dalian Meilun Biotech, China) was used to induce marrow adipogenesis in vivo as previously described (Lazarenko et al. 2007). Briefly, rosiglitazone maleate was supplemented to the standard diet (0.14 mg per 1 g of diet) for 7 weeks. The control group was fed a standard diet. All mice were given free access to water and diet. Food intake and body weight of individual mice were monitored. After 7 weeks, rosiglitazone maleate was withdrawn from the diet. SBC5 cells were injected into the medullary cavity of the distal femur of NSG mice. In particular, 2 × 106 of cells were resuspended in cold PBS and injected in volume of 10 μl, using a microsyringes. After 4 weeks, bone destruction was visulized by microCT.
In vitro adipogenesis
The induction of adipogenesis of OP9 began after cell confluence for 2 days. OP9 differentiation protocol was described as previous studies (Wolins et al. 2006). Briefly, OP9 was treated with ɑ-MEM supplemented with 15% Knockout TM SR (Invitrogen; catalog no. 10828-028), 100 U/ml penicillin, and 100 μg/ml streptomycin. Adipogenesis was stopped when lipid droplet was abundant in the cytoplasm of OP9.
In vivo adipogenesis
Female B-NSG mice (12 weeks old) and female C57BL/6J mice were used in this experiment. Rosiglitazone maleate (Dalian Meilun Biotech, China) was used to induce marrow adipogenesis in vivo as previously described (Lazarenko et al. 2007). Briefly, rosiglitazone maleate was supplemented to the control diet (0.14 mg per 1 g of diet) for 7 weeks. All mice were given free access to water and diet. Food intake and body weight of individual mice were monitored.
In vitro osteoclast differentiation
Bone marrow cells were collected from tibias and femurs of female C57BL/6J mice. To induce osteoclast differentiation, bone marrow cells (1 × 106 cells/24-well plate) were cultured in osteoclast differentiation medium with M-CSF (25 ng/ml) and RANKL (33 ng/ml) (R&D Systems) for 7 days. To elucidate the roles of SBC-5 in osteoclast differentiation, 60% SBC5 condition medium supplemented with these reagents was added after 1 days, 60% SBC3 condition medium was used as control group. At day 8, TRAP staining kit (Sigma Aldrich) was performed to determine in vitro osteoclastogenesis. To explore the indirect effect of SBC5 cells on osteoclastogenesis, indirect co-culture of SBC5 cells and osteocyte-like cell MLO-Y4 was performed. MLO-Y4 osteocytes (1 × 106 cells/24-well plate) were cultured with 80% SBC5 condition medium for 7 days, 80% SBC3 condition medium were used as control group. All these condition media were added at the beginning of the culture. The mRNA expression of RANKL of MLO-Y4 cells in different groups was compared after 7 days.
RNA-seq data processing and analysis
SBC3 and SBC5 cells were sent for transcriptome sequencing, each with three replicates. Total RNA was isolated with Trizol (Life Technologies). RNA-seq was performed on illumina X-10. Sequencing data were aligned to the Homo sapiens genome (GRCh38.p10). HISAT2 was used to count the number of reads mapped to each gene (default options). All libraries showed good quality, with sizes ranging between 5.1 and 5.4 × 107 read counts. The data were analyzed on the free online platform of Majorbio I-Sanger Cloud Platform (https://www.i-sanger.com).
Oil red O staining and ALP staining
The lipid droplets were stained by oil red O, according to the previous protocol (Kraus et al. 2016), with some modifications. Briefly, the cells were fixed with paraformaldehyde, then washed twice in distilled water and stained with oil red O (0.21%) for 15 min. A total of 350 μl of absolute ethanol each well was added to 24-well plate. Eluate was transferred to a new 96-well plate (100 μl/well). Absorption was measured in triplicate at 510 nm on a Multiskan® Spectrum spectrophotometer (Thermo scientific). ALP staining was performed using ALP staining kit (Sigma) according to the manufacturer's instructions.
Direct contact co-culture system
This co-culture system mimics the process that SBC5 colonizes in the BMAs abundant microenvironment. OP9 cells were induced to mature BMAs in 6-well plates, subsequently, cell suspension containing 8000 SBC5 cells were added to each well, the same amount of SBC3 cells were used as control. Dynamic morphological changes of SBC3/SBC5 and OP9 adipocytes were observed with microscope (Olympus, IX71) every 2–3 days. The direct contact co-culture of SBC3/SBC5 and OP9 adipocytes was lasted for 2 weeks. The mRNA level of OP9 adipocytes and SBC5 cells were detected using species-specific primers.
Transwell migration and invasion assay
The 24-well transwell plates with 8.0 μm pore size were used to detect tumor cells migration (matrigel-uncoated inserts) and invasion (matrigel-coated inserts). The cell suspension containing 50,000 tumor cells (SBC3/SBC5) in RPMI-1640 medium containing 1% FBS was placed in the upper chamber of the inserts. Condition medium from OP9 and OP9 adipocytes were added to the lower chamber. RPMI-1640 medium with 10% FBS was used as control group. The transwell plates were incubated for 13 h in migration assay and 36 h in invasion assay. Assays were performed in three replicates and repeated two times. The numbers of migrated and invaded cells were counted in at least five different fields for each insert under a microscope (100 × magnification).
Bone marrow fat quantification by osmium staining and microCT
Osmium tetroxide was used to qualify bone marrow fat in mouse according to the protocol from Scheller et al. (2014), with some modifications. Tibia and femur were fixed for 48 h in 4% paraformaldehyde and decalcified in 20% EDTA, pH 7.4, for 14 days. 2% osmium tetroxide solution 750 μl and 5% Potassium dichromate 750 μl were added to 2-ml microtube, each with two bones inside. To keep the bone intact, we did not cut off the bone but prolong staining time to 60 h at room temperature. Stained bones were then moved to a 50 ml centrifuge tube and repeatedly washed with distilled water for 2 h. The bones were then put in a new 2.0-ml microtube for microCT scanning. The whole intact bone was scanned using a microCT system (Quantum GX, PerkinElmer, USA). Scan settings were as follows: voltage (kV): 90, FOV (mm): acquisition: 36, recon: 25, voxel size: 50 μm, scan mode: high resolution, 14 min, display setting: off. The analysis of the content of BMAs was to assess the osmium densities (gray or white color in bone marrow cavity).
Gene expression analysis
Total RNA from tissues and cells was prepared with Trizol (Life Technologies). RNA was reverse transcribed with PrimeScript™ RT reagent Kit with gDNA Eraser (Takara), and transcripts were quantified by real-time PCR using SYBR Premix Ex Taq II (Takara). Primer sequences were showed below (Tables 1, 2).
Table 1.
Primer sequence (human)
| Target gene | Primer sequence |
|---|---|
| ΒACTIN |
F-CCACGAAACTACCTTCAACTCC R-GTGATCTCCTTCTGCATCCTGT |
| ECAD |
F-GACCATTCAGTACAACGACCCAACC R-ACCGCTTCCTTCATAGTCAAACACG |
| CXCR4 |
F-TTCTACCCCAATGACTTGTG R-ATGTAGTAAGGCAGCCAACA |
| MMP9 |
F- GCACGACGTCTTCCAGTACC R- TCAACTCACTCCGGGAACTC |
| PTHLH |
F- GCTCGGTGGAGGGTCTCA R- TCATGGAGGAGCTGATGTTCAG |
| IL6 |
F- AGGGCTCTTCGGCAAATGTA R- GAAGGAATGCCCATTAACAACAA |
| S100A8 |
F-GACCTGAAGAAATTGCTAGAG R-TGCCACGCCCATCTTTATCAC |
| S100A9 |
F-CACCCAGACACCCTGAAC R-GCCCTCGTCACCCTCGTG |
| LEPR |
F-GCGTTAAAGCTCTCGTGGCA R-CTTTGAGAGTCCAGCAGGCA |
| ADIPOR1 |
F-AATTCCTGAGCGCTTCTTTCCT R-CATAGAAGTGGACAAAGGCTGC |
| ADIPOR2 |
F-TGCAGCCATTATAGTCTCCCAG R-GAATGATTCCACTCAGGCCTAG |
| PTH1R |
F-AGCAGTACCGCTGCTCAAAT R-GGTGTATGGTGTGGCCATGA |
Table 2.
Primer sequence (murine)
| Target gene | Primer sequence |
|---|---|
| Bactin |
F-AGATTACTGCTCTGGCTCCTAGC R-ACTCATCGTACTCCTGCTTGCT |
| Pthlh |
F-TTCGGTGGAGGGGCTTGGCC R-CGGCGGCGCAAGTCTTGGAT |
| S100a8 |
F-GACAATGCAATTAACTTCGAGGAG R-TGTGGCTGTCTTTGTGAGATGC |
| S100a9 |
F-CACAGTTGGCAACCTTTATG R-CAGCTGATTGTCCTGGTTTG |
| Fabp4 |
F-GTGATGCCTTTGTGGGAACCT R-CATGCCTGCCACTTTCCTTG |
| Pparg |
F-CACTCGCATTCCTTTGACATC R-CGCACTTTGGTATTCTTGGAG |
| Pth1r |
F-TCTGCAATGGTGAGGTGCAG R-GCTACTCCCACTTCGTGCTT |
| Ager |
F-ACTGAAGCTTGGAAGGTCCTC R-CGAGTGTTTCTTTGCCATCGG |
| Lep |
F-GGAAAATGTGCTGGAGACCC R-TACCGACTGCGTGTGTGAAAT |
| Adipoq |
F-TGACGACACCAAAAGGGCTC R-GTGGTAAGAGAAGTAGTAGAGT |
| Il6 |
F-TCCAGTTGCCTTCTTGGGAC R-GCCATTGCACAACTCTTTTCTCA |
| Tnfa |
F-CCCACGTCGTAGCAAACCA R-ACAAGGTACAACCCATCGGC |
| Cxcl12 |
F-TCAGATTGTTGCACGGCTGA R-GTTACAAAGCGCCAGAGCAG |
| Tlr4 |
F-CGCTGCCACCAGTTACAGAT R-AGGAACTACCTCTATGCAGGG |
Statistical analyses
All data are presented as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism software (version 6.04) unless otherwise specified. RNA-seq data were analyzed on the free online platform of Majorbio I-Sanger Cloud Platform (https://www.i-sanger.com). Animal number and the replicate number of experiments were indicated in the figure legends. Statistical significance was set as p < 0.05. Two group comparisons were performed using unpaired, two-tailed Student’s t test, and an ANOVA test was used for multiple group comparisons.
Results
OP9 adipocytes promote the invasion of SBC5 cells instead of SBC3 cells
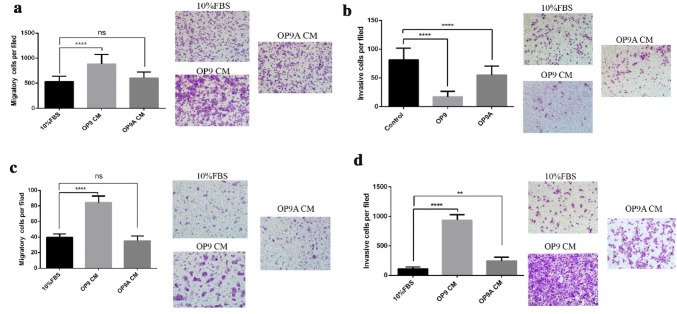
We investigated the mutual influences between SBC5 cells and OP9 adipocytes (OP9 A) according to the “seed” and “soil” hypothesis. SBC3 cells, which were also established from human SCLC but without bone metastatic potential, were used as the control. First, we explored the effect of OP9/OP9 adipocytes on the migration and invasion of SBC3 and SBC5. 10% FBS culture medium was used as the control. OP9 instead of OP9 adipocytes significantly enhanced the migration of SBC3 (Fig. 1a). A similar result was obtained from SBC5 cells (Fig. 1c). In contrast, OP9 and OP9 adipocytes significantly inhibited the invasion of SBC3 cells (Fig. 1b) but enhanced the invasion of SBC5 cells (Fig. 1d).
Fig. 1.
OP9 adipocytes promote the invasion of SBC5 instead of SBC3 in vitro.a The effect of OP9 or OP9 adipocyte condition medium on the migration of SBC3, each with three replicates. b The effect of OP9 or OP9 adipocytes condition medium on the invasion of SBC3, each with three replicates. c The effect of OP9 or OP9 adipocytes condition medium on the migration of SBC5, each with three replicates. d The effect of OP9 or OP9 adipocytes condition medium on the invasion of SBC5, each with three replicates. The above data are represented as mean ± SD, *p < 0.05, **p < 0.01, ****p < 0.0001, ns indicates p ≥ 0.05
SBC5 cells promote the osteoblast, osteoclast differentiation and de-differentiation of mature OP9A
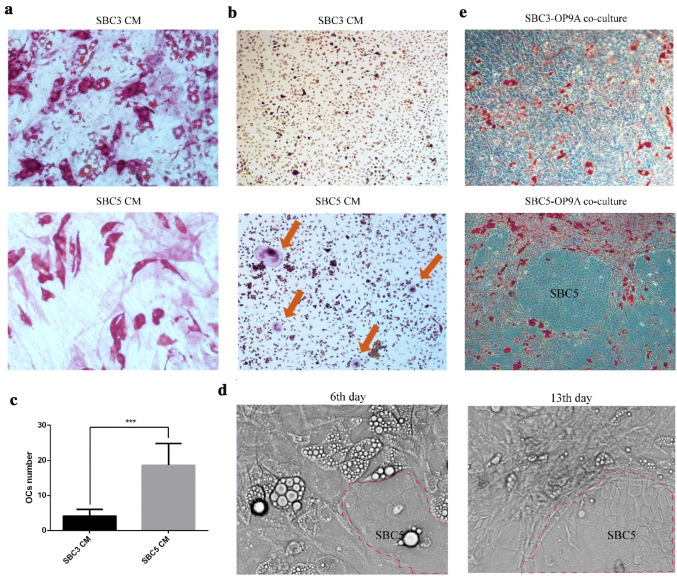
Next, we performed co-culture experiments to explore the effect of SBC5 on osteoblast and osteoclast differentiation and de-differentiation of mature bone marrow adipocytes. We found that SBC5 condition medium (CM) instead of SBC3 CM promoted the osteoblastic differentiation even in the absent of osteoblastic differentiation medium and inhibited the adipogenic differentiation of OP9 (Fig. 3a, Supplementary Fig. 1a, b). Osteoclast is indispensable in the development of bone lesion. Thus, we also investigated the effect of OP9A and SBC3/5 on osteoclast differentiation. SBC5 CM supplemented with M-CSF significantly promoted the formation of TRAP staining-positive mononuclear cells compared with SBC3 CM (Supplementary Fig. 3c). More importantly, SBC5 instead of SBC3 promoted the osteoclast differentiation in the co-culture system (Fig. 3b, c). Similarly, SBC5 instead of SBC3 significantly enhanced the RANKL mRNA expression in MLO-Y4 osteocytes (Supplementary Fig. 3d). We also designed a direct contact co-culture system to mimic the niche microenvironment in which BMAs were abundant. Interestingly, SBC5 promoted the de-differentiation of mature OP9 adipocytes: from the lipid abundant adipocytes into fibroblast-like cells. SBC5 cells initially affected surrounding adipocytes, then spreading to other proximal adipocytes. Eventually, SBC5 cells were closely surrounded by fibroblast-like cells (Fig. 3d). In contrast, SBC3 gradually occupied the original OP9 adipocytes space without obviously affecting their morphology (Fig. 3e).
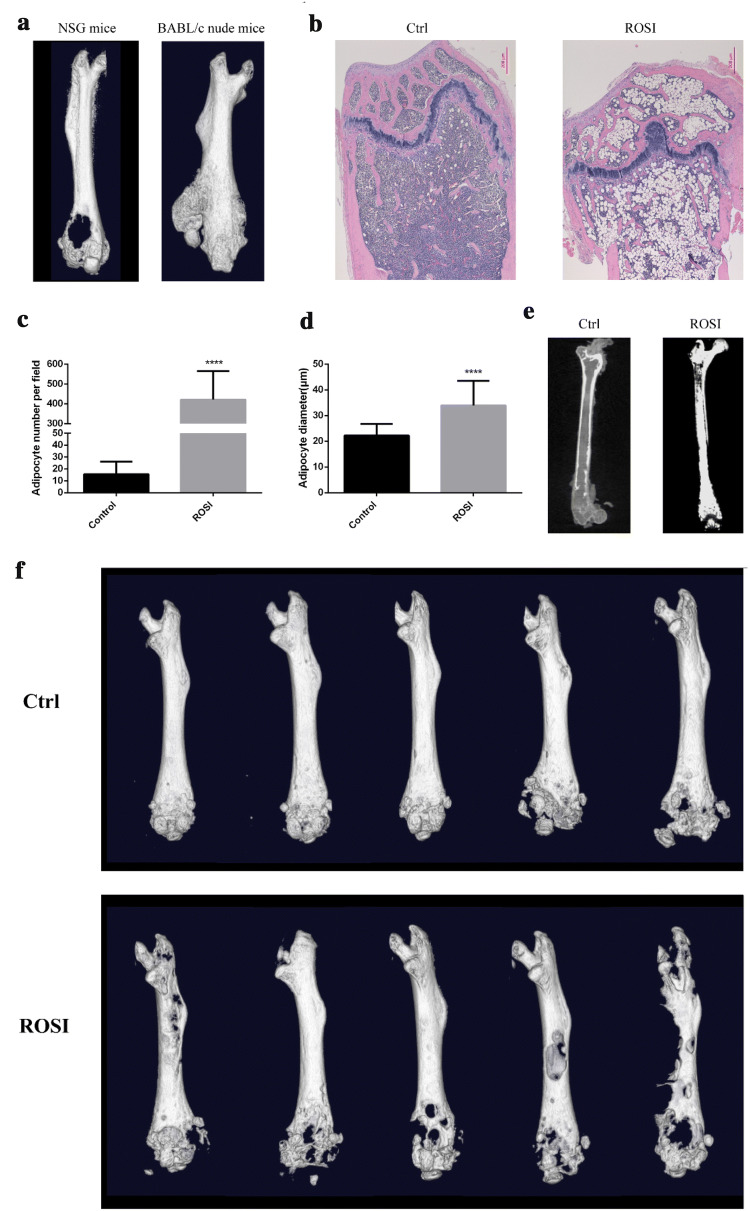
Fig. 3.
Rosiglitazone-induced marrow adipogenesis and enhanced osteolytic bone destruction. a SBC5 cells were injected into the medullary cavity of the distal femur of NSG mice and BABL/c nude mice, bone destruction was visulized using microCT. b C57BL/6J female mice were fed with diet supplemented with rosiglitazone maleate for 7 weeks. Femur was separated for HE staining. scale bars = 200 µm. Adipocyte number (c) and diameter (d) were measured using Image J. Data are represented as mean ± SD. ****p < 0.0001. e B-NSG mice were fed with diet supplemented with rosiglitazone maleate for 7 weeks. Osmium tetroxide staining was performed in the femur and microCT scanning was used to visualize and quantify BMAs. f after B-NSG mice were feed with rosiglitazone for 8 weeks, SBC5 cells were implanted by intra-femoral injection. Bone destruction was observed by microCT after 1 month
Rosiglitazone-induced marrow adiposity enhanced osteolytic bone destruction in vivo
The above data from in vitro experiments indicated that mutual promotion may occur between SBC5 and bone marrow adipocytes. We used intra-femoral inoculation of SBC5 to investigate the relationship between SBC5 and BMAs in vivo. Expectedly, intra-femoral injection of SBC5 reproducibly induced osteolytic lesion in the ends of the femur (Fig. 3a). Unexpectedly, in another group, one of BABL/c mice developed osteoblastic bone lesions in the femur shaft (Fig. 3a). Thus, we found that SBC5 induced osteolytic and osteoblastic lesion in different bone region. The occurrence of this phenomenon may somehow as a result of accidental implantation of SBC5 in the femur shaft. Next, to investigate the effect of bone marrow adipose tissue on bone destruction, we established an in vivo model for bone marrow adipogenesis. We used rosiglitazone to increase the bone marrow adipocytes. Rosiglitazone diet did not affect the food intake and the weight of abdominal adipose tissue (viscous adipose tissue) but increase the interscapular adipose tissue (brown tissue) (Supplementary Fig. 2a–c). Quantitation of the content of bone marrow adipocytes was accomplished by osmium tetroxide staining and HE staining. We found that rosiglitazone greatly increased the number and the diameter of bone marrow adipocytes (Fig. 2b–d, Supplementary Fig. 2d). Similar results were obtained from BABL/c nude mice (Supplementary Fig. 3e) and Biocytogen-NOD/SCID/IL2-receptor gamma-chain knockout (B-NSG) mice (Fig. 3e). SBC5 cells were implanted into B-NSG mice by intra-femoral injection. Micro-CT scanning showed that the rosiglitazone-induced marrow adiposity obviously enhanced the osteolytic destruction (Fig. 3f).
Fig. 2.
SBC5 cells promote the osteoblastic and osteoclastic differentiation and de-differentiation of mature BMAs. a 90%SBC3 or 90%SBC5 condition medium was added to the OP9 for 1 week. ALP and Oil red O double staining were used to investigate osteoblast activity and lipid accumulation, separately (200 ×). b 60%SBC3 or SBC5 condition medium was used to induce osteoclast differentiation. TRAP staining was used to qualify osteoclast (100 ×), arrow indicated multinucleated osteoclast. c the number of osteoclasts was counted in SBC3 and SBC5 group. Data are represented as mean ± SD. ***p < 0.001. d After OP9 was induced to mature adipocytes, SBC3 or SBC5 (1 × 104) was added for direct co-culture with mature OP9 adipocytes in six-well plate. The same field of view was imaged every 2–3 days to observe the dynamic morphological change of OP9 adipocytes. The representative images at the 6th, 13th days were showed. Red dotted line indicated the boundary of SBC5 cell colonies (200 ×). e SBC3 or SBC5 cells co-cultured with mature OP9 adipocytes for 2 weeks, and oil red O staining was performed (100 ×), each 3 replicates
Gene cluster S100A7/A8/A9 are the most highly expressed genes in bone metastatic cell line SBC5
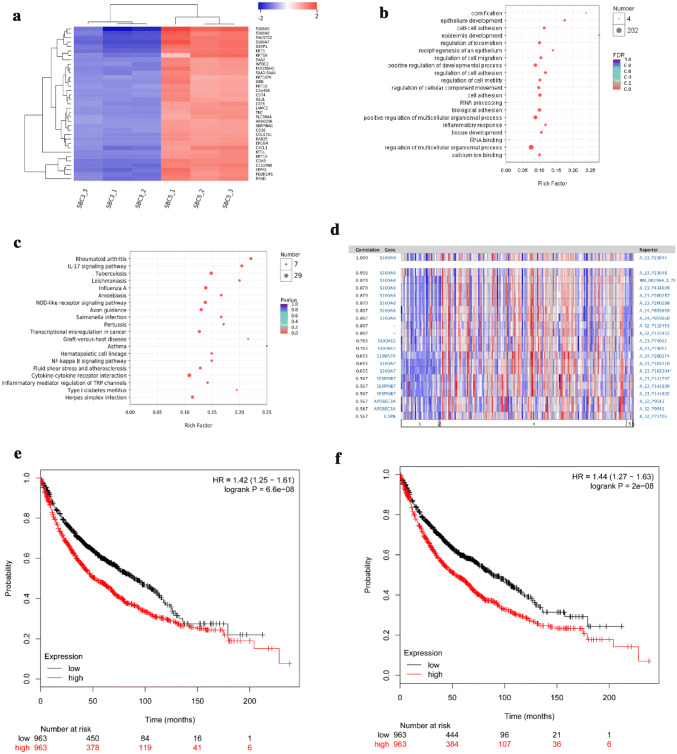
Non-bone metastatic cell line SBC3 and bone metastatic cell line SBC5 have similar genetic background and totally different potential of bone metastasis. The above result indicated the unique crosstalk between SBC5 and OP9A. To investigate the potential mechanism, RNA-seq analysis was performed to compare the transcriptome between SBC3 and SBC5. The result indicated that S100A9, S100A8 and S100A7 genes were the most prominent gene cluster up-regulated in SBC5 cells (Fig. 4a). Gene Ontology (GO) enrichment analysis suggested that the up-regulated genes in SBC5 were mainly involved in cornification, epithelium development and cell–cell adhesion, etc. (Fig. 4b). KEGG enrichment analysis suggested that these genes were mainly involved in signaling pathways of inflammation-related diseases and factor–factor receptor interactions (Fig. 4c). The Oncomine revealed the co-expression of S100A8/S100A9 in non-small cell lung cancer patients in TCGA (Fig. 4d). Similar results were obtained from other studies (data not showed). Survival analysis indicated that high expression of S100A8 and S100A9 was associated with poor prognosis. The risk ratios of lung cancer patients with high expression of S100A8 and S100A9 were 1.42 (Fig. 4e) and 1.44 (Fig. 4f), respectively.
Fig. 4.
RNA-seq analysis of SBC3 and SBC5. a The heatmap represented the high expression of the top 35 genes in SBC5, comparing with SBC3, each with three replicates. b GO enrichment analysis of up-regulated genes in SBC5 (top 20). Ordinate is the enriched GO, including molecular function, biological process, and cellular component. Abscissa is rich factor in which different colors of the nodes represent different FDR(p value_corrected), whereas different node size representes the number of genes enriched in the category. c KEGG analysis of up-regulated genes in SBC5 (Top 20). Ordinate is the enriched pathway of the up-regulated genes. Abscissa is rich factor in which different colors of the nodes represent different FDR (p value_corrected) whereas different node size representes the number of genes enriched in the pathway. The above RNA-seq data a–c were analyzed on the free online platform of Majorbio I-Sanger Cloud Platform (https://www.i-sanger.com). d Gene co-expression of S100A8 and S100A9 in lung cancer patients from TCGA data.Data was obtained and analyzed in https://www.oncomine.com. Data of KM survial plot of S100A8 (e) and S100A9 (f) were obtained and analyzed using the free platform (https://kmplot.com), Affymatrix ID: 202917_s_at, 203535_at
IL6R adjacent to S100A7/A8/A9 in SBC5 was up-regulated by BMAs
The above results indicate that high expression of S100A8/A9 in SBC5 cells may play an important role in the unique crosstalk with OP9A. Thus, we perform direct contact co-cultures to explore the mechanism of crosstalk between BMAs and SBC5. The PCR results showed that, after 2 weeks co-culture, the mRNA expression of adipogenic markers (Pparg and Fabp4) was significantly down-regulated (fold changes of − 0.85, and − 0.85, respectively, p < 0.01) in OP9 adipocytes (Fig. 5a, Supplementary Fig. 3a). MMP9, which could degrade the key components of the extracellular matrix, was significantly up-regulated by 103-fold (p < 0.0001, Fig. 5b) in SBC5 after co-culture, whereas ECAD did not change significantly (Supplementary Fig. 3b).
Fig. 5.
Dysregulated gene expression of OP9 adipocytes and SBC5 in the co-culture system. OP9 cells were induced to mature adipocytes, SBC5 cells (1 × 104) were added for direct co-culture with mature OP9 adipocytes in six-well plate, each with three replicates. After 2 weeks, qRT-PCR was used to determine the relative gene expression of OP9 adipocytes and SBC5 cells. The mRNA level of aPparg (OP9 adipocytes), bMMP9 (SBC5), cIl6 (OP9 adipocytes), dIL6R (SBC5), eS100A8 (SBC5), fS100A9 (SBC5), gPTHLH (SBC5), h the ratio of Pth1r, Ager, Tlr4 (OP9 adipocytes) iPth1r (OP9 adipocytes), jAger (OP9 adipocytes), kTlr4 (OP9 adipocytes) were showed, each with three replicates. The above data are represented as mean ± SD, *p < 0.05, **p < 0.01, ****p < 0.0001, ns indicates p ≥ 0.05
Second, we compared the mRNA expression of ligands (Il6, Lep, Adipoq and Cxcl12) in OP9 adipocytes and their corresponding receptors (IL6R, LEPR, ADIPOR1, ADIPOR2 and CXCR4) on SBC5 cells. The mRNA expression of Lep (Supplementary Fig. 3c) was undetectable in OP9 adipocytes after co-cultured with SBC5 cells. The mRNA expression of Adipoq and Cxcl12 mRNA was significantly down-regulated in OP9 adipocytes (fold changes of − 0.99, − 0.96, separately, p < 0.001, Supplementary Fig. 3d, e). However, their mRNA levels of corresponding receptors (LEPR, ADIPOR1 and ADIPOR2) on SBC5 were unchanged (Supplementary Fig. 3f–h), CXCR4 mRNA was up-regulated by 24-fold (p = 0.015, Supplementary Fig. 3i). Il6 mRNA in OP9 adipocytes and its receptor IL6R mRNA on SBC5 cells were significantly up-regulated by 2.5-fold, 5.1-fold, separately (p = 0.009, 0.018, separately, Fig. 5c, d).
Third, we compared the mRNA expression of ligands (S100A8, S100A9, PTHLH) on SBC5 and their corresponding receptors (Ager, Tlr4, Pth1r) on OP9 adipocytes. S100A8 in SBC5 cells showed an increased trend, but there was no statistical difference (Fig. 5e, p = 0.056). Similar results were obtained from S100A9 (Fig. 5f, p = 0.26). The mRNA level of PTHLH was significantly increased by about 66% (p = 0.002, Fig. 5g). We also compared the mRNA expression levels of Pth1r, Ager, Tlr4 in the co-cultured OP9 adipocytes. Compared with Pthlr mRNA, Tlr4 and Ager mRNA were relatively high expressed on OP9 adipocytes (208.6-fold and 4.4-fold, separately, p < 0.0001, Fig. 5h). However, Pthlr, Ager and Tlr4 mRNAs were significantly down-regulated (− 0.53, − 0.66 and − 0.83, separately, p < 0.05, Fig. 5i–k) after co-culture for 2 weeks.
S100A8/A9-TLR4 pathway regulated the crosstalk between lung cancer cells and BMAs
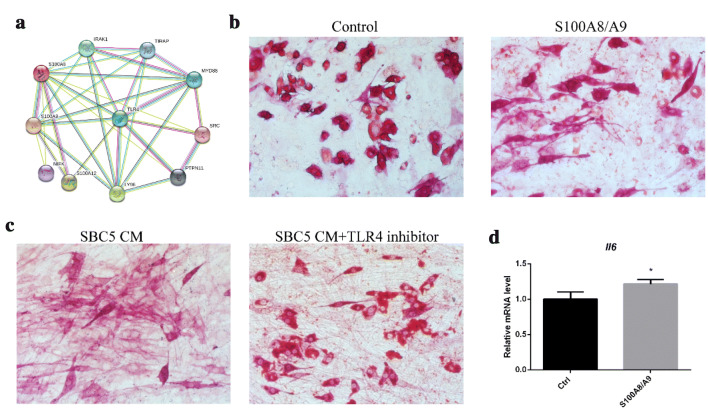
High expression of S100A8/A9 and up-regulation of IL6R in SBC5 were found when co-cultured with OP9A. Unexpectedly, we found that both S100A7/A8/A9 and IL6R were localized on chromosome 1q21.3. More importantly, IL6R is located proximal to S100A7/A8/A9 in 1q21.3, only S100A4 is located between IL6R and S100A7/A8/A9. A similar gene cluster was found in mouse chromosome 3F1. We hypothesized that S100A8/A9 -TLR4 pathway was connected to the crosstalk between lung cancer cells and BMAs. There were two main receptors for S100A8/A9, AGER and TLR4. First, we explored the effect of AGER in co-culture. AGER inhibitor FPS-ZM1 5 μM was added to the co-culture medium. Our results showed that there was no significant difference in mRNA expressions of Pparg, Fabp4 and Il6 (Supplementary Fig. 4a–c). The protein–protein interaction (PPI) network suggested that only TLR4 was closely associated with its ligands S100A8/A9 (Fig. 6a, Supplementary Fig. 4d). Thus, TLR4 in OP9A could be the main receptor for S100A8/A9. To confirm this, we added S100A8/A9 and TLR4 inhibitor TAK242 into the co-culture system. First, S100A8/A9 (0.2 μg/ml and 1 μg/ml) was added to OP9 culture medium. ALP and Oil red O double staining showed that 1 μg/ml S100A8/A9 could obviously enhance the osteoblastic differentiation and inhibit adipogenic differentiation (Fig. 6b). These cells were characterized by elongated shape with positive staining for ALP. As SBC5 CM is potent inducer of osteoblastic differentiation and adipocyte de-differentiation, TLR4 inhibitor TAK242 (10 μmol/l) was added to the SBC5 CM when co-cultured with OP9. We found that there was nearly no invisible Oil red O-positive BMAs in the control group, whereas TLR4 inhibitor group showed abundant lipid droplets (Fig. 6c). To explore the effect of S100A8/A9 on Il6 expression, we added the S100A8/A9 (0.4 μg/ml) to the mature OP9 adipocyte for 24 h. qRT-PCR showed that Il6 mRNA expression in OP9 adipocytes was up-regulated by 21% by S100A8/A9 (Fig. 6d).
Fig. 6.
S100A8/A9-TLR4 is involved in the osteoblastic and adipogenic differentiation. a The PPI network of S100A9 constructed based on STRING DATA (https://string-db.org). Edges between different nodes represent the protein–protein interaction. b S100A8/A9 (1ug/ml) was added to culture medium of OP9 for 7 days, ALP and Oil red O double staining were used to detect osteoblast activity and lipid accumulation, separately (200 ×). c 90% SBC5 condition medium and TLR4 inhibitor TAK242 10 μmol/l were added to OP9 culture medium, ALP and Oil red O double staining were used to determine osteoblast activity and lipid accumulation, separately (200 ×). d S100A8/A9 (0.4 μg/ml) was added to the culture medium for 24 h, each with three replicates, qRT-PCR was used to detect the mRNA expression of Il6 in OP9 adipocytes, *p < 0.05
Discussion
The interaction between lung cancer cells and bone microenvironment is more complicated than the “vicious cycle” theory would suggest. Most previous studies focused on the effects of genes on bone metastasis and neglected the most abundant cell type—BMAs. We found that increased number of BMAs enhanced osteolytic bone destruction. Interactions between lung cancer cells and BMAs modulated the imbalance of bone remodeling, leading to the initiation and development of bone destruction. This crosstalk is regulated by 1q21.3 (S100A8/A9-IL6R)-TLR4 pathway. High expression of S100A8/A9 could be one of the main characteristics of lung cancer cells with bone metastatic potential. S100A8/A9 could bind to its receptor TLR4 on BMAs, leading to enhance release of IL6 from BMAs. IL6R gene located next to S100A8/A9 genes on chromosome 1q21.3 was up-regulated by BMAs. Therefore, our study reveals a complex crosstalk between lung cancer cells and BMAs. S100A8/A9 are important activators in this crosstalk. BMAs cooperated with lung cancer cells in bone marrow niche to create a “vicious cycle”.
One of the most intriguing finding in this study was that SBC5 induced osteolytic lesion at the distal femur and osteoblastic lesion at the femur shaft in vivo. This seemingly conflicted finding was also reported in bone metastatic prostate cancer cell line PCSD1 in vivo (Hirata et al. 2016). In vitro experiments showed that SBC5 significantly promoted osteoblastic and osteoclastic differentiation and adipocyte de-differentiation. Besides, SBC5 significantly promoted the up-regulation of RANKL in MLO-Y4 osteocytes. In contrast, these features are not found in SBC3 cells. More importantly, Lewis cells established from C57BL lung cancer, displayed a similar phenotype. Lewis cell-bearing mice showed increased bone formation before metastasis. The clinical experiment findings are consistent with the mouse model. Non-small cell lung cancer patients without metastasis exhibited an increased trabecular bone density in the thoracic vertebrae; this study identified SiglecFhigh neutrophils as lung cancer promoter but did not show the exact mechanism in the crosstalk between lung cancer and neutrophils (Engblom et al. 2017).
Intertrabecular vertebral metastasis, which is more frequent in SCLC, would not cause osteoblastic or osteolytic bone destruction. Our in vitro results could help to explain this confusing clinical outcome. On one hand, BMAs only enhanced the invasion of bone metastatic SBC5 instead of non-bone metastatic SBC3. On the other hand, SBC5 instead of SBC3 promoted osteoblast and osteoclast differentiation as well as de-differentiation of mature BMAs. This indicated that bone metastasis potential of tumor cells may determine its interaction with bone marrow cells, especially bone marrow adipocytes. Compared with non-bone metastatic SBC3, SBC5 displayed the most robust up-regulation of S100A8/A9 expression (> 1000-fold) showed by RNA-seq analysis. S100A8 and S100A9 are often co-expressed in lung cancer patients. These two proteins could form a heterodimer, namely calprotectin (S100A8/A9). Calprotectin constitutes about 40% of the cytoplasmic proteins in neutrophil (Nacken et al. 2003). Release of calprotectin attracted more neutrophil to the infective site (Hiratsuka et al. 2006). Our studies reveal other potential source of S100A8/A9 in vivo. Only recently, strong S100A8/A9 expression was found in various cancer types, including lung cancer. More intriguingly, all the poorly differentiated squamous cell carcinoma showed S100A9 immunopositivity (Arai et al. 2001). Based on the previous findings, we revealed that S100A8/A9 may play an important role in the crosstalk between lung cancer cells and BMAs. Further analysis showed that the main receptor of S100A8/A9 in BMAs was TLR4 instead of AGER. Activation of TLR4 could induce IL6 production in macrophages (Takeuchi et al. 1999) and MSCs (He et al. 2016). More importantly, TLR4 activation in BMSCs could enhance IL-6 production and promote osteogenic differentiation through Wnt3a and Wnt5a signaling (He et al. 2016). We also observed the up-regulation of Il6 in OP9 adipocytes and IL6R in SBC5 cells in the co-culture system. Previous studies have showed that IL6 is a powerful growth factor for some tumor cells. As we known, BMAs are not the main producers of IL6, however, IL6 is the main inflammatory factors released from BMAs (Luo et al. 2018). Considering the abundance of BMAs in bone marrow, the amount of IL6 from BMAs could be huge. Besides, previous studies showed that paracrine IL-6 promoted autocrine IL-6/STAT3 signaling and cell migration in breast cancer cells (Leslie et al. 2010). Knockout of IL6 receptor in breast cancer cells significantly suppressed the cancer promoting effect from the paracrine source of IL6 (Szczerba et al. 2019). Based on the above results and analysis, we conclude that SBC5 may communicated with BMAs by S100A8/A9-TLR4 -IL6 signaling.
Bone metastasis and bone destruction are not mediated by a single gene, but rather by multiple genes engaging in the crosstalk with bone marrow-resident cells. Goh and colleague found that S100A7/A8/A9 and IL-1 receptor-associated kinase 1 (IRAK1) localized within chromosome 1q21.3 created a positive feedback loop to enhance breast cancer recurrence (Goh et al. 2017). Besides, 1q21 amplification is characterized as a very high-risk genetic feature in relapsed multiple myeloma, which often leads to osteolytic bone destruction (Marchesini et al. 2017). Our results indicated that BMAs enhanced the expression of IL6R. Unexpectedly, IL6R was adjacent to S100A7/A8/A9 in chromosome 1q21.3. As we known, genes may not be randomly distributed on chromosomes. The functional-related genes are more likely to be located in the same genomic regions, defined as “genomic neighbourhood” (De et al. 2010). Our results reveal a cluster of genes that contribute to the development of bone metastasis. 1q21.3 (S100A7/A8/A9-IL6R) may be a potential target for the treatment of bone metastasis in lung cancer patients.
Conclusion
Our study suggest that bone marrow adipocytes could enhance the osteolytic bone destruction in lung cancer. Besides, the crosstalk between bone marrow adipocytes and lung cancer cells in bone marrow microenvironment was activated by 1q21.3 (S100A8/A9-IL6R)-TLR4 pathway. 1q21.3 (S100A8/A9-IL6R) could be a potential target for bone metastasis in lung cancer patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the research core facility of west china hospital at Sichuan university for assistance with histopathology.
Author contributions
GL and MT: methodology, investigation, formal analysis, writing—original draft preparation; QZ, LL, YL, YX and CL: methodology, investigation, data curation, formal analysis; LT and XC: writing—original draft preparation, methodology, visualization; XY: conceptualization, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition. GL and MT contributed equally to this article.
Funding
This work was supported by grants from the National Natural Science Foundation of China (nos. 81770875, 81572639), the Science and Technology Department of Sichuan Province (2018SZ0142), the Sichuan University (no. 2018SCUH0093), and the National Clinical Research Center for Geriatrics of West China Hospital (no. Z2018B05), 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (2020HXFH008, ZYGD18022).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures were approved by the Institutional Animal Care and Use Committee of West China Hospital, Sichuan University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guojing Luo and Mengjia Tang contributed equally to this work.
References
- Arai K et al (2001) Immunohistochemical investigation of S100A9 expression in pulmonary adenocarcinoma: S100A9 expression is associated with tumor differentiation. Oncol Rep 8(3):591–596 [DOI] [PubMed] [Google Scholar]
- Behan JW et al (2009) Adipocytes impair leukemia treatment in mice. Cancer Res 69(19):7867–7874. 10.1158/0008-5472.CAN-09-0800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti G et al (2020) LIGHT/TNFSF14 promotes osteolytic bone metastases in non-small cell lung cancer patients. J Bone Miner Res 35(4):671–680. 10.1002/jbmr.3942 [DOI] [PubMed] [Google Scholar]
- Bullwinkle EM et al (2016) Adipocytes contribute to the growth and progression of multiple myeloma: unraveling obesity related differences in adipocyte signaling. Cancer Lett 380(1):114–121. 10.1016/j.canlet.2016.06.010 [DOI] [PubMed] [Google Scholar]
- De S et al (2010) Genomic neighbourhood and the regulation of gene expression. Curr Opin Cell Biol 22(3):326–333. 10.1016/j.ceb.2010.04.004 [DOI] [PubMed] [Google Scholar]
- D'Oronzo S et al (2019) Metastatic bone disease: pathogenesis and therapeutic options: up-date on bone metastasis management. J Bone Oncol 15:4. 10.1016/j.jbo.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsanipour EA et al (2013) Adipocytes cause leukemia cell resistance to l-asparaginase via release of glutamine. Cancer Res 73(10):2998–3006. 10.1158/0008-5472.CAN-12-4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom C et al (2017) Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science. 10.1126/science.aal5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler IJ et al (2008) The "seed and soil" hypothesis revisited. Lancet Oncol 9(8):808. 10.1016/S1470-2045(08)70201-8 [DOI] [PubMed] [Google Scholar]
- Gandaglia G et al (2015) Impact of the site of metastases on survival in patients with metastatic prostate cancer. Eur Urol 68(2):325–334. 10.1016/j.eururo.2014.07.020 [DOI] [PubMed] [Google Scholar]
- Gimble JM et al (1996) The function of adipocytes in the bone marrow stroma: an update. Bone 19(5):421–428 [DOI] [PubMed] [Google Scholar]
- Goh JY et al (2017) Chromosome 1q213 amplification is a trackable biomarker and actionable target for breast cancer recurrence. Nat Med 23(11):1319–1330. 10.1038/nm.4405 [DOI] [PubMed] [Google Scholar]
- He X et al (2016) TLR4 Activation Promotes Bone Marrow MSC Proliferation and Osteogenic Differentiation via Wnt3a and Wnt5a Signaling. PLoS ONE 11(3):e149876. 10.1371/journal.pone.0149876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herroon MK et al (2013) Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget 4(11):2108–2123. 10.18632/oncotarget.1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T et al (2016) Specific bone region localization of osteolytic versus osteoblastic lesions in a patient-derived xenograft model of bone metastatic prostate cancer. Asian J Urol 3(4):229–239. 10.1016/j.ajur.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka S et al (2006) Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 8(12):1369–1375. 10.1038/ncb1507 [DOI] [PubMed] [Google Scholar]
- Kraus NA et al (2016) Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 5(4):351–358. 10.1080/21623945.2016.1240137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko OP et al (2007) Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology 148(6):2669–2680. 10.1210/en.2006-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie K et al (2010) Differential interleukin-6/Stat3 signaling as a function of cellular context mediates Ras-induced transformation. Breast Cancer Res 12(5):R80. 10.1186/bcr2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G et al (2018) Bone marrow adipocyte: an intimate partner with tumor cells in bone metastasis. Front Endocrinol (Lausanne) 9:339. 10.3389/fendo.2018.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini M et al (2017) ILF2 is a regulator of RNA splicing and DNA damage response in 1q21-amplified multiple myeloma. Cancer Cell 32(1):88–100. 10.1016/j.ccell.2017.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T et al (2000) Bone metastasis model with multiorgan dissemination of human small-cell lung cancer (SBC-5) cells in natural killer cell-depleted SCID mice. Oncol Res 12(5):209–217. 10.3727/096504001108747701 [DOI] [PubMed] [Google Scholar]
- Nacken W et al (2003) S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc Res Tech 60(6):569–580. 10.1002/jemt.10299 [DOI] [PubMed] [Google Scholar]
- Niikura N et al (2011) Treatment outcome and prognostic factors for patients with bone-only metastases of breast cancer: a single-institution retrospective analysis. Oncologist 16(2):155–164. 10.1634/theoncologist.2010-0350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riihimaki M et al (2014) Metastatic sites and survival in lung cancer. Lung Cancer 86(1):78–84. 10.1016/j.lungcan.2014.07.020 [DOI] [PubMed] [Google Scholar]
- Scheller EL et al (2014) Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods Enzymol 537:123–139. 10.1016/B978-0-12-411619-1.00007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafat MS et al (2017) Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood 129(10):1320–1332. 10.1182/blood-2016-08-734798 [DOI] [PubMed] [Google Scholar]
- Sheng X et al (2016) Adipocytes cause leukemia cell resistance to daunorubicin via oxidative stress response. Oncotarget 7(45):73147–73159. 10.18632/oncotarget.12246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczerba BM et al (2019) Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 10.1038/s41586-019-0915-y [DOI] [PubMed] [Google Scholar]
- Tabe Y et al (2017) Bone marrow adipocytes facilitate fatty acid oxidation activating AMPK and a transcriptional network supporting survival of acute monocytic leukemia cells. Cancer Res 77(6):1453–1464. 10.1158/0008-5472.CAN-16-1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O et al (1999) Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11(4):443–451 [DOI] [PubMed] [Google Scholar]
- Trotter TN et al (2016) Adipocyte-lineage cells support growth and dissemination of multiple myeloma in bone. Am J Pathol 186(11):3054–3063. 10.1016/j.ajpath.2016.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolins NE et al (2006) OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. J Lipid Res 47(2):450–460. 10.1194/jlr.D500037-JLR200 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T et al (1996) Intertrabecular pattern of tumors metastatic to bone. Cancer 78(7):1388–1394. 10.1002/(SICI)1097-0142(19,961,001)78:7<1388:AID-CNCR4>3.0.CO;2-H [DOI] [PubMed] [Google Scholar]
- Yamaguchi T (2001) Intertrabecular vertebral metastases: metastases only detectable on MR imaging. Semin Musculoskelet Radiol 5(2):171–175. 10.1055/s-2001-15,676 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.