Significance
This study reports that chronic stress activates the GCR–HMGB2–LDLR axis via cortisol release, leading to abnormal cholesterol metabolism and ultimately promoting esophageal carcinogenesis. Chronic stress is associated with increased ESCC risk, and further exacerbates ESCC progression through HMGB2. Cortisol, by activating GCR, stimulates ESCC cell proliferation. Moreover, GCR activation enhances HMGB2 transcription, which in turn binds to SREBF1, thereby upregulating LDLR transcription and disrupting cholesterol metabolism. Clinical parameters in stressed ESCC patients underscore the relevance of stress management as a pivotal strategy for both the prevention and treatment of this malignancy.
Keywords: ESCC, chronic stress, cortisol, HMGB2, LDLR
Abstract
Recent studies have demonstrated that chronic stress can enhance the development of multiple human diseases, including cancer. However, the role of chronic stress in esophageal carcinogenesis and its underlying molecular mechanisms remain unclear. This study uncovered that dysregulated cholesterol metabolism significantly promotes esophageal carcinogenesis under chronic stress conditions. Our findings indicate that the persistent elevation of glucocorticoids induced by chronic stress stimulates cholesterol uptake, contributing to esophageal carcinogenesis. The activated glucocorticoid receptor (GCR) enrichment at the promoter region of High Mobility Group Box 2 (HMGB2) facilitates its transcription. As a transcription coactivator, HMGB2 enhances Sterol Regulatory Element Binding Transcription Factor 1 (SREBF1) transcription and regulates cholesterol metabolism through LDL particle uptake into cells via Low Density Lipoprotein Receptor (LDLR). These results emphasize the significant impact of chronic stress on esophageal carcinogenesis and establish cholesterol metabolism disorder as a crucial link between chronic stress and the development of ESCC. The implications suggest that effectively managing chronic stress may serve as a viable strategy for preventing and treating ESCC.
Esophageal cancer (EC) is widely recognized as one of the most aggressive malignancies in the world due to lifestyle choices, nutritional deficiencies, lack of effective therapeutic targets, etc. Consequently, esophageal squamous cell carcinoma (ESCC) exhibits a 5-y survival rate below 30% (1–3). Chronic stress has been implicated in mood disorders, depression, immune suppression, and an increasing risk of developing various cancers. Chronic stress mainly activates the hypothalamus–pituitary–adrenal (HPA) axis and the sympathetic nervous system (SNS) to address pressure and maintain homeostatic regulation. In response to stress, the HPA axis becomes activated, prompting the adrenal cortex to synthesize and release cortisol and corticosterone. The SNS is activated to release catecholamine hormones, such as epinephrine and norepinephrine (4–6). Even reports in the literature show that work stress is an important risk factor for colorectal, lung, and esophagus cancers (7–9). Further investigation is required to determine the mechanism between chronic stress and ESCC development and whether it can serve as a significant target for this disease.
Evidences suggest that chronic stress exerts considerable influence on energy metabolism by disrupting key metabolic processes and impairing energy homeostasis, leading to metabolic diseases (10, 11). Cholesterol metabolism plays a crucial role in maintaining both the health and optimal functioning of the body. The uptake of low-density lipoprotein (LDL) through the LDL receptor (LDLR) represents a vital pathway for maintaining cholesterol equilibrium (12, 13). Research indicates that dysregulated cholesterol metabolism may contribute to cancer progression and therapy resistance (14–16). However, further investigation is needed to explore whether chronic stress affects cholesterol metabolism in ESCC and elucidate the underlying mechanisms driving this disorder.
In this study, we uncover how chronic stress facilitates the onset and progression of ESCC by disrupting tumor cell cholesterol metabolism. Under conditions of chronic stress, the activated GCR was mainly located from the cytoplasm into the nucleus where it enriches at the HMGB2 promoter region, leading to its transcriptional activation. We report that silence HMGB2 distinctly inhibited the proliferation of ESCC both in vitro and in vivo. Additionally, knockout of HMGB2 inhibited 4-nitrochinoline-oxide-induced (4NQO)/stress-induced esophageal tumor growth. Upregulation of LDLR expression through HMGB2 binding with transcription factor SREBF1 enhances LDL particle uptake, thereby providing energy or fulfilling other metabolic demands in cancer cells. Overall, our findings reveal a cortisol–HMGB2–LDLR axis that regulates cholesterol metabolism in chronic stress–induced ESCC.
Results
Chronic Stress Promotes ESCC Carcinogenesis.
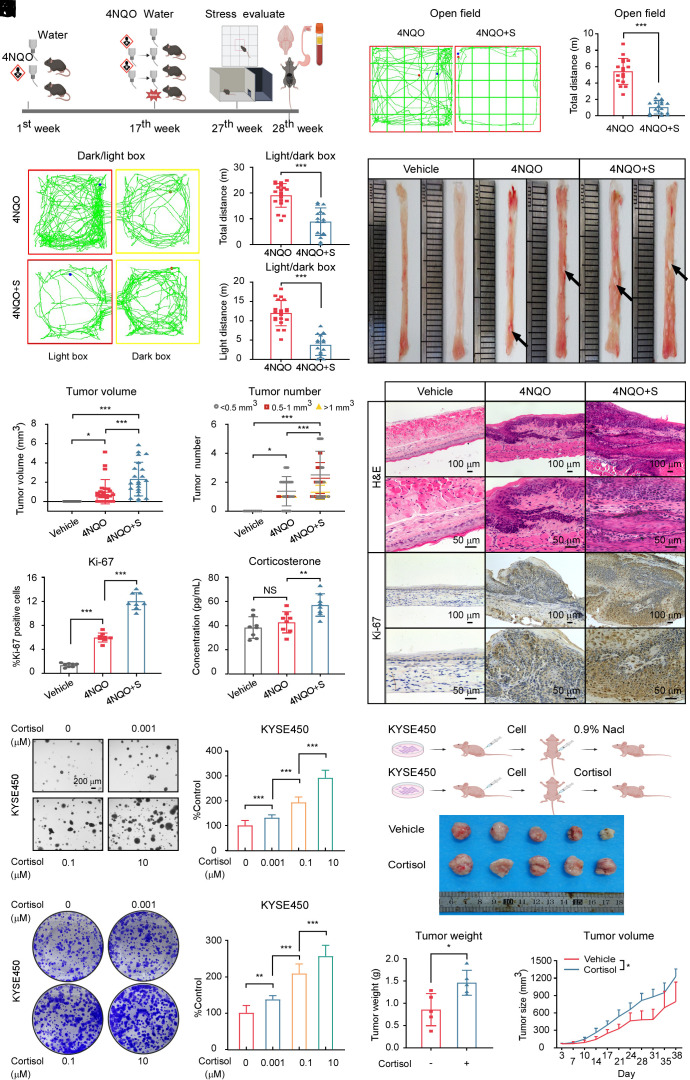
To investigate the potential impact of chronic stress on ESCC tumorigenesis and growth, we established a mice ESCC model induced by chronic stress and 4NQO treatment (Fig. 1A and SI Appendix, Fig. S1A). The establishment of a chronic unpredictable mild stress model was evaluated through open field and light–dark box experiments. Mice exposed to chronic stress exhibited a notable reduction in total distance, center distance, time in the center, and number of times traversing the center (Fig. 1 B and C and SI Appendix, Fig. S1 B–D). In the light–dark box experiment, the total distance and light box distance traveled by the mice in the 4NQO + stress group significantly decreased; however, immobility time substantially increased (Fig. 1 D–F and SI Appendix, Fig. S1E). Anatomical examination of the esophagus revealed that chronic stress–treated mice showed a remarkable increase in tumor volume, number, and the ratio of weight to length, but we found there were no sex differences between male and female mice (Fig. 1 G–I and SI Appendix, Fig. S1 F–H). Esophageal tissue hematoxylin and Eosin (H&E) staining unveiled more invasive tumor alterations in the 4NQO + stress group than in the 4NQO group (Fig. 1J and SI Appendix, Fig. S1 I and J). Moreover, the expression of Ki-67, a cell proliferation marker, was also significantly higher in the 4NQO + stress group than in the 4NQO group (Fig. 1 J and K and SI Appendix, Fig. S1I).
Fig. 1.
Chronic stress facilitates ESCC development. (A) Diagram detailing the 4NQO or 4NQO + stress-induced ESCC model in C57 mice. The figure was created with BioRender.com. (B) Representative activity trajectory of 4NQO and 4NQO + stress-treated mice mapped using the open field test. (C) Comparison of the total distance traveled by the 4NQO (n = 17 mice) and 4NQO + stress (n = 18 mice) groups. (D) Representative distance tracks of 4NQO and 4NQO + stress-treated mice mapped using the light–dark box test. (E and F) Comparison of the total distance (E) and light box distance traveled (F) by the 4NQO (n = 20 mice) and 4NQO + stress (n = 15 mice) groups. (G) Representative macroscopic images of esophageal tumors from mice at the 28th wk. Black arrows indicate in situ tumor lesions. (H and I) Tumor volume (H) and number of esophageal tumors (I) in each group. (Vehicle, n = 10; 4NQO, n = 25; 4NQO + stress, n = 22). (J) Harvested esophageal tissues of male mice were stained with H&E and IHC staining of Ki-67. (Scale bar, 100 μm or 50 μm). (K) Statistical analysis of Ki-67 expression levels of male mice was shown (n = 8/group). (L) The corticosterone levels in the plasma of vehicle, 4NQO, and 4NQO + stress mice were quantified with an ELISA kit. (n = 8/group). (M and N) Cell proliferation after administering cortisol (0, 0.001, 0.1, and 10 μM) to KYSE450 cells was measured using soft agar (M) and crystal violet staining assays (N). (Scale bar, 200 μm). (O) Schematic illustrating the treatment regimen of normal saline or cortisol to KYSE450 CDX mice model. (n = 5/group). The figure was created with BioRender.com. (P) Photograph of the harvested tumors from KYSE450 CDX mice model. (Q) Weight of excised tumors in each group. (n = 5/group). (R) Tumor volume measurements were obtained twice weekly. The formula of tumor volume (mm3) calculation = length × width × height × 0.52. (n = 5/group). *P < 0.05, **P < 0.01, ***P < 0.001, NS = no significance.
To investigate how chronic stress promotes the development of ESCC, we measured the levels of stress hormones in the plasma of mice in the vehicle, 4NQO, and 4NQO + stress groups. We observed that the changes in plasma glucocorticoid concentration were most pronounced. Multiple mammals secrete both corticosterone and cortisol. In humans, cortisol is the primary glucocorticoid, whereas in rodents, corticosterone is the primary glucocorticoid (17, 18). The corticosterone and cortisol levels were dramatically elevated in mice treated with chronic stress compared with mice in both vehicle and 4NQO groups, however, only 4NQO treatment did not influence the glucocorticoid levels (Fig. 1L and SI Appendix, Fig. S1K). The norepinephrine and epinephrine levels in plasma were significantly increased whereas the concentration of plasma 5-HT in mice was reduced sharply in mice treated with chronic stress compared with mice in both vehicle and 4NQO groups (SI Appendix, Fig. S1 L–N). Cortisol markedly enhanced the growth of ESCC cells in a dose-dependent manner (Fig. 1 M and N and SI Appendix, Fig. S1 O and P). Additionally, we sought to confirm our in vivo findings by injecting KYSE450 cells into nude mice and subsequently administering cortisol. The results showed that cortisol significantly promoted tumor growth (Fig. 1 O–R). Together, these results demonstrate that chronic stress–induced glucocorticoid released from the HPA axis plays an important role in esophageal carcinogenesis.
Chronic Stress Promotes ESCC Progression through HMGB2.
To investigate how chronic stress promotes the progression of ESCC, we employed proteomic-sequencing technology to identify differentially expressed proteins using esophageal tissues from the mice ESCC model (Fig. 2A). We identified a total of 6 down-regulated genes and 16 up-regulated genes based on proteomic-sequencing results and literature analysis of proteins related to cancer and chronic stress. (Fig. 2B). By analyzing data from the TCGA public database, we identified two potential candidate proteins, HMGB2, and PGAM1, for subsequent investigation (Fig. 2C and SI Appendix, Fig. S2A). We observed that 4NQO treatment led to an increase in HMGB2 expression; moreover, further elevation of HMGB2 expression was observed after chronic stress exposure. However, there was no significant alteration in the expression of PGAM1 (SI Appendix, Fig. S2B). Consequently, we chose HMGB2 as the target molecule for our study. Immunohistochemistry (IHC) staining also revealed that chronic stress significantly upregulated HMGB2 expression compared to the non-stress-treated group (Fig. 2 D and E and SI Appendix, Fig. S2C). Besides, we found stress treatment increased HMGB2 protein expression compared with the vehicle group (SI Appendix, Fig. S2 D and E).
Fig. 2.
Chronic stress enhances ESCC progression via HMGB2. (A) Schematic illustrating the proteomics assay using the esophageal samples from the 4NQO and 4NQO + stress group. The figure was created with BioRender.com. (B) The heat map displayed differentially expressed proteins between the 4NQO and the 4NQO + stress groups that were selected after reviewing the literature for genes associated with cancer and stress. (C) Plots illustrating HMGB2 transcript expression levels in the normal esophagus and ESCC from the TCGA database. (Normal = 11, Tumor = 81). (D) Statistical analysis of the HMGB2 expression level. (E) Representative IHC staining images illustrating HMGB2 expression in harvested male esophageal tissues. (n = 8/group). (Scale bar, 100 μm or 50 μm). (F) Representative IHC staining images illustrating HMGB2 expression in paired adjacent and tumor tissues from an ESCC human tissue array. (Scale bar, 200 μm or 50 μm). (G) Statistical analysis of HMGB2 expression between cancer tissues and paired adjacent normal tissues. (n = 59). (H) Statistical analysis of HMGB2 expression. (Adjacent, n = 60; Tumor, n = 59). (I) Cell proliferation was measured in mock and shHMGB2 KYSE410 and KYSE450 cells at 0 h, 24 h, 48 h, and 72 h by MTT assay. (J) Cell proliferation was measured in mock and shHMGB2 ESCC cells by soft agar colony formation assays. (Scale bar, 200 μm). (K) Cell proliferation was measured in mock and shHMGB2 ESCC cells by crystal violet staining assay. (L) Cell proliferation of KYSE30 and KYSE150 cells overexpressing HMGB2 was measured by MTT assay. (M) Cell proliferation of ESCC cells overexpressing HMGB2 was measured by soft agar colony formation assays. (Scale bar, 200 μm). (N) Cell proliferation of ESCC cells overexpressing HMGB2 was measured by crystal violet staining assay. (O and P) Schematic illustrating (O) and representative image (P) of tumors derived from the KYSE450 cell-derived xenograft (CDX) mice model exposed to chronic stress. (n = 4/group). The figure (O) was created with BioRender.com. (Q) Statistical analysis of tumor weight. (n = 4/group). (R) Tumor volume measurements were obtained twice weekly. The formula of tumor volume (mm3) calculation = length × width × height × 0.52. (n = 4/group). *P < 0.05, **P < 0.01, ***P < 0.001.
To further determine the potential function of HMGB2 in ESCC, we first assessed the expression of HMGB2 and found that HMGB2 expression was significantly elevated in ESCC tissues and cell lines (Fig. 2 F–H and SI Appendix, Fig. S2 F and G). More importantly, survival analysis indicated that ESCC patients with high HMGB2 expression had a dramatically shorter survival time compared to those with low HMGB2 expression (SI Appendix, Fig. S2H). Overall, these results revealed that chronic stress could promote the expression of HMGB2, which is highly expressed in ESCC.
Next, we sought to identify the oncogenic function of HMGB2 in ESCC by measuring cell proliferation after silencing its expression in KYSE410 and KYSE450 ESCC cells (SI Appendix, Fig. S2 I and J). The results of MTT, soft agar colony formation, and crystal violet staining experiments indicated that cell proliferation was dramatically reduced after HMGB2 silencing (Fig. 2 I–K). Furthermore, we found that HMGB2 overexpression could promote ESCC cell proliferation (Fig. 2 L–N and SI Appendix, Fig. S2K). Until now, our results have indicated that high expression of HMGB2 and ESCC are positively correlated. We next sought to determine whether chronic stress could promote ESCC progression through HMGB2. We constructed an HMGB2 knockdown and mock cell-derived xenograft (CDX) mouse model and challenged mice with chronic stress (Fig. 2O). Our results indicated that the mice’s tumor weight and volume significantly increased after the chronic stress exposure compared to the mock group. In contrast, chronic stress did not promote tumor growth in the HMGB2 knockdown group (Fig. 2 P-R). H&E and IHC staining revealed that chronic stress facilitated the expression of HMGB2 and Ki-67 in the mock group but not in the HMGB2 knockdown group (SI Appendix, Fig. S2 L–N). Next, we treated ESCC cells with cortisol in the presence or absence of HMGB2. The results showed that cortisol did not promote the proliferation of ESCC cells depleted of HMGB2 compared to the control group (SI Appendix, Fig. S2 O and P). Our experimental findings demonstrate that chronic stress promotes the occurrence and development of ESCC by increasing the expression of HMGB2.
Western blot was used to verify the total knockdown efficiency of Hmgb2 KO mice (SI Appendix, Fig. S3A). To investigate the oncogenic role of HMGB2 in vivo, Hmgb2 knockout (KO) and wild-type (WT) mice were treated with 4NQO or 4NQO + stress (SI Appendix, Fig. S3B). WT mice exposed to chronic stress showed a notable reduction in the total distance, center distance, and time in the box center. However, when giving chronic stress to Hmgb2 KO mice, there was no statistically significant difference compared to the Hmgb2 KO mice without stress treatment (SI Appendix, Fig. S3 C–G). In the light–dark box experiment, the total distance and light box distance traveled by WT mice in the 4NQO + stress group significantly decreased, whereas, the immobility time substantially increased. However, these behavioral alterations were not found in Hmgb2 KO mice (SI Appendix, Fig. S3 H–K).
At the end of the 28th wk, mice were killed, and the esophagi were collected. Representative esophageal tumors are illustrated in SI Appendix, Fig. S3L. The tumor volume, number, and the ratio of weight to the length of the esophagus were conspicuously decreased in Hmgb2 KO mice than in WT mice. Chronic stress could promote the growth of ESCC in WT mice but not in Hmgb2 KO mice (SI Appendix, Fig. S3 M–O). Chronic stress boosted ESCC tumorigenesis and the expression of Ki-67 and HMGB2 in WT mice but not in Hmgb2 KO mice, as evidenced by H&E and IHC staining (SI Appendix, Fig. S4 A–C). Taken together, these data also confirm that chronic stress facilitates tumorigenesis in ESCC through enhancing HMGB2 expression.
Cortisol Promotes ESCC Cell Growth through GCR Activation.
Cortisol, one of the primary glucocorticoids, exerts its effects by binding to the GCR and forming activated complexes that enter the cell nucleus, influencing gene transcription and translation (19, 20). Immunofluorescence assays verified after administering 10 μM cortisol to ESCC cells that the GCR was mainly located from the cytoplasm to the nucleus (SI Appendix, Fig. S5 A and B). To investigate how chronic stress affects HMGB2 expression, we initially treated ESCC cells with different concentrations of cortisol. The results showed that cortisol could increase HMGB2 mRNA and protein levels in a dose-dependent manner (SI Appendix, Fig. S5 C and D). Analysis of data provided by the TCGA public database showed that GCR expression was markedly upregulated in ESCC patients compared to normal controls (SI Appendix, Fig. S5E). Next, we found that GCR expression was significantly higher in ESCC tissues and cell lines (SI Appendix, Fig. S5 F–H). Overall, GCR has a high expression in ESCC.
We knocked down NR3C1 (gene name of GCR) in ESCC cells to ascertain whether GCR could affect the proliferation of ESCC cells (SI Appendix, Fig. S5I). We subsequently conducted MTT, soft agar colony formation, and crystal violet staining assays to identify that knockdown of NR3C1 exponentially inhibited ESCC cell proliferation (SI Appendix, Fig. S5 J–L). To determine whether GCR could promote ESCC growth in vivo, KYSE450 cells depleted of NR3C1 via knockdown were subcutaneously injected into nude mice to establish tumor xenografts. Results showed that the tumor weight and tumor volume were significantly reduced in the NR3C1 knockdown groups compared to the mock control (SI Appendix, Fig. S6 A–C). Besides, the noticeably diminished expression of Ki-67 and GCR was observed in NR3C1-knockdown mice (SI Appendix, Fig. S6 D–G). Additionally, a NR3C1 overexpressed plasmid was transfected into ESCC cells to verify the oncogenic function of GCR (SI Appendix, Fig. S6H). As expected, NR3C1 overexpression promoted ESCC cell proliferation, as evidenced by MTT, soft agar colony formation, and crystal violet staining assay results (SI Appendix, Fig. S6 I–K). In summary, GCR activation can promote ESCC cell proliferation. We next sought to confirm whether cortisol facilitates the occurrence and progression of ESCC through GCR. To that end, we treated NR3C1 knockdown ESCC cells with cortisol. Results suggested that cortisol failed to promote the proliferation of ESCC cells depleted of NR3C1 in comparison to the control group (SI Appendix, Fig. S6 L and M). Additionally, the cortisol level did not change significantly in the NR3C1 knockdown or overexpression group compared with the control group in vivo and in vitro (SI Appendix, Fig. S6 N–P). The experimental results above demonstrated that cortisol promotes the occurrence and development of ESCC through GCR activation.
Activated GCR Promotes the Transcription of HMGB2.
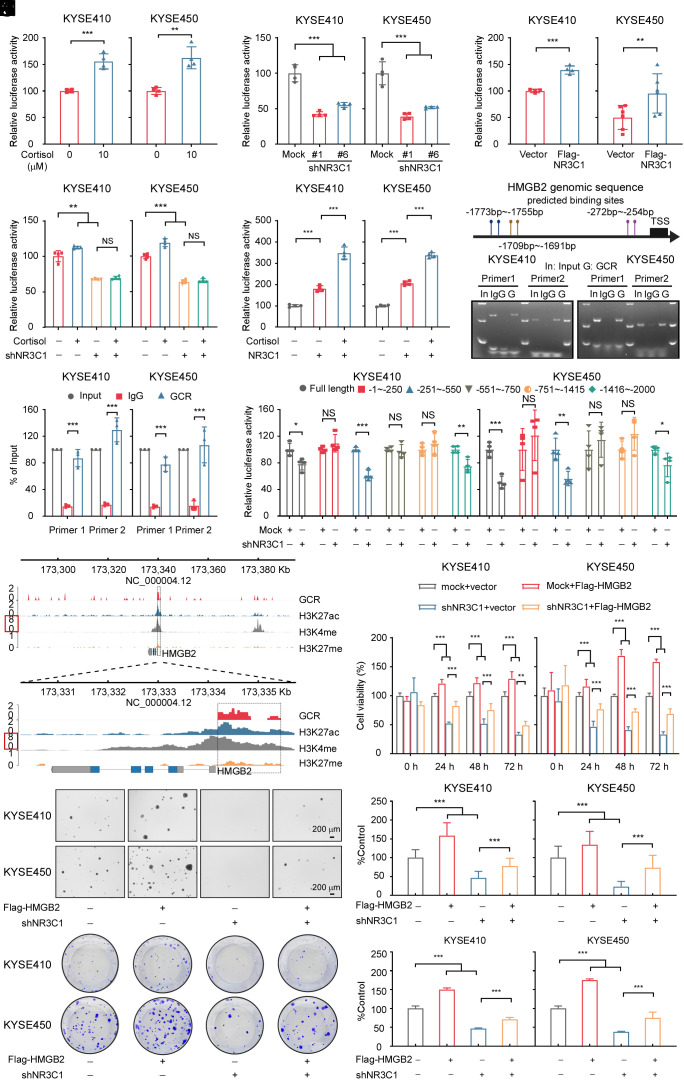
Our experimental findings determined that cortisol could increase the mRNA level of HMGB2. We speculated cortisol could regulate the transcription of HMGB2 and designed a dual-luciferase assay and found an increase in luciferase activity in the HMGB2 promoter transfected cells relative to the control group (Fig. 3A). The analysis using the GEPIA database revealed a correlation between GCR and HMGB2 (SI Appendix, Fig. S7A). To explore this connection further, we transfected HMGB2 promoter plasmids into NR3C1-depleted or NR3C1 overexpressing ESCC cells and subsequently performed dual-luciferase assays. The results showed that knockdown or overexpression of NR3C1 could inhibit or promote HMGB2 transcription, respectively (Fig. 3 B and C). Next, we treated NR3C1-depleted and NR3C1-overexpressing ESCC cells with cortisol. Interestingly, cortisol treatment could not enhance HMGB2 transcription in NR3C1 knockdown ESCC cell lines compared to the control cells (Fig. 3D). Moreover, cortisol treatment of cells overexpressed NR3C1 significantly increased luciferase activity (Fig. 3E). These experimental results suggested that cortisol promotes the transcription of HMGB2 through GCR activation.
Fig. 3.
Activated GCR enhances the transcription of HMGB2. (A) Dual-luciferase activity was detected 48 h after transfecting the HMGB2-promoter into KYSE410 and KYSE450 cells treated with 10 μM cortisol or DMSO for 18 h. (n = 4/group). (B) Relative HMGB2 luciferase promoter activity in GCR-depleted ESCC cells. Dual-luciferase was detected at 48 h after transfection. (n = 4/group). (C) Relative HMGB2 luciferase promoter activity in NR3C1 overexpressing ESCC cells. Dual-luciferase activity was detected at 48 h after transfection. (n = 4/group). (D) shNR3C1 ESCC cells were transfected with the HMGB2 promoter reporter plasmid. Transfected cells were then treated with 10 μM cortisol or DMSO for 18 h and luciferase activity was subsequently measured. (n = 4/group). (E) The HMGB2 promoter reporter plasmid was transfected in ESCC cells with NR3C1 overexpression. Transfected cells were then treated with 10 μM cortisol or DMSO for 18 h and luciferase activity was subsequently measured. (n = 4/group). (F) Schematic illustrating the predicted binding sites used to assess the enrichment of GCR at the HMGB2 promoter. (G) Representative images of GCR enrichment at the HMGB2 promoter were obtained using the CHIP assay in ESCC cells. (H) The gray value analysis of GCR enrichment at the HMGB2 promoter was measured using the CHIP assay. (n = 3/group). (I) Dual-luciferase reporter assays of ESCC cells transfected with HMGB2 truncated promoters or full length. (n = 4/group). (J) Gene tracks of GCR, H3K27ac, H3K4me, and H3K27me enrichment with CUT&TAG analysis at the HMGB2 promoter locus in KYSE450 cells. (K–M) HMGB2 overexpression rescued the cell proliferation defect induced by HMGB2 depletion in ESCC cells, as evidenced by MTT (K), soft agar (L), and crystal violet staining assay (M) results. (Scale bar, 200 μm). *P < 0.05, **P < 0.01, ***P < 0.001, NS = no significance.
To further determine whether GCR could promote HMGB2 transcription, we next conducted a chromatin immunoprecipitation (CHIP) experiment. We designed primers within the 2,000 bp region upstream of the HMGB2 promoter and the results indicated that GCR could be enriched at the HMGB2 promoter region (Fig. 3 F–H). Then, using a luciferase reporter assay, serial truncated analysis of the HMGB2 promoter revealed that the −251 to −550 bp and −1,416 to −2,000 bp region of the HMGB2 promoter is required for GCR-mediated HMGB2 transactivation (Fig. 3I). These results confirmed that GCR could promote the transcription of HMGB2. We further conducted CUT&TAG experiments in KYSE450 cells (SI Appendix, Fig. S7B). We found that GCR, H3K27ac (a marker of active enhancer and promoter), and H3K4me (a marker of an active promoter) histone marks share the same occupied region within the 2,000 bp region downstream of HMGB2, as opposed to H3K27me (a marker of a relatively silent region). These results suggested that the GCR enrichment at the promoter region of HMGB2 promotes its transcription (Fig. 3J). We conducted rescue experiments to explore whether HMGB2 is a functional downstream factor of GCR. As anticipated, HMGB2 overexpression could restore cell survival inhibition caused by NR3C1 depletion (Fig. 3 K–M and SI Appendix, Fig. S7C). These results revealed that cortisol could promote the transcription of HMGB2 through GCR activation.
HMGB2 Promotes ESCC Proliferation through the Cholesterol Metabolism Pathway.
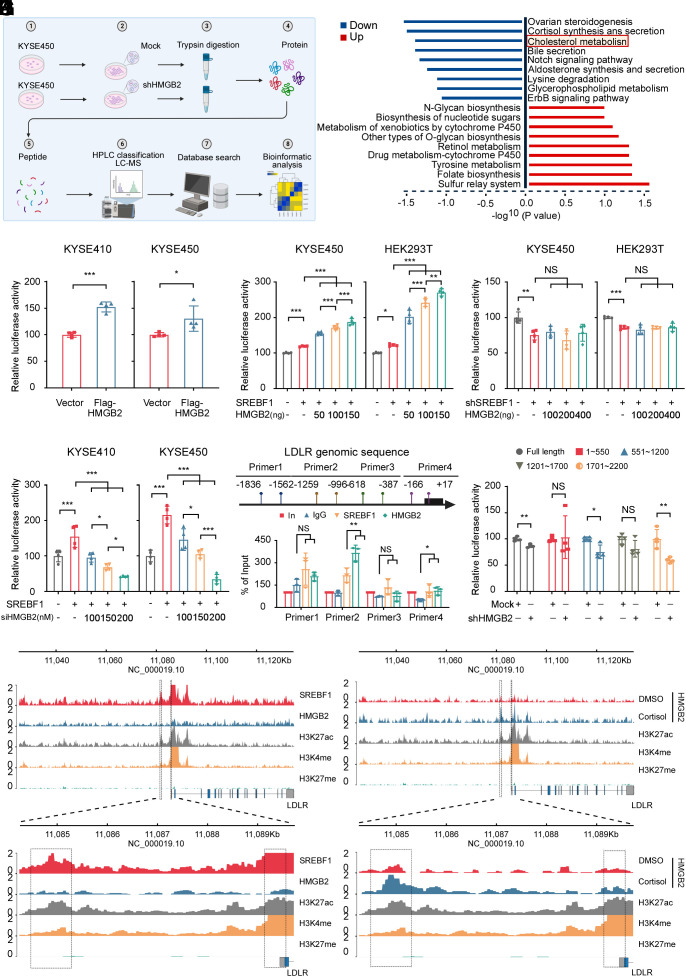
To investigate the mechanism by which HMGB2 promotes ESCC proliferation, we conducted proteomics to identify potential downstream targets of HMGB2 (Fig. 4A). Analysis of KEGG enrichment plots indicated that the cholesterol metabolism pathway exhibited a marked change (Fig. 4B). Subsequently, the transcriptome sequencing results showed that LDLR significantly decreased among the genes of the cholesterol metabolism pathway (SI Appendix, Fig. S8A). Both mRNA and protein levels of LDLR were reduced considerably in HMGB2-depleted KYSE450 cells compared to the mock group, these results validated the accuracy of the sequencing (SI Appendix, Fig. S8 B and C). Moreover, IHC staining revealed that chronic stress facilitated LDLR expression in WT mice but not in Hmgb2 KO mice (SI Appendix, Fig. S8 D and E). Besides, western blot experiments also revealed that chronic stress increased HMGB2 and LDLR protein levels (SI Appendix, Fig. S8F). We subsequently identified that knockdown of LDLR markedly inhibited ESCC cell proliferation in vitro (SI Appendix, Fig. S8 G–I). shLDLR KYSE450 CDX results showed the tumor weight and tumor volume were significantly decreased in the LDLR knockdown groups compared to the mock control (SI Appendix, Fig. S8 J–L). The above results indicated that LDLR could promote ESCC cell proliferation.
Fig. 4.
HMGB2 promotes ESCC proliferation through the cholesterol metabolism pathway. (A) Schematic illustrating the flow of the proteomic sequencing experiments using mock and shHMGB2 in KYSE450 cells. The figure was created with BioRender.com. (B) Differentially enriched protein list detected from the proteomic sequencing of shHMGB2 vs Mock KYSE450 cells. (C) Relative LDLR luciferase promoter activity in KYSE410 and KYSE450 cells overexpressing of HMGB2. Dual-luciferase was detected at 48 h after transfection. (n = 4/group). (D and E) Dual-luciferase reporter assay was used to measure the relative LDLR luciferase promoter activity in KYSE450 and HEK293T cells overexpressing (D) or depleted of SREBF1 (E) 48 h after cotransfecting the LDLR promoter-reporter plasmid and different concentrations of HMGB2. (n = 4/group). (F) Dual-luciferase reporter assay was used to measure the relative LDLR luciferase promoter activity in KYSE410 and KYSE450 cells overexpressing SREBF1 after cotransfecting the LDLR promoter-reporter and siHMGB2 plasmid 48 h. (n = 4/group). (G) Schematic illustrating the designed primers used to assess the enrichment of SREBF1 and HMGB2 at the LDLR promoter. The figure was created with BioRender.com. (H) The gray value analysis of SREBF1 and HMGB2 enrichment at the LDLR promoter was measured in KYSE450 cells using the CHIP assay. (n = 3/group). (I) Dual-luciferase reporter assays of KYSE450 cells transfected with LDLR truncated promoters or full length. (n = 4/group). (J) Gene tracks illustrating SREBF1, HMGB2, H3K27ac, H3K4me, and H3K27me enrichment at the LDLR promoter locus in KYSE450 cells based on CUT&TAG analysis. (K) Gene tracks illustrating HMGB2, H3K27ac, H3K4me, and H3K27me enrichment at the LDLR promoter locus in KYSE450 cells based on CUT&TAG analysis after treatment with DMSO or 10 μM cortisol for 18 h. *P < 0.05, **P < 0.01, ***P < 0.001, NS = no significance.
We next checked the ESCC cells’ cholesterol metabolism change after cortisol treatment. The results indicated that cortisol could enhance cholesterol uptake, total cholesterol, and cholesteryl ester levels in ESCC cells without affecting the free cholesterol level (SI Appendix, Fig. S9 A–D). However, after knocking down HMGB2, there was a significant decrease in cholesterol metabolism in ESCC cells, also there was no significant change observed in free cholesterol (SI Appendix, Fig. S9 E–H), whereas cortisol treatment did not affect cholesterol metabolism in HMGB2-depleted ESCC cells (SI Appendix, Fig. S9 I–L). These data suggest that chronic stress affects cholesterol metabolism through HMGB2. Next, we further verified whether cortisol affects cholesterol metabolism through GCR. The cholesterol metabolism in ESCC cells notably decreased after GCR knockdown, with no significant alteration in free cholesterol (SI Appendix, Fig. S9 M–P). Also, cortisol failed to cause cholesterol metabolism change in NR3C1-depleted ESCC cells (SI Appendix, Fig. S9 Q–T). To this end, we hereafter may suggest chronic stress regulates HMGB2 transcription through the release of cortisol and activation of GCR, leading to abnormal cholesterol metabolism.
To further validate the impact of HMGB2 on LDLR transcription, we designed dual-luciferase reporter assays and the results showed that the overexpression of HMGB2 significantly increased luciferase activity, and vice versa (Fig. 4C and SI Appendix, Fig. S10A). The LDLR transcript level was also markedly decreased in NR3C1-depleted ESCC cells (SI Appendix, Fig. S10B). Based on the results above, we speculated that cortisol and GCR also affect the transcription of LDLR. The dual-luciferase reporter assay results indicated that overexpression of NR3C1 substantially increased luciferase activity, while knockdown of NR3C1 markedly reduced luciferase activity (SI Appendix, Fig. S10 C and D). We also found cortisol did not enhance LDLR transcription in NR3C1-depleted ESCC cell lines compared to the mock group (SI Appendix, Fig. S10E). The results confirmed that GCR promotes the transcription of LDLR in response to cortisol. To determine whether GCR directly or indirectly through HMGB2 promotes LDLR transcription, we designed primers within the 2,000 bp region upstream of the LDLR promoter to conduct CHIP experiments. The results indicated that GCR cannot be enriched at the LDLR promoter region (SI Appendix, Fig. S10 F–H). The results suggested that GCR may promote the transcription of LDLR through HMGB2.
As HMGB2 is a transcription coactivator that requires binding with transcription factors to promote gene transcription, we selected two transcription factors based on Genecards database predictions and literature (21, 22), The SREBF (Sterol Regulatory Element Binding Transcription Factor) family, consisting of three mammalian isoforms (SREBF1a, SREBF1c, and SREBF2), plays a crucial role in regulating cholesterol metabolism (23, 24). Immunoprecipitation assays were performed showing that endogenous HMGB2 can precipitate endogenous SREBF1, and vice versa, but not SREBF2 in KYSE450 cells (SI Appendix, Fig. S10 I and J). Besides, Immunofluorescence assays verified after administering 10 μM cortisol to KYSE450 cells that the SREBF1 was mainly located from the cytoplasm to the nucleus and colocalized in the nucleus with HMGB2 (SI Appendix, Fig. S10K).
To confirm that HMGB2 promotes LDLR transcription through binding with SREBF1, we performed dual-luciferase reporter assays and the results demonstrated that HMGB2 could dose-dependently enhance or decrease the luciferase activity in SREBF1 overexpressed cells, but not in the SREBF1-knockdown cells (Fig. 4 D–F). Moreover, cortisol treatment of cells overexpressing SREBF1 significantly enhanced luciferase activity, but not in SREBF1-depleted ESCC cells (SI Appendix, Fig. S10 L and M). Subsequently, CHIP experiments were conducted to demonstrate that HMGB2 and SREBF1 were coenriched at the LDLR gene promoter region (Fig. 4 G and H and SI Appendix, Fig. S10N). Then, using a luciferase reporter assay, serial truncated analysis of the LDLR promoter revealed that the 551 to 1,200 bp and 1,701 to 2,200 bp region of the LDLR promoter is required for SREBF1 combined HMGB2-mediated LDLR transactivation (Fig. 4I). CUT&TAG experiments also suggested that SREBF1, HMGB2, H3K27ac, and H3K4me histone marks share a similar occupied region within the 5,000 bp region of the upstream LDLR promoter, as opposed to H3K27me (Fig. 4J). Upon treating KYSE450 cells with cortisol, the peak was increased compared to the control cells (Fig. 4K) which indicated that binding with SREBF1 and treating cells with cortisol increased the regulatory effect of HMGB2 on LDLR transcription. Subsequently, we depleted SREBF1 in ESCC cells and assessed cholesterol-related parameters. The study indicated that cholesterol metabolism notably decreased after silencing SREBF1, with no significant alteration in free cholesterol (SI Appendix, Fig. S11 A–D). Also, cortisol treatment did not affect cholesterol metabolism in SREBF1-depleted ESCC cells (SI Appendix, Fig. S11 E–H).
In addition to the increased exogenous cholesterol uptake, enhanced cholesterol biosynthesis is also a key mechanism by which cholesterol metabolism promotes cancer progression, and 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) is the rate-limiting enzyme in this process (13). We checked the change in the cholesterol biosynthesis pathway and the study displayed that neither the mRNA nor the protein levels of HMGCR were changed when ESCC cell lines were treated with cortisol (SI Appendix, Fig. S11 I and J). The activity of HMGCR also did not have a significant change in the esophagus from the 4NQO/stress carcinogenesis model (SI Appendix, Fig. S11K).
Overall, the experimental findings demonstrate that cortisol affects cholesterol metabolism through the GCR–HMGB2–LDLR axis but not through the cholesterol biosynthesis pathway, and HMGB2 promotes the transcription of LDLR by binding to SREBF1 at the LDLR promoter region.
Clinical Relevant Indicators in ESCC Patients under Stress.
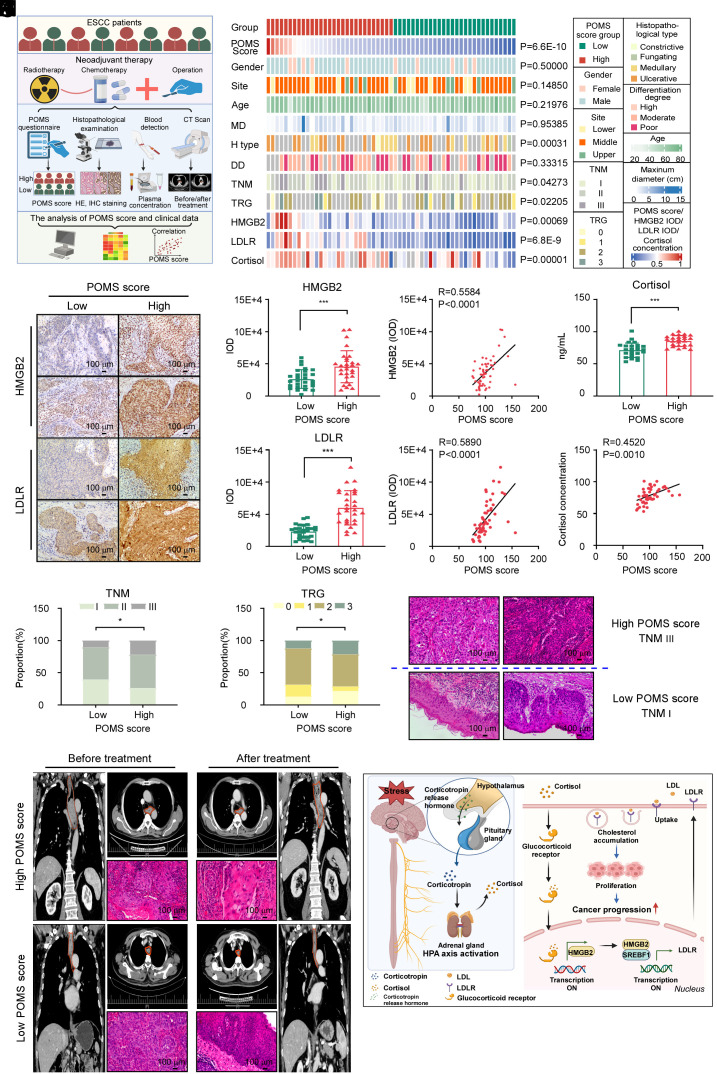
We have observed that chronic stress can promote the occurrence and progression of ESCC, so we aimed to further validate these findings in clinical. First, we conducted a questionnaire using the POMS with 57 ESCC patients and collected their respective clinical information, tumor tissue, and blood samples. The 57 ESCC patients were categorized into two groups based on POMS scores and patients in the high POMS score group had more negative moods, and vice versa. The study analyzed the distribution of gender, tumor site, histopathological type, differentiation degree, TNM stage, TRG classification between the two groups, as well as age, maximum diameter of the tumor, HMGB2 and LDLR expression levels, and cortisol concentration (Fig. 5 A and B). IHC staining suggested that the expression of HMGB2 and LDLR in the high POMS score group was significantly higher than that in the low POMS score group and revealed a positive correlation between HMGB2 expression, LDLR expression, and negative emotional scores (Fig. 5 C–G). Additionally, the analysis showed that ESCC patients with higher POMS scores had higher concentrations of cortisol and there was a positive correlation between cortisol levels and negative emotional scores (Fig. 5 H and I). The plasma 5-HT was dramatically decreased but the concentration of epinephrine and NE were significantly increased in the high POMS score group (SI Appendix, Fig. S12 A–C), and the corticosterone levels displayed no significant change between these two groups (SI Appendix, Fig. S12D). More importantly, compared to the group with lower POMS scores, the group with higher POMS scores harbored a higher proportion of patients in TNM III stage, TRG 3 grade, and ulcerative type (Fig. 5 J and K and SI Appendix, Fig. S12E) and the expression of HMGB2 was positively correlated with the TNM stage. (SI Appendix, Fig. S12F). H&E staining revealed that ESCC patients with high POMS scores exhibited a more severe degree of tumor invasion and differentiation in the TNM III stage than those with low POMS scores in the TNM I stage (Fig. 5L). Comparison of CT and H&E staining before and after neoadjuvant therapy in patients with ESCC at the same TNM II stage revealed that the group with high POMS was significantly insensitive to neoadjuvant therapy, while the group with low POMS was sensitive to neoadjuvant therapy (Fig. 5M). However, there is no statistically significant difference between the POMS low score group and the POMS high score group regarding the distribution of gender, tumor site, age, maximum tumor diameter, differentiation degree, and the positive rate of Ki-67 (SI Appendix, Fig. S12 G–O). These results suggested that the chronic stress–cortisol–HMGB2–LDLR axis may serve as an important marker of prognosis and neoadjuvant treatment efficacy.
Fig. 5.
Clinical relevant markers in ESCC patients experiencing stress. (A) Schematic illustrating the analysis of the clinical patient data. The figure was created with BioRender.com. (B) A heat map illustrating the frequencies of two POMS score groups among 57 ESCC patient samples. Detailed clinical analyses of individual tumor samples were annotated. P values to the right indicate significant nonrandom distributions for each attribute. The chi-square test was used to assess the statistical significance of categorical variables. (C) Representative IHC staining images of HMGB2 and LDLR. (Scale bar, 100 μm or 50 μm). (D and E) The statistical analysis of IHC staining of HMGB2 (D) and LDLR (E) from ESCC patients in the low (n = 28) and high POMS score (n = 29) groups. (F and G) Correlation analysis of the POMS score and the expression of HMGB2 (F) and LDLR (G) of IHC staining from ESCC patients. (n = 57). (H) The analysis of the POMS score and the plasma concentration of cortisol from ESCC patients in the low (n = 25) and high POMS score (n = 25) groups. (I) Correlation analysis of the POMS score and concentration of cortisol from the plasma of ESCC patients. (n = 50). (J) Stacked bar plots illustrating the distribution of TNM stage across low (I = 39.3%, II = 50%, III = 10.7%) and high POMS score (I = 25.9%, II = 51.9%, III = 22.2%). (K) Stacked bar plots illustrating the distribution of TRG classification across low (0 = 12.5%, 1 = 18.8%, 2 = 56.2%, 3 = 12.5%) and high POMS score (0 = 21.43%, 1 = 7.14%, 2 = 50.00%, 3 = 21.42%). *P < 0.05, ***P < 0.001. (L) Representative H&E staining pictures of ESCC patients in the POMS score high in the TNM III stage and low groups in the TNM I stage. (Scale bar, 100 μm or 50 μm). (M) Representative CT scans (coronal plane and horizontal plane) and H&E staining images of two ESCC patients in POMS scores high and POMS scores low group before and after neoadjuvant treatment who have the same TNM II stage. (Scale bar, 100 μm). (N) Graphical abstract of the project. Chronic stress activates the GCR–HMGB2–LDLR axis by releasing cortisol, which can cause abnormal cholesterol metabolism and ultimately promote esophageal carcinogenesis
We previously detected that cortisol could increase the total cholesterol concentration of ESCC cells. We then found that the total cholesterol content was higher in the tumor tissue of ESCC patients with a high POMS score (SI Appendix, Fig. S12P). Additionally, the results indicated that chronic stress could enhance the total cholesterol content in ESCC tissues in two PDX cases; similar to previous findings, free cholesterol levels remained unchanged (SI Appendix, Fig. S12 Q–T). Nevertheless, experimental results indicated that there were no significant differences in plasma total cholesterol and free cholesterol of ESCC patients in comparison to healthy individuals (SI Appendix, Fig. S12 U and V).
We further consider whether the chronic stress–cortisol–HMGB2–LDLR axis could be applied to other types of malignancy. We conducted the questionnaire on 16 colorectal and 15 lung cancer patients. IHC staining suggested that there was no difference and correlation between HMGB2, LDLR, and POMS scores in colorectal and lung cancer patients (SI Appendix, Fig. S13 A–J). What’s more, the studies also showed that neither the mRNA nor the protein levels of LDLR were changed when HMGB2 was knocked down (SI Appendix, Fig. S13 K–N). Besides, the experimental results also displayed that neither the mRNA nor the protein levels of HMGB2 and LDLR were changed when cells were treated with cortisol (SI Appendix, Fig. S13 O–R). Subsequently, we found that there was no significant decrease in total cholesterol in HMGB2-depleted colorectal and lung cancer cells (SI Appendix, Fig. S13 S and T). To summarize, chronic stress did not induce changes in the HMGB2–LDLR axis in other types of cancers.
We conclude that chronic stress promotes the occurrence and development of ESCC by influencing cholesterol metabolism, and the cortisol–HMGB2–LDLR–cholesterol axis plays a positive role in chronic stress–stimulating ESCC tumorigenesis and growth (Fig. 5N).
Discussion
This study unveils cellular and molecular findings connecting chronic stress to promoting ESCC. Initially, we provide important insights into the role of chronic stress in promoting esophageal carcinogenesis. 4NQO is a water-soluble quinoline derivative used to establish an animal model of ESCC in mice and produces similar histological and molecular changes as those seen in human ESCC (25). It has been reported that HMGB2 binds to damaged DNA leading to nucleosome unfolding and subsequent DNA repair (26, 27). We used proteomics to screen differential proteins and selected HMGB2 as a target gene. We found that only stress treatment increased HMGB2 protein expression compared with the vehicle group. Therefore, the cortisol-GCR-HMGB2–SREBF1–LDLR axis was not specific to the presence of DNA damage.
When challenged with chronic stress, the HPA axis and SNS are activated and then glucocorticoid (cortisol, corticosterone), epinephrine, and norepinephrine are released (4) and glucocorticoid is the most representative of these and also as evidenced in clinical ESCC patients. The analysis indicated that ESCC patients with elevated POMS scores exhibited increased cortisol concentrations and a positive association was observed between cortisol levels and negative emotional scores. Yang et al also demonstrated that plasma corticosterone levels were significantly higher in mice experiencing long-term anxiety and/or depression compared to unstressed controls (18). Cui reported that compared to the control group, the stress-induced group exhibited significantly elevated serum levels of epinephrine, and norepinephrine (28). Short-term steroids exert anti-inflammatory effects throughout the body and are often prescribed to treat chronic inflammatory conditions (29). The study reported that increasing active glucocorticoid signaling in CD8+ T cells affects responses to immunotherapy, which reduces immune checkpoint blockade efficacy. Besides conditional deletion of the glucocorticoid receptor could inhibit tumor growth (30). This study observed that cortisol significantly promoted ESCC cell growth in vitro and in vivo. Our study demonstrates a strong link between chronic stress and ESCC.
Second, we found that chronic stress promotes ESCC by causing abnormal cholesterol metabolism. As a hallmark of cancer, increased cholesterol intake supports membrane biogenesis and other functional needs of the fast proliferation cells (13, 14, 31, 32). Cholesterol-derived metabolites have been found to play complex roles in supporting cancer progression and suppressing antitumor immunity (33). Therefore, targeting cholesterol metabolism in cancer therapy has attracted widespread attention and presents a promising therapeutic opportunity. Our study suggested HMGB2 is an oncogene in ESCC and also many other researchers have reported that HMGB2 could promote the progression of cancers (34–37). Our research indicated that LDLR is a downstream target of HMGB2, which could bind to the transcription factors of LDLR, promoting LDLR transcription, increasing cholesterol uptake, leading to abnormal cholesterol metabolism, and facilitating the development of tumors. In our experiments, we observed that cortisol increases cholesterol uptake and the levels of total cholesterol and cholesterol esters in ESCC cells. The findings give insights into the important role of chronic stress inducing esophageal carcinogenesis through abnormal cholesterol metabolism and lay the foundation for future cancer therapy strategies. However, when we examined cholesterol levels in the blood of depressed ESCC patients, we found that there was no significant change. This suggests that chronic stress may only increase the cholesterol uptake capacity of tumor cells, with no significant effect on plasma cholesterol metabolism.
Most importantly, it is established that prolonged stress conditions are theorized to contribute to an increased susceptibility to cancer or the facilitation of tumor development (28, 38, 39). In our study, it was observed that individuals diagnosed with ESCC and presenting higher POMS scores, indicating a more pronounced experience of negative emotions during the assessment, tended to exhibit an advanced clinical stage of ESCC. Neoadjuvant therapy is now commonly used in clinical practice due to its ability to increase patient outcomes and reduce the likelihood of cancer recurrence. Patients with ESCC also benefit from neoadjuvant therapy, but there are still a large number of patients who are resistant to or ineffective in neoadjuvant therapy, which greatly hinders the clinical application of neoadjuvant therapy (40, 41). POMS scores were evaluated on patients undergoing neoadjuvant therapy for ESCC, and patients with high POMS scores had correspondingly poorer tumor regression. This implies that emotional distress, as assessed by POMS scores, played a crucial role as an independent factor influencing both the clinical stage and prognosis of ESCC patients. The findings underscore the importance of recognizing and addressing chronic stress in the overall evaluation and care of individuals grappling with ESCC and chronic stress management and intervention as an important strategy for ESCC prevention and treatment. Some clinical medications can interfere with the cortisol–GCR–HMGB2–LDLR axis. Ketoconazole, the first-line cortisol inhibitor has the antiproliferative and proapoptotic effect in human cancer cells (42). Mifepristone is a glucocorticoid receptor (GCR) antagonist that could inhibit ovarian cancer cell growth in vitro and in vivo (43). However, whether these drugs can be used to treat ESCC patients under stress still requires further experimental research and clinical validation.
Our findings indicate that the cortisol–HMGB2–LDLR–cholesterol axis is crucial in promoting ESCC progression under chronic stress. Control chronic stress could serve as a potent and previously unrecognized means to improve the treatment success of ESCC.
Materials and Methods
All mouse studies were approved by the Ethics Committee of the China-US (Henan) Hormel Cancer Institute. Clinical investigation of cancer patients and healthy volunteers was approved by the Affiliated Cancer Hospital of Zhengzhou University Research Ethics Committee (Zhengzhou, Henan, China; No. 2021-KY-0239-001) as well as the use of human blood and paraffin-embedded esophagus samples. All participants who participated in the study provided their informed consent. 4NQO carcinogenesis model, CDX model, PDX model, H&E and IHC staining, shRNA knockdown, western blot, MTT, soft agar colony formation assay, crystal violet staining assay, immunofluorescence, qRT-PCR, and CHIP assay were done largely as previously described (25, 44–46). Additional details of the experimental procedures are provided in SI Appendix, Materials and Methods. Graphs were designed using GraphPad Prism. Statistical analyses were conducted with SPSS.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 82073075, 81872335, 82203290); the Key projects jointly built by Henan Province and the Ministry of Finance (No. SBGJ202102068, No. SBGJ202102071, No. 242102311187, No. 242300420096); Project of Zhongyuan Scholar Talent (No. 234000510008) and Major Science and Technology Projects in Henan Province (No. 221100310100).
Author contributions
S.Z. and Z.D. designed research; T.W., X.W., M.Y., R.B., Y.Z., Z.Z., F.L., R.W., X.S., L.J., K.L., X.L., and G.J. performed research; T.W., K.W., R.B., Y.Z., and R.W. analyzed data; and T.W., S.Z., and Z.D. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Simin Zhao, Email: smzhao@hci-cn.org.
Zigang Dong, Email: dongzg@zzu.edu.cn.
Data, Materials, and Software Availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (https://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD059578 (47) and PXD059616 (48). The CUT&TAG data generated for this study have been deposited in the GEO database (GSE286200) (49). The RNA-seq data generated for this study have been deposited in the GEO database (GSE286336) (50). All other data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Smyth E. C., et al. , Oesophageal cancer. Nat. Rev. Dis. Primers 3, 17048 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang C. S., Chen X. L., Research on esophageal cancer: With personal perspectives from studies in China and Kenya. Int. J. Cancer 149, 264–276 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H., et al. , Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Dai S., et al. , Chronic stress promotes cancer development. Front. Oncol. 10, 1492 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassamal S., Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 14, 1130989 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goreis A., et al. , Physiological stress reactivity and self-harm: A meta-analysis. Psychoneuroendocrinology 158, 106406 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Russell G., Lightman S., The human stress response. Nat. Rev. Endocrinol. 15, 525–534 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Yuan A., Wang S., Li Z., Huang C., Psychological aspect of cancer: From stressor to cancer progression. Exp. Ther. Med. 1, 13–18 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang T., et al. , Work stress and the risk of cancer: A meta-analysis of observational studies. Int. J. Cancer 144, 2390–2400 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Ip C. K., Herzog H., Zhang L., Chronic stress and energy homoeostasis. Aging 11, 9963–9964 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Kooij M. A., The impact of chronic stress on energy metabolism. Mol. Cell. Neurosci. 107, 103525 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Beloribi-Djefaflia S., Vasseur S., Guillaumond F., Lipid metabolic reprogramming in cancer cells. Oncogenesis 5, e189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang B., Song B. L., Xu C., Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2, 132–141 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Xu H., Zhou S., Tang Q., Xia H., Bi F., Cholesterol metabolism: New functions and therapeutic approaches in cancer. Biochim. Biophys. Acta Rev. Cancer 1874, 188394 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Guillaumond F., et al. , Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. U. S. A. 112, 2473–2478 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng H., Zhou T., Mo X., Liu C., Yin Y., Low-density lipoprotein promotes lymphatic metastasis of esophageal squamous cell carcinoma and is an adverse prognostic factor. Oncol. Lett. 17, 1053–1061 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadel L., et al. , Modulating glucocorticoid receptor actions in physiology and pathology: Insights from coregulators. Pharmacol. Ther. 251, 108531 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang H., et al. , Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat. Med. 25, 1428–1441 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Lightman S. L., Birnie M. T., Conway-Campbell B. L., Dynamics of ACTH and cortisol secretion and implications for disease. Endocr. Rev. 41, bnaa002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meijer O. C., Koorneef L. L., Kroon J., Glucocorticoid receptor modulators. Ann. Endocrinol. 79, 107–111 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Pusterla T., de Marchis F., Palumbo R., Bianchi M. E., High mobility group B2 is secreted by myeloid cells and has mitogenic and chemoattractant activities similar to high mobility group B1. Autoimmunity 42, 308–310 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Starkova T., Polyanichko A., Tomilin A. N., Chikhirzhina E., Structure and functions of HMGB2 protein. Int. J. Mol. Sci. 24, 8334 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao W., Espenshade P. J., Expanding roles for SREBP in metabolism. Cell Metab. 16, 414–419 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis C. A., et al. , SREBP maintains lipid biosynthesis and viability of cancer cells under lipid- and oxygen-deprived conditions and defines a gene signature associated with poor survival in glioblastoma multiforme. Oncogene 34, 5128–5140 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Zhou L., et al. , DUSP4 promotes esophageal squamous cell carcinoma progression by dephosphorylating HSP90β. Cell Rep. 42, 112445 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Hock R., Furusawa T., Ueda T., Bustin M., HMG chromosomal proteins in development and disease. Trends Cell Biol. 17, 72–79 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starkova T. Y., et al. , Structural characteristics of high-mobility group proteins HMGB1 and HMGB2 and their interaction with DNA. Int. J. Mol. Sci. 24, 3577 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui B., et al. , Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J. Clin. Invest. 129, 1030–1046 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta R., et al. , Short-term adverse effects of early subclinical allograft inflammation in kidney transplant recipients with a rapid steroid withdrawal protocol. Am. J. Transplant. 18, 1710–1717 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Acharya N., et al. , Endogenous glucocorticoid signaling regulates CD8(+) T cell differentiation and development of dysfunction in the tumor microenvironment. Immunity 53, 658–671 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopecka J., Godel M., Riganti C., Cholesterol metabolism: At the cross road between cancer cells and immune environment. Int. J. Biochem. Cell Biol. 129, 105876 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Kuzu O. F., Noory M. A., Robertson G. P., The role of cholesterol in cancer. Cancer Res. 76, 2063–2070 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma X., et al. , Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 30, 143–156.e145 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang P., Lu Y., Gao S., High-mobility group box 2 promoted proliferation of cervical cancer cells by activating AKT signaling pathway. J. Cell. Biochem. 120, 17345–17353 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Neubert E. N., et al. , HMGB2 regulates the differentiation and stemness of exhausted CD8(+) T cells during chronic viral infection and cancer. Nat. Commun. 14, 5631 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui G., Cai F., Ding Z., Gao L., HMGB2 promotes the malignancy of human gastric cancer and indicates poor survival outcome. Hum. Pathol. 84, 133–141 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Lu K., et al. , HMGB2 upregulation promotes the progression of hepatocellular carcinoma cells through the activation of ZEB1/vimentin axis. J. Gastrointest. Oncol. 14, 2178–2191 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saul A. N., et al. , Chronic stress and susceptibility to skin cancer. J. Natl. Cancer Inst. 97, 1760–1767 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X., et al. , Chronic stress promotes gastric cancer progression and metastasis: An essential role for ADRB2. Cell Death Dis. 10, 788 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang H., et al. , Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: The NEOCRTEC5010 randomized clinical trial. JAMA Surg. 156, 721–729 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eyck B. M., et al. , Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: The randomized controlled CROSS trial. J. Clin. Oncol. 39, 1995–2004 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Mahler C., Verhelst J., Denis L., Ketoconazole and liarozole in the treatment of advanced prostatic cancer. Cancer 71, 1068–1073 (1993). [DOI] [PubMed] [Google Scholar]
- 43.Goyeneche A. A., Carón R. W., Telleria C. M., Mifepristone inhibits ovarian cancer cell growth in vitro and in vivo. Clin. Cancer Res. 13, 3370–3379 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Q., et al. , BRD4 drives esophageal squamous cell carcinoma growth by promoting RCC2 expression. Oncogene 41, 347–360 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Bondi C. O., Rodriguez G., Gould G. G., Frazer A., Morilak D. A., Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33, 320–331 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Jung Y. H., et al. , Strain differences in the chronic mild stress animal model of depression and anxiety in mice. Biomol. Ther. 22, 453–459 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang T., et al. , Data from “Chronic stress-induced cholesterol metabolism abnormalities promote ESCC tumorigenesis and predict neoadjuvant therapy response.” iProX. http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD059578. Deposited 9 January 2025. [DOI] [PMC free article] [PubMed]
- 48.Wang T., et al. , Data from “HMGB2 promote ESCC tumorigenesis and predict neoadjuvant therapy response.” iProX. http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD059616. Deposited 10 January 2025. [DOI] [PMC free article] [PubMed]
- 49.Wang T., et al. , Data from “Chronic stress-induced cholesterol metabolism abnormalities promote ESCC tumorigenesis and predict neoadjuvant therapy response.” GEO. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE286200. Deposited 9 January 2025. [DOI] [PMC free article] [PubMed]
- 50.Wang T., et al. , Data from “Chronic stress-induced cholesterol metabolism abnormalities promote ESCC tumorigenesis and predict neoadjuvant therapy response.” GEO. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE286336. Deposited 10 January 2025. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (https://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD059578 (47) and PXD059616 (48). The CUT&TAG data generated for this study have been deposited in the GEO database (GSE286200) (49). The RNA-seq data generated for this study have been deposited in the GEO database (GSE286336) (50). All other data are included in the article and/or SI Appendix.