Abstract
Objective
Globally, the occurrence of Metabolic dysfunction-associated steatotic liver disease (MASLD) is on a steady rise. Fish oil has anti-inflammatory effects and can improve lipid metabolism. The article aims to assess the impact of fish oil supplementation on MASLD.
Methods
We conducted a systematic search of Cochrane, Embase, PubMed, and Web of Science up to September 31, 2024, for randomized control trials (RCTs). The risk of bias of the included RCTs was evaluated using the Cochrane Collaboration’s tool. Outcomes measured were aspects of liver injury, lipid profile, insulin resistance, anthropometric measurements, and more.
Results
Seven randomized controlled trials (RCTs) involving 439 participants were incorporated into the analysis. In general, the risk of bias in these RCTs was either low or not clearly defined. Pooled analysis showed that triglycerides [TG, pooled standard mean difference (SMD): −0.40 (95% CI: −0.58 to −0.21)], aspartate transaminase [AST, SMD: −0.29 (95% CI: −0.48 to −0.10)], HOMA-IR [SMD: −2.06 (95% CI: −3.36 to −0.49)] and waist circumference [Waist-C, SMD: −0.31 (95% CI: −0.54 to −0.08)] were significantly improved. But showed no significant benefits on alanine transaminase [ALT, SMD: −0.15 (95% CI: −0.45 to 0.15)], gamma-glutamyl transpeptidase [GGT, SMD: −0.07 (95% CI: −0.26 to 0.12)], body mass index [BMI, SMD: 0.16 (95% CI: −0.34 to 0.02)], high-density lipoprotein cholesterol [HDL, SMD: 0.02 (95% CI: −0.18 to 0.22)], low-density lipoprotein cholesterol [LDL, SMD: −0.01 (95% CI: −0.20 to 0.18)], Total Cholesterol [TC, SMD: −0.34 (95% CI: −0.70 to 0.01)] and so on.
Conclusion
The current evidence supports the fish oil supplementation in improving MASLD. Fish oil supplementation may also regulate blood lipids and improve glucose metabolism disorders.
Systematic review registration
https://www.crd.york.ac.uk/PROSPERO/#myprospero, identifier CRD42024513246.
Keywords: MASLD, fish oil, meta-analysis, NAFLD, NASH
1. Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) represents a significant chronic liver condition, spanning various clinical and pathological manifestations such as fatty degeneration, steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma (1). Up to 30% of adults were affected by MASLD in Western, which implied the obesity epidemic (2). Due to shifting lifestyles and dietary habits, the prevalence of MASLD in China has surged to 25%. Despite being asymptomatic in the early stages, MASLD is positively correlated with the risks of cardiovascular disease (CVDs) and type 2 diabetes (T2DM), as evidenced by substantial evidence (3, 4). Calorie restriction and exercise remain the primary treatments for reducing visceral obesity and liver steatosis (5). Evidence from randomized controlled trials clearly indicates that most patients find it difficult to maintain weight loss (6). Tofogliflozin, Sitagliptin, Semaglutide, Pioglitazone, and Ursodeoxycholic Acid have demonstrated efficacy in improving inflammation, insulin resistance, liver function, and histological features of MASLD (7). Treatment of MASLD remains challenging for the scientific community despite numerous clinical trials, with no approved treatments currently available.
Docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA) are constituents of fish oil, categorized as Omega-3 polyunsaturated fatty acids (n-3 PUFAs). These compounds have demonstrated efficacy in treating cardiovascular disease (CVD) by reducing triglycerides and regulating inflammation (8). ω-3 PUFAs may additionally enhance the observed decrease in total body fat during weight loss induced by diet (9). Studies on total parenteral nutrition have confirmed that prolonged dietary deficiency in ω-3 PUFAs can result in liver steatosis (10–12). Evidence from animal (10, 13) and human (14) studies suggests that ω-3 PUFA dietary supplements may prevent MASLD or reduce liver fat, independent of weight loss (10, 13, 14). ω-3 PUFAs effectively reduce abnormal triglyceride (TAG) levels (15–17). Lipidomics studies have revealed a significant correlation between a high liver N-6:N-3 ratio and MASLD severity (18). Several studies indicate that incorporating n-3 PUFAs into a low-fat diet can decrease steatosis and enhance liver enzymes and metabolic parameters (14, 19). Studies have demonstrated that MASLD patients exhibit significantly elevated levels of n-3 PUFAs, particularly DHA, in their blood compared to healthy subjects. Fish oil supplementation has been found to significantly improve liver function and lipid metabolism in MASLD patients (1, 20).
Numerous clinical trials and studies are investigating the effectiveness of fish oil supplementation in treating MASLD. Nevertheless, the most recent clinical data has not been included in meta-analyses for data aggregation, leading to insufficient evidence-based medicine to support this intervention. Therefore, a meta-analysis was conducted on the supplementation of fish oil in patients with MASLD. Our aim is to evaluate the effects of fish oil on liver injury, lipid profile, insulin resistance, anthropometric measurements, and other relevant parameters.
2. Materials and methods
2.1. Data sources and literature search strategy
This evidence-based analysis followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) 2020 statement (21) and was prospectively registered in PROSPERO (CRD42024513246). We systematically searched the Cochrane, Embase, PubMed, and Web of Science databases up to 31 September, 2024. The search keywords: fish oils, Metabolic dysfunction-associated steatotic liver disease. The search strategy was ((“Fish Oils”[Mesh]) OR ((((((Oils, Fish) OR (Fish Oil)) OR (Oil, Fish)) OR (Fish Liver Oils)) OR (Liver Oils, Fish)) OR (Oils, Fish Liver))) AND ((“Non-alcoholic Fatty Liver Disease”[Mesh]) OR (((((((((((((Non alcoholic Fatty Liver Disease) OR (MASLD)) OR (Nonalcoholic Fatty Liver Disease)) OR (Fatty Liver, Nonalcoholic)) OR (Fatty Livers, Nonalcoholic)) OR (Liver, Nonalcoholic Fatty)) OR (Livers, Nonalcoholic Fatty)) OR (Nonalcoholic Fatty Liver)) OR (Nonalcoholic Fatty Livers)) OR (Nonalcoholic Steatohepatitis)) OR (Nonalcoholic Steatohepatitides)) OR (Steatohepatitides, Nonalcoholic)) OR (Steatohepatitis, Nonalcoholic))). The search results from four databases are presented in Supplementary Table S1. This trial had no language or geographical restrictions.
2.2. Study selection
Inclusion criteria were as follows: (1) The study design is RCT; (2) Participants diagnosed with MASLD; (3) Intervention group received fish oil supplementation, while the control group received placebo or other treatments. Title and abstract screening for eligibility was independently conducted by two authors (L.K.Z and D.M.S). Disagreements were resolved by consulting a senior author (H.Q.B). Reviews, letters, editorial comments, case reports, conference abstracts, non-human studies, unpublished articles, those with incomplete data, and non-English articles were excluded.
2.3. Data extraction
Two authors (L.K.Z and D.M.S) independently screened literature and extracted data from the included trials, including the first author’s last name, number of participants, publication year, country, and outcome data for both intervention and control groups. Any disagreements were resolved by a third investigator (H.Q.B.) for a final decision. The outcomes focused on were: (1) biochemical markers, including serum markers of liver injury (ALT, AST, GGT and CK18-M30) and lipid profiles (TC, TG, HDL and LDL), adiponectin, and UA; (2) insulin, HOMA-IR and FBS; and (3) anthropometric parameters, such as obesity estimated by BMI, waist circumference, hip circumference, and WHR.
2.4. Risk of bias assessment
Two authors (L.K.Z and D.M.S) used tool (22) to assess the risk of bias in the included studies, with the third author (H.Q.B) responsible for confirming the judgment results. RCTs were assessed for high, low, or unclear risk of bias in six domains: randomization method, allocation concealment, blinding, completeness of results data, selective reporting of results, and other sources of bias. If a study did not provide data, it was rated as having an unclear risk of selective reporting bias (see Figures 1, 2).
Figure 1.
Risk of bias graph of included studies.
Figure 2.

Risk of bias summary of included studies.
2.5. Methodological quality evaluation
The methodological quality assessment was based on JADAD score (23), including the following: sequence generation, allocation concealment, blinding, withdrawals and drop outs, and randomization efficacy. The evaluation process was independently performed by two of the authors (L.K.Z and D.M.S). Disagreement was resolved by third author ((H.Q.B; Supplementary Table S2).
2.6. Statistical analysis
All analyses were conducted using Review Manager software version 5.4 (Nordic Cochrane Center, the Cochrane Collaboration, 2020). We anticipated clinical heterogeneity, so we chose a random-effects model. Dichotomous variables are presented as risk ratios (RR) with their 95% confidence intervals (95% CI). Continuous variables are expressed as standardized mean differences (SMD) with their 95% CI. Statistical heterogeneity between studies was assessed by calculating the I2 statistic. An I2 value greater than 50% was considered to indicate significant heterogeneity (24), a random-effect model was used to estimate the combined SMD when significant heterogeneity was detected (I2 > 50%). Otherwise, the fixed-effect model was applied. A p-value less than 0.05 was considered statistically significant. To assess the impact of individual studies on the pooled outcomes exhibiting notable heterogeneity, we further conducted one-way sensitivity analyses. The existence of publication bias was visually assessed using funnel plots generated in Review Manager 5.3 (Cochrane Collaboration, Oxford, United Kingdom). Additionally, Egger’s regression tests (25) were conducted in Stata 12.0 (Stata Corp, College Station, TX, United States) to further evaluate potential bias in outcomes with at least 10 included studies. A p-value less than 0.05 was deemed statistically significant, indicating the presence of publication bias. Our study conducts subgroup analysis based on the dosage of fish oil and the duration of intervention to explore the stability of the results and potential sources of heterogeneity.
3. Results
3.1. Characteristics of included studies
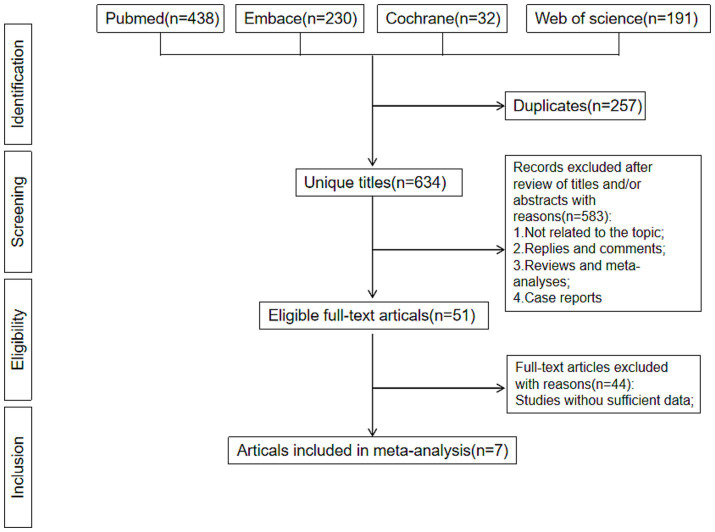
After excluding duplicate literature, 623 literature identified in our research. Figure 3 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart. After screening of titles and abstracts, 616 records were excluded. Seven RCTs, comprising 439 participants, were included after reviewing the full texts. Of these articles, there were 340 males and 99 females, with a minimum sample size of 34 and a maximum sample size of 74, a minimum mean age of 33.6 years and a maximum of 60.88 years, and a minimum mean body mass index of 26.0 and a maximum of 33.3. The study lasted for a minimum of 12 weeks and a maximum of 1 year. The characteristics of the included RCTs (26–32) were presented in Table 1.
Figure 3.
Flowchart of the systematic search and selection process.
Table 1.
Baseline characteristics of include studies.
| Authors | Study period | Country | Study design | Patients (n) | BMI | Age (mean/median) | Male | Time of duration |
|---|---|---|---|---|---|---|---|---|
| Intervention/control | Intervention/control | Intervention/control | Intervention/control | |||||
| Argo, 2015 | 2007-2010 | USA | RCT | 17/17 | 31.6/33.3 | 46.4/47.2 | 6/7 | 1 year |
| Qin, 2015 | 2012-2013 | China | RCT | 36/34 | 26.4/26.0 | 57/55 | 26/25 | 3 months |
| Parker, 2019 | 2011-2013 | Australia | RCT | 25/25 | 27.8/28.0 | 33.6/34.7 | 25/25 | 12 weeks |
| Shojasaadat, 2019 | 2016-2017 | Iran | RCT | 35/34 | 31.48/30.65 | 41.77/42.35 | 18/20 | 12 weeks |
| Song, 2020-a | 2018-2019 | China | RCT | 21/21 | 29.43/27.92 | 46/47 | 19/18 | 12 weeks |
| Song, 2020-b | 2018-2019 | China | RCT | 17/21 | 27.80/27.92 | 44/47 | 16/18 | 12 weeks |
| Cansanção, 2020 | 2018-2020 | Brazil | RCT | 13/11 | 30.77/31.82 | 60.54/60.88 | 8/9 | 6 months |
| Guo, 2022-a | 2019-2021 | China | RCT | 37/37 | 27.6/26.7 | 54.7/56.3 | 22/19 | 3 months |
| Guo, 2022-b | 2019-2021 | China | RCT | 37/37 | 26.2/26.7 | 56.6/56.3 | 20/19 | 3 months |
3.2. Effects of fish oil on serum markers of liver injury
The analysis involved data from 7 RCTs for AST (26–32), 6 for ALT and GGT (27–32), and 2 for CK18-M30 (26, 27). Significant statistical heterogeneity was observed among studies for AST (I2 = 62%, p = 0.007) and CK18-M30 (I2 = 88%, p = 0.005). The meta-analysis revealed that compared to the control group, the fish oil group exhibited a significant improvement in AST (SMD: −0.29, 95% CI: −0.48 to −0.10), whereas no significant differences were observed in ALT (SMD: −0.15, 95% CI: −0.45 to 0.15), GGT (SMD: −0.07, 95% CI: −0.26 to 0.12), or CK18-M30 (SMD: −0.74, 95% CI: −1.95 to 0.47; see Figure 4).
Figure 4.
Forest plots of effects of fish oil on serum markers of liver injury: (A) ALT; (B) AST; (C) GGT; (D) CK18-M30.
3.3. Effect of fish oil on serum lipid profiles
The meta-analysis included data from five RCTs for HDL (27, 29–32), six for LDL (26, 27, 29–32) and total cholesterol (TC) (26, 27, 29–32), and seven for triglycerides (TG) (26–32). Significant heterogeneity was observed among studies for total cholesterol (TC: I2 = 69%, p = 0.002). The fish oil group exhibited a significant improvement in triglycerides (SMD: −0.40, 95% CI: −0.58 to −0.21). However, no significant changes were found in HDL (SMD: 0.02, 95% CI: −0.18 to 0.22), LDL (SMD: −0.01, 95% CI: −0.20 to 0.18), or TC (SMD: −0.34, 95% CI: −0.70 to 0.01; see Figure 5).
Figure 5.
Forest plots of effect of fish oil on serum lipid profiles: (A) HDL-c; (B) LDL-c; (C) TG; (D) total cholesterol.
3.4. Effect of fish oil on fasting blood sugar, insulin and homeostatic model assessment for insulin resistance
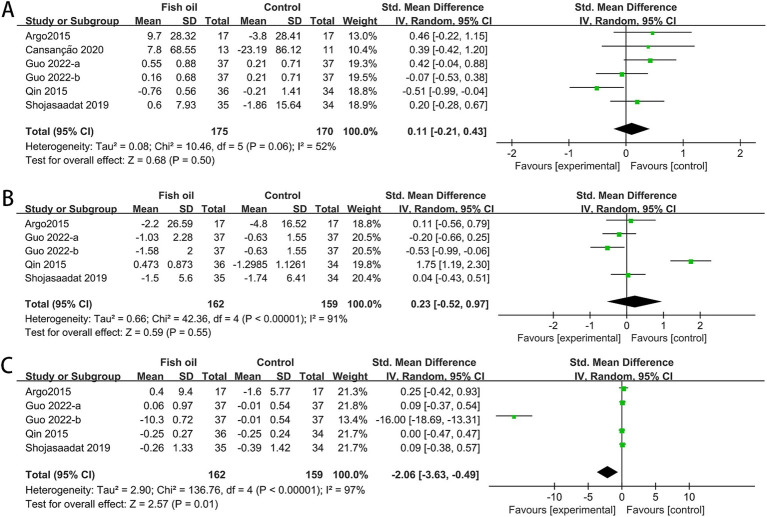
The meta-analysis included data from five for FBS (26, 27, 29, 30, 32), four for insulin and HOMA-IR values (26, 27, 29, 32). Significant heterogeneity was observed among studies for FBS (I2 = 52%, p = 0.06), insulin (I2 = 91%, p < 0.00001), and HOMA-IR (I2 = 97%, p < 0.00001). The meta-analysis showed a significant improvement in HOMA-IR with fish oil supplementation, no significant change in insulin levels, and a significant increase in FBS. The combined SMD for FBS was 0.08 (95% CI: −0.13 to 0.30), the combined SMD for insulin was 0.23 (95% CI, −0.52 to 0.97), and the SMD for HOMA-IR was −2.06 (95% CI, −3.36 to −0.49; see Figure 6).
Figure 6.
Forest plots of effect of fish oil on fasting blood sugar, insulin and homeostatic model assessment for insulin resistance: (A) FBS; (B) insulin; (C) HOMA-IR.
3.5. Effect of fish oil on anthropometric measurements
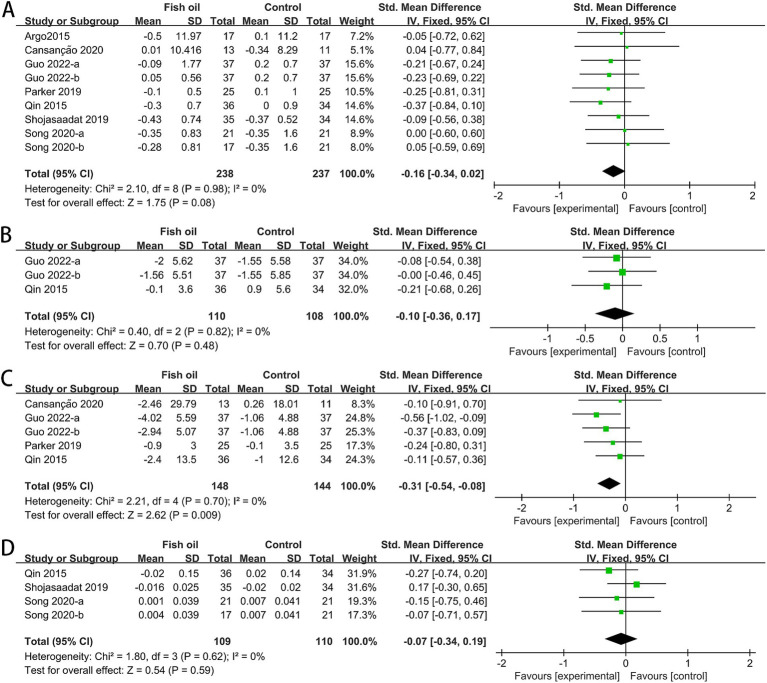
The analysis included data from seven RCTs for BMI (26–32), 2 for hip circumference (Hip-C) (27, 32), four for waist circumference (Waist-C) (27, 28, 30, 32), and three for waist-to-hip ratio (WHR) (27, 29, 31). No statistical heterogeneity was observed among the studies. The meta-analysis revealed a significant improvement in Waist-C (SMD: −0.31, 95% CI: −0.54 to −0.08) in the fish oil group. However, there were no significant improvements in BMI (SMD: 0.16, 95% CI: −0.34 to 0.02), Hip-C (SMD: −0.10, 95% CI: −0.36 to 0.17), or WHR (SMD: −0.07, 95% CI: −0.34 to 0.19; see Figure 7).
Figure 7.
Forest plots of effect of fish oil on anthropometric measurements: (A) BMI; (B) Hip-C; (C) Waist-C; (D) WHR.
3.6. Effect of fish oil on adiponectin, UA and TNF-α
The analysis included data from two RCTs for adiponectin (26, 27), three for uric acid (UA) (27, 31, 32), and three for tumor necrosis factor-alpha (TNF-α) (27, 31, 32). Heterogeneity was observed among studies for adiponectin (I2 = 91%, p = 0.0009) and TNF-α (I2 = 82%, p = 0.0001). The meta-analysis revealed a significant improvement in TNF-α levels in the fish oil group compared to the control group (SMD: −0.76, 95% CI: −1.35 to −0.18). However, there were no significant changes in adiponectin (SMD: 0.70, 95% CI: −0.72 to 2.12) or UA (SMD: −0.14, 95% CI: −0.37 to 0.09; see Figure 8).
Figure 8.
Forest plots of effect of fish oil on adiponectin, UA and TNF-α: (A) adiponectin; (B) UA; (C) TNF-α.
3.7. Publication bias
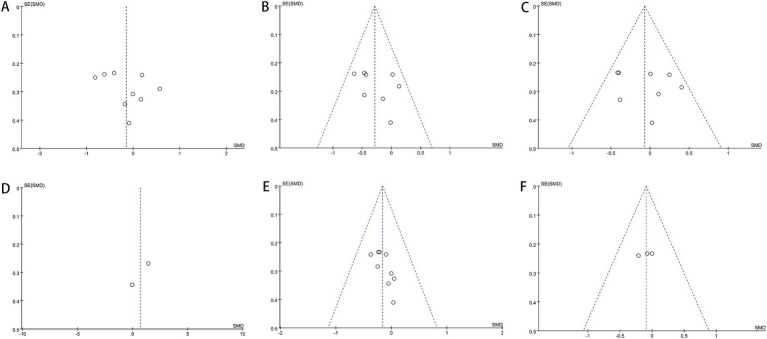
Our investigation found that Egger’s test results indicated no publication bias for certain outcomes: AST (p = 0.302), ALT (p = 0.358), GGT (p = 0.638), HDL (p = 0.452), LDL (p = 0.964), TG (p = 0.387), TC (p = 0.616), Hip-C (p = 0.236), Waist-C (p = 0.475), WHR (p = 0.864), insulin (p = 0.431), FBS (p = 0.449), TNF-α (p = 0.303), and UA (p = 0.051). However, publication bias was suggested by the test for BMI (p = 0.034) and HOMA-IR (p = 0.024). Funnel plots also revealed publication bias in ALT, BMI, FBS, insulin, and HOMA-IR. Figures 9–11 demonstrate the visual assessment of funnel plots.
Figure 9.
Funnel plots of (A) ALT, (B) AST, (C) GGT, (D) adiponectin, (E) BMI and (F) Hip-C.
Figure 11.
Funnel plots of (A) HDL-c, (B) LDL-c, (C) TG, (D) total cholesterol, (E) CK18-M30 and (F) UA.
Figure 10.
Funnel plots of (A) Waist-C, (B) WHR, (C) FBS, (D) insulin, (E) HOMA-IR and (F) TNF-α.
3.8. Sensitivity analysis
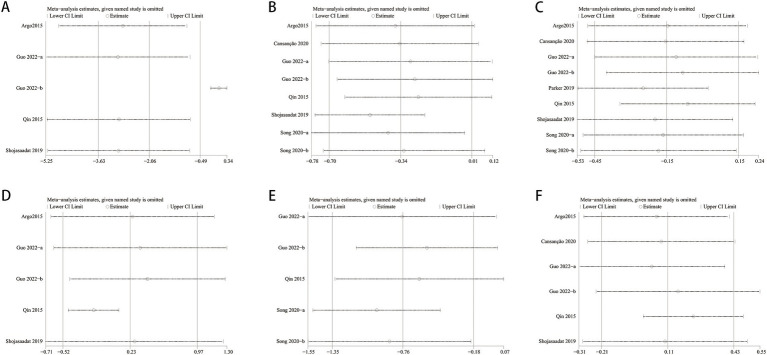
One-way sensitivity analyses were conducted to assess result stability and evaluate heterogeneity’s impact on the study outcomes. ALT, Total Cholesterol, insulin, TNF-α, and HOMA-IR were compared, and each study’s influence on the combined SMD was examined through sequential removal. Sensitivity analyses indicated consistent combined SMDs after excluding individual studies for FBS, ALT, and insulin. Total Cholesterol, TNF-α, and HOMA-IR showed significant fluctuations in the combined SMD, indicating result instability. Exclusion of the Shojasaadat-2019 and Song-2020 studies led to a shift from non-significant to significant findings for Total Cholesterol. Likewise, exclusion of the Qin-2015 and Guo-2022 studies reversed significant findings to non-significant for TNF-α. In terms of HOMA-IR, excluding the Guo-2022 study led to a transition from significant to non-significant outcomes (see Figure 12).
Figure 12.
Sensitivity analysis of (A) HOMA-IR, (B) total cholesterol, (C) ALT, (D) insulin, (E) TNF-a and (F) FBS.
3.9. Subgroup analysis
Subgroup analyses were conducted based on the dosage of fish oil and the intervention duration to explore the stability of the results and potential sources of heterogeneity. For ALT, we found that there was a statistically significant difference when the intervention duration was greater than 12 weeks (p < 0.0001), while there was no statistical significance when it was less than 12 weeks (p = 0.1). The heterogeneity among subgroups decreased (I2 = 0). However, the dose subgroup analysis did not show statistical significance, and heterogeneity decreased in the subgroup less than 2000 mg (I2 = 36%). The subgroup analysis of AST indicated that there was statistical significance when the intervention dose was less than 2000 mg (p = 0.0006) and when the intervention duration was greater than 12 weeks (p = 0.0004); there was no statistical significance when the intervention dose was greater than 2000 mg (p = 0.45) and when the treatment duration was less than 12 weeks (p = 0.56). The subgroup analysis of GGT showed statistical significance when the intervention dose was less than 2000 mg (p = 0.02), but no statistical significance when it was greater than 2000 mg (p = 0.2). The subgroup analysis based on intervention duration did not reveal any statistical significance (see Table 2).
Table 2.
Subgroup analysis.
| Subgroup | ALT | AST | GGT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | SMD [95%CI] | p value | I 2 | Study | SMD [95%CI] | p value | I 2 | Study | SMD [95%CI] | p value | I 2 | |
| Total | 9 | −0.15 [−0.45, 0.15] | 0.33 | 62% | 8 | −0.29 [−0.48, −0.10] | 0.003 | 10% | 8 | −0.07 [−0.26, 0.12] | 0.46 | 30% |
| Dose | ||||||||||||
| ≥2000mg | 5 | −0.06 [−0.57, 0.44] | 0.8 | 73% | 4 | -0.10 [−0.37, 0.17] | 0.45 | 0% | 4 | 0.18 [−0.09, 0.45] | 0.2 | 0% |
| <2000mg | 4 | −0.27 [−0.61, 0.07] | 0.12 | 38% | 4 | −0.46 [−0.72, −0.20] | 0.0006 | 0% | 4 | −0.31 [−0.57, −0.04] | 0.02 | 0% |
| Time of duration | ||||||||||||
| >12 weeks | 5 | −0.50 [−0.74, −0.26] | <0.0001 | 0% | 4 | −0.46 [−0.71, −0.20] | 0.0004 | 0% | 4 | −0.24 [−0.50, 0.01] | 0.06 | 0% |
| ≤12 weeks | 4 | 0.23 [−0.05, 0.51] | 0.1 | 0% | 4 | −0.08 [−0.36, 0.20] | 0.56 | 0% | 4 | 0.14 [−0.14, 0.42] | 0.33 | 16% |
4. Discussion
Metabolic dysfunction-associated steatotic liver disease (MASLD) has become the leading cause of chronic liver disease globally, owing to the rising prevalence of obesity and its related metabolic syndrome, posing a significant public health issue (33). MASLD has a reported prevalence of approximately 30% in Western countries and ranges from 12 to 24% in Asia (34). MASLD comprises a spectrum of liver damage, ranging from simple steatosis to non-alcoholic steatohepatitis, fibrosis, cirrhosis, end-stage liver disease, and occasionally hepatocellular carcinoma. Advanced liver fibrosis is recognized as an independent risk factor for mortality (35). Additionally, MASLD patients are at increased risk of atherosclerosis and cardiovascular disease (CVD), which is the primary cause of death in this population (36). Prior evidence indicates an association among MASLD, insulin resistance, and type 2 diabetes (T2DM) (37, 38). Globally, 37.3% of T2DM patients are affected by MASLD (4). Furthermore, MASLD is associated with an increased risk of extrahepatic cancers, particularly colon cancer, gastric cancer, and certain hormone-related cancers, which pose the highest cancer risks (39). Over the past decade, a growing body of observational studies has revealed an association between MASLD and a heightened prevalence and incidence of chronic kidney disease (CKD) (40, 41). Hence, MASLD poses a considerable public health challenge. Lifestyle modifications remain the mainstay of therapy, proving effective in addressing metabolic syndrome, reducing hepatic fat accumulation, and halting disease progression in individuals. Nonetheless, their implementation and adherence may pose challenges. Various drugs, dietary supplements, and surgical interventions are being investigated and have shown effectiveness in managing MASLD. Our study provides an updated systematic review and meta-analysis of randomized controlled trials (RCTs) assessing the use of fish oil supplements in MASLD treatment.
The analysis results of this article demonstrate significant improvements or trends in liver enzymes, lipid profiles, and body measurements among participants who received fish oil supplementation. However, glycemic metabolism did not show improvement trends in these RCTs. Biochemical data on liver enzymes and metabolic status showed significant improvements in TG, AST, HOMA-IR, and waist circumference. Unlike findings from other meta-analyses, our observations suggest that fish oil supplement intake significantly reduces TNF-α, a crucial pro-inflammatory mediator. This reduction in pro-inflammatory mediators is linked to a decrease in low-grade chronic inflammation, providing favorable outcomes not only for Metabolic dysfunction-associated steatotic liver disease (MASLD) but also for cardiovascular health. Nevertheless, fish oil supplementation did not result in significant benefits for ALT, GGT, FBS, CK18-M30, BMI, HipC, WHR, HDL, LDL, adiponectin, Total Cholesterol, insulin, or UA. Supplementing with fish oil also has an impact on the imaging and biopsy scores of MASLD patients. There have been reports in the literature that after supplementing with fish oil, improvements in steatosis, fibrosis, lobular inflammation, ballooning, and liver fat percentage were confirmed through ultrasound, MR, and liver biopsy (42). However, due to the limited number of relevant studies included in this research, a comprehensive analysis was not possible, therefore, more research is needed in the future.
Variables showing high heterogeneity, including HOMA-IR, Total Cholesterol, ALT, Insulin, and TNF-α, underwent sensitivity analysis. The results indicated instability in HOMA-IR, Total Cholesterol, and TNF-α outcomes, with unclear sources of heterogeneity. Interpretation of these meta-analytical findings should be cautious due to potential confounders. The results of the subgroup analysis on ALT, AST, and GGT show that when the fish oil dosage is less than 2000 mg and the treatment duration is more than 12 weeks, the heterogeneity significantly decreases, indicating that the heterogeneity is mainly related to the dosage and treatment duration. At the same time, statistically, the optimal fish oil treatment dosage may be no more than 2000 mg, and the treatment duration should be more than 12 weeks. However, due to the limited number of studies included in this research, further studies may be needed. The literature included in this study did not analyze the side effects of fish oil, but some articles (43) indicate that the side effects of taking fish oil supplements are minimal and comparable to the control group.
The mechanism by which fish oil effectively alleviates MASLD can be summarized as follows: Fish oil may positively influence cell membrane fluidity. Increased membrane fluidity is positively associated with GLUT4 translocation to the cytoplasm (44, 45). Enhanced membrane fluidity can concurrently reinstate the tyrosine kinase activity of insulin receptor substrates (IRS)-1 and − 2, facilitating insulin signaling transduction (46, 47). Another potential mechanism contributing to the development of MASLD is chronic low-grade inflammation. Hepatic triglyceride accumulation is associated with macrophage recruitment, leading to the synthesis of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. Extensive evidence suggests that fish oil (FO) intervention inhibits the toll-like receptor (TLR)-4 signaling pathway (48, 49). Consequently, pro-inflammatory cytokine production is suppressed. Additionally, fish oil (FO) supplementation may ameliorate MASLD by inhibiting triglyceride (TG) synthesis and promoting TG oxidation. FO modulates various nuclear receptors (PPAR family) and transcription factors (SREBP) responsible for lipid synthesis and metabolism (50, 51). Moreover, polyunsaturated fatty acids (PUFA) regulate transcription factors that control the expression of proteins involved in de novo lipogenesis, such as acetyl-CoA carboxylase (Acc) and fatty acid synthase (Fasn) (52, 53). This process inhibits de novo lipogenesis, which is a major contributor to hepatic steatosis (54).
This study has several limitations. Firstly, heterogeneity among the RCTs and sensitivity analysis indicates instability in some results, with observed publication bias for certain indicators. Secondly, the overall sample size of the seven included RCTs is relatively small, we attempted to conduct a meta-regression, but due to the limited number of studies included, there was a significant imbalance in the results. Thirdly, variations exist in treatment doses, durations, and protocols, although a subgroup analysis was conducted, the results may be uncertain due to the insufficient number of studies included. Further research is necessary to establish the dose-effect relationship of fish oil in treating fatty liver disease. This article represents the first meta-analysis investigating MASLD treatment with fish oil. All included studies are RCTs, providing tightly controlled confounding factors and baseline levels, reflecting a high level of evidence. This analysis highlights the effectiveness of fish oil in treating MASLD, broadening clinical options.
5. Conclusion
Current evidence suggests that fish oil supplementation improves plasma levels of AST, TG, TNF-α, and HOMA-IR, as well as waist circumference in the treatment of MASLD. Further research requires large sample sizes and long-term follow-up in randomized controlled trials to confirm these benefits.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Abbreviations
MASLD, Metabolic dysfunction-associated steatotic liver disease; ALT, plasma alanine transaminase; AST, aspartate transaminase; GGT, gamma-glutamyl transpeptidase; TC, total cholesterol; TG, triglycerides; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; UA, uric acid; HOMA-IR, insulin resistance score; FBS, fasting blood glucose; BMI, body mass index; WHR, hip circumference, and waist-hip ratio; CK18-M30, cytokeratin 18 fragments M30.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LZ: Writing – original draft, Writing – review & editing. DS: Writing – review & editing. HB: Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1524830/full#supplementary-material
References
- 1.Guo XF, Yang B, Tang J, Li D. Fatty acid and non-alcoholic fatty liver disease: meta-analyses of case-control and randomized controlled trials. Clin Nutr. (2018) 37:113–22. doi: 10.1016/j.clnu.2017.01.003, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. (2007) 191:235–40. doi: 10.1016/j.atherosclerosis.2006.08.021, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. (2019) 71:793–801. doi: 10.1016/j.jhep.2019.06.021, PMID: [DOI] [PubMed] [Google Scholar]
- 5.Musso G, Gambino R, Cassader M, Pagano G. A Meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. (2010) 52:79–104. doi: 10.1002/hep.23623, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Franz MJ, Vanwormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. (2007) 107:1755–67. doi: 10.1016/j.jada.2007.07.017, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Kosmalski M, Frankowski R, Ziółkowska S, Różycka-Kosmalska M, Pietras T. What’s new in the treatment of non-alcoholic fatty liver disease (NAFLD). J Clin Med. (1852) 12:12. doi: 10.3390/jcm12051852, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weitz D, Weintraub H, Fisher E, Schwartzbard AZ. Fish oil for the treatment of cardiovascular disease. Cardiol Rev. (2010) 18:258–63. doi: 10.1097/CRD.0b013e3181ea0de0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munro IA, Garg ML. Prior supplementation with long chain omega-3 polyunsaturated fatty acids promotes weight loss in obese adults: a double-blinded randomised controlled trial. Food Funct. (2013) 4:650–8. doi: 10.1039/c3fo60038f, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Alwayn IPJ, Gura K, Nose V, Zausche B, Javid P, Garza J, et al. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr Res. (2005) 57:445–52. doi: 10.1203/01.PDR.0000153672.43030.75, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Buchman A. Total parenteral nutrition-associated liver disease. JPEN J Parenter Enter Nutr. (2002) 26:S43–8. doi: 10.1177/014860710202600512, PMID: [DOI] [PubMed] [Google Scholar]
- 12.Burns DL, Gill BM. Reversal of parenteral nutrition-associated liver disease with a fish oil-based lipid emulsion (Omegaven) in an adult dependent on home parenteral nutrition. JPEN J Parenter Enter Nutr. (2013) 37:274–80. doi: 10.1177/0148607112450301, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Periz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. (2009) 23:1946–57. doi: 10.1096/fj.08-125674, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker HM, Johnson NA, Burdon CA, Cohn JS, O’Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. (2012) 56:944–51. doi: 10.1016/j.jhep.2011.08.018, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Sao R, Aronow WS. Association of non-alcoholic fatty liver disease with cardiovascular disease and subclinical atherosclerosis. Arch Med Sci. (2018) 14:1233–44. doi: 10.5114/aoms.2017.68821, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim EJ, Kim BH, Seo HS. Cholesterol-induced nonalcoholic fatty liver disease and atherosclerosis aggravated by systemic inflammation. PLoS One. (2014) 9:e97841. doi: 10.1371/journal.pone.0097841, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marx N, Duez H, Fruchart JC. Peroxisome proliferator-activated receptors and atherogenesis: regulators of gene expression in vascular cells. Circ Res. (2004) 94:1168–78. doi: 10.1161/01.RES.0000127122.22685.0A, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, et al. Increase in long-chain polyunsaturated fatty acid n – 6/n – 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond). (2004) 106:635–43. doi: 10.1042/CS20030326, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Lu W, Li S, Li J, Wang J, Zhang R, Zhou Y, et al. Effects of omega-3 fatty acid in nonalcoholic fatty liver disease: a meta-analysis. Gastroenterol Res Pract. (2016) 2016:1459790. doi: 10.1155/2016/1459790, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo XF, Wang C, Yang T, Li S, Li KL, Li D. Vitamin D and non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Food Funct. (2020) 11:7389–99. doi: 10.1039/D0FO01095B, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed). (2021) 372:n71. doi: 10.1136/bmj.n71, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, TetzlaffJ ADG. Preferred reporting items for systematic reviews and meta analyses: the PRISMA statement. OpenMed. (2009) 6:e12330. doi: 10.1371/journal.pmed.1000097, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EASL, EASD, EASO. European Association for the Study of the liver; European Association for the Study of diabetes; European Association for the Study of obesity. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. (2016) 64:1388–402. doi: 10.1016/j.jhep.2015.11.004, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-analysis detected by a simple. Graphical Test BMJ (Clinical Res ed). (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Argo CK, Patrie JT, Lackner C, Henry TD, de Lange EE, Weltman AL, et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J Hepatol. (2015) 62:190–7. doi: 10.1016/j.jhep.2014.08.036, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin Y, Zhou Y, Chen SH, Zhao XL, Ran L, Zeng XL, et al. Fish oil supplements lower serum lipids and glucose in correlation with a reduction in plasma fibroblast growth factor 21 and prostaglandin E2 in nonalcoholic fatty liver disease associated with hyperlipidemia: a randomized clinical trial. PLoS One. (2015) 10:e0133496. doi: 10.1371/journal.pone.0133496, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker HM, Cohn JS, O'Connor HT, Garg ML, Caterson ID, George J, et al. Effect of fish oil supplementation on hepatic and visceral fat in overweight men: a randomized controlled trial. Nutrients. (2019) 11:475. doi: 10.3390/nu11020475, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shojasaadat F, Ayremlou P, Hashemi A, Mehdizadeh A, Zarrin R. A randomized controlled trial comparing effects of a low-energy diet with n-3 polyunsaturated fatty acid supplementation in patients with non-alcoholic fatty liver disease. J Res Med Sci. (2019) 24:21. doi: 10.4103/jrms.JRMS_282_18, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cansanção K, Citelli M, Carvalho Leite N, de Las L, Hazas MC, Dávalos A, et al. Impact of long-term supplementation with fish oil in individuals with non-alcoholic fatty liver disease: a double blind randomized placebo controlled clinical trial. Nutrients. (2020) 12:3372. doi: 10.3390/nu12113372, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song L, Zhao XG, Ouyang PL, Guan Q, Yang L, Peng F, et al. Combined effect of n-3 fatty acids and phytosterol esters on alleviating hepatic steatosis in non-alcoholic fatty liver disease subjects: a double-blind placebo-controlled clinical trial. Br J Nutr. (2020) 123:1148–58. doi: 10.1017/S0007114520000495, PMID: [DOI] [PubMed] [Google Scholar]
- 32.Guo XF, Wang C, Yang T, Ma WJ, Zhai J, Zhao T, et al. The effects of fish oil plus vitamin D3 intervention on non-alcoholic fatty liver disease: a randomized controlled trial. Eur J Nutr. (2022) 61:1931–42. doi: 10.1007/s00394-021-02772-0, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. (2011) 34:274–85. doi: 10.1111/j.1365-2036.2011.04724.x, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. (2005) 42:44–52. doi: 10.1002/hep.20734, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in MASLD after up to 33 years of follow-up. Hepatology. (2015) 61:1547–54. doi: 10.1002/hep.27368, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. (2010) 363:1341–50. doi: 10.1056/NEJMra0912063, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. (2001) 50:1844–50. doi: 10.2337/diabetes.50.8.1844, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham heart study. Hepatology. (2010) 51:1979–87. doi: 10.1002/hep.23593, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne CD, Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism. (2020) 111:154170. doi: 10.1016/j.metabol.2020.154170, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis. (2014) 64:638–52. doi: 10.1053/j.ajkd.2014.05.019, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Byrne CD. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat Rev Nephrol. (2017) 13:297–310. doi: 10.1038/nrneph.2017.16, PMID: [DOI] [PubMed] [Google Scholar]
- 42.Scorletti E, Byrne CD. Omega-3 fatty acids and non-alcoholic fatty liver disease: evidence of efficacy and mechanism of action. Mol Asp Med. (2018) 64:135–46. doi: 10.1016/j.mam.2018.03.001, PMID: [DOI] [PubMed] [Google Scholar]
- 43.Calderon Martinez E, Zachariah Saji S, Salazar Ore JV, Borges-Sosa OA, Srinivas S, Mareddy NSR, et al. The effects of omega-3, DHA, EPA, Souvenaid® in Alzheimer's disease: a systematic review and meta-analysis. Neuropsychopharmacol Rep. (2024) 44:545–56. doi: 10.1002/npr2.12455, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manna P, Jain SK. Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-γ-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J Biol Chem. (2012) 287:42324–32. doi: 10.1074/jbc.M112.407833, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bugianesi E, Mccullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. (2005) 42:987–1000. doi: 10.1002/hep.20920, PMID: [DOI] [PubMed] [Google Scholar]
- 46.Gormaz JG, Rodrigo R, Videla LA, Beems M. Biosynthesis and bioavailability of long-chain polyunsaturated fatty acids in non-alcoholic fatty liver disease. Prog Lipid Res. (2010) 49:407–19. doi: 10.1016/j.plipres.2010.05.003, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. (2003) 112:1785–8. doi: 10.1172/JCI20514, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo XF, Gao JL, Li JM, Li D. Fat-1 mice prevent highfat plus high-sugar diet-induced non-alcoholic fatty liver disease. Food Funct. (2017) 8:4053–61. doi: 10.1039/C7FO01050H, PMID: [DOI] [PubMed] [Google Scholar]
- 49.Guo XF, Sinclair AJ, Kaur G, Li D. Differential effects of EPA, DPA and DHA on cardio-metabolic risk factors in high-fat diet fed mice. Prostag Leukotr Ess. (2018) 136:47–55. doi: 10.1016/j.plefa.2017.09.011, PMID: [DOI] [PubMed] [Google Scholar]
- 50.Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (MASLD). Prog Lipid Res. (2009) 48:1–26. doi: 10.1016/j.plipres.2008.08.001, PMID: [DOI] [PubMed] [Google Scholar]
- 51.Takeuchi Y, Yahagi N, Izumida Y, Nishi M, Kubota M, Teraoka Y, et al. Polyunsaturated fatty acids selectively suppress sterol regulatory element-binding protein-1 through proteolytic processing and autoloop regulatory circuit. J Biol Chem. (2010) 285:11681–91. doi: 10.1074/jbc.M109.096107, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jump DB, Clarke SD. Regulation of gene expression by dietary fat. Annu Rev Nutr. (1999) 19:63–90. doi: 10.1146/annurev.nutr.19.1.63, PMID: [DOI] [PubMed] [Google Scholar]
- 53.Jump DB, Tripathy S, Depner CM. Fatty acid-regulated transcription factors in the liver. Annu Rev Nutr. (2013) 33:249–69. doi: 10.1146/annurev-nutr-071812-161139, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. (2005) 115:1343–51. doi: 10.1172/JCI23621, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.