Abstract
Non-coding RNAs (ncRNAs) are reported to be regulators of signaling pathways that are involved in colorectal cancer (CRC) progression. Aiming at finding ncRNAs (miRNAs) that are differentially expressed in tumor versus normal colorectal tissue samples, online RNA-seq data were analyzed. Of between 18 candidate miRNAs, hsa-miR-29b-1 (miR-29b-1) represented the highest fold change of expression level. Hsa-miR-29b-1 is encoded from the third intron of LOC646329 long ncRNA gene. Surprisingly, two miR-29b sponging sites were predicted within exons of LOC646329 gene. Then, dual luciferase assay supported the interaction of miR-29b-1 with LOC646329-variant D transcript. Also, a direct indication of miR-29b-1 with 3′UTR sequence of SMAD3 gene was verified through dual luciferase assay and RT-qPCR analysis. Furthermore, a reverse pattern of expression was detected between miR-29b-1 and LOC646329-variant D transcript in about 25 pairs of CRC tumor samples, detected by RTqPCR. Consistently, overexpression of LOC646329-variant D transcript was followed by increased SMAD3 and p21 genes expression level and downregulation of CyclinD1 genes in HCT116 cells, detected by RT-qPCR, and western analysis. Also, overexpression of it was followed by increased G1 cell population of HCT-116 cells. All of these data suggested a tumor suppressor effect for LOC646329-variant D in CRC tumor tissue samples, consistent to its reduced expression level at late stages of CRC progression. Data also indicated that LOC646329-variant D exerts its suppression effect on CRC progression through sponging miR-29b, which in turn regulates Wnt and TGFB signaling pathways. This makes LOC646329-variant D transcript as a novel potential therapy target.
Keywords: Colorectal cancer, lncRNA, miR-29b, Tgfβ signaling pathway, SMAD3
Introduction
Colorectal cancer (CRC) is the third most common cause of cancer-related death worldwide with an incidence of 8.1–8.3 per 100,000 Iranian men and 6.5–7.5 per 100,000 Iranian women. CRCs are molecularly heterogenous disease, means various genomic and epigenomic modifications are involved (Inamura 2018; Rafiemanesh et al. 2016). Despite the diagnostic and therapeutic advances in CRC, a significant proportion of CRC patients experience cancer recurrence and die within 5 years after surgical treatment of their primary tumor (Kim et al. 2018). In addition to protein-coding mRNAs, it is estimated that substantial portion of non-coding regions of human genome are transcribed and involved in cell activities. Long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) are two important classes of non-coding transcriptome. lncRNAs transcripts are more than 200 bases long, often spliced and polyadenylated whereas, miRNAs are the transcript with 18–25 bases long which is excised from 70- to 100-nt hairpin-shaped precursors (Fang and Fullwood 2016). Reports have indicated that lncRNAs could participate in various aspect of cell function particularly proliferation and the etiology of cancers (Yan et al. (2015)). For example, CCAT-1, MALAT1, BANCR, and OCC-1 long ncRNAs have been linked to CRC incidence (Xiang et al. 2014; Yang et al. 2015; Guo et al. 2014; Najafi et al. 2017).
MicroRNAs are small non-coding RNAs that regulate different cell functions such as proliferation, differentiation, and apoptosis by inhibiting the expression of multiple genes through targeting 3′ UTR sequences on mRNAs. Particularly, miR-143, miR-145, and miR-29b are deregulated in CRC (Masuda et al. 2017; Michael et al. 2003; Inoue et al. 2015). Also, we have reported the involvement of several other miRNAs in CRC incidence elsewhere (Moez et al. 2019; Bakhshmand and Soltani 2019; Fasihi et al. 2018; Dokanehiifard et al. 2017; Saleh et al. 2016). Likewise, miR-29b-1 has been considered as a biomarker of different cancers and is capable of downregulating TGFβ, WNT, and MAPK signaling pathways through targeting SMAD3, β-Catenin, and ERK1/2 genes, respectively (Domingo-Gonzalez et al. 2015; Wang et al. 2014; Subramanian et al. 2014). It is known that lncRNAs as miRNA sponges or competing endogenous RNAs, are able to bind miRNAs and regulate their functions. Several examples of such lncRNA have been reported to date (Militello et al. 2017). Databases like BiBiServ2-RNAhybrid (https://bibiserv2.cebitec.uni-bielefeld.de/rnahybrid) are established to predict which lncRNAs can bind to miRNAs (Das et al. 2014). Linc-Pint is an intergenic tumor suppressor lncRNA which prohibits cancer cell migrations through polycomb repressive complex 2-dependent manner in mice model (Marin-Bejar et al. 2017). It is reported that Linc-Pint is also able to regulate MAPK and TGFβ pathways (Marin-Bejar et al. 2013). Here in this study, we found a novel transcript variant for LOC646329 (it was named LOC646329-variant D) by re-analyzing RNA-sequencing raw data. Furthermore, based on bioinformatic analysis we discovered LOC646329-variant D is able to sponge miR-29b-1 and indirectly reactivate SMAD3 in TGFβ signaling pathway that results in cell arrest.
Methods
Patient samples
25 human CRC tissues and their paired noncancerous samples were obtained from patients at Jam hospital (Tehran, Iran). All the patients were diagnosed with CRC and no radiotherapy or chemotherapy was done prior to the operation. Immediately after resection, specimens were snap-frozen in liquid nitrogen and stored at – 80 °C. Study protocol was approved by research committee at Tarbiat Modares University and all patients provided informed consent for using of specimens in research.
Plasmid construction
The sequence of miR-29b-1 precursor was cloned into peGFP-C1 expression vector under CMV Promoter. The LOC646329-variant D related cDNA was amplified from CRC sample using high-fidelity pfu enzyme and cloned into pTG19-T vector. Then, sequences were verified by golden standard sequencing techniques. Also, to construct the dual reporter vector for demonstrating sponge phenomenon, LOC646329-variant D related cDNA was cloned into the downstream of the Luciferase ORF in pSicheck2 Vector. All of the sequence of the used primer sets is listed in Table 1.
Table 1.
The sequences of oligonucleotides and primers
| Primer | Forward primer | Reverse primer |
|---|---|---|
| lncRNA cloning | CATCTGAACTGTCACGGCAGA | TTGAGTTGGGTCCTTGTTTTC |
| lncRNA expression | CTGCTTTTACACCTACATGTTGCC | CATTAGCATGTTTCCAACCCTGC |
| miR-29b-1 | GCTGGTTTCATATGGTGGT | AACTCAAGGTTCTTCCAGTCACG |
| GAPDH | GCCACATCGCTCAGACAC | GGCAACAATATCCACTTTACCAG |
| SMAD3 | CCTGCTGGAACATCATCTCAG | CTTCCTAAGAGTCAAAGTCCCC |
| p21 | ACCAGCATGACAGATTTCTACCA | ACTAAGGCAGAAGATGTAGAGCG |
| CyclinD1 | CAGAGTGATCAAGTGTGACCC | CGTCGGTGGGTGTGCAAGC |
Cell culture and transfection
Three CRC cell lines (SW480, HT29, and HCT116) were obtained from Pasteur Institute (Tehran, Iran). SW480 was grown in Dulbecco’s modified Eagle’s medium (DMEM) and HCT116 was cultivated in Hepes buffered Dulbecco’s modified Eagle’s medium (RPMI), supplemented with 2 mM l-glutamine (Merck, Germany), 1% penicillin streptomycin (100 U/mL of penicillin and 100 μg/mL of streptomycin) (PS, Gibco, USA), 10% fetal bovine serum (FBS, Gibco, USA) and incubated at 37 °C with 5% CO2. For doing plasmid transfection, the cells were seeded on a 12-well plate and allowed to reach at least 70% confluency in 24 h (h) before transfection. After that, the cells were transfected with constructed vectors using Turbofectamin (Thermo Scientific, Waltham, MA, USA) according the manufacturer’s instructions (Dalby et al. 2004). Transfection efficiency was estimated by fluorescence microscopy. Then, transfectants were harvested 48 h after transfection, to isolate total RNA.
Total RNA extraction and RT-qPCR
Total RNA was extracted from colorectal cancer tissues and cells using TRIzol reagent (Invitrogen, Life Technologies, Carlsbad, CA, USA). The quality and quantity of extracted RNA were analyzed by agarose gel electrophoresis and spectrophotometry, respectively. Then cDNA was synthesized with M-MLV reverse transcriptase kit (Invitrogen, Life Technologies, Carlsbad, CA, USA). RT-qPCR was performed using SYBR Green assay (Takara, Dalian, China). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. The assay was conducted using the ABI 7300 system (Applied Biosystems). All reactions were done in triplicate and expression level of LOC646329-variant D and miR-29b-1 was calculated by 2−∆∆Ct.
Western blot
RIPA buffer including protease inhibitor (Roche, Nutley, NJ, USA) were used to lyse the screened cells. Then, the purified protein samples were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The PVDF membrane was subsequently blocked by 5% skim milk in Tris-buffered saline containing 0.1% Tween for 2 h at room temperature. After that, treated-PVDF membrane was incubated overnight at 4 °C with primary antibody specific for Cyclin D1 (Abcam, Hong Kong, China) and B-Actin (all Cell Signaling Technology, Danvers, MA, USA). The Western blots were visualized with an ECL reaction kit (Beyotime, China), recorded on a Canon EOS 60D and quantitated using CLIQS. Using densitometry Image J (National Institute of Health, City, MD, USA), the protein bands were quantified and normalized to that of the corresponding B-Actin bands.
Dual reporter assay
HEK293T cells were co-transfected with vector ensuring miR-29b-1 expression and either with the psicheck2-Luciferase vector containing LOC646329-variant D or SMAD3 sequences. Cells were harvested 48 h post-transfection. Luciferase activity was measured using the dual-luciferase reporter assay system (Promega, Madison, WI, USA).
Cell cycle analysis
1 × 105 cells/well for each SW480 and HCT116 Cells were transfected with overexpression plasmids of LOC646329-variant D or miR-29b-1 in 24-well plates in triplicate. For each construct, a scrambled vector (1 μg/well) along with untransfected cells were used as negative controls. 48 h after transfection, the transfected cells were harvested. After centrifuging at 1200 rpm in 4 °C for 5 min, the cell pellets were washed twice in PBS. Then, the washed-pellets were fixed in 1 mL ice-cold 70% ethanol for 30 min. Each sample was incubated at room temperature for 30 min after resuspending the cells in 500 µl PBS, containing presidium iodide (PI, Sigma, USA) staining solution (1 mg/mL) and RNase A (0.2% v/v). To calculate the number of cells in each cell cycle phase, the prepared cells were subjected to FACS Calibur flow cytometer (Becton Dickinson), A total of 10,000 events were counted for each sample, and finally analyzed utilizing Cell Quest software (BD Biosciences, USA).
Statistical and RNAseq analysis
To perform statistical analyses, SPSS 25.0 (SPSS, Inc., Chicago, IL, USA) was used. All data were presented as the mean ± SD. The Student’s t test and was used for comparison of two independent group. A p value < 0.05 was considered as statistically significant.
For RNAseq analysis against miRNAs, data’s was downloaded from TCGA data bank using TCGA biolink package of R program In which, sequencing data has been prepared through illumine method. There were 459 patient samples and five healthy ones. Box plot graph exhibited abnormal distribution, then, the data was normalized using deseq2 package to draw heat map and PCA graph in R program. Finally, significant expression differences of miRs was calculated using deseq2 package.
For the analysis of target genes expression, RNA seq raw data was downloaded from SRA NCBI data base. Theses samples were included four normal and four patient. Then, RNA’s terminal adaptors were dissociated using trime galore software and aligned by HISAT2 on the whole genome. Finally, the quality of data were checked using FASTQC tool. The aligned data were rated by htseq count software and, significant expression differences of the genes was calculated by DESEQ2 package.
Results
Bioinformatics analysis of differentially expressed ncRNAs in CRC samples
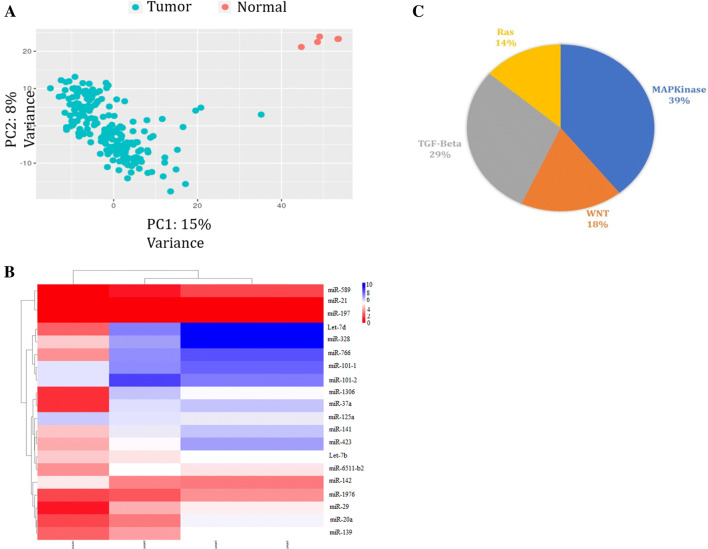
To extract differentially expressed genes (DEGs), several datasets of colorectal polyp and non-polyp miRNA expression samples were downloaded from TCGA database (TCGA ID number: TCGA COAD /TCGA COAD READ) and analyzed via DESq packages (Anders and Huber 2010). Principal component analysis (PCA) of the samples which were used in this study, showed a successful discrimination of normal and tumor specimens (Fig. 1a). In total, 379 of 1883 miRNAs were differentially expressed in 459 tumors versus five normal samples. Top 20 miRNAs showing more than 2-folds de-regulation between normal and CRC samples (Tumor or polyp) were chosen for further examination (Fig. 1b & Table 2). Targets predication for these miRNAs represented that MAPK, TGFβ, Wnt, and RAS are the most prominent signaling pathways which are orchestrated by forenamed miRNAs (Fig. 1c). Based on our observations in primary steps of CRC, MAPK and TGFβ signaling pathways were the strong hot spots for the future of CRC treatments. Since, miR-29b-1 had high fold change of expression level between normal and cases, it was chosen for further investigation. Bioinformatics analyses indicated that miR-29b-1 is capable of targeting the genes in MAPK, TGFβ, and Wnt signaling pathways.
Fig. 1.
Selection of candidate CRC-related miRNAs through bioinformatics analysis of TCGA database. a PCA analysis showed correct differentiation of normal data from tumor data
adopted from TCGA database. b Drawn heat map for the top 20 miRNAs which are differentially expressed in normal and tumor CRC samples using heatmap2 package in R programing. c Target predictions of 10 candidate miRNAs. The MAPK and TGFβ signaling were the major pathways to be targeted by candidate miRNAs
Table 2.
De-regulated miRNAs between normal and CRC samples
| Upregulated miRNAs | Fold change | Downregulated miRNAs | Fold change |
|---|---|---|---|
| hsa-miR-29b-1 | 5.5076 | hsa-miR-589 | − 2.516 |
| hsa-miR-542 | 5.1195 | hsa-miR-324 | − 2.631 |
| hsa-miR-582 | 4.6971 | hsa-miR-484 | − 2.777 |
| hsa-miR-20a | 4.5324 | hsa-miR-671 | − 2.913 |
| hsa-miR-335 | 4.4173 | hsa-miR-378a | − 3.059 |
| hsa-miR-16-1 | 4.2304 | hsa-miR-181a-1 | − 3.409 |
| hsa-miR-182 | 4.1949 | hsa-miR-1296 | − 3.762 |
| hsa-miR-16-2 | 4.0917 | hsa-miR-574 | − 3.851 |
| hsa-miR-379 | 3.7573 | hsa-miR-1976 | − 4.21 |
Identification of a novel splice variant for the host gene of miR-29b-1 in human cell lines
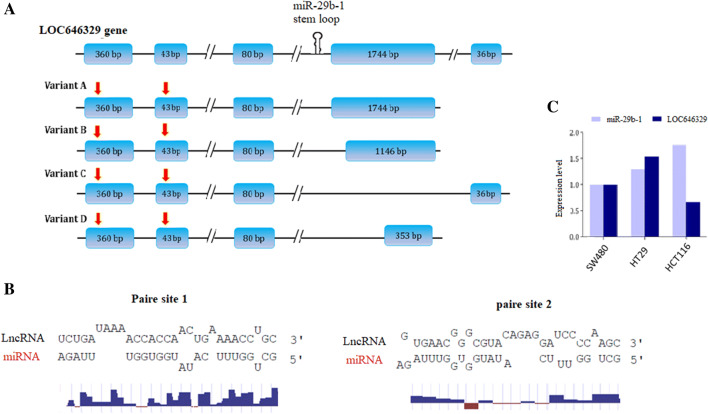
UCSC database analysis indicated that miR-29b-1 is hosted by a ncRNA gene named Linc-pint (LOC646329). There are three reported splice variants (here we have named them as variant-A, B, & C) for Linc-pint gene and interestingly, we found another shorter (719-nt) and novel splice variant in the RNA-sequencing data and named it as LOC646329 splice variant D. Stem loop which is responsible for miR-29b-1 production is located in the third intron of LOC646329 gene. Surprisingly, bioinformatics analysis indicated that miR-29b-1 is sponged by all of its host gene splice variants at the first and second exons (Fig. 2a). Bioinformatics analysis indicated that LOC646329-variant D was capable of sponging miR-29b-1 through near to complete complementary base pairing (Fig. 2b). Then, RT-qPCR analysis indicated that LOC646329-variant D expression was somewhat reverse to the expression of miR-29b-1 in CRC originated cell lines (Fig. 2c).
Fig. 2.
Schematic illustration of different variants of Linc-pint (LOC646329) gene. a Novel LOC646329-variant D has shorter sequence since, its 4th exon is smaller than other splice variants. Hsa-miR-29b-1 is encoded from the third intron of LOC646329 gene (hairpin structure). Sponge sites for miR-29b-1 are illustrated by red vertical arrows on top of the exons 1 and 2 of all transcript variants. b Top: Pairing status of miR-29b-1 with its complementary sequences in LOC646329 transcripts. Bottom; shows the nucleotides that are paired with miR-29b-1, are more conserved than unpaired ones in the sponging sites of miR-29b-1. c The comparative expression of LOC646329-variant D and miR-29b-1 in three CRC-originated cell lines, detected by RT-qPCR analysis. GAPDH and U48 were used as internal controls
LOC646329-variant D and miR-29b-1 expression status in CRC tumor and non-tumor samples
The expression status of miR-29b-1 and its host gene or sponger (LOC646329-variant D) was investigated in three different stages of CRC tissue samples. LOC646329-variant D expression level was significantly higher than normal pairs, at the middle stage of CRC tumors. However, in early stage and late stage of CRC tumors, LOC646329-variant D expression level was substantially lower than in normal. Reverse to the LOC646329-variant D expression, miR-29b-1 expression was significantly higher than the normal pairs at the late stage. Interestingly, while LOC646329-variant D expression was barely detectable in early stage samples, miR-29b-1 was detected in all of the tested samples. Also, at the late stage in which miR-29b-1 was highly detectable, LOC646329-variant D expression level was lower than normal (Fig. 3a). All of these expression levels suggested a reverse expression pattern between miR-29b-1 and LOC646329-variant D expression in CRC tissue samples. Nevertheless, analysis of LOC646329-variant D and miR-29b-1 expression levels in whole tumors versus normal pairs indicated that their expression have been higher than normal (Fig. 3b, c).
Fig. 3.
LOC646329-variant D and miR-29b-1 expression status in three different stages of CRC. aLOC646329-variant D expression level was significantly higher than normal pairs, at the middle stage of CRC tumors, while miR-29b-1 expression was significantly higher than the normal pairs at the late stage, detected by RT-qPCR. b, c Overall, LOC646329-variant D and miR-29b-1 expression levels were higher in CRC tumor samples versus normal pairs, detected by RT-qPCR
Evidences for direct interaction of miR-29b-1 with SMAD3 transcript
Bioinformatics analysis suggested SMAD3 as a bona fide target gene for miR-29b-1 which contains three recognition sites within its 3′UTR sequence. Therefore, miR-29b-1 precursor sequence was cloned in pEGFP expression vector and successful overexpression (~ 50 folds) of mature miR-29b-1 was confirmed through RT-qPCR analysis (Fig. 4a). RT-qPCR analysis indicated that SMAD3 gene expression level has been significantly reduced, following the miR-29b-1 overexpression in HCT116 cells (Fig. 4b). Then, 3′UTR sequence of SMAD3 gene was cloned downstream to the Renilla luciferase gene in the pSicheck2 vector and a direct interaction of miR-29b-1 with SMAD3 gene 3′UTR sequence was investigated through dual luciferase assay. Assay result indicated 1.5-folds decreased luciferase activity when miR-29b-1 was overexpressed along with Luc::3′UTR sequence of SMAD3 construct, compared to the off-target (Luc::3′UTR sequence of SOX3.) construct (Fig. 4c).
Fig. 4.
Evidence for the effect of miR-29b-1 on SMAD3 expression. a Successful overexpression of miR-29b-1 in HCT116 cells, detected by RT-qPCR. U48 was used as internal control. b RT-qPCR results indicated downregulation of SMAD3 gene expression, following the miR-29b-1 overexpression in HCT-116 cells. c Dual luciferase assay indicated occurrence of direct interaction between miR-29b-1 and 3′UTR sequence of SMAD3 gene. 3′UTR sequence of Sox2 was used as an off-target control in the assay
LOC646329-variant D expression effect on TGFβ signaling through sponging miR-29b-1
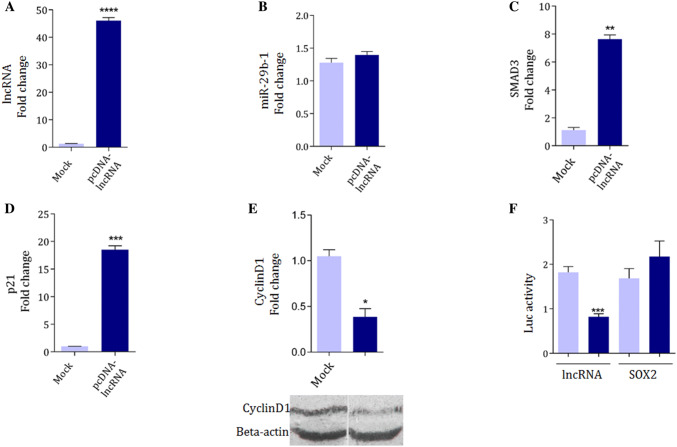
When, LOC646329-variant-D was cloned in pcDNA 3.1 (+) expression vector and successfully overexpressed in HCT116 cells (Fig. 5a), ended in no significant change in miR-29b-1 expression level (Fig. 5b). However, it was followed by increased level of SMAD3 gene expression as a miR-29b-1 target gene, detected through RT-qPCR analysis (Fig. 4c). The p21 and cyclinD1 are known to be regulated by phospho-SMAD3 downstream to the TGFβ signaling pathway. Overexpression of LOC646329-variant-D was followed by p21 gene upregulation detected by RT-qPCR (Fig. 5d) and CyclineD1 gene downregulation, detected by RT-qPCR and western analysis (Fig. 5e).
Fig. 5.
Evidences for LOC646329-variant D expression effect on TGFβ signaling through sponging miR-29b-1. a The successful overexpression of LOC646329-variant D transcripts in HCT116 cells, detected by RT-qPCR. GAPDH was used as internal control. b No significant expression alteration of miR-29b-1 following the LOC646329-variant D overexpression. U48 was used as internal control of the RT-qPCR analysis. c Upregulation of SMAD3 gene expression, following LOC646329-variant D overexpression. GAPDH was used as internal control. d Upregulation of P21 gene expression, following LOC646329-variant D overexpression. GAPDH was used as internal control. e Top: RT-qPCR results of CyclinD1 gene expression downregulation, and Bottom: Western blotting results of Cyclin D1 protein expression reduction, following LOC646329-variant D overexpression in HCT-116 cells. GAPDH and B-actin were used as internal controls. f Dual luciferase assay supported the direct interaction between miR-29b-1 and LOC646329-variant D transcripts. SOX2 gene 3′UTR sequence was used as off target in this experiment
All of these results consistently suggested that miR-29b-1 is sponged by LOC646329-variant D transcripts. Bioinformatics analyses indicated there are two miR-29b-1 recognition sites within the first and second exons of LOC646329-variant D transcript (Fig. 2a). Therefore, a direct interaction between miR-29b-1 and LOC646329-variant D transcript was investigated through dual luciferase assay, as well. To this aim, 719 bp length LOC646329-variant D related cDNA was cloned downstream to the Renilla luciferase in the pSicheck2 vector and was co-transfected in HEK293Tcells along with peGFP vector ensuring miR-29b-1 overexpression. Then, results were compared with the cells that mock control vector was co-transfected along with pSicheck2 recombinant vector. Results of luciferase assay supported a direct interaction between miR-29b-1 and LOC646329-variant D transcript (Fig. 5f). Overall, these results emphasized on the effect of LOC646329-variant D on TGFβ signaling through sponging of miR-29b-1.
LOC646329-variant D overexpression effect on CRC originated cell cycle status
To find the effect of LOC646329-variant D overexpression on cell cycle status, we analyzed the cell cycle progression in SW480 and HCT116 cell lines, which are representing early and middle stages of CRC progression, respectively (Ahmed et al. 2013). No significant alteration of cell cycle progression was detected through PI staining flow cytometry, following the overexpression of LOC646329-variant D in SW480 cell lines, compared to negative control cells (Fig. 6a, b). However, overexpression of LOC646329-variant D in HCT116 cells resulted in a significant elevation in G1 phase cell population and a significant reduction in G2/M phase cell populations (p value < 0.05), compared to the control cells transfected with the empty mock vector (Fig. 6c, d).
Fig. 6.
Effect of LOC646329-variant D overexpression on SW480 and HCT116 cell cycle statuses. a, b PI staining 36 h after transfection of LOC646329-variant D expression plasmid into SW480 CRC cell line. No significant alteration was detected in cell population distribution of SW480 cells following LOC646329-variant D overexpression, compared to the negative control cells transfected by mock vector. Error bars indicate SD of duplicated experiments. c, d Cell cycle distribution of HCT116 cells overexpressing LOC646329-variant D. A significant elevation of G1 population and a significant reduction of G2/M cells population was detected in HCT116 cells, overexpressing LOC646329-variant D, compared to the control cells transfected by mock vector. Error bars indicate SD of duplicated experiments
Discussion
CRC is one of the most common human malignant diseases and remains the third leading cause of cancer-related death worldwide. It is shown that CRC is a molecularly heterogenous disease (Inamura 2018). Cancer incidence is affected by cell signaling pathways such as TGFβ that plays key role in cell propagation, differentiation, and even tumor induction (Tauriello et al. 2018). This pathway contains TGFβ receptors (TGFβR1 and TGFβR2) and SMAD complex proteins (SMAD2, SMAD2, and SMAD4) that are important components of pathway. Dysregulation of TGFβ and Wnt signaling key components are identified in various types of cancers including CRC (Abedini Bakhshmand et al. 2018). On the other hand, signaling pathways are affected by regulatory factors such as ncRNAs including miRNAs, and deregulation of them leads to induction of different cancer types (Najafi et al. 2017; Fang et al. 2018). For example, miR-92b by targeting SMAD3 is a potential oncogene in glioblastomas (Wu et al. 2013). Also, miR-29b by suppressing TCF7L2, Snail, and BCL9L (coactivators of β-Catenin) downregulates Wnt signaling pathway in CRC (Subramanian et al. 2014). High-throughput sequencing techniques such as RNAseq and miRNAseq are useful for investigating the dysregulation of lncRNAs and miRNAs in cancers cells (Strubberg and Madison, 2017). Here, we intended to reanalyze miRNAseq data for colorectal samples to find dysregulated candidate miRNAs involving in CRC incidence.
DEGs investigation suggested 20 differentially expressed miRNAs with more than two-fold changes between normal and tumor CRC samples. Then, target predictions for these miRNAs, suggested TGFβ (29%), Wnt (18%), and MAPK (39%) signaling pathways as the most prominent target pathways (Fig. 1). In between, miR-29b-1 had relatively high fold change among the differentially expressed miRNAs. Hsa-miR-29b-1 is produced from the stem loop which is located in third intron of Linc-pint (LOC646329) gene, based on UCSC data analysis (Fig. 2a). Up to date, three splice variants have been reported for LOC646329, here we named them as variants A, B, and C. Interestingly, we found a shorter novel variant for Linc-pint gene (named Variant-D) that is expressed in different cell lines (Fig. 2c). Furthermore, all of these LOC646329 encoded transcript variants were predicted to be capable of sponging miR-29b-1 at their exon 1 and 2 (Fig. 2a, b). When, the expression status of LOC646329-variantD and miR-29b-1 was investigated in 25 CRC tumor/normal pairs, a reverse expression pattern was deducible in middle stages and late stages of the tested CRC samples (Fig. 3a). Never the less, both miR-29b-1 and its host gene (LOC646329) expression level were increased in tumors versus normal pairs (Fig. 3b, c).
Hsa-miR-29b-1 is reported to be an important regulator of TGFβ signaling pathway through targeting of SMAD3 gene (Qin et al. 2011). Consistently, when miR-29b-1 was overexpressed in HCT-116 cells (Fig. 4a), SMAD3 gene expression was reduced (Fig. 4b) and dual luciferase assay supported direct interaction of this miRNA with 3′UTR sequence of SMAD3 gene (Fig. 4c). On the other hand, overexpression of LOC646329-variant D in HCT-116 cells (Fig. 5a), resulted in increased SMAD3 and P21 genes expression (Fig. 5c, d), and reduced Cyclin D1 protein level (Fig. 5e).
Dual reporter assay is a useful strategy for evidencing direct interaction between lncRNA, which acts as a molecular sponge (or ceRNA), and target miRNA (Shan et al. 2018). When LOC646329-variantD was cloned downstream to the luciferase gene in pSICHECK vector and miR-29b-1 was overexpressed, it ended in reduced luciferase activity, compared to related control (Fig. 5f). All of these results could be interpreted based on the effect of miR-29b-1 against SMAD3 gene expression (Fig. 4c) and sponging effect of LOC646329-variantD against miR-29b-1 (Fig. 5f). Consistently, when LOC646329-variantD was overexpressed in HCT-116 cells, it was followed by significant (p value < 0.05) increased cell population fraction at G1 phase, detected by PI flow cytometry (Fig. 6b). However, overexpression of LOC646329-variantD in SW480 cells did not have such an effect (Fig. 6a). This effect might be interpreted by lower endogenous expression level of LOC646329-variantD in HCT-116 cells, compared to its level in SW480 cells (Fig. 2c). Although both tumor suppressor (Eyholzer et al. 2010) and oncogenic roles (Yan et al. 2015) have been attributed to miR-29b in different cancer types, increased expression level of it particularly at the late stages of tested CRC samples (Fig. 3b, c), makes it oncogenic candidate. Therefore, considering sponging effect of LOC646329-variantD against miR-29b (Fig. 5f), a tumor suppressor effect is deduced for LOC646329-variantD in CRC originated HCT116 cells. This conclusion is consistent with increased G1 cell population of HCT-116 cells, following the overexpression of LOC646329-variantD, while it did not have such an effect in SW480 cells (Fig. 6). It is reported that miR-29b downregulates Wnt signaling (Saleh et al. 2016) and also, it is reported that TGFB signaling promotes cell proliferation in HCT-116 cells and at the late stages of CRC (Abedini Bakhshmand et al. 2018). Accordingly, a tumor suppressor effect was expected for miR-29b and an oncogenic role was expected for the sponger of it which is LOC646329-variantD. This inconsistency could be interpreted based on the effect of LOC646329-variantD on other signaling pathways and also the feedback effect of miR-29b and its host gene LOC646329 on each other.
At the bottom line, in this study we discovered a novel transcript variant of LOC646329 (variant-D), which harbors miR-29b sponging sites. Our results indicated that LOC646329-variant D was capable of directly restraining suppressor activity of miR-29b-1 against TGFβ signaling pathway. Accordingly, existence of LOC646329-variant D sponges miR-29b-1 and as a result, SMAD3 is upregulated and in the presence of SMAD2/4, it will induce the expression level of p21 and conversely downregulate cyclin D1 (Fig. 7).
Fig. 7.
Schematic representation of a deduced regulatory network in which LOC646329-variant D regulates TGFβ signaling pathway through both encoding and also sponging miR-29b-1
Acknowledgements
Authors are thankful to the helps and advices of all lab mate in room 4402 at Genetics Dept. TMU, Tehran, Iran.
Author contributions
AJ performed the experiments and analyzed data, AJ and BS wrote manuscript; AJ and SD and BS designed experiments; BS supervised the study. SD advised the research. MB provided tumor/normal tissues and provided pathology information of them.
Funding
This work was supported by TMU and NIMAD (grant number 943387).
Compliance with ethical standards
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abedini BE, Soltani BM (2018) Regulatory effect of hsa-miR-5590-3P on TGFbeta signaling through targeting of TGFbeta-R1, TGFbeta-R2, SMAD3 and SMAD4 transcripts. Biol Chem [DOI] [PubMed]
- Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknaes M, Hektoen M et al (2013) Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2:e71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11(10):R106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshmand EA, Soltani BM (2019) Regulatory effect of hsa-miR-5590-3P on TGFβ signaling through targeting of TGFβ-R1, TGFβ-R2, SMAD3 and SMAD4 transcripts. Biol Chem 400(5):677–685 [DOI] [PubMed] [Google Scholar]
- Das S, Ghosal S, Sen R, Chakrabarti J (2014) lnCeDB: database of human long noncoding RNA acting as competing endogenous RNA. PLoS ONE ONE 9(6):e98965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokanehiifard S, Yasari A, Najafi H, Jafarzadeh M, Nikkhah M, Mowla SJ et al (2017) A novel microRNA located in the TrkC gene regulates the Wnt signaling pathway and is differentially expressed in colorectal cancer specimens. J Biol Chem 292(18):7566–7577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo-Gonzalez R, Wilke CA, Huang SK, Laouar Y, Brown JP, Freeman CM et al (2015) Transforming growth factor-beta induces microRNA-29b to promote murine alveolar macrophage dysfunction after bone marrow transplantation. Am J Physiol Lung Cell Mol Physiol 308(1):L86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyholzer M, Schmid S, Wilkens L, Müller BU, Pabst T (2010) The tumour-suppressive miR-29a/b1 cluster is regulated by CEBPA and blocked in human AML. Br J Cancer 103(2):275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Fullwood MJ (2016) Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genom Proteom Bioinform 14(1):42–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Huang B, Sun S, Xiao M, Guo J, Yi X et al (2018) miR-27a inhibits cervical adenocarcinoma progression by downregulating the TGF-betaRI signaling pathway. Cell Death Dis 9(3):395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasihi AM, Soltani B, Atashi A, Nasiri S (2018) Introduction of hsa‐miR‐103a and hsa‐miR‐1827 and hsa‐miR‐137 as new regulators of Wnt signaling pathway and their relation to colorectal carcinoma. J Cell Biochem 119(7):5104–5117 [DOI] [PubMed]
- Guo Q, Zhao Y, Chen J, Hu J, Wang S, Zhang D et al (2014) BRAF-activated long non-coding RNA contributes to colorectal cancer migration by inducing epithelial-mesenchymal transition. Oncol Lett 8(2):869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura K (2018) Colorectal cancers: an update on their molecular pathology. Cancers (Basel) 10(1) [DOI] [PMC free article] [PubMed]
- Inoue A, Yamamoto H, Uemura M, Nishimura J, Hata T, Takemasa I et al (2015) MicroRNA-29b is a novel prognostic marker in colorectal cancer. Ann Surg Oncol 22(Suppl 3):S1410–S1418 [DOI] [PubMed] [Google Scholar]
- Kim C, Kim WR, Kim KY, Chon HJ, Beom SH, Kim H et al (2018) Predictive nomogram for recurrence of stage i colorectal cancer after curative resection. Clin Colorectal Cancer 17(3):e513–e518 [DOI] [PubMed] [Google Scholar]
- Marin-Bejar O, Marchese FP, Athie A, Sanchez Y, Gonzalez J, Segura V et al (2013) Pint lincRNA connects the p53 pathway with epigenetic silencing by the polycomb repressive complex 2. Genome Biol 14(9):R104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Bejar O, Mas AM, Gonzalez J, Martinez D, Athie A, Morales X et al (2017) The human lncRNA LINC-PINT inhibits tumor cell invasion through a highly conserved sequence element. Genome Biol 18(1):202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Hayashi N, Kuroda Y, Ito S, Eguchi H, Mimori K (2017) MicroRNAs as biomarkers in colorectal cancer. Cancers (Basel) 9(9):124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael MZ, O’Connor SM, van Holst Pellekaan NG, Young GP, James RJ (2003) Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res 1(12):882–891 [PubMed]
- Militello G, Weirick T, John D, Doring C, Dimmeler S, Uchida S (2017) Screening and validation of lncRNAs and circRNAs as miRNA sponges. Brief Bioinform 18(5):780–788 [DOI] [PubMed] [Google Scholar]
- Moez MJ, Bjeije H, Soltani BM (2019) Hsa-miR-5195-3P induces downregulation of TGFβR1, TGFβR2, SMAD3 and SMAD4 supporting its tumor suppressive activity in HCT116 cells. Int J Biochem Cell Biol 109:1–7 [DOI] [PubMed] [Google Scholar]
- Najafi H, Soltani BM, Dokanehiifard S, Nasiri S, Mowla SJ (2017) Alternative splicing of the OCC-1 gene generates three splice variants and a novel exonic microRNA, which regulate the Wnt signaling pathway. RNA 23(1):70–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM et al (2011) TGF-beta/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol 22(8):1462–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiemanesh H, Pakzad R, Abedi M, Kor Y, Moludi J, Towhidi F et al (2016) Colorectal cancer in Iran: Epidemiology and morphology trends. EXCLI J 15:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh AJ, Soltani BM, Dokanehiifard S, Medlej A, Tavalaei M, Mowla SJ (2016) Experimental verification of a predicted novel microRNA located in human PIK3CA gene with a potential oncogenic function in colorectal cancer. Tumor Biol 37(10):14089–14101 [DOI] [PubMed] [Google Scholar]
- Shan Y, Ma J, Pan Y, Hu J, Liu B, Jia L (2018) LncRNA SNHG7 sponges miR-216b to promote proliferation and liver metastasis of colorectal cancer through upregulating GALNT1. Cell Death Dis 9(7):722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubberg AM, Madison BB (2017) MicroRNAs in the etiology of colorectal cancer: pathways and clinical implications. Dis Model Mech 10(3):197–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian M, Rao SR, Thacker P, Chatterjee S, Karunagaran D (2014) MiR-29b downregulates canonical Wnt signaling by suppressing coactivators of beta-catenin in human colorectal cancer cells. J Cell Biochem 115(11):1974–1984 [DOI] [PubMed] [Google Scholar]
- Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M et al (2018) TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554(7693):538–543 [DOI] [PubMed] [Google Scholar]
- Wang B, Li W, Liu H, Yang L, Liao Q, Cui S et al (2014) miR-29b suppresses tumor growth and metastasis in colorectal cancer via downregulating Tiam1 expression and inhibiting epithelial-mesenchymal transition. Cell Death Dis 5:e1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZB, Cai L, Lin SJ, Lu JL, Yao Y, Zhou LF (2013) The miR-92b functions as a potential oncogene by targeting on Smad3 in glioblastomas. Brain Res 1529:16–25 [DOI] [PubMed] [Google Scholar]
- Xiang JF, Yin QF, Chen T, Zhang Y, Zhang XO, Wu Z et al (2014) Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res 24(5):513–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Guo Q, Fu F, Wang Z, Yin Z, Wei Y et al (2015) The role of miR-29b in cancer: regulation, function, and signaling. Onco Targets Ther 8:539 [DOI] [PMC free article] [PubMed]
- Yan X, Hu Z, Feng Y, Hu X, Yuan J, Zhao SD et al (2015) Comprehensive genomic characterization of long non-coding RNAs across human cancers. Cancer Cell 28(4):529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MH, Hu ZY, Xu C, Xie LY, Wang XY, Chen SY et al (2015) MALAT1 promotes colorectal cancer cell proliferation/migration/invasion via PRKA kinase anchor protein 9. Biochim Biophys Acta 1852(1):166–174 [DOI] [PMC free article] [PubMed] [Google Scholar]