Abstract
Purpose
Baseline tumor size (BTS) and the presence of massive lesions are important for predicting the clinical course of cancer. However, their impact on survival and clinical response in patients with advanced NSCLC undergoing immune checkpoint inhibitor (ICI) treatment has been scarcely investigated.
Methods
We retrospectively reviewed 294 patients who underwent ICI therapy for advanced or recurrent non-small-cell lung cancer (NSCLC) between January 2016 and July 2019.
Results
Of these 294 patients, 284 (96.6%) had at least one measurable lesion. Of these, 263 patients treated with ICI monotherapy were included in the analysis. The median total and maximum target lesion diameters were 96.5 mm and 49.1 mm, respectively. Median progression-free survival (PFS) with massive lesions (max BTS > 50 mm, group A) and without massive lesions (max BTS ≤ 50 mm, group B) was 2.5 months (95% CI 1.8–3.7) and 6.7 months (95% CI 5.1–9.7), respectively. Median overall survival (OS) for groups A and B was 9.5 months (95% CI 5.5–12.3) and 20.0 months (95% CI 13.3–32.0), respectively. The multivariate analysis revealed marked associations between the presence of massive lesions and both PFS and OS.
Conclusion
The presence of massive lesions (max diameters > 50 mm) is an independent prognostic factor in advanced NSCLC treated with ICI monotherapy. Although overall response rates were similar between groups A and B, the disease control rate was significantly poorer for group A. Max BTS might be useful for predicting clinical outcomes for patients undergoing immunotherapy as a parameter reflecting their tumor burden.
Electronic supplementary material
The online version of this article (10.1007/s00432-020-03271-1) contains supplementary material, which is available to authorized users.
Keywords: Tumor size, Non-small-cell lung cancer, Immune checkpoint inhibitor, Overall survival, Progression-free survival
Introduction
Immune checkpoint inhibitors (ICIs), including anti-programmed death-1 (PD-1) antibodies and anti-programmed death-ligand 1 (PD-L1) antibody, are currently approved for unresectable advanced or recurrent non-small-cell lung cancer (NSCLC). Several pivotal clinical trials have demonstrated the clinical benefit [1–4] and revolutionary long-lasting response for some patients [5, 6] that these agents present over docetaxel, which has been the standard of care in second-line therapy. KEYNOTE 024 [7] and 042 [8] trials have demonstrated prolonged survival with pembrolizumab over platinum doublet chemotherapy in first-line settings for PD-L1 positive NSCLC. More recently, the superiority of the combination of ICI and platinum doublet chemotherapy over chemotherapy alone, irrespective of PD-L1 expression, has been shown in several phase III trials [9–11]. Under such circumstances, various clinical factors have been proposed as potential predictive and prognostic factors for patients undergoing ICI therapy [12–14]. However, no firm indicator has been fully established to guide the optimal choice of treatment options. Unlike molecular-targeted agents, such as epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) whose response can be predicted with considerable certainty due to the presence of driver oncogenes, ICIs require that clinicians employ a more multilateral approach to accurately predict outcomes.
Baseline imaging findings are essential for determining the extent of primary and metastatic tumors and for clinical staging according to the tumor, node, and metastasis (TNM) classification [15]. Although clinicians often use the imaging profile (such as the size and number of lesions) for decision making, there is a paucity of data on the impact of the tumor burden and on the outcomes of patients with advanced cancer undergoing ICI therapy. For situations where ICI therapy in combination with other agents is available, there are no efficacy data for ICI monotherapy according to lesion size. A few studies have suggested the usefulness of total baseline tumor size (BTS) according to the revised Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) guidelines for patients with advanced NSCLC [16] and melanoma [17, 18] undergoing ICI monotherapy. However, the impact of the tumor’s maximum BTS (max BTS), including for primary and metastatic sites, on clinical outcomes (i.e., the efficacy of ICI monotherapy for patients with massive lesions) has not been well described in previous studies. Moreover, a large BTS does not necessarily mean the presence of massive lesions. The aim of this study was therefore to investigate the features of max BTS and their impact on survival and radiological response in patients with advanced NSCLC undergoing ICI monotherapy.
Methods
Patients
We retrospectively reviewed the medical records of 294 patients with advanced or recurrent NSCLC who had undergone ICI therapy at the Department of Thoracic Oncology and Respiratory Medicine at the Tokyo Metropolitan Cancer and Infectious Diseases Center of Komagome Hospital (Tokyo, Japan) between January 2016 and July 2019. Patients who met the following criteria were included in the analysis: [1] histologically or cytologically confirmed unresectable advanced (stage III or IV) or recurrent NSCLC; [2] undergoing ICI (atezolizumab, nivolumab, or pembrolizumab) monotherapy (i.e., not combined with other antitumor agents) either as first-line or later-line therapy; and [3] evaluable tumor burden (detailed later) at baseline according to RECIST 1.1 [19]. We also examined the following clinical factors as baseline characteristics: sex, age, Eastern Cooperative Oncology Group-Performance Status (ECOG-PS), histological subtype, epidermal growth factor receptor (EGFR) mutation status, programmed death-ligand 1 (PD-L1) expression, staging (Union for International Cancer Control classification, 8th edition) [15], lines of chemotherapy, serum albumin levels, lactate dehydrogenase (LDH) levels, neutrophil-to-lymphocyte ratio (NLR), and Gustave Roussy Immune Score (GRIm-Score), which is calculated from albumin, LDH, and NLR values [20].
Evaluation of baseline tumor burden
We reviewed all computed tomography (CT) images for each patient immediately before the start of ICI monotherapy. Tumor measurements were determined through axial CT scans with thicknesses of 5.0 mm or less performed with various scanners. Whole body images were acquired with contrast medium unless there were contraindications. All images were stored with a picture archiving and communication system (Yokogawa Electric Co., Ltd., Tokyo, Japan). The investigator assessed the baseline overall tumor burden and the best objective responses to ICI monotherapy for all CT scans using RECIST 1.1. All measurable lesions up to a maximum of 2 lesions per organ and 5 lesions in total were identified, and the target lesions required the largest diameter to be ≥ 10 mm or the short axis to be ≥ 15 mm to be considered a lymph node lesion. Patients who had no measurable lesion were excluded from the analysis.
Statistical analysis
The study employed descriptive statistics to summarize the patients’ baseline characteristics. Using Fisher’s exact tests for categorical data and a Mann–Whitney U test for continuous variables, we assessed the intergroup differences at baseline and the clinical response to ICI monotherapy. We defined progression-free survival (PFS) as the time elapsed from the start of ICI monotherapy to the first documented disease progression or death. Overall survival (OS) was defined as the time elapsed from the start of ICI monotherapy to death, irrespective of the cause of death. Patients with no disease progression or who died at the time of the analysis were censored at the date of last contact. We estimated the survival distributions (PFS and OS) using the Kaplan–Meier method and compared the intergroup differences using a log-rank test. The potential predictors of survival were explored using a Cox regression. Characteristics with a p value < 0.05 after the univariate analysis were included in the multivariate analysis. The overall response rate (ORR) was defined as the proportion of patients with complete response (CR) or partial response (PR) as their best overall response according to RECIST 1.1. The disease control rate (DCR) was defined as the proportion of patients with CR, PR, or stable disease (SD) as their best overall response. All p values in this study are two-sided, and p < 0.05 was considered statistically significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
Of the 294 patients with NSCLC who underwent ICI therapy, 284 (96.6%) had at least one measurable lesion. Of the 284 patients with measurable lesions, 263 underwent ICI monotherapy, excluding those who were administered ICI in combination with other antitumor agents, and were included in the analysis (Supplemental Fig. 1). The median age of these 263 patients was 69 (range 31–88) years. Overall, 165 patients (62.7%) had adenocarcinoma, and 64 (24.3%) had squamous cell carcinoma. Based on the 8th edition of the TNM classification for lung cancer, a total of 16 (6.1%), 50 (19.0%), 115 (43.7%), and 82 (31.2%) patients presented with stage III, stage IVA, stage IVB, and recurrent disease, respectively. As initial ICI therapy, 140 patients (53.2%) were administered nivolumab, 80 (30.4%) were administered pembrolizumab, and 43 (16.3%) were administered atezolizumab. Table 1 provides a summary of the patients’ characteristics.
Table 1.
Baseline Characteristics of Analyzed Patients (n = 263)
| Characteristics | Total (n = 263) | max BTS > 50 mm (n = 128) | max BTS ≤ 50 mm (n = 135) | p |
|---|---|---|---|---|
| Age, median (range), years | ||||
| Median (range), years | 69 (31–88) | 67 (37–87) | 71 (31–88) | 0.004 |
| Age group, n | ||||
| < 75 years | 189 (71.9) | 99 (77.3) | 90 (66.7) | 0.057 |
| ≥ 75 years | 74 (28.1) | 29 (22.7) | 45 (33.3) | |
| Sex, n (%) | ||||
| Female | 82 (31.2) | 41 (32.0) | 41 (30.4) | 0.791 |
| Smoking status | ||||
| Brinkman index ≥ 400 | 199 (75.7) | 90 (70.3) | 109 (80.7) | 0.061 |
| ECOG-PS, n (%) | ||||
| 0/1 | 183 (69.6) | 74 (57.8) | 109 (80.7) | < 0.001 |
| ≥ 2 | 80 (30.4) | 54 (42.2) | 26 (19.3) | |
| Histological subtypes, n (%) | ||||
| Adenocarcinoma | 165 (62.7) | 74 (57.8) | 91 (67.4) | 0.269 |
| Squamous cell carcinoma | 64 (24.3) | 34 (26.6) | 30 (22.2) | |
| NSCLC, NOS | 25 (9.5) | 16 (12.5) | 9 (6.7) | |
| Other | 9 (3.4) | 4 (3.1) | 5 (3.7) | |
| Staging, n (%) | ||||
| III | 16 (6.1) | 6 (4.7) | 10 (7.4) | 0.001 |
| IVA | 50 (19.0) | 32 (25.0) | 18 (13.3) | |
| IVB | 115 (43.7) | 64 (50.0) | 51 (37.8) | |
| Recurrence | 82 (31.2) | 26 (20.3) | 56 (41.5) | |
| Number of organs involved | ||||
| 0 or 1 | 101 (38.4) | 40 (31.2) | 61 (45.2) | 0.023 |
| ≥ 2 | 162 (61.6) | 88 (68.8) | 74 (54.8) | |
| Presence of brain metastasis, n (%) | ||||
| Yes | 66 (25.1) | 38 (29.7) | 28 (20.7) | 0.117 |
| Presence of bone metastasis, n (%) | ||||
| Yes | 86 (32.7) | 56 (43.8) | 30 (22.2) | < 0.001 |
| Presence of liver metastasis, n (%) | ||||
| Yes | 52 (19.8) | 32 (25.0) | 20 (14.8) | 0.044 |
| PD-L1 expression, n (%) | ||||
| < 1% | 43 (16.3) | 23 (18.0) | 20 (14.8) | 0.92 |
| 1–49% | 39 (14.8) | 18 (14.1) | 21 (15.6) | |
| ≥ 50% | 79 (30.0) | 38 (29.7) | 41 (30.4) | |
| Unknown | 102 (38.8) | 49 (38.3) | 53 (39.3) | |
| EGFR mutation status, n (%) | ||||
| Positive | 27 (10.3) | 15 (11.7) | 12 (8.9) | 0.659 |
| Negative | 205 (77.9) | 97 (75.8) | 108 (80.0) | |
| Unknown | 31 (11.8) | 16 (12.5) | 15 (11.1) | |
| Initially chosen ICI, n (%) | ||||
| Atezolizumab | 43 (16.3) | 21 (16.4) | 22 (16.3) | 0.971 |
| Nivolumab | 140 (53.2) | 67 (52.3) | 73 (54.1) | |
| Pembrolizumab | 80 (30.4) | 40 (31.2) | 40 (29.6) | |
| Lines of previous chemotherapy, n (%) | ||||
| 0 | 58 (22.1) | 32 (25.0) | 26 (19.3) | 0.192 |
| 1 | 122 (46.4) | 52 (40.6) | 70 (51.9) | |
| ≥ 2 | 83 (31.5) | 44 (34.4) | 39 (28.9) | |
| Albumin, g/dL | ||||
| Median (IQR) | 3.6 (3.0–4.0) | 3.3 (2.7–3.7) | 3.9 (3.4–4.2) | < 0.001 |
| LDH, U/L | ||||
| Median (IQR) | 214.5 (182.0–292.0) | 239.0 (188.0–374.5) | 202.5 (175.3–244.0) | < 0.001 |
| NLR | ||||
| Median (IQR) | 4.1 (2.5–6.3) | 5.0 (3.2–8.4) | 3.1 (2.1–5.0) | < 0.001 |
| GRIm score, n (%) | ||||
| 0 | 92 (36.4) | 19 (15.3) | 73 (56.6) | < 0.001 |
| 1 | 74 (29.2) | 40 (32.3) | 34 (26.4) | |
| 2 | 64 (25.3) | 47 (37.9) | 17 (13.2) | |
| 3 | 23 (9.1) | 18 (14.5) | 5 (3.9) | |
BTS baseline tumor size, ECOG-PS Eastern Cooperative Oncology Group-performance status, NSCLC non-small-cell lung cancer; NOS not otherwise specified, PD-L1 programmed death-ligand 1, EGFR epidermal growth factor receptor, ICI immune checkpoint inhibitor, IQR interquartile range, LDH lactate dehydrogenase, NLR neutrophil to lymphocyte ratio, GRIm score Gustave Roussy Immune Score
Baseline tumor burden according to RECIST 1.1
The median number of target lesions and involved organs at the start of ICI therapy was 3 [interquartile range (IQR) 2–4] and 2 (IQR 1–3), respectively. The median total and maximum diameters of the target lesions were 96.5 mm (IQR 55.3–144.7) and 49.1 mm (IQR 34.0–69.8), respectively. The most common site for the largest target lesions was the lungs. Table 2 provides a summary of the characteristics of baseline tumor burden according to RECIST 1.1.
Table 2.
Characteristics of baseline tumor burden according to RECIST 1.1
| Number of target lesions | |
| Median [IQR] | 3.0 [2.0–4.0] |
| Number of organs involved | |
| Median [IQR] | 2.0 [1.0–3.0] |
| Total diameter of target lesions (total BTS) | |
| Median [IQR] | 96.5 [55.3–144.7] |
| Maximum diameter of target lesions (max BTS) | |
| Median [IQR] | 49.1 [34.0–69.8] |
| Mean diameter of target lesions | |
| Median [IQR] | 34.7 [24.5–45.0] |
| Site of largest target lesions, n (%) | |
| Primary lesion | 158 (60.1) |
| Bone | 21 (8.0) |
| Pleura | 19 (7.2) |
| Liver | 15 (5.7) |
| Intrathoracic lymph node | 11 (4.2) |
| Adrenal gland | 10 (3.8) |
| Lung (metastasis) | 10 (3.8) |
| Extrathoracic lymph node | 5 (1.9) |
| Brain | 2 (0.8) |
| Other | 12 (4.6) |
RECIST 1.1 Response Evaluation Criteria in Solid Tumors guidelines version 1.1, IQR interquartile range, BTS baseline tumor size
Clinical outcomes of immune checkpoint inhibitor monotherapy in the overall population (n = 263)
The median PFS for the patients who underwent ICI monotherapy was 4.1 months (95% confidence interval [CI] 3.1–5.5), and the median OS was 13.3 months (95% CI 10.9–16.5). The ORR and DCR for all patients to ICI therapy was 26.3% and 43.0%, respectively.
Baseline characteristics of the subgroups with and without massive lesions
The patients with massive lesions defined as maximum diameters > 50 mm (max BTS > 50 mm) tended to have a high median age, poor ECOG-PS, multiple metastatic sites, bone metastasis, liver metastasis, and high GRIm scores (calculated from serum concentrations of albumin and lactate dehydrogenase and from the neutrophil–lymphocyte ratio) compared with those without massive lesions (max BTS ≤ 50 mm) (Table 1).
Clinical outcomes of immune checkpoint inhibitor therapy for the subgroups with and without massive lesions
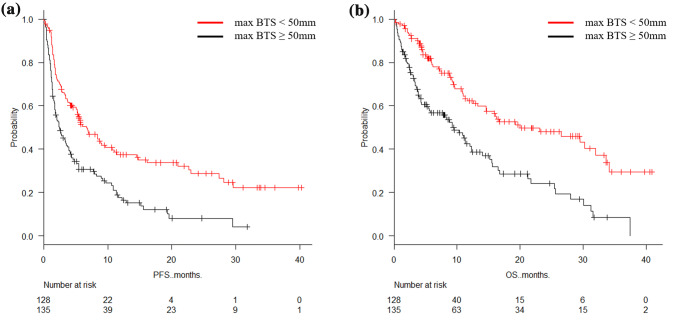
The median PFS for the patients with massive lesions (max BTS > 50 mm) (n = 128) and those without massive lesions (max BTS ≤ 50 mm) (n = 135) was 2.5 months (95% CI 1.8–3.7) and 6.7 months (95% CI 5.1–9.7), respectively (p < 0.001). The median OS for the patients with max BTS > 50 mm and max BTS ≤ 50 mm was 9.5 months (95% CI 5.5–12.3) and 20.0 months (95% CI 13.3–32.0), respectively (p < 0.001) (Fig. 1). The ORR for the patients with max BTS > 50 mm and max BTS ≤ 50 mm was 25.0% and 26.7%, respectively (p = 0.78). The DCR for the patients with max BTS > 50 mm and max BTS ≤ 50 mm was 43.0% and 65.9%, respectively (p < 0.001) (Table 3).
Fig. 1.
Estimated Kaplan–Meier survival curves for a progression-free survival and b overall survival comparing patients with massive lesions (max BTS > 50 mm) (n = 128) against those without massive lesions (max BTS ≤ 50 mm) (n = 135)
Table 3.
Best Overall Response to ICI Monotherapy According to RECIST 1.1 in the Subgroup With and Without Massive Lesions
| Total (n = 263) | max BTS ≤ 50 mm (n = 135) | max BTS > 50 mm (n = 128) | p | |
|---|---|---|---|---|
| CR, % | 0.4 | 0.7 | 0.0 | – |
| PR, % | 25.9 | 26.7 | 25.0 | 0.78 |
| SD, % | 28.5 | 38.5 | 18.0 | < 0.001 |
| PD, % | 41.4 | 29.6 | 53.9 | < 0.001 |
| ORR, % | 26.3 | 27.4 | 25.0 | 0.78 |
| DCR, % | 54.4 | 65.9 | 43.0 | < 0.001 |
ICI immune checkpoint inhibitor; RECIST 1.1 response evaluation criteria in solid tumors guidelines version 1.1, BTS baseline tumor size, CR complete response, PR partial response, SD stable disease, PD progressive disease, ORR overall response rate, DCR disease control rate
Univariate analysis for progression-free survival and overall survival
The univariate analysis for PFS revealed that smoking status, ECOG-PS, number of organs involved, presence of bone metastasis, presence of liver metastasis, PD-L1 expression, EGFR mutation status, initially chosen ICI, lines of previous chemotherapy, GRIm-Score, and maximum diameter of target lesions were significantly associated with PFS. The univariate analysis for OS showed that smoking status, ECOG-PS, number of organs involved, presence of bone metastasis, presence of liver metastasis, EGFR mutation status, initially chosen ICI, GRIm-Score, and maximum diameter of target lesions were significantly associated with OS (Table 4).
Table 4.
Univariate analysis for progression free survival and overall survival for patients with non-small-cell lung cancer treated immune checkpoint inhibitor monotherapy
| Factor | PFS | OS | ||||
|---|---|---|---|---|---|---|
| Survival at 1 year, % (95% CI) | HR (95% CI) | p | Survival at 1 year, % (95% CI) | HR (95% CI) | p | |
| Age group | ||||||
| < 75 years (n = 189) | 27.2 (20.8–34.0) | 1 | 0.47 | 52.9 (44.9–60.2) | 1 | 0.89 |
| ≥ 75 years (n = 74) | 28.8 (16.1–42.7) | 1.13 (0.81–1.59) | 51.1 (35.8–64.5) | 1.03 (0.70–1.51) | ||
| Sex | ||||||
| Female (n = 82) | 26.6 (17.0–37.2) | 1 | 0.21 | 45.4 (33.6–56.4) | 1 | 0.063 |
| Male (n = 181) | 28.3 (21.2–35.7) | 0.82 (0.61–1.11) | 56.1 (47.3–64.0) | 0.72 (0.51–1.02) | ||
| Smoking status | ||||||
| Brinkman index ≥ 400 (n = 199) | 29.6 (22.7–36.8) | 1 | 0.005* | 55.7 (47,4–63.2) | 1 | 0.032* |
| Brinkman index < 400 (n = 64) | 21.7 (12.3–32.8) | 1.59 (1.15–2.17) | 44.1 (30.8–56.6) | 1.49 (1.03–2.16) | ||
| ECOG-PS | ||||||
| 0/1 (n = 183) | 34.8 (27.4–42.4) | 1 | < 0.001* | 61.1 (52.6–68.5) | 1 | < 0.001* |
| ≥ 2 (n = 80) | 12.3 (5.7–21.5) | 1.92 (1.43–2.56) | 33.3 (22.0–45.1) | 2.65 (1.89–3.73) | ||
| Histological subtype | ||||||
| Squamous (n = 64) | 25.8 (15.2–37.7) | 1 | 0.88 | 57.6 (41.9–70.5) | 1 | 0.55 |
| Non-squamous (n = 199) | 28.5 (21.7–35.6) | 0.97 (0.70–1.35) | 51.1 (43.2–58.5) | 1.13 (0.76–1.68) | ||
| Number of organs involved | ||||||
| 0 or 1 (n = 101) | 41.9 (31.0–52.4) | 1 | < 0.001* | 73.1 (61.3–81.7) | 1 | < 0.001* |
| ≥ 2 (n = 162) | 19.4 (13.3–26.3) | 1.89 (1.39–2.56) | 41.0 (32.5–49.2) | 2.19 (1.51–3.18) | ||
| Presence of brain metastasis | ||||||
| No (n = 197) | 26.8 (20.1–33.9) | 1 | 0.34 | 54.6 (46.2–62.3) | 1 | 0.28 |
| Yes n = 66) | 30.6 (19.7–42.1) | 1.17 (0.85–1.62) | 47.2 (34.2–59.1) | 1.22 (0.85–1.76) | ||
| Presence of bone metastasis | ||||||
| No (n = 177) | 33.7 (26.1–41.5) | 1 | < 0.001* | 64.9 (56.1–72.3) | 1 | < 0.001* |
| Yes (n = 86) | 15.8 (8.6–24.9) | 1.87 (1.40–2.51) | 29.3 (19.3–40.0) | 2.84 (2.02–3.99) | ||
| Presence of liver metastasis | ||||||
| No (n = 211) | 31.3 (24.5–38.3) | 1 | < 0.001* | 57.1 (49.0–64.3) | 1 | 0.0021* |
| Yes (n = 52) | 14.6 (6.5–25.7) | 1.79 (1.29–2.49) | 36.3 (23.2–49.7) | 1.80 (1.24–2.61) | ||
| PD-L1 expression | ||||||
| ≥ 50% (n = 79) | 42.4 (30.1–54.1) | 1 | 0.0014* | 60.7 (47.4–71.6) | 1 | 0.28 |
| < 50% or unknown (n = 184) | 21.6 (15.5–28.3) | 1.73 (1.24–2.42) | 49.3 (40.9–57.1) | 1.24 (0.84–1.83) | ||
| EGFR mutation status | ||||||
| Negative or unknown (n = 236) | 31.1 (24.7–37.6) | 1 | < 0.001* | 57.0 (49.5–63.8) | 1 | < 0.001* |
| Positive (n = 27) | NA | 3.99 (2.59–6.14) | 19.2 (7.0–36.0) | 2.64 (1.70–4.10) | ||
| Initially chosen ICI | ||||||
| Atezolizumab (n = 43) | 18.2 (5.0–37.9) | 1 | 0.0034* | 19.6 (4.0–44.0) | 1 | 0.044* |
| Nivolumab (n = 140) | 23.7 (16.9–31.2) | 0.78 (0.52–1.19) | 50.8 (41.8–59.1) | 0.69 (0.42–1.16) | ||
| Pembrolizumab (n = 80) | 41.3 (28.7–53.4) | 0.47 (0.30–0.77) | 65.8 (51.9–76.6) | 0.48 (0.27–0.86) | ||
| Previous lines of chemotherapy | ||||||
| 0 (n = 58) | 39.8 (24.0–55.1) | 1 | 0.015* | 74.0 (58.6–84.4) | 1 | 0.071 |
| ≥ 1 (n = 205) | 24.3 (18.3–30.8) | 1.63 (1.10–2.41) | 48.4 40.7–55.7) | 1.59 (0.96–2.62) | ||
| GRIm score | ||||||
| Low (0/1) (n = 166) | 34.4 (26.6–42.4) | 1 | < 0.001* | 62.1 (53.2–69.7) | 1 | < 0.001* |
| High (2/3) (n = 87) | 17.1 (9.6–26.6) | 1.89 (1.41–2.55) | 37.8 (26.2–49.3) | 2.31 (1.63–3.28) | ||
| Maximum diameter of target lesions | ||||||
| Max BTS ≤ 50 mm (n = 135) | 37.4 (28.6–46.3) | 1 | < 0.001* | 62.3 (52.3–70.7) | 1 | < 0.001* |
| Max BTS > 50 mm (n = 128) | 17.6 (11.0–25.5) | 1.92 (1.44–2.57) | 42.5 (32.8–52.0) | 2.24 (1.59–3.14) | ||
PFS progression free survival, OS overall survival, NSCLC non-small-cell lung cancer, ICI immune checkpoint inhibitor, HR hazard ratio, CI confidence interval, ECOG-PS eastern cooperative oncology group-performance status, PD-L1 programmed death-ligand 1, EGFR epidermal growth factor receptor, GRIm score Gustave Roussy Immune Score, BTS baseline tumor size
Multivariate analysis for progression-free survival and overall survival
The multivariate analysis for PFS revealed that ECOG-PS, PD-L1 expression, EGFR mutation status, and maximum diameter of target lesions were significantly associated with PFS. The multivariate analysis for OS showed that ECOG-PS, presence of bone metastasis, EGFR mutation status, initially chosen ICI, GRIm score, and maximum diameter of target lesions were significantly associated with OS (Table 5).
Table 5.
Multivariate analysis for progression free survival and overall survival for patients with non-small-cell lung cancer treated immune checkpoint inhibitor monotherapy
| HR (95% CI) | p | |
|---|---|---|
| Independent factors for PFS | ||
| ECOG-PS (≥ 2 vs. 0/1) | 1.43 (1.01–2.01) | 0.043 |
| EGFR mutation status (positive vs. negative/unknown) | 3.60 (2.17–5.97) | < 0.001 |
| Maximum diameter of target lesions (max BTS ≤ 50 mm vs. > 50 mm) | 1.44 (1.03–2.02) | 0.032 |
| Independent factors for OS | ||
| ECOG-PS (≥ 2 vs. 0/1) | 1.81 (1.23–1.43) | 0.0027 |
| Presence of bone metastasis (yes vs. no) | 1.86 (1.24–2.78) | 0.0025 |
| EGFR mutation status (positive vs. negative/unknown) | 3.12 (1.81–5.38) | < 0.001 |
| Initially chosen ICI (pembrolizumab vs. atezolizumab) | 0.51 (0.26–0.97) | 0.041 |
| GRIm score (high vs. low) | 1.56 (1.02–2.38) | 0.039 |
| Maximum diameter of target lesions (max BTS ≤ 50 mm vs. > 50 mm) | 1.51 (1.02–2.22) | 0.038 |
PFS progression free survival, OS overall survival, NSCLC non-small-cell lung cancer, ICI Immune Checkpoint Inhibitor, HR hazard ratio, CI confidence interval, ECOG-PS Eastern Cooperative Oncology Group-performance status, EGFR epidermal growth factor receptor, GRIm score Gustave Roussy Immune Score, BTS baseline tumor size
Discussion
Our study assessed the clinical significance of maximum BTS in patients with advanced NSCLC treated with ICI monotherapy. Our findings revealed significant differences in PFS and OS between the patients with and without massive lesions defined as those with maximum diameters > 50 mm. The multivariate analysis revealed that the presence of massive lesions was independently associated with poor PFS and OS. As far as we know, this is the first study to investigate the clinical significance of max BTS in patients with advanced NSCLC treated with ICI therapy.
Preceding studies have recommended setting the cut-off points for total BTS at approximately 100 mm and have indicated the clinical significance of total BTS (sum of diameters of measurable lesions, maximum of 2 lesions per organ and 5 lesions) according to the RECIST criteria as a prognostic factor for patients with melanoma (n = 459) [17, 18] and NSCLC (n = 58) [16] treated with ICI monotherapy. There are only a few available similar analyses (other than for ICI therapy) on the prognostic value of tumor burden for patients with metastatic renal cell carcinoma (RCC) undergoing molecular targeted therapy [21, 22]. A post hoc analysis of our cohort also validated the prognostic importance of total BTS. Our cohort’s median total BTS was 96.5 mm, which was approximately the same as the total BTS in previous studies. The median PFS for the patients with large total BTS (total BTS > 100 mm) (n = 127) and those with small total BTS (total BTS ≤ 100 mm) (n = 136) was 2.2 months (95% CI 1.7–3.4) and 5.8 months (95% CI 4.8–9.2), respectively (p < 0.001). The median OS for patients with total BTS > 100 mm and total BTS ≤ 100 mm was 8.8 months (95% CI 4.3–11.3) and 19.2 months (95% CI 13.3–28.1), respectively (p < 0.001). The ORR for the patients with total BTS > 100 mm and max BTS ≤ 100 mm was 25.2% and 27.2%, respectively (p = 0.78). The DCR for the patients with max BTX > 100 mm and max BTX ≤ 100 mm was 39.4% and 69.1%, respectively (p < 0.001). We also found a certain positive correlation between max BTS and total BTS (Supplemental Fig. 2). Although there are some discrepancies between max BTS and tumor burden, the former could be a useful surrogate prognostic indicator for the latter. The simplicity and readiness of the max BTS measurement could not only help clinicians quickly extrapolate the survival of patients undergoing ICI monotherapy but also reduce interobserver disagreement in the assessment, thereby indicating the possible superiority of max BTS over total BTS.
Based on our study’s results, we should note the following points in the clinical course of the 2 patient groups divided by max BTS: (1) the shorter survival of the patients with massive lesions (max BTS > 50 mm), despite the lack of difference in ORR between the groups and (2) the higher rate of long-lasting response and the “tail-effect” illustrated in the Kaplan–Meier curves observed in the group without massive lesions (max BTS ≤ 50 mm).
The lower DCR and higher PD rate among the patients with max BTS > 50 mm could be one of the characteristics that contributed to such an outcome. To further characterize the differences between the groups, we conducted a post hoc analysis. We defined patients as responders if they achieved either CR, PR, or continuous SD for more than 6 months; otherwise, they were defined as non-responders. For the patients with follow-ups longer than 6 months or who progressed in less than 6 months, we divided those who achieved PFS for more than 6 months (PFS > 6 months) from those who did not (PFS ≤ 6 months). For the patients with follow-ups longer than 12 months or who died in less than 12 months, we divided those who achieved OS of more than 12 months (OS > 12 months) from those who did not (OS ≤ 12 months). We found significantly lower proportions of responders (33.9% vs. 47.1%, p = 0.047) and those who achieved PFS > 6 months (33.7% vs. 56.7%, p = 0.001) and OS > 12 months (27.1% vs. 45.7%, p = 0.004) among the max BTS > 50 mm patients (Supplemental Table 1).
These results also suggest that patients with massive lesions might be less likely to achieve initial disease control and experience early deterioration. In other words, the absence of massive lesions could predict a higher likelihood of a favorable outcome and long-lasting responses with ICI monotherapy. Possible future strategies for patients with massive lesions might include combining ICI therapy with other anticancer agents (e.g., cytotoxic agents, angiogenesis inhibitors, and other ICIs) or radiotherapy. Currently, combined regimens of ICI and platinum doublets are available for first-line treatment for advanced NSCLC irrespective of PD-L1 expression [9–11]. However, no ICI combination therapy is available for second or later-lines of treatment. In the near future, we might need to consider conducting clinical trials for ICI therapy combined with other anticancer agents or radiotherapy as first or later-lines of treatment based on tumor burden. Max BTS (or total BTS) could be another factor worth considering as a simple indicator of tumor burden and as a potential stratification factor or inclusion criteria in future clinical trials for ICIs or other targeted therapies.
The impact of baseline tumor burden according to PD-L1 expression has not been explored in previous studies [16, 17] but it could be an important factor in selecting the optimal treatment. We therefore further stratified the patients according to PD-L1 expression to explore the impact of max BTS. For patients whose PD-L1 expression was low (0–49%) or unknown, the median PFS for the patients with BTS > 50 mm (n = 90) and those with max BTS ≤ 50 mm (n = 94) was 2.0 months (95% CI 1.5–2.7) and 5.4 months (95% CI 2.9–8.7), respectively (p < 0.001). The median OS for the patients with max BTS > 50 mm and those with max BTS ≤ 50 mm was 8.3 months (95% CI 4.1–11.1) and 23.1 months (95% CI 12.6–33.6), respectively (p < 0.001). The ORR for the patients with max BTS > 50 mm and those with max BTS ≤ 50 mm was 14.4% and 22.3%, respectively (p = 0.19). The DCR for the patients with max BTS > 50 mm and those with max BTS ≤ 50 mm was 35.5% and 58.5%, respectively (p = 0.002) (Supplemental Table 2). For the patients with high PD-L1 expression (≥ 50%), the median PFS of those with max BTS > 50 mm (n = 38) and those with max BTS ≤ 50 mm (n = 41) was 8.0 months (95% CI 2.1–15.2) and 10.7 months (95% CI 5.8–not reached), respectively (p = 0.14). The median OS for the patients with max BTS > 50 mm and those with max BTS ≤ 50 mm was 15.5 months (95% CI 5.5–not reached) and 19.2 months (95% CI 10.8–not reached), respectively (p = 0.18). The ORR for the patients with max BTS > 50 mm and those with max BTS ≤ 50 mm was 39.0% and 50.0%, respectively (p = 0.37). The DCR for the patients with max BTS > 50 mm and those with max BTS ≤ 50 mm was 60.5% and 82.9%, respectively (p = 0.043) (Supplemental Table 3). The prognostic impact of max BTS was reproduced in the subgroup with low or unknown PD-L1 expression with statistical significance. For those patients with high PD-L1 expression, the PFS, OS and ORR in the group with max BTS > 50 mm were numerically shorter or lower (but with no statistical significance) compared with those with max BTS ≤ 50 mm. The DCR was significantly lower in the group with max BTS > 50 mm. Although we cannot draw firm conclusions from this exploratory analysis (considering the relatively small number of patients in each subgroup especially for high PD-L1 expression), the magnitude of the impact of max BTS on the clinical outcomes could differ according to PD-L1 expression. In the subgroup analysis of the KEYNOTE-042 trial, for the patients with low (1–49%) PD-L1 expression, there was no significant difference in OS between Pembrolizumab monotherapy and platinum doublet chemotherapy cohorts [median OS: 20.0 vs. 12.2 months, HR: 0.69 (95% CI 0.77–1.11), and the survival curves crossed at 12 months. Putting these results together with the poor disease control by ICI monotherapy in patients low or unknown PD-L1 expression illustrated in our analysis, max BTS may have greater prognostic impact among those with low PD-L1 expression. Hence, we might redeliberate the optimal treatment strategies, including the combination therapies with other agents or radiotherapy, in future clinical trials on immunotherapy by taking into account patients’ tumor size.
Our study has a number of limitations, the first of which was its retrospective, nonrandomized, single-center nature. The likelihood of an unintentional patient selection bias could therefore not be completely ruled out. Second, the radiographical assessment of the baseline CT scan was performed by thoracic oncologists (who were not specialized in radiology) and was based on reading reports from diagnostic radiologists. Third, the influence of prior chemotherapy or radiotherapy was not considered in this study. At present, pembrolizumab monotherapy and in combination with platinum-based regimens is available for patients with advanced PD-L1 positive (≥ 1%) NSCLC in Japan. Prior treatments might have affected the changes in target lesion size during ICI therapy, which could hinder the generalizability of our results. Previous analyses of patients with renal cell carcinoma undergoing molecular-targeted therapy have shown that the second-line tumor burden was an independent prognostic factor, although tumor burden changes between first-line and second-line therapy did not affect survival [21, 23]. We cannot of course simply translate these results to patients with NSCLC treated with ICI. However, such longitudinal changes might need to be considered in future analyses. Fourth, the presence of “multiple” massive lesions was not taken into account. For some patients, measuring total BTS might be appropriate to more accurately predict the clinical outcomes. However, these limitations might be inevitable considering this study’s retrospective nature. Hence, future prospective studies with larger cohorts based on key clinical characteristics (e.g., PD-L1 expression, lines of treatment and combinations with other agents) are warranted to validate this study’s findings.
Conclusion
The presence of massive lesions (defined as those with maximum diameters > 50 mm) is an independent prognostic factor for patients with advanced NSCLC treated with ICI monotherapy. Although the ORR was similar between the max BTS > 50 mm and ≤ 50 mm groups, the DCR was significantly lower for the max BTS > 50 mm group. Max BTS might be a useful tool for predicting the clinical outcomes of patients undergoing immunotherapy as a parameter reflecting their tumor burden.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1. Flow diagram for enrolled patients (TIF 30 kb)
Supplemental Fig. 2. Correlation between maximum baseline tumor size (max BTS) and total baseline tumor size (total BTS) according to RECIST 1.1 (TIF 64 kb)
Acknowledgments
We would like to thank Enago (https://www.enago.jp/) for the English language review.
Author contributions
TH and YH conceptualized this study. TH, RK and SK acquired the clinical data. TH, YH and YO were responsible for the data interpretation. TH and YH drafted the manuscript. All authors have read and approved the current version of the manuscript.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of Interest
YH has received personal fees from AstraZeneca, Eli Lilly Japan, Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, Kyowa Kirin, and CSL Behring, outside the submitted work. YO has received personal fees from Chugai Pharmaceutical and Takeda Oncology, outside the submitted work. No other potential conflicts of interest were reported.
Ethics approval
The study protocol was approved by the Ethics Committee of the Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital and conducted in accordance with the tenets of the Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Basappa NS, Elson P, Golshayan AR, Wood L, Garcia JA, Dreicer R et al (2011) The impact of tumor burden characteristics in patients with metastatic renal cell carcinoma treated with sunitinib. Cancer 117(6):1183–1189 [DOI] [PubMed] [Google Scholar]
- Bigot F, Castanon E, Baldini C, Hollebecque A, Carmona A, Postel-Vinay S et al (2017) Prospective validation of a prognostic score for patients in immunotherapy phase I trials: the gustave roussy immune score (GRIm-Score). Eur J Cancer 84:212–218 [DOI] [PubMed] [Google Scholar]
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae YK, Pan A, Davis AA, Raparia K, Mohindra NA, Matsangou M et al (2016) Biomarkers for PD-1/PD-L1 blockade therapy in non-small-cell lung cancer: is PD-L1 expression a good marker for patient selection? Clin Lung Cancer 17(5):350–361 [DOI] [PubMed] [Google Scholar]
- Chansky K, Detterbeck FC, Nicholson AG, Rusch VW, Vallieres E, Groome P, et al (2017) The IASLC lung cancer staging project: external validation of the revision of the tnm stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 12(7):1109–21 [DOI] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247 [DOI] [PubMed] [Google Scholar]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092 [DOI] [PubMed] [Google Scholar]
- Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M et al (2018) Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol 36(17):1675–1684 [DOI] [PubMed] [Google Scholar]
- Gibney GT, Weiner LM, Atkins MB (2016) Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 17(12):e542–e551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550 [DOI] [PubMed] [Google Scholar]
- Herzberg B, Campo MJ, Gainor JF (2017) Immune checkpoint inhibitors in non-small cell lung cancer. Oncologist 22(1):81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H, Kondo T, Omae K, Takagi T, Izuka J, Kobayashi H et al (2016) The magnitude of best tumor shrinkage during second-line targeted therapy affects progression-free survival but not overall survival in patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 46(6):568–574 [DOI] [PubMed] [Google Scholar]
- Ishihara H, Kondo T, Yoshida K, Omae K, Takagi T, Iizuka J et al (2017) Evaluation of tumor burden after sequential molecular-targeted therapy in patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 47(3):226–232 [DOI] [PubMed] [Google Scholar]
- Joseph RW, Elassaiss-Schaap J, Kefford R, Hwu WJ, Wolchok JD, Joshua AM et al (2018) Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin Cancer Res 24(20):4960–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsurada M, Nagano T, Tachihara M, Kiriu T, Furukawa K, Koyama K et al (2019) Baseline tumor size as a predictive and prognostic factor of immune checkpoint inhibitor therapy for non-small cell lung cancer. Anticancer Res 39(2):815–825 [DOI] [PubMed] [Google Scholar]
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ et al (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393(10183):1819–1830 [DOI] [PubMed] [Google Scholar]
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379(21):2040–2051 [DOI] [PubMed] [Google Scholar]
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A et al (2019) Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 37(7):537–546 [DOI] [PubMed] [Google Scholar]
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389(10066):255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301 [DOI] [PubMed] [Google Scholar]
- Vokes EE, Ready N, Felip E, Horn L, Burgio MA, Antonia SJ et al (2018) Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 29(4):959–965 [DOI] [PubMed] [Google Scholar]
- Warner AB, Postow MA (2018) Bigger is not always better: tumor size and prognosis in advanced melanoma. Clin Cancer Res 24(20):4915–4917 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Flow diagram for enrolled patients (TIF 30 kb)
Supplemental Fig. 2. Correlation between maximum baseline tumor size (max BTS) and total baseline tumor size (total BTS) according to RECIST 1.1 (TIF 64 kb)
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.