Significance
The Hippo pathway is highly conserved in eukaryotes from yeasts to humans. While individuals with mutations in the Hippo kinase gene MST1 experience recurrent infection, little is known about the role of MST1 and its homolog MST2 in innate immunity. Using the bacterial pathogen Legionella pneumophila to investigate host responses, we found that mammalian immune cells proteolytically modify MST1/2 to regulate programmed cell death as a defense mechanism against infection. This posttranslational modification of MST1/2 is not only triggered by pathogens, but also sterile stimuli involved in various inflammatory conditions. Our study provides insight into how MST1/2 function as a key death coordinator that could serve as a drug target to modulate immune responses.
Keywords: type IV secretion, STK3, STK4, pattern recognition receptors, GSDME
Abstract
The mammalian Hippo kinases, MST1 and MST2, regulate organ development and suppress tumor formation by balancing cell proliferation and death. In macrophages, inflammasomes detect molecular patterns from invading pathogens or damaged host cells and trigger programmed cell death. In addition to lytic pyroptosis, the signatures associated with apoptosis are induced by inflammasome activation, but how the inflammasomes coordinate different cell death processes remains unclear. Here, we identify the crucial role of MST1/2 in inflammasome-triggered cell death. Macrophages proteolytically convert full-length MST1/2 into the MST1/2 N-terminal fragments (MST1/2-NT) when the NLRC4 inflammasome detects flagellin from the pathogenic bacterium, Legionella pneumophila. Activation of the NLRP3 inflammasome by the damage-associated molecular pattern, extracellular ATP, also produces MST1/2-NT. Caspase-1, the protease activated by these inflammasomes, directly cleaves MST1/2, and blockage of caspase-1 inhibits MST1/2-NT production in macrophages challenged with L. pneumophila. Importantly, MST1/2-NT production is critical for macrophages to trigger a set of death processes associated with apoptosis upon inflammasome activation and knocking out Mst1/2 causes dysregulated gasdermin protein cleavage for pyroptotic death. Furthermore, macrophages lacking MST1/2 have increased susceptibility to virulent L. pneumophila, revealing that the Hippo kinases are important restriction factors against the pathogen. These findings demonstrate that proteolytic cleavage of MST1/2 induced by inflammatory stimuli is an immune pathway to regulate programmed cell death in macrophages and uncover a unique link between the tumor-suppressive Hippo kinases and the inflammasomes in innate immunity.
Mammalian cells can undergo various forms of programmed cell death, including pyroptosis and apoptosis, in response to invading pathogens (1). Cytosolic pattern recognition receptors, named inflammasomes, in the host cells are immune detectors for pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) (2, 3). During infection, the NLR family CARD domain containing 4 (NLRC4)/NLR family of apoptosis inhibitor proteins (NAIP) inflammasomes sense conserved PAMPs, such as flagellin from pathogenic bacteria, and activate the cysteine protease caspase-1 (4–8). Consequently, caspase-1 cleaves gasdermin D (GSDMD) to liberate the GSDMD N-terminal domain which forms pores in the cell membranes and results in pyroptosis, a lytic form of cell death (9–11). Similarly, the NLR family pyrin domain containing 3 (NLRP3) inflammasome detects DAMPs, e.g., extracellular ATP from damaged host cells, and triggers pyroptosis through caspase-1 mediated GSDMD cleavage (12, 13).
Apoptosis is a form of programmed cell death triggered by the initiator proteases caspase-8 or caspase-9 upon receiving extrinsic signals through the death receptors or release of cytochrome c from mitochondria, respectively. The initiator caspases then convert caspase-3 and caspase-7 into active apoptosis executioners, leading to chromatin DNA fragmentation, cleavage of poly ADP-ribose polymerase 1 (PARP1), and formation of apoptotic bodies. The membrane-encompassed apoptotic bodies limit release of cellular content into extracellular environments at the early stage of apoptosis (14, 15); however, recent discoveries show that caspase-3 can induce lytic pyroptosis by cleaving another pore-forming protein, gasdermin E (GSDME) (16–18).
Detection of flagellin by the NLRC4/NAIP5 inflammasome is a sophisticated host defense against virulent Legionella pneumophila (Lp) in macrophages. The inflammasome is activated upon sensing flagellin from Lp which possesses a key virulence factor, the Dot/Icm type IV secretion system (T4SS), but is not activated by nonvirulent Lp with a defective T4SS (4–6). While pyroptosis is triggered in response to virulent Lp, mouse macrophages expressing the NAIP5/NLRC4 inflammasome also exhibit several features of apoptosis, such as DNA fragmentation and caspase-7 activation (19–21). In a similar manner, delivery of purified flagellin into macrophages by electroporation activates caspase-1 and the apoptotic caspase-8/-3 (22). Notably, replication of virulent Lp remains largely restricted in Gsdmd−/− macrophages with activated NLRC4 inflammasomes due to profound caspase-8 activation and subsequent cell death, indicating the importance of the multiple death pathways triggered by the inflammasome in pathogen restriction (23, 24). The ability of the NLRC4 inflammasome to activate different caspases and death pathways also has a role in Salmonella pathogenesis (25). Interestingly, even when the death routes through caspase-8 and GSDMD are blocked, the NLRC4 inflammasome is capable of inducing apoptosis and cell lysis via caspase-1 (24–26), indicating the existence of another death cascade. However, the signaling components that coordinate these death routes upon NLRC4 activation remain unclear.
Mammalian STE20-like kinase 1 (MST1) and MST2 are the Hippo kinases responsible for initiating the conserved Hippo pathway that is critical for embryonic development in mammals (27–29). Conditional Mst1/2 gene deletions lead to enlarged tissues and tumors (30–32), demonstrating the regulatory activities of MST1/2 in cell survival and proliferation. Likewise, overproduction of MST1 induces apoptosis in cancer cell lines (33–35). The pro-apoptotic activity is highly enhanced when the MST1/2 N-terminal kinase domains (MST1/2-NT) are separated from their C-terminal regulatory domains via proteolytic cleavage (35–37). Moreover, proteolytic separation from the nuclear export signal in the C-terminal domain allows MST1/2-NT accumulation in the nucleus where these Hippo kinase variants promote chromatin condensation and histone phosphorylation, both hallmarks of apoptosis (38–40). While MST1/2 are well known for their importance in development and cancer, humans with loss-of-function mutations in MST1 experience recurrent infection and have low numbers of circulating lymphocytes (41, 42), suggesting that the tumor-suppressive Hippo kinases influence host defense and survival of immune cells.
Here, we report the integral role of MST1/2 in programmed cell death triggered by the inflammasomes. Our study reveals a unique mechanism by which macrophages proteolytically process MST1/2 into MST1/2-NT upon detection of PAMPs/DAMPs by the inflammasomes. Importantly, MST1 and MST2 act as central coordinators for multiple death processes downstream of the inflammasomes and are critical host factors for restriction of pathogenic Lp in macrophages.
Results
Macrophages Cleave MST1/2 in Response to Flagellin from Virulent Lp.
To investigate the role of the Hippo kinases in innate immunity, we first determined the status of MST1/2 in macrophages during Lp infection. Upon challenge of either mouse immortalized bone marrow–derived macrophages (iBMDMs) or RAW264.7 macrophages with the virulent Lp strain (Lp02) expressing a functional T4SS, we observed decreases of MST1/2 full-length (FL) proteins and the appearance of a faster-migrating band with an estimated molecular weight of ~33 kD (Fig. 1A and SI Appendix, Fig. S1A) similar to that described for MST1/2-NT (35, 36). The fact that the monoclonal antibodies used were directed against the amino-termini of MST1/2 suggested that the 33 kDa fragments include the pro-apoptotic kinase domains within the first 300 amino acids (SI Appendix, Fig. S1B). MST1/2-NT were undetectable in either iBMDMs or RAW264.7 macrophages challenged with the T4SS-defective strain Lp03, suggesting that MST1/2 cleavage is a specific response to the presence of a functional Dot/Icm T4SS in Lp (Fig. 1A and SI Appendix, Fig. S1A). Remarkably, MST1/2 remained in the full-length form in human U937 macrophages challenged with Lp02 (SI Appendix, Fig. S1A), indicating that a host factor absent in the U937 cells might be required for production of MST1/2-NT.
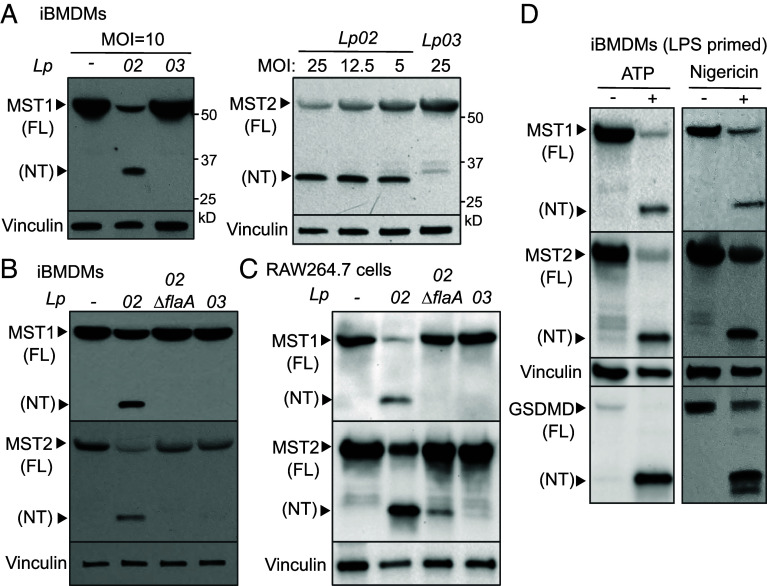
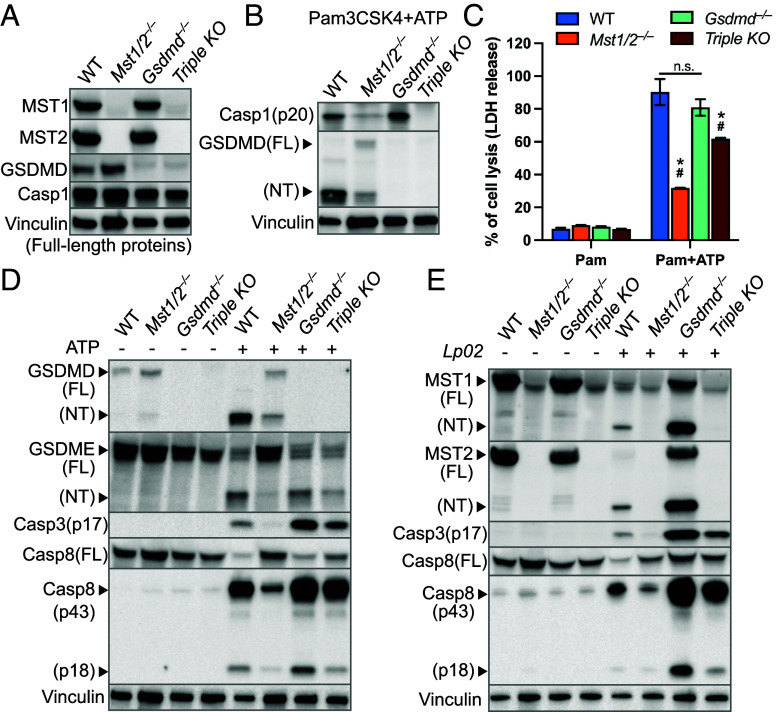
Fig. 1.
Inflammasome activation triggers cleavage of the Hippo kinases. (A) iBMDMs were challenged with the indicated Lp strains for 3 h. Levels of MST1, MST2, and vinculin (as internal controls) in cell lysate were determined by immunoblotting. MOI, multiplicity of infection. (B and C) Macrophages were challenged with the Lp strains for 3 h at MOI =10 for iBMDMs or MOI = 20 for RAW264.7 cells. (D) iBMDMs were primed with LPS for 3 h, followed by ATP stimulation for 3 h or nigericin for 4 h. Levels of MST1, MST2, GSDMD cleavage (as an indicator of NLRP3 activation) and vinculin (as internal controls) were detected by immunoblotting.
U937 cells are highly permissive to Lp intracellular growth and poorly express NAIP for the detection of Lp flagellin (43, 44). Thus, we tested whether MST1/2-NT production is a host response to Lp flagellin. When iBMDMs, RAW264.7, or J774 macrophages expressing the NAIP5/NLRC4 inflammasome were challenged with the Lp strain lacking flagellin (Lp02∆flaA), MST1/2-NT production was minimal or markedly reduced and the level of MST1/2-FL was comparable to that in Lp03-infected macrophages or uninfected control cells (Fig. 1 B and C and SI Appendix, Fig. S1C). Similar results were obtained in human THP-1 cells (SI Appendix, Fig. S1D) expressing higher levels of NAIP for flagellin detection and NLRC4 activation than U937 macrophages (44). Together, these data show that MST1/2-NT are mainly generated under conditions known to result in activation of the NLRC4 inflammasome, pointing toward a possible link between the sensing of the PAMP and MST1/2 processing.
Activation of the NLRP3 Inflammasome by DAMPs Triggers MST1/2 Cleavage.
To assess whether inflammasome activators other than flagellin can trigger MST1/2 processing, iBMDMs were primed with LPS followed by stimulation with DAMPs, including adenosine triphosphate (ATP) or nigericin. The DAMPs triggered GSDMD cleavage, a result of the NLRP3 inflammasome activation, and production of MST1/2-NT (Fig. 1D). MST1-NT was also detected in primed J774 macrophages treated with ATP while the caspase-1 fragment p20 was produced upon NLRP3 activation (SI Appendix, Fig. S1E). Similar results were observed in human THP-1 cells (SI Appendix, Fig. S1F) that have competent NLRP3 inflammasomes responding to nigericin (45). Collectively, MST1/2-NT production occurs in mouse and human macrophages upon activation of the NLRC4 and NLRP3 inflammasomes by PAMPs and DAMPs, respectively.
Mst1 and Mst2 Gene Knockouts in Macrophages.
Using CRISPR-Cas9 gene-editing, we generated Mst1/2 double-knockout (Mst1/2−/−) macrophages. Immunoblotting confirmed the absence of both MST1 and MST2 in several monoclonal iBMDMs (N5, N13, N19, and N12) (SI Appendix, Fig. S2A). Absence of MST1/2 in these iBMDM clones did not affect the levels of other Hippo signaling components, including Mps one binder kinase activator-like 1 (MOB1), large tumor suppressor homolog 1 (LATS1) and Yes-associated protein 1 (YAP1), or non-Hippo-related proteins, such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (SI Appendix, Fig. S2B). Consistent with MST1/2 being responsible for MOB1 phosphorylation (46), Mst1/2−/− iBMDMs had no detectable level of phosphorylated MOB1 (SI Appendix, Fig. S2B). In addition to the Mst1/2−/− clones lacking both kinases, we obtained iBMDMs clones that express either MST1 only (clone N4) or MST2 only (clone N15) (SI Appendix, Fig. S2C). The same approach was used to generate Mst1/2−/− RAW264.7 macrophages (SI Appendix, Fig. S2D).
Mst1/2−/− Macrophages Are Resistant to DAMP-Induced Lytic Cell Death.
Since MST1/2-NT are produced when the inflammasomes are activated, we used a lactate dehydrogenase (LDH) release assay to determine how the lack of MST1/2 affects lytic cell death of LPS-primed iBMDMs upon the DAMP exposure. In contrast to WT iBMDMs, Mst1/2−/− iBMDMs stimulated with ATP for 3 h had markedly reduced LDH release (Fig. 2A). However, the basal levels of NLRP3 in WT and Mst1/2−/− iBMDMs were comparable, and Mst1/2−/− iBMDMs increased NLRP3 and interleukin-1β protein expression upon LPS stimulation as WT iBMDMs did (Fig. 2B). A similar inhibition of ATP-induced cell lysis was observed in Mst1/2−/− macrophages primed with Pam3CSK4 that stimulates NLRP3 expression through a different Toll-like receptor than LPS (Fig. 2A), suggesting that MST1/2 are critical for programmed cell death triggered by the NLRP3 inflammasome.
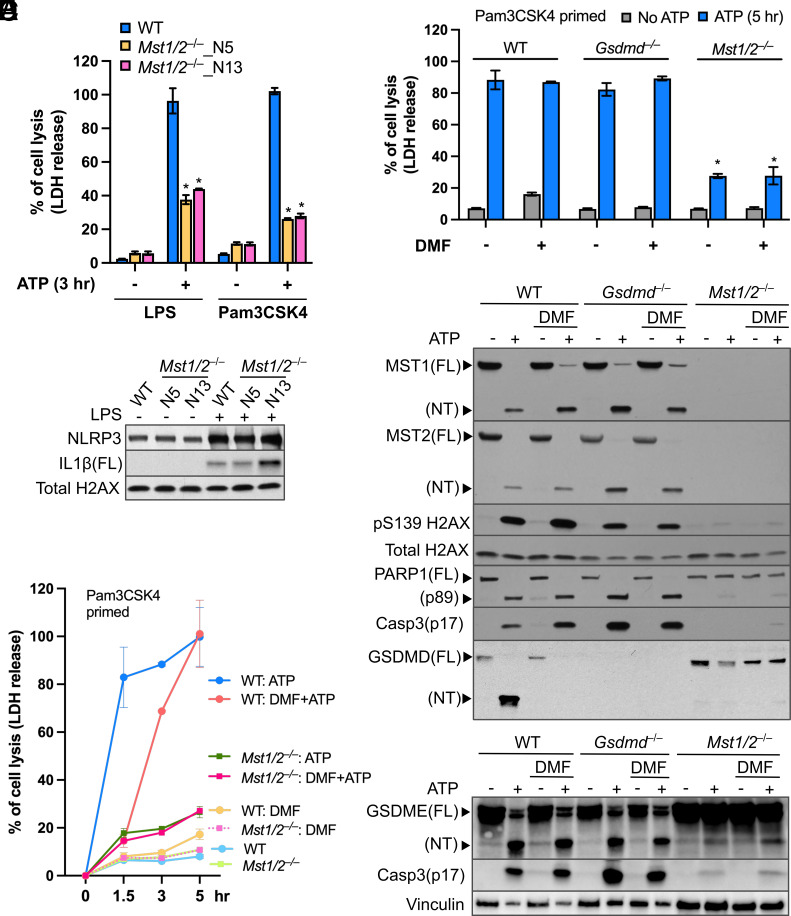
Fig. 2.
The Hippo kinases control lytic cell death in macrophages treated with ATP. (A) LPS- or Pam3CSK4-primed iBMDMs were stimulated with ATP for 3 h. LDH activity within the conditioned media was determined using a colorimetric cytotoxicity assay and converted into percentages of the positive controls (iBMDMs lysed by Triton X-100). Data were presented as mean ± SD of technical triplicates and representative of at least three independent experiments. *P < 0.001, Student’s t test, two tailed; unpaired, compared to WT+ATP. (B) Protein levels of NLRP3 and full-length (FL) IL1β in cell lysate of iBMDMs primed with or without LPS were detected by immunoblotting. Histone H2AX served as an internal control. (C) Time course of ATP-triggered cell lysis in Pam3CSK4-primed iBMDMs with/without the presence of DMF was determined by the LDH release assay described in A. (D) Cell lysis induced by 5-h ATP stimulation in Pam3CSK4-primed iBMDMs treated with/without DMF was measured by the LDH release assay as described in A. *P < 0.001, Student’s t test, two tailed; unpaired, compared to WT iBMDMs receiving the same treatment. (E and F) Levels of the indicated proteins in cell lysates from the experiments in D were determined by immunoblotting. Total H2AX and vinculin served as internal controls.
GSDMD is the main executioner of lytic pyroptosis upon inflammasome activation (10–12). To exclude the possibility that MST1/2-mediated cell death required GSDMD pore-forming activity, we made use of dimethyl fumarate (DMF) that prevents cleavage of GSDMD by caspases (47). While WT iBMDMs stimulated with ATP for 1.5 h had robust LDH release (Fig. 2C), the DMF treatment suppressed cell lysis. Strikingly, DMF-treated WT iBMDMs eventually committed to a delayed cell lysis that was detectable following 3 h of ATP stimulation and reached the same level of cell lysis as macrophages without the DMF treatment after 5 h of stimulation (Fig. 2C). The delayed cell death was also observed in Gsdmd−/− iBMDMs treated with ATP (SI Appendix, Fig. S3A), which is similar to earlier findings (11). This suggests that WT macrophages undergo a cell death that does not require GSDMD pores upon NLRP3 activation. In contrast, LPS- or Pam3CSK4-primed Mst1/2−/− iBMDMs stimulated with ATP did not undergo this delayed cell death regardless of the DMF treatment (Fig. 2 C and D), indicating that MST1/2 modulate ATP-induced cell death independent of GSDMD cleavage.
Death Signatures Associated with Apoptosis Are Diminished in Mst1/2−/− Macrophages Treated with ATP.
Removing the C-terminal regulatory domains allows MST1/2-NT to remain in the cell nucleus and promote phosphorylation of histones, such as histone H2AX on serine 139, a marker of chromatin fragmentation during apoptosis (39, 40). Since NLRP3 activation produces MST1/2-NT, we examined H2AX phosphorylation in ATP-treated macrophages. As expected, phosphorylation of S139 H2AX was strongly enhanced in WT iBMDMs producing MST1/2-NT (Fig. 2E), and additional signatures of apoptosis, including PARP1 cleavage into an 89 kDa fragment and the activated caspase-3 fragment p17, were robustly produced. Consistent with the results in iBMDMs, primary mouse macrophages also triggered MST1/2-NT production, caspase-3 activation, and PARP1 cleavage upon the LPS/ATP treatment (SI Appendix, Fig. S3B). These death signatures and MST1/2-NT were still highly induced by ATP stimulation in WT iBMDMs treated with DMF and Gsdmd−/− iBMDMs (Fig. 2E) but, in contrast, at near-undetectable levels in Mst1/2−/− iBMDMs receiving the same treatments (Fig. 2E), suggesting that MST1/2 are important for these death processes known to be involved in apoptosis.
MST1 and MST2 are highly similar in their protein sequences (SI Appendix, Fig. S1B) and have redundant functions in phosphorylation of the scaffold protein MOB1 (46, 48). To determine whether the two homologous kinases have shared activities in macrophage cell death, we tested the iBMDMs clones that either express MST1 (Mst1+) or MST2 (Mst2+). Upon ATP stimulation, MST1 or MST2 was cleaved in Mst1+ or Mst2+ iBMDMs, respectively, and the cell death processes associated with apoptosis were restored (SI Appendix, Fig. S3C) while Mst1+ iBMDMs induced the death processes at levels comparable to WT iBMDMs. Thus, both Hippo kinases can promote programmed cell death in macrophages treated with ATP, and MST1 is more sufficient in triggering cell death than MST2.
The Hippo Kinases Affect Cleavage of the Gasdermin Proteins for Lytic Cell Death.
Caspase-3 can cause lytic cell death by cleaving gasdermin E (GSDME), another pore-forming protein in the gasdermin family (16, 17). Given that ATP-induced cell lysis and caspase-3 activation are controlled by MST1/2 (Fig. 2 D and E), we sought to determine the role of MST1/2 in GSDME cleavage. With ATP stimulation, WT iBMDMs cleaved both GSDMD and GSDME (Fig. 2 E and F). Blocking GSDMD cleavage by DMF or knocking out GSDMD did not affect caspase-3 activation nor GSDME cleavage, whereas GSDME cleavage was drastically reduced in Mst1/2−/− iBMDMs (Fig. 2F), likely due to low caspase-3 activation. Intriguingly, we found that GSDMD cleavage was also reduced in Mst1/2−/− iBMDMs while WT, Mst1+, or Mst2+ iBMDMs produced high levels of GSDMD-NT upon ATP stimulation (SI Appendix, Fig. S3C). These results demonstrate that MST1/2 influence processing and activation of GSDMD and GSDME in macrophages treated with ATP.
MST1/2 Affect NLRP3 Activation Triggered by ATP.
When exposed to extracellular ATP, macrophages assemble the NLRP3 inflammasome complex to activate caspase-1 that cleaves GSDMD (11, 13). Since GSDMD cleavage is reduced in Mst1/2−/− iBMDMs, we measured production of caspase-1 p20 to determine whether MST1/2 affect NLRP3 activation. Although the levels of full-length caspase-1 were comparable in WT and Mst1/2−/− iBMDMs without ATP stimulation, production of caspase-1 p20 induced by ATP was low in LPS- or Pam3CSK4-primed Mst1/2−/− iBMDMs along with reduced GSDMD cleavage (Fig. 3A). Given that Mst1/2−/− iBMDMs expressed similar levels of NLRP3 to WT iBMDMs (Fig. 2B), the reduction of caspase-1 p20 indicates that MST1/2 regulate activation of the NLRP3 inflammasome. Indeed, when Mst1/2−/− macrophages were transduced to express wildtype MST1, the cells regained the ability to cleave GSDMD upon ATP stimulation (Fig. 3B), which is consistent with the results from the Mst1+, or Mst2+ iBMDM clones (SI Appendix, Fig. S3C).
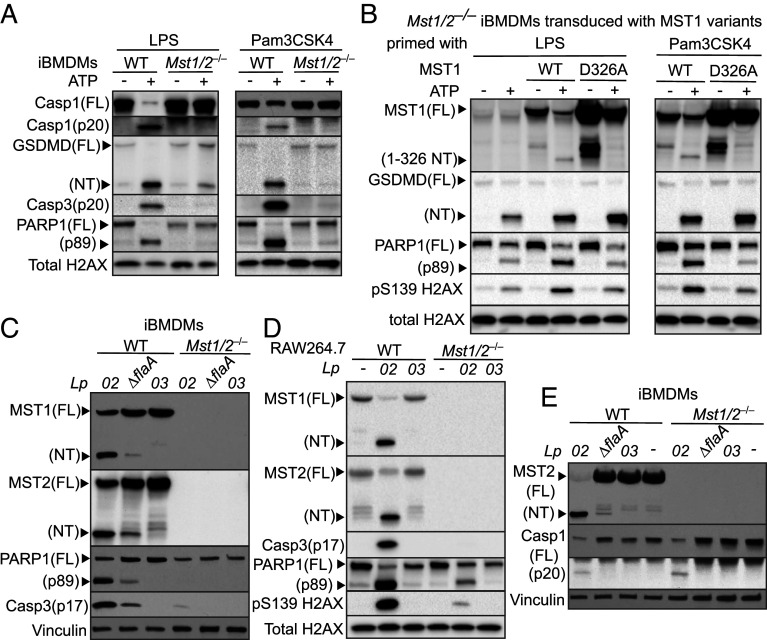
Fig. 3.
The Hippo kinases are critical for various death processes in macrophages upon ATP stimulation and Lp challenge. (A) LPS- or Pam3CSK4-primed iBMDMs were stimulated with ATP for 3 h. (B) Mst1/2−/− iBMDMs were transduced to express WT MST1 or the MST1D326A variant. The transduced iBMDMs were primed with LPS or Pam3CSK4 and stimulated with ATP for 3 h. (C) iBMDMs were challenged with the Lp strains at MOI = 20 for 3 h. (D) RAW264.7 macrophages were challenged with the Lp strains at MOI = 25 for 4.5 h. (E) iBMDMs were challenged with the Lp strains at MOI = 10 for 3 h. Levels and processing of indicated proteins in the cell lysates were analyzed by immunoblotting. Histone H2AX and vinculin served as internal controls.
MST1-NT Controls Distinct Death Processes in ATP-Treated Macrophages.
Inflammasome activation triggers proteolytic cleavage of full-length MST1/2 into MST1/2-NT. Since ATP stimulation activates caspase-1 and the sizes of MST1/2-NT are ~ 33 kD, we speculated that cleavage of MST1/2 is catalyzed by caspase-1 within the region of a.a. 300 to 330. Based on the finding that caspase-1 preferentially cuts the peptide bonds after aspartates (D) in its substrates (49) and MST1 is more effective than MST2 in inducing cell death (SI Appendix, Fig. S3C), we constructed a noncleavable MST1 variant by replacing the D326 residue with an alanine. Although spontaneous fragments larger than the 33 kD MST1-NT were detected in LPS- or Pam3CSK4-primed Mst1/2−/− iBMDMs expressing MST1D326A without ATP stimulation, these large fragments did not trigger cell death (Fig. 3B). Unlike WT MST1 being processed into MST1-NT, MST1D326A was resistant to the ATP-triggered cleavage (Fig. 3B). Remarkably, Mst1/2−/− iBMDMs expressing MST1D326A that failed to produce MST1-NT upon ATP stimulation showed lower levels of PARP1 cleavage and H2AX phosphorylation than Mst1/2−/− iBMDMs expressing WT MST1 (Fig. 3B), while GSDMD cleavage was increased at similar levels in the macrophages expressing WT MST1 or the noncleavable variant (Fig. 3B). Together, the results suggest that full-length MST1/2 are required for NLRP3 activation by ATP and MST1/2-NT production is responsible for the death processes associated with apoptosis.
MST1/2 Regulate Programmed Cell Death in Macrophages Challenged with Lp.
Next, we examined the cell death processes in macrophages during Lp infection. Along with MST1/2-NT production, PARP1 cleavage, caspase-3 activation, and S139 H2AX phosphorylation in WT iBMDMs (Fig. 3C) and RAW264.7 macrophages (Fig. 3D) were strongly induced in response to the virulent Lp02 strain but not the T4SS-null Lp03 strain. In agreement with PARP1 cleavage as a cellular response to DNA fragmentation, challenge of Lp02 caused chromatin DNA laddering in iBMDMs (SI Appendix, Fig. S4A), which also matched with the increased TUNEL positive macrophages upon Lp challenge reported by other groups (19–21). The Lp02 strain without flagellin (Lp02∆flaA) triggered noticeable decreases of MST1/2-NT, PARP1 cleavage, caspase-3 activation, and DNA laddering in iBMDMs (Fig. 3C and SI Appendix, Fig. S4A). In contrast, Mst1/2−/− iBMDMs were defective in activating these cell death processes in response to Lp02 or Lp02∆flaA (Fig. 3C). RAW264.7 macrophages lacking MST1/2 also had highly reduced caspase-3 activation, PARP1 cleavage, and H2AX phosphorylation when challenged with the virulent Lp02 strain (Fig. 3D), reproducibly showing that MST1/2 are critical for these death processes in macrophages. Similar to the responses to ATP, iBMDMs expressing MST1 or MST2 alone restored activation of the death processes upon challenge of Lp02 and higher levels of PARP1 cleavage and caspase-3 activation were detected in Mst1+ iBMDMs than in Mst2+ iBMDMs (SI Appendix, Fig. S4B).
Since knocking out MST1/2 suppressed lytic cell death in macrophages treated with ATP (Fig. 2A), we compared LDH release by WT and Mst1/2−/− iBMDMs challenged with Lp. The LDH release was triggered by Lp in a manner that required the T4SS and flagellin (SI Appendix, Fig. S4C). Surprisingly, unlike the defect in triggering cell lysis in response to ATP, Mst1/2−/− iBMDMs had an increase in LDH release slightly higher than WT iBMDMs when challenged with Lp02 (SI Appendix, Fig. S4C). Recent studies show that macrophages trigger GSDMD cleavage in response to virulent Lp (24, 50), but the impact of GSDME in Lp-induced cell lysis is unclear. We found that Lp02 challenge triggered cleavage of both GSDMD and GSDME in WT iBMDMs (SI Appendix, Fig. S4D). While expressing moderately increased full-length GSDMD, Mst1/2−/− iBMDMs had more GSDMD-NT but less GSDME-NT (SI Appendix, Fig. S4D) than WT iBMDMs upon challenge of Lp02, indicating that the increased GSDMD-NT might compensate the reduction of GSDME-NT in cell lysis. The decreased GSDME cleavage likely resulted from minimal caspase-3 activation in Mst1/2−/− iBMDMs challenged with Lp02 (SI Appendix, Fig. S4D). Intriguingly, the levels of full-length caspase-1 and the activated p20 fragment were similar between Lp02-challenged WT and Mst1/2−/− iBMDMs (Fig. 3E), suggesting that lack of MST1/2 does not affect activation of the NLRC4 inflammasome by virulent Lp.
The NLRC4 Inflammasome Is Required to Trigger MST1/2-NT Production and the Death Signatures Associated with Apoptosis.
The fact that NLRC4 activation was unaffected but the death signatures, for example PARP1 cleavage, were reduced in Lp02-challenged Mst1/2−/− iBMDMs indicates a role of MST1/2 downstream of the NLRC4 inflammasome. Supporting this idea, Nlcr4−/− RAW264.7 macrophages challenged with Lp02 were defective in producing MST1/2-NT and had reduced PARP1 cleavage and H2AX phosphorylation (Fig. 4A). Meanwhile, low levels of MST1/2-NT and the death signatures were detected in Nlcr4−/− macrophages challenged with Lp02 or WT macrophages challenged with Lp02∆flaA (Fig. 4A), indicating that additional host pathways might be involved in MST1/2 cleavage during Lp infection. To further confirm that NLRC4 activation triggers MST1/2 processing, we reconstituted this flagellin-sensing inflammasome in human embryonic kidney (HEK) 293T cells. Upon production of recombinant NLRC4, NAIP5, caspase-1, and Lp flagellin in transiently transfected HEK293T cells, MST1/2-NT and the cell death signatures were readily detectable (Fig. 4B). Moreover, activation of the reconstituted inflammasome by flagellin from Salmonella enterica or Pseudomonas aeruginosa triggered similar levels of MST1/2-NT and cell death as Lp flagellin (Fig. 4C). The data from the reconstitution assays and NLRC4 knockout macrophages demonstrate that MST1/2-NT production is a process downstream of the NLRC4/NAIP5 inflammasome and caspase-1.
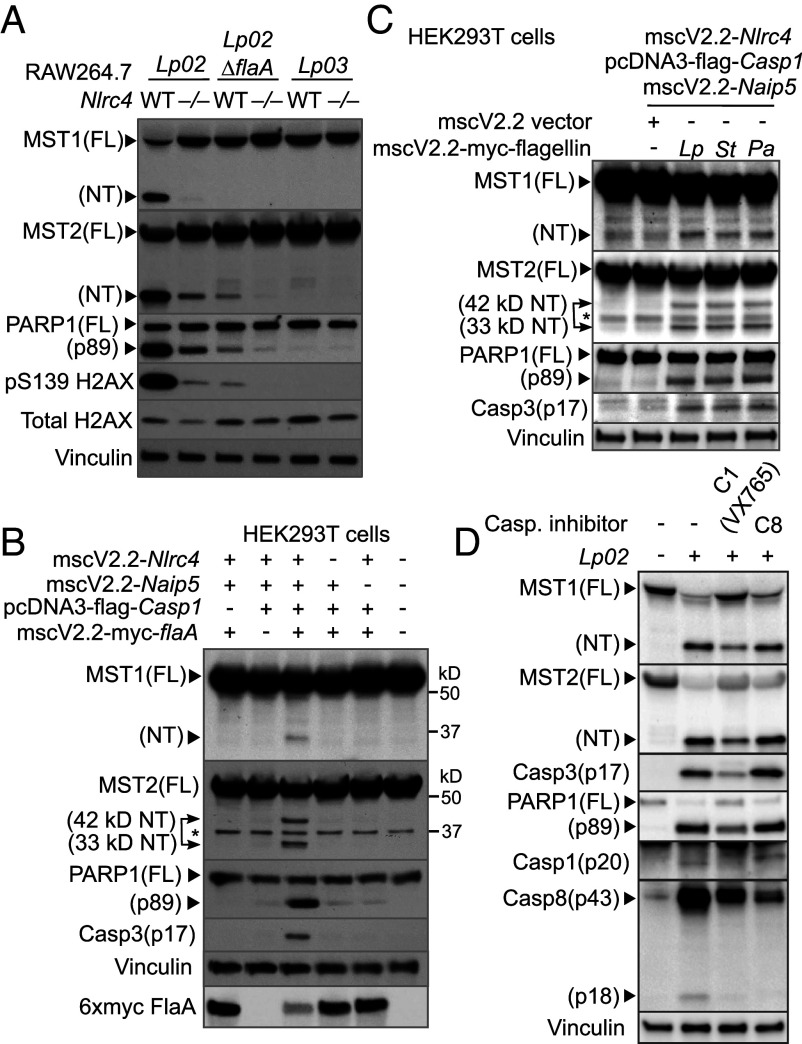
Fig. 4.
Processing of the Hippo kinases is a downstream effect of the NLRC4 inflammasome. (A) WT or Nlrc4−/− RAW264.7 macrophages were challenged with the indicated Lp strains at MOI = 20 for 4.5 h. Levels and processing of the indicated proteins were determined by immunoblotting. (B) Cell lysates from HEK293T cells producing mouse NLRC4, NAIP5, caspase-1, and 6xmyc-tagged Lp flagellin FlaA were collected 24 h after transfection. Levels and processing of the indicated proteins were determined by immunoblotting. *nonspecific, cross-reacting band. An additional 42 kDa MST2-NT was observed in the cells with activated NAIP5/NLRC4 inflammasomes. (C) Mouse NAIP5/NLRC4 inflammasome was reconstituted in HEK293T cells with cotransfection of the flagellin genes encoded by different pathogens using transient transfection as described in B. (D) WT iBMDMs were pretreated with the caspase-1 inhibitor VX765 (100 μM) or caspase-8 inhibitor (200 μM) 1 h prior to challenge of the Lp02 strain (MOI = 20, 3 h). Levels and processing of the indicated proteins were analyzed by immunoblotting. Vinculin served as an internal control.
Caspase-1 Cleaves MST1/2 in Macrophages Challenged with Lp.
Pro-apoptotic agents, such as Fas ligands and staurosporine, induce MST1/2 cleavage via caspase-3 in cancer cells (34, 35). We examined whether caspase-3 is responsible for MST1/2 cleavage in macrophages during Lp infection. When iBMDMs or RAW264.7 cells challenged with Lp02 were treated with the caspase-3 inhibitor Z-DEVD-FMK, production of MST1/2-NT was unaffected (SI Appendix, Fig. S5 A–C). On the other hand, the pan-caspase inhibitor Z-VAD-FMK efficiently prevented MST1/2 processing, suggesting that the MST1/2 cleavage triggered by Lp is mediated by caspases other than caspase-3. Indeed, the caspase-1 inhibitor Z-WEHD-FMK reduced MST1/2 cleavage triggered by Lp although with slightly lower efficiency than the pan-caspase inhibitor (SI Appendix, Fig. S5 B and C), suggesting that caspase-1 and an unidentified cysteine protease are involved in MST1/2 processing. A different caspase-1 inhibitor VX765 that effectively prevented caspase-1 p20 production also suppressed MST1/2 cleavage (Fig. 4D). Consistent with the effects of the caspase-1 inhibitors, RAW264.7 macrophages with caspase-1 knockout had reduced MST1/2-NT when challenged with Lp (SI Appendix, Fig. S5D), confirming the involvement of caspase-1 in MST1/2 cleavage.
Caspase-8 colocalizes with the caspase-1/NLRC4 inflammasome complex during Lp infection (23). Since activated caspase-8 p43 and p18 fragments were detected in WT iBMDMs challenged with Lp02 (Fig. 4D), we tested whether caspase-8 is involved in MST1/2 cleavage. Although the caspase-8 inhibitor Z-IETD-FMK suppressed caspase-8 activation, it did not decrease the levels of MST1/2-NT, PARP1 cleavage, and caspase-3 activation (Fig. 4D). In contrast, these death signatures and caspase-8 activation were reduced in Lp02-infected macrophages by the caspase-1 inhibitors (Fig. 4D and SI Appendix, Fig. S5B) or the pan-caspase inhibitor (SI Appendix, Fig. S5B) along with decreased MST1/2-NT production. Notably, caspase-1 cleaved full-length MST1/2 and generated MST1/2-NT in an in vitro cleavage assay (SI Appendix, Fig. S5E), demonstrating that MST1/2 are direct substrates of caspase-1. The immunoblot detecting the flag tags at the N termini of the recombinant MST1/2 confirmed that these cleavage events occur within MST1/2 (SI Appendix, Fig. S5E). These findings further support that caspase-1 is the main protease for Lp-induced MST1/2 cleavage with a possibility that additional proteases other than caspase-8 or caspase-3 might be involved.
MST1 and MST2 Are the Key Regulators of Pro-Apoptotic Caspase-8 and -3 in Wildtype Macrophages Exposed to ATP or Lp.
While GSDMD cleavage is a route to lytic cell death, we also found that caspase-3 involved in apoptosis can be triggered in WT macrophages with activated NLRP3 or NLRC4 inflammasomes as reported by other groups (51–53). Moreover, activation of the apoptosis initiator, caspase-8, is highly enhanced in Gsdmd−/− macrophages challenged with Lp, which is critical for restriction of Lp in the host cells that cannot undergo GSDMD-mediated cell death (23, 24). To better understand how MST1/2 coordinate activation of these pro-apoptotic caspases in WT and Gsdmd−/− macrophages, we knocked out Mst1 and Mst2 in Gsdmd−/− iBMDMs to construct a triple knockout (TKO) clone (Fig. 5A). While the levels of full-length caspase-1 were similar (Fig. 5A), production of activated caspase-1 p20 was detected in WT and Gsdmd−/− iBMDMs but suppressed in the TKO and Mst1/2−/− iBMDMs (Fig. 5B). Since the Gsdmd−/− iBMDMs were used as the parental cells to make the TKO cells, this result independently confirms the importance of MST1/2 in ATP-induced NLRP3 activation. Interestingly, ATP-induced lytic death in the TKO iBMDMs was at a level lower than in Gsdmd−/− or WT iBMDMs but higher than in Mst1/2−/− iBMDMs (Fig. 5C). Given that the TKO macrophages did not activate caspase-1 but still underwent intermediate cell lysis upon ATP stimulation, this observation indicates that, while MST1/2 are involved, an additional factor contributes to the lytic cell death especially in macrophages lacking GSDMD.
Fig. 5.
The Hippo kinases affect activation of caspases in wildtype macrophages in response to ATP and Lp challenge. (A) Presence or absence of the indicated proteins in the gene knockout iBMDMs was determined by immunoblotting. (B) Pam3CSK4-primed iBMDMs were treated with ATP for 5 h. Activation of caspase-1 and GSDMD cleavage in the cell lysates were analyzed by immunoblotting. (C) Pam3CSK4-primed WT and the gene knockout iBMDMs were stimulated with ATP for 5 h. Cell lysis was determined by the LDH release assay as described in Fig. 2A. Data were presented as mean ± SD of technical triplicates and representative of at least three independent experiments. *P < 0.05 compared to WT+ATP, #P < 0.05 compared to Gsdmd−/−+ATP. Student’s t test, two tailed; unpaired. (D) Cell lysates of the Pam3CSK4-primed iBMDMs after 5 h ATP stimulation were analyzed by immunoblotting. (E) Cell lysates of the iBMDMs challenged with Lp02 (MOI = 20, 3 h) were analyzed by immunoblotting. Vinculin served as an internal control.
Consistent with this idea, Gsdmd−/− iBMDMs treated with ATP robustly activated caspase-8 (p43/p18) and caspase-3 (p17), which was accompanied by GSDME cleavage (Fig. 5D). The TKO iBMDMs showed reduced activation of both caspases and GSDME cleavage when compared to Gsdmd−/− iBMDMs, suggesting that the reduced GSDME cleavage may contribute to the intermediate LDH release and MST1/2 facilitate these death processes when GSDMD is absent. We noticed that the levels of GSDME cleavage and caspase-8/-3 activation in the TKO iBMDMs were relatively comparable to the levels in WT iBMDMs upon ATP stimulation, but the levels of activated caspase-8 (p43/p18) and caspase-3 (p17) were minimal in Mst1/2−/− iBMDMs expressing GSDMD (Fig. 5D). Challenging these macrophages with virulent Lp showed similar phenomena that knocking out Mst1/2 has much stronger impacts on activated caspase-8 (p43/p18) and caspase-3 (p17) in WT iBMDMs expressing GSDMD than in Gsdmd−/− iBMDMs (Fig. 5E). Consistently, the assays using luciferin-conjugated caspase substrates confirmed that the activities of caspase-8 and caspase-3 were both significantly reduced in Mst1/2−/− iBMDMs challenged with the virulent Lp02 strain (SI Appendix, Fig. S6). Together, these results suggest that MST1/2 are particularly critical for activation of caspase-8 and caspase-3 by the inflammasomes in WT macrophages while removal of GSDMD opens an alternative pathway to activate caspase-8 and caspase-3.
MST1/2 Restrict Intracellular Replication of Lp in WT Macrophages.
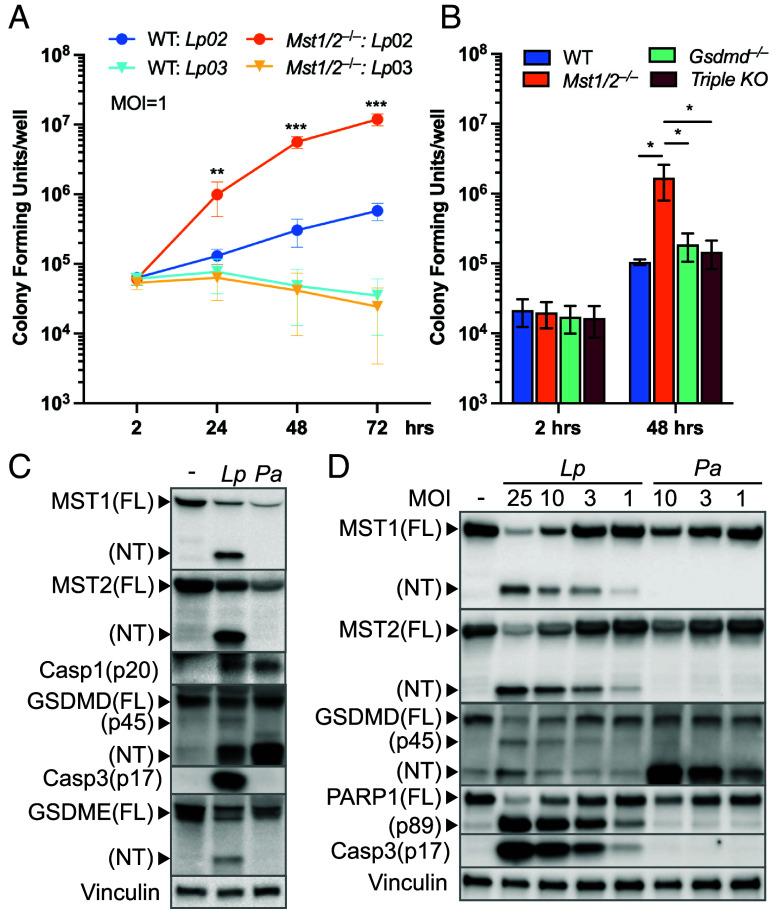
Given the importance of MST1/2 in host programmed cell death, we determined the impacts of MST1/2 on intracellular growth of Lp in macrophages. While WT iBMDMs only allowed mild Lp02 intracellular replication (~ninefold) over 72 h, Mst1/2−/− iBMDMs were severely attenuated in their ability to restrict Lp02 replication, allowing the bacteria to grow more than 150-fold over the same period (Fig. 6A). Notably, both WT and Mst1/2−/− iBMDMs restricted growth of the nonvirulent Lp03 strain (Fig. 6A). Since MST1/2-NT production is only triggered by the virulent Lp02 strain, the data suggest that the cell death processes, such as PARP1 cleavage, caspase-8/-3 activation, and/or GSDME cleavage, mediated by MST1/2-NT play a key role in restriction of pathogenic Lp in WT macrophages expressing GSDMD. Indeed, while MST1 and MST2 contribute to maximal caspase-8/-3 activation in macrophages lacking GSDMD (Fig. 5E), the Gsdmd−/− and TKO iBMDMs that had relatively high levels of activated caspase-8/-3 due to the alternative pathway (Fig. 5E) retained the ability to restrict Lp02 (Fig. 6B).
Fig. 6.
The Hippo kinases are key determinants of host defense against virulent Lp. (A) WT or Mst1/2−/− iBMDMs were challenged with the Lp strains at MOI = 1. iBMDMs were washed at 2 h post infection to remove free Lp. At the indicated time points, iBMDMs were lysed with digitonin to release intracellular Lp. Lp colony-forming units (CFUs) were enumerated by plating assays. Data are the mean ± SD of six biological replicates (N = 6). **P < 0.01, ***P < 0.001 (Student’s t test, two tailed, unpaired, compared to WT: Lp02 at the same time points). (B) Intracellular replication of the virulent Lp02 strain in WT and the gene knockout iBMDMs was determined as described in A. Data are the mean ± SD of four biological replicates (N = 4). *P < 0.05, Student’s t test, two tailed, unpaired. (C and D) WT iBMDMs were challenged with the virulent Lp02 strain or P. aeruginosa at MOI = 10 in C or at various MOIs in D for 3 h. Levels of the indicated proteins in the cell lysates were determined by immunoblotting. Vinculin served as an internal control.
MST1/2-NT Production Triggered by the NLRC4 Inflammasome Is a Unique Host Defense Against Lp.
Since flagellin from different bacterial pathogens trigger MST1/2-NT production and the cell death signatures in HEK293T cells with the reconstituted NLRC4 inflammasome (Fig. 4C), we assessed more closely if processing of MST1/2 also occurs in macrophages challenged with P. aeruginosa that can activate the NLRC4 inflammasome (54). While Lp triggered profound MST1/2-NT production with moderate GSDMD cleavage in WT iBMDMs, P. aeruginosa predominantly induced GSDMD cleavage without detectable MST1/2-NT (Fig. 6C). However, reduction of full-length MST1/2 and similar levels of activated caspase-1 p20 were detected in macrophages challenged with the two pathogens (Fig. 6C), suggesting that the NLRC4 inflammasome chooses between MST1/2 or GSDMD for cleavage depending on the pathogen it encounters. This discrepancy in cleavage substrates remained in macrophages challenged with the pathogens at different MOIs as MST1/2-NT were readily detected in macrophages challenged with low MOIs of Lp but was undetectable in macrophages challenged with P. aeruginosa (Fig. 6D). Moreover, P. aeruginosa-challenged macrophages that preferentially cleaved GSDMD did not activate caspase-3 or cleave PARP1 while Lp robustly induced these death signatures and MST1/2-NT production (Fig. 6D). We noticed that the GSDMD p45 fragment, caused by activated caspase-3 (55), was also detected in iBMDMs challenged with Lp. These results again show that MST1/2-NT production is critical for activation of the death processes associated with apoptosis in macrophages and reveal an interesting mechanism by which the NLRC4 inflammasome can initiate different death processes through cleavage of MST1/2 or GSDMD.
Discussion
The Hippo pathway is highly conserved in animals and plays critical roles in homeostasis and diseases, including organ development and tumorigenesis (27, 28). This study reveals that the Hippo kinases MST1 and MST2 are extensively integrated in the inflammasome network and functioning as regulators for macrophage cell death. Cleaving full-length MST1/2 into MST1/2-NT is a host response to not only the invading pathogen but also exposure of the damage-associated molecular patterns, representing an intriguing mechanism by which macrophages choose different routes to activate various processes of programmed cell death.
Upon a close examination of MST1/2 during Lp infection, we found that the C-terminal regulatory domains are proteolytically removed from the full-length kinases and MST1/2-NT are produced in a manner that requires the virulence factor T4SS and the PAMP flagellin (Fig. 1), leading us to investigate the involvement of host inflammasomes. Supporting the idea that MST1/2 cleavage is an outcome of inflammasome activation, human and mouse macrophages expressing the NLRC4 or NLRP3 inflammasomes trigger MST1/2 cleavage in response to virulent Lp or DAMPs (Fig. 1 and SI Appendix, Fig. S1) together with the results from the Nlrc4−/− macrophages challenged with Lp and the NLRC4 reconstitution assays (Fig. 4 A–C). Importantly, Mst1/2−/− iBMDMs and Mst1/2−/− RAW264.7 macrophages fail to activate a set of molecular processes involved in programmed cell death, including caspase-3 activation, PARP1 cleavage, and histone H2AX phosphorylation, triggered by ATP or Lp infection (Figs. 2 and 3). Moreover, production of MST1-NT in Mst1/2−/− iBMDMs expressing wildtype MST1 restores PARP1 cleavage and histone phosphorylation, whereas these death processes remain minimal in macrophages expressing the MST1D326A variant that is resistant to ATP-induced proteolysis (Fig. 3B), demonstrating that cleavage of the Hippo kinase is a unique way to promote macrophage cell death upon receiving inflammatory stimulation.
Pyroptosis caused by GSDMD cleavage is a lytic cell death triggered by inflammasomes. Previous reports (51–53) and this study (Figs. 2 and 3) show that the death signatures associated with apoptosis, such as PARP1 cleavage and caspase-3 activation, are also induced by DAMPs and bacterial pathogens, highlighting the flexibility of the inflammasomes to activate multiple forms of programmed cell death. This study uncovers MST1/2 as the key regulators downstream of the inflammasomes to promote PARP1 cleavage, caspase-3 activation, and histone phosphorylation (Fig. 3). The analyses of the gasdermin proteins in macrophages challenged with Lp show that the pore-forming gasdermins, GSDMD and GSDME, are cleaved in WT macrophages. In contrast, Mst1/2−/− macrophages have markedly reduced GSDME cleavage, suggesting that MST1/2 can affect pore-forming activity of GSDME by regulating caspase-3 activation (SI Appendix, Fig. S4D). While production of GSDMD-NT (~30 kD) by caspase-1 leads to pore formation, caspase-3 can cleave GSDMD at a different site and produce the intermediate p45 fragment which cannot form pores (55). Consistent with caspase-3 activation by the inflammasome, the GSDMD p45 fragment was also produced in Lp02-infected iBMDMs (Fig. 6D). Interestingly, a recent report shows that primary human macrophages without interferon-priming undergo cell lysis but mainly produce the GSDMD p45 fragment upon challenge of virulent Lp (50), implying that caspase-3 might contribute to lysis of the human cells via cleaving GSDME. Together, these observations indicate a role of MST1/2 in lytic cell death of macrophages in response to virulent Lp by controlling the caspase-3/GSDME route.
While MST1/2 are critical for regulating the caspase-3/GSDME route and PARP1 cleavage downstream of the NLRC4 inflammasome, MST1/2 have an unexpected role in NLRP3 activation by ATP. Using production of caspase-1 p20 as a readout for inflammasome activation, we found that Mst1/2−/− macrophages stimulated with ATP fail to activate the NLRP3 inflammasome, an effect also reproducible in Mst1/2/Gsdmd−/− (TKO) macrophages (Fig. 5B). On the other hand, lack of MST1/2 has no effect on caspase-1 activation upon detection of virulent Lp by the NLRC4 inflammasome (Fig. 3E). In Mst1/2−/− iBMDMs, the defect in NLRP3 activation leads to reduced GSDMD cleavage (Fig. 5B), which is restored in iBMDMs expressing MST1 or MST2 (SI Appendix, Fig. S3C). Since the protein levels of NRLP3 are similar in WT and Mst1/2−/− iBMDMs (Fig. 2B), MST1/2 possibly affect NLRP3 activation through other mechanisms, such as assembly of the NLRP3 complex, posttranscriptional/translational priming, or ATP sensing, that remain to be defined. Intriguingly, Mst1/2−/− iBMDMs expressing the noncleavable MST1D326A variant have minimal PARP1 cleavage and histone phosphorylation, whereas GSDMD cleavage is restored at a level comparable to the level in Mst1/2−/− iBMDMs expressing wildtype MST1 (Fig. 3B). These analyses reveal two distinct activities of the Hippo kinases that production of MST1/2-NT is a way to facilitate the death processes associated with apoptosis while optimal GSDMD cleavage is controlled by full-length MST1/2 in macrophages treated with ATP.
Chemotherapeutic agents induce MST1/2-NT production and apoptosis in cancer cell lines, because proteolytic removal of the C-terminal regulatory domains by caspase-3 highly enhances MST1/2 kinase activity (34–37). Unlike these agents, inflammasome-trigged MST1/2 cleavage is mainly executed by caspase-1 but not caspase-3 (Fig. 4D and SI Appendix, Fig. S5). This agrees with the data where MST1/2 processing requires the presence of caspase-1 in cells with the reconstituted NLRC4 inflammasome (Fig. 4B). The in vitro cleavage assays demonstrate that MST1/2 are direct substrates of caspase-1 (SI Appendix, Fig. S5E), which has not been shown before. We notice that MST1/2 cleavage triggered by Lp challenge is not completely abolished in WT macrophages treated with the caspase-1 inhibitors or in the caspase-1 knockout macrophages (Fig. 4D and SI Appendix, Fig. S5 B-D), suggesting that additional host proteases responding to Lp challenge may also cleave MST1/2. Similarly, in Nlrc4−/− macrophages challenged with Lp02 and WT macrophages challenged with the flagellin-knockout Lp02∆flaA strain, MST1/2-NT production and the death processes are highly attenuated but not completely abolished (Fig. 4A), indicating that host defense pathways other than the NLRC4 inflammasome or stress caused by Lp challenge may activate unidentified proteases to cleave MST1/2. For example, the NLRP3 inflammasome, caspase-11, and caspase-8 can be triggered by the Lp02∆flaA strain in primed macrophages (56–58), and cathepsin proteases are activated upon challenge of wildtype Lp without NLRC4 activation (59). Further investigation is needed to identify additional host proteases for MST1/2 cleavage during Lp infection. The feature of MST1/2-NT production and its contribution in cell death processes triggered by a wide variety of stimuli, including chemotherapeutic agents (34–37, 48), PAMPs and DAMPs, highlights broad implications of MST1/2 in microbial infection, inflammasome-related immune disorders, and cancers.
We identified the Hippo kinases MST1/2 as restriction factors for Lp replication within macrophages (Fig. 6A), aligning with the clinical observation that humans with loss-of-function mutations in MST1 are susceptible to infection (41, 42). Both kinases promote the cell death signatures associated with apoptosis since the MST1/2-NT fragments are highly similar (SI Appendix, Fig. S1B) with MST1 being more effective than MST2 (SI Appendix, Figs. S3C and S4B). Gsdmd−/− macrophages retain the ability to restrict Lp infection because of enhanced caspase-8 activity (23, 24). Our results are in line with these studies and additionally show that MST1/2 facilitate optimal caspase-8 and caspase-3 activation by the inflammasomes while a secondary pathway is likely activating caspase-8/-3 in macrophages lacking GSDMD (Fig. 5 D and E). It is important to note that the Hippo kinases play a more critical role in the death processes associated with apoptosis in WT macrophages than in Gsdmd−/− macrophages since caspase-8/-3 activation are drastically suppressed in Mst1/2−/− macrophages but Gsdmd−/− and the TKO macrophages have caspase-8/-3 activation higher or comparable to WT macrophages due to the secondary pathway caused by lack of GSDMD (Fig. 5 D and E and SI Appendix, Fig. S6). This provides an explanation for the results from the Lp intracellular replication assay that Mst1/2−/− macrophages are permissive to the virulent Lp02 strain while Gsdmd−/− and TKO macrophages remain restrictive (Fig. 6B). Interestingly, Gsdmd/Caspase-8 double-knockout macrophages retain the ability to restrict Lp (24), suggesting caspase-1 can activate a restriction route that is independent of caspase-8 and GSDMD. Given that caspase-1 cleaves MST1/2, this indicates that MST1 and MST2 may be parts of this route.
Collectively, the results illustrate that, particularly in WT macrophages, MST1/2 cleavage by caspase-1 provides a path allowing the inflammasomes to activate the death processes associated with apoptosis (SI Appendix, Fig. S7). This also fits with the findings that, despite the NLRC4 inflammasome being activated at similar levels (i.e., similar caspase-1 p20 production, Fig. 6C), P. aeruginosa triggers strong GSDMD cleavage without detectable MST1/2-NT but Lp induces robust MST1/2-NT production, PARP1 cleavage, and caspase-3 activation (Fig. 6D). While the NLRC4 inflammasome may choose between GSDMD or MST1/2 to activate different death processes, the influence from effectors secreted by the pathogens on the choice of GSDMD or MST1/2 for cleavage remains to be determined. The increased GSDMD cleavage in Mst1/2−/− macrophages challenged with virulent Lp (SI Appendix, Fig. S4D) also implies that caspase-1 activated by the NLRC4 inflammasome may cleave more GSDMD when MST1/2 are not present as a substrate option. Notably, production of GSDMD-NT is reversely correlated with MST1/2-NT production and the death processes associated with apoptosis (Fig. 6D and SI Appendix, Fig. S4D), providing an alternative model that GSDMD-NT may inhibit activation of caspase-8/-3 and PARP1 cleavage. Thus, it is possible that MST1/2 serve as competing substrates for caspase-1 and allows the inflammasomes to activate pro-apoptotic caspase-8 and caspase-3 by reducing GSDMD-NT (SI Appendix, Fig. S7). This substrate-competition model also describes the secondary pathway for highly activated caspase-8/-3 in Gsdmd−/− macrophages and is still compatible with the model that MST1/2-NT is a way to promote caspase-8/-3 activation since knocking out MST1/2 in Gsdmd−/− macrophages reduces the levels of activated caspase-8/-3 (Fig. 5E).
Our study supports that both action models can function in parallel, but that the severity and/or type of the microbial insult ultimately determines which substrate, MST1/2 or GSDMD, is chosen for cleavage upon inflammasome activation (SI Appendix, Fig. S7A). In addition, this study identifies an unanticipated role of the full-length MST1/2 proteins in activation of the NLRP3 inflammasome by ATP and that MST1/2 cleavage is also triggered by the NLRP3 inflammasome to coordinate programmed cell death (SI Appendix, Fig. S7B), revealing the remarkable impacts of the conserved Hippo kinases in innate immunity. While the primary focus of this study is the importance of MST1/2 in cell death of macrophages, arising questions, such as the dynamic relationship between GSDMD and MST1/2 in restriction of Lp pathogenesis and the contribution of MST1/2 in programmed cell death triggered by the inflammasomes besides NLRC4 and NLRP3 remain to be answered.
Materials and Methods
Generating Gene Knockout Macrophages.
The CRISPR/Cas9 technique was used to knock out Mst1 (Stk4), Mst2 (Stk3), or Casp1 genes in macrophages as described previously (48).
Bacterial Cultures.
L. pneumophila Philadelphia-1 strains Lp02, Lp03, and Lp02∆flaA carrying pJB908 plasmids were cultured as reported (48). P. aeruginosa PAO1F strain was cultured in high salt LB broth as described previously (60).
Detailed Materials and Methods used in this study can be found in SI Appendix.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Drs. M.P. Machner and A. Rietsch for feedback on the manuscript, and Dr. J. Kagan for sharing the parental and Gsdmd−/− iBMDMs. We thank members of the Machner lab for their support. This study was funded by the Wayne State University startup funds and University Research Grant 2020-21 (to P.-C.L.), NIH AI182688 (to P.-C.L.), and NIH AI148544 (to Y.H.). D.B. and S.S.L. were supported by the Intramural Research Program of the NIH under Project No. 1ZIAHD008893-12 (to M.P. Machner).
Author contributions
Y.-T.S., S.M.Q., B.M.S.L., and P.-C.L. designed research; Y.-T.S., S.M.Q., B.M.S.L., M.H.A., D.B., S.S.L., and P.-C.L. performed research; M.H.A. and Y.H. contributed new reagents/analytic tools; Y.-T.S., S.M.Q., B.M.S.L., and P.-C.L. analyzed data; P.-C.L. secured funding; and Y.-T.S., S.M.Q., B.M.S.L., and P.-C.L. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All data are included in the manuscript and/or SI Appendix.
Supporting Information
References
- 1.Ketelut-Carneiro N., Fitzgerald K. A., Apoptosis, pyroptosis, and necroptosis—Oh My! The many ways a cell can die. J. Mol. Biol. 434, 167378 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Lamkanfi M., Dixit V. M., Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 28, 137–161 (2012). [DOI] [PubMed] [Google Scholar]
- 3.de Zoete M. R., Palm N. W., Zhu S., Flavell R. A., Inflammasomes. Cold Spring Harb. Perspect. Biol. 6, a016287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren T., Zamboni D. S., Roy C. R., Dietrich W. F., Vance R. E., Flagellin-deficient Legionella mutants evade Caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2, e18 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molofsky A. B., et al. , Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J. Exp. Med. 203, 1093–1104 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamboni D. S., et al. , The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat. Immunol. 7, 318–325 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Kofoed E. M., Vance R. E., Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477, 592–595 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J., Zhao Y., Shi J., Shao F., Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc. Natl. Acad. Sci. U.S.A. 110, 14408–14413 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross C., et al. , Inflammatory caspases: Toward a unified model for caspase activation by inflammasomes. Annu. Rev. Immunol. 40, 249–269 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Shi J., et al. , Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Kayagaki N., et al. , Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015). [DOI] [PubMed] [Google Scholar]
- 12.He W., et al. , Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25, 1285–1298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Y., Hara H., Núñez G., Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 41, 1012–1021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strasser A., O’Connor L., Dixit V. M., Apoptosis signaling. Annu. Rev. Biochem. 69, 217–245 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Elmore S., Apoptosis: A review of programmed cell death. Toxicol. Pathol. 35, 495–516 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers C., et al. , Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 8, 14128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., et al. , Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Jiang M., Qi L., Li L., Li Y., The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 6, 1–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derré I., Isberg R. R., Macrophages from mice with the restrictive Lgn1 allele exhibit multifactorial resistance to Legionella pneumophila. Infect. Immun. 72, 6221–6229 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santic M., Asare R., Doric M., Abu Kwaik Y., Host-dependent trigger of caspases and apoptosis by Legionella pneumophila. Infect. Immun. 75, 2903–2913 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akhter A., et al. , Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 5, e1000361 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee B. L., et al. , ASC- and caspase-8-dependent apoptotic pathway diverges from the NLRC4 inflammasome in macrophages. Sci. Rep. 8, 3788 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascarenhas D. P. A., et al. , Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. PLoS Pathog. 13, e1006502 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonçalves A. V., et al. , Gasdermin-D and Caspase-7 are the key Caspase-1/8 substrates downstream of the NAIP5/NLRC4 inflammasome required for restriction of Legionella pneumophila. PLoS Pathog. 15, e1007886 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doerflinger M., et al. , Flexible usage and interconnectivity of diverse cell death pathways protect against intracellular infection. Immunity 53, 533–547.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang P., et al. , NLRC4 inflammasome-dependent cell death occurs by a complementary series of three death pathways and determines lethality in mice. Sci. Adv. 7, eabi9471 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan D., The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu F.-X., Zhao B., Guan K.-L., Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163, 811–828 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh S., et al. , Crucial role for Mst1 and Mst2 kinases in early embryonic development of the mouse. Mol. Cell Biol. 29, 6309–6320 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou D., et al. , Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song H., et al. , Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc. Natl. Acad. Sci. U.S.A. 107, 1431–1436 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou D., et al. , Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc. Natl. Acad. Sci. U.S.A. 108, E1312–E1320 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ura S., Masuyama N., Graves J. D., Gotoh Y., MST1-JNK promotes apoptosis via caspase-dependent and independent pathways. Genes Cells 6, 519–530 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Lee K.-K., Ohyama T., Yajima N., Tsubuki S., Yonehara S., MST, a physiological caspase substrate, highly sensitizes apoptosis both upstream and downstream of caspase activation. J. Biol. Chem. 276, 19276–19285 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Graves J. D., et al. , Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J. 17, 2224–2234 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kakeya H., Onose R., Osada H., Caspase-mediated activation of a 36-kDa myelin basic protein kinase during anticancer drug-induced apoptosis. Cancer Res. 58, 4888–4894 (1998). [PubMed] [Google Scholar]
- 37.Creasy C. L., Ambrose D. M., Chernoff J., The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J. Biol. Chem. 271, 21049–21053 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Ura S., Masuyama N., Graves J. D., Gotoh Y., Caspase cleavage of MST1 promotes nuclear translocation and chromatin condensation. Proc. Natl. Acad. Sci. U.S.A. 98, 10148–10153 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen W., et al. , MST1 promotes apoptosis through phosphorylation of histone H2AX. J. Biol. Chem. 285, 39108–39116 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung W. L., et al. , Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell 113, 507–517 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Abdollahpour H., et al. , The phenotype of human STK4 deficiency. Blood 119, 3450–3457 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nehme N. T., et al. , MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood 119, 3458–3468 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogel J. P., Andrews H. L., Wong S. K., Isberg R. R., conjugative transfer by the virulence system of Legionella pneumophila. Science 279, 873–876 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Kortmann J., Brubaker S. W., Monack D. M., Cutting edge: Inflammasome activation in primary human macrophages is dependent on flagellin. J. Immunol. 195, 815–819 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zito G., et al. , Cellular models and assays to study NLRP3 inflammasome biology. Int. J. Mol. Sci. 21, 4294 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Praskova M., Xia F., Avruch J., MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr. Biol. 18, 311–321 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Humphries F., et al. , Succination inactivates gasdermin D and blocks pyroptosis. Science 369, 1633–1637 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St. Louis B. M., Quagliato S. M., Su Y.-T., Dyson G., Lee P.-C., The Hippo kinases control inflammatory Hippo signaling and restrict bacterial infection in phagocytes. mBio 15, e03429-23 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamkanfi M., et al. , Targeted peptidecentric proteomics reveals Caspase-7 as a substrate of the Caspase-1 inflammasomes. Mol. Cell. Prot. 7, 2350–2363 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bass A. R., et al. , Human GBP1 facilitates the rupture of the Legionella-containing vacuole and inflammasome activation. mBio 14, e01707-23 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malireddi R. K. S., Ippagunta S., Lamkanfi M., Kanneganti T.-D., Cutting edge: Proteolytic inactivation of poly(ADP-ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. J. Immunol. 185, 3127–3130 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Vasconcelos N. M., et al. , An apoptotic caspase network safeguards cell death induction in pyroptotic macrophages. Cell Rep. 32, 107959 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C., et al. , NLRP3 inflammasome activation triggers gasdermin D–independent inflammation. Sci. Immunol. 6, eabj3859 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miao E. A., Ernst R. K., Dors M., Mao D. P., Aderem A., Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc. Natl. Acad. Sci. U.S.A. 105, 2562–2567 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taabazuing C. Y., Okondo M. C., Bachovchin D. A., Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem. Biol. 24, 507–514.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Case C. L., et al. , Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc. Natl. Acad. Sci. U.S.A. 110, 1851–1856 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Casson C. N., et al. , Caspase-11 activation in response to bacterial secretion systems that access the host cytosol. PLoS Pathog. 9, e1003400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pollock T. Y., Vázquez Marrero V. R., Brodsky I. E., Shin S., TNF licenses macrophages to undergo rapid caspase-1, -11, and -8-mediated cell death that restricts Legionella pneumophila infection. PLoS Pathog. 19, e1010767 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu W., et al. , Sensing cytosolic RpsL by macrophages induces lysosomal cell death and termination of bacterial infection. PLoS Pathog. 11, e1004704 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee P.-C., Zmina S. E., Stopford C. M., Toska J., Rietsch A., Control of type III secretion activity and substrate specificity by the cytoplasmic regulator PcrG. Proc. Natl. Acad. Sci. U.S.A. 111, E2027–2036 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All data are included in the manuscript and/or SI Appendix.