Abstract
Background
Tetrandrine inhibits tumor cell proliferation and demonstrates chemoprevention in cancer models. Speculation on the association between its effects on K+ and Ca2+ channels and cancer chemoprevention has been made. Thapsigargin also affects K+ and Ca2+ conductance. Thapsigargin, however, is a weak tumor promoter in the two-stage model of mouse skin carcinogenesis, yet it can induce apoptosis in androgen-independent prostatic cancer cells. I have postulated that arachidonic acid release from cells in culture is associated with cancer chemoprevention. The effects of tetrandrine and thapsigargin on arachidonic acid release from human colon carcinoma and rat liver cells and prostacyclin production by rat liver cells are compared in the current studies.
Results
Tetrandrine and thapsigargin stimulate arachidonic acid release from human colon carcinoma and rat liver cells and prostacyclin production in rat liver cells. The stimulation by tetrandrine is not affected by incubation with actinomycin D, 100 mM KCl, the [Ca2+]i chelator, 1,2-bis (o-amino-5-fluorophenoxy) ethane-N,N,N',N',-tetraacetic acid tetraacetoxymethylester (BAPTA/AM) or in the absence of extracellular Ca2+. In contrast, stimulation by thapsigargin is inhibited by incubation with actinomycin D, 100 mM KCl, BAPTA/AM or in the absence of extracellular Ca2+.
Conclusion
Both tetrandrine and thapsigargin stimulate arachidonic acid release, but based on the different results obtained in the presence of actinomycin D, the [Ca2+]i chelator, 100 mM KCl and in the absence of extracellular Ca2+, the mechanisms leading to this release and pathways leading to apoptosis and/or cancer chemoprevention may be different. Stimulations by tetrandrine may be mediated by activation of a secretory phospholipase A2, whereas thapsigargin's stimulations may be mediated by the cytoplasmic Ca2+-dependent phospholipase A2.
Background
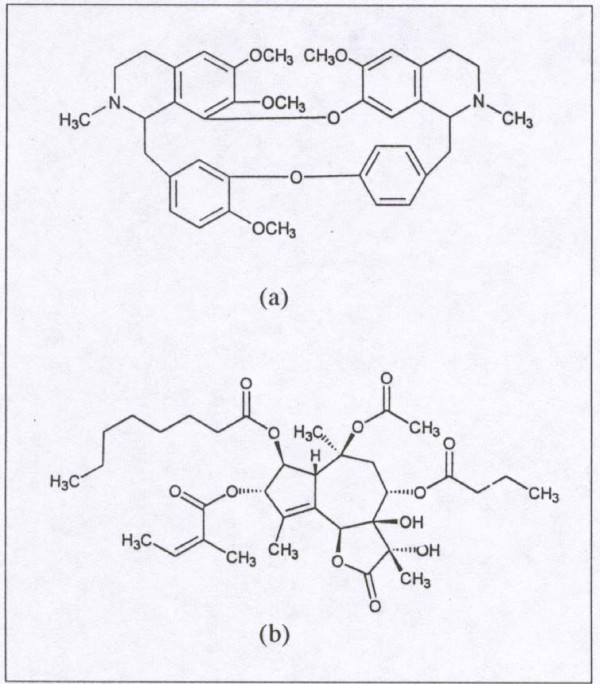
Tetrandrine (TET), a bisbenzylisoquinoline (Fig. 1a), isolated from the root of the plant Stephania tetrandra has a number of potential medicinal properties. These include blockage of voltage-gated Ca2+ channels [1], large-conductance Ca2+ activated K+ (BK) channels, and intracellular Ca2+ pumps [1-6]. TET also has anti-inflammatory [2,7] and anti-cancer activities [8,9]. TET stimulates prostaglandin (PG)E2 production by macrophages [10], probably after first releasing the substrate, arachidonic acid (AA) by altering phospholipase (Plase) activities. TET also induces apoptosis in many cell types including human leukemic (U937), human lung carcinoma (A549), human hepatoblastoma (HEPG2), neuro 2a mouse neuroblastoma and rat glioma cells (C-6) [11-14].
Figure 1.
a): Tetrandrine (TET), isolated from the plant Stephania tetrandra (Structure reproduced with permission from G. Wang [9]) and b): Thapsigargin(THAP), isolated from the plant Thapsia garganica (Structure reproduced with permission from S. B. Christensen [15]).
Thapsigargin (THAP), a hexaoxygenated tetracycle sesquiterpine lactone, (Fig. 1b) isolated from the plant Thapsia garganica, also has a number of potential medicinal applications [15]. However, THAP is classified as a weak tumor promoter as measured in the two-stage model of mouse skin carcinogenesis [16]. Nevertheless, THAP [17] and its enzymatically modified analog [18] have been proposed as targeted therapy for prostate cancer. THAP, like TET, blocks intracellular calcium pumps resulting in increased cytoplasmic Ca2+, ([Ca2+]i) [reviewed in 15]. It also affects ion channels. THAP induces a Ca2+-dependent release of AA from [3H]-AA labelled macrophages and stimulates AA metabolism in the rat peritoneal macrophages [19]. THAP induces apoptosis in many cells including human neuroblastoma, colon cancer and prostate cancer cells and thymocytes [17,20-22].
Based on the stimulation of AA release by known cancer chemopreventative agents, I have proposed that AA release by cells is associated with cancer chemoprevention [23-27], possibly, but not necessarily, by activating a secreted tumor suppressor phospholipase A2 (PLA2) [28,29]. In this report, evidence is presented that TET, a potential cancer chemopreventive compound, and THAP, a weak tumor promoter that also possesses potential cancer preventative properties for androgen-independent prostate cancer, both stimulate AA release from human colon carcinoma and rat liver cells. Both compounds also stimulate prostacyclin (PGI2) production in rat liver cells. The release of AA and AA metabolites appears to be initiated by different mechanisms.
Results
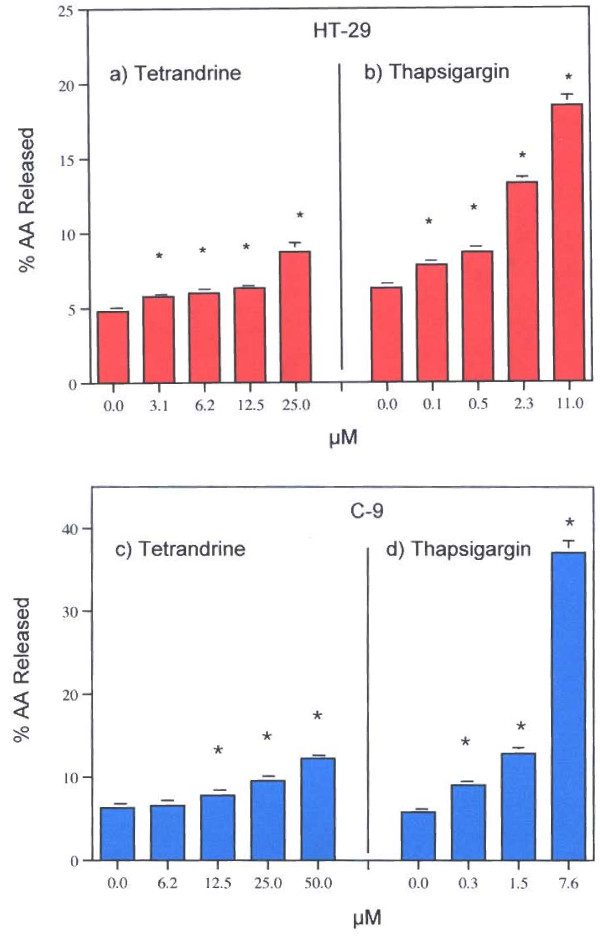
TET and THAP release AA from human colon carcinoma (HT-29) cells and rat liver (C-9) cells in a concentration-dependent fashion (Fig. 2a – 2d respectively). As little as 0.1 to 0.3 μM THAP stimulates AA release. With both HT-29 and C-9 cells, THAP is about 10 to 30 times more potent than TET. Characterizations of these effects are shown in Table 1. Pre-incubation with actinomycin D partially inhibits stimulation by THAP but does not affect stimulation by TET. TET's stimulation of AA release does not require new mRNA synthesis, whereas THAP's stimulation does. As shown below, THAP's stimulation is mediated, in part, by the Ca2+-dependent PLA2 an induced enzyme. The absence of extracellular Ca2+ partially inhibits THAP's stimulations but does not affect the AA release stimulated by TET. Depolarization of the cells with 100 mM KCl does not affect TET's stimulations, but does partially inhibit release of AA stimulated by THAP. Pre-incubation with the L-TYPE Ca2+ channel blocker, diltiazem, has no effect on the actions of either TET or THAP in HT-29 or C-9 cells.
Figure 2.
Effect of a) TET and b) THAP on AA release from HT-29 cells. Effect of c) TET and d) THAP on AA release from C-9 cells. The data are representative of several experiments. The analyses were performed with triplicate or quadruplicate dishes. * = Statistically significant vs MEM/BSA
Table 1.
Effects of Diltiazem (50 μg/ml), Actinomycin D (1 μg/ml), EGTA (1 mM), KCl (100 mM), and BABTA/AM (16 μg/ml) on AA Release from HT-29 or C-9 Cells stimulated by TET or THAP.
| Some biological effects* | Agent tested | Action of test agent | AA Release | |
| HT-29 | C-9 | |||
| TET | Diltiazem | Blocks L-type Ca2+ channels | NI | NI |
| Ion Channels | Actinomycin D | Inhibits RNA synthesis | NI | NI |
| Apoptosis | EGTA | Chelates extracellular Ca2+ | NI | NI |
| Depolarization | 100 mM KCl | Depolarizes | NI | NI |
| [Ca2+]I | BAPTA/AM | Chelates [Ca2+]i | NI | ** |
| THAP | Diltiazem | Blocks L-type Ca2+ channels | NI | NI |
| Ion Channels | Actinomycin D | Inhibits RNA synthesis | ↓ | ↓ |
| Apoptosis | EGTA | Chelates extracellular Ca2+ | ↓ | ↓ |
| Depolarization | 100 mM KCl | Depolarizes | ↓ | ↓ |
| [Ca2+]i | BAPTA/AM | Chelates [Ca2+]I | ↓ | ** |
* = References in text
NI = No Inhibition (or stimulation)
↓ = Inhibition: statistically significant
** = BAPTA/AM (16 μg/ml) stimulates AA release (6.17 ± 0.088 (4)), MEM/BSA control vs (11.6 ± 0.322 (4)), BAPTA/AM (16 μg/ml). Thus, the effect of BAPTA/AM on C-9 cells is not recorded.
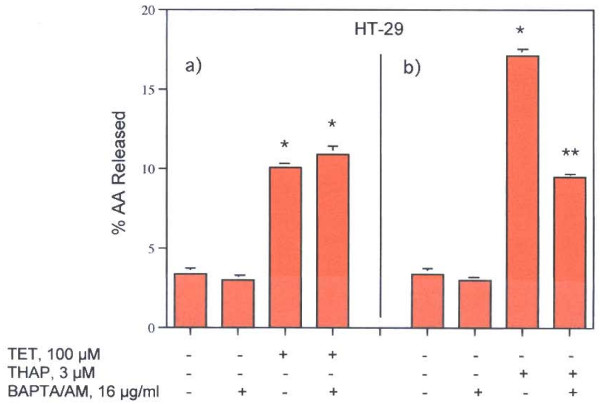
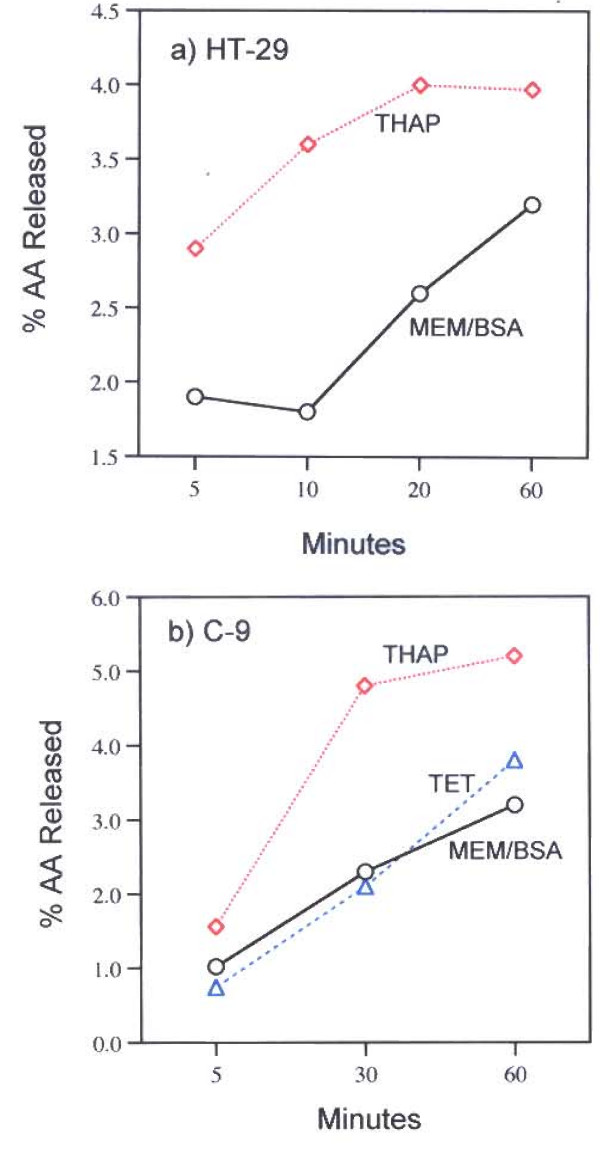
The role of [Ca2+]i in these stimulations is striking. While pre-incubation with the [Ca2+]i chelator, BAPTA/AM (16 μg/ml), does not affect TET's stimulation of AA release from HT-29 cells, such treatment does inhibit THAP's stimulation of AA release from the HT-29 cells (Fig. 3, Table 1). Thus, a role for intracellular Ca2+ pumps is strongly suggested only for the action of THAP. Ohuchi et al [19] had shown that [Ca2+]i increased within 2 min after treatment with THAP as measured by fluorescence changes in Quin 2 loaded peritoneal cells. A similar rise in [Ca2+]i has been measured after administration of THAP to thymocytes, rat liver microsomes [30] and in macrophages, astrocytoma cells, fibroblasts and human tumor lymphocytes [31]. The findings that the increased AA release is stimulated 5 minutes after incubation of HT-29 and C-9 cells with THAP is consistent with a role for increased [Ca2+]i in the activities of THAP (Fig 4a, 4b).
Figure 3.
Effect of BAPTA/AM, 16 μg/ml, on AA release by a) TET and b) THAP from HT-29 cells. The data are representative of two experiments, each with similar results. The analyses were performed on quadruplicate dishes. * = Statistically significant vs MEM/BSA.
Figure 4.
Time-course of AA release from a) HT-29 and b) C-9 cells by TET (50 μM) and THAP (2 μM). The analyses were performed on duplicate dishes. After the 60 minutes incubation, the AA release by TET is not statistically significant. TET's stimulation of AA release from HT-29 cells was not done.
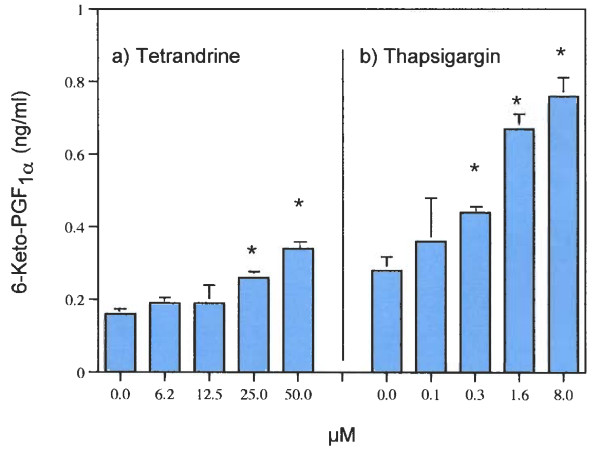
While both cells express COX activity [32,33], only the major product of COX activity (PGI2) can be quantitatively estimated at the low cell densities used in these studies. Both TET and THAP stimulate PGI2 (measured as 6-keto PGF1α) production in these C-9 cells (Fig. 5a, 5b). As with AA release, THAP is at least 10-times more potent at stimulating PGI2 production than TET.
Figure 5.
Effects of a) TET and b) THAP on PGI2 (6-keto-PGF1α) production in rat liver cells (C-9). The data are representative of several experiments. The analyses were performed with triplicate dishes. * = Statistically significant vs MEM/BSA.
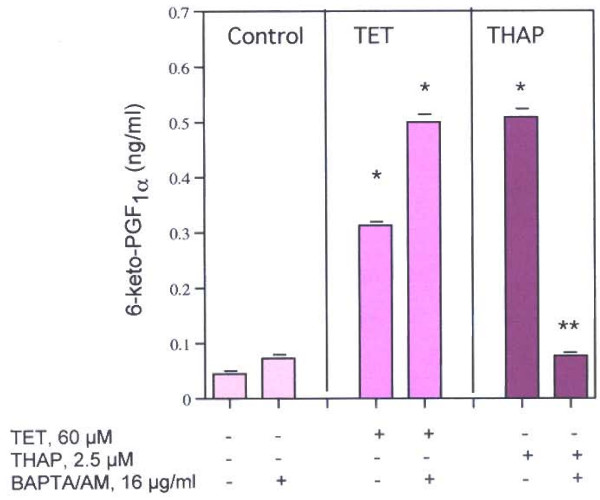
The likely role of [Ca2+]i in the stimulation of PGI2 production by THAP is shown in Fig. 6. Chelation of the increased [Ca2+]i with BAPTA/AM completely inhibits the stimulation by THAP but has no effect on the stimulation with TET.
Figure 6.
Effect of BAPTA/AM, 16 μg/ml on THAP's stimulated PGI2 production by rat liver cells. The data are representative of two experiments, each with similar results. * = Statistically significant vs THAP or BAPTA/AM. ** = Statistically significant vs THAP.
Discussion
It has been proposed that the cancer chemopreventative properties attributed to TET reside in its ability to effect ion channels which leads to inhibition of cell proliferation and apoptosis [9]. Retinoic acids, tamoxifen, PPAR-agonists, (e.g GW-7845), some non-steroidal anti-inflammatory drugs, vitamin D3, anti-oxidants, (e.g resveratrol and caffeic acid phenylester) and statins all release AA from cells in culture [23-26]. All have been shown to have cancer preventative properties. Results of studies on correlation, if any, of AA release and cancer chemoprevention by TET or THAP have not been published. For the purpose of this study, however, I have postulated that the cancer chemopreventive properties of the test agent are causally related to the capacity of that ligand to release AA. I have also suggested that most, if not all, of these agents at μM concentrations, may be intercalating into the cell membrane and releasing AA as a result of activated PLAse activity [25,26].
The mechanism of action of non-steroidal anti-inflammatory drugs that leads to apoptosis and cancer chemoprevention also involves stimulation of AA release and is associated with sphingomyelin to ceramide conversion [34]. The AA release probably results in ceramide-mediated apoptosis [35]. Both TET and THAP stimulate the release of AA from human colon carcinoma and rat liver cells. TET does prevent cancer [reviewed in 8] but THAP is considered to be a weak tumor promotor as measured in the two-stage model of mouse skin carcinogenesis [19]. THAP and some of its analogs, however, do induce apoptosis in androgen-independent prostatic cancer cells [17,18,36]. Apoptotic effects of THAP also have been reported in thymocytes and mouse lymphomas, including the WRH17-2 and WHB12 cell lines [17,20-22].
Clear differences in the pathways of AA release stimulated by TET and THAP were found in this study. TET stimulates AA release (Fig. 2) in HT-29 and C-9 cells and PGI2 production in C-9 cells (Fig. 5). It has been reported that TET blocks voltage-gated Ca2+ channels [1-7] and depolarizes the cells [8], but blockage of these channels does not affect AA release (Table 1). Nor is the AA release stimulated by TET blocked by pre-incubation of the cells with actinomycin D, in the presence of 100 mM KCl or in the absence of extracellular Ca2+. It is interesting to note that cycloheximide (178 μM), did not affect TET's induced apoptosis in human lymphoblasts (CEM-C7) [37]. TET's stimulation of AA release is not affected by the [Ca2+] chelator, BAPTA/AM (Fig. 3). In contrast, THAP's release of AA by HT-29 cells is blocked by BAPTA/AM (Fig. 3), is inhibited by pre-incubation with actinomycin D, is inhibited by EGTA and is inhibited when the cells are incubated in 100 m M KCl (Table 1). That the rise in [Ca2+]i resulting from treatment with THAP appears to be associated with the stimulation of AA release is suggested by the relatively early increase, after 5 minutes, of AA release (Fig. 4). Both TET and THAP stimulate PGI2 production in the C-9 cells (Fig. 5), but only the PGI2 production stimulated by THAP is inhibited by BAPTA/AM (Fig 6).
The relationship between AA release and cancer chemoprevention by TET and THAP could be explained, in part, by implicating the PLA2 enzymes that catalyze the release of AA from phospholipids. The tumor suppressing sPLA2 may be only one of sixteen structurally different PLA2 enzymes [38,39]. If the tumor suppressor genes are overexpressed or activated, at least two cancer preventative pathways may result, one from the activity of the group 11A tumor suppressor [Reviewed in [39]] and a second from the AA released. The proximity of the PLA2 to the AA-esterified phospholipid after treatment of the cells is of importance, and may depend on the location of binding of the test agent. For example, celecoxib binds at the upper hydrocarbon core, close to the phospholipid head groups whereas rofeco
xib binds at the polar head groups of the membrane [40]. Celecoxib and rofecoxib differ in their release of AA [41]. TET may activate a tumor suppressing sPLA2, while THAP induces the Ca2+-dependent cPLA2. Both would lead to AA release.
Conclusion
Cells treated with tetrandrine and thapsigargin share several common features including pathways that lead to induction of apoptosis. These properties probably reflect their interaction with cell membranes and the altered expression of signaling processes. One reaction that does not appear to be shared is deesterification of a phospholipid by a PLA2. Based on the effects of inhibition of mRNA synthesis, 100 mM KCl, extracellular and especially intracellular Ca 2+, thapsigargin's release is mediated, in part, by a Ca 2+-dependent PLA2, whereas tetrandrine's stimulated release is mediated by a secretory PLA2. In addition to the altered signaling properties that accompany the membrane intercalation, both tetrandrine and thapsigargin release biologically active AA.
Methods
The rat liver (C-9 cell line) were purchased from the American Type Culture Collection (Manassas, VA, USA) and the human colon carcinoma (the HT-29 cell line) was obtained from Dr. Basil Rigas, Chief, Division of Cancer Prevention, SUNY at Stony Brook, NY, USA. They were maintained in Eagle's minimum essential medium (MEM) supplemented with 10% fetal bovine serum. [3H] AA (91.8 Ci/mmol) was obtained from NEN Life Science Products, Inc. (Boston, MA, USA). BAPTA/AM, the [Ca2+]i chelator, diltiazem, the L-type Ca2+ channel agonist, tetrandrine and thapsigargin were purchased from BIOMOL International, (Plymouth, PA, USA). All other reagents were from Sigma Chemical Co. (St. Louis, MO, USA) or Calbiochem, (San Diego, CA, USA).
Two days prior to experiments, the HT-29 or C-9 cells were treated with 0.25% trypsin-EDTA and, after addition of minimal essential media (MEM) containing 10% fetal calf serum, the floating cells were seeded on to 35 mm culture dishes. The plating densities varied from 0.1 to 0.5 × 105 cells/35 mm dish. The freshly seeded cultures were incubated for 24-h to allow for cell attachment. After decantation of MEM containing the fetal bovine serum, 1.0 ml fresh MEM containing 10% fetal bovine serum and [3H] AA (0.2 mCi/ml) were added and the cells incubated for another 24-h. The cells were washed 4 times with MEM and incubated for various periods of time with 1.0 ml of MEM containing 1.0 mg BSA/ml (MEM/BSA) and different concentrations of each compound. The culture fluids were then decanted, centrifuged at 2000 × g for 10 min, and 200 μl of the supernate counted for radioactivity. The MEM/BSA values are the control values. Radioactivity recovered in the washes before the incubation was compared to input radioactivity to calculate the % radioactivity incorporated into the cells. For experiments on the effects of actinomycin D, the HT-29 or C-9 cells were washed with MEM 4 times, incubated with actinomycin D (1 μM) for 2-h, washed once and incubated again for 6-h in the presence or absence of actinomycin D (1 μM). For the effects of BAPTA/AM, the pre-incubation was 50 minutes. For PGI2 production, 1.0 ml of MEM supplemented with 10% fetal bovine serum, void of [3H] AA, was added after the first 24-h incubation. The cells were incubated for another 24-h, washed three times with MEM, then incubated with the compounds in MEM/BSA for various periods of time. The culture fluids were decanted and analyzed for 6-keto-PGF1α, the stable hydrolytic product of PGI2, by radioimmunoassay [42].
The [3H] AA release is presented as a percentage of the radioactivity incorporated by the cells. Except for the time-course experiments, which used duplicate dishes, three to five culture dishes were used for each experimental point. The data are expressed as mean values ± SEM. The data were evaluated statistically by the unpaired Student's t-test. A P value < 0.05 was considered significant.
Acknowledgments
Acknowledgements
My thanks to Hilda B. Gjika for preparation of the manuscript and to Dr. Armen H. Tashjian, Jr., Department of Genetic and Complex Diseases, Harvard School of Public Health, for his continuing interest in these studies.
References
- Wang G, Lemos JR. Tetrandrine: a new ligand to block voltage-dependent Ca2+ and Ca+- activated K+ channels. Life Sci. 1995;56:295–306. doi: 10.1016/0024-3205(94)00952-X. [DOI] [PubMed] [Google Scholar]
- Kwan CY, Achike FI. Tetrandrine and related bis-benzylisoquinoline alkaloids from medicinal herbs: cardiovascular effects and mechanisms of action. Acta Pharmacol Sin. 2002;23:1057–1068. [PubMed] [Google Scholar]
- Yao WX, Jiang MX. Effects of tetrandrine on cardiovascular electrophysiologic properties. Acta Pharmacol Sin. 2002;23:1069–1074. [PubMed] [Google Scholar]
- Hagiwara M, Adachi-Akahane S, Nagao T. High-affinity binding of [3H]DTZ323 to the diltiazem-binding site of L-type Ca2+ channels. Eur J Pharmacol. 2003;466:63–71. doi: 10.1016/S0014-2999(03)01547-4. [DOI] [PubMed] [Google Scholar]
- Wu SN. Large-conductance Ca2+- activated K+ channels: physiological role and pharmacology. Curr Med Chem. 2003;10:649–661. doi: 10.2174/0929867033457863. [DOI] [PubMed] [Google Scholar]
- Ransom CB, Liu X, Sontheimer H. BK channels in human glioma cells have enhanced calcium sensitivity. Glia. 2002;38:281–291. doi: 10.1002/glia.10064. [DOI] [PubMed] [Google Scholar]
- Lai JH. Immunomodulatory effects and mechanisms of plant alkaloid tetrandrine in autoimmune diseases. Acta Pharmacol Sin. 2002;23:1093–1101. [PubMed] [Google Scholar]
- Chen YJ. Potential role of tetrandrine in cancer therapy. Acta Pharmacol Sin. 2002;23:1102–1106. [PubMed] [Google Scholar]
- Wang G, Lemos JR, Iadecola C. Herbal alkaloid tetrandrine: from an ion channel blocker to inhibitor of tumor proliferation. Trends Pharmacol Sci. 2004;25:120–123. doi: 10.1016/j.tips.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Pang L, Hoult JR. Cytotoxicity to macrophages of tetrandrine, an antisilicosis alkaloid, accompanied by an overproduction of prostaglandins. Biochem Pharmacol. 1997;53:773–782. doi: 10.1016/S0006-2952(96)00817-9. [DOI] [PubMed] [Google Scholar]
- Lai YL, Chen YJ, Wu TY, Wang SY, Chang KH, Chung CH, Chen ML. Induction of apoptosis in human leukemic U937 cells by tetrandrine. Anticancer Drugs. 1998;9:77–81. doi: 10.1097/00001813-199801000-00009. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kang GH, Kim KC, Kim KM, Park DI, Choi BT, Kang HS, Lee YT, Choi YH. Tetrandrine-induced cell cycle arrest and apoptosis in A549 human lung carcinoma cells. Int J Oncol. 2002;21:1239–1244. [PubMed] [Google Scholar]
- Yoo SM, Oh SH, Lee SJ, Lee BW, Ko WG, Moon CK, Lee BH. Inhibition of proliferation and induction of apoptosis by tetrandrine in HepG2 cells. J Ethnopharmacol. 2002;81:225–229. doi: 10.1016/S0378-8741(02)00082-X. [DOI] [PubMed] [Google Scholar]
- Jin Q, Kang C, Soh Y, Sohn NW, Lee J, Cho YH, Baik HH, Kang I. Tetrandrine cytotoxicity and its dual effect on oxidative stress-induced apoptosis through modulating cellular redox states in Neuro 2a mouse neuroblastoma cells. Life Sci. 2002;71:2053–2066. doi: 10.1016/S0024-3205(02)01989-6. [DOI] [PubMed] [Google Scholar]
- Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases. Trends Pharmacol Sci. 1998;19:131–135. doi: 10.1016/S0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- Hakii H, Fujiki H, Suganuma M, Nakayasu M, Tahira T, Sugimura T, Scheuer PJ, Christensen SB. Thapsigargin, a histamine secretagogue, is a non-12-O-tetradecanoylphorbol-13-acetate (TPA) type tumor promoter in two-stage mouse skin carcinogenesis. J Cancer Res Clin Oncol. 1986;111:177–181. doi: 10.1007/BF00389230. [DOI] [PubMed] [Google Scholar]
- Furuya Y, Lundmo P, Short AD, Gill DL, Isaacs JT. The role of calcium, pH, and cell proliferation in the programmed (apoptotic) death of androgen-independent prostatic cancer cells induced by thapsigargin. Cancer Res. 1994;54:6167–6175. [PubMed] [Google Scholar]
- Denmeade SR, Jakobsen CM, Janssen S, Khan SR, Garrett ES, Lilja H, Christensen SB, Isaacs JT. Prostate-specific antigen-activated thapsigargin prodrug as targeted therapy for prostate cancer. J Natl Cancer Inst. 2003;95:990–1000. doi: 10.1093/jnci/95.13.990. [DOI] [PubMed] [Google Scholar]
- Ohuchi K, Sugawara T, Watanabe M, Hirasawa N, Tsurufuji S, Fujiki H, Christensen SB, Sugimura T. Analysis of the stimulative effect of thapsigargin, a non-TPA-type tumour promoter, on arachidonic acid metabolism in rat peritoneal macrophages. Br J Pharmacol. 1988;94:917–923. doi: 10.1111/j.1476-5381.1988.tb11604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Chow SC, Nicotera P, Orrenius S. Intracellular Ca2+ signals activate apoptosis in thymocytes: studies using the Ca2+-ATPase inhibitor thapsigargin. Exp Cell Res. 1994;212:84–92. doi: 10.1006/excr.1994.1121. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Miyamura A, Takata K, Inden M, Tsuchiya D, Nakamura K, Taniguchi T. Possible involvement of both endoplasmic reticulum-and mitochondria-dependent pathways in thapsigargin-induced apoptosis in human neuroblastoma SH-SY5Y cells. J Pharmacol Sci. 2003;92:228–36. doi: 10.1254/jphs.92.228. [DOI] [PubMed] [Google Scholar]
- He Q, Montalbano J, Corcoran C, Jin W, Huang Y, Sheikh MS. Effect of Bax deficiency on death receptor 5 and mitochondrial pathways during endoplasmic reticulum calcium pool depletion-induced apoptosis. Oncogene. 2003;22:2674–2679. doi: 10.1038/sj.onc.1206363. [DOI] [PubMed] [Google Scholar]
- Levine L. Tamoxifen and the raloxifene analog LY117018: their effects on arachidonic acid release from cell in culture and on prostaglandin I2 production by rat liver cells. BMC Cancer. 2004. [DOI] [PMC free article] [PubMed]
- Levine L. Does the release of arachidonic acid from cells play a role in cancer chemoprevention? FASEB J. 2003;17:800–802. doi: 10.1096/fj.02-0906hyp. [DOI] [PubMed] [Google Scholar]
- Levine L. Tamoxifen stimulates arachidonic acid release from rat liver cells by an estrogen receptor-independent, non-genomic mechanism. BMC Cancer. 2003;3:24. doi: 10.1186/1471-2407-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine L. Statins stimulate arachidonic acid release and prostaglandin I2 production in rat liver cells. Lipids Health Dis. 2003;2:1. doi: 10.1186/1476-511X-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine L. Proteasome inhibitors: their effects on arachidonic acid release from cells in culture and arachidonic acid metabolism in rat liver cells. BMC Pharmacol. 2004;4:15. doi: 10.1186/1471-2210-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier RT, Hong KH, Halberg RB, Hawkins TL, Richardson P, Mulherkar R, Dove WF, Lander ES. Secretory phospholipase Pla2g2a confers resistance to intestinal tumorigenesis. Nat Genet. 1997;17:88–91. doi: 10.1038/ng0997-88. [DOI] [PubMed] [Google Scholar]
- MacPhee M, Chepenik KP, Liddell RA, Nelson KK, Siracusa LD, Buchberg AM. The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin-induced intestinal neoplasia. Cell. 1995;81:957–966. doi: 10.1016/0092-8674(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca 2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Shiff SJ, Rigas B. The role of cyclooxygenase inhibition in the antineoplastic effects of nonsteroidal antiinflammatory drugs (NSAIDs) J Exp Med. 1999;190:445–450. doi: 10.1084/jem.190.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas A, Levine L. Arachidonic acid metabolism by rat liver cells (the C-9 cell line) J Pharmacol Exp Ther. 1984;231:230–235. [PubMed] [Google Scholar]
- Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci U S A. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- Christensen SB, Andersen A, Kromann H, Treiman M, Tombal B, Denmeade S, Isaacs JT. Thapsigargin analogues for targeting programmed death of androgen-independent prostate cancer cells. Bioorg Med Chem. 1999;7:1273–1280. doi: 10.1016/S0968-0896(99)00074-7. [DOI] [PubMed] [Google Scholar]
- Teh BS, Chen P, Lavin MF, Seow WK, Thong YH. Demonstration of the induction of apoptosis (programmed cell death) by tetrandrine, a novel anti-inflammatory agent. Int J Immunopharmacol. 1991;13:1117–1126. doi: 10.1016/0192-0561(91)90163-2. [DOI] [PubMed] [Google Scholar]
- Laye JP, Gill JH. Phospholipase A2 expression in tumours: a target for therapeutic intervention? Drug Discov Today. 2003;8:710–716. doi: 10.1016/S1359-6446(03)02754-5. [DOI] [PubMed] [Google Scholar]
- Diaz BL, Arm JP. Phospholipase A2. Prostaglandins Leukot Essent Fatty Acids. 2003;69:87–97. doi: 10.1016/S0952-3278(03)00069-3. [DOI] [PubMed] [Google Scholar]
- Walter MF, Jacob RF, Day CA, Dahlborg R, Weng Y, Mason RP. Sulfone COX-2 inhibitors increase susceptibility of human LDL and plasma to oxidative modification: comparison to sulfonamide COX-2 inhibitors and NSAIDs. Atherosclerosis. 2004;177:235–243. doi: 10.1016/j.atherosclerosis.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Levine L. Stimulated release of arachidonic acid from rat liver cells by celecoxib and indomethacin. Prostaglandins Leukot Essent Fatty Acids. 2001;65:31–35. doi: 10.1054/plef.2001.0284. [DOI] [PubMed] [Google Scholar]
- Levine L. Measurement of arachidonic acid metabolites by radioimmunoassay. In: Rose NR, Friedman H, Fahey JL, editor. Manual of Clinical Laboratory Immunology. 3. Washington DC: American Society for Microbiology; 1986. pp. 685–691. [Google Scholar]