Abstract
Purpose
Proton therapy could minimize the risk of side effects and, therefore, reduce the possible detrimental effect on health-related quality of life (HRQOL) of re-irradiation. The aim of this study was to determine the effect of re-irradiation with active scanning proton therapy on recurrent glioblastoma (GBM) in terms of HRQOL scored by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-C30 and EORTC Quality of Life Questionnaire Brain Cancer Module (QLQ-BN20).
Methods
Thirty-three patients with recurrent GBM were re-irradiated with active scanning proton therapy. Subscales within the EORTC QLQ-C30 include five functional scales, six single-item scales, and global QoL. The BN20 assessed visual disorders, motor function, communication deficit, various disease symptoms, treatment, toxicity, and future uncertainty. The patients completed the questionnaires before starting proton therapy, the last day of proton therapy, and at every follow-up visit until progression of disease.
Results
The treatment was associated with improvement or stability in most of the preselected HRQOL domains. Global health improved over time with a maximum difference of six points between baseline and 3-months follow-up. Social functioning and motor dysfunction improved over time with a maximum difference of eight and two points, respectively. We showed a non-significant decrease in cognitive and emotional functioning. Fatigue remained stable during the analysis such as the other preselected domains.
Conclusions
Re-irradiation with proton therapy is a safe and effective treatment in patients with recurrent glioblastoma. Proton therapy does not negatively effect on HRQOL, but rather it seems to preserve HRQOL until the time of disease progression.
Keywords: Quality of life, Recurrence glioblastoma, Proton therapy, Re-irradiation
Introduction
Glioblastoma (GBM) is the most common and aggressive primary brain tumor in adults [(Central Brain Tumor Registry of the United States (CBTRUS) 2010)]. Despite advancements in surgery, medical therapy, and radiation therapy (RT), the prognosis remains poor with a median survival of 15 months (Koshy et al. 2012). To date, surgery followed by RT with concomitant and adjuvant temozolomide (TMZ) is the standard of care for newly diagnosed GBM; however, nearly all patients ultimately recur within 2 cm of the resection margin (Minniti et al. 2010). Treatment options at relapse/progression are limited and include surgery, second-line chemotherapy, re-irradiation, or a combination of these. Treatment decision-making is based on type of progression, extent of recurrence, performance, and neurological status of the patient; regardless the type of treatment, median 6-month progression-free survival is 40–50% (Kazmi et al. 2019; Amelio and Amichetti 2012). Re-irradiation is an effective strategy, but is usually employed for small lesions and has the potential risk of both early and late side effects that could adversely affect the quality of life (QoL) of the patient. Among these, radionecrosis is probably the most relevant with an estimated incidence of 5–30% (Amelio and Amichetti 2012; Navarria et al. 2019). Despite the plenty of data regarding GBM re-irradiation, the issue regarding the assessment of patients’ QoL is under evaluated in the majority of series (Scoccianti et al. 2018; Ryu et al. 2014). Proton therapy (PT), thanks to the typical dose fall off of the Bragg peak, could minimize the risk of side effects compared to conventional photon therapy, allowing the treatment of larger recurrent tumors and ultimately reducing the possible detrimental effect of re-irradiation on QoL.
In the present study, we report the outcomes of active scanning PT re-irradiation of recurrent GBM in terms of QoL scored by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-C30 and EORTC Quality of Life Questionnaire Brain Cancer Module (QLQ-BN20). Data have been prospectively collected and analyzed retrospectively. The protocol was reviewed and approved by the local ethics committee (Ethics committee for clinical trials, Azienda Provinciale per i Servizi Sanitari, Trento, Italy).
Materials and methods
Patients and treatment
Between January 2015 and December 2018, 33 consecutive patients with recurrent GBM were re-irradiated with active scanning PT at our institution. Patients aged ≥ 18 years with adequate renal, hepatic, and hematologic function, previously histologically confirmed GBM, KPS (Karnofsky performance status) ≥ 60, time from last radiotherapy ≥ 3 months, were eligible for proton therapy. Exclusion criteria were poor KPS and multifocal progression. All patients had been previously treated with photon radiotherapy (60 Gy in 30 fractions) with concomitant and adjuvant TMZ. Median age and KPS at re-irradiation were 53 years (range 30–68) and 80, (range 60–100), respectively. Target definition was based on computed tomography (CT), magnetic resonance imaging (MRI) with contrast, and fluoro-18-l-dihydroxyphenylalanine PET (18F-DOPA PET) imaging. Gross tumor volume (GTV) included any area of enhancement on MRI plus any pathological PET uptake regions. Clinical target volume (CTV) was generated by adding to the GTV a 3-mm uniform margin manually edited out of anatomical barriers to microscopic tumor spread. The CTV was expanded by 3–4 mm to create the planning target volume (PTV). Median CTV and PTV were 75 and 118 cc, respectively. All patients received 36 GyRBE (RBE: relative biologic effectiveness) in 18 fractions, with concomitant TMZ in 7 patients (25%).
Health-related QOL (HRQOL) assessment
The HRQOL was assessed by European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core-30 (QLQ-C30, version 3) (Aaronson et al. 1993) and EORTC Quality of Life Questionnaire Brain Cancer Module (QLQ-BN20) (Taphoorn et al. 2010a). Subscales within the EORTC QLQ-C30 include five functional scales (physical, role, emotional, cognitive, and social), three symptoms scales (nausea, vomiting, and fatigue), six single-item scales (insomnia, appetite loss, constipation, diarrhea, dyspnea, and financial effect of tumor/treatment), and global QoL. The BN20 is specifically developed for brain patients and assessed visual disorders, motor function, communication deficit, various disease symptoms, treatment, toxicity, and future uncertainty.
The EORTC QLQ-C30 was scored according to the European score manual (Fayers et al. 2001); a higher positive score on a functional scale or on the global health status scale represents improved functioning, whereas higher scores on symptom scales and single-item scales represent a high level of symptomatology/difficulty. The QLQ-BN20 was scored likewise to the QLQ-C30; higher score is associated with clinical deterioration.
The patients completed the EORTC questionnaires before starting PT (t0), the last day of PT (t1), and every follow-up visit [1-month (t2), 3-months (t3), and every 3 months thereafter] until progression of disease. Seven out of 33 patients were not evaluable: 3 experienced disease progression before t3; the remaining 4 patients, because of the neurological and psychological status, were not able or did not agree to fill the questionnaires despite being still without evidence of progression. The twenty-six patients who completed at least three questionnaires before disease progression were included in this analysis. We analyzed Qol data up to 3-month follow-up, because at this time point, most of the patients were free of progression; considering that the median progression-free survival was 5.9 months, the analysis at 6- and 9-month follow-up could be misleading, because most of the patients already experienced tumor progression.
Statistical analysis
Survival (OS) and progression-free survival (PFS) after re-irradiation were calculated from initiation of PT until tumor progression or death (by any cause), according to the Kaplan–Meier method.
HRQOL was measured with two questionnaires: the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life of Cancer Patients (QLQ-C30) and the EORTC QLQ Brain (QLQ-BN20). From these questionnaires, we preselected nine outcome indicators as measures of quality of life on nine domains: physical functioning, cognitive functioning, social functioning, emotional functioning, motor dysfunction, communication deficit, fatigue, insomnia, and global health.
Change during time of the nine outcome domains was tested with repeated measures ANOVA, performed on each domain with linear mixed models. We further used linear mixed models to investigate the effect of different variables (age at proton therapy, CTV and PTV size, chemotherapy, use of steroids at the beginning of the PT, start of steroids use during the PT, and time between two radiation treatments) on the nine outcome domains and their change during time.
All analyses have been performed with R statistical software (R Core Team 2016; Pinheiro et al. 2016).
According to Osoba et al. (1998), a deviation in an individual score by 5–10 points indicated a slight change, by 10–20 points indicated a moderate change, and by > 20 points represented a high clinical relevance. Furthermore, a change of > 10 points was classified as the minimum clinically meaningful change (Minniti et al. 2013).
Results
All patients completed the treatment without breaks. During follow-up, three patients (9%) developed radionecrosis (diagnosed at imaging) with mild symptoms controlled with steroids (grade 2 according to Common Terminology Criteria for Adverse Events version 4.0). The median PFS was 5.9 months, while the 3-, 6- and 9-month PFS rates were 90%, 45% and 11%, respectively. Median OS after PT was 8.7 months, while the 6-, 9- and 12-month survival after PT rates were 100%, 45% and 33%, respectively. One patient did not die due to tumor progression.
Questionnaire response rate and patient characteristics
Before starting PT all patients completed the questionnaires. The response rate among patients was 100% at t1, 89% at t2 (1 month) and 64% at t3 (3 months), which seems an acceptable response rate level considering the histology and the nature of the treatment. The characteristics of the patients who completed HRQOL assessments at baseline (t0) and at least three additional assessment (t1–3) are shown in Table 1.
Table 1.
Patient characteristics
| Number of patients | 26 |
| Gender | |
| Male | 18 |
| Female | 8 |
| Age at re-irradiation (years) | |
| Median | 53.4 |
| Range | 30–69 |
| KPS at re-irradiation | |
| Median | 80 |
| Range | 60–100 |
| Time to previous radiotherapy (months) | |
| Median | 21.3 |
| Range | 5–96 |
| Tumor location | |
| Left side | 13 (50%) |
| Temporal | 4 |
| Frontal | 4 |
| Occipital | 3 |
| Parietal | 2 |
| Right side | 13 (50%) |
| Temporal | 2 |
| Frontal | 7 |
| Occipital | 2 |
| Parietal | 2 |
| Concomitant temozolomide | 7 (25%) |
| Use of corticosteroids during treatment | 19 (76%) |
| Target volumes (cm3) | |
| Median clinical target volume | 75 cc |
| Median planning target volume | 118 cc |
HRQOL changes during proton therapy treatment (t0–t1)
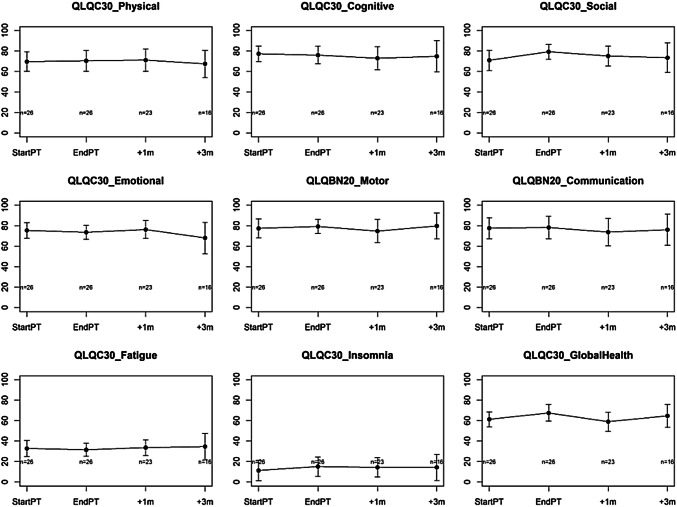
The treatment was associated with a substantial stability in all the preselected HRQOL domains without a detrimental effect on QoL, although there were no statistically significant changes recorded in global QOL for any of the subgroups observed. Despite none of the subscales showing a minimum clinically meaningful change (> 10 points), a near-meaningful positive change (> 5–10 points) was seen in social functioning (8.3 points; p: 0.067); also global health scale increased at the end of PT (6.3 points; p: 0.067). Cognitive and emotional functioning scales were associated with a not significant negative change (< 5 points); the remaining ones were associated with a positive change, although < 5 points (Fig. 1).
Fig. 1.
HRQOL changes. Changes over time in mean health-related quality of life. Questionnaires were completed at the beginning of radiation therapy (StartPT), at the end of radiation therapy (EndPT), one month (+ 1 m) and three months (+ 3 m) after the end of radiation therapy
HRQOL changes during follow-up (t1–t3)
The analysis of HRQOL during follow-up confirmed that there was not a detrimental effect of PT re-irradiation on HRQOL (Fig. 1). None of the domains showed a minimum clinically meaningful change (> 10 points); global health and social functioning scales confirmed a positive change (2.5 points; p: 0.6 and 3.4 points; p: 0.5, respectively) at the last follow-up analyzed (t3) together with a motor dysfunction subscale that showed a positive increase of 2.3 points (p: 0.4).
Patient and treatment characteristics influencing HRQOL
The irradiated CTV had a significant effect on QLQBN_Motor (p = 0.009) and QLQC30_Social (p = 0.041) values; a CTV increase of one cc was associated with an average decrease of 0.27 and 0.26 points on the QLQBN_Motor and QLQC30_Social scale, respectively. Concomitant chemotherapy significantly impacted the QLQC30_Physical values; patients who received PT and concomitant TMZ had clinically better baseline QLQC30_Physical values compared with patients who received PT alone (82.4 vs 65.16 points, respectively); however, they showed a more relevant decrease during the analyzed time points (p = 0.018). The use of steroid before PT was associated with worse values in QLQC30_Physical e QLQBN20_Motor scale at baseline (76.6 and 66 points, respectively) compared with patients who start treatment without steroids (81.4 and 87.2, respectively); however, also in this subgroup of patients, the re-irradiation with PT was not detrimental in terms of QoL (Table 2). Finally, we analyzed the impact of radionecrosis in terms of HRQOL without finding any statistical correlation.
Table 2.
Patient and treatment characteristics influencing HRQOL
| QLQC30_Social | QLQBN20_Motor | |||
|---|---|---|---|---|
| Coeff | p value | Coeff | p value | |
| z = CTV (clinical target volume) | ||||
| Intercept | 61.1 | 96.4 | ||
| Time | ||||
| End of treatment | 26.0 | 0.468 | − 4.3 | 0.445 |
| 1 month after | 21.1 | − 16.3 | ||
| 3 months after | 13.1 | − 3.8 | ||
| z | 0.1 | 0.788 | − 0.27 | 0.009 |
| z × time | ||||
| End of treatment | − 0.2 | 0.041 | 0.09 | 0.110 |
| 1 month after | − 0.2 | 0.1 | ||
| 3 months after | − 0.1 | 0.01 | ||
| QLQC30_Physical | ||
|---|---|---|
| Coeff | p value | |
| z = concomitant chemotherapy | ||
| Intercept | 65.1 | |
| Time | ||
| End of treatment | 0.00 | 0.302 |
| 1 month after | 4.2 | |
| 3 months after | − 10.4 | |
| z | 17.2 | 0.125 |
| z × time | ||
| End of treatment | 2.5 | 0.018 |
| 1 month after | − 15.2 | |
| 3 months after | 12.2 | |
| QLQC30_Physical | QLQBN20_Motor | |||
|---|---|---|---|---|
| Coeff | p value | Coeff | p value | |
| z = steroids before proton therapy | ||||
| Intercept | 81.4 | 87.2 | ||
| Time | ||||
| End of treatment | 2.7 | 0.468 | − 3.5 | 0.448 |
| 1 month after | − 3.6 | − 6.1 | ||
| 3 months after | − 1.7 | − 4.1 | ||
| z | − 25.1 | 0.002 | − 21.1 | 0.031 |
| z × time | ||||
| End of treatment | − 4.3 | 0.123 | 11.4 | 0.483 |
| 1 month after | 7.2 | 4.1 | ||
| 3 months after | − 13.0 | 0.8 | ||
Results from linear mixed models to investigate the effect of patient and treatment characteristics (CTV, concomitant chemotherapy, steroids before proton therapy) on time changes of outcome indicators (QLQC30_Social, QLQBN20_Motor, and QLQC30_Physical). Results most interesting from a clinical point of view are shown. CTV has a negative effect on QLQBN20_Motor and a differential effect on QLQC30_Social time behavior. Concomitant chemotherapy shows a differential effect on QLQC30_Physical time behavior. Steroids before proton therapy have a negative effect on QLQC30_Physical and QLQBN20_Motor
Statistically significant p are in bold (p < 0.05)
Discussion
Despite an aggressive multimodality strategy, almost all patients with GBM inevitably recur. Second-line therapies for these cases are scant and their effectiveness poor. Nowadays, re-irradiation is not only considered as a palliative treatment, but in selected cases, it could have a favorable impact in terms of local control and overall survival, at least comparable to the second-line chemotherapy regimens (Amelio and Amichetti 2012; Scoccianti et al. 2018)]. Due to a risk of acute and late side effects, careful selection of eligible patients for treatment should be done also to preserve the QoL of this population. In general, patients suitable for re-irradiation have small lesions and good performance status. The literature on QoL after re-irradiation of recurrent GBM is sparse, and often, QoL metrics have not been used as the endpoint for clinical trials, but increased interest is emerging and the results seem to highlight the safety and the not detrimental effect of re-irradiation in all HRQOL domains (Ernst-Stecken et al. 2007; Wick et al. 2014).
Ernst-Stecken et al. prospectively evaluated the efficacy and QoL of hypofractionated stereotactic RT for recurrent GBM with a regimen of 7 Gy × 5 fractions in 15 patients with a median PTV of 22.4 cc; QoL remained stable in two-thirds of the patients for a median time of 9 months (Ernst-Stecken et al. 2007). Wick et al. compared in a phase II randomized study re-irradiation (36 Gy; five times 2 Gy/week) vs re-irradiation + APG101 (CD95L-binding protein) in 91 patients in terms of efficacy and toxicity: no clinically meaningful or statistically remarkable differences between the two groups over time in any of the scales were observed (Wick et al. 2014).
Thanks to its better dose distribution compared to conventional photon therapy, PT could minimize the risk of side effects, allowing the treatment of larger recurrent tumors without increasing the possible detrimental effect of re-irradiation on QoL.
To date, only two studies have evaluated the efficacy and safety of fractionated PT for recurrent glioblastoma with encouraging results in terms of local control and toxicity, but without reporting the impact of treatment in QoL (Mizumoto et al. 2013; Galle et al. 2015).
The present analysis is the first that reports the effect of re-irradiation with PT for large recurrent GBM in terms of changes in HRQOL. The preliminary results of this study were presented in 2018 at the National Conference of the Italian Association of Radiotherapy and Clinical Oncology (AIRO) (Scartoni et al. 2018a), at the European Association of Neuro-Oncology meeting (Scartoni et al. 2018b) and at the European Society for Radiotherapy and Oncology meeting in 2019 (Scartoni et al. 2018c).
According with studies mentioned above, our results confirm that re-irradiation PT does not negatively affect HRQOL despite the large size of recurrence; indeed, our irradiated volumes were significantly larger compared with previous published studies (clinical target volume range, 21.8–259.1 cc vs 5–50 cc) (Amelio and Amichetti 2012). All preselected domains remained stable during the analyzed time points, even though without a minimum clinically meaningful change. However, several near-meaningful improvements have been registered, rather suggesting that PT seems to preserve HRQOL until the time of disease progression.
Interestingly, Global Health and Social Functioning score increased at the end of proton therapy (p = 0.067) and at the last follow-up (t3). Increased Global health over time in our group may have been the result of various effects. It is conceivable that RT may preserve HRQOL, but other factors like tumor response to treatment may influence the response rate. Emotional and cognitive scales were associated with a not significant negative change during treatment (t0–t1), probably because patients perform treatment away from home and the nostalgia could have an impact on this together with the need to stop their everyday life (i.e., daily activities and interests).
Moreover, previous studies demonstrated that HRQOL decreased in patients with glioma who suffered from fatigue (Taphoorn et al. 2010b; Aprile et al. 2015)] that typically increases markedly during and after the end of RT (Bitterlich and Vordermark 2017); conversely, in our study, there was not a significant increase in fatigue during and after the end of PT, and fatigue did not have a negative impact in terms of QoL.
Several patient factors, such as tumor location (Hahn et al. 2003), presence of mood disorder (Litofsky et al. 2004), and gender (Mainio et al. 2006), may have an impact on QoL in patients with GBM, although in our analysis, we did not observe any significant correlation between these factors and changes in QoL.
Regarding treatment characteristics, RT may decrease QoL in correlation with adverse effects such as hair loss, fatigue, somnolence, or cognitive problems (Walker et al. 2003), and the negative effects of corticosteroid use on neurocognitive function and/or QoL are well documented in healthy subjects (Lupien et al. 1999; Young et al. 1999); conversely, the addition of TMZ seems to have not a significant negative effect on QoL measures, except on social functioning (Taphoorn et al. 2005).
In our study, larger CTV and the use of steroids before PT were associated with a negative effect on QoL; this observation is not surprising, because use of steroids is frequent in patients with a lower performance status and neurological deficits that, obviously, could negatively impact the patient’s well-being.
Finally, reduction of corticosteroid dependence is reported as an indirect measurement in estimating the effect on QoL as well as neurological improvement (Neider et al. 1999); in our series, one-third of patients reduced the use of steroids during and after the re-irradiation confirming the favorable effect on QoL.
Despite our analysis being comprehensive, there are limitations such as the retrospective nature of the study and small size of patient population. At the same time, is worth noting that enrolled patients had similar characteristics, which were treated homogeneously, and data were collected prospectively.
Conclusion
Re-irradiation with PT is a safe and effective treatment for large recurrent GBM with good performance status; the treatment does not translate into a negative effect on HRQOL; rather, it seems to preserve HRQOL until the time of disease progression. Additional studies are necessary to confirm these results, possibly integrating basic cognitive screening such as Mini-Mental Status Exam, which could make the assessment even more accurate.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by DS, DA, and IG. The first draft of the manuscript was written by DS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
There are no conflict of interest disclosures from any authors.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human and animal participants
This article does not contain any studies with animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aaronson NK, Ahmedzai S, Bergman B et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376 [DOI] [PubMed] [Google Scholar]
- Amelio D, Amichetti M (2012) Radiation therapy for the treatment of recurrent glioblastoma: an overview. Cancers (Basel) 4(1):257–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprile I, Chiesa S, Padua L (2015) Occurrence and predictors of the fatigue in high-grade glioma patients. Neurol Sci 36:1363–1369 [DOI] [PubMed] [Google Scholar]
- Bitterlich C, Vordermark D (2017) Analysis of health-related quality of life in patients with brain tumors prior and subsequent to radiotherapy. Oncol Lett 14(2):1841–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Central Brain Tumor Registry of the United States (CBTRUS) (2010) Statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2007. https://www.cbtrus.org. Accessed 20 Mar 2020
- Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G (2007) Survival and quality of life after hypofractionated stereotactic radiotherapy for recurrent malignant glioma. J Neurooncol 81(3):287–294 [DOI] [PubMed] [Google Scholar]
- Fayers PM, Aaronson NK, Bjordal K et al (2001) European Organization for Research and Treatment of Cancer QLQ-C30 scoring manual, 3rd edn. EORTC, Brussels [Google Scholar]
- Galle J, McDonald M, Simoneaux V, Buchsbaum J (2015) Reirradiation with proton therapy for recurrent gliomas. Int J Part Ther 2(1):11–18 [Google Scholar]
- Hahn CA, Dunn RH, Logue PE, King JH, Edwards CL, Halperin EC (2003) Prospective study of neuropsychologic testing and quality-of life assessment of adults with primary malignant brain tumors. IJROBP 55(4):992–999 [DOI] [PubMed] [Google Scholar]
- Kazmi F, Soon YY, Leong YH, Koh WY, Vellayappan B (2019) Re-irradiation for recurrent glioblastoma (GBM): a systematic review and meta-analysis. J Neurooncol 142(1):79–90 [DOI] [PubMed] [Google Scholar]
- Koshy M, Villano JL, Dolecek TA et al (2012) Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol 107:207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litofsky NS, Farace E, Anderson F Jr, Meyers CA, Huang W, Laws ER Jr (2004) Glioma Outcomes Project Investigators. Depression in patients with highgrade glioma: results of the Glioma Outcomes Project. Neurosurgery 54(2):358–366 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gillin CJ, Hauger RL (1999) Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: a dose-response study in humans. Behav Neurosci 113(3):420–430 [DOI] [PubMed] [Google Scholar]
- Mainio A, Tuunanen S, Hakko H, Niemela A, Koivukangas J, Rasanen P (2006) Decreased quality of life and depression as predictors for shorter survival among patients with low-grade gliomas: a follow-up from 1990 to 2003. Eur Arch Psychiatry Clin Neurosci 256(8):516–521 [DOI] [PubMed] [Google Scholar]
- Minniti G, Amelio D, Amichetti M et al (2010) Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol 97:377–438 [DOI] [PubMed] [Google Scholar]
- Minniti G, Scaringi C, Baldoni A et al (2013) Health-related quality of life in elderly patients with newly diagnosed glioblastoma treated with short-course radiation therapy plus concomitant and adjuvant temozolomide. Int J Radiat Oncol Biol Phys 86(2):285–291 [DOI] [PubMed] [Google Scholar]
- Mizumoto M, Okumura T, Ishikawa E et al (2013) Reirradiation for recurrent malignant brain tumor with radiotherapy or proton beam therapy. Technical considerations based on experience at a single institution. Strahlenther Onkol 189(8):656–663 [DOI] [PubMed] [Google Scholar]
- Navarria P, Minniti G, Clerici E et al (2019) Re-irradiation for recurrent glioma: outcome evaluation, toxicity and prognostic factors assessment. A multicenter study of the Radiation Oncology Italian Association (AIRO). J Neurooncol 142(1):59–67 [DOI] [PubMed] [Google Scholar]
- Neider C, Nestle U, Niewald M, Walter K, Schnabel K (1999) Hyperfractionated reirradiation for malignant glioma. Front Radiat Ther Oncol 33:150–157 [DOI] [PubMed] [Google Scholar]
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16(1):139–144 [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2016) nlme: linear and nonlinear mixed effects models. R package version 3.1-128. https://CRAN.R-project.org/package=nlme. Accessed 20 Mar 2020
- R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org. Accessed 20 Mar 2020
- Ryu S, Buatti JM, Morris A, Kalkanis SN, Ryken TC, Olson JJ (2014) AANS/CNS Joint Guidelines Committee the role of radiotherapy in the management of progressive glioblastoma: systematic review and evidence-based clinical practice guideline. J Neurooncol 118(3):489–499 [DOI] [PubMed] [Google Scholar]
- Scartoni D, Amelio D, Farace P, Widesott L, Fellin F, Giacomelli I, Schwarz M, Amichetti M (2018a) Health related quality of life in patients with recurrence glioblastoma treated with active beam protontherapy. https://www.congressiairo.it/2018/Rimini_files/AIRO%202018%20abstract%20book.pdf. Accessed 20 Mar 2020
- Scartoni D, Amelio D, Giacomelli I, Amichetti M (2018b) Health related quality of life in recurrent glioblastoma treated with re-irradiation with active scanning protontherapy. Neuro-Oncology 20(Suppl_3):iii250 [Google Scholar]
- Scartoni D, Amelio D, Farace P, Widesott L, Fellin F, Giacomelli I, Schwarz M, Amichetti M (2018c) Health related quality of life in large recurrence glioblastoma treated with active beam protontherapy. Radiother Oncol 133:S690 (April 2019) [Google Scholar]
- Scoccianti S, Francolini G, Carta GA et al (2018) Re-irradiation as salvage treatment in recurrent glioblastoma: a comprehensive literature review to provide practical answers to frequently asked questions. Crit Rev Oncol Hematol 126:80–91 [DOI] [PubMed] [Google Scholar]
- Taphoorn MJ, Stupp R, Coens C et al (2005) Health-related quality of life in patients with glioblastoma: a randomised controlled trial. Lancet Oncol 6(12):937–944 [DOI] [PubMed] [Google Scholar]
- Taphoorn MJ, Claassens L, Aaronson NK et al (2010a) An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer 46:1033–1040 [DOI] [PubMed] [Google Scholar]
- Taphoorn MJ, Sizoo EM, Bottomley A (2010b) Review on quality of life issues in patients with primary brain tumors. Oncologist 15:618–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M, Brown J, Brown K et al (2003) Practical problems with the collection and interpretation of serial quality of life assessments in patients with malignant glioma. J Neurooncol 63(2):179–186 [DOI] [PubMed] [Google Scholar]
- Wick W, Fricke H, Junge K et al (2014) A phase II, randomized, study of weekly APG101 + reirradiation versus reirradiation in progressive glioblastoma. Clin Cancer Res 20(24):6304–6313 [DOI] [PubMed] [Google Scholar]
- Young AH, Sahakian BJ, Robbins TW, Cowen PJ (1999) The effects of chronic administration of hydrocortisone on cognitive function in normal male volunteers. Psychopharmacology 145(3):260–266 [DOI] [PubMed] [Google Scholar]