Abstract
Introduction
Sex-based differences in histological subtypes, in frequencies of mutations, and differences in response to the various therapeutic approaches in lung cancer are well studied. In general, the literature is controversial, and the large majority of the investigations may not provide evidence from the last decade.
Objective
The objective of the current study was to reveal timely sex-based differences in patients with lung cancer in the era of immunotherapy and molecularly targeted agents.
Methods
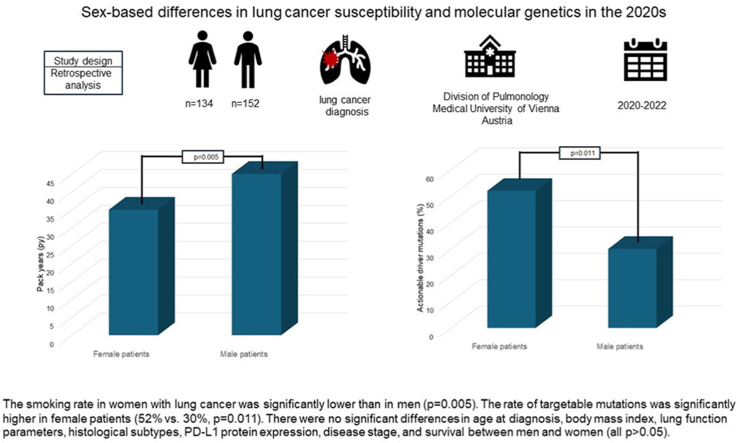
We retrospectively analyzed a consecutive cohort of 286 patients (female:male ratio 134:152/47 %:53 %) who were diagnosed with lung cancer between 2020 and 2022 in the pulmonology department of the Medical University of Vienna, Austria. Demographic characteristics, histological subtypes, the PD-L1 expression on tumor cells, presence of mutations, treatment, and survival of male and female patients were compared.
Results
The smoking rate in women with lung cancer was significantly lower than in men (p = 0.005). The rate of targetable mutations was significantly higher in female patients (52 % vs. 30 %, p = 0.011). There were no significant differences in age at diagnosis, body mass index, lung function parameters, histological subtypes, PD-L1 protein expression, disease stage, and survival between men and women (all p > 0.05).
Conclusion
Female Caucasian patients seem to have a higher susceptibility to lung cancer. Although the rate of genetic alterations is similar in both sexes, actionable driver mutations are significantly more common in women.
Keywords: Lung cancer, Sex-based differences, Lung cancer susceptibility
Graphical abstract
1. Introduction
Lung cancer is the second most common malignancy and the leading cause of cancer mortality in women and men worldwide [[1], [2], [3]]. Whereas lung cancer incidence has been decreasing among men, an increase in incidence rate among women has been reported. Due to improved diagnostics, particularly the introduction of lung cancer screening programs and due to new therapeutic strategies including the introduction of immunotherapy, survival of lung cancer continues to increase for both sexes [4].
In the recent decades, sex-based differences, resulting from different hormonal milieu, physiological and anatomical features as well as sex-specific genes and environmental exposures, have been well studied in lung cancer patients. Thus, many trials reported sex-specific differences in lung cancer susceptibility but also in lung cancer histology, PD-L1 (programmed cell death-ligand 1) status, molecular genetics, and treatment response. Studies published in the 1990s or early 2000s postulated, that women are more likely than men to develop lung cancer despite lower nicotine consumption [5,6], whereas other studies refute this hypothesis [7]. A 2020 published systematic analysis in 40 countries confirmed the assumption of an increasing trend of lung cancer incidence rates in young females that cannot be fully explained by the differences in smoking prevalence between sexes [8]. Furthermore, women are significantly more likely to develop non-smoking-associated lung cancer when compared to men [9]. In the past, squamous cell carcinoma dominated in men, while women had higher rates of adenocarcinoma. In recent years, however, the incidence of squamous cell carcinoma has declined in both sexes, and adenocarcinoma is now the most common subtype of lung cancer in both men and women in industrialized countries [10]. Other studies that investigated patterns in molecular pathology found a predominance of EGFR-mutations [11], and a higher susceptibility to smoking-related KRAS-mutant cancers in women when compared to men [12]. Sex-based differences have been also observed on the subject of response to various treatment modalities. Trials in the 2010s reported that women have a superior response to chemotherapy compared to men [13]. Newer trials focused on the sex-based differences in the response to immunotherapy, that was introduced as a novel treatment option for lung cancer within clinical trials in 2012. These trials demonstrate an inferior response to mono-immunotherapy while a greater benefit of chemo-immunotherapy in females [14,15]. Improved median overall survival of females was also reported in late-stage and in resected lung cancer [[16], [17], [18]].
The literature is controversial and the large majority of the investigations may not provide evidence from the last decade. In the past 10 years, however, epidemiological changes as well as further developments in diagnostics, molecular characterization of tumor tissue, and lung cancer treatment have been documented. This study was conducted in order to reveal sex-based differences in lung cancer patients from 2020 to 2022.
The aim of this retrospective analysis was to compare clinical characteristics, immunohistochemical and molecular pathologic features, therapeutic approaches, and survival of women and men with lung cancer.
2. Materials and methods
2.1. Patient population and study database
In this retrospective analysis performed in 2023, 286 patients (female:male ratio 134:152/47 %:53 %) with newly diagnosed lung cancer from April 2020 to December 2022 at the Division of Pulmonology of the Department of Internal Medicine II at the Medical University of Vienna, Austria, were consecutively enrolled. There were no exclusion criteria for study enrolment. Data were obtained from pneumologist's charts, pathological, radiological, and surgical reports.
The clinical characteristics of male and female patients included sex, age, smoking status, lung function parameters (FEV1 [forced expiratory volume in 1 s], TLCO SB [diffusion capacity for carbon monoxide, single breath]), coexistence of chronic obstructive pulmonary disease (COPD) or pulmonary fibrosis, and body mass index (BMI). Histological subtype, PD-L1 expression, and the presence of cancer mutations (with or without targetable pathways) were collected. All patients were staged according to the 8th TNM edition of lung cancer staging. Therapeutic approaches for all patients had been discussed at our institutional multidisciplinary tumor board.
The study was approved by the Ethics Committee of the Medical University of Vienna (EK 1103/2021) according to the Declaration of Helsinki. Written informed consent was not required due to the retrospective study design.
2.2. Statistical analysis
Data are presented as mean values ± standard deviations (SD) where appropriate. To evaluate differences between women and men with lung cancer, absolute and relative frequencies for men and women were calculated for categorical variables and compared using a Chi-Square test. For continuous variables, mean and standard deviation for men and women were calculated and compared using a t-test. For the skewed variable PD-L1 TPS (tumor proportion score), data was displayed as median and interquartile ranges (IQR), and the Mann-Whitney U test was calculated to compare groups. Differences for all analyses were considered statistically significant for p values < 0.05. Overall survival was calculated as the time from the date of histological confirmation of lung cancer (index date) to the date of the last record (date of death or last follow-up). To compare survival between men and women, Kaplan-Meier curves were plotted and a log-rank test was computed.
Due to the exploratory character of the study, no adjustment for multiplicity was performed. Analyses were performed with R.4.2.
3. Results
In this analysis, clinical characteristics, immunohistochemical and molecular pathologic features, treatment modalities, and the overall survival of all consecutive 152 (53 %) men and 134 (47 %) women who were diagnosed with lung cancer between April 2020 and December 2022 were compared. Baseline demographics and clinical characteristics are shown in Table 1.
Table 1.
Clinical characteristics and demographics of male and female lung cancer patients.
| male patients n = 152 |
female patients n = 134 |
p-value | |
|---|---|---|---|
| Age, mean ± SD | 67.01 ± 8.90 | 66.17 ± 10.92 | 0.477 |
| PY, mean ± SD | 44.74 ± 28.15 | 35.02 ± 28.1 | 0.005 |
| Ever-smoker, % | 92.1 | 86.3 | 0.180 |
| Body mass index, mean ± SD | 25.96 ± 5.11 | 25.19 ± 5.04 | 0.228 |
| Comorbities | |||
| COPD, % | 55.2 | 46.4 | 0.186 |
| Lung fibrosis, % | 1.4 | 2.4 | 0.886 |
| Lung function | |||
| FEV1 %, mean ± SD | 71.2 ± 24.41 | 70.23 ± 22.18 | 0.768 |
| TLCO SB %, mean ± SD | 58.8 ± 20.62 | 56.05 ± 17.88 | 0.373 |
| Histology | 0.190 | ||
| Squamous cell carcinoma, % | 21.1 | 20.9 | 1.000 |
| Adenocarcinoma, % | 50.0 | 58.2 | 0.204 |
| Adenosquamous carcinoma, % | 3.9 | 1.5 | 0.370 |
| Small-cell lung cancer, % | 16.4 | 15.7 | 0.987 |
| Large-cell cancer, % | 3.9 | 0 | 0.056 |
| Carcinoid, % | 2.6 | 3.0 | 1.000 |
| Immunohistochemistry and molecular pathology | |||
| PD-L1 status (%), median (IQR) | 4.0 (1.00, 40.00) | 5.00 (1.00; 51.5) | 0.535 |
| PD-L1≥50 %, % | 23.4 | 26.8 | 0.623 |
| Mutation, % | 71.8 | 73.6 | 0.907 |
| Treatable mutations, % (of all mutations) | 29.7 | 52.2 | 0.011 |
| Stage | 0.349 | ||
| IA1, % | 1.3 | 2.2 | |
| IA2, % | 4.6 | 9.0 | |
| IA3, % | 3.9 | 6.7 | |
| IB, % | 4.6 | 3.7 | |
| IIA, % | 0 | 2.2 | |
| IIB, % | 6.6 | 5.2 | |
| IIIA, % | 11.8 | 11.2 | |
| IIIB, % | 11.8 | 9.0 | |
| IIIC, % | 6.6 | 4.5 | |
| IVA, % | 14.5 | 18.7 | |
| IVB, % | 32.9 | 26.9 | |
| Treatment | |||
| Surgery | 23.8 | 24.6 | 0.991 |
| Radiotherapy | 43.8 | 42.3 | 0.893 |
| Chemotherapy | 51.7 | 44.4 | 0.279 |
| Immunotherapy | 33.6 | 35.2 | 0.879 |
| Targeted treatment | 4.9 | 9.0 | 0.278 |
Concerning smoking history, women with lung cancer had smoked significantly less compared to male patients (35 pack-years vs. 45 pack-years, p = 0.005). The rate of non-smokers among females compared to males was also slightly higher (13.7 % vs. 7.9 %, p = 0.180). Correspondingly, a higher prevalence of coexistent COPD was found among male patients (55 % vs. 46 %, p = 0.186). Regarding lung function, there was no statistically significant difference in the mean FEV1 value between men and women (71 % vs. 70 %, p = 0.768). Similarly, no significant differences in TLCO values could be noted between men and women (59 % vs. 56 %, p = 0.373).
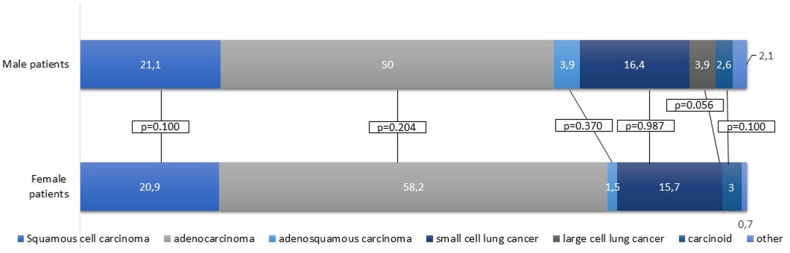
In men and women, there were no significant differences in the proportion of histological subtypes (Table 1 and Fig. 1). Adenocarcinoma was the most common histological subtype in both men and women followed by squamous cell carcinoma (50 % vs. 58 %, p = 0.204 and 21 % vs. 21 %, p = 1.000; respectively). Out of the 134 female patients, 11 subjects were diagnosed at age <50 years. In these, adenocarcinoma was the predominant subtype followed by carcinoid in two women aged <50 years. In male patients, only 4 out of 152 subjects were younger than 50 years, in whom the most common histological subtype was carcinoid in 50 % of the cases.
Fig. 1.
Histological subtypes of lung cancer in male and female patients. In the proportion of histological subtypes, an even distribution was found between men and women. Adenocarcinoma was the most common histological subtype (50 % vs. 58.2 %, p = 0.204) followed by squamous cell carcinoma (21.1 % vs. 20.9 %, p = 1.000). Small-cell lung cancer counted for 16.4 % and 15.7 %, while adenosquamous carcinoma for 3.9 % and 1.5 % of all cases in male and female patients (p = 0.987 and p = 0.370, respectively). Large-cell lung carcinoma was found in men only (p = 0.056). The proportion of carcinoids was 2.6 % and 3 % (p = 1.000) in men and women.
Overall, the median (IQR) PD-L1 TPS with 4 % (1.00–40.00) and 5 % (1.00–51.15) in men and women was similar (p = 0.535) as well as the proportion of patients with a PD-L1 protein expression ≥50 % showed an even distribution between male and female patients (23 % vs. 27 %, p = 0.623).
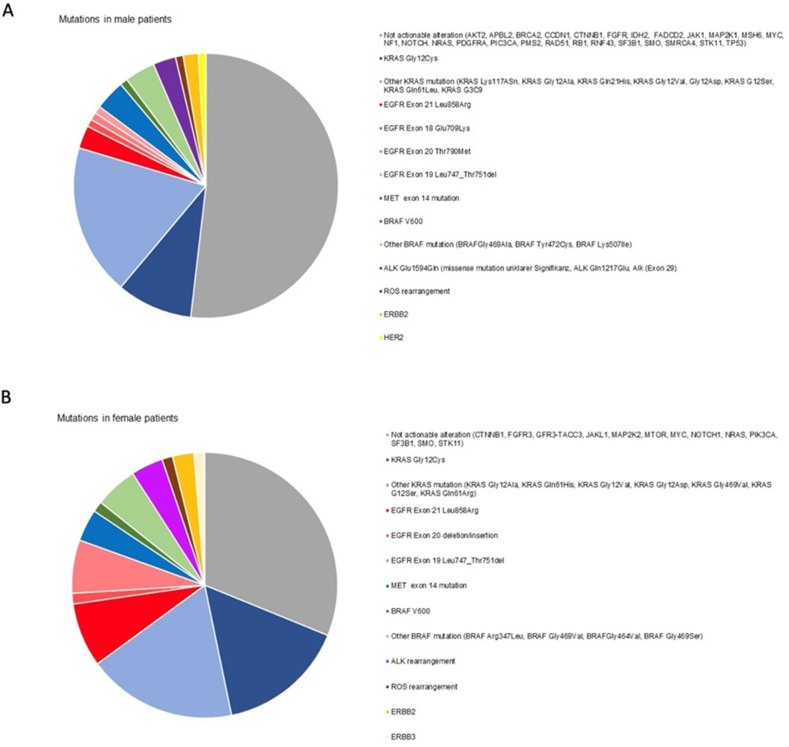
Molecular pathology testing that was performed in 67.8% of all patients (in 67.8% of the male and in 67.9% of the female patients) revealed no significant differences regarding the presence of gene mutations overall (72 % vs. 74 % in men vs. women, p = 0.907). However, only 29.7 % of all detected genetic alterations were treatable in men, whereas in women, 52.2 % of all mutations were target mutations (p = 0.011). Overall, treatable mutations were found in 14.5 % of male and in 26.1 % of female patients, respectively. The majority of genetic alterations were found in adenocarcinomas: in men and women, 95.5 % and 91.4 % of all treatable mutations, respectively. Particularly, treatable EGFR (epidermal growth factor receptor) mutations as well as treatable KRAS-mutant and HER-mutant lung cancers were found more frequently in female patients. All mutations tested and found in men and women are presented in Fig. 2a and b.
Fig. 2.
Mutation analysis in male and female lung cancer patients. Overall, molecular pathology testing revealed no significant differences regarding the presence of gene mutations between men and women (71.8 % vs. 73.6 %, p = 0.907). (A) In male patients, treatable genetic alterations counted for 29.7 % of all detected mutations. (B) Among female patients, 52.2 % of all mutations were target mutations. Treatable EGFR-mutant, treatable KRAS-mutant, and HER-mutant lung cancers were found more frequently in female patients.
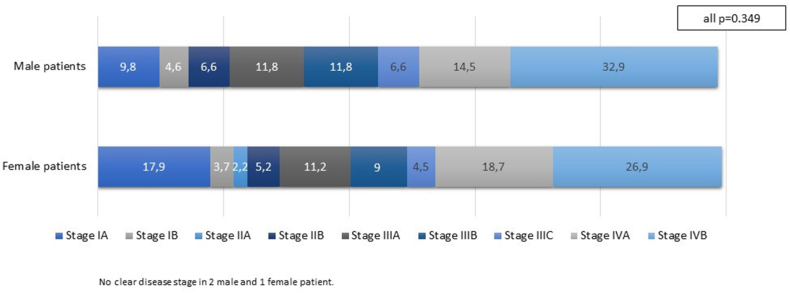
At diagnosis, a high proportion of patients regardless of sex had metastatic disease at presentation (47.4 % vs. 45.6 % in men and women, respectively; Fig. 3). When comparing early-stage disease (stage I and II), locally advanced disease (stage IIIa, IIIB, and IIIC), and metastatic disease (stage IVA and IVB), no significant differences were found between men and women (p = 0.349). Moreover, there were no significant differences in the treatment strategies.
Fig. 3.
Stage of the disease at the time of diagnosis in male and female patients. In the distribution of early-stage disease (stage I and II), locally advanced disease (stage IIIa, IIIB, and IIIC), and metastatic disease (stage IVA and IVB), no significant differences were found between men and women (p = 0.349).
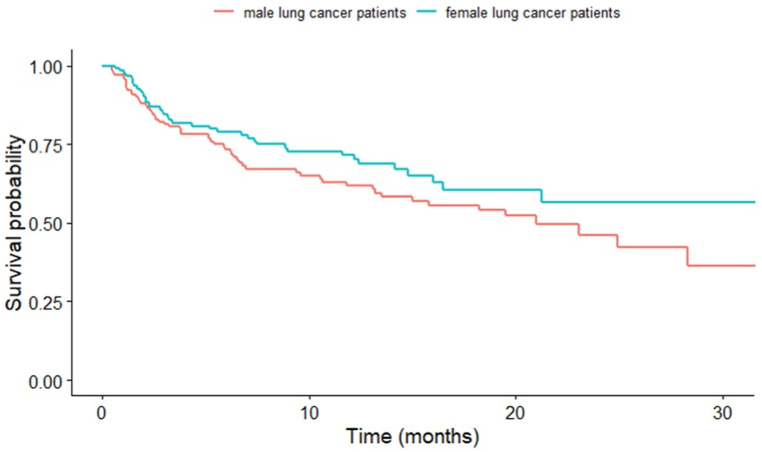
The median overall survival for the entire cohort was 23.4 months (HR 3.86, 95 % CI: 15.8–30.9). In terms of survival, female lung cancer patients experienced a slight survival benefit compared to male lung cancer patients without reaching statistical significance (p = 0.090; Fig. 4).
Fig. 4.
Kaplan-Meier survival analysis was performed for male (n = 152, 53 %) and female patients (n = 134, 47 %) with lung cancer between 2020 and 2022.
4. Discussion
In the recent decades, results of numerous studies on sex-specific differences in lung cancer have been published, but some of them are controversial, some remain speculative, and others cover distant periods. Most studies involved elderly individuals born before the middle of the last century. The risk of lung cancer in these populations may not be representative of the risk among persons born later. Multiple inconsistent statements and hypotheses were observed.
One hypothesis addresses the susceptibility to smoking-associated lung cancer. Several trials reported that both sexes with comparable smoking exposure have the same risk to develop lung cancer [7,[19], [20], [21], [22]]. A meta-analysis published in 2018 including data from 99 cohorts revealed that smoking yields similar risks of lung cancer in women compared with men [21]. Also, Kreuzer et al. reported, that risk estimates did not differ appreciably between sexes [22]. However, there are also several case-control and cohort studies that reported a higher lung cancer risk for smoking women compared to men at a given level of exposure to cigarette smoke [5,6,[23], [24], [25], [26]]. Our findings support this hypothesis, as the cumulative tobacco consumption of women was significantly less intensive than of men in our cohort. While the female-to-male ratio in lung cancer incidence rates have increased during the last two decades, the prevalence of cigarette consumption among women has not yet exceeded the prevalence among men. The crossover from a historically higher incidence of lung cancer among young men to a higher incidence among young women are not fully explained by smoking behaviors between sexes supporting the hypothesis of a higher susceptibility to lung cancer in women. Despite the controversy regarding the susceptibility of smoker-associated lung cancer, it is clear that in non-smokers, lung cancer is more common in women than in men. Worldwide, an estimated 15 % of lung cancer in men versus 53 % in women are not related to smoking [27,28].
Explanations for sex-based differences in lung cancer other than smoking patterns include genetic, molecular, hormonal, and viral factors. A higher susceptibility to lung cancer in women may be explained by a higher expression of cytochrome CYP1A1 genes in female lungs resulting in greater carcinogen activation and/or a higher level of DNA that plays an important role in carcinogenesis initiation [2,[29], [30], [31], [32]]. Moreover, a hormonal influence in the pathogenesis of lung cancer may play a significant role. The over-expression of estrogen receptor (ER) in non-small cell lung cancer, mainly in adenocarcinomas, was reported by several studies with ERβ as the more abundant form of ER in lung cancer [33,34]. ERβ expression was reported to be the highest in pre-menopausal women, while a lower and a minimum expression were observed in post-menopausal women and in men, respectively. Also, premenopausal women were found to develop adenocarcinomas more likely as well as to present with more extensive disease upon diagnosis when compared to postmenopausal women [[35], [36], [37], [38]]. It has been also reported that tobacco smoking may aggravate the effect of estrogen on lung carcinogenesis [39,40]. Some controversies are observed in regard to the relationship of hormone replacement therapy and lung cancer. Some trials reported an increased correlation between lung cancer risk and the use of estrogen hormone replacement therapy [[41], [42], [43], [44]] whereas other studies found a decreased risk and favorable prognosis in women after estrogen hormone therapy [45,46]. A mechanistic relationship between the estrogen receptor (ER) and EGFR has been reported in the pathogenesis of lung cancer, wherein an enhanced antitumor effect of the ER antagonist and the EGFR antagonist on the inhibition of cell migration in lung cancer was observed. Several findings support a rationale for the use of dual lung cancer therapy of estrogen receptor antagonist and EGFR tyrosine kinase inhibitor as the combination has been shown to decrease tumor cell proliferation in vitro and in vivo more profoundly when compared to each drug administered separately [[47], [48], [49]].
In terms of the effect of viral factors, a meta-analysis concluded evidence that human papillomavirus (HPV) infection, particularly HPV 16 and 18, is associated with a significantly greater risk for lung cancer [50]. Other trials also found a significant increase in risk for lung cancer among females with HPV infection, while among men, no significant association was found [51,52].
Most studies report a predominance of adenocarcinomas in women [[53], [54], [55]], which was also evaluated in our study. At 58.2 %, adenocarcinoma was the most common tumor entity in women. Regarding male patients, we also observed a predominance of adenocarcinomas, consistent with the results of various trials [56]. This finding is, however, contrary to the results of other trials postulating squamous cell cancer to be the leading histological subtype in men [53,54]. The histological subtype depends on where and when the study took place and how the smoking behavior of the populations changed over the years. It is noteworthy that studies that describe a predominance of squamous cell carcinomas in men often cover earlier time periods, whereas more recent studies often report a predominance of adenocarcinoma. Among males, squamous cell carcinoma that is associated strictly with the smoking status rates declined 30 % or more in North America and some European countries, whereas adenocarcinoma rates rose among males and females with the increases among males exceeding 50 % in many areas of Europe [57].
Adenocarcinomas are more frequently associated with the presence of driver mutations. Moreover, it is known that oncogenic mutations are more common in women, particularly mutations in the TP53, the KRAS, and the EGFR genes, whereby these sex-associated differences may be even more pronounced considering the ethnic background [[58], [59], [60]]. An observational study that included 17.664 lung cancer patients described a genetic alteration in about 50 % of the tumors, whereby 20–25 % of them were actionable driver mutations [60,61]. In our study, we revealed a similar rate of genetic alterations in men and women (72 % vs. 74 %). However, females more frequently presented with actionable driver mutations, in particular EGFR and KRAS mutations. Therefore, molecular profiling is particularly advisable especially in women who might benefit more from target-oriented stratified medical treatments.
In our analysis, the majority of both men and women had advanced disease stages at the time of diagnosis (IIIB, IIIC, and IV), wherein no significant differences regarding the distribution of disease stage between men and women were observed. Some study cohorts described similar findings [54,62]. However, a database of the United States including 47.706 lung cancer patients revealed more advanced pathologic TNM stages in men compared with women [63]. Also, a Japanese lung cancer registry study with 12.509 cases, women had a significantly higher incidence of stage IA disease than men [64]. In these studies, a more active health-seeking behavior of women was discussed to be the reason for a cancer diagnosis at a lower stage. However, the databases were queried for patients who had undergone a resection of histologically confirmed non-small cell lung cancer and thus presenting particularly the lower disease stages but not the advanced stages. In general, it must be considered that the differences in data collection makes it difficult to compare the various studies.
Due to the introduction of targeted therapies in lung cancer patients with genetic alterations and due to the introduction of immunotherapy, therapeutic strategies and survival has dramatically changed over the last decade. In the current analysis, we did not find a significant difference related to the therapeutic strategies between man and women with lung cancer. Women were found significantly more often to have an actionable driver mutation and more frequently underwent a targeted therapy, however without reaching the significance threshold. It must be considered that lung cancer treatment options are based on different features including the disease stage, patient's lung function, overall health, and the presence of genetic alterations. In our study, we found an equal distribution of disease stages in men and women that may be one reason of similar therapeutic strategies. However, sex-based differences in the response to various treatment options are known: various trials reported a superior response of women to chemotherapy but an inferior response to mono-immunotherapy [13,65]. A higher response rate to first-generation EGFR-inhibitors in women was also observed and improved median overall survival of females was reported in late-stage and in resected lung cancer [66]. Sex-based differences in the development of side effects of systemic therapy were also observed, whereby women seem to have a tendency to experience more chemotherapy- and immunotherapy-related adverse events without negative impacts on the treatment success, however [67]. Sex-based differences are currently not fully understood, so that sex does not have an impact on the therapeutic decisions. Clinical trials evaluating sex-associated differences will help address existing sex-based disparities in lung cancer and may impact therapeutic decisions henceforward.
Evaluating the survival of lung cancer patients, several trials reported a survival benefit of women compared to men [[14], [15], [16],60]. In contrast, sex-related differences in survival of male and female lung cancer patients were not seen when adjusted for known risk factors of survival in a large, prospective Australian study [68].
In our study, female patients also experienced a survival benefit, that, however, did not achieve the threshold for statistical significance. The number of patients in our study is too small to make a reliable statement about gender-specific differences in the survival of lung cancer patients in the era of immunotherapy and targeted therapy, however.
Limitations of this study include the retrospective single-center setting and the short time period with a limited number of patients. Thus, our analysis only gives indications for potential sex-based differences in lung cancer susceptibility and molecular genetics in the 2020s but does not allow to draw definitive conclusions. Moreover, results obtained in a Caucasian population may not be generalizable to other populations.
Further studies are needed to better investigate sex-based differences to clarify controversial findings across the literature, to assess treatment-associated differences in novel therapy options in lung cancer over the past decade, and to optimize lung cancer screening policies.
5. Conclusions
Despite of less smoking exposure, female Caucasian patients seem to have a higher susceptibility to lung cancer with a higher rate of actionable driver mutations.
CRediT authorship contribution statement
Berta Mosleh: Writing – original draft, Visualization, Investigation, Data curation. Pavla Sarova: Writing – review & editing, Visualization, Methodology, Data curation. Sonja Zehetmayer: Software, Formal analysis, Data curation. Felicitas Oberndorfer: Writing – review & editing. Joachim Widder: Writing – review & editing. Helmut Prosch: Writing – review & editing. Marco Idzko: Writing – review & editing. Clemens Aigner: Writing – review & editing. Mir Alireza Hoda: Writing – review & editing, Visualization, Supervision, Methodology, Investigation, Conceptualization. Daniela Gompelmann: Writing – original draft, Visualization, Project administration, Methodology, Investigation, Data curation, Conceptualization.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
The study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and approved by the Institutional Ethics Committee of the Medical University of Vienna (EK 1103/2021). Written informed consent was not required due to the retrospective study design.
Funding
This research received no external funding.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
References
- 1.World Cancer Research Fund International 2023. https://www.wcrf.org/preventing-cancer/cancer-statistics/lung-cancer-statistics/
- 2.Mederos N., Friedlaender A., Peters S., Addeo A. Gender-specific aspects of epidemiology, molecular genetics and outcome: lung cancer. ESMO Open. 2020 Nov;5(Suppl 4) doi: 10.1136/esmoopen-2020-000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barta J.A., Powell C.A., Wisnivesky J.P. Global epidemiology of lung cancer. Ann Glob Health. 2019 Jan 22;85(1):8. doi: 10.5334/aogh.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIH Annual report to the nation: cancer death rates continue to decline, 2022.
- 5.Zang E.A., Wynder E.L. Differences in lung cancer risk between men and women: examination of the evidence. J. Natl. Cancer Inst. 1996 Feb 21;88(3–4):183–192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 6.Kiyohara C., Ohno Y. Sex differences in lung cancer susceptibility: a review. Gend. Med. 2010 Oct;7(5):381–401. doi: 10.1016/j.genm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 7.De Matteis S., Consonni D., Pesatori A.C., Bergen A.W., Bertazzi P.A., Caporaso N.E., Lubin J.H., Wacholder S., Landi M.T. Are women who smoke at higher risk for lung cancer than men who smoke? Am. J. Epidemiol. 2013 Apr 1;177(7):601–612. doi: 10.1093/aje/kws445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fidler-Benaoudia M.M., Torre L.A., Bray F., Ferlay J., Jemal A. Lung cancer incidence in young women vs. young men: a systematic analysis in 40 countries. Int. J. Cancer. 2020 Aug 1;147(3):811–819. doi: 10.1002/ijc.32809. [DOI] [PubMed] [Google Scholar]
- 9.Wakelee H.A., Chang E.T., Gomez S.L., Keegan T.H., Feskanich D., Clarke C.A., Holmberg L., Yong L.C., Kolonel L.N., Gould M.K., West D.W. Lung cancer incidence in never smokers. J. Clin. Oncol. 2007 Feb 10;25(5):472–478. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barta J.A., Powell C.A., Wisnivesky J.P. Global epidemiology of lung cancer. Ann Glob Health. 2019;85(1):8. doi: 10.5334/aogh.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014 Jul 31;511(7511):543–550. doi: 10.1038/nature13385. Epub 2014 Jul 9. Erratum in: Nature. 2014 Oct 9;514(7521):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dogan S., Shen R., Ang D.C., Johnson M.L., D'Angelo S.P., Paik P.K., Brzostowski E.B., Riely G.J., Kris M.G., Zakowski M.F., Ladanyi M. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin. Cancer Res. 2012 Nov 15;18(22):6169–6177. doi: 10.1158/1078-0432.CCR-11-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheatley-Price P., Blackhall F., Lee S.M., et al. The influence of sex and histology on outcomes in non-small-cell lung cancer: a pooled analysis of five randomized trials. Ann. Oncol. 2010;21:2023–2028. doi: 10.1093/annonc/mdq067. [DOI] [PubMed] [Google Scholar]
- 14.Conforti F., Pala L., Bagnardi V., Viale G., De Pas T., Pagan E., Pennacchioli E., Cocorocchio E., Ferrucci P.F., De Marinis F., Gelber R.D., Goldhirsch A. Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. J. Natl. Cancer Inst. 2019 Aug 1;111(8):772–781. doi: 10.1093/jnci/djz094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conforti F., Pala L., Pagan E., Corti C., Bagnardi V., Queirolo P., Catania C., De Pas T., Giaccone G. Sex-based differences in response to anti-PD-1 or PD-L1 treatment in patients with non-small-cell lung cancer expressing high PD-L1 levels. A systematic review and meta-analysis of randomized clinical trials. ESMO Open. 2021 Oct;6(5) doi: 10.1016/j.esmoop.2021.100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakelee H.A., Wang W., Schiller J.H., Langer C.J., Sandler A.B., Belani C.P., Johnson D.H., Eastern Cooperative Oncology Group Survival differences by sex for patients with advanced non-small cell lung cancer on Eastern Cooperative Oncology Group trial 1594. J. Thorac. Oncol. 2006 Jun;1(5):441–446. [PubMed] [Google Scholar]
- 17.Minami H., Yoshimura M., Miyamoto Y., Matsuoka H., Tsubota N. Lung cancer in women: sex-associated differences in survival of patients undergoing resection for lung cancer. Chest. 2000 Dec;118(6):1603–1609. doi: 10.1378/chest.118.6.1603. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida Y., Murayama T., Sato Y., Suzuki Y., Saito H., Nomura Y. Gender differences in long-term survival after surgery for non-small cell lung cancer. Thorac. Cardiovasc. Surg. 2016 Sep;64(6):507–514. doi: 10.1055/s-0035-1558995. [DOI] [PubMed] [Google Scholar]
- 19.Osann K.E., Anton-Culver H., Kurosaki T., Taylor T. Sex differences in lung-cancer risk associated with cigarette smoking. Int. J. Cancer. 1993 Apr 22;54(1):44–48. doi: 10.1002/ijc.2910540108. [DOI] [PubMed] [Google Scholar]
- 20.Bain C., Feskanich D., Speizer F.E., Thun M., Hertzmark E., Rosner B.A., Colditz G.A. Lung cancer rates in men and women with comparable histories of smoking. J. Natl. Cancer Inst. 2004 Jun 2;96(11):826–834. doi: 10.1093/jnci/djh143. [DOI] [PubMed] [Google Scholar]
- 21.O'Keeffe L.M., Taylor G., Huxley R.R., Mitchell P., Woodward M. Peters SAE. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018 Oct 3;8(10) doi: 10.1136/bmjopen-2018-021611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreuzer M., Boffetta P., Whitley E., Ahrens W., Gaborieau V., Heinrich J., Jöckel K.H., Kreienbrock L., Mallone S., Merletti F., Roesch F., Zambon P., Simonato L. Gender differences in lung cancer risk by smoking: a multicentre case-control study in Germany and Italy. Br. J. Cancer. 2000 Jan;82(1):227–233. doi: 10.1054/bjoc.1999.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Risch H.A., Howe G.R., Jain M., Burch J.D., Holowaty E.J., Miller A.B. Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am. J. Epidemiol. 1993 Sep 1;138(5):281–293. doi: 10.1093/oxfordjournals.aje.a116857. [DOI] [PubMed] [Google Scholar]
- 24.Brownson R.C., Chang J.C., Davis J.R. Gender and histologic type variations in smoking-related risk of lung cancer. Epidemiology. 1992 Jan;3(1):61–64. doi: 10.1097/00001648-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Harris R.E., Zang E.A., Anderson J.I., Wynder E.L. Race and sex differences in lung cancer risk associated with cigarette smoking. Int. J. Epidemiol. 1993 Aug;22(4):592–599. doi: 10.1093/ije/22.4.592. [DOI] [PubMed] [Google Scholar]
- 26.Tulinius H., Sigfússon N., Sigvaldason H., Bjarnadóttir K., Tryggvadóttir L. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol. Biomarkers Prev. 1997 Nov;6(11):863–873. [PubMed] [Google Scholar]
- 27.Parkin D.M., Bray F., Ferlay J., Pisani P., Global cancer statistics Explanations for sex-based differences in lung cancer other than smoking patterns include genetic, molecular, hormonal, and viral factors. CA A Cancer J. Clin. 2002;55:74–108. 2005. [Google Scholar]
- 28.North C.M., Christiani D.C. Women and lung cancer: what is new? Semin. Thorac. Cardiovasc. Surg. 2013;25(2):87–94. doi: 10.1053/j.semtcvs.2013.05.002. Summer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uppstad H., Osnes G.H., Cole K.J., Phillips D.H., Haugen A., Mollerup S. Sex differences in susceptibility to PAHs is an intrinsic property of human lung adenocarcinoma cells. Lung Cancer. 2011 Mar;71(3):264–270. doi: 10.1016/j.lungcan.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Wei Q., Cheng L., Amos C.I., Wang L.E., Guo Z., Hong W.K., Spitz M.R. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J. Natl. Cancer Inst. 2000 Nov 1;92(21):1764–1772. doi: 10.1093/jnci/92.21.1764. [DOI] [PubMed] [Google Scholar]
- 31.Mollerup S., Ryberg D., Hewer A., Phillips D.H., Haugen A. Sex differences in lung CYP1A1 expression and DNA adduct levels among lung cancer patients. Cancer Res. 1999 Jul 15;59(14):3317–3320. PMID: 10416585. [PubMed] [Google Scholar]
- 32.Zhan P., Wang Q., Qian Q., Wei S.Z., Yu L.K. CYP1A1 MspI and exon7 gene polymorphisms and lung cancer risk: an updated meta-analysis and review. J. Exp. Clin. Cancer Res. 2011 Oct 20;30(1):99. doi: 10.1186/1756-9966-30-99. PMID: 22014025; PMCID: PMC3212928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hershberger P.A., Stabile L.P., Kanterewicz B., Rothstein M.E., Gubish C.T., Land S., et al. Estrogen receptor beta (ERbeta) subtype-specific ligands increase transcription, p44/p42 mitogen activated protein kinase (MAPK) activation and growth in human non-small cell lung cancer cells. J. Steroid Biochem. Mol. Biol. 2009;116:102–109. doi: 10.1016/j.jsbmb.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Lara V., Pena-Mirabal E., Baez-Saldana R., Esparza-Silva A.L., Garcia-Zepeda E., Cerbon Cervantes M.A., et al. Estrogen receptor beta and CXCR4/CXCL12 expression: differences by sex and hormonal status in lung adenocarcinoma. Arch. Med. Res. 2014;45:158–169. doi: 10.1016/j.arcmed.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Pirie K., Peto R., Green J., et al. Lung cancer in never smokers in the UK million women study. Int. J. Cancer. 2016;139:347–354. doi: 10.1002/ijc.30084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berardi R., Verdecchia L., Paolo M.D., et al. Women and lung cancer: clinical and molecular profiling as a determinate for treatment decisions: a literature review. Crit. Rev. Oncol. Hematol. 2009;69:223–236. doi: 10.1016/j.critrevonc.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Moore K.A., Mery C.M., Jaklitsch M.T. Menopausal effects on presentation, treatment, and survival of women with non-small cell lung cancer. Ann. Thorac. Surg. 2003;76:1789–1795. doi: 10.1016/s0003-4975(03)01024-5. [DOI] [PubMed] [Google Scholar]
- 38.Greiser C.M., Greiser E.M., Dören M. Menopausal hormone therapy and risk of lung cancer-Systematic review and meta-analysis. Maturitas. 2010;65:198–204. doi: 10.1016/j.maturitas.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 39.Meireles S.I., Esteves G.H., Hirata R., Jr., Peri S., Devarajan K., Slifker M., Mosier S.L., Peng J., Vadhanam M.V., Hurst H.E., Neves E.J., Reis L.F., Gairola C.G., Gupta R.C., Clapper M.L. Early changes in gene expression induced by tobacco smoke: evidence for the importance of estrogen within lung tissue. Cancer Prev. Res. 2010 Jun;3(6):707–717. doi: 10.1158/1940-6207.CAPR-09-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng J., Xu X., Mace B.E., Vanderveer L.A., Workman L.R., Slifker M.J., Sullivan P.M., Veenstra T.D., Clapper M.L. Estrogen metabolism within the lung and its modulation by tobacco smoke. Carcinogenesis. 2013;34:909–915. doi: 10.1093/carcin/bgs402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganti A.K., Sahmoun A.E., Panwalkar A.W., Tendulkar K.K., Potti A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J. Clin. Oncol. 2006;24:59–63. doi: 10.1200/JCO.2005.02.9827. [DOI] [PubMed] [Google Scholar]
- 42.Chlebowski R.T., Schwartz A.G., Wakelee H., Anderson G.L., Stefanick M.L., Manson J.E., Rodabough R.J., Chien J.W., Wactawski-Wende J., Gass M., et al. Women's Health Initiative, I. Oestrogen plus progestin and lung cancer in postmenopausal women (Women's Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet. 2009;374:1243–1251. doi: 10.1016/S0140-6736(09)61526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slatore C.G., Chien J.W., Au D.H., Satia J.A., White E. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J. Clin. Oncol. 2010;28:1540–1546. doi: 10.1200/JCO.2009.25.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz A.G., Wenzlaff A.S., Prysak G.M., Murphy V., Cote M.L., Brooks S.C., Skafar D.F., Lonardo F. Reproductive factors, hormone use, estrogen receptor expression and risk of non small-cell lung cancer in women. J. Clin. Oncol. 2007;25:5785–5792. doi: 10.1200/JCO.2007.13.3975. [DOI] [PubMed] [Google Scholar]
- 45.Ayeni O., Robinson A. Hormone replacement therapy and outcomes for women with non-small-cell lung cancer: can an association be confirmed? Curr. Oncol. 2009;16:21–25. doi: 10.3747/co.v16i3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chlebowski R.T., Anderson G.L., Manson J.E., Schwartz A.G., Wakelee H., Gass M., Rodabough R.J., Johnson K.C., Wactawski-Wende J., Kotchen J.M., et al. Lung cancer among postmenopausal women treated with estrogen alone in the Women's Health Initiative randomized trial. J. Natl. Cancer Inst. 2010;102:1413–1421. doi: 10.1093/jnci/djq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nose N., Sugio K., Oyama T., Nozoe T., Uramoto H., Iwata T., Onitsuka T., Yasumoto K. Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J. Clin. Oncol. 2009;27:411–417. doi: 10.1200/JCO.2008. [DOI] [PubMed] [Google Scholar]
- 48.Stabile L.P., Lyker J.S., Gubish C.T., Zhang W., Grandis J.R., Siegfried J.M. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res. 2005;65:1459–1470. doi: 10.1158/0008-5472.CAN-04-1872. [DOI] [PubMed] [Google Scholar]
- 49.Marquez-Garban D.C., Chen H.W., Fishbein M.C., Goodglick L., Pietras R.J. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids. 2007;72:135–143. doi: 10.1016/j.steroids.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong W.M., Xu Q.P., Li X., Xiao R.D., Cai L., He F. The association between human papillomavirus infection and lung cancer: a system review and meta-analysis. Oncotarget. 2017 Oct 9;8(56):96419–96432. doi: 10.18632/oncotarget.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin F.C., Huang J.Y., Tsai S.C., Nfor O.N., Chou M.C., Wu M.F., Lee C.T., Jan C.F., Liaw Y.P. The association between human papillomavirus infection and female lung cancer: a population-based cohort study. Medicine (Baltim.) 2016 Jun;95(23) doi: 10.1097/MD.0000000000003856. Erratum in: Medicine (Baltimore). 2016 Jul 18;95(28):e0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bae J.-M., Kim E.H. Human papillomavirus infection and risk of lung cancer in never-smokers and women: an ‘adaptive’ meta-analysis. Epidemiol Health. 2015;37 doi: 10.4178/epih/e2015052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pesch B., Kendzia B., Gustavsson P., Jöckel K.H., Johnen G., Pohlabeln H., Olsson A., Ahrens W., Gross I.M., Brüske I., Wichmann H.E., Merletti F., Richiardi L., Simonato L., Fortes C., Siemiatycki J., Parent M.E., Consonni D., Landi M.T., Caporaso N., Zaridze D., Cassidy A., Szeszenia-Dabrowska N., Rudnai P., Lissowska J., Stücker I., Fabianova E., Dumitru R.S., Bencko V., Foretova L., Janout V., Rudin C.M., Brennan P., Boffetta P., Straif K., Brüning T. Cigarette smoking and lung cancer--relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int. J. Cancer. 2012 Sep 1;131(5):1210–1219. doi: 10.1002/ijc.27339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wainer Z., Wright G.M., Gough K., Daniels M.G., Russell P.A., Choong P., Conron M., Ball D., Solomon B., Sex-Dependent Staging in Non-Small-Cell Lung Cancer Analysis of the effect of sex differences in the eighth edition of the tumor, node, metastases staging system. Clin. Lung Cancer. 2018 Nov;19(6):e933–e944. doi: 10.1016/j.cllc.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Domagala-Kulawik J., Trojnar A. Lung cancer in women in 21th century. J. Thorac. Dis. 2020 Aug;12(8):4398–4410. doi: 10.21037/jtd-20-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Koning H.J., van der Aalst C.M., de Jong P.A., Scholten E.T., Nackaerts K., Heuvelmans M.A., Lammers J.J., Weenink C., Yousaf-Khan U., Horeweg N., van 't Westeinde S., Prokop M., Mali W.P., Mohamed Hoesein F.A.A., van Ooijen P.M.A., Aerts J.G.J.V., den Bakker M.A., Thunnissen E., Verschakelen J., Vliegenthart R., Walter J.E., Ten Haaf K., Groen H.J.M., Oudkerk M. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 2020 Feb 6;382(6):503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 57.Devesa S.S., Bray F., Vizcaino A.P., Parkin D.M. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int. J. Cancer. 2005 Nov 1;117(2):294–299. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 58.Dearden S., Stevens J., Wu Y.L., Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap) Ann. Oncol. 2013 Sep;24(9):2371–2376. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soh J., Toyooka S., Matsuo K., Yamamoto H., Wistuba I.I., Lam S., Fong K.M., Gazdar A.F., Miyoshi S. Ethnicity affects EGFR and KRAS gene alterations of lung adenocarcinoma. Oncol. Lett. 2015 Sep;10(3):1775–1782. doi: 10.3892/ol.2015.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Isla D., Majem M., Viñolas N., Artal A., Blasco A., Felip E., Garrido P., Remón J., Baquedano M., Borrás J.M., Die Trill M., García-Campelo R., Juan O., León C., Lianes P., López-Ríos F., Molins L., Planchuelo M.Á., Cobo M., Paz-Ares L., Trigo J.M., de Castro J. A consensus statement on the gender perspective in lung cancer. Clin. Transl. Oncol. 2017 May;19(5):527–535. doi: 10.1007/s12094-016-1578-x. [DOI] [PubMed] [Google Scholar]
- 61.Barlesi F., Mazieres J., Merlio J.P., Debieuvre D., Mosser J., Lena H., Ouafik L., Besse B., Rouquette I., Westeel V., Escande F., Monnet I., Lemoine A., Veillon R., Blons H., Audigier-Valette C., Bringuier P.P., Lamy R., Beau-Faller M., Pujol J.L., Sabourin J.C., Penault-Llorca F., Denis M.G., Lantuejoul S., Morin F., Tran Q., Missy P., Langlais A., Milleron B., Cadranel J., Soria J.C., Zalcman G. Biomarkers France contributors. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet. 2016 Apr 2;387(10026):1415–1426. doi: 10.1016/S0140-6736(16)00004-0. [DOI] [PubMed] [Google Scholar]
- 62.Moore R., Doherty D., Chamberlain R., Khuri F. Sex differences in survival in non-small cell lung cancer patients 1974-1998. Acta Oncol. 2004;43(1):57–64. [PubMed] [Google Scholar]
- 63.Wainer Z., Wright G.M., Gough K., Daniels M.G., Russell P.A., Choong P., Conron M., Ball D., Solomon B. Sex-dependent staging in non-small-cell lung cancer; analysis of the effect of sex differences in the eighth edition of the tumor, node, metastases staging system. Clin. Lung Cancer. 2018 Nov;19(6):e933–e944. doi: 10.1016/j.cllc.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 64.Sakurai H., Asamura H., Goya T., Eguchi K., Nakanishi Y., Sawabata N., Okumura M., Miyaoka E., Fujii Y., Japanese Joint Committee for Lung Cancer Registration Survival differences by gender for resected non-small cell lung cancer: a retrospective analysis of 12,509 cases in a Japanese Lung Cancer Registry study. J. Thorac. Oncol. 2010 Oct;5(10):1594–1601. doi: 10.1097/JTO.0b013e3181f1923b. [DOI] [PubMed] [Google Scholar]
- 65.Wheatley-Price P., Ma C., Ashcroft L.F., et al. The strength of female sex as a prognostic factor in small-cell lung cancer: a pooled analysis of chemotherapy trials from the Manchester Lung Group and Medical Research Council Clinical Trials Unit. Ann. Oncol. 2010;21:232–237. doi: 10.1093/annonc/mdp300. [DOI] [PubMed] [Google Scholar]
- 66.Pinto J.A., Vallejos C.S., Raez L.E., Mas L.A., Ruiz R., Torres-Roman J.S., Morante Z., Araujo J.M., Gómez H.L., Aguilar A., Bretel D. Gender and outcomes in non-small cell lung cancer: an old prognostic variable comes back for targeted therapy and immunotherapy? ESMO open. 2018 Jan 1;3(3) doi: 10.1136/esmoopen-2018-000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colli L.M., Morton L.M., Chanock S.J. Sex-related effect on immunotherapy response: implications and opportunities. JNCI J Natl Cancer Inst. 2019;111:749–750. doi: 10.1093/jnci/djz096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu X.Q., Yap M.L., Cheng E.S., Ngo P.J., Vaneckova P., Karikios D., Canfell K., Weber M.F. Evaluating prognostic factors for sex differences in lung cancer survival: findings from a large Australian cohort. J. Thorac. Oncol. 2022 May;17(5):688–699. doi: 10.1016/j.jtho.2022.01.016. Epub 2022 Feb 4. PMID: 35124253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.