Abstract
Background
Thoracic solitary fibrous tumors (TSFTs) are uncommon mesenchymal tumors. The data regarding surgical outcomes and prognostic factors are scarce. This retrospective paper is to analyze surgical outcomes, clinical characteristics and prognosis of TSFT.
Methods
A single-center retrospective study of the data of 70 patients with TSFT who underwent surgical resection in our department between August 2008 and October 2014 was conducted.
Results
A total of 70 TSFTs (58 benign, 12 malignant) were included and all patients underwent complete surgical resection except one recurrent patient with initial treatment. TSFTs originated from the pleura (n = 43), lung (n = 9), mediastinum (n = 16), esophagus (n = 1) and diaphragm (n = 1), respectively. Mass excision was only performed in 29 patients, en bloc excision including surrounding structures was performed in 41 patients. During follow-up, no tumor recurrence occurred in benign TSFT patients. All recurrences occurred in 6 malignant patients, and 5 of them died because of local recurrence and distant metastasis. Median follow-up was 95 months (range, 3–133 months). The 5-year overall survival (OS) of TSFT patients was 94.3%. The 5-year relapse-free survival and OS of malignant TSFT patients were 58.3% and 66.7%, respectively.
Conclusion
The gold standard of TSFT treatment is complete surgical resection. VATS is safe and reliable for treating selected TSFT patients. Aggressive surgical resection could be underwent in such patients of local recurrence or solitary metastatic tumor. A long-term follow-up is necessary due to the risk of recurrence.
Keywords: Solitary fibrous tumor, Malignant solitary fibrous tumor, Surgical resection, Recurrence
Introduction
Solitary fibrous tumors (SFTs) are rare tumors, mostly originate from the fibroblastic mesenchymal cells of pleural submesothelial tissue, though they can originate from extrapleural sites as well (Hanau and Miettinen 1995). Most of SFTs are benign because of the slow-growing and low metastatic potential, 10–20% are malignant (England et al. 1989). SFTs are usually asymptomatic in early stages, mostly detected incidentally by chest imaging. Compared to benign SFTs, malignant tumors have more aggressive behavior with correlatively symptomatic presentation, greater propensity to recur and metastasize, and poorer survival (Cardillo et al. 2009; Harrison-Phipps et al. 2009; Milano et al. 2011). One of the main principles of surgical treatment of the primary tumor is to obtain adequate negative margins. The majority of SFTs have a benign clinical course, and complete surgical resection generally offers satisfactory results. This study revealed a descriptive and retrospective analysis of 70 patients with thoracic solitary fibrous tumors (TSFTs) who underwent surgical treatment in our hospital.
Material and methods
From August 2008 to October 2014, we retrospectively reviewed the clinical records of 76 patients who underwent surgical resection for benign or malignant TSFT in our department.
All the patients received a thorough preoperative evaluation in our hospital, including history, physical examinations, serological tests, chest radiographs, pulmonary function tests, thoracic/brain computed tomography (CT) and technetium bone scanning. Positron emission tomography (PET-CT) or thoracic magnetic resonance imaging were carried out if needed. Percutaneous CT-guided needle biopsies of tumors were done in some cases.
To affirm the original histological diagnoses and ensure uniformity, each case was reviewed by at least two junior pathologists and a senior pathologist to confirm the diagnosis, and malignant SFT was diagnosed when one of following criteria was satisfied (England et al. 1989): (1) increasing mitotic activity (4 mitoses per 10 high-power fields), (2) high cellularity with crowding and overlapping of nuclei, (3) presence of necrosis, or (4) pleomorphism.
In total, 76 patients with TSFT were identified. Of them, six patients were excluded because they were lost to follow-up. Postoperative follow-up was performed every 3–6 months. Evaluation modalities for the follow-up included chest computed tomography, additional abdominal or brain computed tomography. Recent patient status was ascertained using the medical records of outpatient clinics or by telephone interview. The remaining 70 patients were enrolled in this study.
Statistical analysis
Clinicopathological data were analyzed using the Mann–Whitney U test, Pearson’s chi-square and Fisher’s exact test. The effect of all these factors on relapse-free survival (RFS) and overall survival (OS) was evaluated with univariate analysis using the log-rank test. A multivariate analysis of independent prognostic factors was performed using Cox proportional hazards Forward LR model. OS were obtained by the Kaplan–Meier method and comparisons between groups with the log-rank test. A p-value of less than 0.05 was considered statistically significant. Analysis and figures were carried out with SPSS for Windows version 23.0 (SPSS, Inc.Chicago, IL, USA).
Results
Patient characteristics and clinical course
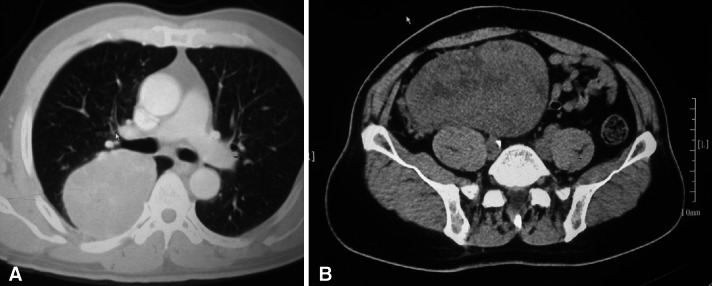
Seventy patients underwent surgical resection for a benign or malignant TSFT in a 6-year period. There were 36 men (51.4%) and 34 women (48.6%) with a mean age of 52.9 ± 11.6 years. No patients had a history of asbestos exposure. Sixty-seven patients had primary TSFTs (benign: 58 vs malignant: 9), including a synchronous double primary benign thoracic and retroperitoneal SFT (size = 10.5 cm, SUVmax = 4.6 and size = 16 cm, SUVmax = 1.9, respectively) (Fig. 1). The others had recurrent tumors (their primary tumors were all malignant TSFTs and they underwent initial surgeries in our hospital). Patients’ clinical characteristics are shown in Table 1.
Fig. 1.
Preoperative CT of a synchronous double primary benign thoracic and retroperitoneal SFT patient: a SFT of pleura (size = 10.5 cm) b retroperitoneal SFT (size = 16 cm)
Table 1.
Patient characteristics (N = 70)
| Characteristic | Number of patients (%) |
|---|---|
| Sex | |
| Male | 36 (51.4%) |
| Female | 34 (48.6%) |
| Age, years (mean ± SD) | 52.9 ± 11.6 |
| Presenting symptoms | 32 (45.7%) |
| Chest pain | 5 |
| Cough | 7 |
| Dyspnea | 4 |
| Weight loss | 1 |
| Abdominal pain | 2 |
| Chest pain plus cough | 4 |
| Chest pain plus fever | 4 |
| Dyspnea plus cough | 4 |
| Cough plus fever | 1 |
| Asymptomatic patients | 38 (54.3%) |
| Localization | |
| Thoracic | 70 |
| Pleura | 43 (61.4%) |
| Lung | 9 (12.9%) |
| Mediastinum | 16 (22.9%) |
| Esophagus | 1 (1.4%) |
| Diaphragm | 1 (1.4%) |
| Peritoneum | 1 |
| Tumor size, cm (mean ± SD) | 8.9 ± 6.2 |
| Tumor size category | |
| < 10 cm | 44 (62.9%) |
| ≥ 10 cm | 26 (37.1%) |
| Tumor type | |
| Benign | 58 (82.9%) |
| Malignant | 12 (17.1%) |
| Surgical approach | |
| Video-assisted thoracoscopic surgery | 27 (38.6%, conversion rate 11.1% [3 of 27]) |
| Standard thoracotomy | 37 (52.9%) |
| Median sternotomy | 6 (8.6%) |
| Surgical procedures | |
| Mass excision | 29 (41.4%) |
| Mass excision with lung resection (wedge resection/lobectomy/pneumonectomy) | 38 (54.3%) |
| Mass excision with enbloc resection of other intrathoracic structures (diaphragm/parietal pleura/ pericardium/rib) | 3 (4.3%) + 2 (redo surgery for re-recurrence) |
Thirty-two patients were symptomatic at the time of diagnosis, and most common symptoms were chest pain and cough. No patient presented with hypoglycemia and hypertrophic osteoarthropathy. The remainder of the patients (54.3%) were asymptomatic, and tumors were incidentally found during chest imaging. The presence of symptoms was correlated with tumor size (p = 0.035) and not with pathological type (p = 0.335). The mean size of symptomatic patients was 10.6 ± 7.2 cm.
Most of preoperative diagnoses were made by the radiologist based on CT. The majority were impressions of a pulmonary mass (lung cancer, mesothelioma, uncertain tumour) or mediastinal tumor (thymoma, fibroma, neurogenic tumor, pericardial cyst, submucosal esophageal tumor). Preoperative percutaneous CT-guided needle biopsy was tried in 12 patients (17.1%, 12/70). The diagnostic rate of SFT and malignant cell-containing tumors that was not definitely diagnosed as malignant SFTP was 33.3% (4/12). No tumor cells were found in the remaining.
Surgical resection was performed through a thoracotomy in 37 patients (52.9%), a video-assisted thoracic surgery (VATS) in 27 patients (38.8%), and a sternotomy in 6 patients (8.6%). VATS was mainly performed in group of < 10 cm (p < 0.001). Mass excision only at its implantation was performed in 29 cases, including one case of esophagus resected by submucosal dissection and one double synchronous primary benign mass excision. But en bloc resection including surrounding structures was performed in the remainder owing to invasion or severe peritumoral inflammatory adhesion. Of them, mass excision with lung resection in 38 patients (wedge resection n = 30, lobectomy: n = 6, pneumonectomy: n = 2). There was no perioperative morbidity or mortality.
Complete surgical resection was performed in all benign patients and nine primary malignant patients. One recurrent patient underwent palliative surgery for regional recurrence in the ipsilateral pleural cavity approximately 8 years after the initial operation. However, he abandoned further treatment and died 3 months later. The second recurrent patient underwent complete resection followed by adjuvant chemotherapy for a solitary lung metastasis approximately 2 years after the initial operation. The third operation was performed in her for re-recurrence after 13 months, and he died 2 months later. The third recurrent patient underwent complete resection for a solitary lung metastasis approximately 6 years after the initial operation. But 66 months after the second surgery on him, re-recurrence and distant metastasis (spleen and bone) occured. Because the recurrent tumor compressed the heart severely, palliative surgery that removed the tumor was performed in him. The patient refused adjuvant chemoradiotherapy and chose instead to receive Chinese traditional treatment. He died 5 months later. The clinical characteristics of patients with malignant TSFTs are shown in Table 2.
Table 2.
Clinical characteristics of patients with malignant TSFT
| No | Age | Sex | Smoking | Symptoms | P/R | Tumor size (cm) | Treatment | RFS (months) | OS(months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | M | Never | None | P | 16 | Mass excision (R0) | 118 + | 118 + |
| 2 | 63 | F | Never | Cough | P | 19 | Neoadjuvant chemotherapy + mass excision with lung wedge resection (R0) | 102 + | 102 + |
| 3 | 72 | F | Never | Dyspnea | P | 40 | Mass excision with lobectomy (R0) | 100 + | 100 + |
| 4 | 19 | F | Never | Chest pain plus fever | P | 13.5 | Mass excision with pneumonectomy (R0) | 95 + | 95 + |
| 5 | 69 | F | Never | Dyspnea plus cough | P |
11.5 18 |
1. Mass excision (R0) 2. Mass excision (R1) + radiotherapy |
8 | 23 |
| 6 | 64 | F | Never | None | P | 14.5 | Mass excision (R0) | 63 + | 63 + |
| 7 | 51 | F | Never | None | P | 7 | Mass excision (R0) | 133 + | 133 + |
| 8 | 62 | F | Never | Cough | P | 10 | Mass excision with lung wedge resection (R0) | 26 | 27 |
| 9 | 43 | F | Never | Dyspnea plus cough | P | 18 |
1. Mass excision with pneumonectomy associated with partial diaphragm and repair (R0) + chemotherapy 2. radiotherapy |
4 | 95 + |
| 10 | 61 | F | Never | Cough | R1 | 7 | Mass excision with lung wedge resection plus partial pericardiectomy and parietal pleura resection (R2) | 3 | 3 |
| 11 | 67 | F | Never | None | R2 |
2 3.5 |
1. Mass excision with lung wedge resection (R0) + chemotherapy 2. Mass excision with lung wedge resection plus parietal pleura biopsy and pericardial nodules biopsy (R2) |
13 | 15 |
| 12 | 58 | M | Never | None | R2 |
1 7 |
1. Mass excision with lung wedge resection (R0) 2. Mass excision with partial costatectomy plus partial pericardiectomy and parietal pleura resection resection (R2) |
66 | 71 |
Surgical margins were categorized as R0: clear (absence of tumor within 1 mm from the edge of the inked specimen); R1: microscopically positive; R2: grossly positive
P primary tumor, R1 regional recurrent tumor, R2 solitary lung metastasis
Relationship between clinical characteristics and pathological type
Out of 70 patients, 58 were proven to be benign and 12 were malignant. Despite the proportion of female patients (24/58) was lower in benign group (41.4%), compared to the malignant group (10/12, 83.3%), the difference was statistically significant (p = 0.008). The mean age in this study was 51.8 ± 10.6 years for benign TSFT and 58.5 ± 14.8 years for malignant tumors (p = 0.018). The mean tumor size was 8 ± 4.6 cm for benign and 13.3 ± 10.2 cm for malignant. The malignant tumors tended to be larger (p = 0.05). Especially in a group of ≥ 10 cm, the malignant tumors had a slight dominance (p = 0.046). There was no significant difference in symptom (p = 0.335), smoking status (p = 0.074), localization (p = 0.233), surgical procedure (p = 0.122) and complete resection (p = 0.171) for benign and malignant. All VATS were performed in benign patients (p = 0.016) (Table 3). Only one incomplete resection occured in reoperation for recurrent malignant patients (Table 2).
Table 3.
Relationship between clinical characteristics and pathological type
| Benign SFT | Malignant SFT | p value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Total | 58 | 82.9 | 12 | 17.1 | |
| Sex | |||||
| Male | 34 | 58.6 | 2 | 16.7 | |
| Female | 24 | 41.4 | 10 | 83.3 | 0.008 |
| Age, (mean ± SD) | 51.8 ± 10.6 | 58.5 ± 14.8 | 0.018a | ||
| Smoking status | |||||
| Smoker | 17 | 29.3 | 0 | 0 | |
| Non-smoker | 41 | 70.7 | 12 | 100 | 0.074 |
| Symptom | |||||
| Symptomatic | 25 | 43.1 | 7 | 58.3 | |
| Asymptomatic | 33 | 56.9 | 5 | 41.7 | 0.335 |
| Localization | |||||
| Pleura | 35 | 60.3 | 8 | 66.7 | |
| Mediastinum | 12 | 20.7 | 4 | 33.3 | |
| Others (lung, esophagus, diaphragm) | 11 | 19 | 0 | 0 | 0.233b |
| Tumor size (mean ± SD) | 8 ± 4.6 | 13.3 ± 10.2 | 0.05a | ||
| Tumor size category | |||||
| < 10 cm | 40 | 69 | 4 | 33.3 | |
| ≥ 10 cm | 18 | 31 | 8 | 66.7 | 0.046a |
| Surgical approach | |||||
| VATS | 24 | 41.4 | 0 | 0 | |
| Non-VATS | 34 | 58.6 | 12 | 100 | 0.016 |
| Surgical procedures | |||||
| Mass excision | 25 | 43.1 | 4 | 33.3 | |
| Mass excision with lung resection | 32 | 55.2 | 6 | 50 | |
| Mass excision with enbloc resection of other intrathoracic structures | 1 | 1.7 | 2 | 16.7 | 0.122b |
| Surgical resection | |||||
| Complete resection | 100 | 100 | 11 | 91.7 | |
| Incomplete resection | 0 | 0 | 1 | 8.3 | 0.171b |
aThese p values were calculated with the Mann–Whitney U test; bthis was calculated using Fisher’s exact test; all others were calculated using Pearson’s chi-square
Long-term follow-up and prognostic analyses
Among all patients, the mean follow-up time was 91 ± 28.7 months. The mean follow-up time for benign TSFT was 95.2 ± 22.8 months and that for malignant TSFT was 70.4 ± 43.8 months (p = 0.08). All benign patients underwent complete surgical resection were alive and no recurrence occurred. All recurrences occurred in 6 malignant patients and 5 of them died because of local recurrence and distant metastasis (Table 2). The median survival of malignant TSFT patients was 83 months (Fig. 2). By univariate analysis, benign vs malignant variant impacted overall survival (p < 0.001). Table 4 outlined the univariate analyses of variables potentially impacting RFS and OS for malignant patients. Significant and borderline significant (p < 0.01) adverse prognostic factors included incomplete surgical resection, recurrent tumor and larger size (≥ 10 cm, for OS but not RFS). But there was no significant prognostic predictor of RFS and OS for malignant patients in multivariate analyses.
Fig. 2.
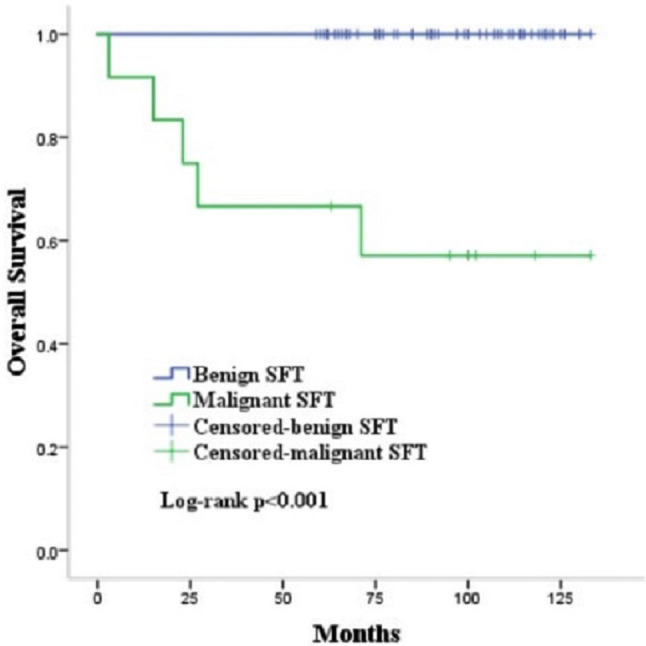
Survival after complete surgical resection of thoracic solitary fibrous tumor. Median survival of malignant TSFT was 83 months. All of the patients with benign TSFT were alive (p < 0.001)
Table 4.
p-values of univariate analyses of factors predicting RFS and OS of malignant TSFT
| Clinical factor | RFS | OS |
|---|---|---|
| Sex, male vs. female | 0.727 | 0.95 |
| Age, < 60 vs. ≥ 60 | 0.619 | 0.124 |
| Symptom, yes vs. no | 0.447 | 0.896 |
| Localization | 0.964 | 0.644 |
| Tumor size, < 10 cm vs ≥ 10 cm | 0.295 | 0.085 |
| Surgical resection, complete vs. incomplete | 0.001 | 0.001 |
| Tumor, primary vs. recurrent | 0.053 | 0.006 |
Discussion
Thoracic solitary fibrous tumor (TSFT) is an uncommon tumor and an estimated age-standardized incidence rate (world standard population) for solitary fibrous tumors of the pleura (SFTp) was 1.4 per million (95% CI, 0.54–2.2) (Thorgeirsson et al. 2010). It peaks at 50–70 years old, although it is seen in all ages (Lococo et al. 2012). The median age was 53 years and malignant TSFTs had an obvious female predominance in this study.
More than 50% of patients are asymptomatic when diagnosed. Common symptoms are chest pain and cough, similar to the results of this study. Okike et al. revealed that symptoms were frequently present in patients with larger tumors or with malignant SFTs (Okike et al. 1978). But there was no statistically significant difference between the proportion of symptomatic patients and pathological type in this study (p = 0.335). Because some symptoms such as chest pain, cough, dyspnea, hemoptysis may occur due to the tumor’s growth-related pressure effect. Paraneoplastic symptoms such as hypoglycemia and hypertrophic osteoarthropathy (the incidence is 4% and 20%, respectively) may also occur due to the secretion of insulin-like growth factor II and the production of a growth hormone-like substance, respectively (Liu et al. 2008).
The size of TSFT varies widely (from 1 to 40 cm) in this study. Malignant tumours tend to be larger than benign ones (p = 0.05). Demicco et al. reported that larger tumours (> 15 cm) had a higher risk of metastasis and death in a risk-assessment model (Demicco et al. 2012). In the study of Gupta et al. malignant SFTs were significantly larger, with median (interquartile range) diameter size of 15.7 (7–17.5) cm compared to 6.05 (3.2–10.9) cm for benign SFTs (p = 0.0291) (Gupta et al. 2017). But the size should not be considered as a predictor of malignancy, some benign TSFTs may reach large sizes and remain asymptomatic.
Because of the rarity of TSFT and its nonspecific radiographic manifestation, the imaging misdiagnosis rate is high (Suehisa et al. 2010). The accuracy rate of preoperative CT diagnosis in this study was only 18.6%. Percutaneous CT-guided needle biopsy is a widely available technique in the diagnosis. Lahon et al. reported a positive predictive value of CT-guided biopsy to be 39% in their study of malignant SFTP; however, they did not specify whether core biopsy or fine-needle aspirate (FNA) only was performed (Lahon et al. 2012). Gupta et al. reported that only 1/13 preoperative FNA made the diagnosis of SFTp and preoperative diagnosis of SFTp was made in all patients (11/11) with core biopsies (Gupta et al. 2017). Cho et al., report that FNA also has the potential for false-positive diagnosis (Cho et al. 2007). Notably, the diagnostic value of core biopsy is higher. In this study, 12 (17.1%) patients underwent percutaneous CT-guided needle biopsy before surgery, and the diagnosis rate is only 33.3%. Generally, it is still not an adequate small biopsy specimen for a definitive preoperative diagnosis. So surgical resection for diagnosis and treatment is acceptable.
It is well known that the gold standard of SFT treatment is complete surgical resection. In this study, complete surgical resection provided a cure in all patients with benign TSFTs. VATS was performed in 27 patients, including three conversion to open approach due to the difficulty of obtaining large free margins and bleeding. We believe VATS has the advantage of effectively checking the blind location of tumors originating from the parietal pleural, such as cupula of pleura, costophrenic angle and mediastinum and dealing with intraoperative adhesion. VATS also has other advantages, such as postoperative pain, reduced respiratory impairment and cosmetic results (Nagahiro et al. 2001). Magdeleinat et al. recommend video-assisted thoracoscopy in the presence of lesions < 5 cm (Magdeleinat et al. 2002). The result is similar to ours (VATS: 5.1 ± 2.4 cm vs non-VATS: 10.9 ± 6.6 cm, p < 0.001). Whether malignant tumors or large tumors, they could be more difficult to be extirpated due to invasive behavior. Lococo et al. proposed that thoracotomy is still the gold standard treatment in sessile, large-sized, invasive, and malignant tumours (Lococo et al. 2012). So thoracotomy or extended resection may be necessary.
The survival outcomes revealed the 5-year RFS and OS of benign patients were all 100%; however, the 5-year RFS and OS of malignant patients was 58.3% and 66.7%, respectively. The 5-year survival of 15 patients with malignant SFTs was reported to be 55% in Memorial Sloan Kettering (Gold et al. 2002), which was similar to us. Bellini et al. (2019) reported that malignant histology (p = 0.03; HR:4.17; 95%CI: 1.15–15.06) and origin from parietal pleura (p = 0.03; HR:3.90; 95%CI: 1.08–14.09) were independent predictors of tumour recurrence and worst RFS (Bellini et al. 2019). A multicenter study reported that positive resection margins, tumors > 10 cm and tumors with a high mitosis rate were the important prognostic factors of SFTs (Houdt et al. 2013). Some literatures reported high p16 expression was significantly associated with malignancy and tumor recurrence (Liu et al. 2008; Liang et al. 2019). Magdeleinat et al. reported a 5 and 10-year survival rate of 22 patients with malignant SFTs (of which only one did not undergo a complete resection) was 89% (Magdeleinat et al. 2002). Milano et al. reported that the actuarial OS and CSS of patients undergoing cancer-directed surgery were similar to those not undergoing surgery beyond 5–6 years (Milano et al. 2011). So the best prognostic factor is probably the completeness of resection, which is in agreement with a multicenter retrospective study (Lococo et al. 2012). In this study, univariate analyses of RFS and OS revealed that complete surgical resection was a significantly prognostic factor for malignant TSFTs. But multivariate analyses revealed no statistically significant difference in this study. Maybe a small number of malignant patients affected the result.
A long, systematic follow-up is mandatory because of the risk of recurrence of TSFTs regardless of the pathological type after surgical resection. A malignant SFTp recurrence has been reported 17 years after surgical resection (Kovacs and Waxman 2019). Postoperative follow-up with CT is recommended every 3–6 months for 2 years and then annually. However, for malignant tumors with a high risk of recurrence, closer clinical follow-up is recommended. In some series of resected malignant SFTP, recurrence occured in 30% (15/50) patients (Lococo et al. 2012). Milano et al. reported 58% of malignant SFTP were found to have regional extension, nodal or distant metastasis at the time of presentation (Milano et al. 2011). Early detection and early treatment are very important. Rena et al. (Rena et al. 2001) reported that local recurrence could be successfully managed by redo surgery. In this study, a solitary lung metastasis occured in an asymptomatic patient with primary malignant TSFT after 6 years postoperative follow-up and the tumour was detected and resected by redo-thoracotomy. When re-recurrence occured in him again, he had been alive beyond 5 years. Thus, aggressive surgical management could be justified in such cases and a satisfactory result also can be obtained.
Because of the limited patient numbers, the role of perioperative adjuvant therapy in SFT is still controversial. Some literatures suggest that malignant tumors can benefit from adjuvant radiotherapy and chemotherapy (Milano et al. 2011; Filosso et al. 2009). The others found no evidence about the possible benefit of adjuvant radiotherapy or chemotherapy after surgical resection of malignant tumors (Cardillo et al. 2000). In this study, one malignant patient received neoadjuvant chemotherapy (epirubicin + ifosfamide + carboplatinum), but the result is response of stable disease. Three malignant patients received postoperative radiotherapy and chemotherapy (Table 2). No conclusion can thus be drawn about the impact of perioperative adjuvant therapies and a larger sample size for further investigation is necessary. Martin-Broto et al. revealed advanced malignant SFTs could benefit from pazopanib through a multi-centre, single-arm, phase II trial (Martin-Broto et al. 2019). Anti-angiogenic therapy would be considered as a new optional adjuvant therapy in the future. Some literatures proposed that England criteria (p = 0.002; HR: 1.98; 95%CI: 1.28–3.07), Diebold (p = 0.008; HR: 1.96; 95% CI: 1.20–3.22) and Tapias (p = 0.003; HR: 1.75; 95% CI: 1.20–2.53) scores were predictors of recurrence (Bellini et al. 2019; Tapias et al. 2015). Maybe prognostic scoring systems could help in postoperative adjuvant therapy decision-making to individuate patients with a higher risk of recurrence equal to TNM stage of lung cancer.
Limitation of the study
First, this was a retrospective single-center study and similar to most of the studies in the literature. Second, due to the rarity of TSFT, despite the patients being collected during 6-year periods, the number of malignant TSFT was too small so that we could not obtain a multivariable survival analysis of RFS and OS for malignant tumors. And also, we could not prove the importance of radical redo surgery for local recurrent tumor and solitary metastatic tumor. However, this study not only proved that complete surgical resection provided a cure in all the patients with benign TSFTs and the majority of primary malignant TSFTs, especially in a synchronous double primary benign thoracic and retroperitoneal SFT (reported first), but also provided valuable information about surgical outcomes and clinical characteristics of TSFTs.
Conclusion
Thoracic solitary fibrous tumors are rare mesenchymal tumors. The majority of TSFTs are rare slow-growing tumors that generally have a favorable prognosis, but about 12% of them are malignant with a possibility of local recurrence and distant metastasis. The preoperative diagnosis of the tumor is a difficult challenge. The best treatment of TSFTs is complete surgical resection. There is still no adequate data to provide significant benefits of radiotherapy and chemotherapy in the treatment of TSFTs. Further study about systemic treatment modality of TSFTs should be performed. A long-term clinical follow-up is highly recommended in all TSFTs because the benign TSFTs also have a malignant behavior.
Funding
The authors declare that they have no funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
This study was approved by the Ethics Committee of Shanghai chest hospital.
Informed consent
Individual written informed consent was obtained from all patients or their legal representatives, as appropriate.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bellini A, Marulli G, Breda C et al (2019) Predictors of behaviour in solitary fibrous tumours of the pleura surgically resected: analysis of 107 patients[J]. J Surg Oncol 120(4):761–767 [DOI] [PubMed] [Google Scholar]
- Cardillo G, Facciolo F, Cavazzana AO et al (2000) Localized (solitary) fibrous tumours of the pleura: an analysis of 55 patients[J]. Ann Thorac Surg 70:1808–1812 [DOI] [PubMed] [Google Scholar]
- Cardillo G, Carbone L, Carleo F et al (2009) Solitary fibrous tumors of the pleura: an analysis of 110 patients treated in a single institution[J]. Ann Thorac Surg 88(5):1632–1637 [DOI] [PubMed] [Google Scholar]
- Cho EY, Han JJ, Han J et al (2007) Fine needle aspiration cytology of solitary fibrous tumours of the pleura[J]. Cytopathology 18(1):20–27 [DOI] [PubMed] [Google Scholar]
- Demicco EG, Park MS, Araujo DM et al (2012) Solitary fibrous tumour: a clinicopathological study of 110 cases and proposed risk assessment model[J]. Mod Pathol 25(9):1298–1306 [DOI] [PubMed] [Google Scholar]
- England DM, Hochholzer L, McCarthy MJ (1989) Localized benign and malignant fibrous tumours of the pleura. A clinicopathologic review of 223 cases[J]. Am J Surg Pathol 13(8):640–658 [DOI] [PubMed] [Google Scholar]
- Filosso PL, Asioli S, Ruffini E et al (2009) Radical resection of a giant, invasive and symptomatic malignant solitary fibrous tumour (SFT) of the pleura[J]. Lung Cancer 64(1):117–120 [DOI] [PubMed] [Google Scholar]
- Gold JS, Antonescu CR, Hajdu C et al (2002) Clinicopathologic correlates of solitary fibrous tumors[J]. Cancer 94(4):1057–1068 [PubMed] [Google Scholar]
- Gupta A, Souza CA, Sekhon HS et al (2017) Solitary fibrous tumour of pleura: CT differentiation of benign and malignant types[J]. Clin Radiol 72(9):796.e9–796.e17 [DOI] [PubMed] [Google Scholar]
- Hanau CA, Miettinen M (1995) Solitary fibrous tumors: histological and immunohistochemical spectrum of benign and malignant variants presenting at different sites[J]. Hum Pathol 26(4):440–449 [DOI] [PubMed] [Google Scholar]
- Harrison-Phipps KM, Nichols FC, Schleck CD et al (2009) Solitary fibrous tumors of the pleura: results of surgical treatment and long-term prognosis[J]. J Thorac Cardiovasc Surg 138(1):19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs T, Waxman J (2019) Recurrence of a malignant solitary fibrous tumor of the pleura 17 years after primary tumor resection—a case report[J]. Respir Med Case Rep 28:100895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahon B, Mercier O, Fadel E et al (2012) Solitary fibrous tumour of the pleura: outcomes of 157 complete resections in a single center[J]. Ann Thorac Surg 94(2):394–400 [DOI] [PubMed] [Google Scholar]
- Liang Y, Heller RS, Wu JK et al (2019) High p16 expression is associated with malignancy and shorter disease-free survival time in solitary fibrous tumor/hemangiopericytoma[J]. J Neurol Surg B Skull Base 80(3):232–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Wang HW, Li FY et al (2008) Solitary fibrous tumors of the pleura: clinicopathological characteristics, immunohistochemical profiles, and surgical outcomes with long-term follow-up[J]. Thorac Cardiovasc Surg 56(5):291–297 [DOI] [PubMed] [Google Scholar]
- Lococo F, Cesario A, Cardillo G et al (2012) Malignant solitary fibrous tumors of the pleura: retrospective review of a multicenter series[J]. J Thorac Oncol 7(11):1698–1706 [DOI] [PubMed] [Google Scholar]
- Magdeleinat P, Alifano M, Petino A et al (2002) Solitary fibrous tumors of the pleura: clinical characteristics, surgical treatment and outcome[J]. Eur J Cardiothorac Surg 21(6):1087–1093 [DOI] [PubMed] [Google Scholar]
- Martin-Broto J, Stacchiotti S, Lopez-Pousa A et al (2019) Pazopanib for treatment of advanced malignant and dedifferentiated solitary fibrous tumour: a multi-centre, single-arm, phase 2 trial[J]. Lancet Oncol 20(1):134–144 [DOI] [PubMed] [Google Scholar]
- Milano MT, Singh DP, Zhang H (2011) Thoracic malignant solitary fibrous tumors: a population-based study of survival[J]. J Thorac Dis 3(2):99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahiro I, Andou A, Aoe M et al (2001) Pulmonary function, postoperative pain and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure[J]. Ann Thorac Surg 72(2):362–365 [DOI] [PubMed] [Google Scholar]
- Okike N, Bernatz PE, Woolner LB (1978) Localized mesothelioma of the pleura: benign and malignant variants[J]. J Thorac Cardiovasc Surg 75(3):363–372 [PubMed] [Google Scholar]
- Rena O, Filosso PL, Papalia E et al (2001) Solitary fibrous tumour of the pleura: surgical treatment[J]. Eur J Cardiothorac Surg 19(2):185–189 [DOI] [PubMed] [Google Scholar]
- Suehisa H, Yamashita M, Komori E et al (2010) Solitary fibrous tumor of the mediastinum[J]. Gen Thorac Cardiovasc Surg 58(4):205–208 [DOI] [PubMed] [Google Scholar]
- Tapias LF, Mercier O, Ghigna MR et al (2015) Validation of a scoring system to predict recurrence of resected solitary fibrous tumors of the pleura[J]. Chest 147(1):216–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson T, Isaksson HJ, Hardardottir H et al (2010) Solitary fibrous tumors of the pleura. An estimation of population incidence[J]. Chest 137(4):1005–1006 [DOI] [PubMed] [Google Scholar]
- van Houdt WJ, Westerveld CM, Vrijenhoek JE et al (2013) Prognosis of solitary fibrous tumors: a multicenter study[J]. Ann Surg Oncol 20(13):4090–4095 [DOI] [PubMed] [Google Scholar]