Summary
The rose is the most cultivated ornamental plant in the world, and one of the reasons is that its fragrance is highly pleasant to humans. This raises the question of which volatile organic compounds (VOCs) emitted by flowers are involved in a rose odor-induced positive emotional response. Here, we invited participants to smell and rate the perceptual characteristics of roses whose VOCs were quantified. We revealed that (1) the more rose-specific the flower perception, the more pleasant the smell and (2) the rosy perception is driven by ionones and to a lesser extent by oxylipins while pleasantness by balanced proportion in the mixture of ionones, oxylipins, and 2-phenylethanol and derivatives. In the mixture, the proportion of some compounds, such as aliphatics and phenolic methyl esters, impact negatively the rose scent. Thus, the pleasure that roses bring to humans could be explained by the non-conscious perception of this unique mixture of compounds.
Subject areas: Natural product chemistry, Molecular biology, Plant biology
Graphical abstract

Highlights
-
•
The more rose-specific the flower perception, the more pleasant the smell
-
•
Rosy perception is driven by ionones and to a lesser extent by oxylipins
-
•
Mainly ionones, oxylipins and 2-phenylethanol drive rose pleasantness
Natural product chemistry; Molecular biology; Plant biology
Introduction
Roses are widely known for their very pleasant smell, color and shape, so much that they are praised in poems, books, music and art. Roses have been present in human history for thousands of years, with the first representation of rose in 1700–1500 BCE.1 Their scent is so appreciated that it is used to odorize our outdoor environment (gardens), indoor environment (house), our beauty products in cosmetics and ourselves with perfumes. In other words, the rose has been cultivated for thousands of years because it is highly appreciated by humans.
Rosa is a genus belonging to the Rosaceae family, comprising about 150 wild species and nearly 30,000 cultivars.2,3 Among these cultivars, there is a lot of diversity, in terms of color and shape but also scent. Some cultivars have little to no smell, and some have a strong fragrance. What accounts for the difference in smell between roses is the diversity of volatile organic compounds (VOCs) emitted.4,5 Hundreds of VOCs are emitted by roses.6,7,8,9 Although the cultivars of roses differ depending on their fragrance and thus their composition of VOCs, the most recurrent ones are monoterpenes, sesquiterpenes, 2-phenylethanol (PEA) and derivatives (phenylpropanoids-related compounds) and fatty acid derivatives or oxylipins.10 Some other compounds are present in minor quantities, sometimes with a very low olfactory detection threshold, such as apocarotenoids including ionones.11 Among VOCs described as contributing to the typical rose scent, PEA, geraniol, β-citronellol and nerol are the most frequently cited.12,13,14,15,16 They are also used to re-create the rose smell.17 These monomolecular odorants are often rated as pleasant,18,19,20,21,22 which is also true for the rose smell.23,24,25 In addition of being attractive, rose smell has an effect on emotional state. Indeed, many studies in both animal models and humans, using mainly rose essential oil, reported positive effects on mood with increased happiness, decreased stress, anxiolytic or anti-conflict effects as well as improvement of the symptoms of depression.23,26,27,28,29,30,31,32 These effects are associated with changes in neural activity in brain areas involved in emotional and motivational behaviors.23 Interestingly, none of these studies used fresh roses and investigated the link between the molecules emitted by the rose and the behavioral effect.
In this context, we set up a study to examine the relationship between the olfactory perception and the compounds emitted by fresh roses. To do so, we selected ten modern roses that were picked in their blooming stage and in the morning when their smell is the strongest.33,34,35 Participants were asked to evaluate blindly odorants using psychophysical tools. Visual analogue scales were used to rate olfactory perception36,37,38,39,40,41 and more particularly odor pleasantness (liking), attractivity (wanting), familiarity and intensity. In addition, the fruity, lemony, floral and rosy notes were questioned. Then, we analyzed the VOCs emitted by roses with headspace method coupled to gas chromatography coupled to mass spectrometry (GC-MS) and investigated the relationship between the olfactory perception of roses and their emitted VOCs. Finally, to better understand the emotional aspect of participants’ relationship to the rose fragrance, natural language processing approach was used.
Results
Rose smell has a strong positive emotional impact
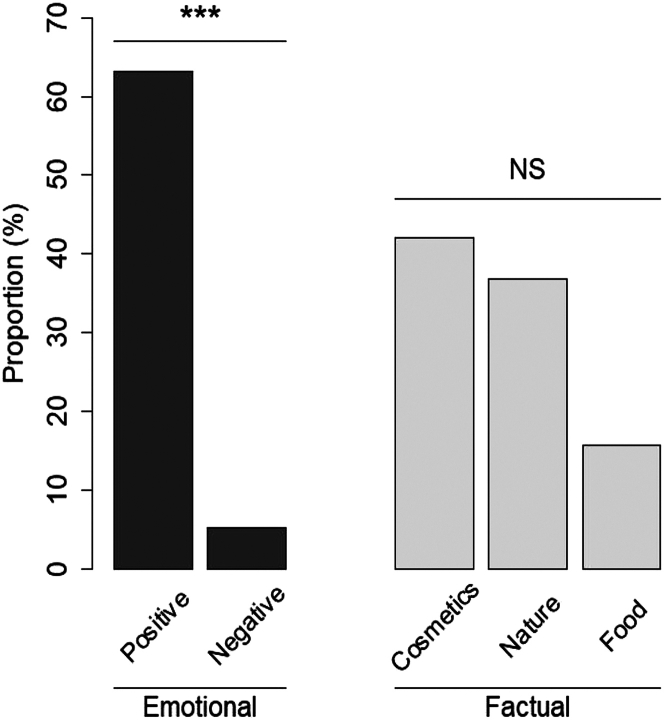
Participants were asked to respond openly to the question “What does the smell of roses make you think of?” This question involves a memory recall and the answer may reflect participants’ relationship to the odor of roses. Participants’ answers were classified into two main categories: answers that refers to (1) the emotional impact of the rose smell on the participants, whether it is positive or negative (i.e., “it is a pleasant, sweet smell”, “it is not one of my favorite fragrances.”), and (2) factual description of the odor. This last category was divided into three subcategories: Cosmetics and perfumes (i.e., “it makes me think of a facial cream.”), green nature (i.e., “it makes me think of a garden) and food (i.e., “it makes me think of rose jam.”). Some of the responses of the participants evoked nostalgia (i.e., childhood, beginning of summer) that we put in the positive category, based on literature and positivity of the associated words.42 The main result is that rose fragrances induce high emotional positive impact, compared to negative one (Figure 1; t-test for proportions, p < 0.001). These positive responses are associated with descriptive elements, especially cosmetics and green nature. Food was also evoked, but to a lesser extend (Figure 1). These data are in line with the literature on the influence of rose smell on well-being.23,27 These descriptions of participants’ response to rose odors were done at the end of olfactory ratings described below, to avoid the influence of the knowledge of the sensory cues by participants.
Figure 1.
Percentages of occurrences given by the participants in response to the question: what does the smell of rose make you think of?
The different roses drive different sensory perception and emotional responses
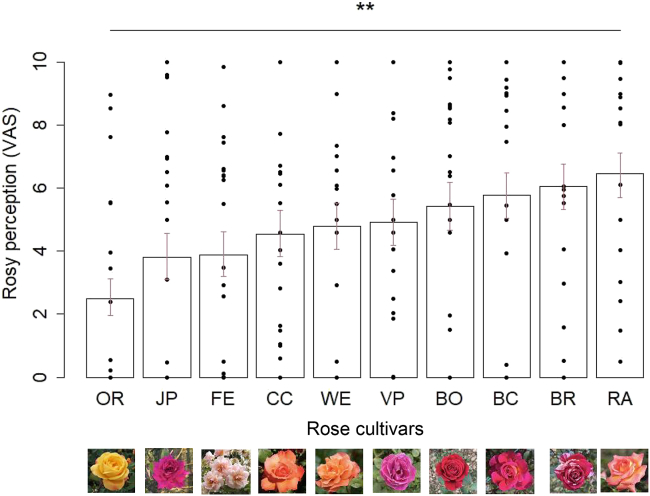
Based on descriptions by rose breeders, we selected 10 rose cultivars with striking differences in scent characteristics (OR: ‘Orientalia’; CC: ‘Christophe Colomb’; BR: ‘Brocéliande’; VP: ‘Violette Parfumée’; FE: ‘Felicia’; BO: ‘Botero’; WE: ‘Westerland’; JP: ‘Jean-Paul Guerlain’; BC: ‘Belle de Clermont’; RA: ‘Roberto Alagna’; Table S1). The scents of some of them are described by breeders as classical old rose, others as more fruity or spicy (Table S1). These roses were presented to 19 participants in opaque flasks, without knowledge of what was in the flasks, so that their ratings were only based on olfactory cues (see methods). Participants were asked to rate how much they smelled rose, on a scale of 0–10. (Figure 2).
Figure 2.
Olfactory evaluation task
(A) Material, example of a freshly picked rose soaked in water-soaked floral foam and placed directly in an opaque flask. All flasks were covered with a piece of polypropylene held in place by a rubber band, so that participants had no visual access to the content.
(B) Participants were asked to smell and rate ten opaque flasks.
We confirmed that the different cultivars of selected roses were perceived significantly different in terms of rosy perception (χ2 = 25.67, df = 9, p = 0.002) (Figure 3). We also asked participants to rate other attributes of the smells, such as pleasantness, attractivity, intensity, familiarity, smell of fruit, lemon and flower (Table S2). Interestingly, the rosy perception of the flower was correlated to pleasantness, attractivity and familiarity. As expected, it was also correlated to flower but not lemon perceptions and negatively correlated to fruity perception. (Table 1). Finally, intensity was not significantly correlated to the rose perception.
Figure 3.
Rating of the 10 rose cultivars in terms of rose perception
VAS: Visual Analogue Scale. OR: ‘Orientalia'. CC: ‘Christophe Colomb'. BR: ‘Brocéliande'. VP: ‘Violette Parfumée'. FE: ‘Felicia'. BO: ‘Botero'. WE: ‘Westerland'. JP: ‘Jean-Paul Guerlain'. BC: ‘Belle de Clermont'. RA: ‘Roberto Alagna'. Bars represent Estimated Marginal Means (±SE) computed from mixed beta regressions and points show individual data. ∗∗p < 0.01.
Table 1.
Correlations of rose perception and others ratings (controlling for the participant effect)
| Rating parameters | r | t | P |
|---|---|---|---|
| Pleasantness | 0.252 | 3.55 | <0.001 |
| Attractivity | 0.256 | 3.61 | <0.001 |
| Familiarity | 0.328 | 4.74 | <0.001 |
| Flower | 0.737 | 14.89 | <0.001 |
| Fruit | −0.317 | −4.57 | <0.001 |
| Intensity | 0.141 | 1.94 | 0.063 |
| Lemon | 0.009 | 0.12 | 0.907 |
r: Pearson’s partial correlation coefficient. t: Pearson’s statistics. P: False Discovery Rate-adjusted P-values.
Rose cultivars have contrasting VOC compositions
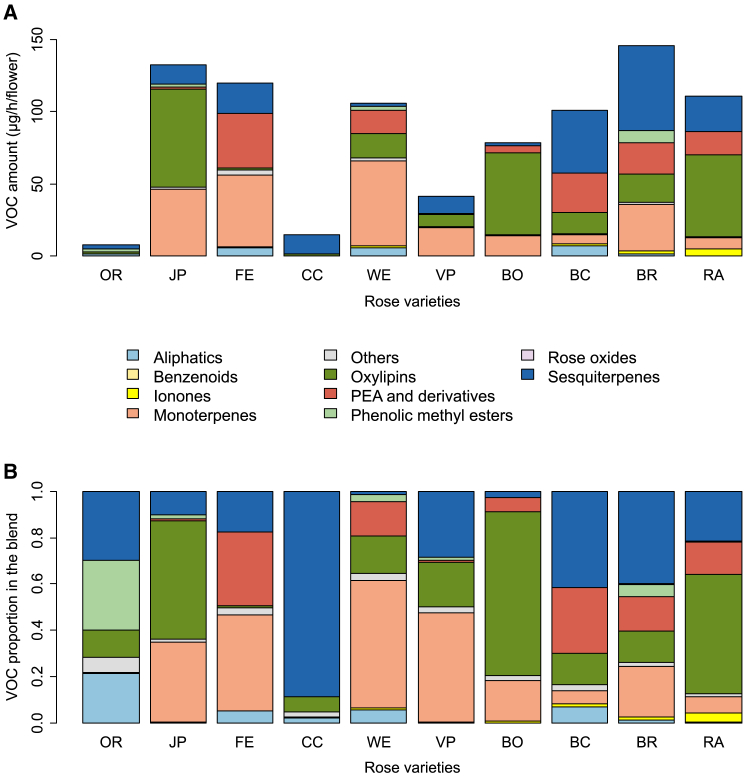
We then captured the VOCs emitted by the ten rose cultivars, using headspace and analyzed their composition by GC-MS. To ensure the validity of the results, between three and six biological replicates per variety were used. Following headspace experiment of the ten rose cultivars, 97 VOCs were detected (Table S3). It is worth noting that the total quantity of VOCs emitted by the roses do not correlate with the weight of the rose (r = 0.121, t = 0.71, df = 34, p = 0.481; Table S4). VOCs were pooled into nine categories of molecules, according to their biosynthesis pathways8,43,44,45,46 (Table S5). We found that the 10 cultivars of roses presented a significantly different pattern of emitted VOCs categories, whatever VOC amounts or relative proportions were considered (variety × VOC category interaction: χ2 = 2576.24, df = 81, p < 0.001 and χ2 = 5736.57, df = 81, p < 0.001, respectively; Figure 4). Total VOC emissions ranged from 11.1 to 149.2 μg/h/flower, while the profile of the bouquet varied markedly. For example, FE and WE emitted a majority of monoterpenes (42.6% and 55.8% respectively) whereas JP, RA, and BO emitted large amounts of oxylipins (50.9%; 51.0% and 70.4% respectively). Ionones and aliphatics were emitted in lesser amounts by the flowers (Table S5).
Figure 4.
VOCs emitted by the roses
Estimated Marginal mean amount (A) or relative proportion (B) of each VOC category in the odor blend emitted by the 10 rose cultivars of this study.
Total amount of VOCs was positively correlated to the familiarity (r = 0.86, df = 8, p = 0.011; Tables S2 and S5) and to the perceived intensity of flowers (r = 0.81, df = 8, p = 0.017; Tables S2 and S5).
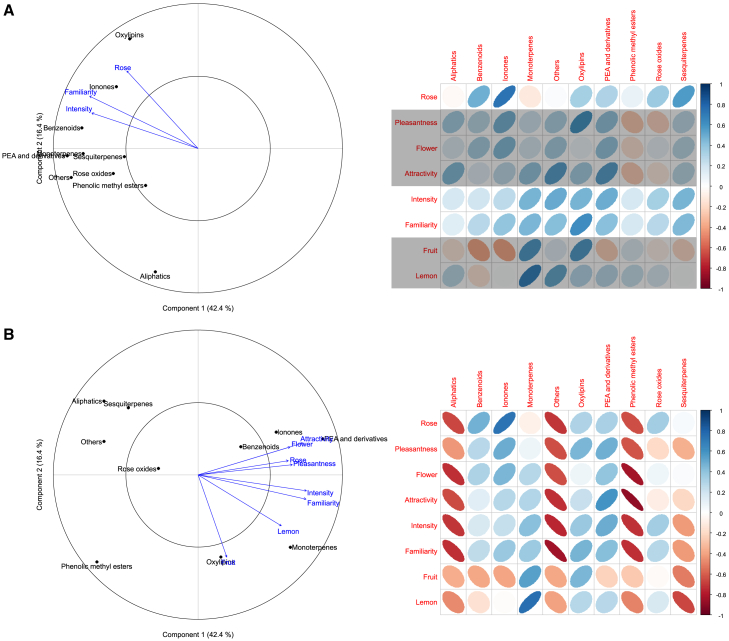
The rosy perception is mainly driven by ionones and oxylipins
Using a combination of multivariate and univariate analyses, we revealed two main categories of VOCs involved in the rosy aspect of perception, ionones and oxylipins. Indeed, first, both multivariate and univariate analyses pointed toward a positive correlation of the perception of rose with the amount of ionones (Figure 5A; Tables 2 and S6). This finding was also true when proportion of ionones were considered (Figures 5B, Tables 2 and S6). Among these ionones, the perception of rose increased when the relative proportion of dihydro-β-ionone in the blend increased (Table S7). Second, multivariate and univariate analyses were also consistent in showing that the perception of rose increased when the relative proportion of hexan-1-ol and to a less extend hexyl acetate, (E)-2-hexenal and (E)-2-hexen-1-ol (oxylipins) in the blend increased (Table S7 and Figure S1). This involvement of oxylipin category was also revealed in the multivariate analysis on VOC amounts (Figure 5A left). Interestingly, while the rosy perception is strongly driven by ionones, the flower perception relies on a more balanced presence of several components such as ionones, PEA and derivatives and benzenoids.
Figure 5.
VOC categories and perceptual ratings
Left: correlation circles from principal component analyses (PCAs) performed on the amount (A) and relative proportion (B) of each VOC category in the odor blend emitted by the 10 rose cultivars of this study. Blue arrows show perceptual ratings that are associated with results of the PCAs with p < 0.1 (Table 2). Right: Ellipses showing univariate correlations between VOC categories and perceptual ratings, in front of the corresponding PCA (color scale: R value, direction and narrowness of the ellipses accordingly). Gray rectangles overlap perceptual ratings which association with the PCA have p > 0.1 (i.e., perceptual ratings not shown on the correlation circle).
Table 2.
Results of permutation tests testing for an association between PCAs on odor blends emitted by the 10 rose cultivars of this study (reduced to VOC categories) and perceptual ratings of these categories
| VOC amounts | VOC relative proportions | |||
|---|---|---|---|---|
| Perceptual rating | r2 | P | r2 | P |
| Rose | 0.591 | 0.043 | 0.539 | 0.070 |
| Pleasantness | 0.449 | 0.126 | 0.579 | 0.051 |
| Flower | 0.271 | 0.335 | 0.596 | 0.039 |
| Attractivity | 0.357 | 0.213 | 0.774 | 0.004 |
| Intensity | 0.667 | 0.019 | 0.777 | 0.010 |
| Familiarity | 0.772 | 0.004 | 0.791 | 0.006 |
| Fruit | 0.056 | 0.831 | 0.489 | 0.086 |
| Lemon | 0.340 | 0.238 | 0.616 | 0.038 |
R2: association coefficient. P: P-value. Bold P-values show significant associations.
Ionones, PEA and derivatives, and oxylipins are the key components for a pleasant rose scent
Pleasantness, attractivity, and familiarity, being correlated to the rosy perception (Table 1), we analyzed the components of these important attributes. Both multivariate and univariate analyses show that PEA and derivatives proportions are associated to attractivity of the rose (Figure 5B and Table S6). Interestingly, pleasantness, like flower perception, is driven by the balanced proportion of multiple molecules, such as ionones, PEA and derivatives and oxylipins. On the contrary, the proportion of some compounds, such as aliphatics and phenolic methyl esters, seem to impact negatively most of the attributes of the rose scent (Figure 5B). It is worth noting that proportion of monoterpenes are associated with the perception of fruit and lemon, both in univariate and multivariate analyses (Figure 5B).
Discussion
Numerous studies report positive effects of the scent of roses, mostly essential oils or individual compounds on emotions and well-being.23,26,29,30,31 Using free-speech analyses in response to the question “What does the smell of roses make you think of?”, we also found that evoking a rose fragrance triggered a positive emotional response. We are well aware that the definition of what is the rose scent could vary according to the country and culture. However, we focused on the determinants of the classical rose scent in Europe, which corresponds to the scent, for example, of the Damask roses and essential oils used for perfumes.47 This is the classical rose scent to which most European people are most familiar, which is the case for our participants. To go further, the aim of this study was to investigate the compounds emitted by fresh roses that are involved in their positive olfactory perception of the rose.
We used garden roses for their complex scent to analyze human response to odors in a more ecological context, compared to monomolecular odorants that are often used in olfactory studies. In our panel of cultivars, we showed that there is not one and unique rose scent but different perceptual representations depending on the variety of the rose. Interestingly, the rosy perception was positively correlated to pleasantness, attractivity, and familiarity. This reveals that the rosy note is important for the positive perception of the flower since the more it smells rose, the more the scent is pleasant and attractive, knowing that these attributes are frequently correlated.18
We then analyzed the quantity of VOCs emitted by the different roses of our panel. In line with a previous study,48 the total amount of VOCs was positively correlated with the intensity of the roses’ scent. We wanted to know if the emblematic individual compounds of the rose were sufficient to describe in its complexity the rose scent. We thus analyzed the perceptual quality of VOCs emitted by the different roses. When authors want to recreate artificial rose perfume, they usually use four compounds (geraniol, nerol, PEA and β-citronellol).17 Unexpectedly, in our study, none of these molecules, neither their categories were found determinant in the rosy perception.
However, our study highlights the role of a major category of molecules, ionones, in the rosy perception. Indeed, we found that both the quantity and proportion of ionones were strongly correlated to the rosy perception, dihydro-β-ionone being the most important driver of this effect. This result is interesting since ionones are highly appreciated by perfumers, and often has a very low odor threshold.49 For example, rose essential oils contains traces of two ionones, β-damascenone and damascone, which have a huge impact on the fragrance of the oil.11 However, it was shown that these molecules are not present in fresh roses, but are degradation products, which appear due to the process of distillation.50 The ionone that we found correlated with perception in our rose cultivars, dihydro-β-ionone, is less known by perfumers and is not described as having rosy odors, but more violet, woody, ambery, and fruity odors (The Good Scent Company Website: http://www.thegoodscentscompany.com/). Contrary to our expectations, the PEA and derivatives category was not correlated to the rosy perception, whereas it is often used to describe and reconstruct the rose scent.17,51 However, proportion of PEA and derivatives was found to be one of the major categories, with ionones and oxylipins, driving pleasantness and attractivity. Our finding is in line with previous studies showing that PEA is frequently judged as pleasant.18,52
Oxylipins are described in the literature as green odors,53,54 and studied for their effect on the emotional state and well-being.55,56,57 Indeed, green odors are known to have an anxiolytic, anti-depressive, anti-fatigue, and anti-stress effects in rodents53,54,56,58,59 and also in humans.60,61 More broadly, green odors are described as pleasant in the literature.62,63,64 Regarding their contribution to the rose smell, green odors reinforce the vegetal character. This is a reason why these compounds are used by perfumers; they bring freshness and greenness to a floral bouquet and thus improve the naturalness of the rose scent.65
In conclusion, it is interesting to note that ionones are the only category of molecules involved both in the rosy and the pleasant perception.
Monoterpenes, are major compounds emitted by rose flowers. Most of them, like geraniol and nerol, when presented as molecular odorants, give the perception of rose66 and are used in simplified model of rose odors.17 Contrary to our expectations, the monoterpene category was not correlated to the floral or rosy fragrance but was associated with the fruity perception, another dimension of the rose perception which is also expected.67,68 These results suggest that the perception of a rose smell in its natural context differs from the one of its individual compounds. Indeed, when included in a mixture, the perception of the compounds can change.69,70 As a consequence, the pleasantness of the mixture can also be different from what is expected from its constituents.21,71,72 This phenomenon is of course occurring for flower extracts.73 Flower scents are complex mixtures composed of hundreds of compounds and rose is no exception. In modern cultivars, rose scents often deviate from the typical rose scent with also fruity notes. Since most studies used monomolecular compounds,20,74,75,76,77 simplified models of rose odor17,73 or essential oils,16 description of olfactory perception can be incomplete or even false.4 Another layer of complexity is due to the fact that, in the mixture, the proportion of some compounds, such as aliphatics and phenolic methyl esters, impact negatively the rose scent. The sweet smell of rose depends thus on the presence, in balanced proportions and quantities, of multiple compounds, some of which are required and others unwanted. Our study also shows that other individual compounds, such as benzaldehyde and isoamyl acetate, may contribute to the pleasantness of the rose perception (Table S7).
The role in the rosy perception of individual compounds could be evaluated more directly by assessing individually each compound during perception using, for example, GC-O.78,79 Furthermore, their impact in the mixture could be evaluated with innovative techniques allowing to add or remove components from a mixture.80
In summary, in addition to the well-known rose-scented phenylpropanoid derivatives, our study illuminates a combination of unexpected molecules emitted by the natural rose and dedicated to its pleasantness, the ionones and oxylipins. These compounds with their interesting properties on emotional behavior contribute to the broad impact of rose scent. Together, these results open new possibilities for the modeling of rose odor, both in fundamental research, remediation (such as aromatherapy), and for industry purposes. In the horticultural sector, rose breeders find it difficult to obtain fragrant roses. They could benefit from our studies, which brings a more comprehensive knowledge of what compounds should be considered in the selection process. Finally, these findings have direct relevance in the field of neuroscience for a better understanding of mixture perception.
Limitations of the study
Some limitations in this study should be noted. First, the sample size for the rose cultivars could be extended, including cultivars of roses with scents that differ even more from the classical rose scent. The second limitation of this study is that the link between VOC abundance and olfactory perception is merely a correlation. Additional experiments where some compounds can be added or withdrawn from the rose scent are needed to determine their role in the perception. Techniques such as GC-GOOD have already been successfully applied to odors.80 Finally, even if the rose is the most beloved flower worldwide, origin and culture of the participants could change olfactory perception and it would be interesting to validate our results on participants from different countries.
Resources availability
Lead contact
Further information and requests for resources and information should be directed to and will be fulfilled by Nathalie Mandairon, nathalie.mandairon@cnrs.fr.
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
This work was supported by the Pack Ambition Recherche of the Région Auvergne Rhône-Alpes. We thank Meilland International for its participation in the project and its support. We thank the CNRS GDR O3 ″Odorant, Odeur, Olfaction" and the GDR "ChemEcol", which facilitated collaboration between the authors. We also thank Lyon Parc de la tête d’or for providing rose cultivars.
Author contributions
J.C.C., M.B., S.B., and N.M. designed research; I.A., A.B., M.T., B.B., and J.D. performed research; I.A., M.H., M.M., S.B., and N.M. analyzed data; I.A., M.H., M.B., J.C.C., S.B., and N.M. edited the paper; I.A., M.H., S.B., and N.M. wrote the paper.
Declaration of interests
The authors declare no competing interests.
Declaration of generative AI in scientific writing
The authors did not use generative AI in scientific writing upon submission of the paper.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Camphor | Sigma | 21293 |
| Porapak Q80/100 | Supelco | 20331 |
| Tenax TA | CJ Lab | MAT-TR-TA |
| nHexane 99% for GC-MS | Carlo Erba | 447212 |
| Software and algorithms | ||
| R software version 4.3.1 | R Core Team7 | https://www.r-project.org/ |
| Mass hunter | Agilent | https://www.agilent.com/en/product/software-informatics/mass-spectrometry-software |
| DataAnalysis | Agilent | https://www.agilent.com/en/product/software-informatics |
Experimental model and study participant details
Participants
Experiments were done following procedures in accordance with the ethical guidelines of the Declaration of Helsinki and the French National Ethics Committee (CPP). Twenty participants from Lyon area in France were tested at the Lyon Neuroscience Research Center. Their olfactory performances were checked using the identification part of the European Test of Olfactory Capabilities (ETOC).81 Ten men and ten women between the ages of 18 and 35 years (mean age: 24 ± 4 years old) who did not present any asthma, odor allergies or olfactory impairment were included. No sex influence on the rating results was found (p = 0.89). Only one participant was finally excluded because he assessed only two flowers out of ten (n = 19). Participants received financial compensation for the experiment and provided written informed consent prior to participation.
Plant material
Ten rose cultivars, ‘Belle de Clermont' (BC), ‘Botéro' (BO), ‘Brocéliande' (BR), ‘Christophe Colomb' (CC), ‘Felicia' (FE), ‘Jean-Paul Guerlain' (JP), ‘Orientalia' (OR), ‘Roberto Alagna' (RA), ‘Violette Parfumée' (VP) and ‘Westerland' (WE), were used in olfactory experiment and for GC-MS analyses (Table S1). Roses were cultivated and harvested in the international rose garden of the Parc de la Tête d'Or in Lyon. All roses were picked on the morning of the experiment except once for one rose, ‘Violette Parfumée', that was kept one time overnight in a cold room due to the lack of fresh flowers available the morning of the experiment. Fully opened flowers were chosen. Roses were chosen for their diversity of scent, as judged by the olfactory descriptions found in catalogs and sites of breeders (Table S1).
Method details
Perceptual rating assessment
Participants were asked to smell and rate ten opaque flasks, which contained the ten freshly picked roses (Table S1). All roses were dipped in a water-soaked floral foam directly placed in the opaque flasks. All flasks were covered by a piece of polypropylene held by a rubber band (deprived of its odor using a 70% alcohol bath) so that participants had no visual access to the content of the flask.
Experiments took place in rooms in which temperature and humidity are controlled. We conducted the experiments in the morning and the afternoon. Participants were asked to sit about 30 cm away from a computer screen and to maintain their position (Figure 2). The 10 flasks containing the ten roses were placed in front of them. Then, 5-s of instructions scrolled on the screen: “Please smell the scented flask in front of you - Be sure to respect the order of presentation” and then, the following instructions indicated to participants when to smell the next flask. These instructions were presented before each new trial. Participants had 1 min to freely smell each odorant and evaluate it. This evaluation was done using a visual analogue scale (VAS) from 0 to 10 with a neutral point at 5. Graduation was not visible and participant had to place a cursor on the scale. Participants had to rate pleasantness (“Rate the pleasantness/unpleasantness of the smell: from very unpleasant to very pleasant”), intensity (“Rate the intensity of the smell: from not at all to very”), familiarity (“Rate the familiarity of the smell: from not at all to very”), attractivity (“How much do you want to experience the smell again: to not at all to very”), fruity smell (“How much do you smell fruit?: from not at all to a lot”), smell of lemon (“How much do you smell lemon?: from not at all to a lot”), smell of flower (“How much do you smell flower?: from not at all to a lot”) and smell of rose (“How much do you smell rose?: from not at all to a lot”) (Figure 2). After 1 min they were instructed to move directly to the next odorant. The order of presentation of the flasks was indicated by a list given to each participant and random between participants.18 We controlled that there was no half-day effect on global perception (Mann-Whitney test; p = 0.65). At the end of the experiment, an additional questionnaire was completed by the participants with the following question: “What does the smell of roses make you think of?” Answers were then categorized into emotional impact (positive or negative) or factual description of the odorant.64 This last category was divided into three subcategories: cosmetics and perfumes, green nature and food. Some of the responses of the participants evoked nostalgia that we put in the positive category, based on literature and positivity of the associated words.42
Biochemical analysis of roses
Volatile organic compounds
VOCs emitted from rose flowers were collected as previously described82 with minor modifications. More precisely, one flower per sample was cut at flower stage 4 (fully opened flower)83 by keeping 3 cm of flower stalk and dipped in a 50 mL beaker filled with tap water placed in a 1 L glass jar equipped with an inlet and outlet (Figure 6). Three to six independent biological replicas were collected for each variety. VOCs were pumped out the jar for 1 h at a flow rate of 100 mL min−1 and trapped on a glass cartridge containing 30 mg of Porapak Q (80/100 mesh, Grace scientific). Inlet air was filtered on metal cartridges filed with 100 mg of Teenax. After the collection, the cartridges were eluted in GC-MS compatible glass vials with 200 μL of a solution of Hexane containing 50 μM of camphor used as internal standard before GC-MS analysis.
Figure 6.

Headspace system used for the extraction of rose
GC-MS analysis
Samples were analyzed on an Agilent 6850 gas chromatogram system connected to an Agilent 5973 mass detector (all components from Agilent Technologies, Santa Clara, USA).82,84 For each sample, 2 μL were injected in a split/splitless injector set at 250°C with a 2:1 split ratio. Separation was realized on a DB-5 capillary column (30 m, 0.25 mm nominal diameter, 0.25 μm film thickness, Agilent) at a flow rate of 2.0 mL min−1 using helium as carrier gas with the following program: initial temperature of 40°C for 3 min followed by a temperature gradient of 3 °C min−1 until reaching the final temperature set at 245°C. Detector temperature was set at 250°C, ionization energy was set at 70 eV and mass spectra were scanned from 35 to 450 amu. MassHunter Unknown Analysis Software (Agilent Technologies) or DataAnalysis Software (Agilent Technologies) were used for data processing. VOCs were identified based on their retention times and their electron ionization mass spectra compared to those present in libraries (Wiley 275, NIST08, the laboratory’s own database). Quantification of each compound emitted by the whole flower was realized by measuring the area of their relative peak compared to those of the internal standard and expressed as μg.H−1.flower−1 which corresponded to the conditions in which participants smelled the samples during the olfactory rating. Compounds were also quantified as their relative proportion to the total blend emission. Compounds were then divided into 9 categories of VOCs, according to their biosynthesis pathways (monoterpenes, PEA and derivatives, oxylipins, ionones, sesquiterpenes, benzenoids, phenyl methyl ethers, rose oxides, aliphatics).43,44 The remaining compounds were grouped into the category “others”. The list of compounds and categories is given in Table S3.
Quantification and statistical analysis
All the statistical analyses were performed with R software version 4.3.1.85 Statistical details of experiments can be found in the figure legends, figures and result section.
Final open question
A Fisher exact tests was used to compare the proportion of participants that gave answers belonging to each ‘factual’ category (i.e., ‘Cosmetics’ vs. ‘Nature’ vs. ‘Food). The same was applied to ‘Positive’ vs. ‘Negative’ emotional answers.
Perceptual ratings
The rosy perception of flowers was compared between rose cultivars using a Wald test applied on a mixed beta regression (link function: logit), where the participant was considered as a random factor (R packages glmmTMB86 and car87). The correlation between the rosy perception and all other ratings was assessed using partial Pearson correlation tests controlling for the effect of the participant, with p-values adjusted for multiple testing with the False Discovery Rate (FDR) method.88
VOCs
The relationship between the total amount of VOCs emitted and the flower weight was assessed using a Pearson correlation test. The pattern of VOCs emitted by flowers was compared between rose cultivars using Wald tests applied on linear mixed models. In these, fixed factors were the VOC category, the variety and the interaction between these two variables, while the biological replicate was considered as a random factor. Response variables were either the total amount of VOCs emitted per category (fourth-root transformed to ensure model fit) or the relative proportion of each category in the blend (square-root transformed). The model fitted on relative proportions excluded the category “Others” to avoid multicollinearity, as relative proportions of all categories sum up to unity by definition. n represents the number of participants.
In all subsequent analyses, mean values per rose variety were used. These were Estimated Marginal Means (EMMeans, R package emmeans89) computed from models detailed above, i.e., mixed beta regressions for perceptual ratings and linear mixed models for VOC amounts/relative proportions. Amounts and relative proportions of individual VOCs were obtained as with VOC categories. The correlation between the total amount of VOCs emitted and all perceptual ratings was assessed using Pearson correlation tests, with FDR-adjusted p-values. The relationship between odor blends and perceptual ratings was assessed as follows: first, a Principal Component Analysis (PCA) was performed on VOC data (either VOC categories or individual compounds) (R package vegan90). VOC amounts were fourth-root transformed then autoscaled, while VOC relative proportions were added a constant of 0.001 (because of zeroes) then transformed using the Centered Log-Ratio transformation.91 In a second step, the association between results of the PCA and perceptual ratings was tested using a dedicated permutation test with 9999 permutations (R package vegan91). Univariate Pearsons’s correlation coefficient was used to complement the interpretation of results from the PCAs. It should be noted that given the number of compounds to be correlated, the limited size of the data (i.e., one value per rose variety) and the necessary correction of the p-values, testing for the significance of these coefficients was precluded. Instead, an arbitrary threshold of 0.5 was used to consider a coefficient as sufficient to be discussed.
Published: December 19, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.111635.
Contributor Information
Sylvie Baudino, Email: sylvie.baudino@univ-st-etienne.fr.
Nathalie Mandairon, Email: nathalie.mandairon@cnrs.fr.
Supplemental information
References
- 1.Caissard J.-C., Adrar I., Conart C., Paramita S.N., Baudino S. Do we really know the scent of roses? Bot. Lett. 2023;170:77–88. doi: 10.1080/23818107.2022.2160807. [DOI] [Google Scholar]
- 2.Leus L., Van Laere K., De Riek J., Van Huylenbroeck J. In: Ornamental crops. Van Huylenbroeck J., editor. Springer International publishing; 2018. Rose; pp. 719–767. [Google Scholar]
- 3.Young M.A., Schorr P. American Rose Society); 2007. Modern Rose 12: The Comprehensive List of Roses in Cultivation or of Historical or Botanical Importance. [Google Scholar]
- 4.Shalit M., Shafir S., Larkov O., Bar E., Kaslassi D., Adam Z., Zamir D., Vainstein A., Weiss D., Ravid U., Lewinsohn E. Volatile compounds emitted by rose cultivars: Fragrance perception by man and honeybees. Isr. J. Plant Sci. 2004;52:245–255. doi: 10.1560/P7G3-FT41-XJCP-1XFM. [DOI] [Google Scholar]
- 5.Feng Y., Cheng X., Lu Y., Wang H., Chen D., Luo C., Liu H., Gao S., Lei T., Huang C., Yu X. Gas chromatography-mass spectrometry analysis of floral fragrance-related compounds in scented rose (Rosa hybrida) varieties and a subsequent evaluation on the basis of the analytical hierarchy process. Plant Physiol. Biochem. 2022;185:368–377. doi: 10.1016/j.plaphy.2022.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L., Yu C., Cheng B., Han Y., Luo L., Pan H., Zhang Q. Studies on the volatile compounds in flower extracts of Rosa odorata and R. chinensis. Ind. Crops Prod. 2020;146 doi: 10.1016/j.indcrop.2020.112143. [DOI] [Google Scholar]
- 7.Feng D., Jian H., Zhang H., Qiu X., Wang Z., Du W., Xie L., Wang Q., Zhou N., Wang H., et al. Comparison of volatile compounds between wild and cultivated roses. Hortscience. 2022;57:657–663. doi: 10.21273/hortsci16473-22. [DOI] [Google Scholar]
- 8.Magnard J.-L., Roccia A., Caissard J.-C., Vergne P., Sun P., Hecquet R., Dubois A., Hibrand-Saint Oyant L., Jullien F., Nicolè F., et al. PLANT VOLATILES. Biosynthesis of monoterpene scent compounds in roses. Science. 2015;349:81–83. doi: 10.1126/science.aab0696. [DOI] [PubMed] [Google Scholar]
- 9.Rusanov K., Kovacheva N., Rusanova M., Atanassov I. Traditional Rosa damascena flower harvesting practices evaluated through GC/MS metabolite profiling of flower volatiles. Food Chem. 2011;129:1851–1859. doi: 10.1016/j.foodchem.2011.05.132. [DOI] [Google Scholar]
- 10.Baudino S., Sun P., Caissard J.C., Nairaud B., Moja S., Magnard J.L., Bony A., Jullien F., Schuurink R., Vergne P., et al. Rose floral scent. Acta Hortic. 2019;1232:69–80. doi: 10.17660/ActaHortic.2019.1232.12. [DOI] [Google Scholar]
- 11.Ohloff G. Importance of minor components in flavors and fragrance. Perfum. Flavor. 1978;3:11–22. [Google Scholar]
- 12.Héthelyi É., Szarka S., Lemberkovics É., Szőke É. SPME-GC/MS identification of aroma compounds in rose flowers. Acta Agron. Hung. 2010;58:283–287. doi: 10.1556/AAgr.58.2010.3.11. [DOI] [Google Scholar]
- 13.Mannschreck A., von Angerer E. The scent of roses and beyond: Molecular structures, analysis, and practical applications of odorants. J. Chem. Educ. 2011;88:1501–1506. doi: 10.1021/ed100629v. [DOI] [Google Scholar]
- 14.Feng L.G., Chen C., Sheng L.X., Liu P., Tao J., Su J.L., Zhao L.Y. Comparative analysis of headspace volatiles of Chinese Rosa rugosa. Molecules. 2010;15:8390–8399. doi: 10.3390/molecules15118390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao C.-Y., Xue J., Cai X.-D., Guo J., Li B., Wu S. Assessment of the key aroma compounds in rose-based products. J. Food Drug Anal. 2016;24:471–476. doi: 10.1016/j.jfda.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao Z., Luo J., Niu Y., Wu M. Characterization of key aroma compounds from different rose essential oils using gas chromatography-mass spectrometry, gas chromatography–olfactometry and partial least squares regression. Nat. Prod. Res. 2018;32:1567–1572. doi: 10.1080/14786419.2017.1389933. [DOI] [PubMed] [Google Scholar]
- 17.Weiss T., Snitz K., Yablonka A., Khan R.M., Gafsou D., Schneidman E., Sobel N. Perceptual convergence of multi-component mixtures in olfaction implies an olfactory white. Proc. Natl. Acad. Sci. USA. 2012;109:19959–19964. doi: 10.1073/pnas.1208110109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalençon L., Thevenet M., Noury N., Bensafi M., Mandairon N. Identification of new behavioral parameters to assess odorant hedonic value in humans: A naturalistic approach. J. Neurosci. Methods. 2022;366 doi: 10.1016/j.jneumeth.2021.109422. [DOI] [PubMed] [Google Scholar]
- 19.Ferdenzi C., Razafindrazaka H., Baldovini N., Poupon D., Pierron D., Bensafi M. Influence of gender and culture on the perception of acidic compounds of human body odor. Physiol. Behav. 2019;210 doi: 10.1016/j.physbeh.2019.112561. [DOI] [PubMed] [Google Scholar]
- 20.Joussain P., Thevenet M., Rouby C., Bensafi M. Effect of aging on hedonic appreciation of pleasant and unpleasant odors. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Y., Tang K., Xu Y., Thomas-Danguin T. A dataset on odor intensity and odor pleasantness of 222 binary mixtures of 72 key food odorants rated by a sensory panel of 30 trained assessors. Data Brief. 2021;36 doi: 10.1016/j.dib.2021.107143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolls E.T., Kringelbach M.L., de Araujo I.E.T. Different representations of pleasant and unpleasant odours in the human brain. Eur. J. Neurosci. 2003;18:695–703. doi: 10.1046/j.1460-9568.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- 23.David O.A., David D.O., Mogoase C., Popescu L.C., Giosan C., Pellegrino A. Psychological effects and brain correlates of a rose-based scented cosmetic cream. J. Sensory Stud. 2019;34 doi: 10.1111/joss.12536. [DOI] [Google Scholar]
- 24.Dmitrenko D., Maggioni E., Brianza G., Holthausen B.E., Walker B.N., Obrist M. Proceedings of the 2020 CHI Conference on Human Factors in Computing Systems, 2020/04/21/ ACM; 2020. CARoma Therapy: Pleasant scents promote safer driving, better mood, and improved well-being in angry drivers; pp. 1–13. [Google Scholar]
- 25.Jirovetz L., Buchbauer G., Stoyanova A., Balinova A., Guangjiun Z., Xihan M. Solid phase microextraction/gas chromatographic and olfactory analysis of the scent and fixative properties of the essential oil of Rosa damascena L. from China. Flavour Fragrance J. 2005;20:7–12. doi: 10.1002/ffj.1375. [DOI] [Google Scholar]
- 26.Abbasijahromi A., Hojati H., Nikooei S., Jahromi H.K., Dowlatkhah H.R., Zarean V., Farzaneh M., Kalavani A. Compare the effect of aromatherapy using lavender and Damask rose essential oils on the level of anxiety and severity of pain following C-section: A double-blinded randomized clinical trial. J. Compl. Integr. Med. 2020;17 doi: 10.1515/jcim-2019-0141. [DOI] [PubMed] [Google Scholar]
- 27.Bradley B.F., Starkey N.J., Brown S.L., Lea R.W. The effects of prolonged rose odor inhalation in two animal models of anxiety. Physiol. Behav. 2007;92:931–938. doi: 10.1016/j.physbeh.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 28.de Almeida R.N., Motta S.C., de Brito Faturi C., Catallani B., Leite J.R. Anxiolytic-like effects of rose oil inhalation on the elevated plus-maze test in rats. Pharmacol. Biochem. Behav. 2004;77:361–364. doi: 10.1016/j.pbb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Dehkordi A.K., Tayebi A., Ebadi A., Sahraei H., Einollahi B. Effects of aromatherapy using the Damask rose essential oil on depression, anxiety, and stress in hemodialysis patients: A clinical trial. Nephro-Urol. Mon. 2017;9 doi: 10.5812/numonthly.60280. [DOI] [Google Scholar]
- 30.Farnia V., Shirzadifar M., Shakeri J., Rezaei M., Bajoghli H., Holsboer-Trachsler E., Brand S. Rosa damascena oil improves SSRI-induced sexual dysfunction in male patients suffering from major depressive disorders: results from a double-blind, randomized, and placebo-controlled clinical trial. Neuropsychiatric Dis. Treat. 2015;11:625–635. doi: 10.2147/NDT.S78696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakinoğlu Oruç F.Ç., Dursun S., Demirli A. Subjective effects of lemon seed, rose and lavender essential oils on humans: A case study from two different age groups. Int J Sec Metabolite. 2017;4:306–311. doi: 10.21448/ijsm.373289. [DOI] [Google Scholar]
- 32.Umezu T., Ito H., Nagano K., Yamakoshi M., Oouchi H., Sakaniwa M., Morita M. Anticonflict effects of rose oil and identification of its active constituents. Life Sci. 2002;72:91–102. doi: 10.1016/S0024-3205(02)02197-5. [DOI] [PubMed] [Google Scholar]
- 33.Picone J.M., Clery R.A., Watanabe N., MacTavish H.S., Turnbull C.G.N. Rhythmic emission of floral volatiles from Rosa damascena semperflorens cv. 'Quatre Saisons. Planta. 2004;219:468–478. doi: 10.1007/s00425-004-1250-5. [DOI] [PubMed] [Google Scholar]
- 34.Helsper J.P.F.G., Davies J.A., Bouwmeester H.J., Krol A.F., van Kampen M.H. Circadian rhythmicity in emission of volatile compounds by flowers of Rosa hybrida L. cv. Honesty. Planta. 1998;207:88–95. doi: 10.1007/s004250050459. [DOI] [Google Scholar]
- 35.Hendel-Rahmanim K., Masci T., Vainstein A., Weiss D. Diurnal regulation of scent emission in rose flowers. Planta. 2007;226:1491–1499. doi: 10.1007/s00425-007-0582-3. [DOI] [PubMed] [Google Scholar]
- 36.Attia T.M., Hamdan A.M. Assessment of the difference in detection of pleasant and unpleasant odors in different grades of hyposmia. Egypt. J. Otolaryngol. 2021;37:18. doi: 10.1186/s43163-021-00070-4. [DOI] [Google Scholar]
- 37.Ayabe-Kanamura S., Schicker I., Laska M., Hudson R., Distel H., Kobayakawa T., Saito S. Differences in perception of everyday odors: a Japanese-German cross-cultural study. Chem. Senses. 1998;23:31–38. doi: 10.1093/chemse/23.1.31. [DOI] [PubMed] [Google Scholar]
- 38.Ferdenzi C., Roberts S.C., Schirmer A., Delplanque S., Cekic S., Porcherot C., Cayeux I., Sander D., Grandjean D. Variability of affective responses to odors: culture, gender, and olfactory knowledge. Chem. Senses. 2013;38:175–186. doi: 10.1093/chemse/bjs083. [DOI] [PubMed] [Google Scholar]
- 39.Hamakawa M., Okamoto T. The effect of different emotional states on olfactory perception: A preliminary study. Flavour Fragrance J. 2018;33:420–427. doi: 10.1002/ffj.3469. [DOI] [Google Scholar]
- 40.Ruser P., Koeppel C.J., Kitzler H.H., Hummel T., Croy I. Individual odor hedonic perception is coded in temporal joint network activity. Neuroimage. 2021;229 doi: 10.1016/j.neuroimage.2021.117782. [DOI] [PubMed] [Google Scholar]
- 41.Zarzo M. Psychologic dimensions in the perception of everyday odors: Pleasantness and edibility. J. Sensory Stud. 2008;23:354–376. doi: 10.1111/j.1745-459X.2008.00160.x. [DOI] [Google Scholar]
- 42.Yang Z., Wildschut T., Izuma K., Gu R., Luo Y.L.L., Cai H., Sedikides C. Patterns of brain activity associated with nostalgia: a social-cognitive neuroscience perspective. Soc. Cognit. Affect Neurosci. 2022;17:1131–1144. doi: 10.1093/scan/nsac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dudareva N., Klempien A., Muhlemann J.K., Kaplan I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013;198:16–32. doi: 10.1111/nph.12145. [DOI] [PubMed] [Google Scholar]
- 44.Knudsen J., Gershenzon J. In: Biology of plant volatiles. Pichersky E., Dudareva N., editors. CRC Presse; 2020. The chemical diversity of floral scent; pp. 57–78. [Google Scholar]
- 45.Lynch J.H., Pichersky E., Dudareva N. In: Biology of plant volatiles. Pichersky E., Dudareva N., editors. CRC Presse; 2020. Floral scent metabolic pathways and their regulation; pp. 147–164. [Google Scholar]
- 46.Sun P., Schuurink R.C., Caissard J.-C., Hugueney P., Baudino S. My Way: Noncanonical biosynthesis pathways for plant volatiles. Trends Plant Sci. 2016;21:884–894. doi: 10.1016/j.tplants.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura S. Scent and component analysis of the hybrid tea rose. Perfum. Flavor. 1987;12:43–45. [Google Scholar]
- 48.Sirotin Y.B., Shusterman R., Rinberg D. Neural coding of perceived odor intensity. eNeuro. 2015;2:e0083. doi: 10.1523/ENEURO.0083-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohloff G., Demole E. Importance of the odoriferous principle of Bulgarian rose oil in flavour and fragrance chemistry. J. Chromatogr. A. 1987;406:181–183. doi: 10.1016/S0021-9673(00)94029-9. [DOI] [Google Scholar]
- 50.Suzuki M., Matsumoto S., Mizoguchi M., Hirata S., Takagi K., Hashimoto I., Yamano Y., Ito M., Fleischmann P., Winterhalter P., et al. Identification of (3S, 9R)- and (3S, 9S)-megastigma-6,7-dien-3,5,9-triol 9-O-beta-D-glucopyranosides as damascenone progenitors in the flowers of Rosa damascena Mill. Biosci. Biotechnol. Biochem. 2002;66:2692–2697. doi: 10.1271/bbb.66.2692. [DOI] [PubMed] [Google Scholar]
- 51.Hua D., Xu P. Recent advances in biotechnological production of 2-phenylethanol. Biotechnol. Adv. 2011;29:654–660. doi: 10.1016/j.biotechadv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Jirovetz L., Buchbauer G., Schmidt E., Denkova Z., Slavchev A., Stoyanova A., Geissler M. Purity, antimicrobial activities and olfactory evaluations of 2-Phenylethanol and some derivatives. J. Essent. Oil Res. 2008;20:82–85. doi: 10.1080/10412905.2008.9699429. [DOI] [Google Scholar]
- 53.Ito A., Miyoshi M., Ueki S., Fukada M., Komaki R., Watanabe T. “Green odor” inhalation by rats down-regulates stress-induced increases in Fos expression in stress-related forebrain regions. Neurosci. Res. 2009;65:166–174. doi: 10.1016/j.neures.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe T., Fujihara M., Murakami E., Miyoshi M., Tanaka Y., Koba S., Tachibana H. Green odor and depressive-like state in rats: Toward an evidence-based alternative medicine? Behav. Brain Res. 2011;224:290–296. doi: 10.1016/j.bbr.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Akutsu H., Kikusui T., Takeuchi Y., Sano K., Hatanaka A., Mori Y. Alleviating effects of plant-derived fragrances on stress-induced hyperthermia in rats. Physiol. Behav. 2002;75:355–360. doi: 10.1016/S0031-9384(01)00670-9. [DOI] [PubMed] [Google Scholar]
- 56.Tokumo K., Tamura N., Hirai T., Nishio H. Effects of (Z)-3-hexenol, a major component of green odor, on anxiety-related behavior of the mouse in an elevated plus-maze test and biogenic amines and their metabolites in the brain. Behav. Brain Res. 2006;166:247–252. doi: 10.1016/j.bbr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Ballanger B., Bath K.G., Mandairon N. Odorants: a tool to provide nonpharmacological intervention to reduce anxiety during normal and pathological aging. Neurobiol. Aging. 2019;82:18–29. doi: 10.1016/j.neurobiolaging.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 58.Nakatomi Y., Yokoyama C., Kinoshita S., Masaki D., Tsuchida H., Onoe H., Yoshimoto K., Fukui K. Serotonergic mediation of the antidepressant-like effect of the green leaves odor in mice. Neurosci. Lett. 2008;436:167–170. doi: 10.1016/j.neulet.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 59.Ueno H., Shimada A., Suemitsu S., Murakami S., Kitamura N., Wani K., Takahashi Y., Matsumoto Y., Okamoto M., Ishihara T. Hexanal inhalation affects cognition and anxiety-like behavior in mice. Z Naturforsch C. 2020;75:409–415. doi: 10.1515/znc-2019-0215. [DOI] [PubMed] [Google Scholar]
- 60.Saito N., Yamano E., Ishii A., Tanaka M., Nakamura J., Watanabe Y. Involvement of the olfactory system in the induction of anti-fatigue effects by odorants. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sano K., Tsuda Y., Sugano H., Aou S., Hatanaka A. Concentration effects of green odor on event-related potential (P300) and pleasantness. Chem. Senses. 2002;27:225–230. doi: 10.1093/chemse/27.3.225. [DOI] [PubMed] [Google Scholar]
- 62.Jacob T.J.C., Fraser C., Wang L., Walker V., O'Connor S. Psychophysical evaluation of responses to pleasant and mal-odour stimulation in human subjects; adaptation, dose response and gender differences. Int. J. Psychophysiol. 2003;48:67–80. doi: 10.1016/S0167-8760(03)00020-5. [DOI] [PubMed] [Google Scholar]
- 63.Lötsch J., Ultsch A., Hähner A., Willgeroth V., Bensafi M., Zaliani A., Hummel T. Data-science based analysis of perceptual spaces of odors in olfactory loss. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-89969-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mantel M., Fournel A., Staedlé I., Oelschlägel A., Carro J., Dubreuil R., Herrier C., Livache T., Haehner A., Hummel T., et al. Using a bio-inspired surface resonance plasmon electronic nose for fundamental research on human olfaction. Sens. Actuators, B. 2022;350 doi: 10.1016/j.snb.2021.130846. [DOI] [Google Scholar]
- 65.Zarzo M. What is a fresh scent in perfumery? Perceptual freshness is correlated with substantivity. Sensors. 2012;13:463–483. doi: 10.3390/s130100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen W., Viljoen A.M. Geraniol — A review of a commercially important fragrance material. South Afr. J. Bot. 2010;76:643–651. doi: 10.1016/j.sajb.2010.05.008. [DOI] [Google Scholar]
- 67.Elsharif S.A., Buettner A. Structure-odor relationship study on geraniol, nerol, and their synthesized oxygenated derivatives. J. Agric. Food Chem. 2018;66:2324–2333. doi: 10.1021/acs.jafc.6b04534. [DOI] [PubMed] [Google Scholar]
- 68.Zhou L., Yu C., Cheng B., Wan H., Luo L., Pan H., Zhang Q. Volatile compound analysis and aroma evaluation of tea-scented roses in China. Ind. Crops Prod. 2020;155 doi: 10.1016/j.indcrop.2020.112735. [DOI] [Google Scholar]
- 69.Coureaud G., Thomas-Danguin T., Sandoz J.-C., Wilson D.A. Biological constraints on configural odour mixture perception. J. Exp. Biol. 2022;225 doi: 10.1242/jeb.242274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marasco A., De Paris A., Migliore M. Predicting the response of olfactory sensory neurons to odor mixtures from single odor response. Sci. Rep. 2016;6 doi: 10.1038/srep24091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grabenhorst F., Rolls E.T., Margot C., da Silva M.A.A.P., Velazco M.I. How pleasant and unpleasant stimuli combine in different brain regions: odor mixtures. J. Neurosci. 2007;27:13532–13540. doi: 10.1523/JNEUROSCI.3337-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laing D.G., Eddy A., Best D.J. Perceptual characteristics of binary, trinary, and quaternary odor mixtures consisting of unpleasant constituents. Physiol. Behav. 1994;56:81–93. doi: 10.1016/0031-9384(94)90264-X. [DOI] [PubMed] [Google Scholar]
- 73.Xiao Z., Luo J., Niu Y., Wang P., Wang R., Sun X. Olfactory impact of esters on rose essential oil floral alcohol aroma expression in model solution. Food Res. Int. 2019;116:211–222. doi: 10.1016/j.foodres.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 74.Anderson A.K., Christoff K., Stappen I., Panitz D., Ghahremani D.G., Glover G., Gabrieli J.D.E., Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat. Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- 75.Jin L., Haviland-Jones J., Simon J.E., Tepper B.J. Influence of aroma intensity and nasal pungency on the ‘mood signature’ of common aroma compounds in a mixed ethnic population. Food Qual. Prefer. 2018;65:164–174. doi: 10.1016/j.foodqual.2017.10.017. [DOI] [Google Scholar]
- 76.Sezille C., Fournel A., Rouby C., Rinck F., Bensafi M. Hedonic appreciation and verbal description of pleasant and unpleasant odors in untrained, trainee cooks, flavorists, and perfumers. Front. Psychol. 2014;5 doi: 10.3389/fpsyg.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao W., Lv Q., Gao X., Sun Z., Yan X., Wei Y. Different brain activation in response to repeated odors of pleasantness and unpleasantness. Chem. Percept. 2020;13:84–91. doi: 10.1007/s12078-019-09270-y. [DOI] [Google Scholar]
- 78.Song H., Liu J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018;114:187–198. doi: 10.1016/j.foodres.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 79.Zhu J., Chen F., Wang L., Niu Y., Yu D., Shu C., Chen H., Wang H., Xiao Z. Comparison of Aroma-Active Volatiles in Oolong Tea Infusions Using GC–Olfactometry, GC–FPD, and GC–MS. J. Agric. Food Chem. 2015;63:7499–7510. doi: 10.1021/acs.jafc.5b02358. [DOI] [PubMed] [Google Scholar]
- 80.Hallier A., Courcoux P., Sérot T., Prost C. New gas chromatography–olfactometric investigative method, and its application to cooked Silurus glanis (European catfish) odor characterization. J. Chromatogr. A. 2004;1056:201–208. [PubMed] [Google Scholar]
- 81.Joussain P., Bessy M., Faure F., Bellil D., Landis B.N., Hugentobler M., Tuorila H., Mustonen S., Vento S.I., Delphin-Combe F., et al. Application of the European Test of Olfactory Capabilities in patients with olfactory impairment. Eur. Arch. Oto-Rhino-Laryngol. 2016;273:381–390. doi: 10.1007/s00405-015-3536-6. [DOI] [PubMed] [Google Scholar]
- 82.Conart C., Bomzan D.P., Huang X.-Q., Bassard J.-E., Paramita S.N., Saint-Marcoux D., Rius-Bony A., Hivert G., Anchisi A., Schaller H., et al. A cytosolic bifunctional geranyl/farnesyl diphosphate synthase provides MVA-derived GPP for geraniol biosynthesis in rose flowers. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2221440120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bergougnoux V., Caissard J.C., Jullien F., Magnard J.L., Scalliet G., Cock J.M., Hugueney P., Baudino S. Both the adaxial and abaxial epidermal layers of the rose petal emit volatile scent compounds. Planta. 2007;226:853–866. doi: 10.1007/s00425-007-0531-1. [DOI] [PubMed] [Google Scholar]
- 84.Conart C., Saclier N., Foucher F., Goubert C., Rius-Bony A., Paramita S.N., Moja S., Thouroude T., Douady C., Sun P., et al. Duplication and specialization of NUDX1 in Rosaceae led to geraniol production in rose petals. Mol. Biol. Evol. 2022;39:msac002. doi: 10.1093/molbev/msac002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.R Core Team (2023). R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing.
- 86.Brooks M., Kristensen K., Benthem K., Magnusson A., Berg C., Nielsen A., Skaug H., Mächler M., Bolker B. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R journal. 2017;9:378. [Google Scholar]
- 87.Fox J., Weisberg S. third Edition. Sage; 2019. An R Companion to Applied Regression. [Google Scholar]
- 88.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 89.Lenth R. Comprehensive R Archive Network (CRAN); 2023. emmeans: Estimated marginal means, aka least-squares meansR package version 1.8. 8.https://CRAN.R-project.org/package=emmeans [Google Scholar]
- 90.Oksanen J., Simpson G., Blanchet F., Kindt R., Legendre P., Minchin P., O’hara R., Solymos P., Stevens M., Szoecs E. Vegan: Community Ecology Package. R package version. 2023;2:6. [Google Scholar]
- 91.Hervé M.R., Nicolè F., Le Cao K.A. Multivariate analysis of multiple datasets: a practical guide for chemical ecology. J. Chem. Ecol. 2018;44:215–234. doi: 10.1007/s10886-018-0932-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.