Abstract
Objective
The primary therapy for intermediate- and high-risk endometrial cancer includes pelvic and paraaortic lymph node evaluation. Laparoscopic surgery is an increasingly popular intervention due to decreased risk and better short-term morbidity; however, a recent study casts doubt on the benefit of this approach in terms of oncological safety. In this cancer registry study, we sought to evaluate the benefit of laparoscopy versus laparotomy and retrospectively compared overall survival, recurrence rates, and recurrence-free survival among patients with intermediate- and high-risk endometrial cancer who underwent either laparoscopic or open surgery.
Methods
This observational study included 419 patients who have been treated from 2011 to 2017. We employed Kaplan–Meier method, and univariable and multivariable Cox-regression to compare overall survival, recurrence rates, and recurrence-free survival in 110 patients, who underwent laparoscopic, with 309 patients, who underwent open surgery. To address the confounding bias in this retrospective study, we also performed a propensity score matching (PSM) analysis including 357 patients (laparoscopy: n = 107; open surgery: n = 250).
Results
We found a benefit for laparoscopic over open surgery in patients with intermediate- and high-risk endometrial cancer for overall survival in both univariable (p = 0.002; PSM: p = 0.016) and multivariable analyses (p = 0.019; PSM: p = 0.007). In contrast, there was no statistically significant difference between both patient groups regarding the cumulative recurrence rates. A univariable analysis identified a significant benefit for laparoscopy regarding recurrence-free survival (p = 0.003; PSM: p = 0.029) but a multivariable analysis failed to confirm this finding (p = 0.108; PSM: p = 0.118).
Conclusions
Our study provides evidence that laparoscopic systematic lymphadenectomy does not present a lower oncological efficacy than open surgery in the treatment of patients with endometrial cancer.
Electronic supplementary material
The online version of this article (10.1007/s00432-019-03122-8) contains supplementary material, which is available to authorized users.
Keywords: Endometrial cancer, Pelvic and paraaortic lymphadenectomy, Laparoscopy, Laparotomy, Overall survival, Observational study, Cancer registry study
Introduction
Laparoscopic surgical procedures become increasingly popular, because in comparison with laparotomic surgery, they are associated with reduced surgical morbidity and a better quality of life (Mourits et al. 2010; Ferguson et al. 2018; Safdieh et al. 2017). Nevertheless, there is a lack of reliable information about this intervention concerning oncological long-term outcomes such as recurrence and survival rate. Clinical research turns to registry studies to obtain empirical evidence to address this limitation. For instance, a recent cancer registry study demonstrated that minimally invasive surgery (MIS) lead to an oncological equivalent outcome (Draeger et al. 2019).
Despite inadequate oncological safety data, laparoscopic access techniques also increasingly replace open surgery in the intervention for uterine malignancies (Park et al. 2016). The recent publication of a randomized international multicenter study in cervical cancer has caused a great uncertainty: the endoscopic radical surgery was associated with a significantly higher risk of recurrence and mortality compared to the open procedure (Ramirez et al. 2018). Furthermore, a recent cohort and registry study from the USA obtained similar results (Melamed et al. 2018). It is not clear why MIS produced inferior outcomes in these studies. Possible explanations are the manipulation of the tumor during endoscopic surgery or the specific conditions of endoscopy (e.g., CO2 pneumoperitoneum).
Prospective randomization confirmed oncological equivalence between open surgery and MIS for patients with early endometrial carcinoma (Zullo et al. 2009), which requires only hysterectomy and adnexectomy; however, there is limited data for intermediate- and high-risk Type 2 endometrial cancer cases, in which systematic lymph node evaluation is part of the primary surgical approach (Park et al. 2016; Kyrgiou et al. 2015). Taking under consideration the results for cervical carcinoma interventions, it is of high clinical relevance whether laparoscopic lymphadenectomy is also detrimental to laparotomic surgery. We tested this hypothesis in a population-based retrospective cohort study based on cancer registry data with long-term follow-up.
Materials and methods
Study design and patient cohort
We conducted a population-based, retrospective cohort study using data from the clinical cancer register at the Regensburg Tumor Center and the University Hospital of Erlangen. The data contained 1066 cases of malignant neoplasia of endometrial carcinoma between January 2011 and December 2017. Data collection and retrospective analysis of patient information were anonymized in accordance with the Declaration of Helsinki, and approved by the Bavarian Law of Cancer Registration.
Patient inclusion and exclusion criteria
We excluded patients with FIGO IV disease (328 patients) and low-risk endometrial carcinoma (221 patients). We further constrained the cohort by patients who underwent surgery (excluding 15 females without surgery), lymphadenectomy (excluding 27 females without lymph node dissection), no conversion surgery (excluding 23 females with conversion from laparoscopy to laparotomy), and who received complete resection (excluding 25 patients with incomplete resection) (Fig. 1). From the remaining 427 patients, we also excluded the ones with unsatisfactory follow-up yielding in 419 patients (39.3% of all cases). From the selected patients, 110 females (26.3%) underwent laparoscopic surgery and 309 females (73.7%) underwent open surgery.
Fig. 1.
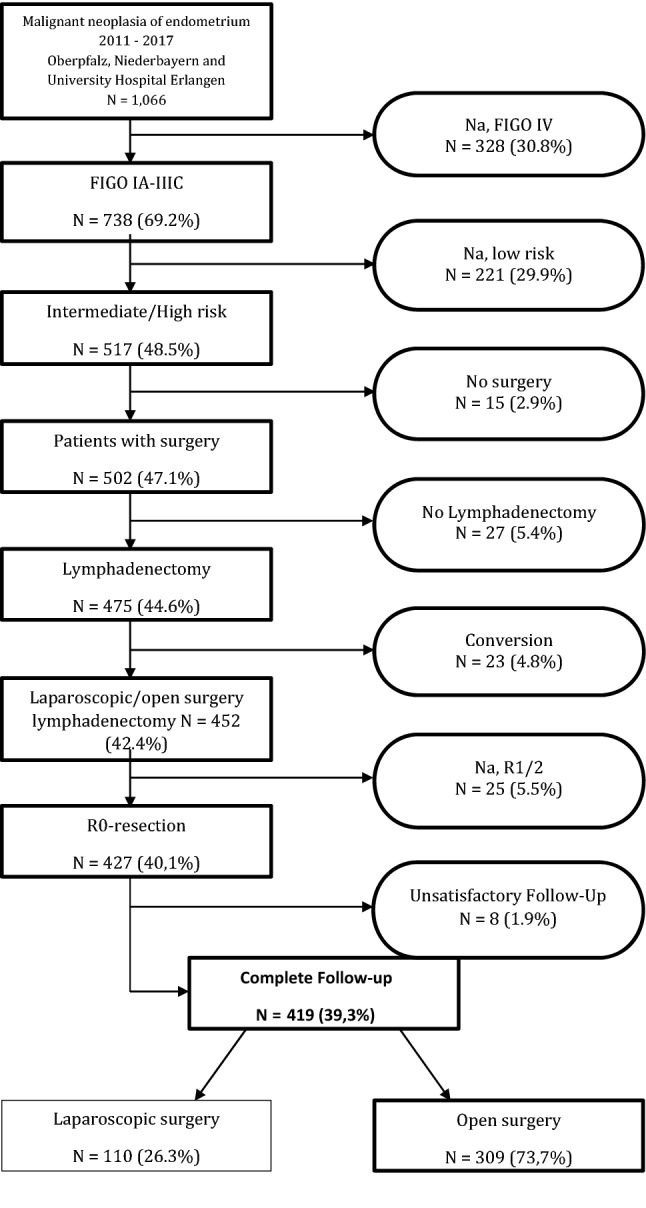
Flowchart for inclusion and exclusion criteria
Statistical analysis
Continuous data are described as means, median, minimum, maximum values, and categorical data are expressed as absolute frequencies and relative percentages. Patient characteristics were compared using two-tailed Student’s t test for continuous data in case of normal distribution, otherwise Mann–Whitney U test was applied. Pearson’s Chi-square test was used for testing independence between categorical variables. Life status for estimating overall survival rates was derived from clinical reports, death certificates, and registration offices. Recurrences were derived from clinical reports and were defined as locoregional relapse and/or recurrence as distant metastases. 5-year-overall survival rates (OS), recurrence-free survival rates (RFS), and cumulative recurrence rates were analyzed from the date of diagnosis until the first event. Patients’ OS and RFS were compared with the Kaplan–Meier method. The follow-up period and survival times were right censored using 31 July 2019 as the cut-off date, rendering a mean follow-up of 4.2 years (median 4.3 years). Survival differences were tested for statistical significance by the two-sided log rank test (Mantel-Cox); the level of significance was set to 0.05. To determine the influence of surgery approach and further covariables on survival, we performed univariable and multivariable regression analyses using Cox proportional hazard models. In multivariable analyses, the hazard ratio (HR) for laparoscopic versus open surgery were adjusted for the covariables, age at diagnosis, comorbidity, FIGO stage, nodal status, histologic type/ grading, risk group, lymph vessel invasion, vein invasion, and perioperative therapy. Comorbidity was adjusted for via Charlson-Comorbidity-Index, categorized in a group with at least one disease and a group without any disease listed in the CCI list. Hazard ratios and corresponding 95% confidence intervals (CI) were estimated and considered statistically significant when the CI excluded 1.0, and a two-sided p value was < 0.05. Additionally, we performed a 1:3 propensity score nearest neighbor matching analysis with caliper 0.2, balancing for the adjustment variables named above. All analyses were performed using IBM SPSS Statistics, version 25.0 (IBM Corp., SPSS for Windows, Armonk, NY, USA).
Results
Patient characteristics in the cancer registry cohort
The cancer registry contained information about 1066 patients diagnosed with malignant neoplasm of endometrium (ICD-10 C54.1) of which 419 females matched the selection criteria for this study (Fig. 1). All selected patients were diagnosed with intermediate- or high-risk endometrial carcinoma and underwent either laparoscopic surgery or laparotomic surgery. Table 1 summarizes the demographic and clinical characteristics of the study cohort. The median age of the patients in the cohort was 69.2 years (mean 68.3, ranging from 29.2 to 91.9 years) with no significant difference between patients who underwent laparoscopic surgery (median 67.4 years with range from 35.3 to 85.4 years) or laparotomic surgery (median 69.6 years with range from 29.2 to 91.9 years, p = 0.052). The mortality 90-day post-intervention was low (0.7%) with only three incidences among the patients who underwent open surgery (1.0%) and none among the patients who underwent laparoscopic surgery.
Table 1.
Patient characteristics according to surgery approach in total cohort
| Surgery approach | X2 | ||||||
|---|---|---|---|---|---|---|---|
| Laparoscopic | Open surgery | Total | |||||
| N | % | N | % | N | % | p | |
| Age at diagnosis | |||||||
| < 65 | 47 | 42.7 | 116 | 37.5 | 163 | 38.9 | 0.338 |
| 65+ | 63 | 57.3 | 193 | 62.5 | 256 | 61.1 | |
| Charlson-Comorbidity-Index | |||||||
| 0 | 76 | 69.1 | 228 | 73.8 | 304 | 72.6 | 0.343 |
| 1+ | 34 | 30.9 | 81 | 26.2 | 115 | 27.4 | |
| FIGO stage | |||||||
| I | 80 | 72.7 | 191 | 61.8 | 271 | 64.7 | 0.075 |
| II | 17 | 15.5 | 54 | 17.5 | 71 | 16.9 | |
| III | 13 | 11.8 | 64 | 20.7 | 77 | 18.4 | |
| Nodal status | |||||||
| N0 | 98 | 89.1 | 251 | 81.2 | 349 | 83.3 | 0.163 |
| N1 | 10 | 9.1 | 47 | 15.2 | 57 | 13.6 | |
| NX/na | 2 | 1.8 | 11 | 3.6 | 13 | 3.1 | |
| Histologic type/grading | |||||||
| G1/G2 and type 1 | 69 | 62.7 | 137 | 44.3 | 206 | 49.2 | 0.001 |
| G3/G4 or type 2 | 41 | 37.3 | 172 | 55.7 | 213 | 50.8 | |
| Risk group | |||||||
| Intermediate risk | 67 | 60.9 | 150 | 48.5 | 217 | 51.8 | 0.026 |
| High risk | 43 | 39.1 | 159 | 51.5 | 202 | 48.2 | |
| Lymph vessel invasion | |||||||
| L0 | 86 | 78.2 | 228 | 73.8 | 314 | 74.9 | 0.619 |
| L1 | 21 | 19.1 | 73 | 23.6 | 94 | 22.4 | |
| LX/na | 3 | 2.7 | 8 | 2.6 | 11 | 2.6 | |
| Vein invasion | |||||||
| V0 | 98 | 89.1 | 275 | 89.0 | 373 | 89.0 | 0.802 |
| V1 | 8 | 7.3 | 26 | 8.4 | 34 | 8.1 | |
| VX/na | 4 | 3.6 | 8 | 2.6 | 12 | 2.9 | |
| Primary therapy | |||||||
| Surgery + Rad + CTX | 17 | 15.5 | 54 | 17.5 | 71 | 16.9 | 0.761 |
| Surgery + Rad | 62 | 56.4 | 158 | 51.1 | 220 | 52.5 | |
| Surgery + CTX | 5 | 4.5 | 12 | 3.9 | 17 | 4.1 | |
| Surgery only | 26 | 23.6 | 85 | 27.5 | 111 | 26.5 | |
| Total | 110 | 100.0 | 309 | 100.0 | 419 | 100.0 | |
In 304 cases, the Charlson-Comorbidity-Index was 0 (72.6%), whereas 115 patients presented a higher value based on at least 1 disease (27.4%). Roughly, two third of the patients (64.7%) displayed cancer of FIGO stage I (n = 271), whereas the remaining patients showed an equal distribution of FIGO stage II (n = 71; 16.9%) and FIGO stage III (n = 77; 18.4%) cancer. The nodal status among the patients was predominantly N0 (n = 83.3%), with few patients displaying N1 (n = 57; 13.6%) and N2 (n = 13; 3.1%). The cohort exhibited a very similar distribution for both lymph vessel invasion and vein invasion among the patients. Half the patients in the cohort were classified as intermediate-risk patients (n = 217; 51.8%) and the other half as high-risk patients (n = 202; 48.2%). Similarly, histology grading was also equally distributed in the cohort with half the patients classified as G1/G2 and type 1 (n = 206; 49.2%) and the other half with G3/4 and type 2 (n = 213; 50.8%).
Overall and recurrence-free survival in the original cohort
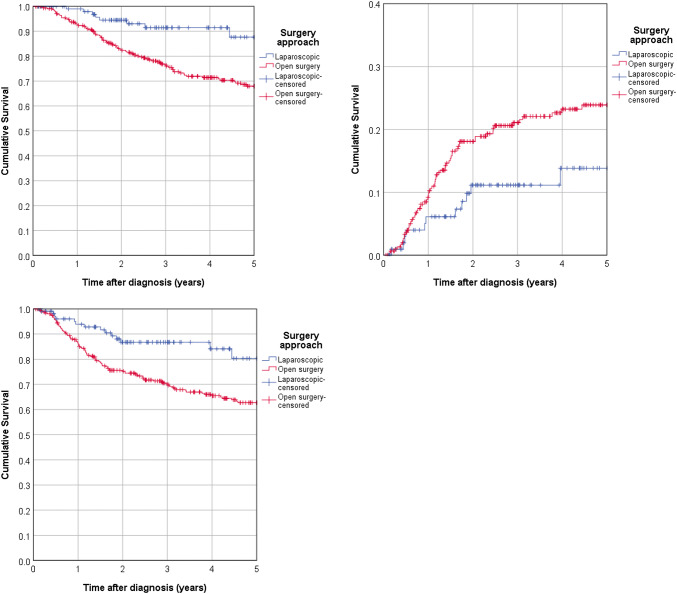
The 5-year-overall survival rate in the total cohort was 72.0%, but was statistically significantly better for laparoscopic surgery (87.6%) than for laparotomic surgery (68.0%, logrank p = 0.002). The univariable Cox-regression presented a hazard ratio (HR) of 0.346 (95% CI 0.174—0.688; p = 0.002) for laparoscopic as opposed to open surgery (Fig. 2a). A multivariable Cox-regression confirmed the benefit for laparoscopic surgery, rendering a HR of 0.435 (95% CI 0.217–0.871; p = 0.019). Table S2 contains an overview of the estimated hazard ratios from uni- and multivariable Cox-regression according to the applied surgical procedure.
Fig. 2.
Patients’ survival according to surgical intervention. a Kaplan–Meier analysis identified a statistically significant benefit in the 5-year overall survival for laparoscopic surgery (log rank p = 0.002). b The 5-year cumulative recurrence rate was higher for open surgery (p = 0.039). c Laparoscopic surgery was superior for 5-year recurrence-free survival (p = 0.003)
Table 2 presents the results from univariable and multivariable Cox-regression on overall survival in subgroups, given as hazard ratios for the effect of laparoscopic approach as opposed to laparotomic surgery. The effects observed in the entire cohort were seen only in subgroups of sufficient case numbers.
Table 2.
Patient characteristics after Propensity Score Matchinga according to surgery approach
| Surgery approach | X2 | ||||||
|---|---|---|---|---|---|---|---|
| Laparoscopic | Open surgery | Total | |||||
| N | (%) | N | (%) | N | (%) | p | |
| Age at diagnosis | |||||||
| < 65 | 45 | 42.1 | 103 | 41.2 | 148 | 41.5 | 0.880 |
| 65+ | 62 | 57.9 | 147 | 58.8 | 209 | 58.5 | |
| Charlson-Comorbidity-Index | |||||||
| 0 | 75 | 70.1 | 190 | 76.0 | 265 | 74.2 | 0.242 |
| 1+ | 32 | 29.9 | 60 | 24.0 | 92 | 25.8 | |
| FIGO stage | |||||||
| I | 77 | 72.0 | 170 | 68.0 | 247 | 69.2 | 0.632 |
| II | 17 | 15.9 | 40 | 16.0 | 57 | 16.0 | |
| III | 13 | 12.1 | 40 | 16.0 | 53 | 14.8 | |
| Nodal status | |||||||
| N0 | 95 | 88.8 | 211 | 84.4 | 306 | 85.7 | 0.535 |
| N1 | 10 | 9.3 | 31 | 12.4 | 41 | 11.5 | |
| NX/na | 2 | 1.9 | 8 | 3.2 | 10 | 2.8 | |
| Histologic type/grading | |||||||
| G1/G2 and Type 1 | 66 | 61.7 | 130 | 52.0 | 196 | 54.9 | 0.092 |
| G3/4 or Type 2 | 41 | 38.3 | 120 | 48.0 | 161 | 45.1 | |
| Risk group | |||||||
| Intermediate risk | 64 | 59.8 | 137 | 54.8 | 201 | 56.3 | 0.382 |
| High risk | 43 | 40.2 | 113 | 45.2 | 156 | 43.7 | |
| Lymph vessel invasion | |||||||
| L0 | 83 | 77.6 | 192 | 76.8 | 275 | 77.0 | 0.949 |
| L1 | 21 | 19.6 | 52 | 20.8 | 73 | 20.4 | |
| LX/na | 3 | 2.8 | 6 | 2.4 | 9 | 2.5 | |
| Vein invasion | |||||||
| V0 | 96 | 89.7 | 226 | 90.4 | 322 | 90.2 | 0.970 |
| V1 | 8 | 7.5 | 18 | 7.2 | 26 | 7.3 | |
| VX/na | 3 | 2.8 | 6 | 2.4 | 9 | 2.5 | |
| Primary therapy | |||||||
| Surgery + Rad + CTX | 16 | 15.0 | 44 | 17.6 | 60 | 16.8 | 0.940 |
| Surgery + Rad | 61 | 57.0 | 140 | 56.0 | 201 | 56.3 | |
| Surgery + CTX | 5 | 4.7 | 11 | 4.4 | 16 | 4.5 | |
| Surgery | 25 | 23.4 | 55 | 22.0 | 80 | 22.4 | |
| Total | 107 | 100.0 | 250 | 100.0 | 357 | 100.0 | |
Rad radio therapy, CTX chemotherapy
aPropensity score 1:3 nearest neighbor matching with caliper 0.2
Next, we also studied the 5-year cumulative recurrence rate, which we assessed as 21.5% in the entire cohort. We found a weak statistically significant difference (p = 0.039) in the 5-year cumulative recurrence rate between the patients who underwent laparoscopic surgery (13.8%) and who underwent laparotomic surgery (23.9%), corresponding to a HR of 0.516 (95% CI 0.272–0.979; p = 0.043) (Fig. 2b). The multivariable Cox-regression did not present any significant differences between both interventions (HR of 0.706 95 CI 0.367–1.358; p = 0.297). There is no robust difference for the 5-year cumulative recurrence rate between both patient groups.
The 5-year recurrence-free survival in the cohort was 66.6%. We obtained a strong statistically significant difference between both interventions (80.3% vs. 62.7%, p = 0.003) indicating that laparoscopic surgery is associated with a better outcome for the patient. The univariable Cox-regression showed a benefit for the laparoscopic intervention (HR of 0.4476 with 95% CI from 0.260 to 0.767; p = 0.003); however, the multivariable Cox-regression failed to present a significant difference between both procedures (HR of 0.635 with 95% CI from 0.365 to 1.104; p = 0.108) (Fig. 3b).
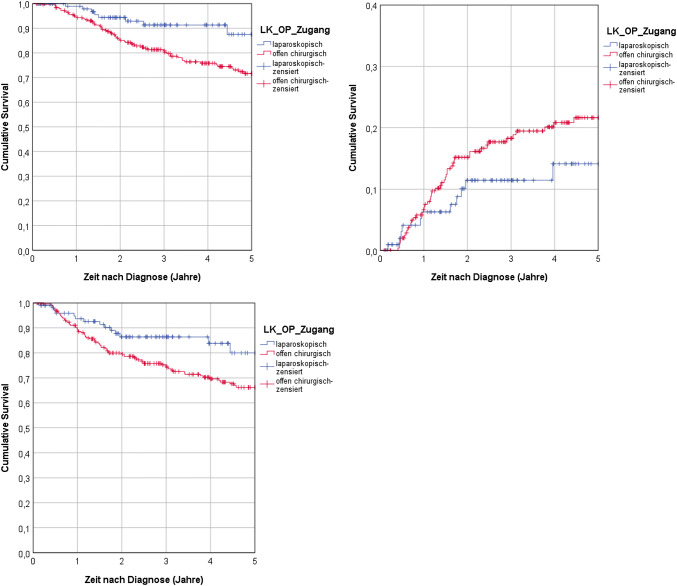
Fig. 3.
Dependence of propensity score matched patient survival on surgical intervention. a According to Mantel-Cox analysis, the 5-year overall survival was statistically significant higher for laparoscopic surgery (p = 0.013). b There was no statistically significant difference between laparoscopic and open surgery (p = 0.144). c Mantel-Cox analysis showed that laparoscopic surgery benefitted significantly the 5-year recurrence-free survival (p = 0.027)
Overall and recurrence-free survival in the cohort after propensity score matching
This cancer registry study is an observational study, thus lacking randomization between the two intervention groups. To reduce the potential impact of putative confounding variables, propensity score matching (PSM) was applied (Rosenbaum and Rubin 1983). This statistical matching technique accounts for parameters that predict the choice of treatment but not necessarily the effect of the treatment. Therefore, we additionally applied PSM analysis on the patients in the cohort to adjust for confounding bias. The 1:3 matched cohort of 357 patients presented a balanced distribution of the characteristics (Table 2).
After employing PSM, we reevaluated the 5-year-overall-survival of patients in the cohort. Survival after laparoscopic surgery (87.5%) was significantly higher than after open surgery (71.6%, p = 0.013, Fig. 3a). Both univariable Cox-regression (HR of 0.422 with 95% CI from 0.210 to 0.849; p = 0.016) and multivariable Cox-regression (HR of 0.362 with 95% CI from 0.174 to 0.757; p = 0.007), confirmed statistical significance, suggesting that laparoscopic surgery is associated with a better patient outcome.
Next, an analyzation of the PSM cohort data was performed for the 5-year cumulative recurrence rates. The rate was lower for laparoscopic surgery (14.1%) than for laparotomic surgery (21.6%). This difference was not significant (Cox-Mantel: p = 0.144). In agreement with this finding, neither the univariable Cox-regression (HR of with 95% CI from 0.320 to 1.188; p = 0.148) nor the multivariable Cox-regression (HR of 0.732 with 95% CI from 0.378 to 1.419; p = 0.356) lead to a statistical significant difference between both interventions (Fig. 3b). By this, there is no difference between both interventions concerning the 5-year cumulative recurrence rate.
Lastly, an analyzation of the recurrence-free survival after PSM was done for both surgical interventions. The 5-year recurrence-free overall survival was significantly (p = 0.027) higher for patients who underwent laparoscopic surgery (80.0%) than for patients who underwent laparotomic surgery (66.2%, Fig. 3c). Univariable Cox-regression analysis revealed only a weak statistical significance (HR = 0.541 with 95% CI from 0.311 to 0.940; p = 0.029) between laparoscopic and laparotomic surgery. Moreover, the multivariable analysis did not present a statistically significant difference (HR of 0.640 with 95% CI from 0.366 to 1.120; p = 0.118) at all. As observed earlier in the entire cohort (see Fig. 2c), we confirmed that the effect in the 5-year recurrence-free survival for laparoscopic surgery over laparotomic surgery is not statistically significant. Table S2 presents an overview of all statistical results after PSM.
Discussion
Generally, MIS reduces complications, operative trauma, and optimize post-surgical care (Kilger et al. 2001), whereas the specific risks of MIS are peritoneal trauma and hypothermia (Peng et al. 2009). Interestingly, recent studies on cervical carcinoma patients showed that MIS yielded an inferior outcome than laparotomy for endoscopic radical surgery (Ramirez et al. 2018; Melamed et al. 2018). These results cause doubt on the benefit of laparoscopic oncological procedures. In our cancer registry study, we compared the outcome of this surgical approach to open surgery in the treatment of intermediate- and high-risk endometrial cancer. The statistical analysis of our cohort data presented that laparoscopy yielded a better overall survival than laparotomy. We confirmed this by multivariable regression analysis and matched pair analysis, which addressed the confounding bias in our retrospective study. Furthermore, we analyzed the outcome in recurrence-free survival for both surgical procedures. Interestingly, recurrence-free survival still showed that laparoscopic surgery is superior to laparotomic surgery; however, this effect was no longer statistically significant, neither for the analysis of the entire cohort nor for the matched pair analysis.
Based on these findings, we conclude that MIS does not reduce oncological safety or efficacy compared to open surgery. We are aware that our findings are in contradiction to the recent study from Ramirez et al. on cervical cancer, which found a poorer outcome for laparoscopic surgery than for laparotomic surgery (Ramirez et al. 2018). There are several reasons for the different outcomes in both studies. First, we conducted an observational cancer registry study, whereas Ramirez et al. performed a prospective randomized study. This could affect the analysis due to the introduction of various biases; however, we addressed this concern by the use of both procedures—multivariable regression methods and matched pair analysis. Second, Ramirez et al. studied early-stage cervical cancer, whereas we examined endometrial carcinoma. Previous studies presented that the treatment outcome differs between different types of cancer: the outcome of MIS vs. open surgery was not inferior for early-stage endometrial cancer, whereas cervical cancer did show this effect (Melamed et al. 2017; Bregar et al. 2017). Lastly, there are unmeasured confounding factor that were not included but that could have affected the patient outcome, for instance, the socioeconomic status of the patient, alcohol consumption, tobacco use or infection with human immunodeficiency virus (HIV) (Melamed et al. 2018; Dryden-Peterson et al. 2016; Coker et al. 2009).
Ramirez et al. argued that the insufflation gas that was applied during laparoscopy could impair patient survival (Ramirez et al. 2018). Indeed, several studies showed that circulating CO2 can cause spillage of cervical cancer in the peritoneal cavity (Lin et al. 2014; Volz et al. 1999). In our study, we examined the recurrence rate among patients who underwent laparoscopy and patients who underwent open surgery but we were not able to identify a statistically significant difference. This indicates that the insufflation gas did not cause tumor spillage in the patients in our cohort. Moreover, this implies that circulating CO2 could easily cause spillage of cervical cancer cells but not of endometrial cancer cells. This would easily explain the observed differences in outcome between our study and the study of Ramirez et al. Additional studies are necessary to explore the different degrees of tumor spillages for different type of cancer during laparoscopic surgery.
In conclusion, the findings of this cancer registry study provide compelling evidence that laparoscopy is not associated with a poorer outcome than open surgery in the treatment of endometrial cancer. Additional prospective trails are necessary to clarify whether laparoscopy is a suitable approach for Type 2 as well as Type 1 endometrial cancer with high risk of recurrence.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflict of interest
Thomas Papathemelis, Helen Oppermann, Stella Grafl, Michael Gerken, Armin Pauer, Sophia Scharl, Anton Scharl, Elisabeth Inwald, Atanas Ignatov, Olaf Ortmann, Monika Klinkhammer-Schalke, Alexander Hein, Matthias W. Beckmann and Michael P. Lux declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments. This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bregar AJ, Melamed A, Diver E, Clemmer JT, Uppal S, Schorge JO, Rice LW, Del Carmen MG, Rauh-Hain JA (2017) Minimally invasive staging surgery in women with early-stage endometrial cancer: analysis of the national cancer data base. Ann Surg Oncol 24(6):1677–1687 [DOI] [PubMed] [Google Scholar]
- Coker AL, DeSimone CP, Eggleston KS, Hopenhayn C, Nee J, Tucker T (2009) Smoking and survival among Kentucky women diagnosed with invasive cervical cancer: 1995–2005. Gynecol Oncol 112(2):365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draeger T, Völkel V, Schnitzbauer V, Gerken M, Benz S, Klinkhammer-Schalke M, Fürst A (2019) Laparoscopic and open resection of rectal cancer-is age an effect modifier for short- and long-term survival? Int J Colorectal Dis 34(5):821–828 [DOI] [PubMed] [Google Scholar]
- Dryden-Peterson S, Bvochora-Nsingo M, Suneja G, Efstathiou JA, Grover S, Chiyapo S, Ramogola-Masire D, Kebabonye-Pusoentsi M, Clayman R, Mapes AC, Tapela N, Asmelash A, Medhin H, Viswanathan AN, Russell AH, Lin LL, Kayembe MKA, Mmalane M, Randall TC, Chabner B, Lockman S (2016) HIV infection and survival among women with cervical cancer. J Clin Oncol 34(31):3749–3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SE, Panzarella T, Lau S, Gien LT, Samouëlian V, Giede C, Steed H, Le T, Renkosinski B, Bernardini MQ (2018) Prospective cohort study comparing quality of life and sexual health outcomes between women undergoing robotic, laparoscopic and open surgery for endometrial cancer. Gynecol Oncol 149(3):476–483 [DOI] [PubMed] [Google Scholar]
- Kilger E, Weis FC, Goetz AE, Frey L, Kesel L, Schütz A, Lamm P, Uberfuhr P, Knoll A, Felbinger TW, Peter K (2001) Intensive care after minimally invasive and conventional coronary surgery: a prospective comparison. Intensive Care Med 27(3):534–539 [DOI] [PubMed] [Google Scholar]
- Kyrgiou M, Swart AM, Qian W, Warwick J (2015) A Comparison of outcomes following laparoscopic and open hysterectomy with or without lymphadenectomy for presumed early-stage endometrial cancer: results from the medical research council ASTEC trial. Int J Gynecol Cancer 25(8):1424–1436 [DOI] [PubMed] [Google Scholar]
- Lin F, Pan L, Li L, Li D, Mo L (2014) Effects of a simulated CO2 pneumoperitoneum environment on the proliferation, apoptosis, and metastasis of cervical cancer cells in vitro. Med Sci Monit 20:2497–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed A, Keating NL, Clemmer JT, Bregar AJ, Wright JD, Boruta DM, Schorge JO, Del Carmen MG, Rauh-Hain JA (2017) Laparoscopic staging for apparent stage I epithelial ovarian cancer. Am J Obstet Gynecol 216(1):50.e1–50.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, Seagle BL, Alexander A, Barber EL, Rice LW, Wright JD, Kocherginsky M, Shahabi S, Rauh-Hain JA (2018) Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med 379(20):1905–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourits MJ, Bijen CB, Arts HJ, ter Brugge HG, van der Sijde R, Paulsen L, Wijma J, Bongers MY, Post WJ, van der Zee AG, de Bock GH (2010) Safety of laparoscopy versus laparotomy in early-stage endometrial cancer: a randomised trial. Lancet Oncol 11(8):763–771 [DOI] [PubMed] [Google Scholar]
- Park DA, Lee DH, Kim SW, Lee SH (2016) Comparative safety and effectiveness of robot-assisted laparoscopic hysterectomy versus conventional laparoscopy and laparotomy for endometrial cancer: a systematic review and meta-analysis. Eur J Surg Oncol 42(9):1303–1314 [DOI] [PubMed] [Google Scholar]
- Peng Y, Zheng M, Ye Q, Chen X, Yu B, Liu B (2009) Heated and humidified CO2 prevents hypothermia, peritoneal injury, and intra-abdominal adhesions during prolonged laparoscopic insufflations. The Journal of Surgical Research. 151(1):40–47 [DOI] [PubMed] [Google Scholar]
- Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, Buda A, Yan X, Shuzhong Y, Chetty N, Isla D, Tamura M, Zhu T, Robledo K, Gebski V, Asher R, Behan V, Nicklin JL, Coleman RL, Obermair A (2018) Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med 379(20):1895–1904 [DOI] [PubMed] [Google Scholar]
- Rosenbaum PR, Rubin DB (1983) The central role of the propensity score in observational studies for causal effects. Biometrika 70(1):41–55 [Google Scholar]
- Safdieh J, Lee YC, Wong A, Lee A, Weiner JP, Schwartz D, Schreiber D (2017) A comparison of outcomes between open hysterectomy and robotic-assisted hysterectomy for endometrial cancer using the national cancer database. Int J Gynecol Cancer 27(7):1508–1516 [DOI] [PubMed] [Google Scholar]
- Volz J, Köster S, Spacek Z, Paweletz N (1999) The influence of pneumoperitoneum used in laparoscopic surgery on an intraabdominal tumor growth. Cancer 86(5):770–774 [PubMed] [Google Scholar]
- Zullo F, Palomba S, Falbo A, Russo T, Mocciaro R, Tartaglia E, Tagliaferri P, Mastrantonio P (2009) Laparoscopic surgery vs laparotomy for early stage endometrial cancer: long-term data of a randomized controlled trial. Am J Obstet Gynecol 200(3):296.e1-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.