Abstract
With the development of antitumor therapies, different treatment methods including monotherapy and combined therapy have achieved clinical efficacy in advanced epidermal growth factor receptor (EGFR) mutant non-small cell lung cancer (NSCLC) patients. Exon 19 deletion (ex19del) and exon 21 L858R mutation are common sensitive subtypes of EGFR mutation. However, potential distinct mechanisms are found from several dimensions including molecular structures, biological behaviors, concomitant mutations, resistance mechanisms and tumor mutation burdens. More evidence indicates the prognostic difference of EGFR subgroups. This review focused on the progress of potential distinct mechanisms and outcomes in clinical trials of advanced NSCLC patients with ex19del or exon 21 L858R mutation.
Keywords: Epidermal growth factor receptor, ex19del, Exon 21 L858R mutation, Non-small cell lung cancer
Overview
Lung cancer is the most common cause of cancer death. Non-small cell cancer (NSCLC) is the major histological categories of lung cancer, which occurs approximately 85% of cases (Siegel et al. 2019; Roskoski 2019; London and Gallo 2020). With the development of epidermal growth factor receptor (EGFR) mutation detection and its targeted therapy, there is a noticeable rise in the survival of advanced NSCLC patients, with a median overall survival (OS) of 38.6 months in single-agent third-generation EGFR-tyrosine kinase inhibitor (EGFR-TKI) and 50.9 months in combination therapy (Ramalingam et al. 2020; Hosomi et al. 2020). However, the mutation rate of EGFR gene varies among countries and ethnicities, which is approximately 50% in Asian patients while only 10–20% in Caucasian patients (Zaric et al. 2014; Harrison et al. 2020). Although there are different types of EGFR mutations, approximately 90% are mutations harboring exon 19 deletion (ex19del) or exon 21 mutation, and L858R mutation is the predominant subtype of exon 21 mutation (Lee 2017; Wang and Li 2019). In spite of both mutations are sensitive to EGFR-TKIs, increasing studies confirmed their differences in therapeutic effects and the optimal therapeutic regimen remains indeterminate (Cross et al. 2014; Riely et al. 2006; Castellanos et al. 2017). This review aimed to explore the distinct potential mechanisms and therapeutic efficacy of advanced NSCLC patients with ex19del and exon 21 L858R mutation to provide optimal first-line treatment strategies for clinical decision.
Distinct mechanisms of sensitive EGFR subtypes
Patients with different EGFR subtypes have specific EGFR-TKI therapeutic effect for the following potential mechanisms. First of all, tracing the difference of molecular structures is fundamental. Ex19del represents in-frame deletions contained amino acid residues 746–750 of the expressed protein while L858R mutation is a single-nucleotide substitution that replaced leucine with arginine at codon 858 (Kumar et al. 2008). EGFR tyrosine kinase domain was comprised of N-terminal lobe, C-terminal lobe and active ATP site lying in the cleft of two lobes (Massarelli et al. 2013). Both of existence of ex19del and L858R mutation could reduce the affinity for ATP and the competition for binding sites, lead to an increased receptor dimerization and activity (Reguart and Remon 2015). Distinctively, certain residues were removed from the loop ATP-binding cleft with a relocation of critical residues in ex19del, while the position of L858R mutation was in the activation loop (A loop), which was distant from ATP-binding cleft, the substitution of arginine contributed to a steady active conformation (Reguart and Remon 2015; Yun et al. 2007; Wee and Wang 2017). The compaction of the ATP-binding site in L858R mutation was looser than that in ex19del, which might account for the affinities and the sensitivity differences to TKIs (Wee and Wang 2017). Second, mutations could alter the biological behaviors of cancer cells. Ex19del cells produced more G1 phase arrest 7 days after receiving gefitinib, while cells with L858R mutation had greater proportion cells in the G2 phase, which suggested a poor degree of inhibition and a further process of mitosis in L858R cells (Zhu et al. 2008; Hong et al. 2019). The EGFR-TKI inhibition level of downstream signals also varies among mutations, the study showed that downstream effectors of phosphorylated EGFR, including Akt and Erk1/2 signals, are more likely to be inhibited by gefitinib in ex19del cells than in L858R cells, which might be associated with the differential sensitivity to EGFR-TKIs (Zhu et al. 2008; Nagano et al. 2018; Sordella et al. 2004; Paez et al. 2004). Besides, L858R mutation could raise cell invasive ability with an up-regulated CXCR4 through CXCL12-CXCR4 pathway, and facilitate the formation of malignant pleural effusion (Tsai et al. 2015). Thirdly, patients with exon 21 mutation are more likely to harbor concomitant mutations compared with ex19del (69% vs. 41%), while the occurrence of concomitant mutations affects prognosis with a significantly reduced objective response rate (ORR) (44% vs. 77%) and survival (median progression-free survival [PFS]: 6.20 months vs. 18.77 months, median OS: 22.70 months vs. not reached) (Hong et al. 2018; Barnet et al. 2017). Fourthly, mechanisms of resistance vary in cells with different mutations, patients with L858R mutations have a higher proportion of unknown resistance mechanisms (32.9% vs. 20.1%) and a lower likelihood of T790M mutation compared with ex19del (36.5% vs. 50.4%), while patients with T790M mutation achieved a better survival than other resistance mechanisms with a median OS of 36.0 months (Ke et al. 2017; Goag et al. 2018). Besides, C797S mutation was a common resistant mechanism to T790M-targeting EGFR-TKIs, which was more frequent in ex19del patients than L858R mutation patients (Wang et al. 2016; Gunther et al. 2016; Thress et al. 2015; Papadimitrakopoulou et al. 2020). Finally, patients with L858R mutation had a higher value of tumor mutation burden (TMB) than patients with ex19del, while TMB was a negative prognostic factor of clinical outcomes for EGFR-mutant NSCLC patients receiving EGFR-TKI (Jiao et al. 2019). Therefore, ex19del and L858R mutation subgroups have specific mechanisms, which accounts for different efficacies to treatments.
Distinct treatments for sensitive EGFR subtypes
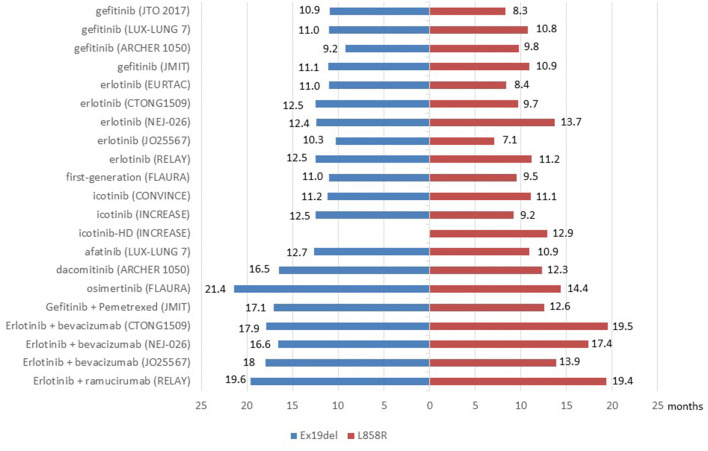
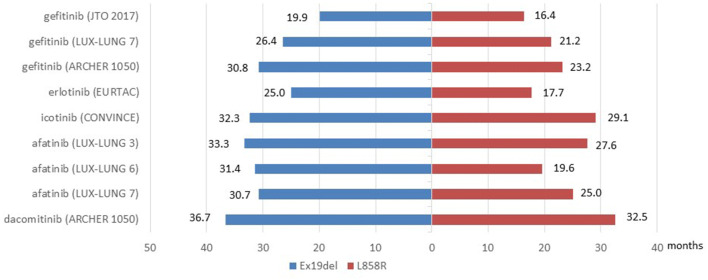
Since both ex19del and L858R mutation subtypes were sensitive to EGFR-TKIs, clinical trials explored the therapeutic efficacy of targeted therapy including monotherapy and combined therapy for advanced EGFR-mutant NSCLC patients, the difference of efficacy between EGFR mutation subtypes was estimated in subgroup analyses. Data was summarized in Fig. 1, Fig. 2 and Table 1.
Fig. 1.
PFS benefits for patients with EGFR ex19del or exon 21 L858R mutation in clinical trials
Fig. 2.
OS benefits for patients with EGFR ex19del or exon 21 L858R mutation in clinical trials
Table 1.
Summarization of clinical trials
| Trial | Intervention arm | Control arm | subgroup | PFS | OS | Citation | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | HR | P | Median | HR | P | |||||
| IPASS | Gefitinib | Carboplatin + paclitaxel | All | 9.5 vs 6.3 | 0.48 (0.36–0.64) | < 0.001 | 21.6 vs 21.9 | 1.00 (0.76–1.33) | 0.99 | Mok et al. 2009; Fukuoka et al. 2011) |
| L858R | 0.55 (0.35–0.87) | / | / | / | / | |||||
| ex19del | 0.38 (0.26–0.56) | / | / | / | / | |||||
| JTO 2017 | Gefitinib | / | All | 9.1 | / | / | 18.2 | / | / | Sutiman et al. 2017) |
| L858R | 8.3 | / | / | 16.4 | / | / | ||||
| ex19del | 10.9 | / | / | 19.9 | / | / | ||||
| EURTAC | Erlotinib | Cisplatin/carboplatin + docetaxel/gemcitabine | All | 9.7 vs 5.2 | 0.37 (0.25–0.54) | < 0.0001 | 22.9 vs 22.1 | / | 0.97 | Rosell et al. 2012; Karachaliou, et al. 2015) |
| L858R | 8.4 vs 6.0 | 0.55 (0.29–1.02) | 0.0539 | 17.7 vs NA | 0.99 (0.56–1.76) | / | ||||
| ex19del | 11.0 vs 4.6 | 0.30 (0.18–0.50) | < 0.0001 | 25.0 vs NA | 0.94 (0.57–1.54) | / | ||||
| CONVINCE | Icotinib | Cisplatin + pemetrexed | All | 11.2 vs 7.9 | 0.61 (0.43–0.87) | 0.006 | 30.5 vs 32.1 | / | 0.885 | Shi et al. 2017) |
| L858R | 11.1 vs 7.8 | 0.76 (0.43–1.33) | 0.331 | 29.1 vs 26.7 | / | 0.526 | ||||
| ex19del | 11.2 vs 8.0 | 0.66 (0.38–1.14) | 0.136 | 32.3 vs 38.8 | / | 0.407 | ||||
| INCREASE | Icotinib-HD | Icotinib | L858R | 12.9 vs 9.2 | 0.75 (0.53–1.05) | < 0.05 | / | / | / | Li et al. 2020) |
| Icotinib | / | ex19del vs L858R | 12.5 vs 9.2 | 0.80 (0.57–1.13) | 0.11 | / | / | / | ||
| LUX-Lung3 | Afatinib | Cisplatin + pemetrexed | All | 11.1 vs 6.9 | 0.58 (0.43–0.78) | 0.001 | 28.2 vs 28.2 | 0.88 (0.66–1.17) | 0.39 | Sequist et al. 2013; Yang et al. 2015) |
| L858R | / | 0.73 (0.46–1.17) | 0.01 | 27.6 vs 40.3 | 1.30 (0.80–2.11) | 0.29 | ||||
| ex19del | / | 0.28 (0.18–0.44) | 0.01 | 33.3 vs 21.1 | 0.54 (0.36–0.79) | 0.0015 | ||||
| LUX-Lung6 | Afatinib | Gemcitabine + pemetrexed | All | 11.0 vs 5.6 | 0.28 (0.20–0.39) | < 0.0001 | 23.1 vs 23.5 | 0.93 (0.72–1.22) | 0.61 | Wu et al. 2014; Yang et al. 2015) |
| L858R | / | 0.32 (0.19–0.52) | / | 19.6 vs 24.3 | 1.22 (0.81–1.83) | 0.34 | ||||
| ex19del | / | 0.20 (0.13–0.33) | / | 31.4 vs 18.4 | 0.64 (0.44–0.94) | 0.023 | ||||
| LUX-Lung7 | Afatinib | Gefitinib | All | 11.0 vs 10.9 | 0.73 (0.57–0.95) | 0.017 | 27.9 vs 24.5 | 0.86 (0.66–1.12) | 0.258 | Park et al. 2016; Paz-Ares et al. 2017) |
| L858R | 10.9 vs 10.8 | 0.71 (0.48–1.06) | / | 25.0 vs 21.2 | 0.91 (0.62–1.36) | 0.6585 | ||||
| ex19del | 12.7 vs 11.0 | 0.76 (0.55–1.06) | / | 30.7 vs 26.4 | 0.83 (0.58–1.17) | 0.2841 | ||||
| ARCHER1050 | Dacomitinib | Gefitinib | All | 14.7 vs 9.2 | 0.59 (0.47–0.74) | < 0.0001 | 41.0 vs 33.6 | 0.75 (0.59–0.95) | 0.0155 | Wu et al. 2017; Mok et al. 2018) |
| L858R | 12.3 vs 9.8 | 0.63 (0.44–0.88) | 0.0068 | 32.5 vs 23.2 | 0.67 (0.47–0.94) | 0.0203 | ||||
| ex19del | 16.5 vs 9.2 | 0.55 (0.41–0.75) | < 0.0001 | 36.7 vs 30.8 | 0.85 (0.62–1.15) | 0.3021 | ||||
| FLAURA | Osimertinib | Gefitinib/erlotinib | All | 18.9 vs 10.2 | 0.46 (0.37–0.57) | < 0.001 | 38.6 vs 31.8 | 0.80 (0.64–1.00) | 0.0462 | Ramalingam et al. 2020; Soria et al. 2018) |
| L858R | 14.4 vs 9.5 | 0.51 (0.36–0.71) | < 0.001 | / | 1.00 (0.71–1.40) | / | ||||
| ex19del | 21.4 vs 11.0 | 0.43 (0.32–0.56) | < 0.001 | / | 0.68 (0.51–0.90) | / | ||||
| JMIT | Gefitinib + pemetrexed | Gefitinib | All | 15.8 vs 10.9 | 0.69 (0.49–0.96) | 0.028 | / | / | / | Cheng et al. 2016) |
| L858R | 12.6 vs 10.9 | 0.58 (0.33–1.01) | 0.054 | / | / | / | ||||
| ex19del | 17.1 vs 11.1 | 0.67 (0.43–1.05) | 0.078 | / | / | / | ||||
| NCT02148380 | Gefitinib + pemetrexed + carboplatin | Gefitinib | All | 17.5 vs 11.9 | 0.48 (0.29–0.78) | 0.003 | 32.6 vs 25.8 | 0.36 (0.20–0.67) | 0.001 | Han et al. 2017) |
| L858R | / | 0.31 (0.15–0.66) | / | / | / | / | ||||
| ex19del | / | 0.60 (0.30–1.21) | / | / | / | / | ||||
| NEJ009 | Gefitinib + carboplatin + pemetrexed | Gefitinib | All | 20.93 vs 11.17 | 0.49 (0.39–0.62) | < 0.001 | 50.90 vs 38.80 | 0.72 (0.55–0.95) | 0.021 | Hosomi et al. 2020) |
| L858R | / | 0.55 (0.38–0.80) | / | / | 0.80 (0.53–1.20) | / | ||||
| ex19del | / | 0.47 (0.34–0.64) | / | / | 0.65 (0.44–0.97) | / | ||||
| CTONG1509 | Erlotinib + bevacizumab | Erlotinib | All | 18.0 vs 11.3 | 0.55 (0.41–0.75) | < 0.001 | / | / | / | Zhou et al. 2019) |
| L858R | 19.5 vs 9.7 | 0.51 (0.33–0.79) | < 0.001 | / | / | / | ||||
| ex19del | 17.9 vs 12.5 | 0.62 (0.41–0.92) | < 0.001 | / | / | / | ||||
| NEJ026 | Erlotinib + bevacizumab | Erlotinib | All | 16.9 vs 13.3 | 0.61 (0.42–0.88) | 0.016 | / | / | / | Saito et al. 2019) |
| L858R | 17.4 vs 13.7 | 0.57 (0.33–0.97) | / | / | / | / | ||||
| ex19del | 16.6 vs 12.4 | 0.69 (0.41–1.16) | / | / | / | / | ||||
| JO25567 | Erlotinib + bevacizumab | Erlotinib | All | 16.0 vs 9.7 | 0.54 (0.36–0.79) | 0.0015 | 47.0 vs 47.4 | 0.81 (0.53–1.23) | 0.3267 | Seto et al. 2014) |
| L858R | 13.9 vs 7.1 | 0.67 (0.38–1.18) | 0.1653 | / | / | / | ||||
| ex19del | 18.0 vs 10.3 | 0.41 (0.24–0.72) | 0.0011 | / | / | / | ||||
| RELAY | Erlotinib + ramucirumab | Erlotinib | All | 19.4 vs 12.4 | 0.59 (0.46–0.76) | < 0.0001 | / | / | / | Nakagawa et al. 2019) |
| L858R | 19.4 vs 11.2 | 0.62 (0.44–0.87) | 0.006 | / | / | / | ||||
| ex19del | 19.6 vs 12.5 | 0.65 (0.47–0.90) | 0.0098 | / | / | / | ||||
EGFR-TKI is more beneficial for ex19del patients than L858R mutation patients
First-generation EGFR-TKI
Clinical trials demonstrated significant prolonged PFS of targeted therapy by comparing first-generation EGFR-TKIs with chemotherapy in advanced EGFR-mutant NSCLC patients (median PFS: gefitinib vs. chemotherapy [IPASS trial], 9.5 months vs. 6.3 months, P < 0.001; erlotinib vs. chemotherapy [EURTAC trial], 9.7 months vs. 5.2 months, P < 0.0001; icotinib vs. chemotherapy [CONVINCE trial] 11.2 months vs. 7.9 months, P = 0.006), however, no benefit was found in OS analyses (Mok et al. 2009; Fukuoka et al. 2011; Rosell et al. 2012; Shi et al. 2017; Karachaliou et al. 2015).
In subgroup analyses, efficacies varied among different subtypes of mutations. For ex19del patients, significant PFS benefit was found in IPASS trial (HR 0.38, 95% CI 0.26–0.56) and EURTAC trial (HR 0.30, 95% CI 0.18–0.50) when comparing first-generation EGFR-TKIs with chemotherapy. While only IPASS trial showed a significant PFS for L858R mutation patients (HR 0.55, 95% CI 0.35–0.87) (Fukuoka et al. 2011; Rosell et al. 2012). Neither of mutation subgroups showed significant OS benefit (Fukuoka et al. 2011; Rosell et al. 2012; Shi et al. 2017; Karachaliou et al. 2015). Meta-analysis showed that the application of first-generation EGFR-TKIs significantly improved PFS for both ex19del patients (HR 0.28, 95% CI 0.20–0.40) and L858R patients (HR 0.44, 95% CI 0.34–0.57), but had no significant benefit on OS compared with chemotherapy (Kuan et al. 2015). Furthermore, several trials conducted a direct comparison between ex19del and L858R mutation patients. In PFS analysis, Sutiman N et al. found that PFS outcome of ex19del patients was superior to patients with L858R mutation after the administration of gefitinib (median PFS: 8.3 months vs. 10.9 months, P = 0.006) in a previous retrospective, observational cohort study(Sutiman et al. 2017). However, no significant PFS difference in a direct comparison between subgroups was shown in IPASS trial or INCREASE trial (Fukuoka et al. 2011; Li et al. 2020). A meta-analysis also conducted a direct comparison and demonstrated an improved PFS for ex19del patients (HR 0.75, 95% CI 0.65–0.85) over L858R mutation patients (Zhang et al. 2014). Besides, INCREASE study further evaluated the efficacy of high dose icotinib (250 mg, thrice daily) for L858R mutation patients, which demonstrated a prolonged PFS than patients receiving regular dose icotinib (125 mg, thrice daily) (12.9 months vs. 9.2 months, HR 0.75, 95% CI 0.53–1.05, P < 0.05) (Li et al. 2020). It suggested that prognosis could be improved for patients with L858R mutations by increasing the dose of EGFR-TKIs with a manageable toxicity. As for OS analysis, only EURTAC trial found a significant OS benefit in the direct comparison between ex19del patients and L858R mutation patients after the application of erlotinib (median OS: 25.0 months vs. 17.7 months, P = 0.001) (Karachaliou, et al. 2015).
In summary, for first-generation EGFR-TKI, clinical trials demonstrated PFS benefit compared with chemotherapy for both sensitive EGFR mutation subtypes. Subgroup analyses revealed a tendency of more potential benefit for ex19del patients than L858R mutation patients.
Second-generation EGFR-TKI
Clinical trials demonstrated the efficacy of irreversible second-generation EGFR-TKI, afatinib in advanced EGFR-mutant NSCLC patients. Significantly prolonged PFS was found when comparing afatinib with chemotherapy (median PFS: LUX-Lung 3 trial, 11.1 months vs. 6.9 months, P = 0.001; LUX-Lung 6 trial, 11.0 months vs. 5.6 months, P < 0.0001) (Sequist et al. 2013; Wu et al. 2014). However, clinical benefit was not presented in OS analyses (Yang et al. 2015). In subgroup analyses, for patients with ex19del, significant prolonged PFS was shown in both studies (LUX-Lung 3 trial, HR 0.28, 95% CI 0.18–0.44; LUX-Lung 6 trial, HR 0.20, 95% CI 0.13–0.33), while only LUX-Lung 6 trial showed significant PFS benefit for patients with L858R mutation (HR 0.32, 95% CI 0.19–0.52) (Sequist et al. 2013; Wu et al. 2014). In OS analyses, patients with ex19del also achieved significant survival benefit (LUX-Lung 3 trial, HR 0.54, 95% CI 0.36–0.79; LUX-Lung 6 trial, HR 0.64, 95% CI 0.44–0.94), while no OS benefit for L858R mutation patients (Yang et al. 2015). Meta-analysis showed both significant PFS (HR 0.24, 95% CI 0.17–0.33) and OS (0.59, 95% CI 0.47–0.73) benefit for patients with ex19del, while no survival benefit for L858R patients was demonstrated when comparing afatinib with chemotherapy (Kuan et al. 2015). The results showed that patients with ex19del had a better tendency of survival compared with L858R mutation patients after administration with afatinib.
Since previous studies had demonstrated better efficacy for EGFR-TKIs than chemotherapy, further explorations on EGFR-TKIs were conducted while setting first-generation EGFR-TKIs (mainly gefitinib or erlotinib) as comparisons. LUX-Lung 7 trial compared afatinib with gefitinib, for sensitive EGFR-mutant NSCLC patients, the survival benefit was found in PFS analysis (median PFS: 11.0 months vs. 10.9 months, P = 0.017), but not in OS analysis. No survival benefit was found in subgroup analyses for patients with ex19del or L858R mutation (Park et al. 2016; Paz-Ares et al. 2017).
Besides, ARCHER 1050 trial demonstrated a significant survival benefit of dacomitinib in both PFS (median PFS, 14.7 months vs. 9.2 months, P < 0.0001) and OS (median OS, 41.0 months vs. 33.6 months, P = 0.0155) analyses for advanced EGFR-mutant patients compared with gefitinib. As for subgroup analyses, survival outcomes differed from afatinib. Patients with L858R mutation achieved both PFS (HR 0.63, 95% CI 0.44–0.88) and OS (HR 0.67, 95% CI 0.47–0.94) benefit, while patients with ex19del only achieved significant PFS benefit (HR 0.55, 95% CI 0.41–0.75) (Wu et al. 2017; Mok et al. 2018). However, the outcomes of OS analysis remained controversial, for ORR did not show a significant result in statistical analysis with a fixed order, besides, OS curves intersected at 12 months which indicated that the potential impact of subsequent treatment effects could not be neglected, thus the credibility of OS results remained questionable. Therefore, although EGFR-mutant patients receiving second-generation EGFR-TKIs showed a survival benefit, subgroup analyses demonstrated more benefit for ex19del patients.
Third-generation EGFR-TKI
As for third-generation EGFR-TKI, significant PFS (median PFS, 18.9 months vs. 10.2 months, P < 0.001) and OS (median OS: 38.6 months vs. 31.8 months, P = 0.0462) benefit was achieved for EGFR-mutant patients receiving osimertinib compared with first-generation EGFR-TKIs. Subgroup analyses showed ex19del patients achieved a significant survival benefit on both PFS (HR 0.43, 95% CI 0.32–0.56) and OS (HR 0.68, 95% CI 0.51–0.90) analyses, while L858R mutation patients only achieved a significant PFS benefit (HR 0.51, 95% CI 0.36–0.71), which indicated a superior benefit for ex19del patients receiving osimertinib (Ramalingam et al. 2020; Soria et al. 2018).
In summary, EGFR-TKI monotherapy could improve the survival of advanced EGFR-mutant NSCLC patients, however subgroup analyses showed distinct survival outcomes. Apart from long-term survival of dacomitinib for L858R mutation patients, ex19del patients could benefit more from monotherapy than patients with L858R mutation. A previous network meta-analysis also suggested that osimertinib was the optimal treatment for ex19del patients considering PFS benefit (Zhao et al. 2019).
The efficacy of combined therapy in subgroups is still controversial
Combination of EGFR-TKI and chemotherapy
Combination therapy further demonstrated clinical benefits compared with EGFR-TKI monotherapy. Several studies explored the clinical efficacy of the combination of EGFR-TKIs and chemotherapy, significant PFS benefit was found in JMIT trial (median PFS: 15.8 months vs 10.9 months, P = 0.028), NCT02148380 trial (median PFS, 17.5 months vs. 11.9 months, P = 0.003) and NEJ009 trial (median PFS, 20.9 months vs. 11.2 months, P < 0.001) when comparing with gefitinib. As for OS analysis, prolonged survival was revealed in both NCT02148380 trial (median OS: 32.6 months vs. 25.8 months, P = 0.001) and NEJ009 trial (median OS: 50.9 months vs. 38.8 months, P = 0.021) (Hosomi et al. 2020; Cheng et al. 2016; Han et al. 2017).
In subgroup analyses, a superior benefit on PFS was demonstrated for L858R patients receiving combination therapy than patients receiving gefitinib in NCT02148380 trial (HR 0.31, 95% CI 0.15–0.66) and NEJ009 trial (HR 0.55, 95% CI 0.38–0.80). NEJ009 trial also showed significant PFS benefit for ex19del patients (HR 0.47, 95% CI 0.34–0.64)(Hosomi et al. 2020; Cheng et al. 2016; Han et al. 2017). A previous network meta-analysis demonstrated treatment efficacy of the combination of gefitinib and chemotherapy for L858R mutation patients in terms of PFS (Zhao et al. 2019). In OS analysis, a significant benefit was achieved only for ex19del patients (HR 0.65, 95% CI 0.44–0.97) in NEJ009 trial (Hosomi et al. 2020). Although survival benefit was revealed in combination therapy of EGFR-TKIs and chemotherapy, the prognosis outcomes in EGFR subtypes were controversial and needed to be further verified.
Combination of EGFR-TKI and antiangiogenic therapy
The combination of EGFR-TKI and antiangiogenic drugs also revealed efficacy in first-line treatment for advanced EGFR-mutant NSCLC patients. Three clinical trials explored the prognosis of the combination of erlotinib and bevacizumab compared with erlotinib, including the CTONG1509 trial, NEJ026 trial and JO25567 trial. All of the trials demonstrated significant PFS benefit for the combination of antiangiogenic therapy and EGFR-TKIs (median PFS: CTONG1509 trial, 18.0 months vs. 11.3 months, P < 0.001; NEJ026 trial, 16.9 months vs. 13.3 months, P = 0.016; JO25567 trial, 16.0 months vs. 9.7 months, P = 0.0015) (Zhou et al. 2019; Saito et al. 2019; Seto et al. 2014). OS results of most trials were immature, and no OS benefit was found in JO25567 trial (median OS: 47.0 months vs. 47.4 months, P = 0.3267) (Seto et al. 2014). Besides, phase 3 trial (RELAY) explored the combination of erlotinib and ramucirumab compared with erlotinib alone, where significant prolonged PFS was also found for combined therapy (median PFS: 19.4 months vs. 12.4 months, P < 0.0001) (Nakagawa et al. 2019). In addition, patients receiving the combination of targeted therapy and antiangiogenic therapy had a less frequency of T790M mutation, as well as other complex mutations and amplifications than patients receiving EGFR-TKI monotherapy at the time of disease progression, which might achieve a better efficacy in subsequent therapies(Zeng et al. 2020; Wang et al. 2018). However, it was reported that combined therapy would bring an increased risk of toxicity, which might bring negative effects on patients’ survival outcomes and quality of life (Zhao et al. 2019).
When considering subgroup analyses, significant PFS benefit of combined therapy was found in CTONG1509 trial (HR 0.51, 95% CI 0.33–0.79), NEJ026 trial (HR 0.57, 95% CI 0.33–0.97) and RELAY trial (HR 0.62, 95% CI 0.44–0.87) for L858R mutation patients, while in CTONG1509 trial (HR 0.62, 95% CI 0.41–0.92), JO25567 trial (HR 0.41, 95% CI 0.24–0.72) and RELAY trial (HR 0.65, 95% CI 0.47–0.90) for ex19del patients. In other words, CTONG1509 trial and NEJ026 trial showed the superiority of combined therapy for L858R mutation patients, while JO25567 trial showed a better survival outcome for ex19del patients(Zhou et al. 2019; Saito et al. 2019; Seto et al. 2014). In RELAY trial, patients with L858R mutation had a slight advantage of progression risk (HR 0.62 vs. 0.65) and a slight disadvantage of median PFS (19.4 months vs. 19.6 months) compared with ex19del patients (Nakagawa et al. 2019). Hence, prognostic outcomes of combined targeted and antiangiogenic therapy were controversial and might bring more potential benefits to L858R mutation patients, which was worthy of further study.
Evidence of differences in therapeutic effects from real-world data
In addition to survival outcomes from clinical trials, real-world studies also showed therapeutic distinctions in different subgroups of advanced sensitive EGFR-mutant NSCLC patients. Most studies focused on first-line EGFR-TKI monotherapy. Lau et al. compared second-generation EGFR-TKI (afatinib) with first-generation EGFR-TKIs (gefitinib, erlotinib), results showed significant OS benefit for patients with ex19del (median OS: 48.8 months vs. 26.4 months), while not for L858R mutation patients (median OS: 25.4 months vs. 20.6 months) (Lau et al. 2019). Further studies focused on direct comparisons between EGFR mutation subgroups. Li et al. explored the efficacy of first-line EGFR-TKIs in a real-world setting, results showed ex19del patients achieved numerically increased time to next treatment (TTNT) and OS compared with L858R mutation patients when both administrated with erlotinib (median TTNT: 14.0 months vs. 12.1 months, median OS: 24.6 months vs. 19.9 months) and afatinib (median TTNT: 12.6 months vs. 11.2 months, median OS: 23.0 months vs. 16.2 months) (Li et al. 2019). Ho et al. also demonstrated significantly superior PFS for ex19del patients when comparing with L858R mutation patients after receiving first-line afatinib (16.0 months vs. 8.7 months, P = 0.001) (Ho et al. 2019). Besides, in real-world clinical practice, NCT03370770 trial demonstrated a prolonged OS (median OS: 45.7 months vs. 35.2 months) when comparing ex19del patients with L858R mutation patients for advanced EGFR-mutant NSCLC who received first-line afatinib and sequential osimertinib after the occurrence of T790M mutation (Hochmair et al. 2019, 2018). Thus, evidence from real-world data also supported the therapeutic distinctions of EGFR mutation subtypes.
Conclusions
More and more evidence showed that although both ex19del and L858R mutation were sensitive EGFR mutations, they had specific molecular mechanisms and should be treated as distinct types of NSCLC. Therapeutic effects reflected the difference. In general, first-line EGFR-TKI monotherapy was beneficial for advanced EGFR-mutant NSCLC patients, subgroup analyses showed monotherapy, especially osimertinib, might be more beneficial for patients with ex19del. Besides, combined therapy was superior to first-generation EGFR-TKI for EGFR-mutant patients, subgroup analyses showed controversial outcomes, generally combination was beneficial for patients with L858R mutation, especially when combining EGFR-TKI with antiangiogenic drugs.
Prospectively, since ex19del and L858R mutation are distinct types of NSCLC, clinicians should administrate optimal treatments for patients with different types of mutations separately. However, current results are still controversial, for instance, both dacomitinib and combined therapy could bring a survival benefit for L858R mutation patients, which is worthy of further comparison. Besides, patients with L858R mutation had a higher TMB value than ex19del patients, the previous retrospective study also demonstrated the efficacy of immune checkpoint blockade treatment for patients with L858R mutation, which was similar to EGFR wild-type patients and better than ex19del patients, thus immunotherapy might be a potential treatment option for L858R mutation patients (Offin et al. 2019; Hastings et al. 2019). Furthermore, our study mainly focused on first-line treatments for patients with advanced EGFR-mutated NSCLC, while current evidence showed that patients with localized diseases could also benefit from EGFR-TKIs (Marquez-Medina and Popat 2016). Retrospective study showed a superior post-recurrence survival (PRS) for ex19del patients compared with L858R mutation patients (median PRS: 27.0 months vs. 21.2 months, P = 0.016), suggesting directions for further researches (Zhang et al. 2018). With the development of next-generation sequencing (NGS), studies on mutation subtypes and correlative mutations will provide a reference for further exploration and the best treatment options for patients with different EGFR mutation subtypes.
Acknowledgements
This work was supported by Nation Key Research and Development Program of China (Grant No. 2016YFC1303800).
Author contributions
The manuscript has been read and approved by all the authors.
Funding
This work was supported by Nation Key Research and Development Program of China (Grant No. 2016YFC1303800).
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Barnet MB, O'Toole S, Horvath LG, Selinger C, Yu B, Ng CC et al (2017) EGFR-Co-mutated advanced NSCLC and response to EGFR tyrosine kinase inhibitors. J Thorac Oncol 12(3):585–590 [DOI] [PubMed] [Google Scholar]
- Castellanos E, Feld E, Horn L (2017) Driven by mutations: the predictive value of mutation subtype in EGFR-mutated non-small cell lung cancer. J Thorac Oncol 12(4):612–623 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Murakami H, Yang PC, He J, Nakagawa K, Kang JH et al (2016) Randomized phase II trial of gefitinib with and without pemetrexed as first-line therapy in patients with advanced nonsquamous non-small-cell lung cancer with activating epidermal growth factor receptor mutations. J Clin Oncol 34(27):3258–3266 [DOI] [PubMed] [Google Scholar]
- Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ et al (2014) AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 4(9):1046–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V et al (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 29(21):2866–2874 [DOI] [PubMed] [Google Scholar]
- Goag EK, Lee JM, Chung KS, Kim SY, Leem AY, Song JH et al (2018) Usefulness of bronchoscopic rebiopsy of non-small cell lung cancer with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor. J Cancer 9(6):1113–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther M, Juchum M, Kelter G, Fiebig H, Laufer S (2016) Lung cancer: EGFR inhibitors with low nanomolar activity against a therapy-resistant L858R/T790M/C797S mutant. Angew Chem Int Ed Engl 55(36):10890–10894 [DOI] [PubMed] [Google Scholar]
- Han B, Jin B, Chu T, Niu Y, Dong Y, Xu J et al (2017) Combination of chemotherapy and gefitinib as first-line treatment for patients with advanced lung adenocarcinoma and sensitive EGFR mutations: a randomized controlled trial. Int J Cancer 141(6):1249–1256 [DOI] [PubMed] [Google Scholar]
- Harrison PT, Vyse S, Huang PH (2020) Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol 61:167–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings K, Yu HA, Wei W, Sanchez-Vega F, DeVeaux M, Choi J et al (2019) EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann Oncol 30(8):1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho GF, Chai CS, Alip A, Wahid MIA, Abdullah MM, Foo YC et al (2019) Real-world experience of first-line afatinib in patients with EGFR-mutant advanced NSCLC: a multicenter observational study. BMC Cancer 19(1):896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmair MJ, Morabito A, Hao D, Yang CT, Soo RA, Yang JC et al (2018) Sequential treatment with afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: an observational study. Future Oncol 14(27):2861–2874 [DOI] [PubMed] [Google Scholar]
- Hochmair MJ, Morabito A, Hao D, Yang CT, Soo RA, Yang JC et al (2019) Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: updated analysis of the observational GioTag study. Future Oncol 15(25):2905–2914 [DOI] [PubMed] [Google Scholar]
- Hong S, Gao F, Fu S, Wang Y, Fang W, Huang Y et al (2018) Concomitant genetic alterations with response to treatment and epidermal growth factor receptor tyrosine kinase inhibitors in patients with EGFR-mutant advanced non-small cell lung cancer. JAMA Oncol 4(5):739–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Wu Q, Zhang J, Zhou Y (2019) Prognostic value of EGFR 19-del and 21–L858R mutations in patients with non-small cell lung cancer. Oncol Lett 18(4):3887–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosomi Y, Morita S, Sugawara S, Kato T, Fukuhara T, Gemma A et al (2020) Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol 38(2):115–123 [DOI] [PubMed] [Google Scholar]
- Jiao XD, He X, Qin BD, Liu K, Wu Y, Liu J et al (2019) The prognostic value of tumor mutation burden in EGFR-mutant advanced lung adenocarcinoma, an analysis based on cBioPortal data base. J Thorac Dis 11(11):4507–4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachaliou N, Mayo-de las Casas C, Queralt C, de Aguirre I, Melloni B, Cardenal F et al (2015) Association of EGFR L858R mutation in circulating free DNA with survival in the EURTAC trial. JAMA Oncol 1(2):149–157 [DOI] [PubMed] [Google Scholar]
- Ke EE, Zhou Q, Zhang QY, Su J, Chen ZH, Zhang XC et al (2017) A higher proportion of the EGFR T790M mutation may contribute to the better survival of patients with exon 19 deletions compared with those with L858R. J Thorac Oncol 12(9):1368–1375 [DOI] [PubMed] [Google Scholar]
- Kuan FC, Kuo LT, Chen MC, Yang CT, Shi CS, Teng D et al (2015) Overall survival benefits of first-line EGFR tyrosine kinase inhibitors in EGFR-mutated non-small-cell lung cancers: a systematic review and meta-analysis. Br J Cancer 113(10):1519–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Petri ET, Halmos B, Boggon TJ (2008) Structure and clinical relevance of the epidermal growth factor receptor in human cancer. J Clin Oncol 26(10):1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SC, Chooback N, Ho C, Melosky B (2019) Outcome differences between first- and second-generation EGFR inhibitors in advanced EGFR mutated NSCLC in a Large population-based cohort. Clin Lung Cancer 20(5):e576–e583 [DOI] [PubMed] [Google Scholar]
- Lee DH (2017) Treatments for EGFR-mutant non-small cell lung cancer (NSCLC): the road to a success, paved with failures. Pharmacol Ther 174:1–21 [DOI] [PubMed] [Google Scholar]
- Li Y, Appius A, Pattipaka T, Feyereislova A, Cassidy A, Ganti AK (2019) Real-world management of patients with epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer in the USA. PLoS ONE 14(1):e0209709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang L, Jiang D, Wang Y, Zang A, Ding C et al (2020) Routinedose and high-dose Icotinib in advanced non-small cell lung cancer patients harboring EGFR Exon 21 L858R mutation: the randomized, Phase II, INCREASE Trial. Clin Cancer Res. 10.1158/1078-0432.CCR-19-3064 [DOI] [PubMed] [Google Scholar]
- London M, Gallo E (2020) Epidermal growth factor receptor (EGFR) involvement in epithelial-derived cancers and its current antibody-based immunotherapies. Cell Biol Int 44:1267–1282 [DOI] [PubMed] [Google Scholar]
- Marquez-Medina D, Popat S (2016) Eventual role of EGFR-tyrosine kinase inhibitors in early-stage non-small-cell lung cancer. Future Oncol 12(6):815–825 [DOI] [PubMed] [Google Scholar]
- Massarelli E, Johnson FM, Erickson HS, Wistuba II, Papadimitrakopoulou V (2013) Uncommon epidermal growth factor receptor mutations in non-small cell lung cancer and their mechanisms of EGFR tyrosine kinase inhibitors sensitivity and resistance. Lung Cancer 80(3):235–241 [DOI] [PubMed] [Google Scholar]
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N et al (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361(10):947–957 [DOI] [PubMed] [Google Scholar]
- Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S et al (2018) Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol 36(22):2244–2250 [DOI] [PubMed] [Google Scholar]
- Nagano T, Tachihara M, Nishimura Y (2018) Mechanism of resistance to epidermal growth factor receptor-tyrosine kinase inhibitors and a potential treatment strategy. Cells 7(11):212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz-Ares L et al (2019) Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20(12):1655–1669 [DOI] [PubMed] [Google Scholar]
- Offin M, Rizvi H, Tenet M, Ni A, Sanchez-Vega F, Li BT et al (2019) Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with egfr-mutant lung cancers. Clin Cancer Res 25(3):1063–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304(5676):1497–1500 [DOI] [PubMed] [Google Scholar]
- Papadimitrakopoulou VA, Han JY, Ahn MJ, Ramalingam SS, Delmonte A, Hsia TC et al (2020) Epidermal growth factor receptor mutation analysis in tissue and plasma from the AURA3 trial: osimertinib versus platinum-pemetrexed for T790M mutation-positive advanced non-small cell lung cancer. Cancer 126(2):373–380 [DOI] [PubMed] [Google Scholar]
- Park K, Tan EH, O'Byrne K, Zhang L, Boyer M, Mok T et al (2016) Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 17(5):577–589 [DOI] [PubMed] [Google Scholar]
- Paz-Ares L, Tan EH, O'Byrne K, Zhang L, Hirsh V, Boyer M et al (2017) Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 28(2):270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y et al (2020) Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 382(1):41–50 [DOI] [PubMed] [Google Scholar]
- Reguart N, Remon J (2015) Common EGFR-mutated subgroups (Del19/L858R) in advanced non-small-cell lung cancer: chasing better outcomes with tyrosine kinase inhibitors. Future Oncol 11(8):1245–1257 [DOI] [PubMed] [Google Scholar]
- Riely GJ, Pao W, Pham D, Li AR, Rizvi N, Venkatraman ES et al (2006) Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 12(3 Pt 1):839–844 [DOI] [PubMed] [Google Scholar]
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13(3):239–246 [DOI] [PubMed] [Google Scholar]
- Roskoski R Jr (2019) Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol Res 139:395–411 [DOI] [PubMed] [Google Scholar]
- Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S et al (2019) Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 20(5):625–635 [DOI] [PubMed] [Google Scholar]
- Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T et al (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31(27):3327–3334 [DOI] [PubMed] [Google Scholar]
- Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y et al (2014) Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 15(11):1236–1244 [DOI] [PubMed] [Google Scholar]
- Shi YK, Wang L, Han BH, Li W, Yu P, Liu YP et al (2017) First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol 28(10):2443–2450 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34 [DOI] [PubMed] [Google Scholar]
- Sordella R, Bell DW, Haber DA, Settleman J (2004) Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 305(5687):1163–1167 [DOI] [PubMed] [Google Scholar]
- Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH et al (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378(2):113–125 [DOI] [PubMed] [Google Scholar]
- Sutiman N, Tan SW, Tan EH, Lim WT, Kanesvaran R, Ng QS et al (2017) EGFR mutation subtypes influence survival outcomes following first-line gefitinib therapy in advanced asian NSCLC patients. J Thorac Oncol 12(3):529–538 [DOI] [PubMed] [Google Scholar]
- Thress KS, Paweletz CP, Felip E, Cho BC, Stetson D, Dougherty B et al (2015) Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 21(6):560–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MF, Chang TH, Wu SG, Yang HY, Hsu YC, Yang PC et al (2015) EGFR-L858R mutant enhances lung adenocarcinoma cell invasive ability and promotes malignant pleural effusion formation through activation of the CXCL12-CXCR4 pathway. Sci Rep 5:13574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Li J (2019) Second-generation EGFR and ErbB tyrosine kinase inhibitors as first-line treatments for non-small cell lung cancer. Onco Targets Ther 12:6535–6548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Tsui ST, Liu C, Song Y, Liu D (2016) EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer. J Hematol Oncol 9(1):59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZF, Ren SX, Li W, Gao GH (2018) Frequency of the acquired resistant mutation T790 M in non-small cell lung cancer patients with active exon 19Del and exon 21 L858R: a systematic review and meta-analysis. BMC Cancer 18(1):148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee P, Wang Z (2017) Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 9(5):52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y et al (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15(2):213–222 [DOI] [PubMed] [Google Scholar]
- Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S et al (2017) Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 18(11):1454–1466 [DOI] [PubMed] [Google Scholar]
- Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N et al (2015) Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 16(2):141–151 [DOI] [PubMed] [Google Scholar]
- Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M et al (2007) Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 11(3):217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaric B, Stojsic V, Kovacevic T, Sarcev T, Tepavac A, Jankovic R et al (2014) Clinical characteristics, tumor, node, metastasis status, and mutation rate in domain of epidermal growth factor receptor gene in serbian patients with lung adenocarcinoma. J Thorac Oncol 9(9):1406–1410 [DOI] [PubMed] [Google Scholar]
- Zeng L, Xiao L, Jiang W, Yang H, Hu D, Xia C et al (2020) Investigation of efficacy and acquired resistance for EGFR-TKI plus bevacizumab as first-line treatment in patients with EGFR sensitive mutant non-small cell lung cancer in a Real world population. Lung Cancer 141:82–88 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sheng J, Kang S, Fang W, Yan Y, Hu Z et al (2014) Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis. PLoS ONE 9(9):e107161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ma Y, Li Y, Shen X, Yu Y, Pan Y et al (2018) Are exon 19 deletions and L858R different in early stage lung adenocarcinoma? J Cancer Res Clin Oncol 144(1):165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Liu J, Cai X, Pan Z, Liu J, Yin W et al (2019) Efficacy and safety of first line treatments for patients with advanced epidermal growth factor receptor mutated, non-small cell lung cancer: systematic review and network meta-analysis. BMJ 367:l5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Wu YL, Cheng Y, Liu Y, Chen G, Cui J et al (2019) CTONG 1509: Phase III study of bevacizumab with or without erlotinib in untreated Chinese patients with advanced EGFR-mutated NSCLC. Ann Oncol 30:603 [Google Scholar]
- Zhu JQ, Zhong WZ, Zhang GC, Li R, Zhang XC, Guo AL et al (2008) Better survival with EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small cell lung cancer patients is due to differential inhibition of downstream signals. Cancer Lett 265(2):307–317 [DOI] [PubMed] [Google Scholar]