Abstract
Objective
To study the surgical outcomes of patients with a second primary lung cancer after the extrapulmonary malignancy.
Materials and methods
Patients who underwent surgical resection for lung cancers between January 2005 and December 2014 were reviewed. Clinical data, imaging characteristics of tumors, surgical approaches, and outcomes were analyzed with a mean follow-up of 97 months.
Results
Of 1075 patients, 166 (15.4%) had a second primary lung cancer after extrapulmonary malignancy. There were no differences in overall 5-year survival rates (81.8% for the group of lung cancer vs. 72.9% for the second primary lung cancer group, p = 0.069) and 5-year disease-free survival (70.1% for the lung cancer group vs. 70.3% for the second primary lung cancer group, p = 0.863) between the two groups. Gender, performance status, tumor size, and maximum standard uptake value (SUVmax) were significantly different between the two groups. After propensity-score matching analysis, patients in the group with lung cancers had better 5-year overall survival (88.1% vs. 72.1% for the group with second primary lung cancers, p = 0.016) and 5-year disease-free survival (80.6% vs. 70.3% for the group with second primary lung cancers; p = 0.054). In the second primary lung cancer group, the patients with preceding breast or thyroid cancers had better prognoses than did those with other extrapulmonary malignancy.
Conclusions
Second primary lung cancers following extrapulmonary malignancies were not uncommon. Surgical resection is considered for early stage secondary primary lung cancer after meticulous work up and result in fair outcome.
Keywords: Second lung cancer, Surgery, Prognosis
Introduction
Lung cancer remains a leading cause of cancer-related deaths worldwide. With the growth of lung cancer screening programs and the rapid evolution of new drugs, patients with lung cancer now have better outcomes with longer survivals. The incidence of multiple synchronous or metachronous cancers has also increased gradually in clinical practice. Lung cancers at earlier stages have the potential for curative treatment. However, the therapeutic strategy depends not only on tumor stage but also on the patients’ performance status. 11% of patients with first lung cancers were reported to develop a second lung cancer (Rheingold et al. 2000). The incidence of second primary lung cancers after an extrapulmonary malignancy has not been studied well. The therapeutic strategy and survival outcome of patients with lung cancer after an extrapulmonary malignancy are controversial (Hofmann et al. 2007; Duchateau and Stokkel 2005; Reinmuth et al. 2014). Most of these studies revealed the prevalence, therapeutic outcome. In addition, theses study did not stratify the early or advanced stage. With improvements in minimally invasive surgery, the surgical mobility and mortality of patients have decreased. It offered the patient who encountered the lung tumor after extrapulmonary malignancy the opportunity of surgical intervention. There have been limited studies on whether surgical resection is effective for lung cancers arising after an extrapulmonary malignancy. In this study, we evaluated the surgical management and prognosis for patients with primary lung cancers (defined here as “lung cancer group”) and those with a second cancer arising in the lung following an extrapulmonary malignancy (defined here as “second primary lung cancer group”).
Materials and methods
All patients who underwent surgical resection for clinical early stage non-small-cell lung cancers (NSCLCs) at Tri-Service General Hospital, Taiwan, between January 2005 and December 2014 were reviewed retrospectively. This study was approved by the Institutional Review Board of our hospital (TSGHIRB 2-108-05-037). Preoperative staging workups for patients with NSCLC included chest CT scans, positron emission tomography (PET) imaging, and abdominal ultrasonography. Magnetic resonance imaging (MRI) of the brain was indicated for patients with clinical stage II or central stage IB NSCLCs. Patients with an underlying malignancy were evaluated for the primary site condition by multidisciplinary conferences before surgery. We excluded patients who had received neoadjuvant therapy, those with metachronous second lung cancers, non-R0 resection patients, and the same histological type of squamous cell carcinoma (SCC) for both pulmonary and nonpulmonary cancers. Determination of cancer stage was based on the tumor–node–metastasis (TNM) classification (8th edition) of the American Joint Committee on Cancer (Detterbeck et al. 2016).
All images were acquired within a single academic medical center using a Philips multidetector CT system (256-slice single-source CT, Brilliance iCT, Philips, Eindhoven, Netherlands). The images were photographed with a section thickness ranging from 1 to 2.5 mm using both a mediastinal (width 350 Hounsfield units, HU; level 30 HU) and lung window (width 1500 HU; level – 600 HU). Tumor size was measured at the largest cross section by averaging the length and width. The ground-glass opacity (GGO) ratio, defined as an area of slight homogenous increase in density that did not obstruct the underlying vascular marking, was assessed by experienced radiologists (Drs. Hsu, Ko, and Chang).
A total of 1075 patients who underwent surgical resection for a NSCLC were enrolled in this study. Anatomic resections included the procedures of lobectomy, bi-lobectomy, and pneumonectomy. Sublobar resections included wedge resection and segmentectomy. All the resected specimens were reviewed by experienced pathologists to confirm the primary lung cancer, which relied on immunochemical staining of specific markers. The patients’ basic profile, underlying malignancy, and treatment were reviewed from their electronic medical records.
Postoperative surveillance included chest CT scans with contrast and measurements of serum tumor markers carcinoembryonic antigen (CEA) level, and anti-squamous cell carcinoma (anti-SCC) antibodies. Follow-up CT scans were performed for tumor surveillance every 6 months. MRI of the brain was performed as indicated clinically. Relapsed tumors (including locoregional recurrences or distant metastases) were documented with imaging or histopathology diagnosis for all patients.
Statistical analysis
Descriptive data are expressed as the mean ± standard deviation. The Mann–Whitney nonparametric U test was used to investigate continuous variables, and the χ2 test was used to compare categorical variables between groups. Overall survival (OS) and disease-free survival (DFS) rates from the date of surgery were calculated using Kaplan–Meier and Cox regression analyses. The variables were selected for multivariable Cox regression analysis when they showed statistically differences in the χ2 and Mann–Whitney U tests. The Cox proportional hazard analysis was used to estimate the level of significance with relative risks and their 95% confidence intervals. Propensity-score matching (PSM) analysis was applied for reducing the bias caused by confounding variables. Gender, tumor size, maximum standard uptake value (SUVmax) of the tumor, performance status (PS) score, and stage were matched. The matching tolerance level was designated as 0.05, and an Omnibus goodness-of-fit test was performed. SPSS v. 22.0 software (IBM, Armonk, NY, USA) was used for all analyses, and statistical significance was defined as p < 0.05.
Results
Of 1075 patients, 166 patients with second primary lung cancers (15.4%) had a prior extrapulmonary malignancies and 909 (84.6%) with lung cancers patients did not have the underlying malignancies. There were no significant differences in patient’s characteristics expect for gender, performance status, tumor size and SUVmax of tumor between the two groups (Tables 1 and 2). Compared with patients in the lung cancer group, patients in the group of second primary lung cancer were predominantly female (71.7% vs. 61.3% for lung cancer group, p = 0.011), poor performance status (PS score of 0 in 64.5% vs.77.4% for lung cancer group, p = 0.004), smaller tumors (1.73 ± 1.14 cm vs. 2.04 ± 1.46 cm for lung cancer group; p = 0.003) and lower SUVmax values of the tumor (4.00 ± 3.90 vs. 5.18 ± 4.73 for lung cancer group; p = 0.020). For patients with a second primary lung cancer, 95 (57.2%) patients underwent anatomic resection; 60 (55.6%) patients had a mutation in the gene encoding the growth factor receptor (EGFR) in resected tumors; 8 patients (4.8%) presented with N1 disease; 8 patients (4.8%) presented with N2 disease. 45 patients (27.1%) were prescribed with adjuvant chemotherapy, and 10 patients (6%) received adjuvant radiotherapy. The most common histological type of tumor was adenocarcinoma (91.6%). The interval between the treatment of extrapulmonary malignancy and diagnosis of second lung cancer is 52.51 months (0–360.1 months). All the patients were categorized as three groups (interval: 0–2 years, 2–5 years, 5 years. In this study, the interval between the treatment if extrapulmonary malignancy and diagnosis of second lung cancer did not affect the results (Tables 3 and 4).
Table 1.
Characteristics of patients with lung cancers or second lung cancers
| Lung cancer | Second lung cancer | p value | |
|---|---|---|---|
| n = 909 (%) | n = 166 (%) | ||
| Gender | |||
| Male | 352 (38.7) | 47 (28.3) | 0.011 |
| Female | 557 (61.3) | 119 (71.7) | |
| Operation | |||
| Anatomic resection | 580 (63.8) | 95 (57.2) | 0.116 |
| Sublobar resection | 329 (36.2) | 71 (42.8) | |
| Differentiation | |||
| Good | 395 (43.4) | 77 (46.2) | 0.104 |
| Moderate | 368 (40.4) | 73 (43.2) | |
| Poor | 146 (16.3) | 16 (10.7) | |
| EGFR gene status | |||
| Wild-type | 318 (35.0) | 48 (28.9) | 0.701 |
| Mutation | 287 (31.6) | 60 (36.2) | |
| Not tested | 304 (33.4) | 58 (34.9) | |
| N status | |||
| N0 | 813 (89.4) | 150 (90.4) | 0.897 |
| N1 | 44 (4.8) | 8 (4.8) | |
| N2 | 52 (5.7) | 8 (4.8) | |
| Histological type | |||
| Adenocarcinoma | 824 (90.6) | 152 (91.6) | 0.82 |
| Squamous cell Carcinoma (SCC) | 46 (5.1) | 9 (5.4) | |
| Others | 39 (4.3) | 6 (3.0) | |
| Performance status (PS) | |||
| 0 | 704 (77.4) | 107 (64.5) | 0.004 |
| 1 | 173 (19.0) | 51 (30.1) | |
| 2 | 31 (3.4) | 9 (5.4) | |
| 3 | 1 (0.1) | 0 (0) | |
| Location | |||
| Central | 522 (57.4) | 87 (52.4) | 0.234 |
| Peripheral | 387 (42.6) | 79 (47.6) | |
| Adjuvant chemotherapy | |||
| Yes | 288 (31.7) | 45 (27.1) | 0.273 |
| No | 621 (68.3) | 121 (72.9) | |
| Adjuvant radiotherapy | |||
| Yes | 68 (7.5) | 10 (6.0) | 0.626 |
| No | 841 (92.5) | 156 (94.0) | |
| Smoking | |||
| Yes | 173 (19.0) | 24 (14.5) | 0.237 |
| No | 660 (72.6) | 131 (78.9) | |
| Ex-smoker | 76 (8.4) | 11 (6.6) | |
| Lymphovascular space invasion (LVSI) | |||
| Absent | 843 (92.7) | 154 (93.3) | 0.871 |
| Present | 66 (7.3) | 11 (6.7) | |
| Visceral pleural invasion(VPI) | |||
| Absent | 871 (95.8) | 158 (95.8) | 0.971 |
| Present | 38 (4.2) | 7 (4.2) | |
| Stage | |||
| 0 | 28 (3.1) | 7 (4.2) | 0.555 |
| I | 700 (77.0) | 135 (81.3) | |
| II | 92 (10.1) | 9 (5.4) | |
| III | 75 (8.3) | 11 (6.6) | |
| IV | 14 (1.5) | 4 (2.4) | |
Statistical significance is defined as p < 0.05 (Bold)
Table 2.
Characteristics of patients with lung cancers or second lung cancers
| Variable | Lung cancer n = 909 | Second lung cancer n = 166 | p value |
|---|---|---|---|
| Age (years) | 61.87 ± 10.92 | 63.07 ± 10.60 | 0.189 |
| SUVmax of tumor | 5.18 ± 4.73 | 4.00 ± 3.90 | 0.020 |
| Tumor size (cm) | 2.04 ± 1.46 | 1.73 ± 1.14 | 0.003 |
| CEA (ng/ml) | 4.14 ± 10.09 | 4.11 ± 7.93 | 0.969 |
| Dissected lymph nodes (n) | 11.46 ± 7.01 | 10.74 ± 6.63 | 0.223 |
| FEV1 (%) | 86.80 ± 16.25 | 84.87 ± 13.94 | 0. 189 |
| GGO ratio | 0.48 ± 0.52 | 0.47 ± 0.41 | 0.886 |
Statistical significance is defined as p < 0.05 (Bold)
Table 3.
Characteristics of patients with lung cancer or second lung cancers after PSM analysis
| Lung cancer | Second lung cancer | p value | |
|---|---|---|---|
| n = 165 (%) | n = 166 (%) | ||
| Gender | |||
| Male | 19 (11.5) | 47 (28.3) | < 0.001 |
| Female | 146 (88.5) | 119 (71.7) | |
| Operation | |||
| Anatomic resection | 108 (65.5) | 95 (57.2) | 0.143 |
| Sublobar resection | 57 (34.5) | 71 (42.8) | |
| Differentiation | |||
| Good | 88 (53.3) | 77 (46.4) | 0.362 |
| Moderate | 60 (36.4) | 73 (44.0) | |
| Poor | 17 (10.3) | 16 (9.6) | |
| EGFR gene status | |||
| Wild-type | 39 (23.6) | 48 (28.9) | 0.253 |
| Mutation | 54 (43.6) | 60 (36.1) | |
| Non-test | 72 (32.7) | 58 (34.9) | |
| N Status | |||
| N0 | 149 (90.3) | 150 (90.4) | 0.558 |
| N1 | 5 (3.0) | 8 (4.8) | |
| N2 | 11 (6.7) | 8 (4.8) | |
| Histological type | |||
| Adenocarcinoma | 152 (92.1) | 152 (91.6) | 0.628 |
| Squamous cell carcinoma (SCC) | 6 (3.6) | 9 (5.4) | |
| Others | 7 (4.2) | 5 (3.0) | |
| Performance status (PS) | |||
| 0 | 92 (55.8) | 107 (64.5) | 0.219 |
| 1 | 56 (33.9) | 50 (30.1) | |
| 2 | 16 (9.7) | 9 (5.4) | |
| 3 | 1 (0.6) | 0 (0) | |
| Location | |||
| Central | 72 (43.6) | 87 (52.4) | 0.124 |
| Peripheral | 93 (56.4) | 79 (47.6) | |
| Adjuvant chemotherapy | |||
| Yes | 52 (31.5) | 45 (27.1) | 0.400 |
| No | 113 (68.5) | 121 (72.9) | |
| Adjuvant radiotherapy | |||
| Yes | 12 (7.3) | 10 (6.0) | 0.666 |
| No | 153 (92.7) | 156 (94.0) | |
| Smoking | |||
| Yes | 22 (13.3) | 24 (14.5) | 0.166 |
| No | 139 (84.2) | 131 (78.9) | |
| Ex-smoker | 4 (2.4) | 11 (6.6) | |
| Lymphovascular space invasion (LVSI) | |||
| Absent | 152 (92.1) | 154 (93.3) | 0.833 |
| Present | 13 (7.9) | 11 (6.7) | |
| Visceral pleural invasion (VPI) | |||
| Absent | 163 (98.8) | 158 (95.8) | 0.174 |
| Present | 2 (1.2) | 7 (4.2) | |
| Stage | |||
| 0 | 2 (1.2) | 7 (4.2) | 0.283 |
| I | 139 (84.2) | 135 (81.3) | |
| II | 5 (3.0) | 9 (5.4) | |
| III | 16 (9.7) | 11 (6.6) | |
| IV | 3 (1.8) | 4 (2.4) | |
Statistical significance is defined as p < 0.05 (Bold)
Table 4.
Characteristics of patients with lung cancer or second lung cancers after PSM analysis
| Variable | Lung cancer n = 165 | Second lung cancer n = 166 | p value |
|---|---|---|---|
| Age (years) | 64.62 ± 10.35 | 63.07 ± 10.60 | 0.180 |
| SUVmax of tumor | 2.57 ± 2.80 | 4.00 ± 3.90 | 0.002 |
| Tumor size (cm) | 1.51 ± 0.78 | 1.73 ± 1.14 | 0.045 |
| CEA (ng/ml) | 4.20 ± 9.39 | 4.10 ± 7.93 | 0.922 |
| Dissected lymph nodes (n) | 10.99 ± 6.75 | 10.74 ± 6.63 | 0.741 |
| FEV1 (%) | 85.11 ± 16.60 | 84.87 ± 13.94 | 0.892 |
| GGO ratio | 0.50 ± 0.40 | 0.47 ± 0.41 | 0.619 |
Statistical significance is defined as p < 0.05 (Bold)
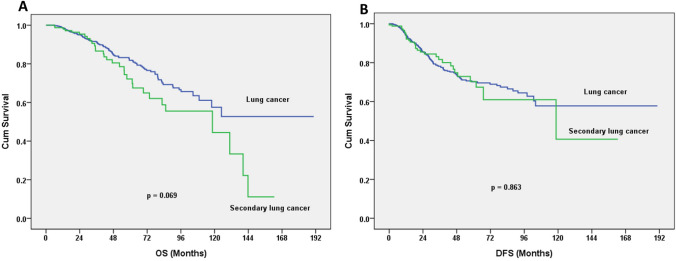
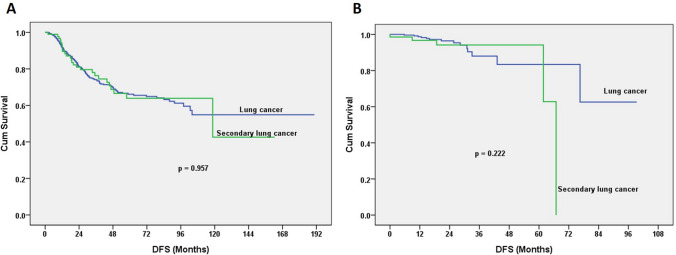
The pathological stage of patients with second primary lung cancers revealed 4.2% with stage 0; 81.3% with stage I; 5.4% with stage II; 6.6% with stage III; and 2.4% with stage IV. Compared with patients of lung cancer without an underlying malignancy, there were no significant differences. The 5-year OS was 81.8% for the group with a lung cancer vs. 72.9% for the group with a second primary lung cancer (log-rank test p = 0.069). The 5-year DFS was 70.1% for the group with a lung cancer vs. 70.3% for the group with a second primary lung cancer (log-rank test; p = 0.863; Fig. 1). 32 patients developed with lung cancer relapse after operation for second lung cancer. Seven of 166 patients developed with extrapulmonary malignancy related relapse (2 breast cancer patients, 3 UCC of bladder). Five Patients died of non-cancer death, including two liver transplantation patients, one sepsis patients, two stoke patients. For patients undergoing anatomic resection, the 5-year OS was 82.0% in the group with a lung cancer vs. 71.8% in the group with a second primary lung cancer (log-rank test; p = 0.063; Fig. 2a). For patients undergoing sublobar resection, the 5-year OS was 82.5% in the group with a lung cancer vs. 73.2% in the group with a second primary lung cancer (log-rank test; p = 0.795; Fig. 2b) When anatomic resection was performed, the 5-year DFS was 66.6% in the group with a lung cancer vs. 63.9% in the group with a second primary lung cancer (log-rank test; p = 0.957; Fig. 3a) For those undergoing sublobar resection, the 5-year DFS was 83.4% in the group with a lung cancer vs. 94.2% in the group with a second primary lung cancer (log-rank test; p = 0.222; Fig. 3b).
Fig. 1.
a Overall survival (OS) curves for the first and second lung cancer groups. The 5-year OS was 81.8% for the lung cancer group, and 72.9% for the second lung cancer group (log-rank test; p = 0.069). b The 5-year disease-free survival (DFS) rates were 70.1% for the lung cancer group vs. 70.3% for the second lung cancer group (log-rank test; p = 0.863)
Fig. 2.
a OS curves after stratifying for surgical procedure. Anatomic resection produced a 5-year OS of 82.0% for the lung cancer group, and 71.8% for the second lung cancer group (log-rank test; p = 0.063). b For those undergoing sublobar resection, there was 5-year OS of 82.5% for the lung cancer group, and 73.2% for the second lung cancer group (log-rank test; p = 0.795)
Fig. 3.
a Disease-free survival (DFS) curves after stratifying for surgical procedure. Anatomic resection gave a 5-year DFS of 66.6% for the lung cancer group, and 63.9% for the second lung cancer group (log-rank test; p = 0.957). b Sublobar resection produced a 5-year DFS of 83.4% for the lung cancer group and 94.2% for the second lung cancer group (log-rank p = 0.222)
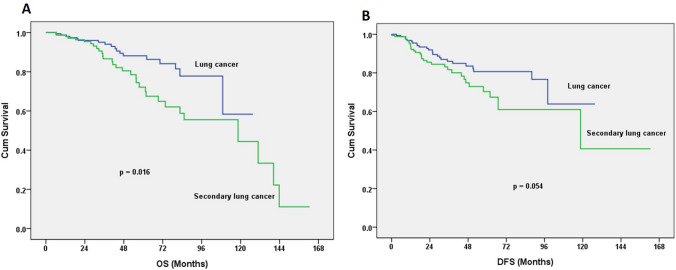
After PSM analysis was performed based on stage, tumor size, gender, PS of patients and SUVmax values of tumors, 165 patients with primary lung cancers were enrolled. Comparisons of the two groups of patients after PSM still showed some characteristic differences in gender distribution, tumor size, and the SUVmax values of tumors. The 5-year OS was 88.1% for the group with a lung cancer vs. 72.9% for the group with a second primary lung cancer (log-rank test; p = 0.016; Fig. 4a). The 5-year DFS was 80.6% for the group with a lung cancer vs. 70.3% for the group with a second primary lung cancer (log-rank test; p = 0.054; Fig. 4b) In this second primary lung cancer group, 71 patients (42.8%) had underlying breast cancers; 28 patients (16.9%) had gastrointestinal (GI) tract malignancies; 20 patients (12.1%) had genitourinary (GU) tract malignancies; 11 patients (6.6%) had thyroid cancers; 8 patients (4.8%) had hepatic malignancies; and 28 patients (16.9%) had other site of malignancies. We clarified the cancer-specific prognoses and surgical approaches. The 5-year OS for patients with breast cancers developing a second cancer in the lungs was 76.7%; 43.1% for patients with GI tract cancers, 86.9% for patients with GU tract cancers, 100% for patients with thyroid cancers, 46.7% for patients with a hepatocellular carcinoma (HCC), and 77.8% for patients with other types of cancer (log-rank test; p = 0.008; Fig. 5a). Likewise, the 5- year DFS for patients with breast cancers developing a second cancer in the lungs was 73.1%, 58.9% for patients with GI tract cancers, 76.9% for patients with GU tract cancers, 87.5% for patients with thyroid cancers, 54.7% for patients with HCC, and 64.4% for patients with other types of cancer (log-rank test; p = 0.498; Fig. 5b).
Fig. 4.
Results are shown after PSM analysis of age, tumor size, and stage. a The 5-year OS was 88.1% for the lung cancer group, and 72.9% for second lung cancer group (log-rank test; p = 0.016). b The 5-year DFS was 80.6% for the lung cancer group and 70.3% for the second lung cancer group (log-rank test; p = 0.054)
Fig. 5.
Cancer-specific survival curves for patients who developed a second lung cancer. a The 5-year OS for patients with breast cancer was 76.7%; 43.1% for those with a GI tract cancer, 86.9% for those with GU tract cancer; 100% for those with thyroid cancer; 46.7% for those with HCC, and 77.8% for those with other types of cancers (log-rank test; p = 0.008). b The 5- year DFS for patients with breast cancer was 73.1%; 58.9% for those with GI tract cancer, 76.9% for those with GU tract cancer; 87.5% for those with thyroid cancer; 54.7% for those with HCC; and 64.4% for those with other types of cancers (log-rank test; p = 0.498)
Multivariable Cox regression analysis revealed that differentiation of tumor was an independent prognostic factor for postoperative relapse with a hazard ratio (HR) of 5.269 after adjusting for other factors in patients with second primary lung cancers. A SUVmax value > 3.3 (HR of 2.157) revealed marginal significance for postoperative relapse. The EGFR gene mutation status, tumor size, surgical procedure, and N1 lymph node status had no significant influence on postoperative relapse (Table 5). No patients were prescribed with immunotherapy in enrolled patients. In second lung cancer group, 7 patients were prescribed with EGFR target therapy.
Table 5.
Multivariable Cox-regressions model for second lung cancer patients with or without relapse
| Factor | OR (95% CI) | p value |
|---|---|---|
| SUVmax > 3.3 | 2.157 (0.923–5.042) | 0.076 |
| Tumor size > 2 cm | 1.369 (0.597–3.142) | 0.458 |
| Differentiation | ||
| Good | Reference | |
| Moderate | 4.262 (1.527–11.901) | 0.006 |
| Poor | 5.269 (1.408–19.714) | 0.014 |
| LVSI | 0.845 (0.330–2.167) | 0.845 |
| EGFR mutation | 0.599 (0.291–1.234) | 0.164 |
| Operation | ||
| Anatomic resection | 0.749 (0.194–2.891) | 0.675 |
| Status of N1 node | ||
| Positive | 1.219 (0.509–2.918) | 0.656 |
OR odds ratio, CI confidence interval
Discussion
The incidence of second primary cancers might be expected to rise because of the persisting effects of genetic or behavioral factors, side effects of chemotherapy and radiotherapy, and better prognosis (Preyer et al. 2017). The etiology, therapeutic strategy, and surgical outcomes of patients with lung cancers with prior extrapulmonary malignancies have not been studied well. Metachronous second lung cancers or second lung cancers arising following a diagnosis of a primary head and neck cancer have been reported (Griffioen et al. 2015). Several studies have shown the diversity of the prognosis for patients of lung cancers with a prior extrapulmonary malignancy (Griffioen et al. 2015; Lim et al. 2018; Lu et al. 2016; Hsu et al. 2015; Ha et al. 2015). Most of these studies did not clarify the difference in subgroups of patients with a second tumor either preceding or following an NSCLC. Patients with a second tumor tend to have overall better survival than patients without second primaries (Duchateau and Stokkel 2005). However, the histological subtype and therapeutic strategy (chemotherapy or surgery) have not been studied clearly (Duchateau and Stokkel 2005). Here, the patients’ histological subtypes and surgical procedures were studied to clarify their survival difference. Anatomic or sublobar resection for these patients revealed fair 5-year OS and 5-year DFS for the second lung cancer group, although anatomic resection is the gold standard in the surgical treatment of patients with resectable NSCLCs (Spira and Ettinger 2004). Mortality following surgical resection is most often associated with tumor relapse (Scott et al. 2007). One study showed that this approach had a trend for equal OS and DFS when tumor size was less than two centimeters (Yendamuri et al. 2013). The therapeutic strategy depends not only on stage and tumor size, but also on the comorbidities and performance and status. Lobectomy is still a valid choice for patients with a metachronous secondary primary lung cancer. Sublobar resection showed equivalent outcomes in tumors size more than two centimeters (Yang et al. 2018). However, the role of surgery for patients with lung cancers with a prior extrapulmonary malignancy has not been studies clearly. In our study, there were no significant differences in the GGO ratios of tumors between the two groups of patients. The Cox regression analysis revealed no significant differences in tumor size or GGO ratios of tumor in the patients’ prognosis, although the comorbidity of a prior malignancy could result in a surgeon’s hesitation to proceed with resection. Meticulous assessment, staging workup, and definitive resection still resulted in a fair prognosis among these patients with a second primary lung cancer. In this study, 32 patients developed with lung cancer relapse after operation for second lung cancer. Seven of 166 patients developed with extrapulmonary malignancy related relapse. Five patients died of non-cancer death. It was challenge to clarify the leading cause of these patients. We tried to clarify the issue of underlying malignancy. The results of underlying cancer-specific survival and surgical strategy (anatomic or sublobar resection) were presented.
PET scans are part of the standard workup for the staging of lung cancers. The 18F-fluorodeoxyglucose metabolic activity of tumors measured by SUVmax is associated with prognosis (Cerfolio et al. 2005). PET scans are important in the workup, especially for patients with prior malignancies. Here, there was a significantly lower SUVmax in the second primary cancer group. After PSM analysis there was still a lower SUVmax in the second primary cancer group. The possible reason was that these patients had regular cancer surveillance after treatment for their underlying malignancy. The Cox regression analysis revealed that a SUVmax of > 3.3 had a marginally significant effect on postoperative outcomes but did not predict the prognosis for this group of patients. When patients with an extrapulmonary malignancy developed a subsequent pulmonary nodule or mass, the PET/CT scans provided the surveillance needed before surgical intervention for the lesion.
The risk of developing subsequent primary malignancies is high in patients with lung cancers (8%) (Yang et al. 2018). The incidence of second lung cancers after prior extrapulmonary malignancies is not clear, although the clinical outcomes have been discussed (Lu et al. 2016). Patients with lung cancer had better prognosis compared with those with prior or synchronous malignancies. The median OS was 12.2 months for 99 patients who developed secondary lung cancers following prior malignances; most of these patients had advanced diseases without surgical intervention. The prognosis for these patients was poor. In our present study, we discuss the therapeutic strategy for patients with clinical early stage cancers after extrapulmonary malignancies. All the patients underwent surgical resection of pulmonary malignancies. For patients with a second primary lung cancer, only 57.2% underwent anatomic resection. The size of tumor is 1.73 cm (mean) with GGO ratio of 0.47. In addition, the performance status of two groups was no significantly different (p = 0.219, Table 3). In our institute, the indications of sublobar resection included tumor size less than 2 cm, pure GGO, port-solid nodule with GGO ratio more than 50%. Patients undergo sublobar resection if meet the criteria for both primary lung cancer. Regarding of or second lung cancer, it was challenge for surgeons to make the wise opinion when performing operation for these patients. There was no significant difference of PFS between anatomic or sublobar resection. We revised it in the paragraph of discussion 45 patients (27.1%) received postoperative chemotherapy and 10 patients (6%) received postoperative radiotherapy. The 5-year OS and DFS results were not significantly different between the two groups of patients. However, the 5-year OS was significantly poorer in the second primary lung cancer group after PSM analysis. There was a similar trend in the 5-year DFS for this group of patients, but with marginal significance. The prior malignancies were categorized into six groups. Among the patients with second primary lung cancers, breast cancer was the most common underlying malignancy (42.8%). In an Asian study, the incidence of second primary lung cancers among patients with breast cancers was 1.78% and the incidence of second primary cancers in the lungs was significantly higher in those treated with radiotherapy than in a non-radiotherapy group (Huang et al. 2017). Estrogen plays an important role in lung carcinogenesis and prognosis in lung and breast cancers. Lung cancer patients who received anti-estrogen therapy had a longer cancer-specific survival than those who did not (Hsu et al. 2015). In our present study, radiotherapy and anti-estrogen therapy had no significant differences in the groups. Our studied population with second primary lung cancers had a high percentage of an underlying breast malignancy. The second most common primary cancer type is breast cancer (14.8%) (Preyer et al. 2017). With improvements in hormone therapy and aggressive surveillance work up after breast cancer; these patients had better survival after second lung cancers. The second most common prior malignancy was GI tracts and others (16.9%). Here, SCCs of the head and neck were categorized as “other”. Patients with metachronous second primary lung cancers following a diagnosis of primary head and neck cancers had a poor prognosis compared with those with synchronous primary lung and head and neck cancers (Griffioen et al. 2015). That cigarette smoking contributes to the formation of aerodigestive SCCs was well known. This was the reason for the high incidence of synchronous or metachronous SCCs in the lung and head and neck. However, it was difficult to differentiate the primary from metastatic lesions with the same histology in different locations. In this study, the histology of the second lung cancers after head and neck SCC showed them to be adenocarcinomas. We excluded these patients with SCC for both head/neck and lung to avoid any confusion between second or metastatic neoplasms.
EGFR is a transmembrane receptor tyrosine kinase involved in the signaling pathway regulating cell proliferation, angiogenesis, and invasion (Mok et al. 2009). The role of EGFR gene mutations as predictive factors for tyrosine kinase inhibitor (TKI) therapy in lung adenocarcinoma was established by the Iressa Pan-Asia Study (IPASS trial) (Maemondo et al. 2010). Most EGFR mutations have been found in adenocarcinomas from female patients, nonsmokers, and Asians (Paez et al. 2004), The percentage of EGFR mutations was the same in both groups of patients. After PSM analysis, the result was still the same. The EGFR mutation is an indicator for TKI therapy; it is not a prognostic factor in lung adenocarcinomas. Mutation status cannot predict postoperative outcomes in second primary lung cancer. In the present study, breast cancer was the majority of underlying malignancies. Molecular studies of the estrogen receptor, progesterone receptor and Human epidermal growth factor receptor 2 (HER2) are important for patients with breast cancers. Women with early stage breast cancers normally received postoperative adjuvant chemotherapy, radiotherapy, hormonal therapy. The role of HER2 medication in the development of lung cancers has not been studied well and needs to be clarified.
This study had some limitations. First, all enrolled patients were operable, and this retrospective study did not analyze patients with nonresectable tumors or advanced disease. Further studies focused with the histopathological characteristics of the tumors and molecular biomarkers might provide more detail results. Second, the decision on the type of operation (anatomic or sublobar resection) was made by individual surgeons, so there was some bias in determining the surgical outcomes. Third, most patients with second lung cancers had preceding breast cancers. It would not be valid to apply these results to different institutions with different patient populations. Fourth, the status of extrapulmonary malignancies played a greater role in patients’ survival. It was challenge to clarify the cancer-specific outcome for patients with multiple cancers. In addition, the advanced molecular medicine and side effect of drug may confound the outcome.
Conclusions
Second primary lung cancers following extrapulmonary malignancies were not uncommon. Surgical resection is considered for early stage secondary primary lung cancer after meticulous work up and result in fair outcome.
Acknowledgements
This research was supported by the Cancer Registry Group, Tri-Service General Hospital. We thank Miss Chia-Ling Yu, who made a significant contribution to analyzing the patients’ survival data.
Funding
The work was supported by the Tri-Service General Hospital (TSGH-C108-112, TSGH-C04-109-031).
Compliance with ethical standards
Conflict of interest
There were no substantial direct or indirect commercial financial incentives associated with this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Brock MV, Alberg AJ, Hooker CM, Kammer AL, Xu L, Roig CM, Yang SC (2004) Risk of subsequent primary neoplasms developing in lung cancer patients with prior malignancies. J Thorac Cardiovasc Surg 127(4):1119–1125 [DOI] [PubMed] [Google Scholar]
- Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA (2005) The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg 130:151–159 [DOI] [PubMed] [Google Scholar]
- Detterbeck FC, Chansky K, Groome PB et al (2016) Staging I, Prognostic Factors Committee Advisory Board. Participating I The IASLC Lung Cancer Staging Project: methodology and validation used in the development of proposals for revision of the stage classification of NSCLC in the forthcoming (eighth) edition of the TNM classification of lung cancer. J Thorac Oncol. 11:1433–1446 [DOI] [PubMed] [Google Scholar]
- Duchateau CS, Stokkel MP (2005) Second primary tumors involving non-small cell lung cancer: prevalence and its influence on survival. Chest 127:1152–1158 [DOI] [PubMed] [Google Scholar]
- Gazdar AF (2009) Personalized medicine and inhibition of EGFR signaling in lung cancer. N Engl J Med 361(10):1018–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffioen GH, Louie AV, de Bree R, Smit EF, Paul MA, Slotman BJ, Leemans CR, Senan S (2015) Second primary lung cancers following a diagnosis of primary head and neck cancer. Lung Cancer 88(1):94–99 [DOI] [PubMed] [Google Scholar]
- Ha D, Choi H, Chevalier C, Zell K, Wang XF, Mazzone PJ (2015) Survival in patients with metachronous second primary lung cancer. Ann Am Thorac Soc 12(1):79–84 [DOI] [PubMed] [Google Scholar]
- Hofmann HS, Neef H, Schmidt P (2007) Primary lung cancer and extrapulmonary malignancy. Eur J Cardiothorac Surg 32:653–658 [DOI] [PubMed] [Google Scholar]
- Hsu LH, Feng AC, Kao SH, Liu CC, Tsai SY, Shih LS, Chu NM (2015) Second primary lung cancers among breast cancer patients treated with anti-estrogens have a longer cancer-specific survival. Anticancer Res 35(2):1121–1127 [PubMed] [Google Scholar]
- Huang YJ, Huang TW, Lin FH, Chung CH, Tsao CH, Chien WC (2017) Radiation therapy for invasive breast cancer increases the risk of second primary lung cancer: a nationwide population-based cohort analysis. J Thorac Oncol 12(5):782–790 [DOI] [PubMed] [Google Scholar]
- Lim MC, Won YJ, Lim J, Salehi T, Yoo CW, Bristow RE (2018) Second primary cancer after primary peritoneal, epithelial ovarian, and fallopian tubal cancer: a retrospective study. BMC Cancer 18(1):800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MS, Chen MF, Huang YK, Liu HP, Tsai YH (2016) Clinical outcome in lung cancer with a second malignancy: the time sequence matters. Medicine (Baltimore) 95:e5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362(25):2380–2388 [DOI] [PubMed] [Google Scholar]
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P et al (2009) Gefitinib or carboplatin paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–995 [DOI] [PubMed] [Google Scholar]
- Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304(5676):1497–1500 [DOI] [PubMed] [Google Scholar]
- Preyer O, Concin N, Obermair A, Concin H, Ulmer H, Oberaigner W (2017) The relative risk of second primary cancers in Austria’s western states: a retrospective cohort study. BMC Cancer. 17(1):699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinmuth N, Stumpf P, Stumpf A et al (2014) Characteristics of lung cancer after a previous malignancy. Respir Med 108:910–917 [DOI] [PubMed] [Google Scholar]
- Rheingold SR, Neugut AI, Meadows AT (2000) Second cancers: incidence, risk factors and management. In: Bast RC, Kufe DW, Pollock RE, et al. (eds) Cancer medicine. B.C. Decker, Hamilton, pp 2399–2406 [Google Scholar]
- Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K, American College of Chest Physicians (2007) Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines. Chest 132:234S–242S [DOI] [PubMed] [Google Scholar]
- Spira A, Ettinger DS (2004) Multidisciplinary management of lung cancer. N Engl J Med 350:379–392 [DOI] [PubMed] [Google Scholar]
- Yang X, Zhan C, Li M, Huang Y, Zhao M, Yang X, Lin Z, Shi Y, Jiang W, Wang Q (2018) Lobectomy versus sublobectomy in metachronous second primary lung cancer: a propensity score study. Ann Thorac Surg 106(3):880–887 [DOI] [PubMed] [Google Scholar]
- Yendamuri S, Sharma R, Demmy M, Groman A, Hennon M, Dexter E, Nwogu C et al (2013) Temporal trends in outcomes following sublobar and lobar resections for small (≤ 2 cm) non-small cell lung cancers—a Surveillance Epidemiology End Results database analysis. J Surg Res 183(1):27–32 [DOI] [PubMed] [Google Scholar]