Abstract
Purpose
Multiple lung lesions found in a single patient at the time of diagnosis often pose a diagnostic dilemma: are these lesions independent primary tumors (IPT) or the result of intrapulmonary metastases (IPM)? While traditional pathological methods sometimes have difficulty distinguishing IPM from IPT, modern molecular profiling based on next-generation sequencing techniques may provide a new strategy.
Methods
Sixteen patients with multiple tumors were enrolled in this study. We performed targeted deep sequencing (~ 2000 × coverage) on a total of 40 tumors and matched blood samples. We compared mutational profiles between tumors within each patient and across patients to evaluate if they were genetically related. Computed tomographic images and histological staining were also used to validate tumor relationships.
Results
A total of 125 mutations were identified in 16 patients. Twelve out of fourteen patients whose histological diagnoses favored IPT did not have any shared mutations in their multiple tumors. The other two showed discrepancies: Pt01 had a shared EGFR exon19 deletion in the two lung tumors found, and Pt16 had one common mutation (BRAFD594G) in two out of five lung tumors. Pt14 with lung metastasis from salivary gland adenoid cystic carcinoma had shared mutations; and Pt15 with suspected intrapulmonary metastasis (IPM) had identical mutations between the two tumors. Visualized data can be readily accessed through the website: mlc.opengene.org.
Conclusion
Analysis of overlapping mutations among different tumors assists physicians in distinguishing IPM from IPT. Our findings demonstrate that DNA sequencing can provide additional evidence in clinical practice when pathology is inadequate to make a conclusive diagnosis.
Electronic supplementary material
The online version of this article (10.1007/s00432-020-03227-5) contains supplementary material, which is available to authorized users.
Keywords: Independent primary tumors, Intrapulmonary metastasis, Targeted deep sequencing
Background
Lung cancer is one of the most genetically heterogeneous cancers, second only to malignant melanoma (Lawrence et al. 2013). While early screening with low-dose computed tomography (CT) has been shown to reduce lung cancer mortality (Team TNLSTR 2011), accurate diagnosis for early lung cancers remains challenging, especially when multiple pulmonary tumors are found (Pomplun 2006; Macmahon et al. 2017). For example, some of these tumors are actually unrelated even when histological images looks similar, and could be diagnosed either as independent primary tumors (IPT) or as intrapulmonary metastases (IPM). Patients with IPT and IPM have distinct staging and require distinct therapeutic strategies (Chen et al. 2018a; Fabian et al. 2011). Currently in clinics, pathologists use Martini–Melamed criteria(Martini and Melamed 1975) and modified versions of it to distinguish IPT and IPM, which was reported to have 90% consistency with molecular characterization in one study (Girard et al. 2009). However, histological diagnoses rely heavily on the training and experience of pathologists; while, molecular genomic profiling should be more objective. The development of Next Generation Sequencing (NGS) techniques has allowed genomic analysis to be used in translational and clinical research. A previous study on only six patients indicated that mutation profiles of individual tumors were different in IPT (Liu et al. 2016); but IPM samples were not examined in this study. Using NGS techniques to distinguish mutational profiles of IPT and IPM has yet to be investigated. We hypothesized that IPT would not have overlapping mutations, while IPM would share common mutations. To this end, we performed targeted deep sequencing (~ 2000 ×) to assess mutational profiles of 40 tumors from 16 patients (Table 1). Through comparison of NGS results with the corresponding pathology reports and CT images, we have confirmed that mutational profiling can help distinguishing IPM from IPT in lung.
Table 1.
.
| Sample no. | Sex/age | Clinical conditions | Location | Size/mm | Type | Smoking history | Pathological TNM | Clinical prediction | Genomic prediction |
|---|---|---|---|---|---|---|---|---|---|
| Pt01 | |||||||||
| T1 | F/74 | None | Right breast | 20 | DCIS | Never smoker | T2b(2)N0M0 | Independent primary | |
| T2 | RUL | 40 | IA | Related | |||||
| T3 | LUL | 20 | IA | ||||||
| Pt02 | |||||||||
| T1 | M/62 | Coughing with blood | RUL | 18 | IA | > 30 pack-years | T3(2)N0M0 | Independent primary | Unrelated |
| T2 | Left liver | 57 | HCC | ||||||
| T3 | LLL | 55 | Carcinosarcoma | ||||||
| Pt03 | |||||||||
| T1 | M/34 | None | Left thyroid | 24 | Papillary | Never smoker | Tis(2)N0M0 | Independent primary | Unrelated |
| T2 | RLL | 5 | AIS | ||||||
| T3 | RUL | 6 | AIS | ||||||
| Pt04Pt04 | |||||||||
| T1 | M/46 | None | LLL | 2.5 | AAH | Former smoker | T1mi(3)N0M0 | Independent primary | Unrelated |
| T2 | LLL | 4.5 | MIA | ||||||
| T3 | LUL | 4.5 | MIA | ||||||
| Pt05 | |||||||||
| T1 | F/54 | Coughing with sputum | RUL | 7 | AIS | Never smoker | T1a(2)N0M0 | Independent primary | Unrelated |
| T2 | RML | 7 | IA | ||||||
| Pt06 | |||||||||
| T1 | F/55 | None | RUL | 5 | MIA | Never smoker | T1mi(3)N0M0 | Independent primary | Unrelated |
| T2 | RUL | 4 | MIA | ||||||
| T3 | RLL | 5 | MIA | ||||||
| Pt07 | |||||||||
| T1 | F/58 | None | LLL | 17 | IA | Never smoker | T1b(2)N0M0 | Independent primary | Unrelated |
| T2 | LUL | 8 | IA | ||||||
| Pt08 | |||||||||
| T1 | F/59 | Chest pain for 20 yrs | RLL | 6 | IA | Never smoker | T1a(2)N0M0 | Independent primary | Unrelated |
| T2 | RUL | 4 | IA | ||||||
| Pt09 | |||||||||
| T1 | M/52 | Coughing | RML | 2.5 | MIA | Never smoker | T1b(2)N0M0 | Independent primary | Unrelated |
| T2 | RUL | 22 | IA | ||||||
| Pt10 | |||||||||
| T1 | F/53 | None | RUL | 10 | IA | Never smoker | T1b(2)N0M0 | Independent primary | Unrelated |
| T2 | RLL | 18 | IA | ||||||
| Pt11 | |||||||||
| T1 | F/66 | None | RML | 10 | IA | Never smoker | T1a(2)N0M0 | Independent primary | Unrelated |
| T2 | RUL | 5.5 | AIS | ||||||
| Pt12 | |||||||||
| T1 | M/58 | None | RLL | 5 | AIS | Never smoker | T1a(2)N0M0 | Independent primary | Unrelated |
| T2 | LLL | 8 | IA | ||||||
| Pt13 | |||||||||
| T1 | F/63 | None | RLL | 17 | IA | Never smoker | T1b(2)N0M0 | Independent primary | Unrelated |
| T2 | RUL | 8 | AIS | ||||||
| Pt14 | |||||||||
| T1 | F/60 | RUL | 11 | ACC | Never smoker | Metastasis | Lung metastasis | Related | |
| T2 | Mouth | 33 | ACC | ||||||
| Pt15 | |||||||||
| T1 | F/65 | Hypertension for 20 yrs | RUL | 2 | IA | Never smoker | T2a(2)N2M1 | Undetermined, favor IPM | Related |
| T2 | RML | 35 | IA | ||||||
| Pt16 | |||||||||
| T1 | F/62 | None | RLL | 5 | AIS | Never smoker | T1(5)N0M0 | Independent primary | Related |
| T2 | RLL | 9 | AIS(MIA) | ||||||
| T3 | RML | 6 | AAH | ||||||
| T4 | RML | 5 | AIS | ||||||
| T5 | RML | 8 | AIS(MIA) | ||||||
| L1 | No malignant cells | ||||||||
RUL right upper lobe, RML right middle lobe, RLL right lower lobe, LUL left upper lobe, LLL left lower lobe, DCIS ductal carcinoma in situ, HCC hepatocellular carcinoma, AIS adenocarcinoma in situ, AD adenocarcinoma, MIA minimally invasive adenocarcinoma, IA invasive adenocarcinoma, AAH atypical adenomatous hyperplasia, ACC adenoid cystic carcinoma
Patients and methods
Patients
Sixteen patients (Table 1) with multiple lesions were selected for this study. Clinical samples were obtained through the Peking University Shenzhen Hospital Institutional Review Board-approved informed consent process; and no procedures were conducted for the exclusive purpose of research. Among the 16 patients, two patients (Pt01 and Pt02) were also found to have tumors at other sites at the time of lung tumor diagnosis; Pt03 had a history of thyroid cancer; and Pt14’s lung cancer was diagnosed to be lung metastasis of salivary gland adenoid cystic carcinoma. All other patients were found to have multiple lung lesions.
Sample processing
Tumor tissue DNA was isolated from Formalin-fixed paraffin-embedded (FFPE) samples with the QIAamp DNA FFPE tissue kit (Qiagen), blood DNA from white blood cells (WBC) was extracted with the RelaxGene Blood DNA system (Tiangen Biotech), and cell-free DNA from plasma was isolated with the QIAamp circulating nucleic acid kit (Qiagen). Extracted DNA was fragmented using enzyme dsDNA Fragmentase (New England Biolabs) and quantified using the Qubit 2.0 fluorometer (Thermo Fisher Scientific). Sequencing libraries were prepared with the KAPA library prep kit (Kapa Biosystems) following the manufacturer’s protocol. Targeted enrichment was performed with a 464-gene panel (HaploX, see Table S1 for full gene list). DNA libraries were sequenced using 150-bp paired-end runs on the NovaSeq 6000 system (Illumina).
Bioinformatic analysis
Sequencing data were filtered by fastp v0.18.0 (Chen et al. 2018c) and aligned to the hg19 genome (GRch37) using Burrows–Wheeler Aligner (Li and Durbin 2010) v0.7.15-r1140 with default settings. Duplicated reads were removed by Gencore v0.12.0 (Chen et al. 2019). We used Samtools v0.1.19 to generate pileup files of properly paired reads with mapping quality ≥ 60 (Li et al. 2009). Somatic variants were called using VarScan2 v2.3.8 (Koboldt et al. 2012) with the following parameters: the minimum read depth at a position = 20, variant allele frequency (VAF) threshold ≥ 0.01, strand–filter ≥ 1; otherwise default.
Results
Distribution of somatic mutations
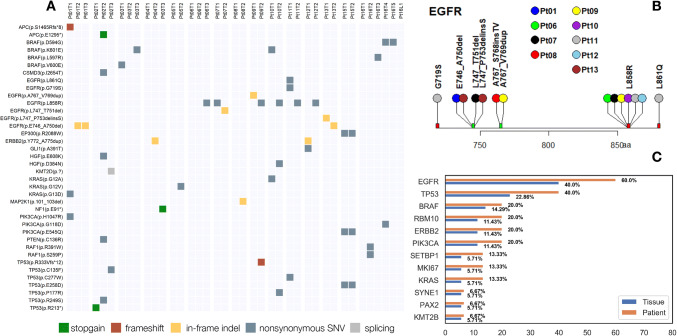
We analyzed 40 tumors and 1 lymph node from 16 patients (Table 1). A total of 125 nonsynonymous mutations (Table S2) were identified in 35 tumors, including 97 single nucleotide variants (SNVs), 13 in-frame indels, 12 frameshift mutations and 3 aberrant splicing isoforms. Figure 1a depicts the mutation types and distributions in canonical cancer related genes across all samples. One carcinosarcoma (Pt02T3) was found to have the highest number (20) of mutations, probably due to Pt02’s history of heavy smoking (Figure S1) (Govindan et al. 2012). Five tumors (Pt03T2, Pt04T1, Pt05T1, Pt06T1 and Pt16T1) had no mutations identified in the panel (Fig. 1a and Figure S1), presumably because of a small tumor size and low tumor purity. EGFR (Fig. 1b) was the most frequently mutated gene both in tissues (n = 13, 37.14%) and in patients (n = 9, 60%). The ten most frequently mutated genes in the lung cancer samples examined (Pt14, Pt01T1, Pt02T2, Pt03T1 were excluded) are shown in Fig. 1c. Mutations were also frequently found in these genes in a published report (Sweeney et al. 2017). Note that different tumors from a single individual often harbor different mutations, leading to higher mutation frequency calculated by patient than by tumor. Due to this high tumor heterogeneity, extra caution should be exercised when interpreting gene testing for diagnosis and targeted therapy.
Fig. 1.
Mutational profiles in canonical genes. a Nonsynonymous mutations in known cancer genes in 15 lung cancer patients with multiple tumors. bEGFR mutations in all samples. c The ten most frequently mutated genes in lung tumors. Mutation frequencies are shown by patient and by tissue
Discussion
Mutational heterogeneity in independent primary tumors (IPT)
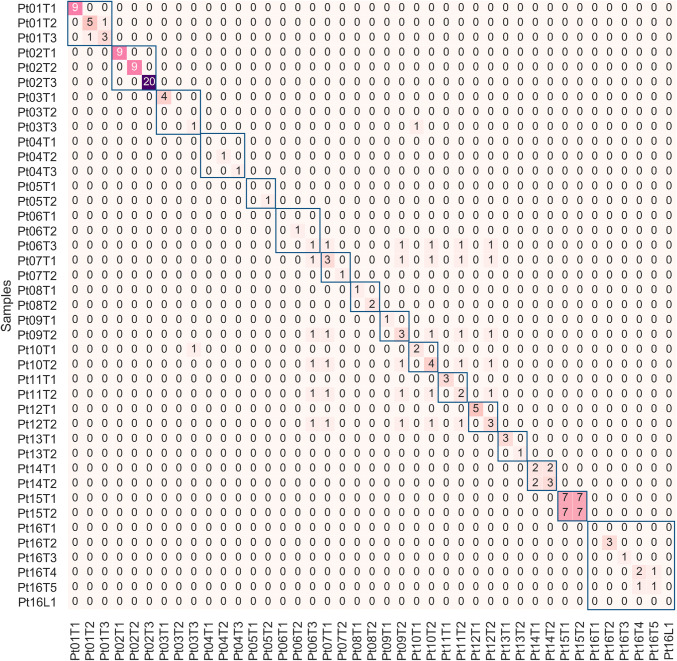
All patients exhibited either no symptoms or mild symptoms when their lung tumors were found by preventative medical examination. Fourteen patients (Table 1, Pt01–Pt13 and Pt16) were clinically predicted to have multiple IPT based on tumor size, location, and histology (see Figure S2 for representative images); and thus were classified as either Stage I or Stage II according to TNM standards. NGS results identified no shared mutations in 12 (Pt02–Pt13, Fig. 2) of the 14 patients with IPT. The patients’ distinct mutational profiles supported our hypothesis that IPT would not have overlapped mutations, as they arose from different clones. Similar results were also reported in a previous study with a smaller cohort (Liu et al. 2016).
Fig. 2.
Similarity among different lesions within each individual. The heatmap shows the mutations shared by 40 tumors and one lymph node (Pt16L1) from 16 patients with multiple lung lesions. Mutation numbers are shown and tumors from the same patients are indicated by blue boxes. NGS identified shared mutations in several pathologically independent tumors
In Pt01, the pathologist found the T2 (RUL) tumor to have a glandular, papillary, and nest-like structure; and the T3 (LUL) tumor to have an acinar structure. Based on these different morphologies, the pathologist inferred that they were IPT. However, DNA sequencing revealed that EGFR p.E746_A750del was shared by both tumors. This mutation was reported to be mutated in 6.95% of lung adenocarcinoma patients (Sweeney et al. 2017). Although the presence of this mutation alone was not sufficient to establish a metastatic relationship of these two tumors, the sequencing results suggested that they were related. We believe that clinical follow-up with extra attention should be given to such patients.
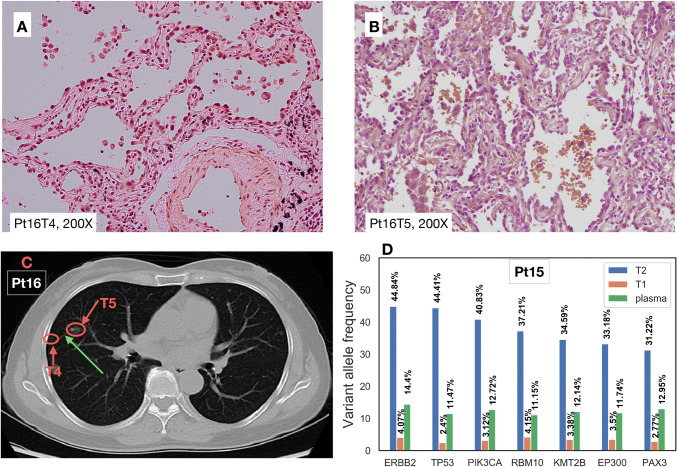
Another discrepancy was found in Pt16. Five lung tumors and several lymph nodes were removed during surgery. No cancer cells were identified in the lymph nodes. The patient’s tumors were pathologically classified as adenocarcinoma in situ (AIS), but minimally invasive adenocarcinoma (MIA) could not be ruled out (see Table 1). NGS showed that the BRAFD594G mutation was shared between T4 and T5; while, no shared mutation was found in other tumors. The BRAFD594G mutation was previously reported to be present in only 0.16% of all non-small cell lung cancer patients (Sweeney et al. 2017). As all the other IPT in our cohort did not share any mutations, we speculated that T4 and T5 were related in some way. Histological examination (Fig. 3a, b) illustrated that the tumor cells lined the surface of the alveolar airways. After carefully reviewing the CT images (Fig. 3c), we found a blood vessel connecting the two tumors (green arrow in Fig. 3c). Considering that the airways (trachea, bronchus, bronchiole) were accompanied by blood vessels, that no mutations were identified in lymph nodes, and that no blood vessel or lymph node invasion was identified in histology, aerogenous metastasis was likely. Aerogenous metastasis is a unique type of metastasis in the lung (Li et al. 2018; Gaikwad et al. 2014), and therefore staging of this patient should reflect the likelihood of aerogenous metastasis. It was also reported previously that aerogenous metastasis without lymph node invasion has a much more favorable prognosis than other stage III lung cancers (Aokage et al. 2007). This clinical case demonstrated the significance of combining pathology, imaging, and NGS results for accurate diagnosis.
Fig. 3.
Patients with intrapulmonary metastasis. a, b Histological images of Pt16T4 and Pt16T5 (HE staining). c One CT image slice from Pt16 showing the blood vessel between T4 and T5. d Seven mutations identified in Pt15T1, Pt15T2, and Pt15 plasma
NGS helps confirming intrapulmonary metastasis (IPM)
Pt15 had one small tumor (T1, 2 mm) and one large invasive adenocarcinoma (T2, 35 mm) with lymph node metastasis and the CT scan did not identify other lesions in the patient’s body. The pathologist therefore suspected the small tumor to be ipsilateral metastasis, but with no confirmative evidence. DNA sequencing revealed identical mutational profiles (Fig. 2, Figure S1) in these two tumors in different lobes, strongly indicating that they are related. To evaluate systemic disease in the patient in the absence of evidence of such from CT images, plasma cell-free DNA was also sequenced using the same panel with an average depth of around 20,000 ×. Circulating tumor DNA (ctDNA) was found in the plasma, suggesting vascular invasion and uncontrolled systemic disease. Variant allele fractions in each patient sample are shown in Fig. 3d. A total of 7 mutations were identified in both tumors, as well as in plasma. As T1 was only 2 mm in size, normal tissue contamination was inevitably introduced during sampling, leading to very low VAF in the sequencing result.
Previous studies on differentiating multiple lung tumors mostly focused on IPT (Liu et al. 2016; Wu et al. 2015), which were more common at the time of first diagnosis, or IPM with other distant metastases (Murphy et al. 2014). To our knowledge, this is the first report of combined analysis by NGS, pathology, and CT images to identify IPM without symptoms, or distant metastasis, at the time of diagnosis.
Mutational similarity in primary tumors and metastasis
One patient (Pt14) was confirmed by histology to have lung metastasis of ACC from the salivary gland, which was resected 4 years before. As the histology of ACC was distinct from lung adenocarcinoma, this metastasis was unambiguously diagnosed. DNA sequencing results showed that the two mutations present in the metastatic lung sample were both also found in the primary tumor (Fig. 2, Figure S1). This demonstrated the efficacy of using targeted deep sequencing to establish a relationship between the primary tumor and the metastatic growth.
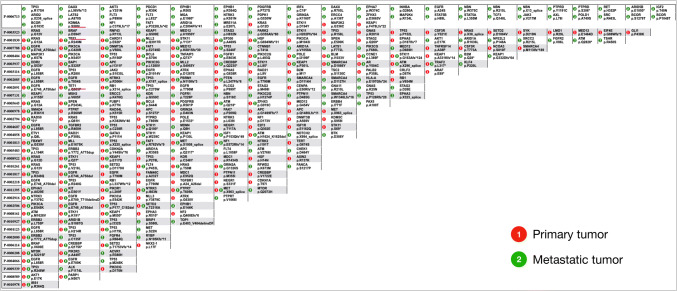
To further validate our hypothesis, we analyzed MSK-IMPACT data (Zehir et al. 2017) with matched primary and metastatic tumors (data available at https://www.cbioportal.org/study/summary?id=msk_impact_2017). In the study, tumors from over 10,000 cancer patients were sequenced using a panel of 410 cancer-associated genes(Cheng et al. 2015) with an average unique depth of 718 ×. In this dataset, we identified 34 lung adenocarcinoma patients with distant metastasis, and compared the point mutations and indels within each patient (Fig. 4). Thirty-one of them (91.2%) have shared mutations between their primary lung tumor and the metastatic growth. We reviewed the clinical information of the three patients (underlined in Fig. 4) with no mutations shared between the primary tumor and metastatic growth, and found that patient P-0006713 had lower DNA input and that only four mutations were identified in primary tumor. We suspect the low DNA input as the reason why no shared mutations were identified. Without detailed pathological information on these patients, it was difficult to judge whether or not the tumors were truly metastatic. Regardless, the results of this analysis strongly support our hypothesis.
Fig. 4.
Mutational comparison of primary lung adenocarcinoma and metastatic growth from MSK-IMPACT study
Mutations with low variant allele frequency (VAF)
Variability in mutation frequencies is often attributed to tumor heterogeneity and normal tissue contamination. Some gene mutations are important in tumorigenesis and early tumor development, but are easily missed because of low VAF. To identify such mutations in early cancers, we utilized targeted deep sequencing to analyze mutational profiles of all the tumors. Among the 125 mutations collectively identified in these samples (Figure S3A), 38 of them occurred at frequencies lower than 5%, and 14 of them were canonical cancer mutations (Figure S3B). The identities of these mutations are not only important for understanding tumorigenesis, but also have clinical significance for selection of the best therapeutic strategy. For example, lung cancers in Asian populations are known to display high EGFR mutation frequencies, especially in non-smokers (Yatabe et al. 2015). Early detection of EGFR mutations would allow patients to receive targeted therapies when applicable.
Conclusions
The increased use of CT has no only improved lung cancer patient survival in general, but also brought new challenges for early clinical diagnosis. The combination of traditional histology and NGS has become a possible solution to these challenges as the cost of NGS decreases. In this pilot study of lung cancer patients with multiple lesions, targeted NGS results can help clinical physicians to confirm suspected IPM, and to identify related tumors when pathology suggests otherwise. The scarcity of data and follow-up clinical information on multiple lung cancers makes this type of data difficult to interpret, making research on multiple lung cancers challenging. We are committed to facilitating data sharing with the greater scientific and clinical communities. Accordingly, we have deposited the results discussed here on the readily accessible website at mlc.opengene.org. This study is a first step in our continuing acquisition and sharing of data on multiple lung cancers. The above website will, therefore, be updated regularly with new data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (PDF 128207 kb)
Acknowledgements
We acknowledge the generous gift of clinical specimens from our patients, to whom we dedicate our work. We thank Jinhua Zhong for clinical sample coordination.
Abbreviations
- IPT
Independent primary tumor
- IPM
Intrapulmonary metastasis
- NGS
Next generation sequencing
- CT
Computed tomography
- ctDNA
Circulating tumor DNA
- VAF
Variant allele frequency
- RUL
Right upper lobe
- LUL
Left upper lobe
- AIS
Adenocarcinoma in situ
- MIA
Minimally invasive adenocarcinoma
- ACC
Adenoid cystic carcinoma
Author contribution
JL, YL, SC and DW designed the study; LT performed the histological examination; JL, GM, XP, JW, XL and RL analyzed and interpreted the patient clinical data; MX and TH carried out the sequencing experiment and collected data; YL, WW, JZ and SC analyzed the bioinformatic data; YL, JL, XL and SC were major contributors in writing manuscript; SC and DW supervised the study.
Funding
We thank grants from the Natural Science Foundation of Guangdong Province of China (2017A030310641), the Medical Scientific Research Foundation of Guangdong Province of China (A2017327) for study design and data collection, the Science and Technology Innovation Committee of Shenzhen Municipality (JCYJ20180228175531145) for data collection and analysis, the Shenzhen Strategic Emerging Industry Development Special Fund (20170922151538732) and the PUHSC-UMHS Joint Institute Project (2019020(PUSH)-r1) for interpretation and writing.
Availability of data and materials
To access the supplementary material accompanying this article, visit the online version of the journal at https://www.springer.com/journal/432/. Raw datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval and consent to participate
Clinical samples were obtained through the Peking University Shenzhen Hospital Institutional Review Board-approved informed consent process. No procedures were conducted for the exclusive purpose of research.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jixian Liu, Guangxian Mao, and Yingmei Li contributed equally to this work.
Contributor Information
Shifu Chen, Email: chen@haplox.com.
Da Wu, Email: wuda0125@hotmail.com.
References
- Aokage K, Ishii G, Nagai K et al (2007) Intrapulmonary metastasis in resected pathologic stage IIIB non-small cell lung cancer: possible contribution of aerogenous metastasis to the favorable outcome. J Thorac Cardiovasc Surg 134(2):386–391 [DOI] [PubMed] [Google Scholar]
- Chen K, Chen W, Cai J et al (2018a) Favorable prognosis and high discrepancy of genetic features in surgical patients with multiple primary lung cancers. J Thorac Cardiovasc Surg 155(1):371–379 [DOI] [PubMed] [Google Scholar]
- Chen S, Zhou Y, Chen Y et al (2019) Gencore: an efficient tool to generate consensus reads for error suppressing and duplicate removing of NGS data. BMC Bioinforma 20(Suppl 23):606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhou Y, Chen Y, Gu J (2018c) Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34(17):i884–i890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Mitchell TN, Zehir A et al (2015) Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 17(3):251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian T, Bryant AS, Mouhlas AL, Federico JA, Cerfolio RJ (2011) Survival after resection of synchronous non-small cell lung cancer. J Thorac Cardiovasc Surg 142(3):547–553 [DOI] [PubMed] [Google Scholar]
- Gaikwad A, Souza CA, Inacio JR et al (2014) Aerogenous metastases: a potential game changer in the diagnosis and management of primary lung adenocarcinoma. Am J Roentgenol 203(6):W570–W582 [DOI] [PubMed] [Google Scholar]
- Girard N, Pao W, Deshpande C et al (2009) Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am J Surg Pathol 33(12):1752–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan R, Ding L, Griffith M et al (2012) Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 150:1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koboldt DC, Zhang Q, Larson DE et al (2012) VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22(3):568–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Polak P et al (2013) Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499:214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R (2010) Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 26(5):589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A et al (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25(16):2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Li X, Xue R et al (2018) Early metastasis detected in patients with multifocal pulmonary ground-glass opacities (GGOs). Thorax 73(3):290–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang J, Li L et al (2016) Genomic heterogeneity of multiple synchronous lung cancer. Nat Commun. 7:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmahon H, Naidich DP, Goo JM et al (2017) Guidelines for management of incidental pulmonary nodules MacMahon et al. Radiol Radiol 284:228–243 [DOI] [PubMed] [Google Scholar]
- Martini N, Melamed MR (1975) Multiple primary lung cancers. J Thorac Cardiovasc Surg 70(4):606–612 [PubMed] [Google Scholar]
- Murphy SJ, Aubry MC, Harris FR et al (2014) Identification of independent primary tumors and intrapulmonary metastases using DNA rearrangements in non-small-cell lung cancer. J Clin Oncol 32(36):4050–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomplun S (2006) Pathology of lung cancer. Lung Cancer 32(4):12–26 [Google Scholar]
- Sweeney SM, Cerami E, Baras A et al (2017) AACR project genie: powering precision medicine through an international consortium. Cancer Discov 7(8):818–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team TNLSTR (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 365(5):395–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Zhao C, Yang Y et al (2015) High discrepancy of driver mutations in patients with NSCLC and synchronous multiple lung ground-glass nodules. J Thorac Oncol. 10(5):778–783 [DOI] [PubMed] [Google Scholar]
- Yatabe Y, Kerr KM, Utomo A et al (2015) EGFR mutation testing practices within the Asia pacific region: results of a multicenter diagnostic survey. J Thorac Oncol 10(3):438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehir A, Benayed R, Shah RH et al (2017) Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23(6):703–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (PDF 128207 kb)
Data Availability Statement
To access the supplementary material accompanying this article, visit the online version of the journal at https://www.springer.com/journal/432/. Raw datasets analyzed during the current study are available from the corresponding author upon reasonable request.