Abstract
Purpose
Approximately 30% of NSCLC patients cannot obtain tissue sample or sufficient tissue sample for molecular subtyping. Cell-free circulating tumor DNA (ctDNA) in plasma is a potential alternative specimen type to assess genomic variants in patients with non-small cell lung cancer (NSCLC). The purpose of this study was to identify the genomic alteration profile of ctDNA in real-world Chinese NSCLC patients.
Methods
A total of 325 subjects with pathological diagnosis of NSCLC were enrolled. 10 ml Peripheral blood was collected in streck tube, and ctDNA NGS analysis was carried out using an Ampliseq-based 11-gene panel.
Results
295 out of 325 patients (90.8%) had detected ctDNA results. In 62.1% (183/295) of these cases, at least one genomic alterations was detected. Frequency altered genes were EGFR (27.8%), TP53 (22.7%), KRAS (21.36%), and PIK3CA (4.75%). EGFR mutation was associated with female, younger age (< 65 years), and adenocarcinoma. The most common mutations in EGFR were L858R (39.4%), exon19 deletions (31.73%), and T790M (18.3%); G13S was the most common alterations in KRAS. TP53 mutation was most occurred in exon7 and exon8. TP53 mutation was significantly more common in patients with history of radiochemotherapy/chemotherapy therapy, and T790M was mainly found in patients with TKIs treatments. Co-existence EGFR mutation with KRAS and different multiple gene co-mutation panels were detected.
Conclusion
In Chinese NSCLC patients, EGFR mutation was significantly associated with female, younger age (< 65 years), and adenocarcinoma. Genomic profiles of NSCLC were associated with the treatment history; TP53 mutation was significantly more frequent in the patients with history of radiochemotherapy/chemotherapy therapy. Various multiple genes co-mutation panels, especially EGFR and KRAS co-mutation, were observed in the ctDNA of Chinese NSCLC patients.
Keywords: Non-small cell lung cancer, Next-generation sequencing, Circulating tumor DNA, Target therapy
Introduction
Lung cancer still remains one of the leading causes of cancer-related mortality worldwide. Non-small cell lung cancer (NSCLC) is the most common type, which accounts for approximately 85% of all lung cancer cases (Bray et al. 2018). Targeted therapy has shown great effectiveness in increasing the survival of advanced NSCLC. Currently, the treatment with matched targeted therapies has become a part of routine clinical practice for NSCLC patients. The National Comprehensive Cancer Network (NCCN) guidelines recommend broad molecular profiling, including screening for the presence of activating alterations in EGFR, ALK, ROS1, MET, ERBB2, and RET to inform selection of effective targeted therapies for NSCLC patients.
In routine clinical practice, the gold standard source for genomic testing is tumor tissue. However, tumor tissue sampling is often unfeasible for different reasons in advanced NSCLC patients. Approximately 30% of them cannot obtain a tissue sample even for biomarker testing, either at diagnosis or at disease progression (Normanno et al. 2017). In addition, repeat tissue biopsy is recommended to evaluate acquired resistance mechanisms in patients whose cancers progress on the first-line target therapy (Etiinger et al. 2015). Tissue biopsy is an invasive procedure not always accept by patients, and does not adequately represent tumor heterogeneity (Gerlinger et al. 2012; Jiang et al. 2016). Circulating tumor DNA (ctDNA) from peripheral blood as a non-invasive option is a useful material to solve those problems. Several studies in different tumor types have demonstrated that ctDNA testing (so-called “liquid biopsy”) can detect the actionable genetic alterations to predict the response of patients to targeted therapies (Denis et al. 2019; Torquato et al. 2019; Kato et al. 2019). Furthermore, ctDNA testing can monitor in real time the genetic status of cancers during treatment to identify mechanisms of resistance (Minari et al. 2018; Jia et al. 2019), and ctDNA may capture a more global picture of tumor heterogeneity than tissue biopsy (Peled et al. 2017; Lebofsky et al. 2015).
Allele-specific PCR, droplet digital PCR (ddPCR), and Amplified Refractory Mutation System (ARMS) PCR are the most commonly used technologies to detect the variants in ctDNA (Del Re et al. 2017). However, those technologies are typically limited to detect specific well-defined mutations in a few genes, and cannot interrogate the full spectrum of mutations that may emerge in the setting of acquired resistance during targeted therapy (Drilon et al. 2015). Next-generation sequencing (NGS) for the detection of ctDNA allows a much broader scope of variants to be identified in each sample on a single platform. Several groups reported the feasibility of mutation detection by clinical plasma-based ctDNA NGS for NSCLC patients (Thompson et al. 2016; Schwaederle et al. 2017). The objective of this study was to identify the genomic alteration profile of ctDNA in real-world Chinese NSCLC patients using an 11-gene Ampliseq-based NGS panel and to evaluate the value of Ampliseq-based NGS ctDNA analysis in real-world NSCLC patients.
Materials and methods
Patients
This retrospective study included 325 NSCLC patients who underwent clinical ctDNA NGS testing in CapitalBio Medlab (Beijing, China) from January 2018 to May 2019. Informed consent was obtained from all patients and all patients in the study had a diagnosis of NSCLC confirmed with pathological examinations by a board-certified pathologist. All patients were initially diagnosed advanced disease or progressed to metastatic disease, or at progression after one or more treatment lines. ctDNA analysis was ordered as clinically indicated by the treating physician of patients. Patients of various ages were included and patients with benign lung tumor or other malignancies were excluded.
Blood samples and cfDNA isolation
Peripheral blood was collected in 10 ml cell-free DNA BCT® blood collection tubes (Streck, La Vista, Nebraska) during routine phlebotomy and shipped at ambient temperature to CapitalBio Medlab (Beijing) no more than 72 h. Blood was centrifuged upon receipt at 1600×g 4 °C for 10 min. The supernatant was transferred to 2 ml sterile Eppendorf tubes (Eppendorf, Hamburg, Germany) and centrifuged at 16000×g 4 °C for 10 min. Plasma was immediately used for ctDNA extraction or stored at − 80 °C until further analysis.
Cell-free DNA (cfDNA) was extracted from 3.5 ml plasma using the MagMax Cell Free DNA isolation kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. cfDNA concentrations were quantified using the Qubit® dsDNA HS assay kit on Qubit fluorometer 3.0 (Life Technologies, Carlsbad, CA, USA). cfDNA quality was confirmed using Bioanalyzer High Sensitivity DNA Analysis kit on Agilent 2100 bioanalyzer (Agilent, Santa Clara, CA, USA) to confirm that the quantitated DNA fragment size was consistent with cfDNA. Samples with genomic DNA fragments and total ctDNA < 10 ng were excluded.
cfDNA sequencing and data analysis
Amplicon libraries were generated from 10 to 100 ng of cfDNA using Oncomine Lung cfDNA assay (Thermo Fisher Scientific, Waltham, MA, USA). This 11-gene panel (ALK, BRAF, EGFR, ERBB2,KRAS, MAP2K1, MET, NRAS, PIK3CA, ROS1, and TP53) enables to analyze > 150 single-nucleotide variants (SNVs) and short indels that are frequently mutated in NSCLC. Target amplification, barcode library preparation, and size selection were performed according to the manufacturer’s protocols. Library quantification was carried out using the Ion Library TaqMan® Quantitation Kit (Thermo Fisher Scientific, Waltham, MA, USA) on Applied Biosystems7500 Real-Time PCR System. If library concentration was < 0.1 nmol/l, the sample was considered to have failed the library preparation step. The library was sequenced with ion PI chip on BES4000 (Capitalbio, Beijing, China) according to the manufacturer’s instructions.
Data analysis
Sequencing reads were mapped to Human Genome Build 19 (Hg19)/GRCh 37 reference sequence for sequence alignment and base calling by Torrent Suite software v5.2 (Life Technologies). Variant calling and annotation of mutations were accomplished by variantCaller plugin, Ion reporter, and Oncomine Knowledgebase Reporter (Thermo Fisher Scientific, Waltham, MA, USA). A sample was deem to be positive if allele frequencies ≥ 0.1% with a median read coverage >25,000 and median molecular coverage > 2500. All statistical analyses were performed by SPSS software version 22.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
In this study, 325 NSCLC patients’ peripheral blood samples were collected. 30 (9.2%) of 325 patients were failed to get variant calling for variable reasons: existing big fragment genomic DNA, insufficient total DNA, lower library concentration, or poor sequencing quality. Most (83.4%, 25/30) were detected big fragment genomic DNA. In total, 295 patients (90.8%) had a successful NGS ctDNA test performed on their plasma, which was similar to previously study (Zugazagoitia et al. 2019). The subjects included 168 male and 127 female, and had a median age of 65 years (range, 29–88 years). Of 295 patients, 93.9% (277/295) had a diagnosis of lung adenocarcinoma (Table 1).
Table 1.
Demographic and clinical characteristics of patients
| Characteristic | N (%) | Mutation detected in ctDNA (%) | No-mutation detected in ctDNA (%) | P | EGFR mutation | P | TP53 mutation | P | KRAS mutation | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||||
| Male | 168 (56.9) | 96 (57.2) | 72 (42.8) | 0.046 | 23 (13.7%) | 0 | 44 (26.2%) | 0.101 | 38 (22.6%) | 0.543 |
| Female | 127 (43.1) | 87 (68.5) | 40 (31.6) | 60 (47.2%) | 23 (18.1%) | 25 (19.7%) | ||||
| Age (years) | ||||||||||
| ≥ 65 | 153 (51.9) | 92 (60.1) | 61 (39.9) | 0.484 | 35 | 0.037 | 37 (24.2%) | 0.531 | 35 (22.88%) | 0.508 |
| < 65 | 142 (48.1) | 91 (64.1) | 51 (35.9) | 48 | 30 (21.1%) | 28 (19.72%) | ||||
| Histology | ||||||||||
| Adenocarcinoma | 277 (93.9) | 174 (62.8) | 103 (37.2) | 0.278 | 82 (29.6%) | 0.028 | 62 (22.4%) | 0.597 | 61 (22.0%) | 0.274 |
| Others | 18 (6.1) | 9 (50.0) | 9 (50.0) | 1 (5.6%) | 5 (27.8%) | 2 (11.1%) | ||||
| Metastasis | ||||||||||
| Yes | 45 (15.3) | 25 (55.6) | 20 (44.4) | 0.331 | 13 (28.9%) | 0.903 | 9 (20.0%) | 0.9 | 6 (13.3%) | 0.154 |
| Unknown | 250 (84.7) | 158 (63.2) | 92 (36.8) | 70 (28.0%) | 48 (19.2%) | 57 (22.8%) | ||||
| Site | ||||||||||
| Left lung | 25 (8.5) | 16 (64.0) | 9 (36.0) | 0.316 | 6 (24.0%) | 0.349 | 7 (28.0%) | 0.091 | 3 (12.0%) | 0.49 |
| Right lung | 40 (13.6) | 29 (72.5) | 11 (27.5) | 15 (37.5%) | 14 (35.0%) | 9 (22.5%) | ||||
| Unknown | 230 (77.9) | 138 (60.0) | 92 (40.0) | 62 (27.0%) | 46 (20.0%) | 51 (22.2%) | ||||
| Previous treatment | ||||||||||
| Un-treatment | 37 (12.5) | 24 (64.9) | 13 (35.1) | 0.08 | 11 (29.7%) | 0 | 5 (13.5%) | 0.03 | 5 (13.5%) | 0.24 |
| Targeted treatment | 32 (10.8) | 26 (81.3) | 6 (18.7) | 20 (63.5%) | 3 (9.3%) | 4 (12.5%) | ||||
| Radio/chemotherapy | 20 (6.7) | 13 (65.0) | 7 (35.0) | 4 (20.0%) | 8 (40.0%) | 6 (30.0%) | ||||
| Unknown | 206 (69.8) | 119 (57.8) | 87 (42.2) | 48 (23.3%) | 51 (24.8%) | 48 (23.3%) | ||||
Distribution of genomic alterations identified in NSCLC
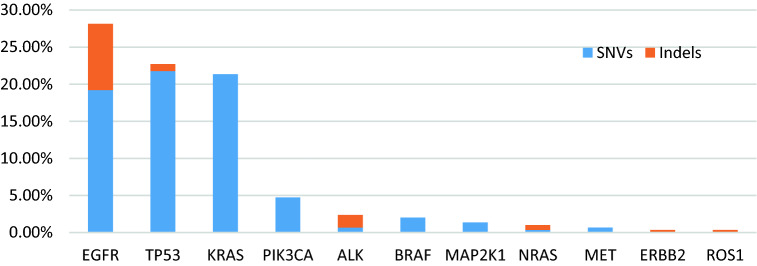
One hundred and eighty-three patients (62.1%) had one or more detectable genomic alterations. Genomic alterations were often found in female patients than male (P = 0.046 < 0.05). The most frequency alterations discerned were in the following genes: EGFR (27.8% of patients), TP53 (22.7%), KRAS (21.36%), and PIK3CA (4.75%) (Fig. 1). The EGFR mutation was significantly more common in female (P < 0.001), < 65 years (P = 0.037 < 0.05), and patients with adenocarcinoma (P = 0.028 < 0.05) (Table 1).
Figure 1.
Long tail of genes altered in ctDNA from patients with NSCLC. Frequent alterations given as a percentage of all (N = 295) patients
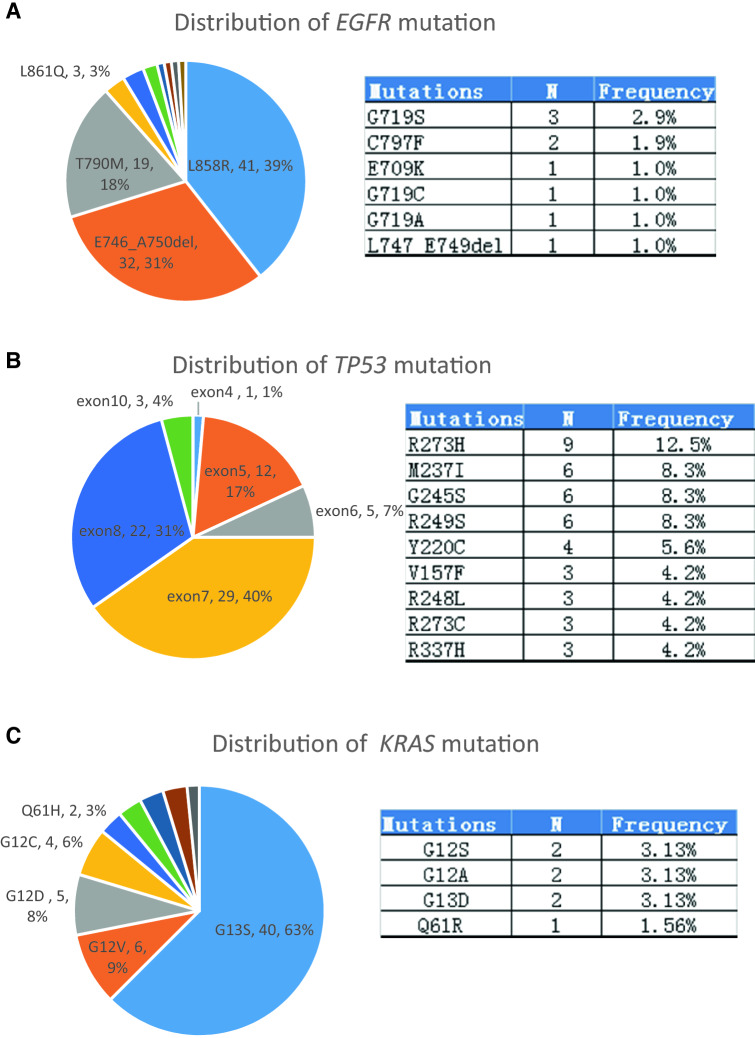
In total, 278 individual variants were detected in the ctDNA of 62.1% (183/295) patients, including 233 (83.9%) SNVs and 45 (16.2%) Indels (insertion/deletion). 104 alterations were found in EGFR, and the most common EGFR alterations were L858R (41 cases, 39.4%), followed by exon 19 deletions (33 cases, 31.73%), and T790M (19 cases, 18.3%) (Fig. 2a). Most TP53 mutations were located in exon 5 (12/72), exon 6 (5/72), exon 7 (29/72), and exon 8 (22/72). R273H (9/72), R249S (6/72), G245S (6/72), and R249S (6/72) were the most common point mutations in TP53 (Fig. 2b). 65.7% (42/64) KRAS mutations occurred in codon 13, and G13S (62.5%, 40/64) was the most frequently alterations observed in 64 KRAS alteration (Fig. 2c); PIK3CA alterations: E542K (6 cases), H1047R (5 cases), and E545K (3 cases) were identified in 14 cases. Seven ALK alterations, including R1275fs (3 cases), F1245fs (2 cases), L1196M (1 case), and F1174L (1 case), were detected in seven cases. BRAF alterations occurred at six patients and were V600E (5 cases) and G466A (1 case).
Fig. 2.
Distribution of EGFR, TP53, and KRAS mutation in NSCLC patients. a Distribution of EGFR mutation that were detected in 83 NSCLC patients (left); detailed mutation types that were not showed in left pie chart (right). b Exons of TP53 mutation that were detected in 67 NSCLC patients (left); top 9 mutation types in TP53 (right). c Distribution of KRAS mutation that were detected in 63 NSCLC patients (left); detailed mutation types that were not showed in left pie chart (right)
Genomic alteration distribution in different previous treatment patients
Among 37 untreated patients, the most common genomic alterations were EGFR-sensitizing mutation (18.92%, 7/37), KRAS (13.51%, 5/37), TP53 (13.51%), and EGFR-sensitizing and KRAS co-mutation (10.81%, 4/37) (Fig. 3a); Among 20 patients who progressed on radiochemotherapy/chemotherapy, the most genomic alteration was TP53 mutation (25%, 5/20), following by KRAS & TP53 co-mutation (10%, 2/20), KRAS (10%, 2/20), and EGFR-sensitizing mutation (10%, 2/20) (Fig. 3b). The TP53 mutation was significantly common in the patients with history of radiochemotherapy/chemotherapy therapy (P = 0.03 < 0.05); among 32 patients who progressed on the first-line EGFR-TKIs, the most common alterations were EGFR-sensitizing mutation + T790M co-mutation (46.88%, 15/32), KRAS (9.38%, 3/32). In total, the EGFR T790M resistance mutation was detected in 50% (16/32) patients progressed on EGFR-TKIs, and the reported less common secondary resistance mutations EGFR D761Y, T854A, L747S (Nguyen et al. 2009) were not found in this series samples (Fig. 3c).
Figure 3.
Genomic alteration prevalence (%) in circulating tumor DNA (ctDNA) from NSCLC patients with different previous treatments. a Untreated patients. b Progression on radiochemotherapy/chemotherapy. c Progression on EGFR-TKIs EGFRS:EGFR-sensitizing
Multiple gene mutations in ctDNA of NSCLC patients
Out of 183 patients with genetic variations, in 58.5% (107/183), one alteration was detected, in 34.4% (63/183), two alterations were detected, and in 7.1% (13/183), three or more alterations were detected. Of the 63 double alternations, 16 EGFR double mutations: 15 were EGFR-sensitizing mutation + T790M and 1 was G719S + L861Q; 10, EGFR + KRAS mutation; 2 EGFR + PIK3CA mutation; 1 ALK + MAP2K mutation; 1 BRAF + KRAS mutation; 1 EGFR + MET mutation; 1KRAS double mutation; 1 KRAS + ALK mutation; 17 TP53 with other genes co-mutation; 3 TP53 double mutation (Table 2); of the 13 with 3 or more alterations, 5 patients carried 2 EGFR mutations (sensitizing + resistant including 2 C797F) + RAS(4 cases) or PIK3CA mutation. And EGFR + KRAS + ALK, EGFR + BRAF + PIK3CA, EGFR + KRAS + PIK3CA + ALK, etc. were observed.
Table 2.
ctDNA-based NGS assay detects multiple gene mutations in NSCLC
| Number | Mutation #1 | Mutation #2 | Mutation #3 | Mutation # 4 | Mutation # 5 |
|---|---|---|---|---|---|
| 10 | EGFR: p.E746_A750del | EGFR p.T790M | – | – | – |
| 4 | EGFR: p.L858R | EGFR p.T790M | – | – | – |
| 1 | EGFR: p.G719S | EGFR p.T790M | – | – | – |
| 1 | EGFR: p.G719S | EGFR p.L861Q | – | – | – |
| 3 | EGFR: p.E746_A750del | KRAS p.G13S | – | – | – |
| 2 | EGFR: p.L858R | KRAS p.G13S | – | – | – |
| 2 | EGFR: p.L858R | KRAS p.G12V | – | – | – |
| 1 | EGFR: p.L858R | KRAS p.G12A | – | – | – |
| 1 | EGFR: p.L858R | KRAS p.G12D | – | – | – |
| 1 | EGFR: p.L861Q | KRAS p.Q61R | – | – | – |
| 1 | EGFR: p.E746_A750del | PIK3CA: p.E545K | – | – | – |
| 1 | EGFR: p.E746_A750del | PIK3CA: p.E542K | – | – | – |
| 1 | EGFR: p.L858R | MET:p.Y1253D | – | – | – |
| 1 | BRAF: p.(G466A) | KRAS p.(G12D) | – | – | – |
| 1 | KRAS: p.G12V | KRAS: p.G13S | – | – | – |
| 1 | KRAS: p.G13S | ALK:p.L1196M | – | – | – |
| 1 | ALK:p.R1275fs | MAP2K1:p.P124Q | – | – | – |
| 9 | TP53 | EGFR | – | – | – |
| 10 | TP53 | KRAS | – | – | – |
| 2 | TP53 | NRAS | – | – | – |
| 3 | TP53 | PIK3CA | – | – | – |
| 1 | TP53 | ROS1 | – | – | – |
| 1 | TP53 | MAP2K1 | – | – | – |
| 1 | TP53 | ERBB2 | – | – | – |
| 3 | TP53 | TP53 | – | – | – |
| 1 | EGFR:p.T790M | KRAS:p.G13S | TP53:p.G245S | – | – |
| 1 | EGFR:p.746_750del | EGFR:p.C797F | PIK3CA:p.E545K | – | – |
| 1 | EGFR:p.L858R | EGFR:p.T790M | KRAS:p.G13S | – | – |
| 1 | EGFR:p.L858R | EGFR:p.C797F | KRAS:p.Q61fs | – | – |
| 1 | EGFR:p.746_750del | TP53:p.R282R | TP53:p.G245S | – | – |
| 1 | EGFR:p.746_750del | EGFR:p.T790M | NRAS:p.Q61fs | – | – |
| 1 | KRAS:p.G13S | TP53:p.R282W | TP53:p.R337H | – | – |
| 1 | BRAF:p.V600E | EGFR:p.(L858R) | KRAS:p.(G13S) | – | – |
| 1 | BRAF:p.V600E | KRAS:p.(G13S) | ALK:p.(R1275fs) | – | – |
| 1 | EGFR:p.G719S | BRAF:p.V600E | PIK3CA:p.H1047R | MAP2K1:p.Q56P | – |
| 1 | EGFR:p.L858R | EGFR:p.T790M | KRAS:p.G13S | TP53:p.V272Vfs | MAP2K1:p.P124L |
| 1 | EGFR:p.E746_A750del | KRAS:p.G13S | TP53:p.G245S | ALK:p.F1245fs | MET:p.H1112fs |
| 1 | EGFR:p.E746_A750del | KRAS:p.(G13S) | PIK3CA:p.E542K | ALK:p.(R1275fs) | – |
Discussion
In this study, we have reported the analysis of 325 NSCLC patients using Amplicon-based commercially cell-free DNA assay, and 9.3% (30/325) were failed to get variant calling for variable reasons: high-molecular-weight DNA fragment contamination, insufficient total cfDNA, etc. A wide range of pre-analytical variables may impact cfDNA analysis, including specimen type, collection tube, transit time and temperature, centrifuge speeds, etc. Plasma is the optimal specimen type for ctDNA analysis in blood. The amount of normal DNA released from leukocytes lysis during clotting is much lower in plasma than serum, especially if the peripheral blood is drawn into cell stabilizing blood collection tubes (Lee et al. 2001; Page et al. 2006). Streck tubes can stabilize the cfDNA and gDNA for up to 14 days at 6–37 °C according to the manufacturer’s instructions. The main function of Streck tubes is to stabilize cfDNA and blood cells in the peripheral blood. However, storage of these tubes at ≤ 10 °C was shown to be associated with an increase of genomic DNA level and lower plasma volumes (Medina et al. 2016), and has a negative effect on sensitivity of ctDNA analysis (Sorber et al. 2018). In our study, all blood samples were required to draw in Streck tubes and shipped at room temperature within 72 h (from the time of blood collection to our lab). Given the great shipping infrastructure in China now, it is safe to assume that the majority of samples can be transferred within 72 h. It has been demonstrated that multistep, high-speed centrifugation could increase plasma volume and decrease gDNA contamination (Sherwood et al. 2016). The two-step centrifugation protocol, 1600×g for 10 min followed by 16,000×g for 10 min, which used in our study, is widely used to get plasma from whole blood samples nowadays.
Although a number of approaches can be used to interrogate cfDNA, NGS still is an attractive platform for lots of advantages: low nucleic acid input, multiplex sequencing ability, reducing cost, labor and turnaround time, etc. The present study was conducted to explore the whole genomic profiling of ctDNA in Chinese NSCLC patients using a commercial Ampliseq-based target NGS panel. Overall, 62.1% of patients had at least one detectable genomic alterations, and the most frequent alterations detected were in: EGFR (27.8%), TP53 (22.7%), KRAS (21.36%), and PIK3CA (4.75%), which were similar to the previous studies based on ctDNA analysis (Schrock et al. 2019). In comparison, the Cancer Genome Atlas (TCGA) showed rates of tissue alterations of TP53 (46%), EGFR (17%), KRAS (36%), and PIK3CA (7%) (Cancer Genome Atlas Research Network 2014). The rates of EGFR and KRAS gene alterations differed between ctDNA and tissue: EGFR alterations were more common in ctDNA and KRAS were more common in tissue. The variances could be related to the different patients populations studied. We also observed that EGFR alterations were more common in female (P < 0.001), < 65 years (P = 0.037 < 0.05), and adenocarcinoma, consistent with the previously reported in analyses performed on tissue (Cancer Genome Atlas Research Network 2014; Tokumo et al. 2005). Previous studies (Rosell et al. 2009; Yang et al.2013) have demonstrated that the most commonly EGFR mutations in NSCLC were exon 19 deletions and point mutation L858R in exon 21, which were predictive of treatment benefit from EGFR-TKIs’ therapy. L861Q, G719X, S768I, etc. were less commonly mutation found in NSCLC (Weber et al. 2014) and also associated with responsiveness to EGFR TKI therapy. These mutations were referred to as EGFR-sensitizing mutation and found in up to 50% Asian patients with NSCLC (Hirsch et al. 2009). In our study patient cohort, L858R was the most common mutation, following by exon19 del. EGFR T790M was common acquired resistance mutation to EGFR-TKIs therapy and has been reported in about 60% patients with disease progression after the first or second EGFR-TKIs therapy (Finlay et al. 2014; Gainor et al. 2013). Study suggested that T790M may also occur in patients without TKIs therapy history, although it was rare (Rosell et al. 2011). 18.3% T790M resistance mutation was detected, most in the patients with TKIs therapy history, and two cases with C797F mutation were detected in our study. KRAS, PIK3CA, ALK, and BRAF mutations have been approved to associate with resistance to EGFR-TKIs therapy in NSCLC (Ohashi et al. 2012; Kosaka et al. 2011; Rolfo et al. 2018), but their importance on EGFR-TKIs’ therapy selection is currently not well understood. To date, the presence mutation in these genes cannot be used to directly guide therapy decisions in NSCLC except the BRAF V600E. KRAS mutations are prognostic marker, and predictive of shorter survival in NSCLC patients. Several KRAS point mutations have been detected in NSCLC and most commonly occurred at codon 12 (Shepherd et al. 2013). We found that majority of KRAS mutations were in codon 13, and G13S was the most common mutation type. These discrepancies may related to: different specimen type, ctDNA versus tissue, or the clonal hematopoiesis of indeterminate potential (CHIP) (Jaiswal et al. 2014; Coombs et al. 2017).
Preclinical results showed that several different point mutations in ALK, e.g., L1196M and S1206Y, had resistance to crizotinib but nor ceritinib (Katayama et al.2014). Seven cases were detected harboring ALK mutations, including one case with L1196M in this study. BRAF V600E mutation was a validated and actionable driver mutation in NSCLC (Planchard et al. 2017), which was resistance to EGFR-TKIs and response to BRAF&MEK inhibitor combined therapy, dabrafenib plus trametinib (Planchard et al. 2016, 2017). V600E was the most common of BRAF point mutations, and occured in 1–2% of patients with lung adenocarcinoma (Davies et al. 2002). The similar result was found in our study.
Initial detection of genomic alterations as well as monitoring the appearance of resistance mutation during treatment may be an important application of ctDNA analysis in NSCLC patients. Several studies have monitored EGFR alterations in NSCLC patients with TKIs treatment using plasma (Zheng et al. 2016; Sueoka-Aragane et al. 2016; Del Re et al. 2017; Que et al. 2016). And T790M was the main resistance mechanism to first or second TKIs, 47% (55/117) EGFR T790M was detected in 117 patients who acquired TKIs resistance in Zheng study, and 40% in Sueoka-Aragane’s study. 50% of patients with the first-line TKI-therapy progression carried EGFR T790M resistance mutation in our study. Different mutational pictures were observed in patient populations with different treatment history in our study. We found that TP53 mutation was significantly more common in the patients with history of radiochemotherapy/chemotherapy therapy. However, the mechanism was not clear, still in need of further investigation.
In lung cancer, EGFR, KRAS, ROS1, or ALK mutations did not usually coexistence (Sholl et al. 2015; Ali et al. 2014; Hrisch et al. 2009), and KRAS and BRAF V600E mutation were mutually exclusive as previously reported (Rajagopalan et al. 2002; Li et al. 2014). However, various multiple genes’ co-mutation panels, e.g., EGFR + KRAS + PIK3CA + ALK, KRAS + BRAF V600E + ALK, and EGFR + BRAF + PIK3CA, were observed in our study. This finding suggested that single gene alteration test alone may not be sufficient for guiding TKIs therapy, and further investigation and more clinical trials data were needed.
A limited number of patients were enrolled in this study, and most patients lacked the clinical characteristic and treatment information. Only SNV and small indels in 11 genes were detected, and it could not decipher the comprehensive molecular maps in ctDNA of Chinese NSCLC patients. Further studies based on broaden gene and variants types were needed to decipher the whole molecular profile in ctDNA of Chinese NSCLC patients and evaluate the clinical utility of ctDNA in clinical practice.
In conclusion, Ampliseq-based NGS ctDNA analysis could detect genomic alterations in the majority of NSCLC patients successfully. In Chinese NSCLC patients, EGFR mutation was significantly associated with female, younger age (< 65 years), and adenocarcinoma. L858R and G13S were the most common mutation points in EFGR and KRAS, respectively. Mutational pictures were associated with the treatment history of NSCLC, and TP53 mutation was significantly more frequent in the patients with history of radiochemotherapy/chemotherapy therapy. And various multiple genes’ co-mutation panels, especially EGFR&KRAS co-mutation, were observed in the ctDNA of Chinese NSCLC patients. NGS-based ctDNA analysis may provide valuable information to guide NSCLC treatment and monitor the treatment resistance. Furthermore, wider application of ctDNA analysis could be greatly beneficial for patients in which an invasive procedure cannot be performed, or repeat genomic assessment for disease progression.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alì G, Proietti A, Pelliccioni S et al (2014) ALK rearrangement in a large series of consecutive non-small cell lung cancers: comparison between a new immunohistochemical approach and fluorescence in situ hybridization for the screening of patients eligible for crizotinib treatment. Arch Pathol Lab Med 138(11):1449–1458 [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424 [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network (2014) Comprehensive molecular profiling of lung adenocarcinoma. Nature 511(7511):543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs CC, Zehir A, Devlin SM et al (2017) Therapy-related clonal therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell 21(3):374–382.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C et al (2002) Mutations of the BRAF gene in human cancer. Nature 417(6892):949–954 [DOI] [PubMed] [Google Scholar]
- Del Re M, Tiseo M, Bordi P et al (2017) Contribution of KRAS mutations and c.2369 C%3eT(p.T790M)EGFR to acquired resistance to EGFR-TKIs in EGFR mutant NSCLC: a study on circulating tumor DNA. Oncotarget 8(8):13611–13619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis MG, Lafourcade MP, Le Garff G et al (2019) Circulating free tumor-derived DNA to detect EGFR mutations in patients with advanced NSCLC: French subset analysis of the ASSESS study. J Thorac Dis 11(4):1370–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drilon A, Wang L, Arcila ME et al (2015) Broad, hybrid capture-based next-generation sequencing identifies actionable genomic alterations in lung adenocarcinomas otherwise negative for such alterations by other genomic testing approaches. Clin Cancer Res 21(16):3631–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger DS, Wood DE, Akerley W et al (2015) Non-small cell lung cancer, version 6.2015. J Natl Compr Canc Netw 13(5):515–524 [DOI] [PubMed] [Google Scholar]
- Finlay MR, Anderton M, Ashton S et al (2014) Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J Med Chem 57(20):8249–8267 [DOI] [PubMed] [Google Scholar]
- Gainor JF, Shaw AT (2013) Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J Clin Oncol 31(31):3987–3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlinger M, Rowan AJ, Horswell S et al (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366(10):883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch FR, Bunn PA Jr (2009) EGFR testing in lung cancer is ready for prime time. Lancet Oncol 10(5):432–433 [DOI] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J et al (2014) Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 371(26):2488–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia N, Sun Z, Gao X, Cheng Y et al (2019) Serial monitoring of circulating tumor DNA in patients with metastatic colorectal cancer to predict the therapeutic response. Front Genet 10:470. 10.3389/fgene.2019.00470.eCollection [DOI] [PMC free article] [PubMed] [Google Scholar]
- JiangY QY, Minn AJ, Zhang NR (2016) Assessing intratumor heterogeneity and tracking longitudinal and spatial clonal evolutionary history by next-generation sequencing. Proc Natl Acad Sci USA 113(37):E5528–E5537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama R, Friboulet L, Koike S et al (2014) Two novel ALK mutations mediate acquired resistance to the next generation ALK inhibitor alectinib. Clin Cancer Res 20(22):5686–5696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Schwaederlé MC, Fanta PT et al (2019) Genomic assessment of blood-derived circulating tumor DNA in patients with colorectal cancers: correlation with tissue sequencing, therapeutic response, and survival. JCO Precis Oncol. 10.1200/PO.18.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Yamaki E, Mogi A, Kuwano H (2011) Mechanisms of resistance to EGFR TKIs and development of a new generations of drugs in non-small-cell lung cancer. J Biomed Biotechnol 2011:165214. 10.1155/2011/165214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebofsky R, Decraene C, Bernard V et al (2015) Circulating tumor DNA as a non-invasive substitute to metastasis biopsy for tumorgenotyping and personalized medicine in a prospective trial across all tumor types. Mol Oncol 9(4):783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Montalvo L, Chrebtow V, Busch MP (2001) Quantitation of genomic DNA in plasma and serum samples:higher concentrations of genomic DNA found in serum than in plasma. Transfusion 41(2):276–282 [DOI] [PubMed] [Google Scholar]
- Li S, Li L, Zhu Y, Huang C et al (2014) Coexistence of EGFR with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: a comprehensive mutation profiling from 5125 Chinese cohorts. Br J Cancer 110(11):2812–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina Diaz I, Nocon A, Mehert DH, Fredebohm J, Diehl F, Holtrup F (2016) Performance of streck cfDNA blood collection tubes for liquid biopsy testing. PLoS ONE 11(11):e0166354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minari R, Bordi P, Del Re M et al (2018) Primary resistance to osimertinib due to SCLC transformation: Issue of T790M determination on liquid re-biopsy. Lung Cancer 115:21–27 [DOI] [PubMed] [Google Scholar]
- Nguyen KS, Kobayashi S, Costa DB (2009) Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer 10(4):281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanno N, Denis MG, Thress KS, Ratcliffe M, Reck M (2017) Guide to detecting epidermal growth factor receptor(EGFR) mutations in ctDNA of patients with advanced non-small-cell lung cancer. Oncotarget 8(7):12501–12516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Sequist LV, Arcila ME et al (2012) Lung cancers with acquired resistance to EGFR resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci USA 109(31):E2127–E2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K, Powles T, Slade MJ, De Bella MT, Walker RA, Coombes RC, Shaw JA (2006) The importance of careful blood processing in isolation of cell-free DNA. Ann NY Acad Sci 1075:313–317 [DOI] [PubMed] [Google Scholar]
- Peled N, Roisman LC, Miron B et al (2017) Subclonal therapy by two EGFR TKIs guided by sequential plasma cell-free DNA in EGFR-mutated lung cancer. J Thorac Oncol 12(7):e81–e84 [DOI] [PubMed] [Google Scholar]
- Planchard D, Besse B, Groen HJM et al (2016) Dabrafenib plus trametinib in patients with previously treated BRAF( V600E) mutant metastatic non-small cell lung cancer: an open label, multicenter phase 2 trial. Lancet Oncol 17(7):984–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchard D, Smit EF, Groen HJM et al (2017) Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 18(10):1307–1316 [DOI] [PubMed] [Google Scholar]
- Que D, Xiao H, Zhao B et al (2016) EGFR mutation status in plasma and tumor tissues in non-small cell lung cancer serves as a predictor of response to EGFR-TKI treatment. Cancer Biol Ther 17(3):320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan H, Bardelli A, Lengauer C et al (2002) Tumorigenesis:RAF/RAS oncogenes and mismatch -repair status. Nature 418(6901):934 [DOI] [PubMed] [Google Scholar]
- Rolfo C, Mack PC, Scagliotti GV et al (2018) Liquid biopsy for advanced non-small cell lung cancer( NSCLC): a statement paper from the IASLC. J Thorac Oncol 13(9):1248–1268 [DOI] [PubMed] [Google Scholar]
- Rosell R, Moran T, Queralt C et al (2009) Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 361(10):958–967 [DOI] [PubMed] [Google Scholar]
- Rosell R, Molina MA, Costa C et al (2011) Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutations. Clin Cancer Res 17(5):1160–1168 [DOI] [PubMed] [Google Scholar]
- Schrock AB, Welsh A, Chung JH et al (2019) Hybrid capture-based genomic profiling of circulating tumor DNA from patients with advanced Non-Small Cell Lung Cancer. J Thorac Oncol 14(2):255–264 [DOI] [PubMed] [Google Scholar]
- Schwaederlé MC, Patel SP, Husain H et al (2017) Utility of genomic assessment of blood-derived circulating tumor DNA (ctDNA) in patients with advanced lung adenocarcinoma. Clin Cancer Res 23(17):5101–5111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd FA, Domerg C, Hainaut P et al (2013) Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-small- cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol 31(17):2173–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood JL, Corcoran C, Brown H, Sharpe AD, Musilova M, Kohlmann A (2016) Optimised pre-ana lytica methods improve KRAS mutation detection in circulating TumourDNA (ctDNA) from patients with non-small cell lung cancer (NSCLC). PLoS ONE 11(2):e0150197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl LM, Aisner DL, Varella-Garcia M et al (2015) Multi-institutional oncogenic driver mutation analysis in lung adenocarcinoma: the lung cancer mutation consortium experience. J Thorac Oncol 10(5):768–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorber L, Zwaenepoel K, De Winne K, Van Casteren K et al (2018) A multicenter study to assess EGFR mutational status in plasma: focus on an optimized workflow for liquid biopsy in a clinical setting. Cancers (Basel) 10(9):E290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka-Aragane N, Katakami N, Satouchi M et al (2016) Monitoring EGFR T790M with plasma DNA from lung cancer patients in a prospective observational study. Cancer Sci 107(2):162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JC, Yee SS, Troxel AB et al (2016) Detection of therapeutically targetable driver and resistance mutations in lung cancer patients by next-generation sequencing of cell-free circulating tumor DNA. Clin Cancer Res 22(23):5772–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumo M, Toyooka S, Kiura K et al (2005) The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res 11(3):1167–1173 [PubMed] [Google Scholar]
- Torquato S, Pallavajjala A, Goldstein A et al (2019) Genetic alterations detected in cell- free DNA are associated with enzalutamide and abiraterone resistance in castration-resistant prostate cancer. JCO Precis Oncol. 10.1200/PO.18.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B, Hager H, Sorensen BS et al (2014) EGFR mutation frequency and effectiveness of erlotinib:a prospective observational study in Danish patients with non-small cell lung cancer. Lung Cancer 83(2):224–230 [DOI] [PubMed] [Google Scholar]
- Yang JC, Hirsh V, Schuler M et al (2013) Symptom control and quality of life in LUX-lung 3: Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutation. J Clin Oncol 31(27):3342–3350 [DOI] [PubMed] [Google Scholar]
- Zheng G, Lin MT, Lokhandwala PM et al (2016) Clinical mutational profiling of bone metastases of lung and colon carcinoma and malignant melanoma using next generation sequencing. Cancer Cytopathol 124(10):744–753 [DOI] [PubMed] [Google Scholar]
- Zugazagoitia J, Ramos I, Trigo JM et al (2019) Clinical utility of plasma-based digital next-generation sequencing in patients with advance-stage lung adenocarcinomas with insufficient tumor samples for tissue genotyping. Ann Oncol 30(2):290–296 [DOI] [PubMed] [Google Scholar]