Abstract
Background
It is highly controversial that how to deal with the lateral lymph-node metastasis in patients with rectal cancer. Although lateral lymph node can be detected by preoperative MRI, the metastasis status cannot be accurately determined following standard total mesorectal excision (TME) in low-risk patients. This study was to assess the correlation between preoperative MRI detected lateral lymph-node (LLN) features and prognosis in patients with non-preradiated low recurrence risk rectal cancers.
Materials and methods
This retrospective study included 593 low-risk rectal cancers underwent TME without neoadjuvant chemo-radiotherapy from January 2013 to December 2015. The features of the largest LLN were retrospectively reviewed on preoperative MRI. The relationship of MR-LLN features with overall survival, metastasis-free survival, and local relapse-free survival was analyzed.
Results
A total of 593 patients including 415 cases of pN0, 86 cases of pN1, and 92 cases of pN2 were enrolled in this study. In pN0 patients, at least one visible LLN was detected in 104 patients on primary MRI. The MR-T staging, postoperative therapy status, the presence of MR-LLN, and short axis (SA) of MR-LLN were significantly correlated with the recurrence in pN0 patients (all p < 0.05). The OS and MFS were significantly lower in patients with MR-LLN SA ≥ 8 mm than SA < 8 mm (p < 0.01, HR = 4.35, 95% CI = 1.48–12.77). The OS and MFS of patients with pN0-LLN(+) and SA ≥ 8 mm were similar to pN2-LLN(−) patients. The location of MR-LLN showed no significant impact on prognosis.
Conclusion
For low-risk rectal cancers without neoadjuvant chemo-radiotherapy, the presence of MR-LLN is associated with poor prognosis. The pN0-LLN(+) SA ≥ 8 mm patients might be concerned as pN2 patients and receive more intensive neoadjuvant or adjuvant treatment.
Electronic supplementary material
The online version of this article (10.1007/s00432-019-03100-0) contains supplementary material, which is available to authorized users.
Keywords: Magnetic resonance imaging, Rectal cancer, Lateral lymph node, Prognosis
Introduction
National comprehensive cancer network (NCCN) categorized lymph nodes (LN) outside the mesorectal envelope should be categorized as distant diseases (M1) (NCCN Clinical Practice Guidelines in Oncology 2019). Contrarily, The Japanese Colorectal Cancer Classification (JCCC) categorized lateral lymph nodes (LLN) as locoregional LNs located in internal iliac region, obturator, and external iliac regions (Akiyoshi et al. 2012). A retrospective multicenter study in Japan reported that the incidence of lateral pelvic lymph-node metastasis in patients with T3 or T4 lower rectal cancer was 18.1% (Sugihara et al. 2006). In Japan, LLN metastasis is considered as a local disease, and the standard procedure for low rectal cancer is total mesorectal excision (TME) plus lateral lymph-node dissection (LLND) (Fujita et al. 2017). A recent published study indicated that patients could benefit from TME plus LLND for a better local control (Ogura et al. 2019).
According to the previous study, LLN metastasis is the main site of local recurrence after standard total mesorectal excision (TME), and the overall survival of patients with LLN metastasis is significantly lower than those without LLN metastasis, suggesting that LLN metastasis is a poor prognostic indicator (Taylor et al. 2011). The management of LLNs remains controversial. Western studies considered that LLN metastasis of rectal cancer as a systemic disease and LLND was not sufficient to improve the overall clinical outcomes (Kusters et al. 2017). The ESMO guidelines for rectal cancer did not recommend LLND as routine practice (Glimelius et al. 2017). According to the American Joint Committee on Cancer (AJCC) (Jäger et al. 2017), N staging is diagnosed based on regional LNs in which internal iliac region LNs were included. Since LLND is not considered as the routine procedure, the presence of internal iliac region LNs metastasis would directly change the pathological N staging and preoperative strategy. Above all, there is no consensus on this issue.
Beyond the anatomy-based cTNM staging system, the ESMO guidelines recommend stratified treatments based on the risk of recurrence of rectal cancers (Glimelius et al. 2017). Recurrence risk assessment is mainly by preoperative MRI, including tumor infiltration depth (cT), lymph-node metastasis status (cN), the distance of the tumor margin to the anus, the mesorectal fascia (MRF) status, extramural venous invasion (EMVI), etc. According to the risk of recurrence, patients were divided into very-low-risk, low-risk, medium-risk, high-risk, and very-high-risk groups. For patients in very-low-risk (≤ cT1N0) and low-risk group (≤ cT3bN0, i.e., MRI evaluation of tumor invasion depth less than 5 mm, or ≤ cN1 when the tumor is in a high position), who have no MRF invasion and no vascular invasion, TME should be considered without preoperative chemotherapy. If postoperative pathology proved no adverse prognostic factors (such as LN metastasis and circumferential resection margin involved), postoperative chemo-radiotherapy (CRT) should not be applied (Glimelius et al. 2017).
Although LLNs can be detected by preoperative MRI, the metastasis status cannot be accurately determined following standard TME and it is impossible to evaluate the pathological status of the LLNs in low-risk patients. However, preoperative MRI examination can be used to evaluate the presence, location, size, and number of lymph nodes (Brown et al. 2003).
There lacks for data that whether the MRI features of LLNs are the prognostic factors of patients with low-risk rectal cancers. Therefore, the relationship between MR-LLN features and prognosis needs to be further evaluated. This retrospective study aim to explore the correlation between preoperative MR detected LLN features and prognosis of patients with low-risk rectal cancers, so as to provide a basis for a more optimal clinical strategy.
Materials and methods
Patients
This retrospective study was approved by the ethics committee of our institution and the requirement for informed patient consent was waived. The patients in this study were included from the prospective database of our hospital for rectal cancer. MRIs of all consecutive patients who underwent operation in our institution from January 2013 to December 2015 were collected.
The inclusion criterias were as follows: patients who had biopsy-proven primary rectal adenocarcinoma, tumor > 1 mm to the MRF, MRI showed no evidence of EMVI, were early MRI T stage(≤ T3b stage) on pretreatment MRI,no distant metastasis on preoperative MRI, no neoadjuvant CRT, underwent TME after preoperative MRI.
The exclusion criteria were patients who had a history of a concurrent other malignancy, had insufficient MRI quality for assessment, had pathological determined mucinous adenocarcinoma, pathological proven > pT3, circumferential resection margin (CRM) positive, pathological EMVI (pEMVI) positive after TME surgery or failed to attend the first outpatient interview (3 months after surgery), and were lost to follow up. Preoperative MRI was retrospectively assessed in all the patients. Pathological proved that pN1/2 patients who had MR-LLNs visible were also excluded.
MRI acquisition
All patients underwent MRI examinations within 1 week before surgery. MRI examinations were performed with a 3.0-T MR unit (Discovery 750; GE Healthcare, Waukesha, Wis) using an eight-channel phased-array body coil in the supine position. Without any bowel preparation, patients were injected intramuscularly with 20 mg of scopolamine butylbromide 30 min prior to imaging to reduce colonic motility. The rectal MRI protocol included axial, axial oblique, coronal, and sagittal T2-weighted images; transverse T1-weighted images; diffusion-weighted images; and gadolinium-enhanced T1-weighted images, as summarized in supplement materials (Table S1).
Surgical treatment and pathological assessment
Surgery based on the principle of TME was recommended 8 weeks or more after the initiation of treatment. The types of resection were intraoperatively decided by surgeons’ discretion. Adjuvant chemotherapy was routinely recommended to the patients.
The 8th edition of the American Joint Committee on Cancer TNM system was used for pathological staging (AJCC cancer staging handbook. 7th ed. 2011). Histopathological results were reviewed by a pair of pathologists. The status of the CRM was assessed following the protocol described by Quirke et al. (2003).
Evaluation of MR-LLN features
All MR images were retrieved from the picture archiving and communication system (PACS) for further evaluation. We concluded the reported MRI features of LNs from previous studies (Brown et al. 2003; Kim et al. 2004; Zhang et al. 2017), including LN diameters (short axis, SA), LN location, border contour status,signal intensity, and chemical shift effect (CSE). Imaging features assessment were based on the largest LLN detected on MRI by one radiologist (with 6 years of experience in radiologic diagnosis for rectal cancer). Conventional MR features of the primary tumor were also evaluated on MRI of all the patients, including the relationship of the tumor lowest edge to the peritoneal reflection (PR), MRF status, MR-TN staging, as well as postoperative therapy status are shown in Table 1. Examples of MR-LLNs in different regions are shown in Fig. 1.
Table 1.
MR feature assessment
| Features | Assessment | Classification |
|---|---|---|
| MR-T stage | Staging of rectal cancer at MRI based on short-axial T2-weighted images |
T1: tumor invades submucosa T2: tumor invades muscularis propria T3: tumor invades through the muscularis propria into pericolorectal tissues |
| Mesorectal-N status | Nodes with irregular borders, mixed signal intensity, or both are suspicious, and uniform nodes smaller than 10 mm with homogeneous signal intensity are not suspicious |
N0: No. of suspicious LN N1: No. of suspicious LNs ≤ 3 N2: No. of suspicious LNs > 3 |
| Tumor location | Tumor relationship with PR |
Above: the lowest edge of the tumor is above the PR Below: the lowest edge of the tumor is below the PR |
| MR-LLN visible | LLN visible in high b-value DWI images (SA ≥ 3 mm) |
MR-LLN(+): visible LLN MR-LLN(−): no visible LLN |
| MR-LLN location | According to the lateral border of the main trunk of the iliac vessels; unilateral/bilateral |
Internal iliac region, obturator and external iliac region (Fig. 1a–c) Left side, right side and bilateral (Fig. 1a–c) |
| MR-LLN features | Border contour | Smooth/irregular |
| Signal intensity | Homogeneous/heterogeneous | |
| Chemical shift effect | Chemical shift effect at the border of normal lymph nodes along the frequency-encoding gradient |
CSE positive CSE negative |
PR peritoneal reflection, SA short axis, LLN lateral lymph node, CSE chemical shift effect
Fig. 1.
Examples of MR-detected lateral lymph nodes in different regions. a In a pT2N0 male patient, MR detected a 5 × 6 mm lymph node (arrow) located in the internal iliac region. b In a pT2N0 female patient, MR detected a 12 × 15 mm lymph node (arrow) located in the obturator region. The internal iliac artery (arrow head) was identified behind the enlarged lymph node. c Two lymph nodes (7 × 8 mm and 8 × 9 mm, respectively) were detected bilaterally in the external iliac region in a pT2N0 male patient. The two MR-detected lateral lymph nodes were located next to the lateral border of external iliac vein (arrow head)
Fifty cases were randomly selected and blinded for testing the reproducibility of MR-LLN imaging features. The same radiologist conducted the second assessment over 6 months after the initial reading. The reproducibility was assessed using k coefficient for all MR-LLN imaging features. If a k coefficient larger than 0.60 was obtained, then the initial assessment was used for the following analysis.
Follow-up
Follow-up consisted of outpatient interviews at 3 month intervals for 2 years, then at 6 month intervals for 3 years, and finally at 12 month intervals until death. MRI examination date, last follow-up, distant metastasis or local-recurrence date, and death date and cause were collected. Metastasis-free survival (MFS), overall survival (OS), and local-recurrence free survival (LRFS) were measured from the surgery until existence of distant metastasis, death, or local recurrence. Metastasis-free or alive or local-recurrence free were censored at the last follow-up. The cut-off date was 31 December 2018. Twenty-seven patients were lost to follow up. The median follow-up was 60 months (6–78 months).
Statistical analysis
Our primary aim was to investigate the role of MR-detected LLN features on the multiple survival outcomes. The second aim was to detect if LLN-defined subgroups predict different survival outcomes. Continuous variables were described as mean ± standard deviation, and categorical variables were described as numbers and percentages. Kaplan–Meier method with log-rank estimate was conducted to achieve our aims. Univariate cox regression was performed to obtain the hazard ratios (HR). The short axis was dichotomized by the cut-off value obtained by survivalROC analysis of OS. Chi-square test was used to describe the relationship between recurrence and MR-LLN features. Bonferroni correction was used for multiple comparisons. Kappa coefficient was calculated to evaluate reproducibility; 0–0.40, 0.41–0.75, and > 0.75 indicated poor, moderate, or good reproducibility, respectively. P values less than 0.05 indicated statistically significant. Calculations were performed using the Statistical Package for Social Sciences Program, version 22.0 (SPSS, Chicago, IL).
Results
A total of 593 patients with rectal adenocarcinoma were enrolled in this study (Fig. 2), including 415 cases of pN0, 86 cases of pN1, and 92 cases of pN2. There were 365 males (61.6%) and 228 females (38.4%), with an average age of 60.6 ± 11.4 years (23–87 years). The relationship between MRI-TN and pathological TN staging is shown in the supplement materials (Table S2).
Fig. 2.
Flowchart summarizes patient inclusion criteria. TME total mesorectal excision, CRT chemo-radiation therapy, CRM circumferential margin, EMVI extramural venous invasion, LLN lateral lymph node
Correlation of MR-LLN features and prognosis in pN0 patients
In pN0 patients, at least one visible LLN was detected in 104 patients (25.1%) on primary MRI, 30 patients of which were located in the internal iliac compartment and 74 patients were located in the obturator and external iliac compartment. The median size of the largest MR-LLN was 7.2 mm in SA. The largest MR-detected LLN in each patient was analyzed.
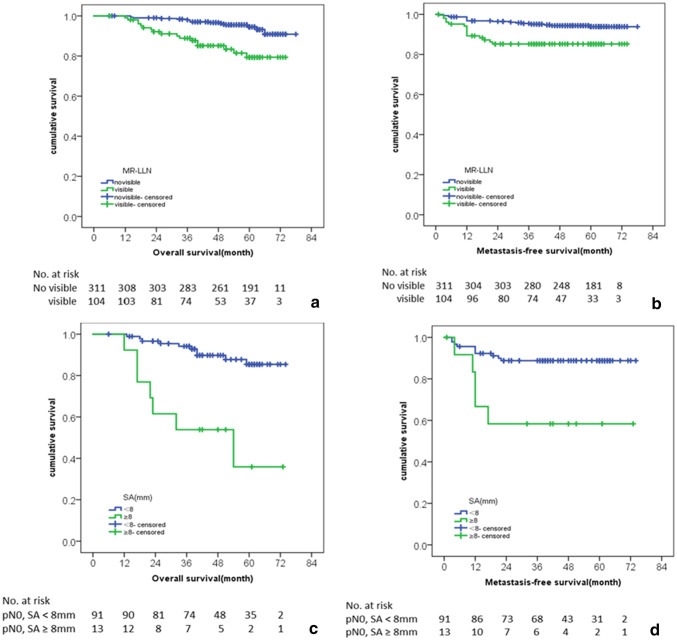
The correlation between MRI features of pN0 patients and the recurrence is shown in Table 2. The results of univariate analysis suggested that in the patients of low recurrence risk group (pN0), MR-T staging, postoperative therapy status, the presence of MR-LLN, and SA of MR-LLN were significantly correlated with the recurrence (all p < 0.05). Kaplan–Meier curve of the correlation to OS and MFS is shown in Figs. 3 and 4. There was no significant difference between the recurrence and MRI features such as MR-LLN border contour, signal intensity, MR-LLN location distribution, CSE status, age, gender, and the relationship between tumor lowest edge and PR.
Table 2.
Association between MRI features and recurrence in pN0 patients
| No. of patients | No. of recurrence | Recurrence | p | |
|---|---|---|---|---|
| Age | ||||
| 18–60 | 196 | 173 | 23 | 0.55 |
| ≥ 61 | 219 | 189 | 30 | |
| Sex | ||||
| Male | 254 | 217 | 37 | 0.17 |
| Female | 161 | 145 | 16 | |
| MR-LLN | ||||
| No visible | 311 | 281 | 30 | < 0.01 |
| Visible | 104 | 81 | 23 | |
| MR-T staging | ||||
| T1 | 94 | 89 | 5 | < 0.01 |
| T2 | 115 | 105 | 10 | |
| T3a + b | 206 | 168 | 38 | |
| Tumor relationship with PR | ||||
| Above | 349 | 34 | 10 | 0.90 |
| Below | 66 | 47 | 13 | |
| Postoperative therapy | ||||
| No | 308 | 279 | 29 | < 0.01 |
| Yes | 107 | 83 | 24 | |
| SA of LLN | ||||
| < 8 mm | 92 | 75 | 16 | <0.01 |
| ≥ 8 mm | 12 | 6 | 7 | |
| Location of LLN | ||||
| Internal iliac | 30 | 21 | 9 | 0.22 |
| Beyond internal iliac | 74 | 60 | 14 | |
| Unilateral/bilateral | ||||
| Left side | 45 | 33 | 12 | 0.65 |
| Right side | 26 | 21 | 5 | |
| Bilateral | 33 | 27 | 6 | |
| Border contour | ||||
| Smooth | 57 | 44 | 13 | 0.81 |
| Irregular | 47 | 37 | 10 | |
| Signal intensity | ||||
| Homogeneity | 37 | 29 | 8 | 0.81 |
| Heterogeneity | 67 | 52 | 15 | |
| CSE | ||||
| Negative | 87 | 67 | 20 | 0.99 |
| Positive | 17 | 14 | 3 | |
Recurrence includes metastasis, recurrence, and death
PR peritoneal reflection, SA short axis, LLN lateral lymph node, CSE chemical shift effect
Fig. 3.
Kaplan–Meier curves for the association between the presence of MR-LLN and the (a) OS and (b) MFS in pN0 patients. In pN0 patients with MR-LLN(+), Kaplan–Meier curves for the association between short axis of the largest MR-LLN and the (c) OS and (d) MFS
Fig. 4.
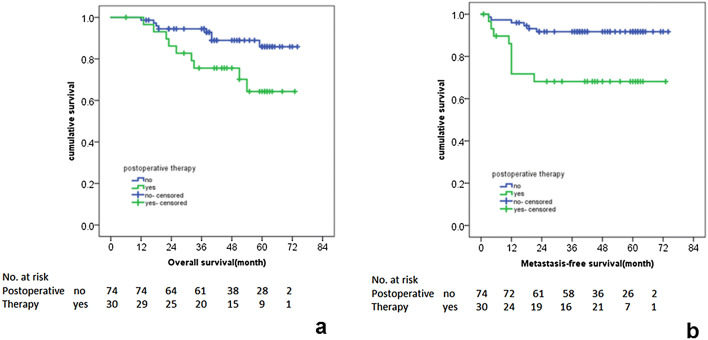
Kaplan–Meier curves for the association between postoperative therapy status and the (a) OS and (b) MFS in pN0 patients with MR-LLN (+)
Univariate analysis results of clinical characteristics, MR-LLN features, and the multiple survival rates of pN0 patients are shown in Table 3. In pN0 patients, MR-T staging was significantly correlated with the OS (p < 0.01). MFS of the group with postoperative therapy was significantly lower than that of the group without postoperative therapy (81.7% vs 95.1%, p < 0.01), and there was no significant correlation between the postoperative treatment and OS and LRFS.
Table 3.
Relationship of clinical and MRI features to pN0 patient survival rates
| No. | OS rate | P | MFS rate | P | LRFS rate | p | |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| 18–60 | 196 | 87.8 | 0.61 | 92.5 | 0.76 | 98.6 | 0.81 |
| ≥ 61 | 219 | 88.7 | 91.0 | 98.5 | |||
| Sex | |||||||
| Male | 254 | 86.1 | 0.25 | 91.9 | 0.66 | 98.4 | 0.83 |
| Female | 161 | 91.0 | 91.2 | 97.9 | |||
| MR-LLN status | |||||||
| No visible | 311 | 90.9 | < 0.01 | 93.8 | < 0.01 | 98.9 | 0.10 |
| Visible | 104 | 79.4 | 85.2 | 97.1 | |||
| MR-T stage | |||||||
| T1 | 94 | 90.9 | < 0.01 | 96.8 | 0.07 | 98.9 | 0.86 |
| T2 | 115 | 92.8 | 92.2 | 98.0 | |||
| T3a + b | 206 | 86.4 | 90.1 | 98.0 | |||
| Postoperative therapy | |||||||
| No | 308 | 97.4 | 0.44 | 95.1 | < 0.01 | 97.9 | 0.48 |
| Yes | 107 | 89.0 | 81.7 | 99.1 | |||
| Postoperative therapy in MR-LLN + | |||||||
| No | 30 | 64.3 | 0.02 | 68.1 | < 0.01 | 96.6 | 0.86 |
| Yes | 74 | 85.9 | 91.7 | 97.3 | |||
| SA of LLN | |||||||
| < 8 mm | 92 | 85.4 | < 0.01 | 88.8 | < 0.01 | 97.8 | 0.27 |
| ≥ 8 mm | 12 | 35.9 | 58.3 | 92.3 | |||
LLN lateral lymph node, SA short axis
In pN0, MR-LLN (+) patients, the OS and MFS of the patients with MR-LLN SA ≥ 8 mm were 35.9% and 58.3%, respectively, which were significantly lower than the patients with MR-LLN SA < 8 mm (OS and MFS were 85.4% and 88.8%, respectively, HR = 4.35, 95% CI = 1.48–12.77, p < 0.01). OS and MFS in patients with postoperative therapy were significantly lower than those without postoperative therapy (p = 0.02 and < 0.01, respectively). There was no significant correlation between the postoperative therapy status and LRFS.
Survival comparison of pN0, MR-LLN (+) versus pN + , and MR-LLN (−) patients
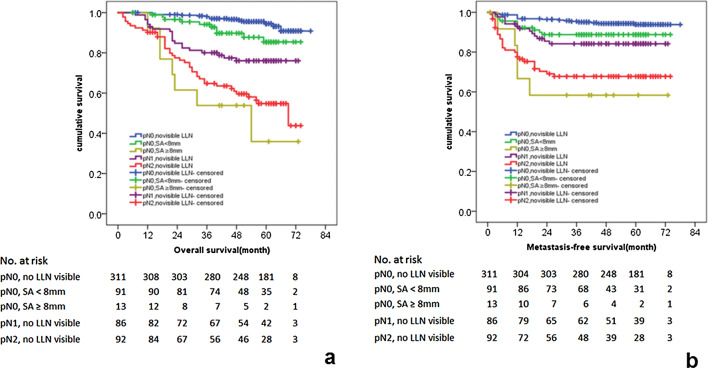
According to the MR-LLN characteristics and prognostic factors: the presence of MR-LLN and the maximum SA of MR-LLN, we divided the patients into three risk layers: pN0-LLN(−), pN0-LLN(+) SA < 8 mm, and pN0-LLN (+) SA ≥ 8 mm. Univariate Kaplan–Meier analysis (Fig. 5a, b) showed significant different prognosis (p < 0.05). The OS and MFS of pN0-LLN (−) patients was significantly better than pN0-LLN (+) SA < 8 mm and pN0-LLN (+) SA ≥ 8 mm patients. The OS and MFS of patients with pN0-LLN (+) and SA ≥ 8 mm were significantly lower than those with pN0-LLN (−) and SA < 8 mm.
Fig. 5.
Kaplan–Meier curves for the association between (a) OS and (b) MFS of patients in different pN and MR-LLN status
In addition, compared with pN1-LLN(−) patients, the OS and MFS of pN0-LLN(+) and SA ≥ 8 mm patients were significantly poorer (p < 0.05). Compared with pN2-LLN(−) patients, there was no significant difference in OS and MFS of pN0-LLN(+) and SA ≥ 8 mm patients (Fig. 5a, b). The survival rates according to pN and MR-LLN status are showed in Table 4.
Table 4.
Patients’ survival rates according to pN and MR-LLN status
| Groups | Overall survival (%) | ||||
|---|---|---|---|---|---|
| 1 year | 2 years | 3 years | 4 years | 5 years | |
| pN0, no LLN visible | 100.0% | 99.0% | 98.4% | 96.6% | 95.3% |
| pN0, SA < 8 mm | 100.0% | 96.6% | 94.1% | 89.6% | 85.4% |
| pN0, SA ≥ 8 mm | 100.0% | 61.5% | 53.8% | 53.8% | 40.4% |
| pN1, no LLN visible | 96.5% | 84.8% | 80.1% | 77.5% | 75.9% |
| pN2, no LLN visible | 91.3% | 77.8% | 65.1% | 61.4% | 55.1% |
| Groups | Metastatic-free survival (%) | ||||
|---|---|---|---|---|---|
| 1 year | 2 years | 3 years | 4 years | 5 years | |
| pN0, no LLN visible | 98.7% | 96.4% | 95.4% | 94.3% | 94.3% |
| pN0, SA < 8 mm | 95.6% | 88.6% | 88.6% | 88.6% | 88.6% |
| pN0, SA ≥ 8 mm | 84.0% | 58.8% | 58.8% | 58.8% | 58.8% |
| pN1, no LLN visible | 94.1% | 85.4% | 84.1% | 84.1% | 84.1% |
| pN2, no LLN visible | 80.0% | 70.4% | 67.7% | 67.7% | 67.7% |
Effect of MR-LLN location
MR-LLN location was divided to internal iliac region and region beyond internal iliac (including obturator and external iliac region). There was no significant difference in OS and MFS between the internal iliac group and the non-internal iliac group, p = 0.22 (Figure S1).
Discussion
This study confirms the value of preoperative MR, which is helpful for clinical treatment strategies. Results of this study indicated that MRI can provide high-accuracy evidence in preoperative decision-making. Patients enrolled in this study were of low risk defined in preoperative MRI and pathology. Results of this study suggested that the survival of pN0 patients with MR-LLN SA ≥ 8 mm is significantly poorer than that of pN1-LLN (−) patients, which indicated that TME alone is insufficient.
The study of JCOG 0212 (Fujita et al. 2012) emphasized the importance of extended resection for LLN, in the expense of more blood loss and prolonged operation time. In terms of efficacy, TME plus LLND had a lower local recurrence compared to TME alone for clinical stage II/III lower rectal cancer (Fujita et al. 2017). However, a more optimal method of local control for locally advanced rectal cancer might be neoadjuvant CRT, for its proven efficacy, safety, and decreasing toxicity with modern radiation technique. Watanabe et al. (2002) reported similar outcomes in patients after LLN resection or neoadjuvant CRT. Thus, prophylactic LLN resection is highly controversial in either locally advanced rectal cancer. The therapeutic LLN resection for locally advanced rectal cancer after neoadjuvant CRT is still debated for the technical difficulties and the low positive rate of harvested LLNs.
The prognostic value of baseline LLN is rarely studied. The recommendation for low-risk rectal cancer in ESMO guideline was based on the data from the Mercury trial. Taylor et al. (2011) demonstrated the ability of MRI to select patients who are likely to have a good outcome with primary surgery alone. The present study used similar criteria to define low-risk rectal cancers, however, stratified some systemic high-risk patients through the assessment of baseline status of MR-LLN.
Previous studies had confirmed that in patients with MR-LLN SA ≥ 7 mm after preoperative neoadjuvant therapy (CRT), the addition of LLND results in a significantly lower local-recurrence rate (Fujita et al. 2012, 2017; Ogura et al. 2019). Previous studies have concluded that MR features of LNs, such as mixed internal signals, irregular border, and CSE, play a role in the assessment of metastatic LNs (Brown et al. 2003; Kim et al. 2004; Zhang et al. 2017). However, for the assessment of LLNs in this study, except for the SA, there is no significant difference between the MR-LLN features and prognosis. This study focused on the relationship between MR-LLN features and survival in patients with low recurrence risk. Results of this study confirmed that the presence and SA of MR-LLN were the poor prognostic indicators of survival in low-risk rectal cancer patients. The chosen of SA is more reliable and widely used in clinical measurement.
pN0 patients were selected as the main evaluated cohort to reduce prognostic factors and exclude the influence of pN + on survival. Comparison between the survival of pN0-LLN (+) and pN1/2-LLN (−) indicated that the survival of pN0-LLN (+) SA ≥ 8 mm patients was poorer than that of pN1/2-LLN (-), and close to that of pN2-LLN (−). For patients with high-risk factors (poor histological differentiation, preoperative intestinal obstruction/perforation, and insufficient LNs detected in specimens) after surgery, postoperative adjuvant chemotherapy was conducted in our institution. Among the 12 patients with pN0-LLN (+) and SA ≥ 8 mm, six patients received postoperative adjuvant chemotherapy in this institution, suggesting that the present treatment strategies might be still insufficient.
AJCC defined LN located in internal iliac region as regional LN. Obturator and external iliac region LN were divided to distant metastasis (Jäger et al. 2017). Results of this study indicated that there is no significant prognostic difference in patients with different MR-LLN sites. By our results, all enlarged LLNs SA > 8 mm in low-risk rectal cancer should be considered as M1 disease, ignoring its location and adjacent vessels.
Patients enrolled in this study were of low recurrence risk (≤ pT3, pCRM-, pEMVI-); distant metastases were not identified in primary MRI. Whether MR-LLN should be considered as regional or distant disease is still controversial.
The limitations of this study are as follows: first, previous studies have been focused on the correlation between LLNs and LR. Since all the patients enrolled in this study were of low recurrence risk, the LR rate was 1.01% (6/593) in the whole cohort. Therefore, the relationship between MR-LLNs and LR could not be evaluated. Second, patients with enlarged LLN should be concerned about lateral metastasis and lateral dissection which were recommended in some countries. However, eligible patients were consecutively retrieved from a prospective database that included all treated rectal cancer in our hospital, which reflected the real treatment choice of our institution. Lateral LNs’ dissection was not routinely performed in our hospital, for the low positive rate after LNs resection and its undetermined oncological benefit in the expense of significant higher blood loss, prolonged operation time, and increased postoperative complication rate. This risk of LNs dissection also led to the fact that the present study lacks pLLN information. The surgeons had tendencies to perform standard TME in this low-risk group; the pathological status of the enlarged MR-LLNs could not be confirmed. Thirdly, the retrospective nature of this study weakens its power to provide high-level evidence. However, eligible patients were consecutively retrieved from a prospective database that included all treated rectal cancer in our hospital, which reflect the treatment choice in the real world. Despite these limitations, we used the present database to show the importance of lateral LNs assessment even in local low-risk rectal cancer. We hope that these results may not only be read by radiologists but also by surgeons, to remind them to have more optimal preoperative decision-making.
This study considered that the presence of MR-LLN would reduce the OS and MFS of rectal cancer. There is no significant difference between pN0-LLN(+) SA ≥ 8 mm and pN2 patients in prognosis, suggested that MR-LLN(+) may potentially result in a poor prognosis. Present clinical strategies may be unlikely sufficient. MRI-LLN evaluation should be emphasized in routine MR reports to support individualized treatment strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. ZYLX201803), Beijing Municipal Administration of Hospitals’ Youth Program (Code: QML20181103) and was supported by Beijing Municipal Science & Technology Commission (Z171100001017102), National Natural Science Foundation (81971584, 91959116), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. ZYLX201803), Beijing Natural Science Foundation (7172049), Beijing Municipal Science & Technology Commission (Z171100001017102), Beijing Hospitals Authority Youth Program (Code: QML20181103), National Key R&D Program of China (2017YFC1309101, 2017YFC1309104).
Abbreviations
- NCCN
National Comprehensive Cancer Network
- JCCC
Japanese Colorectal Cancer Classification
- AJCC
American Joint Committee on Cancer
- LLN
Lateral lymph node
- CRT
Chemo-radiatherapy
- SA
Short axis
- TME
Total mesorectal excision
- LLND
Lateral lymph-node dissection
- MRF
Mesorectal fascia
- EMVI
Extramural venous invasion
- CRM
Circumferential resection margin
- CSE
Chemical shift effect
- PR
Peritoneal reflection
- MFS
Metastasis-free survival
- OS
Overall survival
- LRFS
Local-recurrence free survival
Author contribution
YSS: conception and design. RJS, LW, and QYL: collection and assembly of data. YSS, RJS, LW, XTL, and XYZ: development of methodology. RJS, XTL, ZG, and YSS: data analysis and interpretation.
Compliance with ethical standards
Conflict of interest
The authors declare no potential conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rui-Jia Sun and Lin Wang have contributed equally to this work.
References
- Akiyoshi T et al (2012) Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: is it regional or distant disease? Ann Surg 255:1129–1134. 10.1097/SLA.0b013e3182565d9d [DOI] [PubMed] [Google Scholar]
- Brown G et al (2003) Morphologic predictors of lymph node status in rectal cancer with use of high spatial resolution MR imaging with histopathologic comparison. Radiology 227(2):371–377. 10.1148/radiol.2272011747 [DOI] [PubMed] [Google Scholar]
- Fujita S, Akasu T, Mizusawa J, Saito N, Kinugasa Y, Kanemitsu Y, Ohue M, Fujii S, Shiozawa M, Yamaguchi T, Moriya Y, Colorectal Cancer Study Group of Japan Clinical Oncology Group (2012) Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol 13:616–621. 10.1016/S1470-2045(12)70158-4 [DOI] [PubMed] [Google Scholar]
- Fujita S et al (2017) Colorectal Cancer Study Group of Japan Clinical Oncology Group. Mesorectal excision with or without lateral lymph node dissection for clinical stage II/III lower rectal cancer (JCOG0212): a multicenter, randomized controlled, noninferiority trial. Ann Surg 266:201–207. 10.1097/SLA.0000000000002212 [DOI] [PubMed] [Google Scholar]
- Glimelius B, Tiret E, Cervantes A, Arnold D, ESMO Guidelines Working Group (2017) Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment, and follow-up. Ann Oncol 28(Suppl 4):iv22–iv40. 10.1093/annonc/mdx224 [DOI] [PubMed] [Google Scholar]
- Jäger T et al (2017) Applicability of American Joint Committee on Cancer and College of American pathologists regression grading system in rectal cancer. Dis Colon Rectum 60:815–826. 10.1097/DCR.0000000000000806 [DOI] [PubMed] [Google Scholar]
- Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG (2004) High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol 52(1):78–83. 10.1016/j.ejrad.2003.12.005 [DOI] [PubMed] [Google Scholar]
- Kusters M, Uehara K, Velde CJHV, Moriya Y (2017) Is there any reason to still consider lateral lymph node dissection in rectal cancer? rationale and technique. Clin Colon Rectal Surg 30:346–356. 10.1055/s-0037-1606112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCN Clinical Practice Guidelines in Oncology (2019) Rectal Cancer (Version 2. 2019). https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed 17 June 2019
- Ogura A et al (2019) Neoadjuvant (Chemo) radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: results of the multicenter lateral node study of patients with low cT3/4 rectal cancer. J Clin Oncol 37(1):33–43. 10.1200/JCO.18.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirke P (2003) Training and quality assurance for rectal cancer: 20 years of data is enough. Lancet Oncol 4(11):695–702. 10.1016/S1470-2045(03)01248-8 [DOI] [PubMed] [Google Scholar]
- Sugihara K et al (2006) Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum 49:1663–1672. 10.1007/s10350-006-0714-z [DOI] [PubMed] [Google Scholar]
- Taylor FG et al (2011) Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg 253(4):711–719. 10.1097/SLA.0b013e31820b8d52 [DOI] [PubMed] [Google Scholar]
- Watanabe T et al (2002) Extended lymphadenectomy and preoperative radiotherapy for lower rectal cancers. Surgery 132(1):27–33. 10.1067/msy.2002.125357 [DOI] [PubMed] [Google Scholar]
- Zhang H et al (2017) Chemical shift effect predicting lymph node status in rectal cancer using high-resolution MR imaging with node-for-node matched histopathological validation. Eur Radiol 27(9):3845–3855. 10.1007/s00330-017-4738-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.