Abstract
Purpose
This prospective study evaluated whether peripheral blood biomarkers and metabolic parameters on F-18 fludeoxyglucose positron emission tomography/computed tomography (F-18 FDG PET/CT) could be associated with clinical outcome in non-small cell lung carcinoma (NSCLC) patients treated with immune checkpoint inhibitors (ICI).
Methods
Data from 33 patients with NSCLC and treated with ICI were collected. Complete blood cell counts before and at the first restaging were measured. All patients underwent F-18 FDG PET/CT at baseline, while 25 patients at the first restaging. Progression-free survival (PFS) and overall survival (OS) were determined and compared using the Kaplan–Meier and the log-rank test. The median follow-up was 11.3 months (range 1–17 months).
Results
Multivariate analyses demonstrated that low neutrophil-to-lymphocyte ratio (NLR < 4.9) and low total lesion glycolysis (TLG < 541.5 ml) at the first restaging were significantly associated with PFS (both p = 0.019) and OS (p = 0.001 and p = 0.048, respectively). An immune-metabolic-prognostic index (IMPI), based on post-NLR and post-TLG was developed, categorizing 3 groups: high risk, 2 factors; intermediate risk, 1 factor; low risk, 0 factors. Median PFS for low, intermediate and high risk was 7.8 months (95% CI 4.6–11.0), 5.6 months (95% CI 3.8–7.4), and 1.8 months (95% CI 1.6–2.0) (p < 0.001) respectively. Likewise, median OS was 15.2 months (95% CI 10.9–19.6), 13.2 months (95% CI 5.9–20.3), and 2.8 months (95% CI 1.4–4.2) (p < 0.001), respectively.
Conclusion
IMPI at the first restaging, combining both inflammatory and metabolic biomarkers, was correlated with PFS and OS. IMPI can be a potentially valuable tool for identifying NSCLC patients who are likely to benefit from ICI.
Keywords: Non-small cell lung cancer, Checkpoint inhibitors, Metabolic response, Neutrophil-to-lymphocyte ratio, F-18 FDG PET/CT
Introduction
In the last years, the use of immune checkpoint inhibitors (ICI) has become a concrete therapeutic option in different tumor types, including advanced non-small cell lung carcinoma (NSCLC) (Topalian et al. 2012; Borghaei et al. 2015; Herbst et al. 2016; Rittmeyer et al. 2017). Notwithstanding the encouraging results, approximately 60% of patients do not respond to treatment with ICI. So far, tumor PD-L1 expression is the only biomarker used for ICI treatment decision, although its expression varies in time or among multiple sites of lesions, and may be influenced by previous treatments (i.e. chemotherapy, radiotherapy) (Chae et al. 2016). Therefore, there is a compelling need to identify and validate predictive biomarkers of therapeutic response to perform the best patients’ selection for immunotherapy.
It is now accepted that immune response against cancer cells is a multistep process involving several factors (i.e. cellular and soluble molecules). Recent studies have investigated the predictive role of various blood markers of systemic inflammation during ICI treatment. For instance, a pro-inflammatory status, characterized by elevated levels of circulating white blood cells, absolute neutrophil, and platelets count is associated with poor outcome in different cancers (McMillan et al. 2013; Paramanathan et al. 2014; Templeton et al. 2014; Petrelli et al. 2015). Moreover, the ratio derived from circulating inflammatory parameters, such as neutrophil-to-lymphocyte ratio (NLR), has been studied in various tumors, NSCLC included (Templeton et al. 2014). This ratio has some advantages: it is easy to calculate, combines two different counts of blood cells, and is largely available in clinical practice. Unlike melanoma, where interesting results have been observed, the correlation between peripheral blood parameters and clinical outcome in patients with NSCLC treated with ICI is still limited (Bagley et al. 2017).
Although F-18 fludeoxyglucose (F-18 FDG) positron emission tomography/computed tomography (PET/CT) remains a fundamental tool in clinical oncology, its role in the ICI scenario is not completely elucidated (Rossi et al. 2017, 2018; Aide et al. 2019). Most researches, investigating the role of metabolic parameters to predict response to ICI, have been focused on melanoma patients, whereas only few studies have been conducted on patients with advanced NSCLC (Kaira et al. 2018; Spigel et al. 2018). Yet the prognostic significance of tumor metabolism on F-18 FDG PET is well-recognized (Liu et al. 2016).
These premises led us to conclude that the combination of clinical and metabolic information might be helpful to assess the response during immunotherapy. Here we prospectively investigate whether metabolic parameters evaluated by F-18 FDG PET/CT and peripheral blood biomarkers can be associated with clinical outcome in patients with advanced NSCLC treated with ICI.
Materials and methods
Study design and patients
In this prospective study, data from 33 patients (21 male, 12 female, mean age 75) with NSCLC and treated with ICI from April 2017 to December 2018 were collected. The inclusion criteria were as follows: (a) pathological diagnosis of NSCLC; (b) patients candidate to immunotherapy with either nivolumab (3 mg/kg every 2 weeks) or pembrolizumab (200 mg/ every 3 weeks); (c) F-18 FDG PET/CT scan performed before and at the first restaging after three or four cycles depending on ICI administered; d) total white blood cell counts, absolute neutrophil counts, absolute lymphocyte counts, and platelet counts were analyzed before and at first restaging. Patients were excluded if they had (1) clinical contraindication for ICI, (2) evidence of other concurrent malignancies, (3) refuse written consent refused, or if (4) plasma glucose level was ≥ 200 mg/dL before F-18 FDG PET/CT.
The study was approved by the local ethics committee of the Humanitas Clinical and Research Hospital, and according to the Helsinki Declaration. All subjects signed a written informed consent. The trial was registered at https://www.clinicaltrials.gov, identifier NCT03563482. Patient’s epidemiologic and clinical characteristics are reported in Table 1.
Table 1.
Patients characteristics
| N (%) | |
|---|---|
| Age median [range] | 75 [51–86] |
| Gender | |
| Male | 21 (63.6) |
| Female | 12 (36.4) |
| Smoking history | |
| Yes | 12 (36.4) |
| Former | 17 (51.5) |
| Never | 4 (12.1) |
| ECOG performance status | |
| 0 | 17 (51.5) |
| ≥ 1 | 16 (48.5) |
| Line of treatment | |
| 0 | 8 (24.2) |
| 1 | 14 (42.4) |
| ≥ 2 | 11 (33.4) |
| Histology | |
| Adenocarcinoma | 21 (63.6) |
| Squamous cell carcinoma | 7 (21.2) |
| Other | 5 (15.1) |
| Tumor PD-L1 expression level | |
| Positive | 11 (33.3) |
| Negative | 8 (24.2) |
| Indeterminate, not evaluable, or missing | 14 (42.5) |
| Mutational status | |
| EGFR | 2 (6.0) |
| ALK | 3 (9.0) |
| KRAS | 8 (24.2) |
| None | 13 (39.4) |
ECOG, Eastern Cooperative Oncology Group; EGFR, Epidermal Growth Factor Receptor; ALK receptor tyrosine kinase;
F-18 FDG PET/CT imaging protocol
PET/CT scans were acquired approximately 60 min after tracer administration in fasting patients. An activity of 250–500 MBq depending on weight was administered. Whole body images were obtained from the skull base to mid-thigh by means of GE Discovery PET/CT 690, with an integrated 64-slice CT. Reconstructed images were then displayed on a GE ADW4.6 workstation (GE Healthcare, Waukesha, WI, USA) and interpreted by experienced nuclear medicine physicians. EANM Research Ltd (EARL) program accredits the scanner used in this study, and image analysis was performed using standardized acquisitions (Boellaard et al. 2015). The threshold for the definition of the volumes of interest (VOIs) was set at 0.5-threshold weight on PETVCAR (GE Healthcare, Waukesha, WI, USA). SUVmax was defined as the value of the highest pixel and SUVmean was defined as the mean SUV related to the tumor burden. Tumor burden was calculated with three-dimensional volumes of interest (VOIs) drawn on the tumor-related activity volume by applying a percentage threshold of 41%: maximum standardized uptake value (SUVmax) and SUVmean were defined as the highest pixel value and the mean uptake, respectively. When required, the contouring was adapted manually by the operator. Volumetric parameters included metabolic tumor volume (MTV), estimated from VOI SUV isoactivity contours automatically drawn, and total lesion glycolysis (TLG), obtained as SUVmean × MTV ml.
Response assessment
Tumor response was assessed according to the immunotherapy-related Response Evaluation Criteria in Solid Tumours (iRECIST) (Seymour et al. 2017). In particular, for new lesions at the first restaging, the result was assigned as unconfirmed progression (iUPD) until confirmed progression (iCPD) was recorded at next radiological evaluation (i.e. appearance of additional new lesions or increase in size of new lesions recorded).
Metabolic response on PET/CT was defined as a reduction of F-18 FDG uptake of 25% or greater according to the European Organization for Research and Treatment of Cancer (EORTC) criteria (Young et al. 1999). More specifically, patients were categorized as CMR (complete metabolic response), PMR (partial metabolic response), SMD (stable metabolic disease) or PMD (progressive metabolic disease. The objective response rate (ORR) was defined as the addition of CMR and PMR, whereas the disease control rate (DCR) was defined as the addition of objective response and SMD (CMR + PMR + SMD).
Statistical analysis
Descriptive statistics were performed using conventional metrics (mean, median, range). The NLR was defined as the ratio between absolute neutrophils and lymphocytes counts. The percentage of variation for all PET parameters (ΔSUVmax, ΔSUVmean, ΔMTV, ΔTLG) during immunotherapy was assessed for the statistical analysis. Kruskal–Wallis test were used when appropriate. All parameters were statistically analyzed with respect to clinical outcome over a median follow-up of 11.3 months (range 1–17). Progression-free survival (PFS) was calculated as the interval from the date of initiation of ICI to the date of either disease progression or death, whereas overall survival (OS) was calculated as the duration between the date of initiation of immunotherapy and the date of death from any cause. Duration of response (DoR) was defined as the time from a best overall response of partial or complete response until the date progressive disease was documented or death. PFS, OS, and DoR were analyzed using the Kaplan–Meier method. A Cox proportional hazards regression analysis was used to evaluate factors independently associated with OS and PFS. Variables included in the final multivariate analysis were selected according to their clinical relevance and statistical significance in a univariate model (cut-off, p < 0.10). All statistical analyses were carried out using the Statistical Package for Social Sciences, version 23.0, for Windows (SPSS, Chicago, IL), and p values < 0.05 were considered to be statistically significant.
Results
Eight patients (24.2%) presented at diagnosis with advanced metastatic NSCLC, whereas the other twenty-five patients (75.8%) started ICI treatment due to disease recurrence after either one or two lines of systemic therapy. Most patients had adenocarcinoma (63.6%) and were former (51.5%) or current smokers (36.4%) with only a minority of non-smokers (12.1%). Eight patients had KRAS mutated cancers, three human epidermal growth factor 2 (HER2) mutated cancers, and two with epidermal growth factor receptor (EGFR) activating mutations.
Twenty-one patients (63.6%) received nivolumab, and twelve patients (36.4%) received pembrolizumab. The median number of immunotherapy cycles was 9 (range: 1–39). One patient performed only 1 cycle of ICI and four patients 2 cycles because of the rapid disease progression or worsening of clinic conditions.
Metabolic response and NLR
The descriptions of baseline and percentage changes in metabolic PET/CT parameters and peripheral blood biomarkers are reported in Table 2. 25 out of 33 patients underwent to PET/CT response evaluation at the first restaging. According to EORTC criteria, PMR occurred in 8 patients (32%), SMD in 11 patients (44%), whereas 6 patients (24%) experienced PMD. The ORR and DCR were 32% (8 of 25 patients) and 76% (19 of 25), respectively. The median DoR was not reached.
Table 2.
Distribution of peripheral blood and metabolic parameters before and at the first restaging during immunotherapy
| Median (range) | |
|---|---|
| SUVmax | |
| Baseline | 13.8 (4.9–35.7) |
| First restaging | 12.7 (3.6–40.6) |
| SUVmean | |
| Baseline | 6.0 (3.2–9.8) |
| First restaging | 5.6 (3.0–8.9) |
| MTV | |
| Baseline | 68.0 (8.3–1772.0) |
| First restaging | 91.3 (2.5–479.1) |
| TLG | |
| Baseline | 392.0 (31.0–2504.1) |
| First restaging | 541.5 (7.6–4035.2) |
| NLR | |
| Baseline | 4.1 (0.81–32.0) |
| First restaging | 4.9 (1.14–67.0) |
SUV standardized uptake value, MTV metabolic tumor volume, TLG total lesion glycolysis, NLR neutrophil–lymphocyte ratio
When comparing percentage changes of NLR between baseline and at the first restaging (ΔNLR), ΔNLR increased progressively from PMR to PMD, and median values resulted statistically different among the three groups: -19.8% (range -58.6% + 68.9%) for PMR, + 29.6% (range -55.6% + 130%) for SMD, and + 180.8% (range + 12.2% + 328.6%) for PMD (p = 0.03) (Fig. 1). Moreover, ΔNLR and ΔTLG were significantly lower in patients with DCR compared to patients without DCR (p = 0.009 for both parameters).
Fig. 1.

Graphical box-plots with medians for metabolic response on F-18 FDG PET/CT and percentage changes of NLR between baseline and the first restaging (ΔNLR)
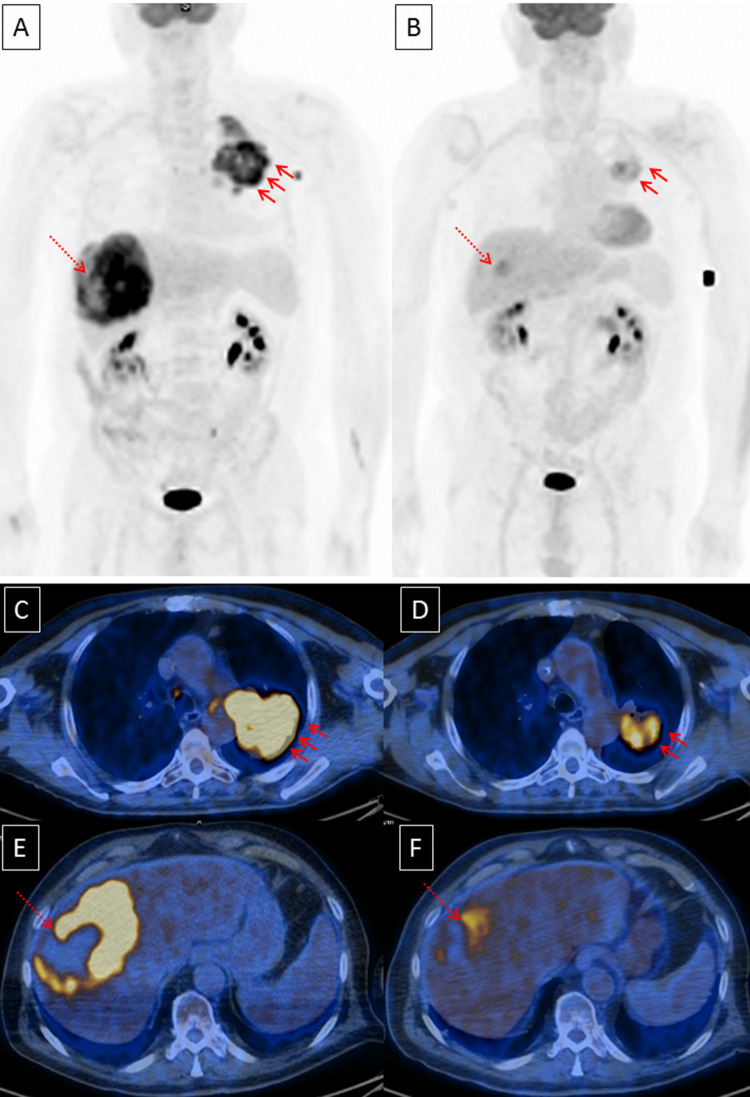
Pictorial example of metabolic response at the first restaging is shown in Fig. 2.
Fig. 2.
Pictorial example of response to checkpoint inhibitors: a baseline MIP (maximal intensity projection) and c, e axial sections of lung and liver metastases (arrows). b MIP and d, f axial sections at the first restaging. Furthermore, NLR decreased from 5.67 to 2.35 and patient was still alive without evidence of progression disease after 15 months since checkpoint inhibitor started
Survival outcomes
For the entire group of patients included in the analysis median PFS and median OS were 4.9 months (95% CI 1–8.8) and 8.1 months (95% CI 2.5–13.7), respectively. At univariate analysis, median NLR < 4.9 at the first restaging (post-NLR) was significantly associated with both longer PFS (HR 0.262, 95% CI 0.100–0.687; p = 0.006) and longer OS (HR 0.135, 95% CI 0.043–0.428; p = 0.001). Likewise, among metabolic PET/CT parameters, median TLG < 541.5 at the first restaging (post-TLG) was significantly associated with longer PFS (HR 0.257, 95% CI 0.095–0.696; p = 0.008) and OS (HR 0.194, 95% CI 0.058–0.646; p = 0.008). Moreover, at the univariate analysis for PFS no significant differences were found with respect to patient age, gender, histology, smoking status, ECOG status, baseline NLR, and baseline PET/CT parameters. At the univariate analysis for OS male gender (HR 0.354, 95% CI 0.130–0.964; p = 0.042) and ECOG status = 0 (HR 0.175, 95% CI 0.050–0.613; p = 0.006) were associated with longer OS.
Multivariate analysis showed that post-NLR < 4.9 (HR 0.257, 95% CI 0.095–0.696; p = 0.019) and post-TLG < 541.5 (HR 0.295, 95% CI 0.107–0.817; p = 0.019) were independently associated with PFS. Likewise, post-NLR < 4.9 (HR 0.044, 95% CI 0.007–0.275; p = 0.001) and post-TLG < 541.5 (HR 0.204, 95% CI 0.042–0.980; p = 0.048) were also associated with better OS. Finally, among all clinical variables investigated, only ECOG status = 0 (HR; 0.091, 95% CI 0.016–0.510; p = 0.006) was associated with OS. The results of univariate and multivariate analyses are summarized in Table 3.
Table 3.
Univariate and multivariate survival analysis
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| Progression-free survival (PFS) | |||||||
| Age | Per year | 0.973 | 0.925–1.024 | 0.296 | |||
| Gender | Male vs female | 0.454 | 0.192–1.075 | 0.073 | |||
| ECOG at the first restaging | 0 vs ≥ 1 | 0.414 | 0.158–1.081 | 0.072 | |||
| Smoking | Never/smokers | 1.897 | 0.551–6.539 | 0.310 | |||
| Line of treatment | 0 or 1 vs ≥ 2 | 1.129 | 0.470–2.712 | 0.786 | |||
| Tumor PD-L1 | Neg vs pos | 0.882 | 0.268–2.906 | 0.836 | |||
| Histology | Adeno vs other | 0.954 | 0.399–2.280 | 0.915 | |||
| Post-SUVmax | < 12.7 vs ≥ 12.7 | 0.489 | 0.188–1.276 | 0.144 | |||
| Post-SUVmean | < 5.6 vs ≥ 5.6 | 0.425 | 0.162–1.116 | 0.082 | |||
| Post-MTV | < 91.3 vs ≥ 91.3 | 0.257 | 0.095–0.696 | 0.008 | |||
| Post-TLG | < 541.5 vs ≥ 541.5 | 0.257 | 0.095–0.696 | 0.008 | 0.295 | 0.107–0.817 | 0.019 |
| Post-NLR | < 4.9 vs ≥ 4.9 | 0.262 | 0.100–0.687 | 0.006 | 0.257 | 0.095–0.696 | 0.019 |
| Overall survival (OS) | |||||||
| Age | Per year | 0.985 | 0.937–1.037 | 0.570 | |||
| Gender | Male vs female | 0.354 | 0.130–0.964 | 0.042 | |||
| ECOG at the first restaging | 0 vs ≥ 1 | 0.175 | 0.050–0.613 | 0.006 | 0.091 | 0.016–0.510 | 0.006 |
| Smoking | Never/smokers | 2.321 | 0.633–8.508 | 0.204 | |||
| Line of treatment | 0 or 1 vs ≥ 2 | 1.415 | 0.555–3.603 | 0.467 | |||
| Tumor PD-L1 | Neg vs pos | 0.033 | 0.574–5.446 | 0.642 | |||
| Histology | Adeno vs other | 0.714 | 0.281–1.813 | 0.479 | |||
| Post-SUVmax | < 12.7 vs ≥ 12.7 | 0.506 | 0.183–1.403 | 0.191 | |||
| Post-SUVmean | < 5.6 vs ≥ 5.6 | 0.403 | 0.136–1.192 | 0.100 | |||
| Post-MTV | < 91.3 vs ≥ 91.3 | 0.194 | 0.058–0.646 | 0.008 | |||
| Post-TLG | < 541.5 vs ≥ 541.5 | 0.194 | 0.058–0.646 | 0.008 | 0.204 | 0.042–0.988 | 0.041 |
| Post-NLR | < 4.9 vs ≥ 4.9 | 0.135 | 0.043–0.428 | 0.001 | 0.044 | 0.007–0.275 | 0.001 |
Immune-metabolic prognostic index (IMPI)
Post-NLR < 4.9 and TLG < 541.5, both independently associated with PFS and OS in the Cox proportional hazard analyses were combined. This allowed us to define an immune-metabolic-prognostic index (IMPI). Eight patients were excluded from the analysis of the index because they were not evaluated with F-18 FDG PET/CT post-treatment. The combination of the abovementioned parameters make it possible to identify 3 groups with different risk as follows: low (neither post-NLR ≥ 4.9 nor TLG ≥ 541.5, IMPI = 0), intermediate (post-NLR ≥ 4.9 or TLG ≥ 541.5, IMPI = 1), and high IMPI (post-NLR ≥ 4.9 and TLG ≥ 541.5, IMPI = 2).
Among the 25 patients evaluable for IMPI, 8 (32%) had low IMPI score, 8 (32%) had intermediate IMPI, and 9 (36%) had high IMPI. Median number of immunotherapy cycles was significantly lower for patients with IMPI = 2 compared to the other groups (4 vs 12 vs 11, p = 0.017). In addition, median PFS was 8 months (95% CI 4.6–11 months), 5.5 months (95% CI 3.8–7.4 months), and 1.8 months (95% CI 1.6–2 months) respectively for the low, intermediate, and high IMPI groups (p < 0.001) (Fig. 3a). Similarly, median OS was 15.2 month (95% CI 11–19.5 months), 13 months (95% CI 6–20 months), 2.8 months (95% CI 1.4–4 months) for the low, intermediate, and high IMPI groups, respectively (p < 0.001) (Fig. 3b).
Fig. 3.
Kaplan–Meier curves with log rank (Mantel-Cox) test obtained for: a PFS and b OS
Tumor PD-L1 expression and circulating-metabolic parameters
PD-L1 expression was evaluable only for 19 patients (57.6%), whereas for the remnant 14 (42.4%) patients tumor PD-L1 status either was not needed to start ICI therapy or was quantitatively insufficient from biopsied material. Association between PD-L1 status and clinical-metabolic parameters is shown in Table 4. In our analysis, tumor PD-L1 positive were associated with higher SUVmax (p = 0.001), SUVmean (p = 0.007) and only a trend for MTV (p = 0.051) at baseline, while no association was found for other parameters, including TLG and NLR.
Table 4.
Association between tumor PD-L1 expression and circulating-metabolic variables
| Variable | Tumor PD-L1 positive n = 11 |
Tumor PD-L1 negative n = 8 |
p |
|---|---|---|---|
| SUVmax | 16.6 (9.4–28.9) | 9.9 (5.3–13.1) | 0.001 |
| SUVmean | 6.2 (3.7–9.8) | 4.8 (3.3–6.5) | 0.007 |
| MTV | 173.6 (8.3–1772) | 15.4 (9.3–209.4) | 0.051 |
| TLG | 424.4 (31–2504.1) | 67.7 (31.8–1368.9) | 0.109 |
| White blood count | 9.9 (5.2–24.5) | 7.1 (3.8–10.3) | 0.075 |
| Neutrophils | 6.1 (2.4–20.9) | 4.6 (2–8.3) | 0.177 |
| NLR | 4.1 (1.3–32) | 5.2 (1.7–8.4) | 0.968 |
| dNLR | 2.4 (0.9–12.9) | 2.5 (1.1–4.3) | 0.717 |
| Platelets | 292 (184–492) | 222 (118–449) | 0.442 |
Discussion
Our study demonstrated that post-NLR and post-TLG at the first restaging during ICI treatment were significantly associated with PFS in patients with advanced NSCLC. In addition, the same parameters along with good performance status (ECOG = 0) resulted as independent prognostic factors for OS.
To date, PD-L1 expression is used as a criterion for the selection of patients to be treated with ICI; although, different studies have demonstrated clinical benefits also in patients with low or absent PD-L1 expression (Borghaei et al. 2015; Motzer et al. 2015; Castello et al. 2018). These evidences claimed the challenge to find the best predictors of response in the era of immunotherapy.
In the last years, it has become apparent that immune cells are fundamental to drive the destiny of several cancers. Cytotoxic CD8+ T cells within tumor landscape are associated with improved outcome, whereas the presence of neutrophils, M2-polarized macrophages and FOXP3+ regulatory T cells is closely correlated with poor prognosis and tumor progression (Tao et al. 2012; Diakos et al. 2014; Yuan et al. 2015). Similarly, systemic cancer-associated inflammation has been demonstrated to be associated with negative outcome. These findings are widely demonstrated in patients treated with “classical” chemotherapy and targeted therapies (Song et al. 2016). On the other hand, the effect of systemic inflammatory status during therapy with ICI remains unclear and limited to melanoma patients treated with ipilimumab or pembrolizumab (Khoja et al. 2016; Weide et al 2016). The NLR is another prognostic factor investigated in patients with NSCLC. Our results are in line with those from a recent study of Suh et al (2018) where NLR ≥ 5 after 6 weeks of treatment with anti-PD-1 antibody was associated with worse outcome. Moreover, also in melanoma patients post-treatment NLR below the median value, after 7 weeks of ipilimumab, was found to be associated with better survival (Di Giacomo et al. 2013). Similarly, although at baseline, Bagley et al. found that NLR ≥ 5 was associated with worse prognosis in a retrospective monocentric study including 175 NSCLC patients treated with nivolumab (Bagley et al. 2017).
Lymphocytes are thought to play a central role in the action of ICI, as their increase suggests likely a re-activation of antitumor activity induced by checkpoint inhibitors. In addition, because ICI block negative regulators of T cell response, it is possible that changes in the proportion of circulating lymphocytes could influence the efficacy of immunotherapy (Templeton et al. 2014; Brummelman et al. 2018). On the other hand, neutrophils are known to influence negatively the response to checkpoint inhibitors and different pathways have been proposed to explain their action. It has been ascertained that the production of Transforming Growth Factor-β favorites the generation of an immunosuppressive environment (Kargl et al. 2017). In our opinion NLR, combining two different cell subtypes, could be a valuable marker of antitumor response in patients receiving ICI.
F-18 FDG PET/CT has become a standard modality for staging and monitoring treatment response in lung cancer, while only few studies and/or case reports have investigated its role during therapy with checkpoint inhibitors. Recently, Kaira et al. (2018) evaluated whether PET/CT could predict therapeutic response after 1 month of nivolumab in a cohort of 24 NSCLC patients. According to PERCIST criteria and semi-quantitative parameters, the value of F-18 FDG PET/CT to predict the response was significantly higher than that of CT. Moreover, at multivariate analysis tumor burden parameters (i.e. TLG) after administration of nivolumab has been confirmed as an independent predictor of PFS and OS. This result is in line with that of our findings, where we demonstrated that post-TLG below the median was a predictor factor both for PFS (HR 0.295; p < 0.001) and OS (HR 0.204; p < 0.001), suggesting that TLG could be useful to monitor the efficacy of ICI therapy. In contrast to semi-quantitative metabolic parameters, Anwar et al. (2018) proposed the PET Response Evaluation Criteria for Immunotherapy (PERCIMT), based on the absolute number (≥ 4) of newly emerged lesions with high F-18 FDG uptake as marker for disease progression in a cohort of melanoma patients treated with ipilimumab. At the same time, however, the Authors highlight the importance of metabolic parameters (e.g. SUVs) in the treatment evaluation to checkpoint inhibitors.
Starting from evidences that immune response is the result of numerous interactions between T cells and other regulatory cells (Tanizaki et al. 2018), and that F-18 FDG PET/CT can provide useful information on the metabolic state of tumor microenvironment (Lopci et al. 2016), here we proposed a model for predicting clinical outcome based on the combination of peripheral blood biomarkers (i.e. NLR) and metabolic PET/CT parameter (i.e. TLG) at the first restaging from treatment start. As observed from Kaplan Meier curves (Fig. 1), the 32% of patients within high-risk group (IMPI score = 2) had both shorter PFS (median, 1.8 months) and OS (median, 2.8 months) than those within intermediate (IMPI = 1) or low risk (IMPI = 0) (p < 0.001). Our results are consistent with those recently published by Seban et al. (2019), who demonstrated that the combination of baseline MTV and derived-NLR identified three groups with significantly different OS and clinical benefit in a cohort of NSCLC patients treated with ICI. Since NLR calculation is immediate, reproducible, with no additional costs, and F-18 FDG PET/CT is routinely performed in clinical oncology, our data can aid clinicians to identify patients benefiting from treatment with ICI and to resolve doubtful cases, such as discriminating between tumor flares and true progression. Hence, our data highlight a possible correlation between changes in peripheral blood leukocytes and metabolic response as shown in Fig. 2, suggesting that a decrease of ΔNLR can reflect antitumor response induced by ICI therapy.
Finally, we demonstrated higher SUVmax and SUVmean values in patients with positive expression of PD-L1, confirming the peculiar metabolic tumor shift already arisen in our preliminary study, where a high glycolytic metabolism in NSCLC seems to be associated with a higher probability of response to ICI (Grizzi et al. 2018).
Our study has some limitations. First, it lacks a control group of patients who did not receive checkpoint inhibitors and includes a relative small number of patients. Furthermore, the cut-off values used as well as the immune-metabolic score index require further validation.
In conclusion, NLR and metabolic parameter by F-18 FDG PET/CT (i.e. TLG) at the first restaging are independent predictors of clinical outcomes (PFS and OS) in patients with advanced NSCLC treated with ICI. Moreover, their combination allows to early identification of patients who might benefit from immunotherapy, sparing them from potential immune-related adverse events on one hand, and reducing costs for health system on the other hand.
Acknowledgements
The Italian Association for Research on Cancer (AIRC-Associazione Italiana per la Ricerca sul Cancro) is acknowledged for the support on this research with the Grant no. 18923.
Funding
This study is supported by Italian Association for Research on Cancer (AIRC-Associazione Italiana per la Ricerca sul Cancro) (Grant 18923-2016).
Compliance with ethical standards
Conflict of interest
EL is the winner of the individual Grant no. 18923 provided by AIRC. AC and EM are supported with fellowships related to the Grant no. 18923.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aide N, Hicks RJ, Le Tourneau C, Lheureux S, Fanti S, Lopci E (2019) FDG PET/CT for assessing tumour response to immunotherapy: report on the EANM symposium on immune modulation and recent review of the literature. Eur J Nucl Med Mol Imaging 46:238–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar H, Sachpekidis C, Winkler J et al (2018) Absolute number of new lesions on F-18 FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging 45:376–383 [DOI] [PubMed] [Google Scholar]
- Bagley SJ, Kothari S, Aggarwal C et al (2017) Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab treated patients with advanced non-small-cell lung cancer. Lung Cancer 106:1–7 [DOI] [PubMed] [Google Scholar]
- Boellaard R, Delgado-Bolton R, Oyen WJ et al (2015) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 42:328–354 [DOI] [PMC free article] [PubMed]
- Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelman J, Mazza EMC, Alvisi G et al (2018) High-dimensional single cell analysis identifies stem-like cytotoxic CD8+ T cells infiltrating human tumors. J Exp Med 215:2520–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello A, Lopci E (2018) Non-small cell lung carcinoma: understanding cancer microenvironment to drive immunotherapy and patients’ selection. Transl Cancer Res 7(Suppl 5):S568–S572 [Google Scholar]
- Chae YK, Pan A, Davis AA et al (2016) Biomarkers for PD-1/PD-L1 blockade therapy in non-small-cell lung cancer: is PD-L1 expression a good marker for patient selection? Clin Lung Canc 17:350–361 [DOI] [PubMed] [Google Scholar]
- Di Giacomo AM, Calabrò L, Danielli R et al (2013) Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother 62:1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 15:e493–e503. [DOI] [PubMed]
- Grizzi F, Lopci E, Castello A (2018) Is it time to change our vision of tumor metabolism prior to immunotherapy? Eur J Nucl Med Mol Imaging 45:1072–1075 [DOI] [PubMed] [Google Scholar]
- Herbst RS, Baas P, Kim DW et al (2016) Pembrolizumab versus docetaxel for previously treated, PDL1 positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550 [DOI] [PubMed] [Google Scholar]
- Kaira K, Higuchi T, Naruse I et al (2018) Metabolic activity by (18)F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging 45:56–66 [DOI] [PubMed] [Google Scholar]
- Kargl J, Busch SE, Yang GH et al (2017) Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun 8:14381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoja L, Atenafu EG, Templeton A et al (2016) The full blood count as a biomarker of outcome and toxicity in ipilimumab-treated cutaneous metastatic melanoma. Cancer Med 5:2792–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dong M, Sun X, Li W, Xing L, Yu J. Prognostic value of F-18 FDG PET/CT in surgical non-small cell lung cancer: a meta-analysis. PLoS One 11:e0146195. [DOI] [PMC free article] [PubMed]
- Lopci E, Toschi L, Grizzi F et al (2016) Correlation of metabolic information on FDG-PET with tissue expression of immune markers in patients with non-small cell lung cancer (NSCLC) who are candidates for upfront surgery. Eur J Nucl Med Mol Imaging 43:1954–1961 [DOI] [PubMed] [Google Scholar]
- McMillan DC (2013) The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 39:534–540 [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Escudier B, McDermott DF et al (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramanathan A, Saxena A, Morris DL (2014) A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol 23:31–39 [DOI] [PubMed] [Google Scholar]
- Petrelli F, Cabiddu M, Coinu A et al (2015) Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol 54:961–970 [DOI] [PubMed] [Google Scholar]
- Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Toschi L, Castello A, Grizzi F, Mansi L, Lopci E (2017) Clinical characteristics of patient selection and imaging predictors of outcome in solid tumors treated with checkpoint-inhibitors. Eur J Nucl Med Mol Imaging 44:2310–2325 [DOI] [PubMed] [Google Scholar]
- Rossi S, Castello A, Toschi L, Lopci E (2018) Immunotherapy in non-small-cell lung cancer: potential predictors of response and new strategies to assess activity. Immunotherapy 10:797–805 [DOI] [PubMed] [Google Scholar]
- Seban RD, Mezquita L, Berenbaum A et al (2019) Baseline metabolic tumor burden on FDG PET/CT scans predicts outcome in advanced NSCLC patients treated with immune checkpoint inhibitors. Eur J Nucl Med Mol Imaging. 10.1007/s00259-019-04615-x [DOI] [PubMed] [Google Scholar]
- Seymour L, Bogaerts J, Perrone A et al (2017) iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18:e143–e152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YJ, Wang LX, Hong YQ et al (2016) Lymphocyte to monocyte ratio is associated with response to first-line platinum-based chemotherapy and prognosis of early-stage non-small cell lung cancer patients. Tumour Biol 37:5285–5293 [DOI] [PubMed] [Google Scholar]
- Spigel DR, Chaft JE, Gettinger S et al (2018) FIR: efficacy, safety, and biomarker analysis of a phase II open-label study of atezolizumab in PD-L1-selected patients with non-small-cell lung cancer. J Thorac Oncol 13:1733–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh KJ, Kim SH, Kim YJ et al (2018) Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother 67:459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizaki J, Haratani K, Hayashi H et al (2018) Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol 13:97–105 [DOI] [PubMed] [Google Scholar]
- Tao H, Mimura Y, Aoe K et al (2012) Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer 75:95–101 [DOI] [PubMed] [Google Scholar]
- Templeton AJ, McNamara MG, Šeruga B et al (2014) Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 106:dju124 [DOI] [PubMed]
- Templeton AJ, Ace O, McNamara MG et al (2014) Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 23:1204–1212 [DOI] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weide B, Martens A, Hassel JC et al (2016) Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res 22:5487–5496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H, Baum R, Cremerius U et al (1999) Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 35:1773–1782 [DOI] [PubMed]
- Yuan A, Hsiao YJ, Chen HY et al (2015) Opposite effects of M1 and M2 macrophage subtypes on lung cancer progression. Sci Rep 5:14273 [DOI] [PMC free article] [PubMed] [Google Scholar]