Summary
Chimeric antigen receptor T cell (CAR T) therapy has been successfully used to treat hematological malignancies. Nonetheless, its application to solid tumors remains challenging. Our previous analysis of the ongoing clinical trial (NCT03874897) demonstrated promising results in patients with advanced CLDN18.2-positive gastric cancer who received CT041 CAR T treatment. Here, we collected peripheral blood and ascites from five patients from the clinical trial 3 and 7 days (d) after CT041 infusion. Patients with a high proportion of naïve-like T cells were more likely to benefit from CT041 treatment. We found that high expression of CLDN18 in ascites epithelial cells correlated with a favorable prognosis, whereas ascites epithelial cells with high MYC expression and strong interactions between tumor cells and T cells were adverse prognostic factors for CT041 treatment. These findings may provide theoretical evidence for the screening of populations that can benefit from CAR T therapy and improve the efficacy of CAR T therapy.
Subject areas: Health sciences, Oncology, Immune response, Transcriptomics
Graphical abstract

Highlights
-
•
ScRNA-seq reveals characteristics of CAR T cells targeting CLDN18.2 in gastric cancer
-
•
Naive-like T cells in peripheral blood and ascites predict better therapeutic outcomes
-
•
CLDN18 overexpression in epithelial cells indicates positive prognosis for CAR T therapy
Health sciences; Oncology; Immune response; Transcriptomics
Introduction
In recent years, chimeric antigen receptor T cell (CAR T) therapy has been widely used for treating hematologic malignancies.1,2,3,4 Nonetheless, its application in solid tumors remains challenging,5,6,7 owing to the insufficient infiltration of CAR T cells into tumor tissues, lack of specific tumor-associated antigens, and an immunosuppressive tumor microenvironment.8,9,10,11 We previously reported that the overall response rate and disease control rate were 48.6% and 73.0%, respectively, in an open-label single-arm phase 1 clinical trial of CT041 CAR T treatment in CLDN18.2-positive patients with digestive system cancer (NCT03874897), thus confirming the safety and efficacy of CAR T therapy in solid tumors.12 Although our study showed that CAR T therapy achieved favorable clinical responses in solid tumors, almost 50% of the CLDN18.2-positive patients did not benefit from the treatment. Thus, it is crucial to investigate the dynamic evolution of CAR T cells in the body and determine their impact on therapeutic efficacy. Gastric cancer (GC) metastases are mostly located in the abdominal cavity, retroperitoneal lymph nodes, or peritoneum, making them difficult to biopsy. Although the on-target off-tumor effect of edema in the gastric mucosa following CT041 transfusion leads to a low sampling success rate, frequent biopsies are not in line with ethical regulations. Therefore, we collected ascites specimens and integrated the results with those from patients’ peripheral blood testing for analysis.
In this study, specimens from five patients with advanced gastric cancer and ascites in our cohort were collected, with sampling before treatment as well as 3 and 7 days after treatment. Using single-cell transcriptome sequencing technology, we analyzed the cell types and proportions in the blood and ascites of patients after CAR T therapy and examined the therapeutic outcomes. High CLDN18 expression in ascitic-fluid epithelial cells (ECs) was an indicator of good prognosis following CAR T therapy. In contrast, high MYC expression in ascitic ECs and a strong interaction between tumor cells and T cells were adverse prognostic factors. These findings can support the development of screening methods for patients suitable for CAR T therapy and improve its efficacy.
Results
Clinical information of patients treated with CT041
Our samples were collected during an ongoing clinical trial (NCT03874897) targeting CLDN18.2 using CT041 CAR T cells in patients with gastric cancer and ascites. Five patients with CLDN18.2-positive gastric cancer were enrolled and received an infusion of 2.5 × 108 CAR T cells. The clinical information is presented in Table 1. According to the RECIST 1.1 criteria, case 5 achieved a partial response (PR), whereas the other four patients had progressive disease (PD).
Table 1.
Clinical information
| Number | Gender | Age | Stage | 18.2 express | Dose | PFS (d) | OS (d) | Clinical evaluation | Peritoneal dropsy |
|---|---|---|---|---|---|---|---|---|---|
| Case1 | Female | 63 | pT4aN0 | 2+, 80% | 2.5∗108 | 58 | 85 | PD | PD’ |
| Case2 | Male | 45 | pT4aN3bM1 | 2+, 70% | 2.5∗108 | 143 | 143 | PD | PD’ |
| Case3 | Male | 59 | cTxNxM1 | 3+, 90% | 2.5∗108 | 78 | 91 | PD | PR’ |
| Case4 | Female | 57 | cT4aN2M1 | 3+, 90% | 2.5∗108 | 54 | 140 | PD | PR’ |
| Case5 | Female | 31 | pT3N1 | 3+, 90% | 2.5∗108 | 596 | 680 | PR | PR’ |
PFS, progression-free survival; OS: overall survival; PD, progressive disease; PR, partial response.
We examined blood samples from these five patients (cases 1–5, Table 1) at different time points: before treatment, 3 days after treatment, 7 days after treatment, 2 weeks (w) after treatment, 4 weeks after treatment and 8 weeks after treatment. CAR copy-number detection revealed that the CAR T cells expanded rapidly in the peripheral blood after infusion. We also collected ascites samples from patients before and after treatment as much as possible, and we found that many CAR T cells were present in the ascites, considerably higher than that in peripheral blood (Figure 1A). However, the CAR copy number gradually decreased in most patients 7 days post-infusion (Figures 1A and S1A). The expression of cytokines, including interferon gamma (IFN-γ) and interleukin-2 (IL-2), increased along with CAR T cell expansion and peaked before decreasing and gradually plateauing, whereas that of others, such as IL-6 and IL-8, increased and then decreased in expression (Figures 1B and S1B). Computed tomography (CT) imaging after treatment with CT041 revealed a reduction in peritoneal thickening in case 5, which indicated a positive effect and demonstrated the efficacy of the treatment (Figure 1B and S2). Thus, we classified case 5 as a partial response (PR), whereas the four other cases were classified as a progressive disease (PD). Interestingly, we found that before treatment, case 5 had more cytotoxic T cells and fewer suppressor T cells in the peripheral blood than the other patients, whereas the other T cell subsets were unrelated to therapeutic efficacy (Figures 1C and S1C).
Figure 1.
Dynamic changes in patient clinical indicators after CAR T therapy
(A) Patient CAR gene copy-number measurements in ascites (left) and blood (middle) samples. The abscissa indicates days after CAR T therapy and the ordinate indicates CAR T copies/μg DNA. For the ascites and peripheral blood samples, the difference in CAR copies 7 days after treatment with CT041 is shown (right). ∗p < 0.05 (Mann-Whitney test).
(B) Abdominal enhanced CT scans at three time points (baseline and 2 and 6 months post-treatment).
(C) Lymphocyte subsets in the blood. Abscissa, different patients; ordinate, contribution of each cell subset to the total number of peripheral blood cells (as percentage).
There are multiple cell types in peripheral blood after CAR T therapy
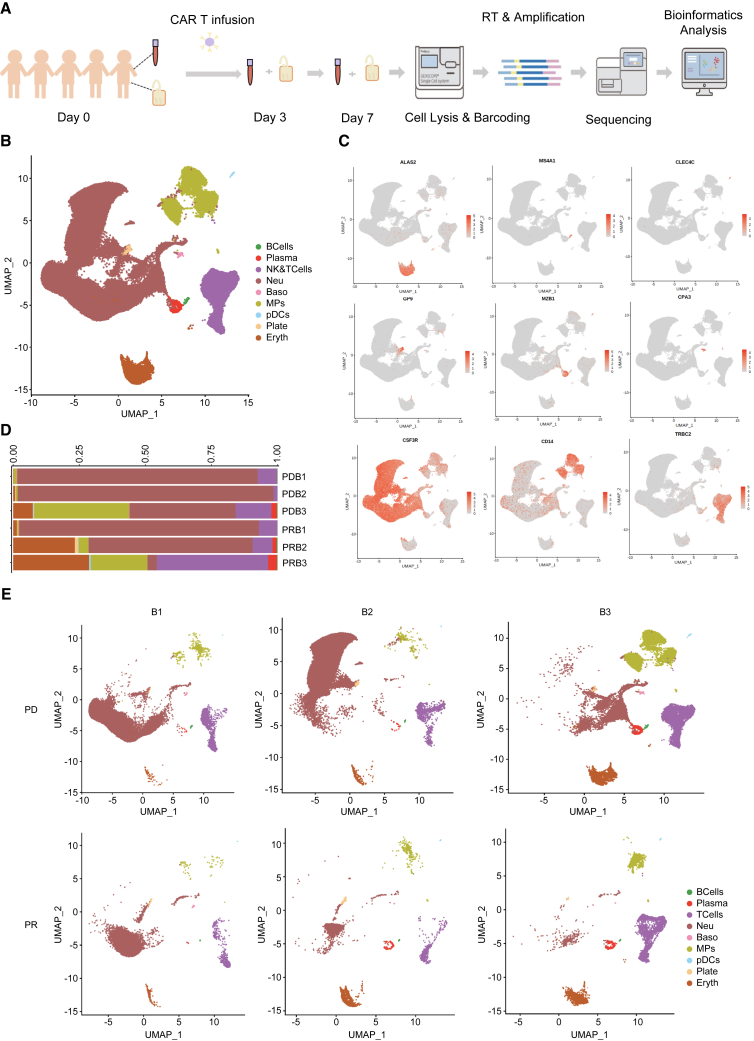
To investigate the dynamic process of infused CAR T cells, 15 peripheral blood mononuclear cell (PBMC) samples from five patients (cases 1–5) were measured before treatment (Blood 1, B1) and 3 days (Blood 2, B2) and 7 days post-treatment (Blood 3, B3) before being used for single-cell sequencing and data analysis (Figure 2A). Nine different cell types were indentified: T cells ,B cells, macrophages (MPs), etc. (Figures 2B, 2C, S2A, and Table 2). The proportion of NK cells, T cells, MPs, and plasma cells in the PBMC in case 5 gradually increased over the course of treatment, whereas the other four cases (PD group) showed a reduction in these cells and a higher proportion of neutrophils in the blood during the early treatment phase (Figures 2D, 2E, and S1).
Figure 2.
Cellular profiling of patient blood samples
(A) Experimental design flow chart.
(B) Cell-type diagram color coded to illustrate the 15 blood samples.
(C) Feature plot of cell-type marker genes in the blood samples.
(D) Cell-type composition of the PD and PR groups.
(E) Subpopulations of cells in the PD and PR groups are shown in a uniform manifold approximation and projection (UMAP). B1–3, blood samples before treatment, 3 days after CAR T therapy, and 7 days after CAR T therapy, respectively.
Table 2.
The cell type, abbreviation, and marker gene of blood samples
| Abbreviation | Cell type | Markers |
|---|---|---|
| TCells | T cells | CD2, CD3D, TRAC, TRBC2 |
| BCells | B cells | CD79A, CD79B, MS4A1 |
| PlasmaCells | Plasma cells | JCHAIN, MZB1, CD79A, IGHG1 |
| MPs | Mononuclear phagocytes | LYZ, CD14, VCAN, CD1C, FCER1A |
| Erythrocytes | Erythrocytes | HBB, HBA1, ALAS2, SNCA, CA1 |
| Neutrophils | Neutrophils | CSF3R, CXCR2, FCGR3B, LCN2, CAMP |
| Platelets | Platelets | PPBP, TUBB1, PF4, GP9 |
| Basophils | Basophils | CLC, GATA2, CPA3 |
| pDCs | Plasmacytoid dendritic cells | IL3RA, CLEC4C, LILRB4, TCF4, TCL1A |
| NaiveT | Naive T cells | CCR7, SELL, LEF1, TCF7, CD4, CD8A |
| CD8Teff | CD8+ effector T cells | CD8A, NKG7, GZMB, GNLY |
| Treg | Regulatory T cells | FOXP3, IL2RA, CTLA4, IKZF2 |
| HelperT | T helper cells | CD40LG, ICOS, GATA3, IL7R |
| NK | Natural killer cells | NKG7, KLRF1, NCR3, CD3D |
| Monocytes | Monocytes | CD14, VCAN, FCN1 |
| cDC2 | Conventional type 2 dendritic cells | CD1C, CD1E, FCER1A, CLEC10A |
| HSCs | Hematopoietic stem cells | CD34, SPINK2, HOPX |
CCR7 expression in naive T cells predicted better treatment efficacy
We subdivided all cells in the NK and T cell clusters in the blood into five different subsets: naive T cells, CD8+ effector T cells, regulatory T cells, T helper cells, and NK cells (Figures 3A–3C and S2B). The proportion of all clusters increased in all patients after treatment, with the most significant increase in CD8A positive T cells. We also observed that the proportion of naive T cells expressing CCR7 was present in the peripheral blood of all patients and gradually decreased after treatment; however, the proportion in the PR group was higher than that in the PD group at all time points (Figures 3D and S2C). The case 5 patient with the best efficacy had a higher proportion of naive T cells and T helper cells but lower NK cells before infusion (Figure S2D).
Figure 3.
Characterization of the immune-cell component of the patient’s blood samples
(A) T cell subset cluster uniform manifold approximation and projection (UMAP) of the 15 blood samples.
(B) Feature plot of T cell subset markers in blood samples.
(C) Heatmap of the top 10 most differentially genes expressed in each subset (abscissa, different cell subsets; red, high expression; blue, low expression).
(D) Histogram showing the contribution of each T cell subset to total T cells, as percentage (top); histogram of the contribution of each T cell subset to all cells in the peripheral blood, as percentage (below). PD, progressive disease group; PR, partial response group; B1–3, blood samples before treatment, 3 days after CAR T therapy, and 7 days after CAR T therapy, respectively.
Malignant epithelial cell number is a poor prognostic factor for CT041 therapy efficacy
In addition to the peripheral blood of the patient, we also evaluated the condition of their ascites because ascites control is closely related to quality of life in patients with advanced ascites. Of the five patients, cases 1 and 2 did not experience relief from ascites after treatment and were classified as PD’, whereas cases 3, 4, and 5 experienced a significant reduction in ascites after treatment and were classified as PR’. We collected 15 ascites samples from these five patients at the same time points as the blood collection and found a large number of CAR T cells in the ascites. Interestingly, we observed that the CAR copy number was significantly higher in the ascites than that in the peripheral blood at the same time points (Figure 1A).
We also conducted single-cell sequencing analysis of ascites samples collected before treatment (P1) and 3 and 7 days after treatment (P2 and P3). A total of 13 different cell subsets clustered together, including T cells, plasma cells, MPs, plasmacytoid dendritic cells, and ECs (Figure 4A), whereas MPs and ECs expressing EpCAM were present in large numbers in the ascites samples (Figures 4C and 4D). We observed a large number of ECs in the PD’ group before CT041 treatment, which gradually decreased after CT041 treatment (Figures 4B, S3A, S3B, and Table 3). The proportion of plasma cells and fibroblasts was higher in the PR’ group than in the PD’ group. In particular, the proportion of T cells in the PR’ group was significantly higher than that in the PD’ group (Figure S3C), which was consistent with the patterns observed in the blood and ascites samples (Figures S3A and 2D). Therefore, we conducted further analyses of T cells and ECs in the ascites to explore the differences in treatment efficacy.
Figure 4.
Cellular profiling of the patients’ ascites samples
(A) Color-coded uniform manifold approximation and projection (UMAP) and marker gene feature plot of cell types from the 15 ascites samples.
(B) Histogram of ascites cell composition. PD, progressive disease group; PR, partial response group; P1–3, ascites samples before transfusion, 3 days after transfusion, and 7 days after transfusion, respectively.
(C) The UMAP showed the expression of signature genes of cell types.
(D) Top 5 most differentially expressed genes, presented as point maps for each ascites cell subgroup (horizontal coordinate, gene name; vertical coordinate, cell subgroup; red, high expression; blue, low expression).
Table 3.
The cell type, abbreviation, and marker gene of ascites samples
| Abbreviation | Cell type | Markers |
|---|---|---|
| TCells | T cells | CD2, CD3D, TRAC, TRBC2 |
| PlasmaCells | Plasma cells | JCHAIN, MZB1, CD79A, IGHG1 |
| MPs | Mononuclear phagocytes | LYZ, CD14, VCAN, C1QA, CD68, CD1C, CCR7, LAMP3 |
| pDCs | Plasmacytoid dendritic cells | LYZ, IL3RA, CLEC4C, LILRB4, TCF4, TCL1A |
| Neutrophils | Neutrophils | CSF3R, CXCR2, FCGR3B, G0S2 |
| Fibroblasts | Fibroblasts | DCN, COL1A1, COL1A2 |
| EpithelialCells | Epithelial cells | EPCAM, CLDN4, KRT18, KRT8 |
| MastCells | Mast cells | TPSAB1, TPSB2, CPA3 |
| NaiveT | Naive T cells | CCR7, LEF1, SELL, TCF7, CD8A, CD4 |
| CD8Teff | CD8+ effector T cells | CD8A, NKG7, GZMA, GNLY |
| Treg | Regulatory T cells | FOXP3, IL2RA, CTLA4, IKZF2 |
| HelperT | T helper cells | CXCR6, CD4, GATA3, CD40LG |
| NK | Natural killer cells | NKG7, XCL1, XCL2, KLRF1, CD3D |
| Basophils | Basophils | CLC, CPA3, MS4A2, GATA2 |
| Macrophages | Macrophages | MRC1, C1QA, CXCL10, CD68 |
| Monocytes | Monocytes | LYZ, CD14, VCAN, FCN1 |
| cDC1 | Conventional type 1 dendritic cells | XCR1, IDO1, CLEC9A, IRF8 |
| cDC2 | Conventional type 2 dendritic cells | CD1C, CD1E, FCER1A, CLEC10A |
| MatureDCs | Mature dendritic cells | CCR7, LAMP3, CCL22 |
Increased number of naive T cells detected in ascites of responders
In the ascites samples, 17 different cell subsets were identified, including cluster 1 (C1), which expressed CCR7, and cluster 6, which expressed FOXP3. The CD8+ effector T cells were mainly found in clusters 2, 5, and 8 (Figures 5A and S4A). There were mainly C2, C3, and C4 in the PD’ group and C1 in the PR’ group, which gradually decreased after treatment (Figure 5B). After CT041 infusion, T cells were elevated to different degrees in the ascitic fluid samples (Figure S4b). Similar to the blood sample results, the PR’ group had a higher proportion of naïve-like T cells in the ascites samples, which gradually decreased with treatment duration. To distinguish CAR T cells from other T cells in vivo, we used RNA sequencing (RNA-seq) to identify CAR T cells and their specific genes, including KLRC1 and GZMA (Figure 5C). Previous studies have reported differences between CAR T cells and non-CAR T cells in hematological malignancies.13 Therefore, we compared our data with hematological malignancy datasets to identify genes that specifically identified CAR T cells (Figure 5D). Using these genes to generate a CAR score uniform manifold approximation and projection (UMAP), we found that the CAR score was highest in CD4+ (clusters 3, 4, 5, 9, 11, and 12) and CD8+ (clusters2, 5, 7, 8, and 13) T cell subsets, as well as in cluster 10, which expressed NKG7 (Figures 5E and S4C).
Figure 5.
Characterization of the immune-cell component of the patients’ ascites samples
(A) Uniform manifold approximation and projection (UMAP) of T cell clusters in the 15 ascites samples.
(B) Histogram of T cell population proportions in the ascites samples (left) and subgroups (right). PD, progressive disease group; PR, partial response group; P1–3, ascites samples before transfusion, 3 days after transfusion, and 7 days after transfusion, respectively.
(C) Volcano plot of CAR+ vs. CAR− T cells.
(D) Venn diagram: left, previously published genes associated with CAR T cells; right, genes identified in this study.
(E) CAR T cell marker gene score UMAP (higher scores are indicated by darker red).
(F) Boxplot of CAR T cell marker gene expression in the different cell subsets.
CLDN18 overexpression in epithelial cells is a good prognostic indicator for CT041
We further clustered the ECs into 14 subsets (Figure 6A). Most ECs originated from case 1, whereas EC 1 and EC 2 were mainly from cases 3 and 4, which were from the PR’ group, and case 5 had relatively fewer ECs (Figures 5A and 5B). Overall, ECs were relatively abundant in the PD’ group when compared to those in the PR’ group (Figure 6B). The cells in clusters 1 and 2 were mainly from the PR’ group and highly expressed CLDN18, which is the target of CT041. We further found that patients in the PR’ group showed a higher proportion and expression of CLDN18 during the treatment process (Figures 6C, 6D, S5C, and S5D). Furthermore, we performed inferred copy-number variation (CNV) analysis and found that malignant ECs with high CLDN18 expression had distinct CNV patterns with weaker mutations on chromosomes 8q+ and 8p+, whereas other tumor cells from patients with PD’ had more enrichment on chromosome 8 and higher expression of MYC genes (Figures 6E, 6F, and S5E), indicating that there is a distinct genome and transcriptome signature that may be an important indicator for CT041 clinical responses.
Figure 6.
Characterization of the epithelial cell (EC) component of patients’ ascites samples
(A) Uniform manifold approximation and projection (UMAP) of staining clusters in ascites EC types.
(B) Histogram of the cell composition of EC subsets (left) and ECs grouped by therapeutic effect, color-coded by UMAP clustering (right).
(C) Point map showing the top five most expressed genes for the ascites EC subsets. Horizontal coordinate, gene name; vertical coordinate, cell subsets. Red, high expression; blue, low expression. Dot size represents percentage expression.
(D) Violin plot of CLDN18 expression in different ascites samples.
(E) inferCNV diagram of the EC subsets. Top: expression in normal cells; bottom: expression in ECs. Genes are arranged from left to right across the entire chromosome. Red, chromosome amplification; blue, chromosome deletion.
(F) Violin plots of EC MYC expression in the progressive disease (PD) and partial response (PR) groups, p < 0.05(Wilcoxon rank-sum test).
The relationship between cell communication and CT041 efficacy
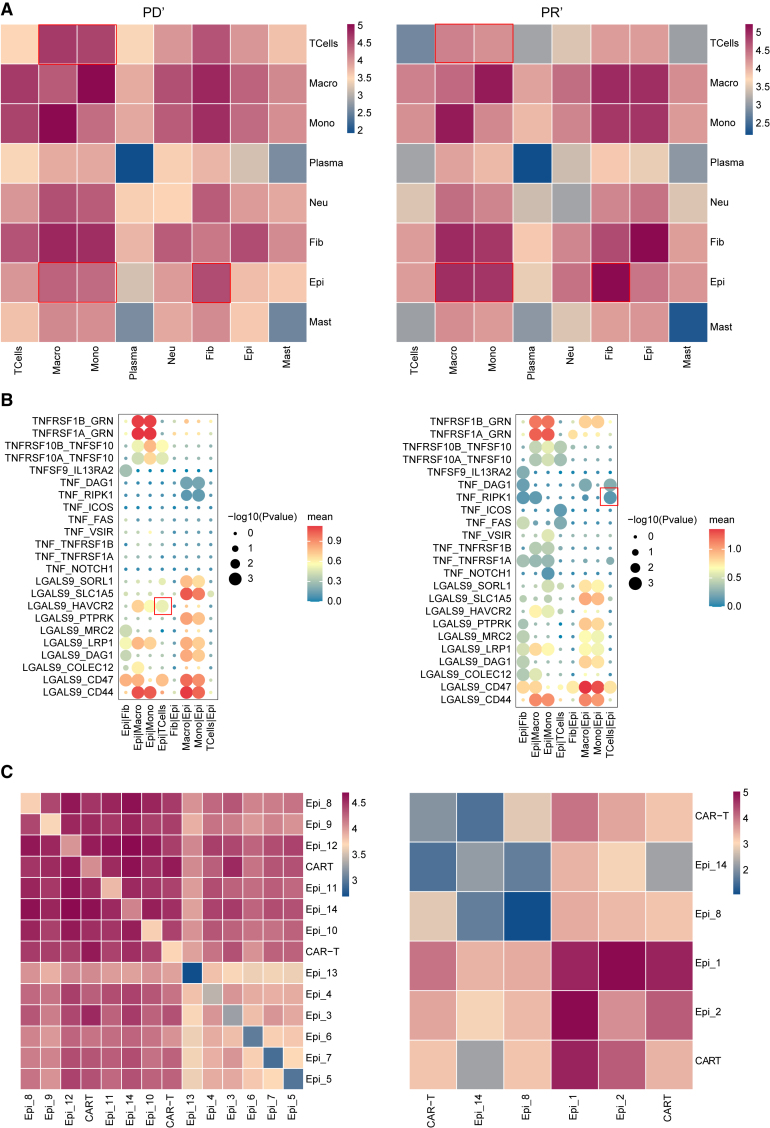
We investigated the interactions between different cell types in the ascites. The results showed that the interactions among ECs and MPs, monocytes, and fibroblasts were significantly enhanced in the PR’ group compared to those in the PD’ group. However, interactions between T cells and MPs/monocytes were significantly weaker in the PR’ group (Figure 7A). The interaction of tumor necrosis factors (TNF) was highly expressed in the PR’ group, and the TNF-RIPK1 interaction promoted apoptosis and necrosis, thereby inhibiting tumor cell growth and metastasis.14 In the PD’ group, there was a significant increase in the interaction between T cells and ECs. LGALS9 and TIM-3 interactions promoted tumor growth and immune escape, making it difficult for immune cells to attack and eliminate tumor cells (Figure 7B).15,16 We found that there was a strong interaction between CAR T cells and epi1/epi2 in the PR’ group when compared with the PD’ group, which was an important factor for patient response to the CT041 treatment (Figure 7C).
Figure 7.
Cell-cell communication and interactions between cell subsets in patients’ ascites samples
(A) The communication between cells in ascites samples represented as a heatmap for the progressive disease (PD) (left) and partial response (PR) (right) groups. Increasing red, stronger interaction; increasing blue, weaker interaction.
(B) Spot diagram of ascites against different cell types. Red, high average expression; blue, low average expression. Larger dots, more significant interactions.
(C) Heatmap illustrating the communication between cell subsets in ascites samples in the PD (left) and PR (right) groups.
Discussion
Almost 1,000 clinical trials for solid tumors are ongoing as of April 2022 (ClinicalTrials.gov), indicating that CAR T therapy is gradually broadening its scope in the field of solid tumors17,18. The trending targets include Claudin18.2, GPC3, and MSLN. Most studies on solid tumors are still in the preclinical stage, with CT041-CG4006 being the first and largest clinical cohort targeting Claudin18.2-positive solid tumors. This study originated from a clinical trial to investigate the dynamic evolution of CT041 in patients. In these patients, in vivo CAR T cell expansion peaked 7 days after treatment, and CAR T cell expansion was considerably higher in the ascites than in the peripheral blood. Patients with a high pre-treatment proportion of peripheral blood T cells, including many cytotoxic T cells and few inhibitory T cells, were more likely to benefit from CT041 therapy.
Different cell subsets play important roles in cell therapy for hematological malignancies. Different CAR T cell subsets can lead to different expansion patterns and differentiation into different phenotypes19,20 that result in different clinical outcomes. A recent study examined two patients with chronic lymphocytic leukemia, who remained in complete remission for 10 years; their peripheral blood contained many cytotoxic CD4+ T cells that continued expressing Ki67 long after CAR T therapy.21 In our study, all patients exhibited an elevated proportion of CD8A+ effector T cells after treatment, and CCR7-expressing naïve-like T cells were present in the peripheral blood. Patients with this subset of cells had a significantly longer duration of benefit, and the cytotoxic function of CAR T cells was likely to be better in patients with naïve-like T cells in their ascites before treatment. This suggests that enriching specific subsets of T cells may improve the efficacy of CAR T therapy, providing theoretical support for more accurate patient selection.
Target selection and expression are crucial for CAR T therapy, and tumor heterogeneity often limits the efficacy of the treatment of solid tumors.22 In our study, although CLDN18.2 expression was consistent in the patients’ gastric cancer tissue samples, the efficacy of CAR T therapy differed between them, and patients with stronger CLDN18 expression in ascites ECs benefited more from treatment. Differences in target expression between metastatic lesions lead to different clinical outcomes; it is therefore crucial to select patients with high and uniform target-gene expression throughout the body. Target loss is an important factor influencing CAR T therapy: the loss of CD19 has been reported to result in relapse and drug resistance following CAR T therapy.23 Frequent sampling of solid tumors can damage tissues, making it difficult to dynamically monitor changes in multiple sites within the body; therefore, noninvasive targeted detection may become a new practice. We previously examined a nuclear-probe labeling method24 to detect target expression at multiple sites within the body; this probe effectively resolved this problem.
The role of the tumor immune microenvironment during CAR T therapy should not be ignored because it is not conducive to CAR T cell infiltration into tumor tissues.25 Current targeted therapies focus primarily on tumor tissue, often ignoring interactions between stromal and tumor tissue in the tumor microenvironment.26,27 Ascites in the peritoneal cavity also have a unique tumor immune microenvironment; we found that there is a large infiltration of MPs in the ascites, and the interaction between ECs and MPs/monocytes was significantly enhanced in the PR’ group. The interaction between ECs and fibroblasts was also significantly enhanced, whereas that between T cells and MPs/monocytes was significantly weakened, which has not been reported in previous literature.
This study dynamically collected blood and ascites samples from patients with gastric cancer treated with CAR T cells, analyzed the cell types and their proportions in the blood and ascites samples after CAR T cell therapy using single-cell transcriptome sequencing technology, and explored the differences in treatment efficacy among patients. This study lays a solid foundation for selecting suitable patients for and improving the efficacy of CAR T therapy in the future.
Limitations of the study
The main limitation of this study was the relatively small sample size, which was limited by the number of patients with gastric cancer and ascites enrolled in our clinical trials. Indeed, there was only one subject in the PR group, which may have caused a bias. To reduce the risk of gastric perforation, we did not perform single-cell sequencing analysis of gastric tumor tissues, which also limited the scope of this study. Future expansion of the cohort can be combined with multi-omics technology for more in-depth exploration.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Changsong Qi (changsongqi@bjmu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
All single-cell RNA-seq data have been deposited at China National Center for Bioinformation with the number of HRA006971(https://www.cncb.ac.cn/).
-
•
This study does not report any original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
We thank the patients, their families, and the study personnel involved in this trial. We thank Yan Jiang and Jiaxin Xiong for helping with the single-cell sequencing data analysis. This study was funded by the National Key R&D Program of China (2022YFA0912400), the Science Foundation of Peking University Cancer Hospital (2022-14), Beijing Hospitals Authority Youth Programme (QMS20201101), Science Foundation of Peking University Cancer Hospital (JC202303, JC202406), Clinical Medicine Plus X - Young Scholars Project of Peking University, Peking University Clinical Scientist Training Program, the Fundamental Research Funds for the Central Universities and by CARsgen Therapeutics Co., Ltd.
Author contributions
The manuscript was written by M.M. and C.L. and critically reviewed and revised by L.J., D.L., P.Z., M.T., M.Z., J.G., Z.P., X.T., J.L., C.Z., M.D., and C.Q. All authors contributed to the analysis and interpretation of the data. The authors affirm the accuracy and completeness of the data.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Gastric cancer blood sample | Peking University Cancer Hospital | NA |
| Gastric cancer ascites sample | Peking University Cancer Hospital | NA |
| Chemicals, peptides, and recombinant proteins | ||
| PBS(add Ca2+/Mg2+) | BasicMedia | REF B320KJ |
| PBS | HycloneTMcytiva | SH30258.01 |
| Ficoll-PaqueTM PLUS | HycloneTMcytiva | Cat.No17144003 |
| Trypan Blue Dye 0.4% | BIO-RED | Cat. #14500013 |
| Critical commercial assays | ||
| GEXSCOPE® Single Cell RNA Library Kits | Singleron | 1100170006 |
| Deposited data | ||
| All single-cell RNA-sequencing data generated by this study | This paper:Genome sequence archive for human (GSA-human) | HRA006971 |
| DEG | GitHub | https://github.com/felon02/paperData/blob/main/HRA006971_DEG.xlsx ) |
| Software and algorithms | ||
| CeleScope | Singleron | https://github.com/singleron-RD/CeleScope |
| SynEcoSys database | Singleron | https://www.synecosys.cn/#/ |
| Seurat | Satija et al.28 | https://github.com/satijalab/seurat |
| Harmony | Korsunsky et al.29 | https://github.com/immunogenomics/harmony |
| InferCNV | Kumar et al.30 | https://github.com/broadinstitute/InferCNV |
| CellPhoneDB | Efremova et al.31 | https://github.com/Teichlab/cellphonedb |
Experimental model and study participant details
Samples and ethical statement
The experimental samples were obtained from an ongoing, open-label, single-arm, phase 1 clinical trial (NCT03874897) of CLDN18.2-targeted CT041 CAR T-cell treatment in patients previously treated for CLDN18.2-positive digestive-system cancers. The investigator initiated the study. The study protocol was approved by the local ethics committee of the Department of Gastrointestinal Oncology at the Peking University Cancer Hospital (Approval Number: 2018YJZ75). Written informed consent was obtained from all patients. This study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. The patients received compensation for travel and meals during the visit.
Method details
Retention of patient samples
Five patients with gastric cancer and ascites, all of whom had received 2.5 × 108 CAR T-cells, were selected. The detailed clinical information is presented in Table S1. Peripheral blood and paired ascites samples (via peritoneal dropsy) were collected from these patients before, 3 d after, and 7 d after treatment. CAR T-cell expansion was detected in the samples, and peripheral blood cytokine release was dynamically monitored for 8 weeks. Other clinical information was obtained from hospital laboratory reports. Patients with no radiographic recurrence after the 6-month follow-up were defined as responders. Those with no initial response after 28 d or with disease progression before the 6 month follow-up were defined as non-responders. An improvement in the ascites index alone indicated effective control of ascites; an increase in ascites index indicated nonresponsive ascites.
qPCR analysis of CAR expression in CLDN18.2 cell expansion and persistence
Persistence of CAR-CLDN18.2 cells was determined via qPCR at the central laboratory of CARsgen Therapeutics Co., Ltd (Xuhui, China). Clinical samples (8 mL peripheral blood and 10 mL ascites) were collected in K2EDTA tubes and gDNA was extracted using the QIAamp DNA Midi Kit (Qiagen, Hilden, Germany). The number of transgene copies per microgram of genomic DNA was determined using a 7500 Fast Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA) in triplicate for each sample. The limit of detection for this assay was 40 copies/g of genomic DNA.
Tissue dissociation and preparation
After passing the ascites samples through a 40 μm sterile strainer, they were centrifuged at 350 ×g for 5 min. The precipitate was then resuspended in 1 mL PBS (HyClone, Logan, UT). The red blood cells in ascites and blood samples were removed using GEXSCOPE red blood cell lysis buffler (RCLB) (Singleron Biotechnologies, Cologne, Germany) at a cell : RCLB ratio of 1:2 (v/v) at 25°C for 5–10 min. Ficoll-Paque Plus medium (GE Healthcare, Chicago, IL) was used to isolate PBMCs via density-gradient centrifugation, and PBMCs were washed with Ca/Mg-free PBS. After removing the red blood cells, the samples were centrifuged sequentially (500 ×g for 5 min, at 400 ×g for 5 min at 4°C, and 400 ×g for 10 min at 4°C) and the supernatant was discarded. The PBMCs were resuspended in PBS to obtain a single-cell suspension. Finally, Trypan Blue was used to evaluate cell viability under a microscope.
RT & amplification & library construction
Single-cell suspensions (2×105 cells/mL) with PBS (HyClone) were loaded onto microwell chip using the Singleron Matrix® Single Cell Processing System. Barcoding Beads are subsequently collected from the microwell chip, followed by reverse transcription of the mRNA captured by the Barcoding Beads and to obtain cDNA, and PCR amplification. The amplified cDNA is then fragmented and ligated with sequencing adapters. The scRNA-seq libraries were constructed according to the protocol of the GEXSCOPE® Single Cell RNA Library Kits (Singleron),32 Individual libraries were diluted to 4 nM, pooled, and sequenced on Illumina novaseq 6000 with 150 bp paired end reads.
Primary analysis of raw reads data
Raw reads from scRNA-seq were processed to generate gene expression matrixes using CeleScope (https://github.com/singleron-RD/CeleScope) v1.9.0 pipeline. Briefly, raw reads were first processed with CeleScope to remove low quality reads with Cutadapt v1.1733 to trim poly-A tail and adapter sequences. Cell barcode and UMI were extracted. After that, we used STAR v2.6.1a34 to map reads to the reference genome GRCh38 (ensembl version 92 annotation). UMI counts and gene counts of each cell were acquired with featureCounts v2.0.135 software, and used to generate expression matrix files for subsequent analysis.
Quality control, dimension-reduction, and clustering
Cells were filtered by gene counts below 200 and the top 2% gene counts and the top 2% UMI counts. Cells with over 50% mitochondrial content were removed. After filtering, 173495 (blood)/126368 (peritoneal dropsy) cells were retained for the downstream analyses, with on average 843(blood)/2034 (peritoneal dropsy) genes and 2825(blood)/7053 (peritoneal dropsy) UMIs per cell. We used functions from Seurat v3.1.228 for dimension-reduction and clustering. Then we used NormalizeData and ScaleData functions to normalize and scale all gene expression, and selected the top 2000 variable genes with FindVariableFeautres function for PCA analysis. Using the top 20 principle components, we separated cells into multiple clusters with FindClusters. Batch effect between samples was removed by Harmony.29 Finally, UMAP algorithm was applied to visualize cells in a two-dimensional space.
Batch effect removal
To remove the batch effect among samples, Harmony v1.0 was used with the top 20 principal components from the PCA.
Cell type annotation and analysis
The cell type identity of each cluster was determined with the expression of canonical markers found in the DEGs using SynEcoSys database. Heatmaps/dot plots/violin plots displaying the expression of markers used to identify each cell type were generated by Seurat v3.1.2 DoHeatmap/DotPlot/Vlnplot. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were used with the “clusterProfiler” R package 3.16.1.36 Pathways with p_adj value less than 0.05 were considered as significantly enriched. The InferCNV package was used to detect the CNAs in 18216 malignant cells.30 5000 non-malignant cells were used as baselines to estimate the CNAs of malignant cells. Genes expressed in more than 20 cells were sorted based on their loci on each chromosome. The gene set score was evaluated using R package UCell v 1.1.0. The UCell score is based on Mann Whitney U statistics, ranking the query genes in order of their expression levels in individual cells. The cell-cell interaction analysis was performed by CellPhoneDB v2.1.031 based on known receptor–ligand interactions between two cell types/subtypes. Cluster labels of all cells were randomly permuted for 1000 times to calculate the null distribution of average ligand-receptor expression levels of the interacting clusters.
Quantification and statistical analysis
Single-cell RNAseq data analysis were conducted using R version 3.5.1. The statistical testing methods can be found in the legends. In Figure 1A, the Mann-Whitney test is used with a significance level defined as ∗p < 0.05, in Figure 6F, the Wilcox.test is used with p < 0.05. The statistical testing method used for single-cell data analysis is not specified and is referred to as "Wilcox" (Wilcox non-parametric rank sum test).
Additional resources
Published: January 7, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.111768.
Supplemental information
References
- 1.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y., et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullard A. FDA approves fourth CAR T-cell therapy. Nat. Rev. Drug Discov. 2021;20:166. doi: 10.1038/d41573-021-00031-9. [DOI] [PubMed] [Google Scholar]
- 4.Munshi N.C., Anderson L.D., Jr., Shah N., Madduri D., Berdeja J., Lonial S., Raje N., Lin Y., Siegel D., Oriol A., et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 2021;384:705–716. doi: 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 5.Newick K., O’Brien S., Moon E., Albelda S.M. CAR T-cell therapy for solid tumors. Annu. Rev. Med. 2017;68:139–152. doi: 10.1146/annurev-med-062315-120245. [DOI] [PubMed] [Google Scholar]
- 6.Berger T.R., Maus M.V. Mechanisms of response and resistance to CAR T-cell therapies. Curr. Opin. Immunol. 2021;69:56–64. doi: 10.1016/j.coi.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Hong M., Clubb J.D., Chen Y.Y. Engineering CAR T-cells for next-generation cancer therapy. Cancer Cell. 2020;38:473–488. doi: 10.1016/j.ccell.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Larson R.C., Maus M.V. Recent advances and discoveries in the mechanisms and functions of CAR T-cells. Nat. Rev. Cancer. 2021;21:145–161. doi: 10.1038/s41568-020-00323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou A.J., Chen L.C., Chen Y.Y. Navigating CAR T-cells through the solid-tumour microenvironment. Nat. Rev. Drug Discov. 2021;20:531–550. doi: 10.1038/s41573-021-00189-2. [DOI] [PubMed] [Google Scholar]
- 10.Shah N.N., Fry T.J. Mechanisms of resistance to CAR T-cell therapy. Nat. Rev. Clin. Oncol. 2019;16:372–385. doi: 10.1038/s41571-019-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow A., Perica K., Klebanoff C.A., Wolchok J.D. Clinical implications of T-cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2022;19:775–790. doi: 10.1038/s41571-022-00689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi C., Gong J., Li J., Liu D., Qin Y., Ge S., Zhang M., Peng Z., Zhou J., Cao Y., et al. Claudin18.2-specific CAR T-cells in gastrointestinal cancers: phase 1 trial interim results. Nat. Med. 2022;28:1189–1198. doi: 10.1038/s41591-022-01800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haradhvala N.J., Leick M.B., Maurer K., Gohil S.H., Larson R.C., Yao N., Gallagher K.M.E., Katsis K., Frigault M.J., Southard J., et al. Distinct cellular dynamics associated with response to CAR T therapy for refractory B cell lymphoma. Nat. Med. 2022;28:1848–1859. doi: 10.1038/s41591-022-01959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taft J., Markson M., Legarda D., Patel R., Chan M., Malle L., Richardson A., Gruber C., Martín-Fernández M., Mancini G.M.S., et al. Human TBK1 deficiency leads to autoinflammation driven by TNF-induced cell death. Cell. 2021;184:4447–4463.e20. doi: 10.1016/j.cell.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monney L., Sabatos C.A., Gaglia J.L., Ryu A., Waldner H., Chernova T., Manning S., Greenfield E.A., Coyle A.J., Sobel R.A., et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 16.Jin H.T., Anderson A.C., Tan W.G., West E.E., Ha S.J., Araki K., Freeman G.J., Kuchroo V.K., Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saez-Ibañez A.R., Upadhaya S., Partridge T., Shah M., Correa D., Campbell J. Landscape of cancer cell therapies: trends and real-world data. Nat. Rev. Drug Discov. 2022;21:631–632. doi: 10.1038/d41573-022-00095-1. [DOI] [PubMed] [Google Scholar]
- 18.Pang N., Shi J., Qin L., Chen A., Tang Y., Yang H., Huang Y., Wu Q., Li X., He B., et al. IL-7 and CCL19-secreting CAR T-cell therapy for tumors with positive glypican-3 or mesothelin. J. Hematol. Oncol. 2021;14:118. doi: 10.1186/s13045-021-01128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheih A., Voillet V., Hanafi L.A., DeBerg H.A., Yajima M., Hawkins R., Gersuk V., Riddell S.R., Maloney D.G., Wohlfahrt M.E., et al. Clonal kinetics and single-cell transcriptional profiling of CAR T-cells in patients undergoing CD19 CAR T immunotherapy. Nat. Commun. 2020;11:219. doi: 10.1038/s41467-019-13880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vormittag P., Gunn R., Ghorashian S., Veraitch F.S. A guide to manufacturing CAR T-cell therapies. Curr. Opin. Biotechnol. 2018;53:164–181. doi: 10.1016/j.copbio.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Melenhorst J.J., Chen G.M., Wang M., Porter D.L., Chen C., Collins M.A., Gao P., Bandyopadhyay S., Sun H., Zhao Z., et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature. 2022;602:503–509. doi: 10.1038/s41586-021-04390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maalej K.M., Merhi M., Inchakalody V.P., Mestiri S., Alam M., Maccalli C., Cherif H., Uddin S., Steinhoff M., Marincola F.M., Dermime S. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol. Cancer. 2023;22:20. doi: 10.1186/s12943-023-01723-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain M.D., Ziccheddu B., Coughlin C.A., Faramand R., Griswold A.J., Reid K.M., Menges M., Zhang Y., Cen L., Wang X., et al. Whole-genome sequencing reveals complex genomic features underlying anti-CD19 CAR T-cell treatment failures in lymphoma. Blood. 2022;140:491–503. doi: 10.1182/blood.2021015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y., Hou X., Li D., Ding J., Liu J., Wang Z., Teng F., Li H., Zhang F., Gu Y., et al. Development of a CLDN18.2-targeting Immuno-PET probe for non-invasive imaging in gastrointestinal tumors. J. Pharm. Anal. 2023;13:367–375. doi: 10.1016/j.jpha.2023.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafiq S., Hackett C.S., Brentjens R.J. Engineering strategies to overcome the current roadblocks in CAR T-cell therapy. Nat. Rev. Clin. Oncol. 2020;17:147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smyth M.J., Ngiow S.F., Ribas A., Teng M.W.L. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 2016;13:143–158. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 27.Joyce J.A., Pollard J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satija R., Farrell J.A., Gennert D., Schier A.F., Regev A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 2015;33:495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korsunsky I., Millard N., Fan J., Slowikowski K., Zhang F., Wei K., Baglaenko Y., Brenner M., Loh P.R., Raychaudhuri S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods. 2019;16:1289–1296. doi: 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar M., Bowers R.R., Delaney J.R. Single-cell analysis of copy-number alterations in serous ovarian cancer reveals substantial heterogeneity in both low- and high-grade tumors. Cell Cycle. 2020;19:3154–3166. doi: 10.1080/15384101.2020.1836439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efremova M., Vento-Tormo M., Teichmann S.A., Vento-Tormo R. CellPhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat. Protoc. 2020;15:1484–1506. doi: 10.1038/s41596-020-0292-x. [DOI] [PubMed] [Google Scholar]
- 32.Dura B., Choi J.-Y., Zhang K., Damsky W., Thakral D., Bosenberg M., Craft J., Fan R. scFTD-seq: freeze-thaw lysis based, portable approach toward highly distributed single-cell 3′ mRNA profiling. Nucleic Acids Res. 2019;47:e16. doi: 10.1093/nar/gky1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. j. 2011;17:10–12. [Google Scholar]
- 34.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 36.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS A J. Integr. Biol. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All single-cell RNA-seq data have been deposited at China National Center for Bioinformation with the number of HRA006971(https://www.cncb.ac.cn/).
-
•
This study does not report any original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.