Abstract
Purpose
To evaluate a radiomic approach for the stratification of diffuse gliomas with distinct prognosis and provide additional resolution of their clinicopathological and molecular characteristics.
Methods
For this retrospective study, a total of 704 radiomic features were extracted from the multi-channel MRI data of 166 diffuse gliomas. Survival-associated radiomic features were identified and submitted to distinguish glioma subtypes using consensus clustering. Multi-layered molecular data were used to observe the different clinical and molecular characteristics between radiomic subtypes. The relative profiles of an array of immune cell infiltrations were measured gene set variation analysis approach to explore differences in tumor immune microenvironment.
Results
A total of 6 categories, including 318 radiomic features were significantly correlated with the overall survival of glioma patients. Two subgroups with distinct prognosis were separated by consensus clustering of radiomic features that significantly associated with survival. Histological stage and molecular factors, including IDH status and MGMT promoter methylation status were significant differences between the two subtypes. Furthermore, gene functional enrichment analysis and immune infiltration pattern analysis also hinted that the inferior prognosis subtype may more response to immunotherapy.
Conclusion
A radiomic model derived from multi-parameter MRI of the gliomas was successful in the risk stratification of diffuse glioma patients. These data suggested that radiomics provided an alternative approach for survival estimation and may improve clinical decision-making.
Keywords: Radiomics, Diffuse glioma, Tumor immune microenvironment, Survival estimation
Introduction
Diffuse gliomas, the most common type of malignant brain tumor, are currently stratified across three malignancy grades: II, III, and IV (Olar et al. 2015). Although the morbidity of diffuse gliomas is not very high among all tumors, it is associated with substantial mortality and exerts a large burden on families and society (Bray et al. 2018; Ferlay 2018). The highly distinct clinical and biological characteristics of diffuse gliomas create an insurmountable obstacle for precision medicine. Histological criteria are used to classify adult diffuse gliomas into the following types: oligodendroglioma, oligoastrocytoma, astrocytoma, and glioblastoma (Ceccarelli et al. 2016). However, there is substantial inter-observer variability in the histopathological diagnosis of diffuse gliomas (van den Bent 2010). With advance in molecular detection techniques, discoveries over the past decade have identified several genetic molecular biomarkers that are useful in the diagnostic and prognostic management of patients with gliomas (Eckel-Passow et al. 2015; Wen and Huse 2017). Genomic and epigenomic landscape suggest that gliomas have pronounced heterogeneity (Binder et al. 2019; Eder and Kalman 2014). As a result, in 2016, the WHO classification of central nervous system tumors integrated molecular alterations and histopathological diagnosis for the precision diagnosis and grading of diffuse gliomas (Louis et al. 2016).
The significant heterogenetic nature of diffuse gliomas means that effective risk stratification is required for patient management. Previously, many studies developed molecular subtype classifiers to provide a useful approach for survival stratification (Noushmehr et al. 2010; Patel et al. 2014; Phillips et al. 2006). However, the invasive availability of specimens limited the clinical application of these findings. Hence, a reproducible, non-invasive medical imaging approach may have great advantages. The broad spectrum of pathologies in diffuse gliomas and complex brain parenchyma poses unique demands on medical imaging. Radiomics, a rapidly evolving field, allows the conversion of medical images to quantitative features (Aerts et al. 2014; Lambin et al. 2012). Radiomics has contributed a great deal to the application of medical imaging in tumor characteristic quantification. Recently, the radiomic analysis of diffuse gliomas has been used to characterize the clinical and biological characteristics of tumors efficiently and has provided a non-invasive window allowing the dynamic monitoring of tumors in real time (Akkus et al. 2017; Bi et al. 2019). More importantly, imaging phenotype changes are the reflection of deep molecular mechanisms (Tan et al. 2019; Zhang et al. 2018). Hence, the ability to identify genotype signatures based on radiomic analysis becomes increasingly appreciated in influencing tumor behavior and clinical outcome. However, global survival impact and the multi-omics data relationships of radiomics in gliomas require further exploration.
Here, we developed novel diffuse glioma classification system by identifying specific prognostic subtypes on the basis of radiomic profiles and comprehensively analyzed radiogenomic phenotype using multi-omics molecular approaches. The diffuse glioma classification system had distinct molecular and clinical features and provided a further understanding of the novel molecular characteristics of diffuse glioma medical imaging phenotypes.
Methods
Medical imaging data
All patient data included in the study were acquired from a public online database The Cancer Imaging Archive (TCIA) (Clark et al. 2013; Spyridon Bakas et al. 2017a, b). One patient was removed owing to a lack of clinical information. A total of 166 glioma patients, 101 glioblastoma (GBM) patients and 65 lower grade glioma (LGG) patients, were included. Follow-up information was obtained from TCGA pan-cancer project (https://gdc.cancer.gov/about-data/publications) (Liu et al. 2018). Overall survival (OS) was chosen as the clinical endpoint. Other clinical and multi-omics genomic information were acquired from previous study (Ceccarelli et al. 2016). Patients’ average age was 52.4 years with a range of 18–85 years. Out of the 166 diffuse gliomas patients, 54% (90/166) were male and 46% (76/166) were female. The majority of samples were grade IV tumors (n = 101, 60.8%), whereas 28 (16.9%) and 37 (22.3%) were grade II and III tumors, respectively. Patient clinical and molecular characteristics have been summarized in Table 1.
Table 1.
The general characteristics of patients included in the study
| Features | Number of patients |
|---|---|
| Total | 166 |
| Age | |
| ≤ 50 | 66 |
| > 50 | 100 |
| Histology | |
| Astrocytoma | 21 |
| Glioblastoma | 101 |
| Oligoastrocytoma | 18 |
| Oligodendroglioma | 26 |
| Grade | |
| G2 | 28 |
| G3 | 37 |
| G4 | 101 |
| Gender | |
| Male | 90 |
| Female | 76 |
| IDH status | |
| Mutant | 57 |
| Wildtype | 90 |
| NA | 19 |
| 1p/19q codeletion | |
| Codel | 13 |
| Non-codel | 148 |
| NA | 5 |
| MGMT promoter status | |
| Methylated | 79 |
| Unmethylated | 48 |
| NA | 39 |
Radiomics analysis
Implementation details of the medical image processing used in the study have been documented previously (Bakas et al. 2017). Further detailed information on the diversity of imaging sequences has been included in Table 2. Briefly, pre-operative multimodal magnetic resonance imaging (MRI), included the following types: pre-contrast T1-weighted (T1), post-contrast T1-weighted (T1-Gd), T2-weighted (T2), and T2 fluid-attenuated inversion recovery (T2-FLAIR), were first submitted to various pre-processing steps, including re-oriented and co-registered, skull-stripped and de-noised: Multimodal MRI volumes were re-oriented to left-posterior-superior coordinate system and co-registered to the same T1 anatomic template using affine registration (Jenkinson et al. 2002; Jenkinson and Smith 2001). Skull-stripping processes were conducted using the Brain Extraction Tool (Smith 2002). If the effect was unsatisfactory, the Multi-Atlas Skull-Stripping (MASS) method was used (Doshi et al. 2013). The Smallest Univalue Segment Assimilating Nucleus, an approach to low-level image processing, was used to reduce noise (Smith and Brady 1997). Then, three sub-regions, including the enhancing part of the tumor core (ET), the non-enhancing part of the tumor core (NET), and the peritumoral edema (ED) were delineated using computer-aided and experts manually corrected by experts. Computer-aided segmentation was performed using the GLioma Image SegmenTation and Registration (GLISTR) software (Bakas et al. 2016; Gooya et al. 2012). Finally, segmentation manual revisions and/or corrections were applied by two computational imaging scientists and a medical doctor with rich medical imaging experience.
Table 2.
Detail imaging parameters for the radiomic analysis
| Parameters | T1 | T1-Gd | T2 | T2_FLAIR |
|---|---|---|---|---|
| Slice thickness | 1–5 mm | 1–5 mm | 1–5 mm | 2-5 mm |
| Repetition time | 4.98–3379.57 ms | 5.87–5500 ms | 5.10–6650 ms | 1000–11000 ms |
| Echo time | 2.1–20 ms | 2.1–17 ms | 1.46–252.05 ms | 74–155 ms |
| Slice spacing | 1–7.5 mm | 1–7.5 mm | 0.98–10 mm | 2–7.5 mm |
| Slices number | 16–359 | 16–339 | 9–235 | 20–178 |
Based on manually corrected tumor sub-region segmentation, a total of 704 radiomic features were extracted volumetrically (in 3D), including intensity, volumetric, morphologic, histogram-based, textural parameters, spatial features, and glioma diffusion properties extracted from glioma growth models.
Survival analysis
Several radiomic features with missing values were imputed using k-nearest neighbor method in “impute” software (Hastie 2019). Then, we applied the Z-score method to normalize the radiomic profile. Univariate COX analysis was performed to reveal the relationships between radiomic features and the OS of gliomas patients. Radiomic features with significant prognostic value were used for further subtype classification.
For unsupervised clustering analysis, 318 survival associated radiomic features were submitted to identify prognosis-based subtypes. Consensus clustering was performed to determine subgroups of gliomas using the ConcensusClusterPlus package in R software (Wilkerson and Hayes 2010). Unsupervised consensus clustering of 318 features resulted in a robust two-cluster consensus solution. In the clustering process, 80% of the tumor samples were sampled 1000 times by adopting the resampling program. Distance similarity between samples was calculated using Euclidean distance, and clustering algorithm adopts k-means to find reliable subgroup classification. We selected the number of clusters that yielded the most stable consensus matrices that have marked distinction in the matrix heatmap.
Gene functional and tumor immune microenvironment
In total, RNA-seq data from 85 patients was obtained from the TCGA pan-cancer atlas project (https://gdc.cancer.gov/about-data/publications). Gene expression profile was further normalized by log2 (normalized value + 1). Differentially expressed genes (DEGs) between C1 and C2 subtypes were identified using limma package in R software (defined as a fold change > 2 and FDR < 0.05) (Ritchie et al. 2015). Then, genes up-regulated in C1 subtype and genes up-regulated in C2 subtype were submitted to gene functional enrichment analysis using The Database for Annotation, Visualization and Integrated Discovery (DAVID) version 6.8.
Considering that tumor immune microenvironment actively participated in the gliomas progression and related to immunotherapy response (Mirzaei et al. 2017), we further explored the difference in immune cell infiltrates between the distinct subtypes. The relative abundance of 16 immune cell infiltrates was computed from the RNA-seq data 85 patients. We used gene set variation analysis (GSVA) to calculate the relative abundance of immune cells (Hanzelmann et al. 2013). The metagenes of 16 immune cell populations were acquired from previous study (Tamborero et al. 2018).
Statistical analysis
All statistical analyses were performed using the R software version 3.6.0. Associations between radiomic subtypes and biological variables were evaluated using a Chi-square test. The distributions of two sets of any continuous variable were compared using the Mann–Whitney U test. Kaplan–Meier curves were used to compare survival time differences. Statistical significance was set at P-value < 0.05.
Results
Prognostic value of radiomic features
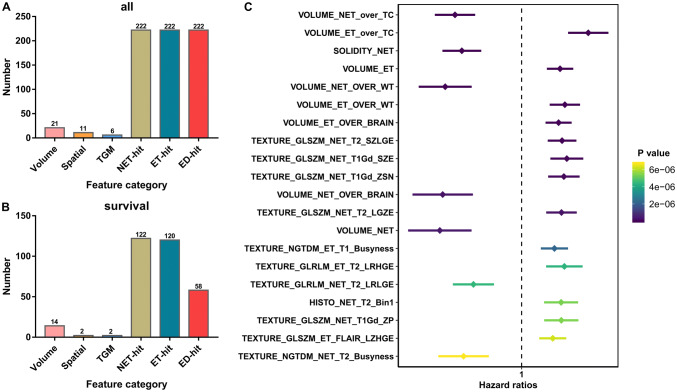
A total of 704 features were extracted from the multi-channel MRI data of 166 diffuse glioma patients (Fig. 1a). Features were mainly subcategorized into 6 subcategories, including: 21 features of volumetric type, 11 features of spatial type, 6 tumor growth model parameter, 222 histogram-based, intensity and textural features of the NET (NET-hit), 222 histogram-based, intensity and textural features of the enhancing part of the ET (ET-hit) and 222 histogram-based, intensity and textural features of the ED (ED-hit).
Fig. 1.
Survival-associated radiomic features. a A total of 704 radiomic features divided into six categories were included in the present stud. b The distribution of survival associated radiomic features. c The top 20 most significant survival-associated radiomic features
Using univariate COX analysis, we found that 318 features were significantly correlated with the OS of gliomas patients. Survival-associated features included 14 features of volumetric type, 2 features of spatial type, 2 tumor growth model parameter and 300 histogram-based, intensity and textural features (122 NET-hit, 120 ET-hit and 58 ED-hit; Fig. 1b). The top 20 most significant survival-associated features have been listed in Fig. 1c.
Identification of subtypes with distinct clinical outcome
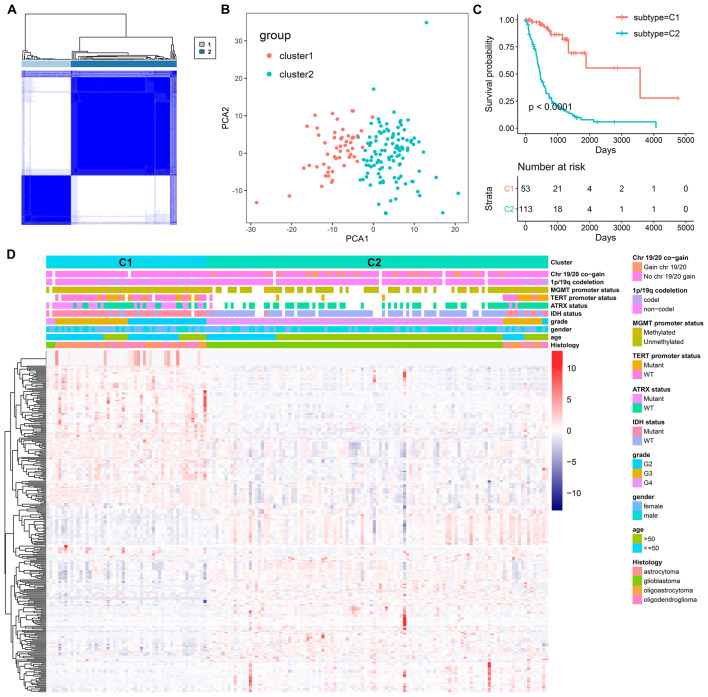
We found that the profiles of survival-associated radiomic features varied considerably at the individual level and partly reflected the OS of glioma patient. Hence, the 318 survival-associated radiomic features may have the potential to identify distinct patterns of patients. Then, consensus cluster analysis based on the prognosis-associated radiomic feature was conducted to identify distinct prognostic radiomic subtypes of diffuse glioma. To obtain a distinct comparison between patients, they were separated into two groups. As shown in Fig. 2a, the consensus clustering matrix showed a cohort of patients with two distinct radiomic subtypes: C1 (n = 53, 31.9%) and C2 (n = 113, 68.1%). Principal component analysis with survival-associated radiomic features was conducted to examine the appearance of glioma subtypes (Fig. 2b). A Kaplan–Meier plot showed that patients in cluster 2 suffered an inferior OS compared to patients in cluster 1 (P < 0.0001, Fig. 2c).
Fig. 2.
Diffuse glioma subtypes associated with prognosis and molecular subtypes. a A consensus matrix heatmap defined two clusters of samples for which consensus values range from 0 (in white, samples never clustered together) to 1 (dark blue, samples always clustered together). b A principal component analysis plot indicates that the two subgroups have distinct different radiomic profiles. c A Kaplan–Meier survival analysis of patients within different clusters on overall survival. Depicted P values are from log-rank tests. d A heatmap of the survival-associated radiomic features by cluster, with clinical and molecular annotations associated with each cluster
The relationships of subtypes with clinical and molecular data have been shown in Fig. 2d. The distribution of clinical parameters and important molecular factors in diffuse gliomas between clusters was not random and the two subtypes showed significant, characteristic clinical and molecular differences.
Radiomic subtypes related to prognosis and molecular subtypes
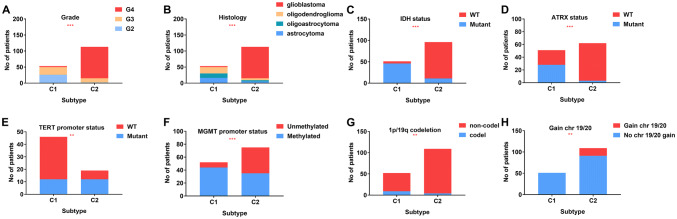
We compared differences in several clinical and molecular factors between C1 and C2 subtypes. Tumors classified as C1 were more frequently LGG (G2 + G3) while C2 were more frequently GBM (G4) (Fig. 3a, b). Furthermore, tumors classified as C1 were more frequently IDH1 mutant (Fig. 3c), ATRX mutant (Fig. 3d), TERT promoter wildtype (Fig. 3e) and MGMT promoter methylated (Fig. 3f). 1p/19q codeletion (Fig. 3g) and gain chr 19/20 status (Fig. 3h) also different between C1 and C2.
Fig. 3.
Clinical and molecular characteristics of the two subtypes. a Grade; (b) histology; (c) IDH status; (d) ATRX status; (e) TERT promoter status; (f) MGMT promoter status; (g) 1p/19q codeletion; (H) gain chr 19/20
Taken together, these findings suggested that the radiomic features of gliomas vary considerably. This was, in part, due to the molecular characteristics of the primary tumor, which affect clinical outcomes.
Differentially expressed genes
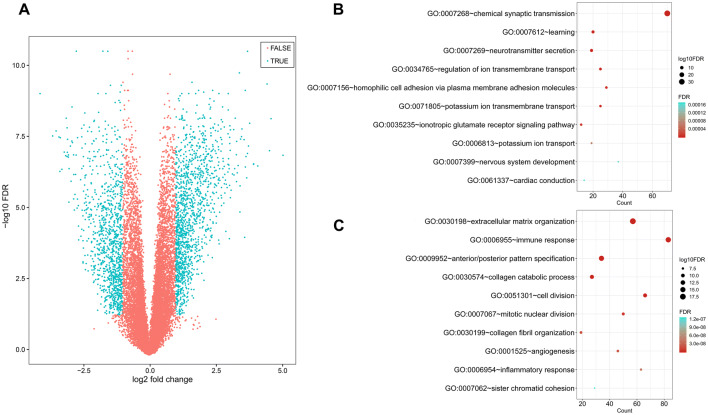
To further explore global alterations in gene expression profiles, the RNA-seq data of 85 patients (51 with the C1 subtype and 34 with the C2 subtype) were submitted to screened DEGs. A total of 2365 DEGs were identified, including 1333 genes that were up-regulated in the C2 subtype and 1032 genes that were up-regulated in the C1 subtype (Fig. 4a). Gene functional enrichment analysis revealed that C1-specific genes were mainly enriched in “chemical synaptic transmission”, “learning”, and “neurotransmitter secretion”, and C2-specific genes were mainly significantly correlated with “extracellular matrix organization”, immune response” and “anterior/posterior pattern specification”. The top 10 most significant biological process terms for C1 and C2 subtypes have been summarized in Fig. 4b, c.
Fig. 4.
Gene functional enrichment analysis of subtype-specific genes. a Volcano plot shows the differentially expressed genes between the two subtypes. b Biological process of C1-specific genes. c Biological process of C2-specific genes
Tumor immune microenvironment
Gene functional enrichment analysis indicated that immune response may be significantly involved in the C2 subtype. We further explored immune cell profiles in the 85 patients. The immune landscape suggested patients into two clusters (Fig. 5a), and we observed that the immune infiltration pattern of C2 clearly deviated from that of C1 cluster. Regulatory T cells, Gamma delta T cells, activated CD8 T cells, aDC, neutrophils, and macrophages were significantly up-regulated in C2. In contrast, Tfh cells, Tem cells, Tcm cells and T helper cells were significantly up-regulated in C1 (Fig. 5b). To investigate the potential response to immunotherapy treatment, we found that PD-1, PD-L1, and CTLA4 mRNA were overexpressed in the C2 subtype (Fig. 5c).
Fig. 5.
The immune landscape of gliomas. a The global immune landscape of C1 and C2 subtypes. b Differences in 16 types of immune cells between the two subtypes. PD1 (c), PD-L1 (d) and CTLA4 (e) are up-regulated in C2 subtypes
Discussion
Precision medicine relies on the accurate classification of patients according to their prognostic characteristics. Radiomic analysis provides a novel approach that quantifies the heterogeneity of tumors (Bi et al. 2019; Lambin et al. 2017). Here we demonstrated the prognostic potential of radiomic profiles for the prognosis stratification of diffuse gliomas and found a strong cancer survival-specific pattern of radiomic landscape. The clustering of diffuse glioma classes within similarly shaped clinical and molecular signature. And the multi-layer molecular data were applied to explore the relationships between radiomic and molecular data.
Many molecular classification systems based on genomics and epigenomics data have showed excellent performance in risk stratification. Previously, Yan et al. developed three major molecular clusters of gliomas based on whole genome gene expression profiling by consensus clustering (Yan et al. 2012). However, there is an urgent need for an objective, radiomic-based classifier for gliomas, which has been highlighted by the invasiveness of existing techniques and the difficulty of replicating results based on current molecular and histopathologic classification systems (Brennan 2011; Sulman and Aldape 2011). The emergence of radiomics has brought about alternative choices and may enhance the effectiveness of subtype classification. In addition, radiomics has exhibited excellent performance in stratifying tumor patients. For example, researchers have shown that radiomic features can be used to predict survival effectively (Nie et al. 2019; Prasanna et al. 2017). Furthermore, several studies suggested that radiomics could be useful in predicting the molecular characteristics of gliomas (Tan et al. 2019; Wu et al. 2019).
In the present study, we explored the relationships between global radiomic feature profiles and the OS of gliomas patients. Compared with radiomic features extracted from NET and ET region, the prognostic value of radiomics extracted from ED region was slightly lower. This may due to the lower variant of radiomic features extracted from the ED region. We found that diffuse glioma patients could be separated into two groups with distinct clinical outcome using unsupervised cluster. Consensus clustering determined different subtypes that have their own idiosyncrasies. Hence, it could provide a stratification model with high intra-class consistency to guide personalized medicine. Itakura H et al. also found that radiomics could be effective in glioma patients’ stratification (Itakura et al. 2015). However, the study on which this finding was based only used a T1 post-contrast MR slice. Our study focused on the stratification performance of prognostic radiomic features. We found that radiomic features effectively separated gliomas into two subgroups with distinct prognosis. Patients in the C1 subtype had superior survival and a low rate of malignant behavior. Genomic data supported the suggestion that the C1 subgroup patients may have a higher probability of IDH mutation. However, the C2 subgroup exhibited the opposite behavior. IDH1 mutations are early events in the development of gliomas (Hartmann et al. 2009; Watanabe et al. 2009). Considering the IDH mutation status difference between the two subtypes, our classifier may be useful in reflecting the development of diffuse gliomas.
By analyzing global RNA-seq data between C1 and C2 subtype, we identified DEGs between the two groups. Gene expression landscape between the two subtypes indicated that patients in the C2 subgroup were correlated with an immune response and some proliferation-associated pathways. One of the most fundamental characteristics of cancer cells is their ability to maintain chronic proliferation (Hanahan and Weinberg 2011). We also investigated tumor immune cell infiltration and immunotherapy biomarkers in diffuse gliomas and found a significant difference of these immune factors between different radiomic subtypes.
In cases of glioblastoma, PD-L1 is expressed in some patients (Berghoff et al. 2015), and the immune checkpoint blockers treatment has shown promise (Agarwalla et al. 2012; Fecci et al. 2007). Previously, Garber et al. reported that tumor-infiltrating lymphocytes (TIL) and PD-L1 expression were significantly correlated with tumor grade (Garber et al. 2016). Berghoff et al. found that IDH-wt gliomas display a more prominent TIL infiltration and higher PD-L1 expression than IDH-mut cases (Berghoff et al. 2017). Kohanbash also found that IDH mutations in glioma cells lead to impact CD8 + T cell accumulation (Kohanbash et al. 2017). These results suggested that immune cells infiltration actively participated in the progression of gliomas. Here, we systematically analyzed the relationships between radiomic features and immune landscape and noticed the different immune profiles between the two subtypes. Patients in C2 radiomic subtype harbored higher CD8 + T cells, PD1, PD-L1, and CTLA4. These findings hinted that patients in the C2 subtype may respond to immunotherapy.
However, some limitations might decrease the scientific evidence of our results. First, future larger external validation is required to validate our findings. Second, although we included multiple parameter MRI imaging data, some other modalities, such as diffusion-weighted imaging and magnetic resonance spectroscopy, are also expected to be included for the precision imaging oncology. Third, the underlying molecular mechanisms of these radiomic features involved in the prognosis of diffuse gliomas are presently unclear. Although correlationships could provide some hints, further research is needed to explore exact biological characteristics of radiomic features.
Conclusions
The use of multi-parameter MRI radiomics features is an effective approach for predicting the survival of glioma patients and identifying glioma subtypes. Multi-layered molecular data revealed distinct clinical features and molecular characteristics between the two groups, which may lead to future precision medicine treatment. These findings based on radiomic analysis maximize the value of information contained in medical imaging. Developing and refining a moderate radiomic approach to estimate clinical outcome and molecular characteristics non-invasively and pre-operatively may be valuable in treatment planning for patients with diffuse glioma.
Acknowledgements
The results shown here are parts based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga and TCIA database: https://www.cancerimagingarchive.net/. Special thanks to the authors (Spyridon Bakas, Hamed Akbari, Aristeidis Sotiras, Michel Bilello, Martin Rozycki, Justin S. Kirby, John B. Freymann, Keyvan Farahani & Christos Davatzikos) of “Advancing The Cancer Genome Atlas glioma MRI collections with expert segmentation labels and radiomic features”. The public online available data are valuable for related research work.
Author contributions
Conception and design: HY and GC. Development of methodology: PL, YTP, and RZG. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, and computational analysis): YW, XJL, SNH, YYF, and ZXW. Writing, review, and/or revision of the manuscript: PL, ZGH, HY, and GC. Study supervision: HY and GC.
Funding
This research was supported by Guangxi Degree and Postgraduate Education Reform and Development Research Projects, China (JGY2019050), Guangxi Zhuang Autonomous Region Health and Family Planning Commission Self-financed Scientific Research Project (Z20180979).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aerts HJ et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006. 10.1038/ncomms5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwalla P, Barnard Z, Fecci P, Dranoff G, Curry WT Jr (2012) Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J Immunother 35:385–389. 10.1097/CJI.0b013e3182562d59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkus Z, Ali I, Sedlar J, Agrawal JP, Parney IF, Giannini C, Erickson BJ (2017) Predicting deletion of chromosomal arms 1p/19q in low-grade gliomas from mr images using machine intelligence. J Digit Imaging 30:469–476. 10.1007/s10278-017-9984-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakas S et al (2016) GLISTRboost: combining multimodal MRI segmentation registration, and biophysical tumor growth modeling with gradient boosting machines for glioma segmentation. Brainlesion 9556:144–155. 10.1007/978-3-319-30858-6_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakas S et al (2017) Advancing the cancer genome atlas glioma MRI collections with expert segmentation labels and radiomic features. Sci Data 4:170117. 10.1038/sdata.2017.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghoff AS et al (2015) Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol 17:1064–1075. 10.1093/neuonc/nou307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghoff AS et al (2017) Correlation of immune phenotype with IDH mutation in diffuse glioma. Neuro Oncol 19:1460–1468. 10.1093/neuonc/nox054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi WL et al (2019) Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin 69:127–157. 10.3322/caac.21552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder H et al (2019) DNA methylation, transcriptome and genetic copy number signatures of diffuse cerebral WHO grade II/III gliomas resolve cancer heterogeneity and development. Acta Neuropathol Commun 7:59. 10.1186/s40478-019-0704-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Brennan C (2011) Genomic profiles of glioma. Curr Neurol Neurosci Rep 11:291–297. 10.1007/s11910-011-0198-7 [DOI] [PubMed] [Google Scholar]
- Ceccarelli M et al (2016) Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 164:550–563. 10.1016/j.cell.2015.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K et al (2013) The cancer imaging archive (TCIA): maintaining and operating a public information repository. J Digit Imaging 26:1045–1057. 10.1007/s10278-013-9622-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi J, Erus G, Ou Y, Gaonkar B, Davatzikos C (2013) Multi-atlas skull-stripping. Acad Radiol 20:1566–1576. 10.1016/j.acra.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel-Passow JE et al (2015) Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372:2499–2508. 10.1056/NEJMoa1407279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder K, Kalman B (2014) Molecular heterogeneity of glioblastoma and its clinical relevance. Pathol Oncol Res 20:777–787. 10.1007/s12253-014-9833-3 [DOI] [PubMed] [Google Scholar]
- Yan W et al (2012) Molecular classification of gliomas based on whole genome gene expression: a systematic report of 225 samples from the Chinese glioma cooperative group. Neuro Oncol 14:1432–1440. 10.1093/neuonc/nos263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecci PE et al (2007) Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res 13:2158–2167. 10.1158/1078-0432.CCR-06-2070 [DOI] [PubMed] [Google Scholar]
- Ferlay J et al (2018) (2019) Estimating the global cancer incidence and mortality in GLOBOCAN sources and methods. Int J Cancer 144:1941–1953. 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- Garber ST et al (2016) Immune checkpoint blockade as a potential therapeutic target: surveying CNS malignancies. Neuro Oncol 18:1357–1366. 10.1093/neuonc/now132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooya A, Pohl KM, Bilello M, Cirillo L, Biros G, Melhem ER, Davatzikos C (2012) GLISTR: glioma image segmentation and registration. IEEE Trans Med Imaging 31:1941–1954. 10.1109/TMI.2012.2210558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Hanzelmann S, Castelo R, Guinney J (2013) GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform 14:7. 10.1186/1471-2105-14-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann C et al (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1010 diffuse gliomas. Acta Neuropathol 118:469–474. 10.1007/s00401-009-0561-9 [DOI] [PubMed] [Google Scholar]
- Hastie T TR, Narasimhan B, Chu G (2019) impute: impute: Imputation for microarray data. R package version 1.60.0.
- Itakura H et al. (2015) Magnetic resonance image features identify glioblastoma phenotypic subtypes with distinct molecular pathway activities. Sci Transl Med 7:303ra138. Doi:10.1126/scitranslmed.aaa7582. [DOI] [PMC free article] [PubMed]
- Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. 10.1016/s1361-8415(01)00036-6 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. 10.1016/s1053-8119(02)91132-8 [DOI] [PubMed] [Google Scholar]
- Kohanbash G et al (2017) Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest 127:1425–1437. 10.1172/JCI90644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambin P et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446. 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambin P et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762. 10.1038/nrclinonc.2017.141 [DOI] [PubMed] [Google Scholar]
- Liu J et al (2018) An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell 173(400–416):e411. 10.1016/j.cell.2018.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN et al (2016) The World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- Mirzaei R, Sarkar S, Yong VW (2017) T Cell Exhaustion in Glioblastoma: Intricacies of Immune Checkpoints. Trends Immunol 38:104–115. 10.1016/j.it.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Nie D et al (2019) Multi-channel 3D deep feature learning for survival time prediction of brain tumor patients using multi-modal. Neuroimages Sci Rep 9:1103. 10.1038/s41598-018-37387-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noushmehr H et al (2010) Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17:510–522. 10.1016/j.ccr.2010.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olar A et al (2015) IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II–III diffuse gliomas. Acta Neuropathol 129:585–596. 10.1007/s00401-015-1398-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AP et al (2014) Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344:1396–1401. 10.1126/science.1254257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HS et al (2006) Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9:157–173. 10.1016/j.ccr.2006.02.019 [DOI] [PubMed] [Google Scholar]
- Prasanna P, Patel J, Partovi S, Madabhushi A, Tiwari P (2017) Radiomic features from the peritumoral brain parenchyma on treatment-naive multi-parametric MR imaging predict long versus short-term survival in glioblastoma multiforme: preliminary findings. Eur Radiol 27:4188–4197. 10.1007/s00330-016-4637-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:e47. 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Brady JM (1997) SUSAN—A new approach to low level image processing. Int J Comput Vision 23:45–78 [Google Scholar]
- Spyridon Bakas HA et al (2017a) Segmentation labels and radiomic features for the pre-operative scans of the TCGA–GBM collection The Cancer Imaging Archive. Nat Sci Data 4:170117. 10.1038/sdata.2017.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyridon Bakas HA et al (2017b) Segmentation labels and radiomic features for the pre-operative scans of the TCGA–LGG collection The Cancer Imaging Archive. Nat Sci Data 4:170117. 10.1038/sdata.2017.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulman EP, Aldape K (2011) The use of global profiling in biomarker development for gliomas. Brain Pathol 21:88–95. 10.1111/j.1750-3639.2010.00456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamborero D et al (2018) A pan-cancer landscape of interactions between solid tumors and infiltrating immune cell populations. Clin Cancer Res 24:3717–3728. 10.1158/1078-0432.CCR-17-3509 [DOI] [PubMed] [Google Scholar]
- Tan Y et al (2019) A radiomics nomogram may improve the prediction of IDH genotype for astrocytoma before surgery. Eur Radiol 29:3325–3337. 10.1007/s00330-019-06056-4 [DOI] [PubMed] [Google Scholar]
- van den Bent MJ (2010) Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician's perspective. Acta Neuropathol 120:297–304. 10.1007/s00401-010-0725-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Nobusawa S, Kleihues P, Ohgaki H (2009) IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol 174:1149–1153. 10.2353/ajpath.2009.080958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen PY, Huse JT (2017) 2016 World health organization classification of central nervous system. Tumors Continuum (Minneap Minn) 23:1531–1547. 10.1212/CON.0000000000000536 [DOI] [PubMed] [Google Scholar]
- Wilkerson MD, Hayes DN (2010) ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics 26:1572–1573. 10.1093/bioinformatics/btq170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Meng J, Yu Q, Li P, Fu S (2019) Radiomics-based machine learning methods for isocitrate dehydrogenase genotype prediction of diffuse gliomas. J Cancer Res Clin Oncol 145:543–550. 10.1007/s00432-018-2787-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X et al (2018) Radiomics strategy for molecular subtype stratification of lower-grade glioma: detecting IDH and TP53 mutations based on multimodal MRI. J Magn Reson Imaging 48:916–926. 10.1002/jmri.25960 [DOI] [PubMed] [Google Scholar]