Abstract
Purpose
Polypharmacy is a common problem among older adults. However, its prevalence and impact on the clinical outcomes of anticancer treatment, such as survival and adverse events, in older patients with advanced cancer have not been well investigated.
Methods
We retrospectively reviewed data from Japanese patients treated with an immune checkpoint inhibitor (ICI) for advanced or recurrent non-small-cell lung cancer (NSCLC) between 2016 and 2019.
Results
Among 157 older (aged ≥ 65 years) patients, the prevalence of polypharmacy, defined as ≥ 5 medications, was 59.9% (94/157). The prevalence of potentially inappropriate medication use, according to the screening tool of older people’s prescription (STOPP) criteria version 2, was 38.2% (60/157). The median progression-free survival (PFS) in patients with and without polypharmacy was 3.7 and 5.5 months, respectively (P = 0.0017). The median overall survival (OS) in patients with and without polypharmacy was 9.5 and 28.1 months, respectively (P < 0.001). Multivariate analysis revealed marked associations between polypharmacy and OS, but no significant associations between polypharmacy and PFS. Polypharmacy was not associated with immune-related adverse events but was associated with higher rate of unexpected hospitalizations during ICI treatment (59.6% vs. 31.7%, P < 0.001).
Conclusion
Polypharmacy is an independent prognostic factor in older patients with advanced NSCLC treated with ICI. Also, polypharmacy could be utilized as a simple indicator of patients’ comorbidities and symptoms or as a predictive marker of unexpected hospitalizations during ICI treatment.
Electronic supplementary material
The online version of this article (10.1007/s00432-020-03252-4) contains supplementary material, which is available to authorized users.
Keywords: Polypharmacy, Non-small cell lung cancer, Overall survival, Geriatric oncology, Comorbidity
Introduction
Polypharmacy is a problem commonly occurring among the older adults, and its potential impact on health-related problems is being increasingly recognized (Ferner and Aronson 2006; Field et al. 2001; LeBlanc et al. 2015; Lees and Chan 2011). Polypharmacy can contribute to undesirable clinical outcomes such as adverse drug events, drug–drug interactions, and reduced adherence to drugs. Moreover, polypharmacy should reflect the burden of comorbidity or physical symptoms in individuals who require drug administration to a certain extent. Although the definition (i.e., the cut-off number of drugs) is not formally unified, polypharmacy is a simple summarization of prescription status in terms of the number of drugs concurrently prescribed to one patient. Potentially inappropriate medication (PIM) use is another concept which reflects the appropriateness of prescription, not just in terms of the numbers of drugs prescribed, generally in the older adults. Several criteria are used to evaluate PIM use, such as the screening tool of older people’s prescriptions (STOPP), the screening tool to alert to right treatment (START) (O'Mahony et al. 2015), the Beers Criteria (American Geriatrics Society Beers Criteria Update Expert P 2015), and the inappropriate prescribing in the elderly tool (IPET) (Rosai 2000).
Lung cancer has a median onset age of around 70, and is therefore often observed in the older adults (Vora and Reckamp 2008). The mortality of lung cancer is high compared with other cancers because only small portion of lung cancer cases are diagnosed at the early stage (Liam et al. 2006; Wang et al. 2013). Approximately 30%–40% of non-small-cell lung cancer (NSCLC) patients present with metastatic disease at the time of diagnosis (Matsuda et al. 2014; Little et al. 2007). The most common metastatic site is bone, followed lung, brain, adrenal gland, liver, and lymph node (Tamura et al. 2015). Distant metastatic lesions along with a primary lesion and its invasion to adjacent structures (trachea, bronchus, chest wall, major vessels, among others) can cause uncomfortable symptoms such as pain and dyspnea. The control of physical symptoms by medications besides systemic anticancer therapy is therefore often required in advanced NSCLC patients.
Older advanced NSCLC patients are naturally prone to polypharmacy due to the relatively high prevalence of comorbidity with aging or cancer-related symptoms. However, the prevalence and clinical impact of polypharmacy or PIM use on the clinical outcomes of anticancer treatment, such as survival and adverse events, in advanced cancer patients have not yet been well investigated. In particular, the data on patients treated with novel anticancer agents, including immune checkpoint inhibitor (ICI) or targeted therapy, are scant. Also, the clinical significance of polypharmacy or PIM use as a prognostic factor in advanced cancer patients has not been well described in prior studies. Moreover, a prior study focused on breast cancer patients who received neoadjuvant chemotherapy showed some comedications influenced immune infiltration and pathological response (Hamy et al. 2020). This preliminary result could arise the hypothesis that polypharmacy which literally means high burdens of comedications can have some impacts on immunotherapy. Hence, this study aims to investigate the prevalence and impact of polypharmacy or PIM use on the clinical outcomes of older patients treated with ICI for advanced NSCLC.
Methods
Patients
In this single-institute study, we retrospectively reviewed the medical records of 250 patients with advanced or recurrent thoracic cancer who had received ICI treatment between 2016 and 2019 at Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital (Tokyo, Japan). Patients who fulfilled the following criteria were included in analysis: (Ferner and Aronson 2006) aged 65 years or older; (Field et al. 2001) histologically or cytologically confirmed unresectable advanced (stage III or IV) or recurrent NSCLC; (LeBlanc et al. 2015) received ICI (nivolumab, pembrolizumab, or atezolizumab) monotherapy at recommended doses either as the first-line or later-line therapy (Fig. 1). In addition, we examined the following clinical factors of patients as the baseline characteristics: sex, age, smoking status indicated by Brinkman index (daily number of cigarettes x years), Eastern Cooperative Oncology Group-Performance Status (ECOG-PS), serum albumin, lactate dehydrogenase (LDH), neutrophil-to-lymphocyte ratio (NLR), Gustav Roussy Immune Score (GRIm-Score) which is calculated from the albumin, LDH, NLR values (Bigot et al. 2017), estimated glomerular filtration rate (eGFR) using the Cockcroft–Gault equation, histological subtype, epidermal growth factor receptor (EGFR) mutation status, programmed death-ligand 1 (PD-L1) expression, staging (UICC classification, 8th edition), lines of chemotherapy, and concomitant medications.
Fig. 1.
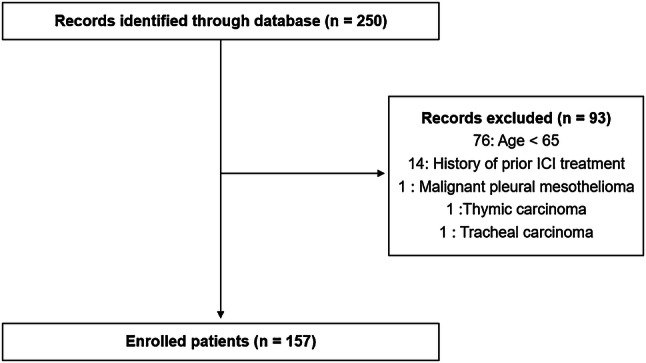
Flow diagram of enrolled patients
Evaluation of concomitant medications
We reviewed all medications administered to each patient at the time of ICI treatment initiation. A concomitant medication was defined as any therapeutic drug used to manage a comorbid condition besides lung cancer between the last visit and day 1 of ICI treatment. Oral, injection (e.g., intravenous, intramuscular, and subcutaneous), suppository, and inhalant medications were included. As-needed medications and topical medications were excluded. Polypharmacy was defined as five or more medications. As for compounding agents, each ingredient was counted separately. PIM use was measured using the STOPP version 2 criteria (O'Mahony et al. 2015), a screening tool used to detect potentially inappropriate prescribing in older adults (defined as those who are aged 65 or older). STOPP version 2 is composed of 13 sections categorized by organ system with a total of 80 checkpoints.
Evaluation of adverse drug reactions and adverse events
Adverse drug reactions induced by ICI treatment were monitored according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0 from the starting date of ICI treatment until the first documented disease progression or the date of death. Immune-related adverse events (irAEs) were defined as adverse events with a potential immunologic basis which include organ-specific irAEs (colitis, hepatitis, pneumonitis, hypothyroidism, etc.) and general adverse events related to immune activation (fatigue, diarrhea, rash, etc.). The highest-grade toxicities during each therapy were recorded. In addition, we reviewed any unexpected inpatient hospitalizations from the time of ICI treatment initiation to the date of PD or death. For those who received ICI treatment in inpatient settings, we also regarded prolongations of the hospitalization duration as unexpected hospitalization events. The investigators defined unexpected hospitalization events as either of the followings: (Ferner and Aronson 2006) exacerbation of lung cancer which needed inpatient management despite of ICI treatment; (Field et al. 2001) occurrence of irAEs which needed inpatient management; (LeBlanc et al. 2015) development of complications unrelated to lung cancer or ICI treatment which needed inpatient management.
Statistical analysis
In this study, we used descriptive statistics to summarize the patients’ baseline characteristics. Using Fisher’s exact tests for categorical data and a Mann–Whitney U test for continuous variables, we assessed the between-group difference at the baseline or the incidence of adverse events during ICI treatment. We defined progression-free survival (PFS) as the time from the start of ICI treatment to the first documented disease progression or the date of death. The overall survival (OS) was determined from the date of starting ICI treatment to the date of death, irrespective of the cause of death. Patients who had no disease progression or had died at the time of the analysis were censored at the date of the last contact. We estimated the survival distributions (PFS and OS) using the Kaplan–Meier method and compared the differences between the groups using a log-rank test. The potential predictors of survival were explored using a Cox regression. Characteristics with a P value < 0.05 after the univariate analysis were included in the multivariate analysis. All P values in this study are two-sided, and P < 0.05 was considered statistically significant. All statistical analyses were performed using the JMP 11 software (SAS Institute, Cary, NC).
Results
Baseline characteristics
Of the 250 NSCLC patients who received ICI, 157 (62.8%) patients (57 females and 100 males) aged at least 65 at the time of ICI treatment initiation were included in the analysis (Fig. 1). The median age of these patients was 73 (range: 65–88) years. Based on the 8th edition of the tumor, node, metastasis (TNM) classification for lung cancer, 15 (9.6%), 37 (23.6%), 54 (34.4%), and 51 (32.5%) patients presented with stage III, stage IVA, stage IVB, and recurrent disease, respectively. Overall, 96 patients (61.1%) had adenocarcinoma, and 43 (27.4%) had squamous cell carcinoma. As initial ICI treatment, 92 patients (58.6%) were treated with nivolumab, 50 (31.8%) with pembrolizumab, and 15 (9.6%) with atezolizumab. Table 1 summarizes the patients’ characteristics.
Table 1.
Baseline characteristics of analyzed patients (n = 157)
| Characteristics | PP (+) (n = 94) | PP (−) (n = 63) | P |
|---|---|---|---|
| Number of concomitant medications, n | |||
| Median (IQR) | 7.0 (6.0–9.0) | 2.0 (0.5–3.0) | < 0.001 |
| Age, years | |||
| Median (range) | 73 (65–88) | 71 (65–82) | 0.68 |
| Age group, n (%) | |||
| 65–74 | 56 (59.6) | 45 (71.4) | 0.17 |
| ≥ 75 | 38 (40.4) | 18 (28.6) | |
| Sex, n (%) | |||
| Female | 36 (38.3) | 21 (33.3) | 0.61 |
| Smoking status, n (%) | |||
| Brinkman index < 400 | 22 (23.4) | 16 (25.4) | 0.85 |
| Brinkman index ≥ 400 | 72 (76.6) | 47 (74.6) | |
| ECOG-PS, n (%) | |||
| 0/1 | 56 (59.6) | 51 (81.0) | 0.018 |
| 2 | 25 (26.6) | 7 (11.1) | |
| 3/4 | 13 (13.8) | 5 (7.9) | |
| Histological subtypes, n (%) | |||
| Adenocarcinoma | 58 (61.7) | 38 (60.3) | 0.33 |
| Squamous cell carcinoma | 22 (23.4) | 21 (33.3) | |
| NSCLC, not other specified | 10 (10.6) | 3 (4.8) | |
| Other | 4 (4.3) | 1 (1.6) | |
| Staging, n (%) | |||
| III | 9 (9.6) | 6 (9.5) | 0.40 |
| IVA | 18 (19.1) | 19 (30.2) | |
| IVB | 36 (38.3) | 18 (28.6) | |
| Recurrence | 31 (33.0) | 20 (31.7) | |
| Presence of brain metastasis, n (%) | |||
| Yes | 21 (22.3) | 9 (14.3) | 0.22 |
| Presence of bone metastasis, n (%) | |||
| Yes | 31 (33.0) | 12 (19.0) | 0.068 |
| Presence of liver metastasis, n (%) | |||
| Yes | 18 (19.1) | 9 (14.3) | 0.52 |
| PD-L1 expression, n (%) | |||
| < 1% | 14 (14.8) | 6 (9.5) | 0.72 |
| 1%–49% | 12 (12.8) | 10 (15.9) | |
| ≥ 50% | 29 (30.9) | 18 (28.6) | |
| Unknown | 39 (41.5) | 29 (46.0) | |
| EGFR mutation status, n (%) | |||
| Positive | 9 (9.6) | 9 (14.3) | 0.14 |
| Negative | 78 (83.0) | 44 (69.8) | |
| Unknown | 7 (7.4) | 10 (15.9) | |
| Initially chosen ICI, n (%) | |||
| Nivolumab | 58 (61.7) | 34 (54.0) | 0.65 |
| Pembrolizumab | 28 (29.8) | 22 (34.9) | |
| Atezolizumab | 8 (8.5) | 7 (11.1) | |
| Lines of previous chemotherapy, n | |||
| Median (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.60 |
| eGFR (mL/min) | |||
| Median (IQR) | 66.2 (57.3–87.8) | 70.8 (58.0–82.1) | 0.95 |
| Alb (g/dL) | |||
| Median (IQR) | 3.3 (2.9–3.8) | 3.8 (3.4–4.2) | 0.001 |
| LDH (U/L) | |||
| Median (IQR) | 219 (187–326) | 196 (166–255) | 0.007 |
| NLR | |||
| Median (IQR) | 4.4 (2.9–7.6) | 3.0 (1.9–5.2) | 0.001 |
| GRIm-Score | |||
| Median (IQR) | 1.0 (0.0–2.0) | 1.0 (0.0–1.0) | 0.007 |
Prevalence of polypharmacy and potentially inappropriate medications among older non-small-cell lung cancer patients
The median number of concomitant medications was six (mean 5.5, range 0–20, interquartile range (IQR) 3–8) at the time of ICI treatment initiation. The prevalence of polypharmacy, defined as at least five concomitant medications, was 94/157 (59.9%) (Fig. 2a, b). According to the STOPP criteria, 60 (38.2%) patients took at least one medication that were judged as PIM (Fig. 2c–e). Among the patients with PIM use (n = 60), 17 (28.3%) violated three or more checkpoints in the STOPP criteria (Table 2).
Fig. 2.
The number of concomitant medications in overall elderly patients (n = 157) (a, b) and potentially inappropriate medications (PIM) according to the STOPP ver. 2 criteria in c overall elderly patients (n = 157), d PIM (+) patients (n = 60) and e PIM (−) patients (n = 97)
Table 2.
Reasons for potentially inappropriate medication judgments according to STOPP criteria among the patients with PIM use (n = 60)
| Criteria in STOPP ver2 (classification number) | n (%) |
|---|---|
| Benzodiazepines (K1) | 29 (48.3) |
| Benzodiazepines for ≥ 4 weeks (D5) | 28 (46.7) |
| Neuroleptic drugs (K2) | 13 (21.7) |
| Hypnotic Z-drugs (K4) | 13 (21.7) |
| Use of regular opioids without concomitant laxative (L2) | 7 (11.7) |
| Any duplicate drug class prescription (A3) | 6 (10.0) |
| Any drug prescribed without an evidence-based clinical indication (A1) | 3 (5.0) |
| Loop diuretic for dependent ankle edema without clinical, biochemical evidence or radiological evidence of heart failure, liver failure, nephrotic syndrome or renal failure (B7) | 3 (5.0) |
| NSAID and vitamin K antagonist, direct thrombin inhibitor or factor Xa inhibitors in combination (C10) | 3 (5.0) |
| First-generation antihistamines (D14) | 3 (5.0) |
| NSAID if eGFR < 50 mL/min/1.73m2 (E4) | 3 (5.0) |
| Vasodilator drugs with persistent postural hypotension (K3) | 3(5.0) |
| Benzodiazepines with acute or chronic respiratory failure (G4) | 2 (3.3) |
| NSAID with concurrent corticosteroids without PPI prophylaxis (H8) | 2 (3.3) |
| Digoxin for heart failure with preserved systolic ventricular function (B1) | 1 (1.7) |
| ACE inhibitors or Angiotensin Receptor Blockers in patients with hyperkalemia (B11) | 1 (1.7) |
| Aspirin in combination with vitamin K antagonist, direct thrombin inhibitor or factor Xa inhibitors in patients with chronic atrial fibrillation without a clear indication for aspirin (C5) | 1 (1.7) |
| Neuroleptics as hypnotics, unless sleep disorder is due to psychosis or dementia (D10) | 1 (1.7) |
| Non-COX-2-selective NSAID with history of peptic ulcer disease or gastrointestinal bleeding, unless with concurrent PPI or H2 antagonist (H1) | 1 (1.7) |
NSAID non-steroidal anti-inflammatory drug, PPI proton pump inhibitor, ACE angiotensin converting enzyme, COX-2 cyclooxygenase 2, H2 histamine H2 receptor
Clinical outcomes of immune checkpoint inhibitor treatment in older patients
The median follow-up time from the time of ICI treatment initiation was 7.1 months. The median PFS time of all older NSCLC patients treated with ICI treatment was 4.3 months [95% confidence interval (CI) 2.8–5.5], and the median OS time was 14.0 months (95% CI 9.7–16.8).
Clinical outcomes of immune checkpoint inhibitor treatment in the subgroup with and without polypharmacy
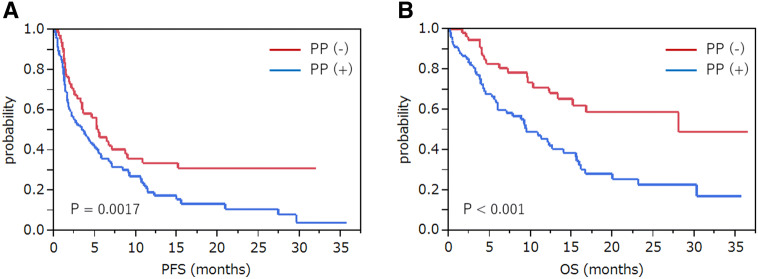
The median PFS time of patients with polypharmacy [PP ( +)] (n = 94) and those without polypharmacy [PP( −)] (n = 63) was 3.7 months (95% CI 1.8–5.4) and 5.5 months (95% CI 3.4–9.0), respectively (P = 0.0017). The median OS time for PP ( +) and PP ( −) patients was 9.5 months (95% CI 6.0–14.0) and 28.1 months (95% CI 13.3–not reached), respectively (P < 0.001) (Fig. 3).
Fig. 3.
Survival analysis of patients with polypharmacy and those without polypharmacy. Estimated Kaplan–Meier survival curves for the a progression-free survival and b overall survival comparing patients with polypharmacy [PP (+)] (n = 94) and without polypharmacy [PP (−)] (n = 63)
Clinical outcomes of immune checkpoint inhibitor treatment in the subgroups with and without potentially inappropriate medication use
The median PFS time of patients with PIM use [PIM (+)] (n = 60) and those without PIM use [PIM (−)] (n = 97) was 3.7 months (95% CI 1.7–5.5) and 5.2 months (95% CI 3.2–8.4), respectively (P = 0.22). The median OS time for PIM (+) and PIM (−) patients was 9.4 months (95% CI 6.0–15.6) and 16.7 months (95% CI 10.9–not reached), respectively (P = 0.029) (Supplemental Fig. 1).
Univariate analysis for progression-free survival and overall survival
The univariate analysis for PFS revealed that smoking status, ECOG-PS, presence of liver metastasis, PD-L1 expression, EGFR mutation status, initially chosen ICI, GRIm-Score, and polypharmacy were significantly associated with PFS. The univariate analysis for OS showed that ECOG-PS, presence of bone metastasis, presence of liver metastasis, PD-L1 expression, EGFR mutation status, GRIm-Score, and polypharmacy were significantly associated with OS (Supplemental Table 1).
Multivariate analysis for progression-free survival and overall survival
Multivariate analysis for PFS revealed that smoking status, ECOG-PS, PD-L1 expression, EGFR mutation status, and GRIm-Score were significantly associated with PFS, whereas significant associations were not observed between polypharmacy and PFS (Table 3). Multivariate analysis for OS showed that polypharmacy, ECOG-PS, and EGFR mutation status were significantly associated with OS (Table 4). Predictability of the multivariate analysis models for PFS and OS was verified either by likelihood ratio test, Wald test, and score log-rank test (P < 0.001).
Table 3.
Multivariate analysis of progression-free survival in elderly patients treated with immune checkpoint inhibitors
| Variants | HR | 95% CI | P |
|---|---|---|---|
| Smoking status (Brinkman index ≥ 400 vs. < 400) | 0.69 | 0.43–1.10 | 0.13 |
| ECOG-PS (≥ 2 vs. 0, 1) | 1.68 | 1.09–2.59 | 0.018* |
| Presence of liver metastasis (yes vs. no) | 0.99 | 0.60–1.64 | 0.97 |
| PD-L1 (positive vs. negative/unknown) | 0.51 | 0.26–0.97 | 0.041* |
| EGFR (positive vs. negative/unknown) | 3.55 | 1.89–6.63 | < 0.001* |
| Initially chosen ICI (nivolumab vs. atezolizumab) | 0.79 | 0.39–1.60 | 0.51 |
| Initially chosen ICI (pembrolizumab vs. atezolizumab) | 1.05 | 0.37–2.95 | 0.93 |
| GRIm (high vs. low) | 1.83 | 1.17–2.87 | 0.0084* |
| Concomitant medications (≥ 5 vs. < 5) | 1.44 | 0.95–2.18 | 0.085 |
Table 4.
Multivariate analysis of overall survival in elderly patients treated with immune checkpoint inhibitors
| Variants | HR | 95% CI | P |
|---|---|---|---|
| ECOG-PS (≥ 2 vs. 0, 1) | 2.09 | 1.26–3.47 | 0.0042* |
| Presence of bone metastasis (yes vs. no) | 1.25 | 0.72–2.16 | 0.41 |
| Presence of liver metastasis (yes vs. no) | 1.23 | 0.69–2.18 | 0.48 |
| PD-L1 (positive vs. negative/unknown) | 0.64 | 0.37–1.10 | 0.10 |
| EGFR (positive vs. negative/unknown) | 2.57 | 1.36–4.88 | 0.0038* |
| GRIm (high vs. low) | 1.54 | 0.89–2.69 | 0.13 |
| Concomitant medications (≥ 5 vs. < 5) | 1.97 | 1.14–3.42 | 0.015* |
Adverse events during immune checkpoint inhibitor treatment in older patients
During ICI treatment, 27 (17.2%) and 6 (3.8%) patients experienced ≥ Grade 2 and ≥ Grade 3 irAE, respectively. Furthermore, 76 (48.4%) patients had at least one unexpected hospitalization, and 22 (14.0%) had two or more. There were 44 (28.0%) patients hospitalized due to exacerbation of lung cancer, 15 (9.6%) for irAE, and 29 (18.5%) for complications unrelated to lung cancer or irAE.
Adverse events during immune checkpoint inhibitor treatment in the subgroups with and without polypharmacy
There was some tendency of higher incidence of irAE in PP ( +) patients compared with PP ( −) patients; however, no significant difference was seen between the two groups (≥ Grade 2: 20.2% vs. 12.7%, P = 0.28, ≥ Grade 3: 5.3% vs. 1.6%, P = 0.4). The proportion of overall unexpected hospitalizations during ICI treatment for any reason was greater in PP ( +) patients compared with PP ( −) patients (59.6% vs. 31.7%, P < 0.001). PP ( +) patients experienced more frequent unexpected hospitalizations due to exacerbation of lung cancer than PP ( −) patients (34.0% vs. 19.0%, P = 0.047) or irAE (13.8% vs. 3.2%, P = 0.028), whereas there was no significant difference in frequency of unexpected hospitalization due to complications unrelated to lung cancer or irAE between PP ( +) and PP ( −) patients (22.3% vs. 12.7%, P = 0.15) (Table 5).
Table 5.
Adverse events during immune checkpoint inhibitor treatment in the subgroups comparing PP ( +) patients (n = 94) and PP ( −) patients (n = 63)
| Adverse events | PP ( +) | PP ( −) | P |
|---|---|---|---|
| Incidence of irAE, n (%) | |||
| ≥ Grade 2 | 19 (20.2) | 8 (12.7) | 0.28 |
| ≥ Grade 3 | 5 (5.3) | 1 (1.6) | 0.4 |
| Reasons for unexpected hospitalizations, n (%) | |||
| Exacerbation of lung cancer (A) | 32 (34.0) | 12 (19.0) | 0.047* |
| Complications unrelated to lung cancer (B) | 21 (22.3) | 8 (12.7) | 0.15 |
| irAE (C) | 13 (13.8) | 2 (3.2) | 0.028* |
| Any reasons (A + B + C) | 56 (59.6) | 20 (31.7) | < 0.001* |
Discussion
This study assessed the clinical significance of polypharmacy and PIM use in older Japanese advanced NSCLC patients treated with immune checkpoint inhibitors. Polypharmacy and PIM use were observed in approximately 60% and 40% of patients, respectively, among this population at the baseline. In addition, our findings revealed significant differences in the OS between patients with and without polypharmacy. The multivariate analysis revealed that baseline polypharmacy is independently associated with OS. Furthermore, polypharmacy was associated with higher incidence of unexpected hospitalizations or the prolongation of hospitalization during ICI treatment.
Although polypharmacy is a common problem among older adults, it has not been well described in the field of oncology. Previous studies on this problem among patients with cancer showed relatively high numbers of prescription drug use in this population (Cashman et al. 2010; Petrini et al. 2010; Jorgensen et al. 2012; Kotlinska-Lemieszek et al. 2014; Maggiore et al. 2014; Raijmakers et al. 2013; Sokol et al. 2007; Todd et al. 2013; Hong et al. 2019; Oldak et al. 2019; Mohamed et al. 2020). In a study using a large population-based national cancer registry in Denmark (Jorgensen et al. 2012), 35% of older (≥ 70 years) cancer patients including various stages used ≥ 5 concomitant medications at diagnosis. However, only a few studies, with small numbers of patients, have focused on certain clinical settings, such as staging, age, and the sites or types of cancer. Considering the heterogeneity of symptoms or patients’ characteristics among such various situations, the application possibilities of these results to certain clinical situations may be impaired. Moreover, limited data on the prevalence of polypharmacy in advanced NSCLC patients are currently available (Todd et al. 2013). In this study, we focused on the prescription status among older advanced NSCLC patients treated with ICIs. Furthermore, we searched the prevalence and details of PIM use according to the STOPP ver. 2 criteria among this population. To the best of our knowledge, no prior study has described these issues.
A high prevalence of polypharmacy was observed among older advanced NSCLC patients. Our findings are mostly consistent with prior studies on populations with metastatic solid-tumor malignancy that included relatively small numbers of NSCLC patients (Cashman et al. 2010; Petrini et al. 2010; Jorgensen et al. 2012; Kotlinska-Lemieszek et al. 2014; Maggiore et al. 2014; Raijmakers et al. 2013; Sokol et al. 2007; Todd et al. 2013; Hong et al. 2019; Oldak et al. 2019; Mohamed et al. 2020). With modest differences among the studies, probably due to the differences in designs, the median numbers of concomitant medications range from 4 to 9. It seems that advanced NSCLC patients do not take higher numbers of medications compared with patients with other types of advanced cancer. However, simple comparisons with prior studies might be inappropriate because of different thresholds in the numbers of medications or the time slots (e.g., simultaneous, cumulative, continuous) used to measure polypharmacy. Numerous thresholds defining polypharmacy have been proposed, but no consensus has been reached. Considering the comparability with preceding data, we applied the “five medications threshold”, which has been more commonly used than others. On the other hand, the high prevalence of polypharmacy defined as ≥ 5 concomitant medications among older advanced cancer patients may implicate the potential utility of other thresholds. Further discussions are needed to estimate the comprehensive health risk of polypharmacy while avoiding the risk of underuse of necessary medications among this specific population. As for the time slot of polypharmacy, we applied simultaneous polypharmacy, which corresponds to the number of medications concurrently taken by patients at the start of ICI treatment. Although this measure may fail to cover all the medications through a period, such as a total clinical course of ICI treatment, we think the simplicity is essential for future application in clinical practice.
PIM use was also common among older advanced NSCLC patients. There are several published studies on PIM use in elderly cancer patients that apply criteria such as the 2012 or 2003 Beers Criteria (American Geriatrics Society Beers Criteria Update Expert P 2012), or the Zhan criteria (Zhan et al. 2001). As far as we know, no study is available that investigates PIM using the STOPP ver. 2 criteria (O'Mahony et al. 2015), which was newly proposed in 2014 as a screening tool based on up-to-date reviews. According to these prior studies, the prevalence of PIM use ranges from 11 to 29%. Utilizing the STOPP ver.2 criteria, we found a higher prevalence of PIM use (60 of 157 patients; 38.2%) among our advanced NSCLC cohort. The most common PIM were psychoactive medications such as benzodiazepines (29 of 60 patients; 48.3%) and antipsychotics (13 of 60 patients; 21.7%). Use of regular opioids without concomitant laxative use was also relatively common (7 of 60 patients; 11.7%) among the patients with PIM use. The reasons these medications were judged as PIMs may reflect the characteristic symptoms (e.g., dyspnea, pain, insomnia, anxiety) of advanced NSCLC. The use of aspirin or calcium channel blockers, which are commonly identified as PIM in prior studies, were less common PIMs according to the STOPP ver. 2 criteria. These differences seemed to largely derive from the differences in the criteria used to judge PIM use rather than only from the specific characteristics of older advanced NSCLC patients.
Existing criteria for evaluating PIM use and polypharmacy target the general older adults and do not necessarily include use in older cancer patients. These measures may have room for improvement to be optimally used in oncology settings. In the context of immunotherapy with ICI treatment, the concomitant medications which could potentially impair its efficacy (e.g., corticosteroids (Ricciuti et al. 2019), antibiotics (Derosa et al. 2018; Elkrief et al. 2019; Pinato et al. 2019; Hakozaki et al. 2019)) may also need to be considered. Interestingly, a prior study on comedications showed the impacts of psychoactive medications on the reduction of tumor growth in breast cancer bearing mice and increased anticancer activity of cyclophosphamide in a T-cell-dependent manner. This result as well as high prevalence of psychoactive medications as PIM observed in our cohorts raise a question whether psychoactive medications are simplistically ‘inappropriate’ or not (Hamy et al. 2020).
In this study, we observed marked associations between OS and polypharmacy in older advanced NSCLC patients receiving ICI treatment. Although we did not see significant differences in PFS using multivariate analysis and the prevalence of irAE between those with polypharmacy and without polypharmacy, we found another interesting fact: the proportion of overall unexpected hospitalizations during ICI treatment was greater in patients with polypharmacy. Furthermore, patients with polypharmacy experienced more frequent unexpected hospitalizations due to exacerbation of lung cancer or irAE, whereas there was no significant difference between patients with polypharmacy and those without in the frequency of unexpected hospitalizations due to medical conditions unrelated to lung cancer or irAE.
These results implicate the following clinical points considering the problem of polypharmacy. First, polypharmacy might not affect the antitumor effects or adverse events of ICI, which implies the pharmacodynamics of ICI may not be affected by the presence of polypharmacy. Second, and more importantly, polypharmacy might be useful as a simple surrogate indicator of comorbidities or as a predictive marker of negative cancer or treatment-related events during ICI treatment, not just as a prognostic factor in older advanced NSCLC patients. Some measures do exist to evaluate comorbidities in general populations, such as the Charlson Comorbidities Index (CCI) (Charlson et al. 1987), the age-adjusted Charlson Comorbidity Index (ACCI) (Charlson et al. 1994), and the Elixhauser Comorbidity Index (ECI) (Elixhauser et al. 1998). As a post hoc exploratory analysis, we compared the mean scores of CCI between PP (+) and PP ( −) patients. There was significant difference between them (6.6 vs. 6.1, P = 0.017). This may support that polypharmacy can in part reflect the burdens of comorbidities. However, using these criteria, advanced cancer patients tend to be classified to the high-risk group just because they have metastatic cancer. Moreover, the burden of symptoms in advanced cancer patients is not reflected. There are some scales to assess symptoms, such as Numerical Rating Scale (NRS), but polypharmacy might be a simpler objective indicator and easy for clinicians to recognize symptoms or comorbidities as a whole. We believe, therefore, that polypharmacy could be another viewpoint worthy of attention as a simple indicator or approximate sum of the burden of physical or psychiatric symptoms along with comorbidities.
This study has several limitations. First, this was a retrospective, non-randomized study that was conducted at a single institution with a relatively small number of patients; thus, the possibility of unintentional selection bias in the selection of patients cannot be entirely excluded. Second, we did not investigate the use of over-the-counter medications, herbal medicinal products, supplements, or use-as-needed medications. This may underestimate the need of medical interventions using medications. However, we prioritized the simplicity in counting concomitant medications considering future application in clinical practice. Third, concordances between polypharmacy and comorbidities or symptoms were not fully examined in this study. Hence, future prospective studies with larger cohorts are warranted to validate the findings of this study.
Conclusions
Polypharmacy is a common problem and an independent prognostic factor in older Japanese patients with advanced or recurrent NSCLC treated with ICI. Although polypharmacy did not have significant impacts on the objective response or occurrence of irAE, it could be utilized as a simple indicator of patients’ comorbidities and symptoms or as a predictive marker of unexpected hospitalizations during ICI treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file2 (TIF 79 kb) Supplemental figure 1. Survival analysis of patients with PIM and those without PIM. Estimated Kaplan–Meier survival curves for (A) progression-free survival and (B) overall survival, comparing PIM (+) patients (n = 60) and PIM (−) patients (n = 97)
Acknowledgements
We would like to thank Enago (https://www.enago.jp/) for the English language review.
Author contributions
TH and AS conceptualized this study. TH, AS, and RK acquired the clinical data. TH, YH, AS, KM, and YO were responsible for the interpretation of the data. TH and YH drafted the manuscript. All authors have read and approved the current version of the manuscript.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
YH has received personal fees from AstraZeneca, Eli Lilly Japan, Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, Kyowa Kirin, and CSL Behring, outside the submitted work. YO has received personal fees from Chugai Pharmaceutical and Takeda Oncology, outside the submitted work. No other potential conflicts of interest were reported.
Ethics approval
The study protocol was approved by the Ethics Committee of the Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital (approval number: 2342) and conducted in accordance with the tenets of the Declaration of Helsinki.
Availability of data and material
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
Prior presentation
Presented in part at European Society for Medical Oncology Asia Congress 2019, Singapore, November 23, 2019.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- American Geriatrics Society Beers Criteria Update Expert P (2012) American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 60(4):616–631 [DOI] [PMC free article] [PubMed]
- Bigot F, Castanon E, Baldini C, Hollebecque A, Carmona A, Postel-Vinay S et al (2017) Prospective validation of a prognostic score for patients in immunotherapy phase I trials: the Gustave Roussy Immune Score (GRIm-Score). Eur J Cancer 84:212–218 [DOI] [PubMed] [Google Scholar]
- By the American Geriatrics Society Beers Criteria Update Expert P (2015) American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 63(11):2227–2246 [DOI] [PubMed]
- Cashman J, Wright J, Ring A (2010) The treatment of co-morbidities in older patients with metastatic cancer. Support Care Cancer 18(5):651–655 [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383 [DOI] [PubMed] [Google Scholar]
- Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47(11):1245–1251 [DOI] [PubMed] [Google Scholar]
- Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H et al (2018) Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 29(6):1437–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elixhauser A, Steiner C, Harris DR, Coffey RM (1998) Comorbidity measures for use with administrative data. Med Care 36(1):8–27 [DOI] [PubMed] [Google Scholar]
- Elkrief A, El Raichani L, Richard C, Messaoudene M, Belkaid W, Malo J et al (2019) Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology 8(4):e1568812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferner RE, Aronson JK (2006) Communicating information about drug safety. BMJ 333(7559):143–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field TS, Gurwitz JH, Avorn J, McCormick D, Jain S, Eckler M et al (2001) Risk factors for adverse drug events among nursing home residents. Arch Intern Med 161(13):1629–1634 [DOI] [PubMed] [Google Scholar]
- Hakozaki T, Okuma Y, Omori M, Hosomi Y (2019) Impact of prior antibiotic use on the efficacy of nivolumab for non-small cell lung cancer. Oncol Lett 17(3):2946–2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamy AS, Derosa L, Valdelievre C, Yonekura S, Opolon P, Priour M et al (2020) Comedications influence immune infiltration and pathological response to neoadjuvant chemotherapy in breast cancer. Oncoimmunology 9(1):1677427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Lee JH, Chun EK, Kim KI, Kim JW, Kim SH et al (2019) Polypharmacy, inappropriate medication use, and drug interactions in older korean patients with cancer receiving first-line palliative chemotherapy. Oncologist [DOI] [PMC free article] [PubMed]
- Jorgensen TL, Herrstedt J, Friis S, Hallas J (2012) Polypharmacy and drug use in elderly Danish cancer patients during 1996 to 2006. J Geriatr Oncol 3(1):33–40 [Google Scholar]
- Kotlinska-Lemieszek A, Paulsen O, Kaasa S, Klepstad P (2014) Polypharmacy in patients with advanced cancer and pain: a European cross-sectional study of 2282 patients. J Pain Symptom Manage 48(6):1145–1159 [DOI] [PubMed] [Google Scholar]
- LeBlanc TW, McNeil MJ, Kamal AH, Currow DC, Abernethy AP (2015) Polypharmacy in patients with advanced cancer and the role of medication discontinuation. Lancet Oncol 16(7):e333–e341 [DOI] [PubMed] [Google Scholar]
- Lees J, Chan A (2011) Polypharmacy in elderly patients with cancer: clinical implications and management. Lancet Oncol 12(13):1249–1257 [DOI] [PubMed] [Google Scholar]
- Liam CK, Pang YK, Leow CH, Poosparajah S, Menon A (2006) Changes in the distribution of lung cancer cell types and patient demography in a developing multiracial Asian country: experience of a university teaching hospital. Lung Cancer 53(1):23–30 [DOI] [PubMed] [Google Scholar]
- Little AG, Gay EG, Gaspar LE, Stewart AK (2007) National survey of non-small cell lung cancer in the United States: epidemiology, pathology and patterns of care. Lung Cancer 57(3):253–260 [DOI] [PubMed] [Google Scholar]
- Maggiore RJ, Dale W, Gross CP, Feng T, Tew WP, Mohile SG et al (2014) Polypharmacy and potentially inappropriate medication use in older adults with cancer undergoing chemotherapy: effect on chemotherapy-related toxicity and hospitalization during treatment. J Am Geriatr Soc 62(8):1505–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda A, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H et al (2014) Cancer incidence and incidence rates in Japan in 2008: a study of 25 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 44(4):388–396 [DOI] [PubMed] [Google Scholar]
- Mohamed MR, Ramsdale E, Loh KP, Arastu A, Xu H, Obrecht S et al (2020) Associations of polypharmacy and inappropriate medications with adverse outcomes in older adults with cancer: a systematic review and meta-analysis. Oncologist 25(1):e94–e108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldak S, Ioannou S, Kamath P, Huang M, George S, Slomovitz B et al (2019) Polypharmacy in patients with ovarian cancer. Oncologist 24(9):1201–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P (2015) STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 44(2):213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini J, Yousry M, Rickenlund A, Liska J, Hamsten A, Eriksson P et al (2010) The feasibility of velocity vector imaging by transesophageal echocardiography for assessment of elastic properties of the descending aorta in aortic valve disease. J Am Soc Echocardiogr 23(9):985–992 [DOI] [PubMed] [Google Scholar]
- Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T et al (2019) Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol [DOI] [PMC free article] [PubMed]
- Raijmakers NJ, van Zuylen L, Furst CJ, Beccaro M, Maiorana L, Pilastri P et al (2013) Variation in medication use in cancer patients at the end of life: a cross-sectional analysis. Support Care Cancer 21(4):1003–1011 [DOI] [PubMed] [Google Scholar]
- Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM (2019) Immune Checkpoint Inhibitor Outcomes for Patients With Non-Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J Clin Oncol 37(22):1927–1934 [DOI] [PubMed] [Google Scholar]
- Rosai J (2000) A Message from the New Editorial Team. Int J Surg Pathol 8(1):1 [DOI] [PubMed] [Google Scholar]
- Sokol KC, Knudsen JF, Li MM (2007) Polypharmacy in older oncology patients and the need for an interdisciplinary approach to side-effect management. J Clin Pharm Ther 32(2):169–175 [DOI] [PubMed] [Google Scholar]
- Tamura T, Kurishima K, Nakazawa K, Kagohashi K, Ishikawa H, Satoh H et al (2015) Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol 3(1):217–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd A, Williamson S, Husband A, Baqir W, Mahony M (2013) Patients with advanced lung cancer: is there scope to discontinue inappropriate medication? Int J Clin Pharm 35(2):181–184 [DOI] [PubMed] [Google Scholar]
- Vora N, Reckamp KL (2008) Non-small cell lung cancer in the elderly: defining treatment options. Semin Oncol 35(6):590–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BY, Huang JY, Cheng CY, Lin CH, Ko J, Liaw YP (2013) Lung cancer and prognosis in Taiwan: a population-based cancer registry. J Thorac Oncol 8(9):1128–1135 [DOI] [PubMed] [Google Scholar]
- Zhan C, Sangl J, Bierman AS, Miller MR, Friedman B, Wickizer SW et al (2001) Potentially inappropriate medication use in the community-dwelling elderly: findings from the 1996 Medical Expenditure Panel Survey. JAMA 286(22):2823–2829 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file2 (TIF 79 kb) Supplemental figure 1. Survival analysis of patients with PIM and those without PIM. Estimated Kaplan–Meier survival curves for (A) progression-free survival and (B) overall survival, comparing PIM (+) patients (n = 60) and PIM (−) patients (n = 97)