Summary
Lepalvir, derived from inflamed rabbit skin inoculated with vaccinia virus, has potential neuroprotective and anti-inflammatory effects. We conducted a phase II, multicenter, randomized, blind, placebo-controlled trial investigating the efficacy and safety of Lepalvir for acute ischemic stroke (AIS). Participants aged 18–80 years with AIS in the anterior circulation and a National Institutes of Health Stroke Scale (NIHSS) score of 4–24 within 48 h post-onset were randomized to receive high-dose (192U), low-dose (96U) Lepalvir, or saline placebo for 14 days. The primary outcome was the proportion of patients achieving a modified Rankin Scale (mRS) score ≤ 1 at day 90 (D90) post-randomization. Among 238 patients, no significant difference in mRS score at D90 was observed across groups, yet a higher percentage in the high-dose group achieved a mRS score ≤ 1 at D90, compared to the control and low-dose group. No significant safety concerns were noted. While functional improvement was not significantly different at D90, Lepalvir showed a favorable safety profile and potential at the higher dosage, warranting further phase III investigation.
Subject areas: health sciences, natural sciences, applied sciences
Graphical abstract

Highlights
-
•
Lepalvir is the extract obtained from inflamed rabbit skin inoculated with vaccinia virus
-
•
Lepalvir has been explored to possess neuroprotective and anti-inflammatory effects
-
•
This is a randomized controlled phase 2 trial in acute ischemic stroke (AIS) patients
-
•
Lepalvir was safe at both 96 U and 192 U dosages but had no effect on the primary endpoint
Health sciences; Natural sciences; Applied sciences
Introduction
Stroke is the second leading cause of death and the third leading cause of disability in the world.1,2 Acute ischemic stroke (AIS) accounted for 69.6%–70.8% of all stroke cases in China, causing a massive health and social economic burden.3,4,5
The pathogenesis of AIS is complex and involves multiple pathophysiological processes, including oxidative and nitrosative stress, neuroinflammation, excitotoxicity, apoptosis, and blood-brain barrier disruption.6,7 Early reperfusion therapy, such as intravenous thrombolysis (IVT) and endovascular treatment (EVT), has become the key to effectively reducing disability and mortality in AIS patients. However, a subset of AIS patients may not be eligible for reperfusion therapy due to out-of-time windows or hemorrhagic transformation risk. Moreover, reperfusion therapy may lead to an ischemia-reperfusion injury.8,9,10,11,12 Therefore, neuroprotection has emerged as a potential therapeutic approach for AIS. However, numerous neuroprotective agents have failed to demonstrate clinical benefit in the treatment of AIS patients,13,14,15,16,17,18 underscoring the urgent need for new therapeutic options.
The extract from rabbit skin inflamed by vaccinia virus has been widely used for treating neuropathic pain and as an anti-allergy remedy.19,20,21 A series of preclinical studies have shown its potential in the treatment of cognitive impairment,22,23,24 demyelinating diseases,25,26,27 spinal cord injury (SCI),28 and hypoxic-ischemic brain injury.29 Cumulative evidence indicated that the extract from rabbit skin inflamed by vaccinia virus may exert neuroprotective and restorative effects through multiple mechanisms, including anti-inflammation by suppressing pro-inflammatory cytokines and leukocyte infiltration, inhibiting apoptosis, modulating cytokines, stimulating neuronal expression of brain-derived neurotrophic factor (BDNF), inhibiting demyelination, and so on.28,30,31,32 Thus, it can be hypothesized that the extract may hold the potential for treating AIS. However, clinical data of the extract obtained from rabbit skin inflamed by vaccinia virus (Lepalvir) for the treatment of AIS are still lacking.
This phase II, multicenter, randomized, blinded, parallel, placebo-controlled, dose-finding study was designed to evaluate the effectiveness and safety of Lepalvir at different doses in patients with AIS.
Results
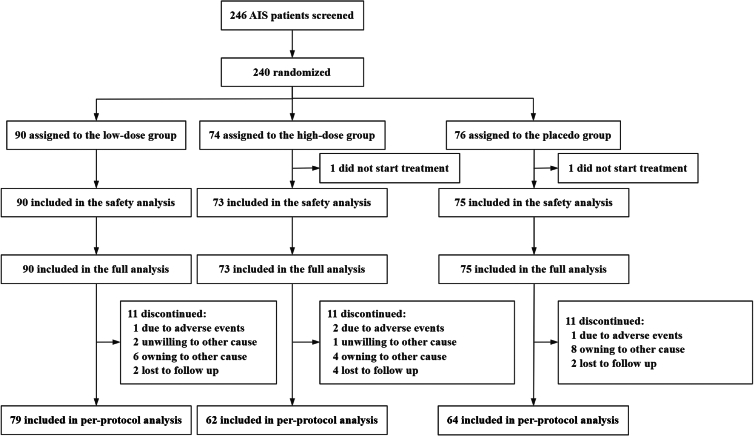
From October 2022 through December 2023, 246 AIS patients were screened at 16 centers in China. Among these patients, 6 were excluded due to consent withdrawal (1 patient) or withdrawal by the enrolling physician before treatment due to an error in assessing patient eligibility (4 patients) or in randomization (1 patient) (Figure 1). Finally, 240 patients were randomized, among whom 2 did not receive any treatment, and thus 238 patients (90 received low-dose Lepalvir, 73 received high-dose Lepalvir, and 75 received placebo normal saline) were included in the safety analysis and full analysis. Of the remaining 238 patients, 205 (79 received low-dose Lepalvir, 62 received high-dose Lepalvir, and 64 received placebo normal saline) completed the study according to the protocol, who were finally included in the per-protocol analysis (Figure 1).
Figure 1.
Flowchart
Baseline characteristics
The baseline demographic and clinical characteristics of patients are listed in Table 1. As shown in Table 1, there were no significant differences among these three groups at baseline, except for the family history of stroke (p = 0.027) and current smoking (p = 0.035).
Table 1.
Baseline characteristics of patients included in the safety analysis
| Characteristics | Low-dose group (n = 90) | High-dose group (n = 73) | Control group (n = 75) |
|---|---|---|---|
| Age, y; median (IQR) | 66.0 (59.0–70.0) | 65.0 (55.0–68.0) | 64.0 (56.0–70.0) |
| <65 | 41 (45.6%) | 36 (49.3%) | 38 (50.7%) |
| ≥65 | 49 (54.4%) | 37 (50.7%) | 37 (49.3%) |
| Men, n (%) | 57 (63.3%) | 55 (75.3%) | 58 (77.3%) |
| Ethnicity, n (%) | |||
| Han | 84 (93.3%) | 71 (97.3%) | 74 (98.7%) |
| Others | 6 (6.7%) | 2 (2.7%) | 1 (1.3%) |
| Body mass index, kg/m2; mean (SD) | 24.5 (3.6) | 24.4 (3.5) | 24.6 (3.4) |
| Medical history, n (%) | |||
| Stroke | 46 (51.1%) | 28 (38.4%) | 34 (45.3%) |
| Hypertension | 59 (65.6%) | 53 (72.6%) | 52 (69.3%) |
| Hyperlipidemia | 28 (31.1%) | 25 (34.2%) | 23 (30.7%) |
| Diabetes | 24 (26.7%) | 21 (28.8%) | 19 (25.3%) |
| Heart disease | 32 (35.6%) | 27 (37.0%) | 27 (36.0%) |
| Atrial fibrillation | 6 (6.7%) | 1 (1.4%) | 3 (4.0%) |
| Family history of stroke, n (%)a. | 4 (4.4%) | 6 (8.2%) | 0 (0.0%) |
| Current smoking, n (%)a. | 33 (36.7%) | 37 (50.7%) | 42 (56.0%) |
| Current heavy drinking, n (%) | 23 (25.6%) | 23 (31.5%) | 31 (41.3%) |
| NIHSS score at baseline, median (IQR) | 7.0 (5.0–8.0) | 6.0 (5.0–8.0) | 7.0 (6.0–8.0) |
| BI score, median (IQR) | 50.0 (35.0–65.0) | 50.0 (35.0–60.0) | 45.0 (35.0–60.0) |
| MoCA score, mean (SD) | 16.1 (7.1) | 17.3 (7.0) | 15.9 (6.9) |
| SS-QOL score, median (IQR) | 145.0 (125.0–171.0) | 147.0 (128.5–169.5) | 140.0 (123.0–171.0) |
| TOAST subtype, n (%) | |||
| LAA | 51 (56.7%) | 48 (65.8%) | 53 (70.7%) |
| SAO | 31 (34.4%) | 18 (24.7%) | 19 (25.3%) |
| CE | 5 (5.6%) | 2 (2.7%) | 2 (2.7%) |
| Other | 3 (3.3) | 5 (6.8%) | 1 (1.3%) |
IQR indicates interquartile range; SD, standard deviation; NIHSS, National Institutes of Health Stroke Scale; BI, Barthel Index; MoCA, Montreal Cognitive Assessment Scale; and SS-QOL, Stroke Specific Quality; TOAST, Trial of ORG 10172 in Acute Stroke Treatment; LAA, large artery atherosclerosis; SAO, small artery occlusion; and CE, cardiogenic embolism. a. p < 0.05.
Efficacy outcomes
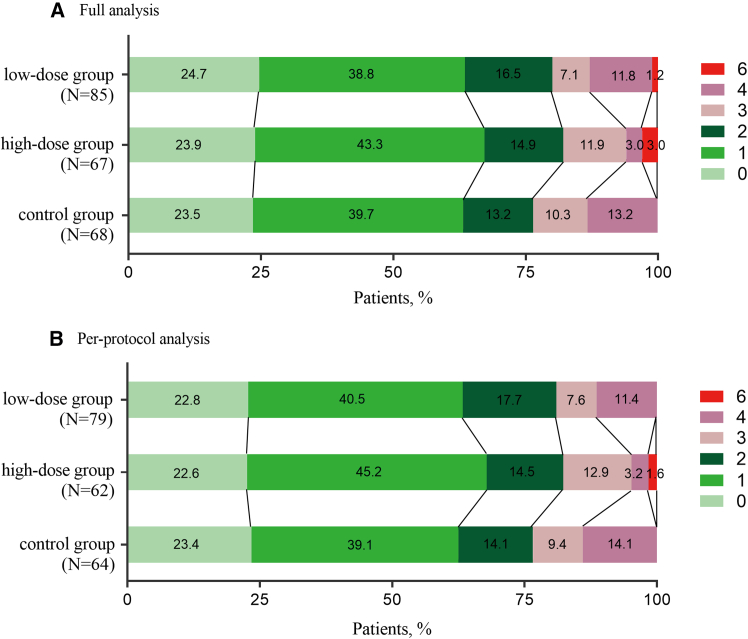
The overall distribution of mRS scores is shown in Figure 2. In the full analysis set (FAS), there was no significant difference in the primary outcome measured as the proportion of patients with an mRS score ≤ 1 at 90 days, which occurred in 54 of 85 patients (63.5%) in the low-dose group, 45 of 67 patients (67.2%) in the high-dose group, and 43 of 68 patients (63.2%) in the control group (p = 0.775). In the per-protocol analysis set (PPS), 50 of 79 patients (63.3%) in the low-dose group, 42 of 62 patients (67.7%) in the high-dose group, and 40 of 64 patients (62.5%) in the control group achieved the primary outcome of an mRS score ≤ 1 at 90 days (p = 0.647). Results of sensitivity analyses for the primary outcome showed no significant difference (p = 0.775).
Figure 2.
Distribution of mRS scores at 90 days in the FAS (A) and PPS (B)
Analyses of secondary efficacy endpoints showed that the following metrics were higher in the high-dose group than in the control group in both the FAS (Table 2) and the PPS (Table S1): the proportion of patients with mRS score ≤ 2 on D90; the proportion of patients with NIHSS score improved by 4 or more or recovered to ≤ 1 on D7, D14, D30, and D90; change in National Institutes of Health Stroke Scale (NIHSS) scores relative to baseline; the mean Montreal Cognitive Assessment (MoCA) score; the mean stroke specific quality of life (SS-QOL) score; and the proportion of BI ≥ 95 on D14, D30, or D90. The results suggested that the 192U dose might be beneficial in restoring the quality of daily life and cognitive function and reducing neurological deficits in patients with AIS but without significant differences.
Table 2.
Secondary efficacy outcomes (FAS)
| Secondary Efficacy Outcomes | Low-dose group | High-dose group | Control group |
|---|---|---|---|
| mRS ≤ 2 on D90, no./total no. (%) | 68/85 (80.0%) | 55/67 (82.1%) | 52/68 (76.5%) |
| NIHSS score improvement of ≥ 4 points or recovery to ≤ 1 point (NIHSS 0–1, no./total no. (%)) | |||
| D7 | 13/90 (14.4%) | 17/71 (23.9%) | 11/71 (15.5%) |
| D14 | 33/82 (40.2%) | 30/68 (44.1%) | 24/66 (36.4%) |
| D30 | 50/84 (59.5%) | 46/68 (67.6%) | 41/68 (60.3%) |
| D90 | 68/84 (81.0%) | 51/65 (78.5%) | 52/68 (76.5%) |
| Changes of NIHSS score from baseline, median (IQR) | |||
| D7 | −2.0 (−3.0 to −1.0) | −2.0 (−3.0 to −1.0) | −1.0 (−2.0 to 0.0) |
| D14 | −3.0 (−4.0 to −2.0) | −3.0 (−5.0 to −2.0) | −3.0 (−4.0 to −2.0) |
| D30 | −4.0 (−6.0 to −3.0) | −4.0 (−5.0 to −3.0) | −4.0 (−5.0 to −2.0) |
| D90 | −5.0 (−6.0 to −4.0) | −5.0 (−6.0 to −4.0) | −5.0 (−6.0 to −3.5) |
| BI score, median (IQR) | |||
| D7 | 60.0 (45.0–90.0) | 65.0 (45.0–85.0) | 65.0 (45.0–80.0) |
| D14 | 85.0 (60.0–95.0) | 85.0 (57.5–95.0) | 80.0 (60.0–95.0) |
| D30 | 95.0 (80.0–100.0) | 95.0 (77.5–100.0) | 90.0 (80.0–100.0) |
| D90 | 100.0 (90.0–100.0) | 100.0 (95.0–100.0) | 100.0 (90.0–100.0) |
| BI score ≥ 95, no./total no. (%) | |||
| D7 | 16/89 (18.0%) | 8/71 (11.3%) | 10/71 (14.1%) |
| D14 | 24/82 (29.3%) | 25/68 (36.8%) | 19/65 (29.2%) |
| D30 | 44/84 (52.4%) | 38/68 (55.9%) | 33/68 (48.5%) |
| D90 | 61/84 (72.6%) | 51/65 (78.5%) | 50/68 (73.5%) |
| MoCA, median (IQR) | |||
| D7 | 18.0 (13.0–23.0) | 21.0 (18.0–25.0) | 20.0 (14.0–23.0) |
| D14 | 21.0 (16.0–24.5) | 24.0 (19.0–26.0) | 22.5 (16.5–25.0) |
| D30 | 21.0 (14.0–25.0) | 24.0 (19.5–27.0) | 22.0 (16.5–26.0) |
| D90 | 23.0 (16.0–26.0) | 24.0 (20.0–28.0) | 22.0 (18.0–27.0) |
| SS-QOL, median (IQR) | |||
| D7 | 174.0 (147.5–195.5) | 173.0 (154.0–206.0) | 174.0 (138.0–200.0) |
| D14 | 194.0 (163.0–212.0) | 197.0 (172.0–224.0) | 196.0 (163.0–210.0) |
| D30 | 209.0 (188.0–226.5) | 212.0 (189.0–234.5) | 208.5 (185.0–223.0) |
| D90 | 220.0 (200.0–234.0) | 226.0 (201.0–239.0) | 225.5 (204.5–237.0) |
D7 indicates day 7; D14, day 14; D30, day 30; D90, day 90; IQR, interquartile range; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; BI, Barthel Index; MoCA, Montreal Cognitive Assessment Scale; and SS-QOL, Stroke Specific Quality of Life Scale.
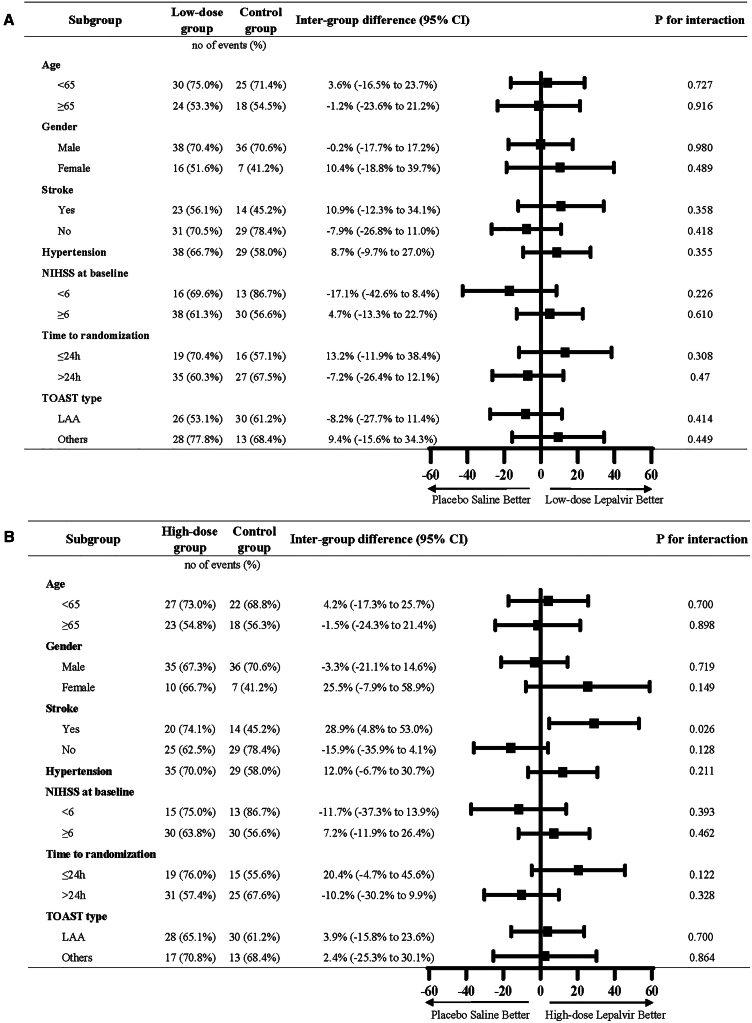
The results of the prespecified subgroup analyses of the primary outcome in the FAS are presented in Figure 3. Notably, factors such as time to receive treatment, gender, baseline NIHSS scores, history of stroke, and hypertension may influence functional outcomes. Among patients with baseline NIHSS score ≥6, the high-dose group showed a slightly better trend than that of the control group (63.8% vs. 56.6%, odds ratio [OR] = 1.343 [95% confidence interval [CI], 0.600–3.003], p = 0.578), which was similar to the main analysis. In addition, female patients, patients with a history of stroke, or patients receiving the treatment agents within 24 h of AIS onset appeared to be more likely to have better functional outcomes in both the low-dose and high-dose groups.
Figure 3.
Odds ratios (ORs) for the primary outcome in prespecified subgroups (FAS)
(A) The low-dose group compared to the control group; (B) the high-dose group compared to the control group. Data are represented as no of events (%). NIHSS, National Institutes of Health Stroke Scale; LAA, large artery atherosclerosis; and TOAST, Trial of ORG 10172 in acute stroke treatment.
Safety outcomes
The analyses of safety outcomes indicated that the three groups had similar incidences of adverse events, serious adverse events, the number of deaths, and similar laboratory test values (Table 3). All serious adverse events and deaths were determined by the investigators to be unrelated to the trial drug.
Table 3.
Safety outcomes (SAS)
| Safety Outcomes | Low-dose group (n = 90) | High-dose group (n = 73) | Control group (n = 75) |
|---|---|---|---|
| All-cause death within 90 days, n (%) | 1 (1.1%) | 2 (2.7%) | 0 (0.0%) |
| Hemorrhagic transformation after stroke within 90 days, n (%) | 0 (0.0%) | 2 (2.7%) | 1 (1.3%) |
| Recurrent ischemic stroke within 90 days, n (%) | 4 (4.4%) | 2 (2.7%) | 2 (2.7%) |
| AEs, n (%) | 75 (83.3%) | 57 (78.1%) | 61 (81.3%) |
| SAEs, n (%) | 9 (10.0%) | 4 (5.5%) | 7 (9.3%) |
| Number of deaths, n (%) | 1 (1.1%) | 2 (2.7%) | 0 (0.0%) |
AEs indicate adverse events; SAEs, serious adverse events.
Discussion
In this phase II, multicentre, randomized, double-blind, multiple-dose, placebo-controlled clinical trial, Lepalvir at different doses were compared for efficacy and safety. Our result showed that Lepalvir was safe and well tolerated at all doses, although no significant improvement in functional outcomes was observed.
There were no significant differences in the efficacy and safety outcomes among the low-dose Lepalvir group, the high-dose Lepalvir group, and the control group in patients with anterior circulation AIS. However, the high-dose Lepalvir group exhibited a slight trend toward superior effectiveness compared to the control group. The proportion of patients with an mRS score ≤ 1 at 90 days was slightly higher in the high-dose (192U Lepalvir) group than in the control group. In subgroup analyses, the results suggested that the AIS patients with more severe neurological deficits (baseline NIHSS score ≥ 6) might benefit from the 192U dose. Besides, female patients, patients with a history of stroke, or receiving the treatment agents within 24 h of AIS onset appeared to be more likely to have better functional outcomes in the Lepalvir groups. Therefore, it will be a logical step to further test the 192U Lepalvir versus the placebo in a well-powered phase III study, and this trial may provide some reference value for it.
Since the 1980s, researchers have conducted numerous animal experiments to explore the therapeutic effects of neuroprotective agents on ischemic stroke, and have achieved encouraging results. However, subsequent clinical trials have mostly reported disappointing results, mainly including no significant improvement in the efficacy of the treatment group compared with the control group, or a higher incidence of adverse events in the treatment group.14,33 The exploratory analysis of the ESCAPE-NA1 (efficacy and safety of nerinetide for the treatment of acute ischemic stroke) trial indicated a potential efficacy of nerinetide for patients who did not receive treatment of alteplase, which was a global multicenter clinical trial with a narrow treatment time window within 12 h, incorporating neuroimaging assessments for participant eligibility.34 Moreover, edaravone dexborneol, a novel multitargeted neuroprotectant, was reported to have significant benefits in improving functional outcomes for patients with AIS in the TASTE (Edaravone Dexborneol vs. Edaravone alone for the treatment of acute ischemic stroke) trial.35 Recently, a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial confirmed that ginkgo diterpene lactone meglumine (GDLM) significantly improved neurological deficits in patients within 48 h of AIS onset and the proportion of patients achieving favorable clinical outcomes at 90 days compared with placebo.36 These previously mentioned trials indicated, to some extent, that multitargeted neuroprotectants might be more promising for AIS treatment than those targeting a single pathway, partially due to the complex process of ischemic damage. In addition, all of them were large-scale clinical trials with a larger sample size.
Previous research data indicated that the nonprotein extract from rabbit skin inflamed by vaccinia virus, consisting of multiple active ingredients, might exert the neuroprotective and restorative effects through multiple targets. An animal study28 showed that it could decrease the expression levels of proinflammatory cytokines such as interleukin 6, thymus chemokine-1 (TCK-1), and lipopolysaccharide-induced CXC chemokine (LIX), whereas increase the levels of prorepair cytokine hepatocyte growth factor and adiponectin. Also, by inhibiting the TLR4/MyD88/NF-κB inflammatory signaling pathway, the extract ameliorated cognitive impairment in vascular dementia (VD) mice.23 On the other hand, preclinical studies suggested that the extract might exert its neuroprotective effects by influencing specific signaling pathways, such as enhancing the expression of neurotrophins by activating autocrine pathways which depend on TrkB activity,37 facilitating the TrkA-mediated neurotrophin signaling pathway.38 Another study found that it improved cognitive function in chronic cerebral hypoperfusion (CCH) rats, possibly through the activation of the Akt/GSK3β pathway, which in turn reduced β-amyloid (Aβ) accumulation and tau phosphorylation in the hippocampus.24
In this clinical trial, no significant improvement in functional outcomes was observed. Possible causes for the results may include but are not limited to the limited sample size, patient characteristics, treatment time window, dosage used, and so on. The primary efficacy outcome in this trial was the proportion of patients with mRS score ≤ 1 at 90 days post-randomization which served as an indicator of neurological functional recovery. The information of infarct core and penumbra volumes on imaging was absent. The assessment of Lepalvir’s effects was not comprehensive. Further studies need to incorporate neuroimaging metrics and biological information. Nevertheless, this trial provided a reference for further research, and offers a preliminary exploration of the effective dosage of Lepalvir for the treatment of AIS.
Limitations of the study
Several limitations in the present study need to be addressed. First, because the trial primarily involved patients of Han Chinese ethnicity, caution should be taken when generalizing these findings to other ethnic groups. Second, patients receiving IVT or EVT were excluded from this study. Given the increasing use of recanalization therapy and the associated risk of ischemia-reperfusion injury, further research is needed to evaluate the efficacy and safety of Lepalvir in patients undergoing recanalization therapy. Third, the sample size in our study tended to be small, and therefore, the results must be interpreted with caution. Additionally, the potential mechanisms of Lepalvir were not explored in this study. Most studies on the specific mechanisms of the extract’s neuroprotective effects were focused on cellular and animal models. Further studies may consider incorporating the biomarkers detection to better explore the pathways involved in its neuroprotective mechanisms.
Conclusions
This phase II, multicenter, randomized, blind, parallel, placebo-controlled clinical trial showed that there were no significant differences in good functional and safety outcomes among the high-dose Lepalvir group, the low-dose Lepalvir group, and the control group. The efficacy still needs to be explored in a large trial. The 192U Lepalvir might be the optimal dose for a phase III clinical trial.
Resource availability
Lead contact
Further information and requests should be directed to and will be fulfilled by the lead contact: Haiqing Song (songhq@xwhosp.org).
Materials availability
This study did not generate new materials.
Data and code availability
-
•
This article includes all data generated or analyzed during this study.
-
•
This paper does not report the original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
We would like to thank the patients and their families for participating in this trial. This trial was funded by a grant from Vanworld Pharmaceutical Rugao Co., Ltd. (Jiangsu, China).
Author contributions
S.H. and W.Y. conceived and designed the study. D.K., M.Q., M.Y., G.A., L.R., L.J., Z.H., Y.Q., Y.W., and S.Y. supplied patients and collected the data with the CRO. Peking University Clinical Research Institute did the statistical analysis. Z.B. wrote the first draft of the manuscript, and all authors provided critical review, revised the text, and approved the final version for publication. S.H., W.Y., and Z.B. had full access to all study data and accepted the final responsibility for the decision to submit for publication. S.H. and W.Y. revised and approved the manuscript.
Declaration of interests
Vanworld Pharmaceutical Rugao Co., ltd. sponsored and funded this study, including providing investigational drug, funding to the investigator team, and sponsoring investigator meetings. There are no other potential conflicts of interest relevant to this article to report.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| SAS 9.4 | SAS | https://www.sas.com/ |
Experimental model and study participant details
The trial (Treatment of Acute Ischemic Stroke with different doses of Lepalvir) was a phase II, multicenter, randomized, blind, parallel, placebo-controlled study conducted at 16 centers in China. Patients were eligible to participate in this trial if they met the following criteria: aged 18 to 80 years, diagnosis of AIS in the anterior circulation according to the 2018 Chinese Guidelines for the Diagnosis and Treatment of AIS,39 ability to receive the study agents within 48 hours of AIS onset, having a National Institute of Health Stroke Scale (NIHSS) score between 4 and 24, and a total score of upper and lower limbs on motor deficits ≥ 2 at admission. The exclusion criteria included: thrombolytic or endovascular therapy; intracranial hemorrhagic disease; transient ischemic attack (TIA); severe consciousness disturbances, dementia, or other diseases affecting evaluation; recurrent ischemic stroke with a mRS score > 1 prior to the current episode; a blood glucose concentration of < 2.8 mmol/L or > 16.8 mmol/L; systolic pressure of ≥ 220 mmHg or diastolic pressure ≥ 120 mmHg after treatment or history of hypotension or three consecutive measurements of blood pressure less than 90/60 mmHg; heart rate < 40 beats/min or > 120 beats/min; acute myocardial infarction or undergoing intervention treatment within 6 months; heart failure, the New York Heart Association (NYHA) Class III-IV; alanine aminotransferase (ALT) or aspartate aminotransferase (AST) > 2.0 × upper limit of normal (ULN) or acute hepatitis, chronic active hepatitis, cirrhosis; severe kidney disease; iatrogenic stroke; malignant tumors; severe systemic diseases; other neuroprotective agents already in use; other conditions that make participation inappropriate.
The ethics committee from each study center approved this study, and all patients or their legally acceptable surrogates provided informed consent before they were assigned to treatment. The study was conducted in accordance with the principles of the Declaration of Helsinki, and all the authors of this article assume responsibility for the accuracy and completeness of the data. The study was registered with https://www.chictr.org.cn/ (unique identifier: ChiCTR2200063819).
Method details
Drugs
Lepalvir is the extract obtained from rabbit skin inflamed by vaccinia virus, and offered by Vanworld Pharmaceutical Rugao Co., Ltd. (Jiangsu, China). There are 6 oligopeptides analyzed by Nano LC-MS/MS. Since the patent application is still in progress, the sequences of peptides are confidential.
Based on our previous safety studies of Lepalvir in healthy individuals, it was safe and well tolerated at single administration doses ranging from 18 to 318 U, and at daily doses of 36 to 240 U administered over consecutive 10 days. Pharmacodynamic studies in the rat middle cerebral artery occlusion (MCAO) model demonstrated that a dose range of 10-40 U/kg significantly reduced the infarct area, improved the neurological function, and reduced cerebral edema, with a clear dose-response relationship. Using the body surface area (BSA) normalization method,40 the estimated human equivalent dose (HED) ranged from 98.4 U∼393 U. In addition, most studies on the extract were conducted over 10- to 14-day periods, with effect observed after 10 days of continuous administration.41,42 Thus, we selected 96 U as the low-dose group and 192 U as the high-dose group, with both doses administered once daily for 14 days.
Randomization and Masking
AIS patients were randomized in a 1:1:1 ratio into three groups: the control group, the low-dose group (Lepalvir, 96U/dose), and the high-dose (Lepalvir, 192U/dose) group. The randomization numbers were assigned using an automated centralized randomization system.
Researchers and other blind researchers, subjects, and sponsors will not be allowed to access any information about the grouping of experimental drugs and related documents. Independent statisticians who have nothing to do with the final statistics of the experiment use a fixed number of seeds to generate a blind bottom through SAS software and upload it to the central random system after the research is officially launched. In the course of the study, the blind bottom was stored in the central random system until the end of the study.
The allocation of drugs was conducted by professional nurses independently, and blinding was performed by statisticians who were not related to this clinical trial. The treatment drug "Lepalvir" was dissolved by "0.9% normal saline", looking a nearly colorless to yellowish clear solution, which was slightly different from the placebo "0.9% normal saline". Therefore, we used a black light-proof bag to be added to the infusion bag to ensure a blind state. Besides, during the trial, the non-blinded personnel and non-blinded inspectors responsible for the management and allocation of experimental drugs in this trial have signed a confidentiality agreement.
Procedures
The study drug, a kind of extract from rabbit skin inflamed by vaccinia virus (Lepalvir®), was obtained from Vanworld Pharmaceutical Rugao Co. Ltd (Jiangsu, China). In this trial, patients were randomly assigned to three groups: control group (normal saline, 160ml for intravenous infusion, once a day, continued for 14 days), low-dose group (Lepalvir, 96U/dose [Lepalvir® 80ml+normal saline 80ml], once a day, continued for 14 days), and high-dose group (Lepalvir, 192U/dose [Lepalvir® 160ml], once a day, continued for 14 days). All the groups' drugs were diluted to 250ml with 0.9% saline.
The patients routinely received standard secondary prevention therapy for AIS in accordance with guidelines,43 including antiplatelet or anticoagulant therapy, and lipid-lowering medications. The use of other neuroprotective agents (e.g., edaravone, edaravone dexborneol, butylphthalide, and ginkgo biloba extract), defibrinogen therapy, and volume expansion therapy were prohibited.
Endpoints
The primary efficacy outcome was the proportion of patients with modified Rankin Scale (mRS) score ≤ 1 at 90 days after randomization. Subgroup analyses for the primary outcome were conducted based on age (< 65 to ≥ 65 years), sex (male and female), medical history, baseline NIHSS score (< 6 and ≥ 6), AIS onset time to randomization (≤ 24 and > 24 hours), and TOAST subtype. The interaction effects of treatment by each of the aforementioned variables for the primary efficacy were tested.
The secondary outcomes included the proportion of patients with mRS score ≤ 2 on D90; mRS score distribution on D7, D14, D30, and D90; NIHSS score on D7, D14, D30, and D90; NIHSS score change from baseline to D7, D14, D30, and D90; the proportion of patients with NIHSS score improved by 4 or more or recovered to ≤ 1 on D7, D14, D30, and D90; Barthel Index (BI) score on D7, D14, D30, and D90; the proportion of patients with BI ≥ 95 on D7, D14, D30, and D90; Montreal Cognitive Assessment (MoCA) score on D7, D14, D30, and D90; Stroke Specific Quality of Life Scale (SS-QOL) score on D7, D14, D30, and D90. The mRS was measured by investigators who were trained, tested, and certified in the use of the mRS system.
Safety outcomes included the all-cause mortality within 90 days; the proportion of patients with hemorrhage transformation after stroke within 90 days; and the recurrence rate of stroke within 90 days. Patients were assessed for the occurrence of any adverse events (AEs) based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0.44
Quantification and statistical analysis
Sample size
The superiority margin in our study was defined as 30%, and the statistical power was 80%. A superiority test with a 1-sided significance level of 0.025 was used. Considering a 10% loss to follow-up rate, 80 participants were required for each group, with a total of 240 participants for the trial.
Statistical analysis
All statistical analyses were prespecified. The main analyses of efficacy outcomes were the full analysis and per-protocol analysis. The full analysis included the randomized patients who received at least once study treatment. The per-protocol analysis excluded patients who were disqualified according to protocol quality control, such as patients who did not use the study drug strictly in accordance with the protocol and patients whose records did not contain primary outcome data. All the patients who underwent randomization and received at least once study treatment were included in the analysis of safety outcomes.
The baseline characteristics among the groups were compared. Categorical variables were presented as counts with percentages and compared using the χ2 test or Fisher exact test. Continuous variables were presented as mean, standard deviation (SD), median, maximum, minimum, and interquartile range (IQR) and compared using analysis of variance (ANOVA) or Kruskal-Wallis test according to data distribution among the groups. Two-group comparisons were conducted using either independent samples t-test or the Wilcoxon test, and within-group comparisons of pre-and post-treatment outcomes were performed by paired t-test or Wilcoxon test.
Differences in efficacy and safety outcomes among the groups were analyzed. Missing data for efficacy and safety outcome variables were not filled and all analyses were based on observed data. For sensitivity analyses of primary outcome, missing data were handled using a multiple-filling method based on missing at random (MAR). Binary outcomes, including primary efficacy outcome (mRS ≤1 at 90 days), secondary efficacy outcomes (mRS ≤ 2 on D90; NIHSS score improved by 4 or more or recovered to ≤ 1; BI ≥ 95), and all safety outcomes, were examined by χ2 test or Fisher exact test. Odds ratios (ORs) with 95% CI were calculated using binary logistic regression analysis, where a common OR in favor of Lepalvir would be >1.0. For continuous outcomes, including BI, NIHSS, MoCA, and Stroke Impact Scale score on D7, D14, D30, and D90, changes in the NIHSS score from baseline to D7, D14, D30, and D90, means or medians were calculated for each group, and mean differences (95% CI) among the groups were estimated by ANOVA or Kruskal-Wallis test. In addition, the primary outcome was analyzed among several prespecified subgroups by testing the treatment-by-subgroup interaction effect with the use of logistic regression models.
Statistical tests in this study were all 2-sided, and P<0.05 was considered statistically significant. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC).
Additional resources
This clinical trial was registered at the Chinese Clinical Trial Registry (ChiCTR), Identifier: ChiCTR2200063819. https://www.chictr.org.cn/.
Published: December 17, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.111621.
Contributor Information
Yuan Wang, Email: wilma0106@xwhosp.org.
Haiqing Song, Email: songhq@xwhosp.org.
Supplemental information
References
- 1.American Heart Association Correction to: Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;136:e196. doi: 10.1161/CIR.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 2.Feigin V.L., Krishnamurthi R.V., Parmar P., Norrving B., Mensah G.A., Bennett D.A., Barker-Collo S., Moran A.E., Sacco R.L., Truelsen T., et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990-2013: The GBD 2013 Study. Neuroepidemiology. 2015;45:161–176. doi: 10.1159/000441085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2019 Risk Factors Collaborators Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2019 Stroke Collaborators Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD 2019 Viewpoint Collaborators Five insights from the Global Burden of Disease Study 2019. Lancet. 2020;396:1135–1159. doi: 10.1016/S0140-6736(20)31404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 7.Chamorro Á., Dirnagl U., Urra X., Planas A.M. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 8.Bai J., Lyden P.D. Revisiting cerebral postischemic reperfusion injury: new insights in understanding reperfusion failure, hemorrhage, and edema. Int. J. Stroke. 2015;10:143–152. doi: 10.1111/ijs.12434. [DOI] [PubMed] [Google Scholar]
- 9.Li X., Cheng S., Hu H., Zhang X., Xu J., Wang R., Zhang P. Progranulin protects against cerebral ischemia-reperfusion (I/R) injury by inhibiting necroptosis and oxidative stress. Biochem. Biophys. Res. Commun. 2020;521:569–576. doi: 10.1016/j.bbrc.2019.09.111. [DOI] [PubMed] [Google Scholar]
- 10.Takagi J., Otake K., Nakao N., Takashashi M., Hirooka Y. Urinary excretion of aquaporin-2 and inappropriate secretion of vasopressin in hyponatremic patients after cerebral infarction. Horm. Metab. Res. 2003;35:62–66. doi: 10.1055/s-2003-38393. [DOI] [PubMed] [Google Scholar]
- 11.Tu W., Xu X., Peng L., Zhong X., Zhang W., Soundarapandian M.M., Balel C., Wang M., Jia N., Zhang W., et al. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell. 2010;140:222–234. doi: 10.1016/j.cell.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam C.K., Yoo T., Hiner B., Liu Z., Grutzendler J. Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature. 2010;465:478–482. doi: 10.1038/nature09001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin R.H., Yeatts S.D., Hill M.D., Moy C.S., Ginsberg M.D., Palesch Y.Y., ALIAS Parts 1 and 2 and NETT Investigators ALIAS (Albumin in Acute Ischemic Stroke) Trials: Analysis of the Combined Data From Parts 1 and 2. Stroke. 2016;47:2355–2359. doi: 10.1161/STROKEAHA.116.012825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Collins V.E., Macleod M.R., Donnan G.A., Horky L.L., van der Worp B.H., Howells D.W. 1,026 experimental treatments in acute stroke. Ann. Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 15.Saver J.L., Starkman S., Eckstein M., Stratton S.J., Pratt F.D., Hamilton S., Conwit R., Liebeskind D.S., Sung G., Kramer I., et al. Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N. Engl. J. Med. 2015;372:528–536. doi: 10.1056/NEJMoa1408827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diener H.-C., Lees K.R., Lyden P., Grotta J., Davalos A., Davis S.M., Shuaib A., Ashwood T., Wasiewski W., Alderfer V., et al. NXY-059 for the treatment of acute stroke: pooled analysis of the SAINT I and II Trials. Stroke. 2008;39:1751–1758. doi: 10.1161/STROKEAHA.107.503334. [DOI] [PubMed] [Google Scholar]
- 17.Chamorro A., Amaro S., Castellanos M., Segura T., Arenillas J., Martí-Fábregas J., Gállego J., Krupinski J., Gomis M., Cánovas D., et al. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): A randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014;13:453–460. doi: 10.1016/S1474-4422(14)70054-7. [DOI] [PubMed] [Google Scholar]
- 18.Elkins J., Veltkamp R., Montaner J., Johnston S.C., Singhal A.B., Becker K., Lansberg M.G., Tang W., Chang I., Muralidharan K., et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2017;16:217–226. doi: 10.1016/S1474-4422(16)30357-X. [DOI] [PubMed] [Google Scholar]
- 19.Eguchi Y., Aoki Y., Yamashita M., Fujimoto K., Sato T., Abe K., Sato M., Yamanaka H., Toyoguchi T., Shimizu K., et al. Clinical Efficacy of Neurotropin for Lumbar Spinal Stenosis with Low Back Pain. Pain Ther. 2023;12:461–473. doi: 10.1007/s40122-022-00472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu M., Zhou F., Li L., Yin Q., Qiu M., Zhang Y. Success with neurotropin in treating pediatric lower extremity pain induced by spinal cord injury after epidural anesthesia. J. Pain Res. 2017;10:1391–1394. doi: 10.2147/JPR.S135037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikawa T., Yasuda S., Minoda S., Ibuki T., Fukuhara K., Iwanaga Y., Ariyoshi T., Sasaki H. Neurotropin(®) ameliorates chronic pain via induction of brain-derived neurotrophic factor. Cell. Mol. Neurobiol. 2015;35:231–241. doi: 10.1007/s10571-014-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang W.-L., Zhao D.-Q., Wang F., Li M., Fan S.-N., Liao W., Zheng Y.-Q., Liao S.-W., Xiao S.-H., Luan P., Liu J. Neurotropin® alleviates hippocampal neuron damage through a HIF-1α/MAPK pathway. CNS Neurosci. Ther. 2017;23:428–437. doi: 10.1111/cns.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou H., Chen X., Lu J., Zhou W., Zou X., Wu H., Li Z., Zhou X. Neurotropin alleviates cognitive impairment by inhibiting TLR4/MyD88/NF-κB inflammation signaling pathway in mice with vascular dementia. Neurochem. Int. 2023;171 doi: 10.1016/j.neuint.2023.105625. [DOI] [PubMed] [Google Scholar]
- 24.Ye L.-L., Huang Y.-L., Cheng X.-E., Shi Y.-Q., Liu Z.-Y., Xiong Y.-F. Effect of neurotropin on Alzheimer’s disease-like changes and cognitive function in rats with chronic cerebral hypoperfusion. Neuroreport. 2023;34:170–177. doi: 10.1097/WNR.0000000000001875. [DOI] [PubMed] [Google Scholar]
- 25.Chen J., Zhou X.-J., Sun R.-B. Effect of the combination of high-frequency repetitive magnetic stimulation and neurotropin on injured sciatic nerve regeneration in rats. Neural Regen. Res. 2020;15:145–151. doi: 10.4103/1673-5374.264461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuoka H., Tanaka H., Sayanagi J., Iwahashi T., Suzuki K., Nishimoto S., Okada K., Murase T., Yoshikawa H. Neurotropin(®) Accelerates the Differentiation of Schwann Cells and Remyelination in a Rat Lysophosphatidylcholine-Induced Demyelination Model. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimoto S., Okada K., Tanaka H., Okamoto M., Fujisawa H., Okada T., Naiki M., Murase T., Yoshikawa H. Neurotropin attenuates local inflammatory response and inhibits demyelination induced by chronic constriction injury of the mouse sciatic nerve. Biologicals. 2016;44:206–211. doi: 10.1016/j.biologicals.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Yao X., Sun C., Fan B., Zhao C., Zhang Y., Duan H., Pang Y., Shen W., Li B., Wang X., et al. Neurotropin exerts neuroprotective effects after spinal cord injury by inhibiting apoptosis and modulating cytokines. J. Orthop. Translat. 2021;26:74–83. doi: 10.1016/j.jot.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hishiyama S., Kotoda M., Ishiyama T., Mitsui K., Matsukawa T. Neuroprotective effects of neurotropin in a mouse model of hypoxic-ischemic brain injury. J. Anesth. 2019;33:495–500. doi: 10.1007/s00540-019-02655-z. [DOI] [PubMed] [Google Scholar]
- 30.Sun C., Li B., Duan H., Tao B., Zhao C., Li W., Pang Y., Fan B., Feng S. Cytokine expressions of spinal cord injury treated by neurotropin and nafamostat mesylate. Ann. Transl. Med. 2021;9:489. doi: 10.21037/atm-21-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukuda Y., Nakajima K., Mutoh T. Neuroprotection by Neurotropin through Crosstalk of Neurotrophic and Innate Immune Receptors in PC12 Cells. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21186456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Y., Fang W., Fan S., Liao W., Xiong Y., Liao S., Li Y., Xiao S., Liu J. Neurotropin inhibits neuroinflammation via suppressing NF-κB and MAPKs signaling pathways in lipopolysaccharide-stimulated BV2 cells. J. Pharmacol. Sci. 2018;136:242–248. doi: 10.1016/j.jphs.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt-Pogoda A., Bonberg N., Koecke M.H.M., Strecker J.-K., Wellmann J., Bruckmann N.-M., Beuker C., Schäbitz W.-R., Meuth S.G., Wiendl H., et al. Why Most Acute Stroke Studies Are Positive in Animals but Not in Patients: A Systematic Comparison of Preclinical, Early Phase, and Phase 3 Clinical Trials of Neuroprotective Agents. Ann. Neurol. 2020;87:40–51. doi: 10.1002/ana.25643. [DOI] [PubMed] [Google Scholar]
- 34.Hill M.D., Goyal M., Menon B.K., Nogueira R.G., McTaggart R.A., Demchuk A.M., Poppe A.Y., Buck B.H., Field T.S., Dowlatshahi D., et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395:878–887. doi: 10.1016/S0140-6736(20)30258-0. [DOI] [PubMed] [Google Scholar]
- 35.Xu J., Wang A., Meng X., Yalkun G., Xu A., Gao Z., Chen H., Ji Y., Xu J., Geng D., et al. Edaravone Dexborneol Versus Edaravone Alone for the Treatment of Acute Ischemic Stroke: A Phase III, Randomized, Double-Blind, Comparative Trial. Stroke. 2021;52:772–780. doi: 10.1161/STROKEAHA.120.031197. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q., Wang A., Xu Q., Xia X., Tian X., Zhang Y., Li X., Yang X., Wang X., Peng J., et al. Efficacy and Safety of Ginkgo Diterpene Lactone Meglumine in Acute Ischemic Stroke: A Randomized Clinical Trial. JAMA Netw. Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukuda Y., Berry T.L., Nelson M., Hunter C.L., Fukuhara K., Imai H., Ito S., Granholm-Bentley A.-C., Kaplan A.P., Mutoh T. Stimulated neuronal expression of brain-derived neurotrophic factor by Neurotropin. Mol. Cell. Neurosci. 2010;45:226–233. doi: 10.1016/j.mcn.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Fukuda Y., Fukui T., Hikichi C., Ishikawa T., Murate K., Adachi T., Imai H., Fukuhara K., Ueda A., Kaplan A.P., Mutoh T. Neurotropin promotes NGF signaling through interaction of GM1 ganglioside with Trk neurotrophin receptor in PC12 cells. Brain Res. 2015;1596:13–21. doi: 10.1016/j.brainres.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 39.Zhong D., Zhang S., Wu B. Interpretation of the "Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke 2018". Chin. J. Modern Neurol. 2019;19:897–901. [Google Scholar]
- 40.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 41.De Reuck J., Decoo D., Vanderdonckt P., Dallenga A., Ceusters W., Kalala J.P., De Meulemeester K., Abdullah J., Santens P., Huybrechts J. A double-blind study of neurotropin in patients with acute ischemic stroke. Acta Neurol. Scand. 1994;89:329–335. doi: 10.1111/j.1600-0404.1994.tb02643.x. [DOI] [PubMed] [Google Scholar]
- 42.De Reuck J., Decoo D., Boon P., Van der Linden C. Neurotropin treatment of brain edema accompanying acute middle cerebral artery infarction. Acta Neurochir. Suppl. 1994;60:332–334. doi: 10.1007/978-3-7091-9334-1_89. [DOI] [PubMed] [Google Scholar]
- 43.Powers W.J., Rabinstein A.A., Ackerson T., Adeoye O.M., Bambakidis N.C., Becker K., Biller J., Brown M., Demaerschalk B.M., Hoh B., et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 44.2017. Common Terminology Criteria for Adverse Events (CTCAE) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
This article includes all data generated or analyzed during this study.
-
•
This paper does not report the original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.