Abstract
Objective
To ascertain the safety and efficacy of radioactive iodine-125 seed implantation (RISI) for the treatment of thoracic tumors.
Methods
Clinical patients with primary or metastatic tumors in the chest treated with RISI were analyzed. The RISI process included the following stages: preoperative planning, template design and 3D printing, CT-guided RISI assisted by a template, and postoperative dosimetric verification. The prescribed dose was ≥ 80 Gy. The main analytic measures were the local control (LC) rate and toxicity.
Results
From April 2015 to July 2018, a total of 92 patients, including 41 with lung cancer and 51 with lung metastases, were analyzed. The median lesion diameter was 5 cm. The median postoperative D90 was 142.6 Gy. The median follow-up was 10.7 months. The overall survival rates at 1 year and 3 years were 59.7% and 22.2%, respectively. The LC rates at 1 year and 3 years were 64.9% and 32.8%, respectively. The LC rates at 3 years for patients with D90 < 140 Gy and D90 > 140 Gy were 23.1% and 54.3%, respectively (P = 0.014). The LC rate of metastatic lung cancer was more favorable than that of primary lung cancer. The multivariate analyses showed that the dose and lesion type were independent factors for LC (P < 0.05). No factors were related to OS. The incidence of pneumothorax and hemoptysis was 35.8% and 3.2%, respectively. Few cases of radiotherapy-related toxicity effects were observed.
Conclusions
RISI may be safe and efficacious and is associated with few complications during the treatment of thoracic tumors. If patients need local treatment and surgery or radiotherapy is not available, RISI could be considered.
Keywords: Thoracic malignancy, Radioactive I-125 seed brachytherapy, 3D printing template, Dose, Toxicity
Introduction
The mainstream local treatments for malignant tumors (including primary or metastatic thoracic tumors) are surgery and external beam radiotherapy (EBRT) (NCCN 2019). However, in clinical practice, the real situation is more complicated, because the tumors may be unresectable, the patients may not be able to tolerate surgery or EBRT, recurrence may develop after EBRT, and palliative treatment can be applied for advanced tumors. Under these circumstances, radioactive iodine-125 seed implantation (RISI) provides another treatment option (Stewart et al. 2016). However, few studies have been conducted on solid tumors, and most of these studies have been conducted with large differences between doses and outcomes (Martinez-Monge et al. 2008; Huang et al. 2013; Jiang et al. 2015). In recent years, the introduction of three-dimensional (3D) printing technology into template design and computed tomography (CT)-guided RISI has improved puncture accuracy. The postoperative target dose could almost completely meet the requirements of the preoperative plan, which makes it possible to explore the dose–outcome relationship (Ji et al. 2017). The purpose of this study was to further clarify the efficacy and safety of RISI in thoracic tumors. We wished to explore the relationships among dose, efficacy, and toxicity, and to help determine the prescription dose.
Materials and methods
Patient selection
We collected the clinical data of patients with thoracic tumors who received 3D template-assisted CT-guided RISI. In our center, the indications for RISI were as follows: primary or metastatic tumors diagnosed by pathology; unresectable tumors or tumors unsuitable for surgery or EBRT; a tumor diameter ≤ 7 cm and with an appropriate puncture path; no bleeding tendency; and good general physical status [Karnofsky Performance Status (KPS) score > 70]. Informed consent was obtained from all individual participants included in the study. The study was approved by the Peking University Third Hospital Medical Science Research Ethics Committee.
Treatment process
Preoperative planning design
All patients underwent CT 2 days before surgery. According to the lesion location, we selected a suitable fixation position for each patient. All patients were fixed with a vacuum pad and marked by a surface positioning line. Next, the CT data were transmitted to a brachytherapy treatment planning system (BTPS) to design the preoperative plan, which included the following steps: delineating the gross tumor volume (GTV) and adjacent organs at risk (OARs); setting the prescription dose and seed radioactivity; determining the puncture pathway (direction, distribution, depth); calculating the quantity of seeds; simulating the spatial distribution of the seeds; and performing the dose calculations for the GTV and OARs (spinal cord, large blood vessels, cavity viscera). We optimized 90% of the dose to the GTV (GTV D90) to match the prescribed dose. According to American Brachytherapy Society guidelines, the recommended prescription dose is ≥ 80 Gy (Stewart et al. 2016).
Individualized design and production of a 3D printing template (3D-PT)
The BTPS data were imported into 3D imaging and reverse-engineering software for individualized digital modeling of a template. 3D-PTs were obtained by a 3D curing rapid-prototyping machine and the material processing of medical curing resins. The 3D-PT contained information such as body-surface characteristics, localization markers, and a simulated needle pathway of the treatment area (Ji et al. 2017).
Puncture and RISI
Local infiltration anesthesia alone or in combination with intercostal nerve-block anesthesia was administered to all patients. The 3D-PT was aligned precisely with the positioning laser line, body-surface positioning line of the patient, template alignment reference line, and characteristics of the patient’s external contours. Then, the accuracy of the template location was verified by real-time CT. The seed needle punctured the skin to a predetermined depth through the template guiding hole. During the puncture, CT was performed to verify the accurate positioning of the implant needle. Finally, the seeds were implanted based on the preoperative plan and actual depth of each needle in the target area. After implantation, the CT was repeated to observe the actual distribution of the seeds, i.e., whether the seeds were distributed evenly, if the seeds had been shed or displaced, and if pneumothorax or bleeding had occurred.
Postoperative verification of dosimetry
The postoperative images were transmitted to the BTPS for the postoperative evaluation of dosimetry. We used D90 to evaluate the tumor target dose. The evaluation of the OARs (rectum, urethra, and bladder) was performed according to the criteria for brachytherapy for prostate cancer, including D0.1 cc and D2 cc (the dose delivered to volumes of 0.1 cm3 and 2 cm3 in the OARs, respectively) and Dmean (the mean dose delivered to the OARs) (Zaorsky et al. 2017). For thoracic tumors, the OARs were the lungs, blood vessels, spinal cord, esophagus, trachea, heart, and skin.
Follow-up
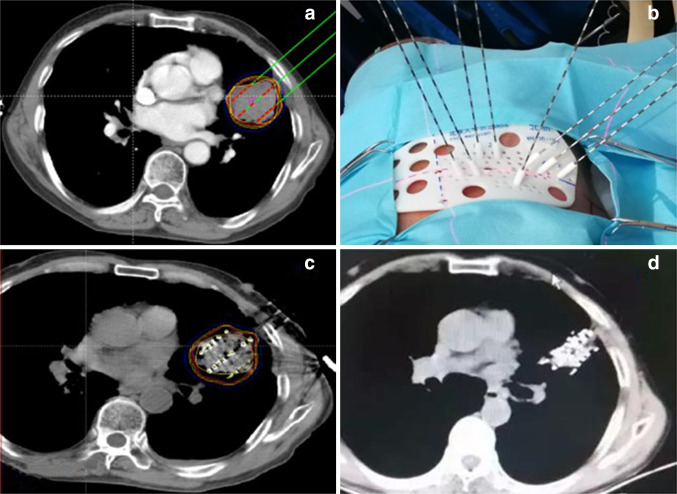
Patients were followed up at 3, 6, 9, and 12 months after the operation and every 6 months thereafter. At each visit, CT was performed to observe the target area and evaluate the overall tumor status. The treatment flowchart is shown in Fig. 1.
Fig. 1.
Treatment flowchart. a Design of the preoperative plan. b Template printing and puncture guidance. c RIS implantation. d Follow-up 3 months after RIS implantation
Parameters
The primary indices that we concerned were local control (LC) and toxicity effects. The secondary index that we measured was overall survival (OS). The evaluation of efficacy was based on the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 (Watanabe et al. 2009), as complete response (CR; disappearance of the target lesion), partial response (PR; reduction of target lesion volume by at least 30% from baseline), progressive disease (PD; increase in target lesion volume by at least 20%), and stable disease (SD; between PR and PD). Adverse reactions were evaluated by the Common Terminology Criteria for Adverse Events (CTCAE) v4.0 (CTCAE 2010).
Statistical analyses
SPSS v20 was used for statistical analyses. We used the Chi-square test to compare ratios. The Kaplan–Meier method was employed to evaluate LC and OS. The log-rank test was used for the univariate analysis and a Cox regression model was used for the multivariate analysis.
Results
From April 2015 to July 2018, 92 patients were enrolled in the present study. All patients had primary or metastatic solid thoracic malignant tumors with a median diameter of 5 (range 1.4–7.0) cm. The general information of the patients is shown in Table 1. The median OS duration was 15 months. The OS rates at 1 year and 3 years were 59.7% and 22.2%, respectively. The median duration of LC for the lesions was 16.4 months. The LC rates at 1 year and 3 years were 64.9% and 32.8%, respectively.
Table 1.
General information of the entire study cohort
| Characteristic | Cases | Percentage |
|---|---|---|
| Sex | ||
| Male | 60 | 65.2 |
| Female | 32 | 34.8 |
| Age (years) | Median 62 (17–88) | |
| KPS score | Median 80 (70–100) | |
| Lesion type | ||
| Primary | 41 | 44.6 |
| Metastatic | 51 | 55.4 |
| Previous RT | 0.0 | |
| No | 36 | 39.1 |
| Yes | 56 | 60.9 |
| Previous RT dose | Median 60 (40–120) | |
| Implantation site | ||
| Lung | 64 | 69.6 |
| Chest wall | 21 | 22.8 |
| Mediastinum | 4 | 4.3 |
| RIS radioactivity | Median 0.63 (0.48–0.8) | |
| RIS number | Median 48.5 (7–189) | |
| Lesion diameter (cm) | Median 5 (1.4–7.0) | |
| Lesion volume (cm3) | Median 33.1 (1.5–287.0) | |
| D90 | Median 142.6 (81–241.8) | |
| Short-term efficacy | ||
| PR | 29 | 31.5 |
| SD | 32 | 34.8 |
| PD | 31 | 33.7 |
KPS Karnofsky Performance Scale, RIS radioactive 125I seeds, D90 90% of the dose received by the GTV, PR partial response, SD stable disease, PD progressive disease
In terms of toxicity effects, there were three cases (3.3%) of grade ≥ 2 radiation pneumonia, including two cases (2.2%) of grade 2 and one case (1.1%) of grade 3 radiation pneumonia. Two patients (2.2%) had grade ≥ 2 radiation esophagitis, including one case (1.1%) of grade 2 and one case of grade 3 radiation esophagitis. One patient (1.1%) had an esophageal fistula. Two patients (2.2%) had tracheal fistulae. Five patients (5.4%) had grade 2 radiation skin reactions. One patient (1.1%) had chest wall pain. Three patients (3.3%) had hemoptysis. Defined radiation myelitis or cardiotoxicity was not observed during the follow-up (Table 2). Thirty-four patients (35.8%) had pneumothorax, including six patients (6.5%) with severe pneumothorax that necessitated closed drainage.
Table 2.
Adverse reactions
| Adverse reactions | Cases | Percentage |
|---|---|---|
| Radiation pneumonia (grade ≥ 2) | 3 | 3.3 |
| Radiation esophagitis (grade ≥ 2) | 2 | 2.2 |
| Esophageal fistula | 1 | 1.1 |
| Tracheal fistula | 2 | 2.2 |
| Radiation myelitis | 0 | 0.0 |
| Radiation skin reaction (grade ≥ 2) | 5 | 5.4 |
| Chest wall pain | 1 | 1.1 |
| Hemoptysis | 3 | 3.2 |
| Pneumothorax | 34 | 35.8 |
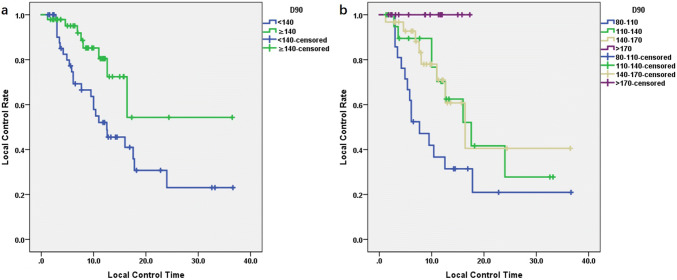
The prognostic factors evaluated were target area, lesion volume, target dose, lesion type, and previous EBRT. The univariate analysis revealed that the LC of patients with D90 ≥ 140 Gy seemed to be better than that of patients with < 140 Gy. The LC of metastatic cancer was better than that of primary cancer, and the difference was significant (P < 0.05). The LC rates at 1 year and 3 years for patients with D90 ≥ 140 Gy were 80.5% and 54.3%, respectively. The LC rate at 1 year for patients with D90 > 170 Gy was ≤ 100% (Table 3, Fig. 2). Regarding the target area of treatment, LC rate in the chest wall was slightly better than that in the mediastinum and lung, but the difference was not significant (P = 0.126). In addition, lesion volume and previous EBRT did not show a significant correlation with LC (P > 0.05) (Table 3).
Table 3.
Univariate and multivariate analyses of local control
| Factor | Median (month) | 1 year (%) | 3 years (%) | UA | MA | ||
|---|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | ||||
| Treatment site | 0.126 | ||||||
| Lung | 16 | 63.5 | 24 (2 years) | ||||
| Mediastinum | 7.7 | 25 | 25 (1 years) | ||||
| Chest wall | None | 82.4 | 61.8 | ||||
| Lesion volume (cm3) | 0.372 | ||||||
| < 33 | 17.6 | 66.1 | 43.2 (2 years) | ||||
| > 33 | 16.4 | 63.8 | 26.1 | ||||
| D90 (Gy) | 0.014 | 0.393 | 0.175–0.884 | 0.024 | |||
| ≥ 140 | 12.5 | 52 | 23.1 | ||||
| < 140 | None | 80.5 | 54.3 | ||||
| Lesion type | 0.020 | 0.456 | 0.223–0.934 | 0.032 | |||
| Primary lung cancer | 12.5 | 51.9 | 19.5 (2 years) | ||||
| Metastatic lung cancer | 24 | 74.6 | 43.9 | ||||
| Previous RT | 0.650 | ||||||
| No | 24 | 66.2 | 29.4 | ||||
| Yes | 16.4 | 64 | 33 | ||||
UA univariate analysis, MA multivariate analysis
Fig. 2.
Relationship between the duration of local control and D90: a the local control rate of patients with D90 > 140 Gy was significantly better than that of patients with D90 < 140 Gy (P < 0.05). b The local control rate of patients with D90 > 170 Gy was 100%
The multivariate analysis revealed that the target dose and lesion type were independent factors for LC (P < 0.05) (Table 3). A subgroup of 41 patients with primary lung cancer was enrolled [26 patients with squamous cell carcinoma (SCC) and 15 patients with adenocarcinoma]. The median durations of LC in the SCC group and adenocarcinoma group were 12.6 months and 10 months, respectively, but the difference between groups was not significant (P = 0.186). The median durations of LC in the group with D90 < 140 Gy and those of the group with D90 ≥ 140 Gy were 11 months and 16.4 months, respectively, and the difference was not significant (P = 0.343) (Fig. 3).
Fig. 3.
Primary lung cancer. a Duration of local control of squamous cell carcinoma was slightly better than that of adenocarcinoma, but the difference was not significant (P > 0.05). b There was no significant difference in the duration of local control between the group with D90 ≥ 140 Gy and the group with D90 < 140 Gy (P > 0.05)
Factors that influenced survival time were not found in the whole study cohort or in the subgroup with primary lung cancer (P > 0.05). There was a low prevalence of toxicity in the entire study cohort, so dosimetry parameters that could influence the factors related to toxicity could not be evaluated.
Discussion
The clinical application of RISI in thoracic tumors has been performed for more than 70 years (Hilaris and Martini 1979). The aims are to improve LC by administering a high local dose and avoid severe damage to healthy tissues. In addition to being used for patients with positive gross/pathologic margins (Mutyala et al. 2010), RISI is also used for palliative treatment in patients who have an unresectable tumor to alleviate symptoms and improve quality of life (Heelan et al. 1987).
However, few studies have reported the application of RISI in solid thoracic tumors or the efficacy and safety of RISI. The incorporation of 3D printing technology into RISI has markedly improved the accuracy of the treatment for solid tumors. Previously, a study showed that CT-guided RISI assisted by 3D-PTs could enable the postoperative actual target dose to meet the design requirements of the preoperative plan (Ji et al. 2017). Based on such quality assurance, we can give a specific dose to tumors to analyze the safety and efficacy of RISI in the treatment of thoracic tumors.
We found that the LC rates at 1 year and 3 years were 64.9% and 32.8%, respectively, which are less favorable than those of stereotactic body radiotherapy (SBRT) (80–90%) (Bradley et al. 2010; Ricardi et al. 2012). However, 60.8% of patients in our study had previously undergone EBRT, and the remainder of the patients were unsuitable for EBRT due to their physical status. That is, the patients enrolled in our study had limited treatment options and a relatively poor prognosis. The finding that 32.8% of patients had LC at 3 years shows that RISI provides a novel opportunity for patients with disease refractory to treatment. Moreover, we found that patients with D90 ≥ 140 Gy had good LC rates (80.5% at 1 year and 54.3% at 3 years). Among these patients, the LC rate of patients with D90 > 170 Gy reached 100%, which suggests the critical role of the dose on the effect of RISI. The other advantage of RISI is that this procedure is performed just once. Upon implantation into the tumor, the radioactive seeds could work for a long time. Patients had a satisfactory treatment experience without the need for multiple rounds of subsequent treatment or travel back and forth to the hospital.
The main problem of using RISI is the lack of a standard prescription dose due to the absence of prospective randomized studies. The reference values from studies have been limited due to the heterogeneity of disease and tumor location and large dose range. Fernando and colleagues performed a phase III randomized clinical study to compare the resection of lung lobes with the resection of lung lobes + radioactive seed implantation for resectable lung cancer. The authors found that the LC rate at 5 years was slightly better for the group that underwent lung-lobe resection + RISI than for the group that under resection only. Because the prevalence of local recurrence was low, the difference between the two groups was not significant. In addition, Fernando and colleagues did not provide detailed information about the dose (Fernando et al. 2014). Parashar et al. compared the efficacy of wedge resection, wedge resection + implantation of radioactive seeds, and SBRT for early lung cancer, and found that the LC rates were similar among groups. Because the patients who underwent surgery + radioactive seed implantation were at high risk of recurrence according to the preoperative and intraoperative evaluation, we hypothesize that the implantation of radioactive seeds might reduce the prevalence of recurrence. The prescribed dose in that study was 80–120 Gy (Parashar et al. 2015). In some studies, the prescription dose was 100–140 Gy, but the dose was based on clinical experience (Martinez-Monge et al. 2008; Huang et al. 2013; Jiang et al. 2015; Ji et al. 2017; Li et al. 2015; Yu et al. 2015; Zhang et al. 2015; Jiang et al. 2018); these studies showed that the LC rate was 25–80%. The present study showed that the dose was positively correlated with LC. The higher the dose, the better the LC was. Based on our study, we recommend that the prescribed dose for solid tumors should be ≥ 140 Gy. Our follow-up studies also considered further increasing the dose from 140 Gy. However, we found no specific dose inflection point in the subgroup with primary lung cancer. The reason may be because the primary lung cancer is more likely to grow in an invasive way than metastatic cancer, and patients with primary cancer more likely to have marginal recurrence than those with metastatic cancer. Hence, further studies are needed to focus not only on the dose but also on the target area, such as the clinical target volume (CTV).
Except for one patient who had grade 3 radiation pneumonia (1.1%) and three patients with esophageal/tracheal fistulae (3.3%), there were no other defined grade 3 toxicity effects. Recently, some studies have reported the toxicity effects of RISI for head and neck tumors. Although the OARs of head and neck cancer are different from those of thoracic tumors, there were no obvious toxicity effects of grade 3 or higher (Jiang et al. 2018), thereby suggesting that the safety of RISI. Radiation pneumonia is a relatively specific toxicity effect of chest EBRT. Studies have shown that the prevalence of grade 3 radiation pneumonia is ~ 20% in thoracic reirradiation, even with SBRT (Kelly et al. 2010; Trovo et al. 2014). However, most of the patients in our study had previously received EBRT, and the prevalence of radiation pneumonia was not high. Only one patient who had grade 3 radiation pneumonia had not previously undergone EBRT. In fact, a study showed that radiation pneumonia might be associated with autoimmune function or infectious pneumonia (Li et al. 2016).
The D0.1 cc and D2 cc variables might be more meaningful for the esophagus and trachea, because these organs are tandem organs. For example, the data for RISI in tumors of the prostate gland showed a limited dose to a tandem organ (rectum) with D0.1 cc ≤ 200 Gy and D2 cc ≤ 145 Gy (Salembier et al. 2007; Georg et al. 2014). In the present study, the D0.1 cc and D2 cc for one patient with an esophageal fistula who had previously undergone EBRT (total dose = 56 Gy) were 38.92 Gy and 31.22 Gy, respectively. Two patients had tracheal fistulae. The D0.1 cc and D2 cc for one patient who had previously undergone EBRT (total dose = 56 Gy) were 105.32 Gy and 68.88 Gy, respectively. The D0.1 cc and D2 cc for another patient who had previously undergone EBRT (total dose = 74 Gy) were 23.56 Gy and 16.02 Gy, respectively. However, from the data of the whole study cohort, some patients had no serious esophageal or tracheal toxicity effects despite receiving high doses. Thus, we could not ascertain the dose-tolerance relationship of the esophagus and trachea.
Conclusions
RISI, as a favorable salvage treatment, may be safe and efficacious and is associated with few complications during the treatment of thoracic tumors. The LC rate at 1 year was 64.9% and that at 3 years was 32.8%. The prevalence of radiation-related adverse reactions was low, whereas the prevalence of the common adverse reactions associated with EBRT, such as radiation pneumonia, esophagitis, and skin reactions, was not high after RISI. The higher the target dose (D90), the better the LC was. We found a significant correlation between the target dose and LC, and a prescribed dose of more than 140 Gy may lead to better outcomes.
Funding
None.
Compliance with ethical standards
Conflict of interest
None of the authors have any conflicts of interest.
Ethical approval
All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This retrospective study was approved by the Peking University Third Hospital Medical Science Research Ethics Committee and registered in the Chinese Clinical Trial Registry (ChiCTR) (http://www.chictr.org.cn) with the registration number ChiCTR1800019945.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bradley JD, El Naqa I, Drzymala RE, Trovo M, Jones G, Denning MD (2010) Stereotactic body radiation therapy for early-stage non-small-cell lung cancer: the pattern of failure is distant. Int J Radiat Oncol Biol Phys 77(4):1146–1150 [DOI] [PubMed] [Google Scholar]
- Common Terminology Criteria for Adverse Events (CTCAE) (2010) version 4.03. National Cancer Institute (NCI). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
- Fernando HC, Landreneau RJ, Mandrekar SJ, Nichols FC, Hillman SL, Heron DE et al (2014) Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non–small-cell lung cancer. J Clin Oncol 32(23):2456–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georg D, Hopfgartner J, Gora J, Kuess P, Kragl G, Berger D et al (2014) Dosimetric considerations to determine the optimal technique for localized prostate cancer among external photon, proton, or carbon-ion therapy and high-dose-rate or low-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 88(3):715–722 [DOI] [PubMed] [Google Scholar]
- Heelan RT, Hilaris BS, Anderson LL, Nori D, Martini N, Watson RC et al (1987) Lung tumors: percutaneous implantation of I-125 sources with CT treatment planning. Radiology 164(3):735–740 [DOI] [PubMed] [Google Scholar]
- Hilaris BS, Martini N (1979) Interstitial brachytherapy in cancer of the lung: a 20 years experience. Int J Radiat Oncol Biol Phys 5(11–12):1951–1956 [DOI] [PubMed] [Google Scholar]
- Huang Q, Chen J, Chen Q, Lai Q, Cai S, Luo K et al (2013) Computed tomographic-guided iodine-125 interstitial implants for malignant thoracic tumors. Eur J Radiol 82(11):2061–2066 [DOI] [PubMed] [Google Scholar]
- Ji Z, Jiang Y, Guo F, Sun H, Fan J, Zhang L et al (2017) Dosimetry verification of radioactive seed implantation for malignant tumors assisted by 3D printing individual templates and CT guidance. Appl Radiat Isot 124:68–74 [DOI] [PubMed] [Google Scholar]
- Jiang G, Li Z, Ding A, Zhou F, Jiao W, Tang D et al (2015) Computed tomography-guided iodine-125 interstitial implantation as an alternative treatment option for lung cancer. Indian J Cancer 51(Suppl 2):e9–e12 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Ji Z, Guo F, Peng R, Sun H, Fan J et al (2018) Side effects of CT-guided implantation of (125)I seeds for recurrent malignant tumors of the head and neck assisted by 3D printing non co-planar template. Radiat Oncol 13(1):18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P, Balter PA, Rebueno N, Sharp HJ, Liao Z, Komaki R et al (2010) Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int J Radiat Oncol Biol Phys 78(5):1387–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Guan J, Yang L, Zheng X, Yu Y, Jiang J (2015) Iodine-125 brachytherapy improved overall survival of patients with inoperable stage III/IV non-small cell lung cancer versus the conventional radiotherapy. Med Oncol 32(1):395 [DOI] [PubMed] [Google Scholar]
- Li P, Wang X, Liu Z, Liu H, Xu T, Wang H et al (2016) Single nucleotide polymorphisms in CBLB, a regulator of T-cell response, predict radiation pneumonitis and outcomes after definitive radiotherapy for non-small-cell lung cancer. Clin Lung Cancer 17(4):253–262 [DOI] [PubMed] [Google Scholar]
- Martinez-Monge R, Pagola M, Vivas I, Lopez-Picazo JM (2008) CT-guided permanent brachytherapy for patients with medically inoperable early-stage non-small cell lung cancer (NSCLC). Lung Cancer 61(2):209–213 [DOI] [PubMed] [Google Scholar]
- Mutyala S, Stewart A, Khan AJ, Cormack RA, O’Farrell D, Sugarbaker D et al (2010) Permanent iodine-125 interstitial planar seed brachytherapy for close or positive margins for thoracic malignancies. Int J Radiat Oncol Biol Phys 76(4):1114–1120 [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (NCCN) (2019) Clinical practice guidelines in oncology: non-small cell lung cancer, version 2. https://www.nccn.org/professionals/physician_gls/default.aspx#nscl
- Parashar B, Port J, Arora S, Christos P, Trichter S, Nori D et al (2015) Analysis of stereotactic radiation vs wedge resection vs wedge resection plus Cesium-131 brachytherapy in early stage lung cancer. Brachytherapy 14(5):648–654 [DOI] [PubMed] [Google Scholar]
- Ricardi U, Filippi AR, Guarneri A, Ragona R, Mantovani C, Giglioli F et al (2012) Stereotactic body radiation therapy for lung metastases. Lung Cancer 75(1):77–81 [DOI] [PubMed] [Google Scholar]
- Salembier C, Lavagnini P, Nickers P, Mangili P, Rijnders A, Polo A et al (2007) Tumour and target volumes in permanent prostate brachytherapy: a supplement to the ESTRO/EAU/EORTC recommendations on prostate brachytherapy. Radiother Oncol 83(1):3–10 [DOI] [PubMed] [Google Scholar]
- Stewart A, Parashar B, Patel M, O’Farrell D, Biagioli M, Devlin P et al (2016) American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy 15(1):1–11 [DOI] [PubMed] [Google Scholar]
- Trovo M, Minatel E, Durofil E, Polesel J, Avanzo M, Baresic T et al (2014) Stereotactic body radiation therapy for re-irradiation of persistent or recurrent non-small cell lung cancer. Int J Radiat Oncol Biol Phys 88(5):1114–1119 [DOI] [PubMed] [Google Scholar]
- Watanabe H, Okada M, Kaji Y, Satouchi M, Sato Y, Yamabe Y et al (2009) New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1). Gan To Kagaku Ryoho 36(13):2495–2501 [PubMed] [Google Scholar]
- Yu X, Li J, Zhong X, He J (2015) Combination of Iodine-125 brachytherapy and chemotherapy for locally recurrent stage III non-small cell lung cancer after concurrent chemoradiotherapy. BMC Cancer 15:656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaorsky NG, Davis BJ, Nguyen PL, Showalter TN, Hoskin PJ, Yoshioka Y et al (2017) The evolution of brachytherapy for prostate cancer. Nat Rev Urol 14(7):415–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Wang DQ, Wu YF (2015) Sodium glycididazole enhances the efficacy of combined iodine-125 seed implantation and chemotherapy in patients with non small-cell lung cancer. Oncol Lett 9(5):2335–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]