Abstract
Purpose
The recurrence after curative hepatectomy is common. Limited data have investigated the effect of transcatheter arterial chemoembolization (TACE) combined with ablation in treating recurrent intermediate-stage hepatocellular carcinoma (HCC) after hepatectomy. We aim to compare the efficacy of TACE combined with ablation versus TACE alone in treating recurrent intermediate-stage HCC after hepatectomy.
Methods
A total of 183 patients with recurrent intermediate-stage HCC after hepatectomy were enrolled at Sun Yat-sen University Cancer Centre, including 111 patients who underwent TACE alone and 72 patients who underwent TACE combined with ablation (TACE–Ablation). Overall survival (OS) and progression-free survival (PFS) were compared by the log-rank test. Propensity score matching (PSM) was used to reduce the confounding bias.
Results
Before PSM, the 5-year OS rates were 43.3% vs. 27.9% (P = 0.001), and the 5-year PFS rates were 21.7% vs. 13.0% (P < 0.001) for TACE–Ablation and TACE-alone groups, respectively. After PSM, TACE–Ablation still resulted in better 5-year OS (41.6% vs. 30.2%, P = 0.028) and 5-year PFS rate (21.3% vs. 15.8%, P = 0.024) than that of TACE alone. Patients in TACE–Ablation group exhibited similar major complication rates to TACE-alone group but higher minor complication rates both before and after PSM. Cox regression analysis identified TACE-alone modality as an independently unfavourable predictor for OS and PFS (both P < 0.05).
Conclusion
TACE combined with ablation is safe and superior to TACE alone in tumour control and prolonging overall survival in recurrent intermediate-stage HCC after hepatectomy.
Electronic supplementary material
The online version of this article (10.1007/s00432-020-03254-2) contains supplementary material, which is available to authorized users.
Keywords: Recurrent intermediate-stage hepatocellular carcinoma, Transcatheter arterial chemoembolization, Transcatheter arterial chemoembolization combined with ablation, Survival
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer death worldwide, with approximately 84,000 new diagnosed cases and 78,000 deaths annually (Bray et al. 2018). Partial hepatectomy remains one of the mainstay choices for early-stage HCC (Bruix and Llovet 2002). Unfortunately, the probability recurrence rate at 5 years after curative hepatectomy is approximately 70% and about 30% of recurrent cases are intermediate-stage HCC, causing a major cause of treatment failure and cancer-related death (Portolani et al. 2006). Therefore, the management of optimal treatments for recurrent intermediate-stage HCC (defined as: intrahepatic recurrence with two to three lesions where at least one was > 3 cm in size or more than three tumours and without extrahepatic metastasis or tumour thrombosis) is urgently required.
Transcatheter arterial chemoembolization (TACE) is the recommended first-line therapy for patients with intermediate-stage primary and recurrent HCC(Galle et al. 2018; Marrero et al. 2018). Although patients with primary intermediate-stage HCC could benefit from TACE according to several randomised clinical trials (Llovet et al. 2002; Lo 2002), TACE is not a radical therapy and only up to 50% patients achieve tumour control (Llovet et al. 2003). Therefore, clinicians have taken efforts to explore other effective treatments for intermediate-stage HCC, including radioembolization, TACE combined with sorafenib or ablation (Raoul et al. 2011). Radioembolization is a promising alternative treatment for intermediate-stage HCC, which deserves further prospective studies (Sangro et al. 2012). Previous studies have shown that TACE combined with sorafenib could achieve longer survival comparing with TACE alone both in primary and recurrent intermediate-stage HCC (Chao et al. 2015; Peng et al. 2019).
TACE combined with ablation (TACE–Ablation) has been reported as an alternative treatment for HCC due to the synergistic effects (Rossi et al. 2000). Previous studies have shown that TACE–Ablation provided better tumour control and longer survival than TACE alone in patients with primary intermediate-stage HCC (Azuma et al. 2016; Yin et al. 2014; Xu et al. 2013; Zheng et al. 2018), and could achieve comparable outcomes to hepatectomy in primary early-stage HCC(Kagawa et al. 2010). In terms of the recurrent HCC, TACE–Ablation is superior to ablation alone in prolonging the survival and is as effective as hepatectomy in recurrent early-stage HCC (Peng et al. 2012, 2018). However, whether TACE–Ablation remains more effective than TACE alone for recurrent intermediate-stage HCC is unclear. Therefore, in the present study, we aimed to investigate the effect of TACE–Ablation versus TACE alone on survival outcomes in patients with recurrent intermediate-stage HCC. In addition, propensity score matching analysis (PSM) was performed to reduce the bias due to the confounding variables at baseline between the two groups.
Materials and methods
Patients
From Jan 2007 to Dec 2015, we retrospectively identified patients with recurrent HCC after curative hepatectomy of primary HCC (He et al. 2018) at Sun Yat-sen University Cancer Center (SYSUCC). The inclusion criteria were as follows: (a) first intrahepatic recurrent HCC after initial hepatectomy; (b) recurrent intermediate-stage HCC (two to three lesions where at least one was > 3 cm in size or more than three tumours); (c) absence of extrahepatic metastasis or portal vein or hepatic vein tumour thrombosis (d) Child–Pugh class A or B; (e) Eastern Cooperative Oncology Group performance scores ≤ 2. The exclusion criteria were as follows: (a) history of other malignancies; (b) systemic therapy history (including molecular targeted therapy or immunotherapy); and (c) severe dysfunction of heart, kidney, lung, or other vital organs.
This study complied with the standards of the 1964 Helsinki Declaration and was approved by SYSUCC Research Ethic Committee. Key raw data in our study have been uploaded onto the Research Data Deposit public platform (www.researchdata.org.cn). The study protocol was approved by the Institutional Review Board of SYSUCC, and informed consent for the data to be used for clinical researches was obtained from all enrolled patients.
TACE procedure
TACE was performed according to the protocol as previously described (Yang et al. 2019). In this study, the TACE procedure was performed by radiologists with more than 5 years of experience in interventional therapy for HCC. First, a 5-F catheter was introduced to assess liver vascular anatomy, and portal vein patency by visceral angiography and patients with an arteriovenous shunt were excluded. Distal super-selective catheterization of the hepatic arteries was performed by a 2.9-F microcatheter (Terumo, Tokyo, Japan) if the 5-F catheter could not advance into the tumour-feeding artery. Hepatic artery infusion chemotherapy was performed using 300 mg of carboplatin (Bristol Myers Squibb, New York, NY). Then, chemolipiodolization for the tumour-feeding arteries was proceeded by a mixture of 50 mg of epirubicin (Pharmorubicin; Pfizer, Wuxi, China), 8 mg of mitomycin C (Zhejiang Hisun Pharmaceutical, Taizhou, China), and 5–10 ml of lipiodol (Lipiodol Ultra-Fluide; André Guerbet Laboratories, Aulnay Sous-Bois, France). Embolization was performed with absorbable gelfoam sponge particles (1–2 mm in diameter, Gelfoam) or polyvinyl alcohol particles (350–560 µm in diameter, Alicon Pharm SCT&TEC). Finally, X-ray imaging of the chest and abdomen was performed to verify the distribution of lipiodol and to exclude ectopic embolization.
Ablation procedure
The indication of additional ablation followed TACE for intermediate-stage HCC in this study was similar to the previous studies (Azuma et al. 2016; Shimose et al. 2019; Yin et al. 2014; Zheng et al. 2018). Briefly, in our enrolled patients, patients with tumour diameter less than 7 cm and tumour number less than 7 were treated by additional ablation, when the nodules could be detected by ultrasonography and available for ablation technology. Treatment decision was made by consensus of two or more hepatologists. Ablation followed TACE within 4–6 weeks (Median: 26 days, range 2–46 days). The ablation treatments consisted of radiofrequency ablation (RFA) and microwave ablation (MWA) in this study, and were performed by hepatologists who had more than 5 years of experience in interventional therapy of HCC. Both therapies were performed under real-time ultrasound (MyLab 90; Esaote SpA, Firenze, Italy) guidance. The ablation procedures were detailed in our previous study (Liu et al. 2018). Briefly, a radiofrequency system (RF 2000; RadioTherapeutics, Mountain View, USA) and a microwave system (ECO-100C; ECO Microwave Electronic Institute, Nanjing, China) were used for RFA and MWA, respectively. All the intrahepatic tumours shown under ultrasound were treated by ablation. At the end of the procedure, the needle track was ablated to prevent bleeding from the liver surface.
Assessment of treatment and follow-up
In both groups, tumour response was evaluated 4–6 weeks after treatment using the modified Response Evaluation Criteria in Solid Tumours (mRECIST) depending on enhanced computed tomography scan (CT), or magnetic resonance imaging (MRI) scans (Gordic et al. 2017). The objective response rate (ORR) was defined as the sum of complete response and partial response, and the disease control rate (DCR) was defined as the sum of complete response, partial response and stable disease (Lencioni and Llovet 2010). Two independent radiologists evaluated the tumour response to the treatments with blinding to each other and discussion was made if any inconsistency. Complications were observed by two independent radiologists, and any disagreements were settled by discussion. Major complications were defined as clinical events leading to additional therapeutic treatments or prolonged hospitalisation; while, minor complications were defined adverse clinical events requiring no or nominal therapies (Ahmed and Technology Assessment Committee of the Society of Interventional R 2014). The first follow-up was performed 1 month after treatment, and then every 2–3 months until death or dropout. Physical examination, serum alpha-fetoprotein and at least one abdominal imaging scan (enhanced CT or MRI) were performed in each follow-up. Overall survival (OS) was defined as the survival time from the diagnostic date of first recurrence after initial hepatectomy to the date of death or the last follow-up. Progression-free time (PFS) was defined as the interval between the diagnostic date of first recurrence after initial hepatectomy and the date of tumour progression according to the mRECIST, death or the last follow-up.
Propensity score matching analysis
To reduce the selection bias, propensity scores for all patients were performed by a logistic regression model using the following baseline characteristics: (a) Age, gender, and viral hepatitis; (b) Barcelona Clinic Liver Cancer (BCLC) stage, albumin–bilirubin (ALBI) grade, serum alpha-fetoprotein (AFP), microvascular invasion (MVI), and tumour differentiation of primary HCC; (c) time to recurrence, tumour size and tumour number of recurrence, and AFP, albumin, total bilirubin, alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase, prothrombin time, white blood cell, platelet, haemoglobin at recurrence. A one-to-one nearest-neighbour matching algorithm with an optimal calliper of 0.2 without replacement was used to generate 65 pairs of patients (Austin 2011; Johnson et al. 2018).
Statistical analysis
Continuous variables were summarised as the median and range. Binary variables were compared using the Chi-squared test, and ordinal categorical variables were compared by the Kruskal–Wallis test. The Kaplan–Meier method and log-rank test were used to compare survival curves. Variables with P value less than 0.10 in the univariate analysis were introduced into the multivariate Cox proportional hazard model. All analyses were two sided, and P values less than 0.05 were considered significant. Statistical analyses were performed using the R program (R version 3.5.0; R Foundation for Statistical Computing, Vienna, Austria) (Team 2013).
Results
Patients
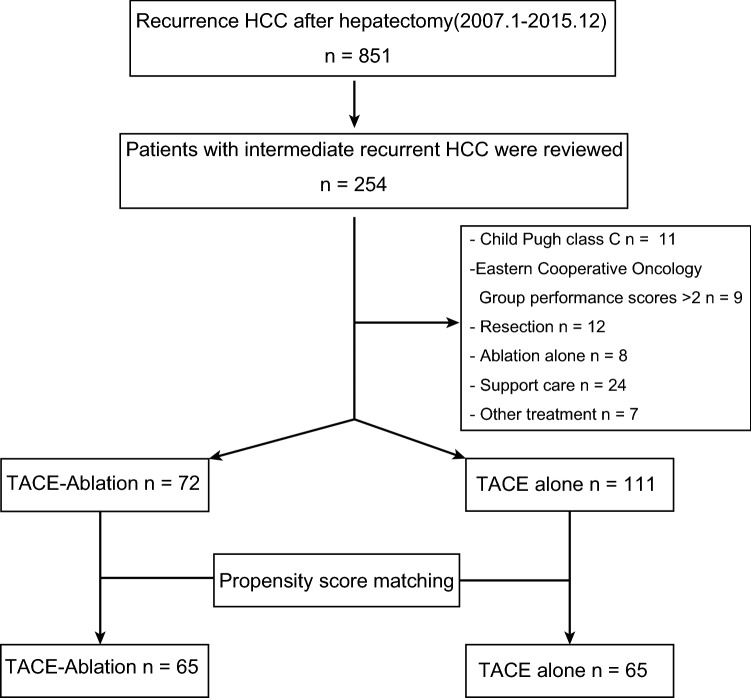
A total of 254 patients with recurrent intermediate-stage HCC were included from 851 patients with postoperative recurrence. Then, we excluded 71 patients according to the criteria. Finally, a total of 183 patents were enrolled in this study, including 111 patients in the TACE-alone group and 72 patients in the TACE–Ablation group (Fig. 1). The baseline characteristics of the overall cohort and matched cohort are summarised in Table 1. There were no significant differences between TACE group and TACE–Ablation group in clinical characteristics of primary HCC, including BCLC stage, ALBI grade, AFP, MVI, and tumour differentiation (all P > 0.05). Early recurrence (within 2 years), size of recurrence, and ALBI I grade at recurrence (all P > 0.05) were not significantly different between the two groups, either. However, patients in the TACE group were younger (48.0 vs 58.0 years, P < 0.001) and suffered more tumours (tumour number > 3; 66.7% vs 54.2%; P < 0.001) and higher level of AFP at recurrence (58.00 vs 11.83 ng/ml, P < 0.001). However, no significant different covariates were found between the two groups after matching (Table 1).
Fig. 1.
Flowchart of the patient selection. HCC hepatocellular carcinoma. TACE transcatheter arterial chemoembolization
Table 1.
Demographic and clinical characteristics of the enrolled patients
| Variable | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| TACE (n = 111) | TACE–Ablation (n = 72) | P | TACE (n = 65) | TACE–Ablation (n = 65) | P | |
| Gender (male/female) | 106 (95.5)/5 (4.5) | 63 (87.5)/9 (12.5) | 0.089 | 56 (86.2)/9 (13.8) | 56 (86.2)/9 (13.8) | 1.000 |
| Age (y) | 48.00 (42.50,57.00) | 58.00 (49.50,64.00) | <0.001 | 53.00 (43.25,59.75) | 54.00 (43.50,62.50) | 0.453 |
| Viral hepatitis (yes) | 106 (95.5) | 69 (95.9) | 0.679 | 61 (93.8) | 62 (95.4) | 0.554 |
| HBV | 105 (94.6) | 67 (93.1) | 61 (93.8) | 60 (92.3) | ||
| HCV | 1 (0.9) | 2 (2.8) | 0 (0.0) | 2 (3.1) | ||
| Antiviral treatment (yes/no) | 62 (55.9)/49 (44.1) | 51 (70.8)/21 (29.2) | 0.060 | 47 (72.3)/18 (28.7) | 47 (72.3)/18 (28.7) | 1.000 |
| Initial hepatectomy stage data | ||||||
| AFP (ng/ml) | 63.6 (10.2,1933.50) | 35.91 (8.07,498.48) | 0.108 | 17.40 (8.62,215.9) | 40.70 (8.20,540.40) | 0.612 |
| BCLC (0-A/B) | 80 (72.1)31 (27.9) | 55 (76.4)/17 (23.6) | 0.634 | 48 (73.8)/17 (26.2) | 48 (73.8)/17 (26.2) | 1.000 |
| ALBI grade (I/II) | 92 (82.9)/19 (17.1) | 53 (73.6)/19 (26.4) | 0.185 | 55 (84.6)/10 (15.4) | 47 (72.3)/18 (27.7) | 0.135 |
| Cirrhosis (yes/no) | 87 (78.4)/24 (21.6) | 53 (73.6)/19 (26.4) | 0.572 | 50 (76.9)/15 (23.1) | 47 (72.3)/18 (27.7) | 0.687 |
| MVI (yes/no) | 45 (40.5)/66 (59.5) | 25 (34.7)/47 (65.3) | 0.525 | 23 (35.4)/42 (64.6) | 24 (36.9)/41 (63.1) | 1.000 |
| Tumour differentiation (I–II/III–IV) | 90 (81.1)/21 (18.9) | 57 (79.2)/15 (20.8) | 0.898 | 50 (76.9)/15 (23.1) | 52 (80.0)/13 (20.0) | 0.831 |
| Anatomic resection (yes/no) | 33 (29.7)/78 (70.3) | 13 (18.1)/59 (81.9) | 0.109 | 17 (26.1)/48 (73.8) | 15 (23.0)/50 (76.9) | 0.822 |
| Resection margin (cm) | 0.80 (0.50,1.50) | 0.85 (0.50,1.50) | 0.868 | 0.70 (0.50,1.50) | 0.80 (0.50,1.50) | 0.912 |
| Recurrence stage data | ||||||
| Time to recurrence (years) (≤ 2/> 2) | 99 (89.2)/12 (10.8) | 58 (80.6)/14 (19.4) | 0.156 | 57 (87.7)/8 (12.3) | 54 (83.1)/11 (16.9) | 0.620 |
| Size of recurrence (cm) | 2.90 (1.80,3.45) | 3.00 (1.67,3.40) | 0.993 | 3.00 (1.80,3.40) | 2.95 (1.65,3.50) | 0.970 |
| Number of recurrence | <0.001 | 0.863 | ||||
| ≤ 3 | 37 (33.3) | 33 (45.8) | 32 (49.2) | 29 (44.6) | ||
| 3–5 | 38 (34.3) | 35 (48.6) | 29 (44.6) | 32 (49.2) | ||
| > 5 | 36 (32.4) | 4 (5.6) | 4 (6.2) | 4 (6.2) | ||
| HBV DNA levels at recurrence (IU/mL) (< 100/≥ 100) | 68/43 (61.3/38.7) | 48/24 (66.7/33.3) | 0.558 | 40/25 (61.5/38.5) | 42/23 (64.6/35.4) | 0.855 |
| ALB at recurrence (g/L) | 43.00 (40.80,45.10) | 42.75 (40.98,44.23) | 0.431 | 42.60 (40.00,44.70) | 42.70 (40.90,44.20) | 0.972 |
| TBIL at recurrence (µmol/L) | 12.60 (9.45,15.00) | 13.15 (10.50,17.22) | 0.138 | 12.70 (9.70,15.30) | 13.50 (10.70,17.30) | 0.234 |
| ALT at recurrence (U/L) | 34.20 (23.80,51.90) | 35.75 (24.78,45.35) | 0.977 | 30.40 (23.20,41.80) | 34.60 (23.20,41.60) | 0.701 |
| AST at recurrence (U/L) | 32.80 (26.65,45.00) | 31.40 (25.73,41.97) | 0.487 | 30.30 (25.60,39.50) | 31.00 (25.40,40.30) | 0.935 |
| ALP at recurrence (U/L) | 87.10 (72.0,109.15) | 93.85 (69.9,108.68) | 0.692 | 83.30 (71.20,106.60) | 90.40 (69.20,105.40) | 0.832 |
| GGT at recurrence (U/L) | 52.20 (34.00,78.40) | 41.90 (30.08,72.60) | 0.148 | 51.10 (30.70,74.70) | 41.50 (30.40,66.30) | 0.420 |
| WBC at recurrence (× 109/L) | 5.80 (4.79,6.79) | 5.30 (4.47,6.13) | 0.084 | 5.68 (4.72,6.74) | 5.38 (4.50,6.30) | 0.373 |
| PLT at recurrence (× 109/L) | 146.0 (106.0,187.0) | 131.5 (110.2,158.0) | 0.089 | 141.0 (100.5,190.2) | 131.5 (112.2,162.75) | 0.763 |
| HGB at recurrence (g/L) | 144.0 (133.0,151.0) | 143.5 (136.0,153.0) | 0.439 | 145.5 (134.0,151.0) | 143.0 (136.00,155.50) | 0.749 |
| AFP at recurrence (ng/mL) | 58.00 (6.80,785.95) | 11.38 (3.84,44.95) | <0.001 | 10.80 (4.20,56.40) | 11.11 (3.55,51.35) | 0.941 |
| CRE at recurrence (µmol/L) | 73.90 (66.65,81.65) | 77.25 (66.78,87.52) | 0.204 | 75.55 (66.02,86.58) | 77.45 (66.95,88.35) | 0.687 |
| PT at recurrence (s) | 11.90 (11.30,12.55) | 11.90 (11.47,12.53) | 0.793 | 11.90 (11.30,12.50) | 11.90 (11.40,12.57) | 0.961 |
| Performance status at recurrence (0/1) | 110 (99.1)/1 (0.9) | 71 (98.6)/1 (1.4) | 1.000 | 64 (98.5)/1 (1.4) | 65 (100.0)/0 (0.0) | 1.000 |
| ALBI at recurrence (I/II–III) | 95 (85.6)/16 (14.4) | 63 (87.5)/9 (12.5) | 0.882 | 55 (84.6)/10 (15.4) | 57 (87.7)/8 (12.3) | 0.800 |
Values are presented as the median (interquartile range) or n (%)
TACE transcatheter arterial chemoembolization, HBV hepatitis B virus, HCV hepatitis C virus, AFP alpha-fetoprotein, ALBI albumin–bilirubin, BCLC Barcelona Clinic Liver Cancer stage, MVI microvascular invasion, ALB albumin, TBIL total bilirubin, ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, GGT γ-glutamyl transpeptidase, CRE creatinine, PT prothrombin time, WBC white blood cell, PLT platelet, HGB haemoglobin
Outcomes in the overall cohort
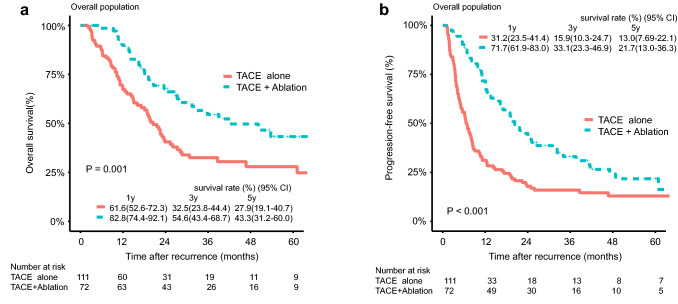
The median follow-up duration was 34.78 months in the TACE-alone group and 32.66 months in the TACE–Ablation group, respectively. The median OS duration was 14.43 months (range, 1.00–97.73 months) in the TACE-alone group and 26.72 months (range 3.00–105.67 months) in the TACE–Ablation group, respectively. During the follow-up, 63 (56.8%) patients in the TACE-alone group and 33 (45.8%) patients in the TACE–Ablation group had died (P = 0.196). The 1-, 3- and 5-year OS rates in the TACE-alone and TACE–Ablation groups were 61.6%, 32.5%, 27.9%, and 82.8%, 54.6%, 43.3%, respectively (P = 0.001) (Fig. 2a). Tumour progression was observed in 92 (82.9%) patients in TACE-alone group and 52 (72.2%) patients in TACE–Ablation group (P = 0.125). The 1-, 3- and 5-year PFS rates in the TACE-alone and TACE–Ablation groups were 31.2%, 15.9%, 13.0%, and 71.7%, 33.1%, 21.7%, respectively (P < 0.001) (Fig. 2b). Treatments for HCC progression in the two groups are shown in Supplementary Table 1.
Fig. 2.
Overall survival (OS) and Progression-free survival (PFS) curves with risk tables for patients with intermediate-stage recurrent HCC after hepatectomy who underwent TACE or TACE combined with ablation. a OS curves before propensity score matching, b PFS curves before propensity score matching. TACE transcatheter arterial chemoembolization, CI confidence interval
Prognostic factors for OS and PFS
The predictors for OS and PFS in univariate and multivariate analyses were shown in Table 2. Univariate analysis indicated that AFP at the primary HCC, MVI of the primary HCC, size and number of recurrence, HBV DNA levels at recurrence, AFP at recurrence, and treatment modality of recurrence were all associated with OS (all P < 0.05); whereas, AFP at the primary HCC, MVI of the primary HCC, size and number of recurrence, HBV DNA levels at recurrence, AFP at recurrence, GGT at recurrence, and treatment modality of recurrence were association with PFS (all P < 0.05). To avoid the effect of co-linearity with the AFP levels at recurrence, the AFP levels at the primary tumour were not included in the multivariate model. Multivariate analysis showed that MVI of the primary HCC (hazard ratio [HR] = 1.92; 95% CI 1.22–2.99, P = 0.004), number of recurrence (HR = 2.26; 95% CI 1.21–4.22, P = 0.010), HBV DNA levels at recurrence (HR = 1.56; 95% CI 1.01–2.41, P = 0.042) and treatment modality of recurrence (TACE alone vs TACE–Ablation, HR = 2.02; 95% CI 1.28–3.12, P = 0.002) were independent prognostic factors for OS, and MVI of the primary HCC (HR = 2.00; 95% CI 1.37–2.90, P < 0.001), number of recurrence (HR = 1.68; 95% CI 1.05–2.68, P = 0.029), and treatment modality of recurrence (TACE alone vs TACE–Ablation, HR = 2.09; 95% CI 1.46–3.00, P < 0.001) were the significant prognostic factor for PFS.
Table 2.
Univariate and multivariate cox regression analyses of the prognostic factors for overall survival and progression-free survival after treatment
| Variables | Overall survival | Progression-free survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Gender (male/female) | 1.34 (0.62–2.90) | 0.453 | 1.43 0.73–2.80) | 0.301 | ||||
| Age, y (> 60/≤ 60) | 0.65 (0.42–1.00) | 0.059 | 0.89 (0.55–1.44) | 0.656 | 0.82 (0.58–1.2) | 0.277 | ||
| Hepatitis (yes/no) | 1.50 (0.47–4.70) | 0.492 | 1.44 (0.11–6.00) | 0.850 | ||||
| Antiviral treatment (yes/no) | 0.81 (0.53–1.20) | 0.33 | 0.77 (0.55–1.10) | 0.138 | ||||
| Initial hepatectomy stage data | ||||||||
| AFP, ng/mL (> 200/≤ 200) | 1.55 (1.00–2.30) | 0.039 | 1.46 (1.00–2.00) | 0.029 | ||||
| BCLC (B/0-A) | 1.28 (0.82–2.00) | 0.27 | 1.22 (0.85–1.80) | 0.281 | ||||
| Cirrhosis (yes/no) | 0.86 (0.55–1.40) | 0.517 | 0.93 (0.64–1.40) | 0.72 | ||||
| MVI (yes/no) | 2.14 (1.40–3.20) | < 0.001 | 1.92 (1.22–2.99) | 0.004 | 2.03 (1.40–2.90) | < 0.001 | 2.00 (1.37–2.90) | < 0.001 |
| Tumour differentiation (III–IV/I–II) | 0.85 (0.50–1.40) | 0.548 | 0.89 (0.59–1.40) | 0.602 | ||||
| Anatomic resection (yes/no) | 0.79 (0.51–1.20) | 0.304 | 0.94 (0.64–1.40) | 0.751 | ||||
| Resection margin, cm (< 1/≥ 1) | 0.94 (0.63–1.40) | 0.745 | 1.10 (0.79–1.50) | 0.579 | ||||
| Recurrence stage data | ||||||||
| Time to recurrence, y (> 2/≤ 2) | 0.55 (0.29–1.10) | 0.076 | 0.85 (0.44–1.71) | 0.685 | 0.68 (0.42–1.10) | 0.114 | ||
| Size of recurrence (cm) (> 3/≤ 3) | 0.63 (0.42–0.96) | 0.033 | 1.07 (0.64–1.89) | 0.812 | 0.81 (0.58–1.10) | 0.027 | 1.11 (0.71–1.73) | 0.647 |
| Number of recurrence (> 3/≤ 3) | 2.25 (1.40–3.50) | < 0.001 | 2.26 (1.21–4.22) | 0.010 | 1.70 (1.20–2.40) | 0.002 | 1.68 (1.05–2.68) | 0.029 |
| HBV DNA levels at recurrence (IU/mL) (≥ 100/< 100) | 1.87 (1.2–2.8) | 0.003 | 1.56 (1.01–2.41) | 0.042 | 1.54 (1.1–2.2) | 0.015 | 1.29 (0.90–1.85) | 0.162 |
| ALB at recurrence, g/L (< 35/≥ 35) | 1.64 (0.60–4.50) | 0.331 | 0.98 (0.40–2.40) | 0.972 | ||||
| TBIL at recurrence, µmol/L (> 17.1/≤ 17.1) | 0.97 (0.60–1.60) | 0.916 | 1.10 (0.74–1.60) | 0.643 | ||||
| ALT at recurrence, U/L (> 50/≤ 50) | 1.50 (0.94–2.40) | 0.087 | 1.17 (0.70–1.96) | 0.533 | 1.15 (0.79–1.70) | 0.468 | ||
| AST at recurrence, U/L (> 40/≤ 40) | 1.40 (0.91–2.10) | 0.123 | 1.26 (0.89–1.80) | 0.196 | ||||
| ALP at recurrence, U/L (> 125/≤ 125) | 1.40 (0.81–2.40) | 0.234 | 1.39 (0.89–2.20) | 0.144 | ||||
| GGT at recurrence, U/L (> 60/≤ 60) | 1.25 (0.83–1.90) | 0.287 | 1.42 (1.00–2.00) | 0.041 | 1.38 (0.97–1.97) | 0.070 | ||
| WBC at recurrence, 109/L (≤ 4/> 4) | 1.27 (0.70–2.3) | 0.432 | 1.03 (0.62–1.70) | 0.915 | ||||
| PLT at recurrence, 109/L (≤ 100/> 100) | 0.68 (0.40–1.20) | 0.161 | 0.80 (0.53–1.20) | 0.292 | ||||
| HGB at recurrence, g/L (≤ 130/> 130) | 1.56 (0.92–2.60) | 0.1 | 1.43 (0.93–2.20) | 0.107 | ||||
| AFP at recurrence, ng/mL (> 200/≤ 200) | 1.98 (1.30–3.10) | 0.002 | 1.17 (0.69–1.96) | 0.563 | 1.75 (1.20–2.50) | 0.003 | 1.04 (0.68–1.58) | 0.858 |
| PT at recurrence sec (> 13.5/≤ 13.5) | 1.01 (0.51–2.00) | 0.987 | 0.83 (0.46–1.5) | 0.544 | ||||
| Performance status at recurrence (1/0) | 1.05 (0.15–7.50) | 0.962 | 1.51 (0.37–6.10) | 0.562 | ||||
| Treatment of recurrence (TACE/TACE–Ablation) | 1.99 (1.30–3.00) | 0.001 | 2.02 (1.28–3.12) | 0.002 | 2.00 (1.40–2.80) | < 0.001 | 2.09 (1.46–3.00) | < 0.001 |
HR hazard ratio, CI confidence interval, TACE transcatheter arterial chemoembolization, HBV hepatitis B virus, HCV hepatitis C virus, AFP alpha-fetoprotein, ALBI albumin–bilirubin, BCLC Barcelona Clinic Liver Cancer stage, MVI microvascular invasion, ALB albumin, TBIL total bilirubin, ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, GGT γ-glutamyl transpeptidase, CRE creatinine, PT prothrombin time, WBC white blood cell, PLT platelet, HGB haemoglobin
Complications and efficacy of the treatments
We summarised the efficacy of the two treatments for patients with recurrent intermediate-stage HCC in Table 3. Overall survival curves stratified with the mRECIST criteria are presented in Supplementary Fig. 1. The complete response (CR) rate, partial response (PR) rate, stable disease (SD) rate and progressive disease (PD) rate in the TACE–Ablation and TACE-alone groups were 36.1%, 38.9%, 13.9%, 11.1%, and 16.2%, 27.9%, 32.4%, 23.4%, respectively. The objective response rate (ORR) based on the mRECIST Criteria in the TACE–Ablation group was higher than that in the TACE-alone group (TACE–Ablation, 75.0% vs TACE alone, 44.1%; P < 0.001). In addition, the disease control rate (DCR) based on the mRECIST Criteria in the TACE–Ablation group was higher than that in the TACE-alone group (TACE–Ablation, 88.9% vs TACE alone, 76.6%; P = 0.057).
Table 3.
Summary of outcomes according to mRECIST Criteria
| Main outcome | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| TACE (n = 111) | TACE–Ablation (n = 72) | P | TACE (n = 65) | TACE–Ablation (n = 65) | P | |
| Tumour response | < 0.001 | 0.020 | ||||
| CR | 18 (16.2) | 26 (36.1) | 11 (16.9) | 23 (35.4) | ||
| PR | 31 (27.9) | 28 (38.9) | 22 (33.8) | 26 (40.0) | ||
| SD | 36 (32.4) | 10 (13.9) | 16 (24.6) | 8 (12.3) | ||
| PD | 26 (23.4) | 8 (11.1) | 16 (24.6) | 8 (12.3) | ||
| ORR | 49 (44.1) | 54 (75.0) | < 0.001 | 33 (50.7) | 49 (75.4) | 0.006 |
| DCR | 85 (76.6) | 64 (88.9) | 0.057 | 49 (75.4) | 57 (87.7) | 0.113 |
Except where indicated, data values represent the number of patients and data in parentheses are percentages
mRECIST modified Response Evaluation Criteria in Solid Tumours, TACE transcatheter arterial chemoembolization, CR complete response, PR partial response, SD stable disease, PD progressive disease, ORR objective response rate, = CR + PR, DCR disease control rate, = CR + PR + SD
Complications after treatment are shown in Table 4. One treatment-related death occurred in the TACE–Ablation group and no death in the TACE-alone group. In addition, one moderate ascites, one gastrointestinal haemorrhage, and one intraperitoneal haemorrhage cases occurred in the TACE-alone group; while, one moderate ascites and two intraperitoneal haemorrhage cases occurred in the TACE–Ablation group (Table 4). Major complications were not significantly different in the two groups. Pain occurred more commonly in the TACE–Ablation group (33 of 72 patients) than the TACE-alone group (26 of 111 patients) (P = 0.003). Patients in the TACE–Ablation group exhibited higher vomiting rate than that of the TACE-alone group (TACE–Ablation, 19 of 72 patients vs TACE alone, 10 of 111 patients; P = 0.003).
Table 4.
Complications after treatment
| Variable | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| TACE (n = 111) | TACE–Ablation (n = 72) | P | TACE (n = 65) | TACE–Ablation (n = 65) | P | |
| Major complication | ||||||
| Death | 0 (0.0) | 1 (1.4) | – | 0 (0.0) | 1 (1.5) | – |
| Liver failure | 0 (0.0) | 1 (1.4) | – | 0 (0.0) | 1 (1.5) | – |
| Moderate ascites | 1 (0.9) | 1 (1.4) | 0.998 | 1 (1.5) | 1 (1.5) | 0.999 |
| Gastrointestinal haemorrhage | 1 (0.9) | 0 (0.0) | – | 1 (1.5) | 0 (0.0) | – |
| Intraperitoneal haemorrhage | 1 (0.9) | 2 (2.8) | 0.562 | 1 (1.5) | 2 (3.1) | 0.998 |
| Minor complication | ||||||
| Fever (> 38.5) | 34 (30.6) | 24 (33.3) | 0.825 | 19 (29.2) | 22 (33.8) | 0.706 |
| Pain | 26 (23.4) | 33 (45.8) | 0.003 | 13 (20.0) | 28 (43.1) | 0.008 |
| Diarrheal | 6 (5.4) | 4 (5.6) | 1.000 | 6 (9.2) | 4 (6.2) | 0.744 |
| Vomiting | 10 (9.0) | 19 (26.4) | 0.003 | 2 (3.1) | 18 (27.7) | 0.001 |
Except where indicated, data values represent the number of patients and data in parentheses are percentages
– There are not enough patients to do statistics, TACE:transcatheter arterial chemoembolization
Outcomes in the matched cohort
After PSM, there was no significantly different between the two groups in terms of baseline characteristics (Table 1). During the follow-up, 39 (60.0%) patients in the TACE-alone group and 32 (49.2%) patients in the TACE–Ablation group had died (P = 0.291). Tumour progression was observed in 53 (81.5%) patients in TACE-alone group and 48 (73.8%) patients in TACE–Ablation group (P = 0.399). The 1-, 3- and 5-year OS rates in the TACE-alone and TACE–Ablation groups were 64.9%, 36.6%, 30.2%, and 81.2%, 52.4%, 41.6%, respectively (P = 0.028) (Fig. 3a). The 1-, 3- and 5-year PFS rates in the TACE-alone and TACE–Ablation groups were 41.3%, 22.0%, 15.8%, and 70.1%, 32.4%, 21.3%, respectively (P = 0.024) (Fig. 3b).
Fig. 3.
Overall survival (OS) and Progression-free survival (PFS) curves with risk tables for patients with intermediate-stage recurrent HCC after hepatectomy who underwent TACE or TACE combined with ablation after propensity score matching. a OS curves after propensity score matching, b DFS curves after propensity score matching. TACE transcatheter arterial chemoembolization, CI confidence interval
Similar to the results before PSM, major complications were not significantly different in the matched two groups. Patients in the TACE–Ablation group exhibited higher pain and vomiting rate and longer hospital stay than that of the TACE-alone group (all P <0.05) (Table 4).
After PSM, the CR, PR, SD and PD rates in the TACE–Ablation and TACE-alone groups were 35.4%, 40.0%, 12.3%, 12.3%, and 16.9%, 33.8%, 24.6%, 24.6%, respectively. The ORR in the TACE–Ablation groups was higher than that in the TACE-alone group (TACE–Ablation, 75.4% vs TACE alone, 50.7%; P = 0.006). In addition, the DCR in the TACE–Ablation group was higher than that in the TACE-alone group (TACE–Ablation, 87.7% vs TACE alone, 75.4%; P = 0.113).
Discussion
High recurrence rate after curative hepatectomy is the main cause of treatment failure and cancer-related death, with 5-year recurrence rate of 70%(Poon et al. 2001). The appropriate management of recurrent HCC is of vital importance. Although TACE is considered as the first-line strategy for intermediate-stage HCC, the efficacy of TACE is modest (Galle et al. 2018; Llovet et al. 2002, 2003; Lo 2002; Marrero et al. 2018). Combination treatment, such as TACE combined with ablation, has shown better survival outcomes than TACE alone in patients with primary intermediate-stage HCC (Azuma et al. 2016; Yin et al. 2014; Xu et al. 2013); while, it remains vague in recurrent intermediate-stage HCC after hepatectomy. To bridge the gap, we explored the question and found that TACE–Ablation was superior to TACE alone with respect to both OS and PFS in patients with recurrent intermediate-stage HCC. However, the TACE group was associated with younger age and heavier tumour burden before treatment. Then, we applied PSM to reduce the confounding bias at baseline and found that TACE–Ablation still showed better both OS and PFS than TACE alone in enrolled patients. To the best of our knowledge, this is the first time to assess whether TACE combined with ablation is more effective than TACE alone for recurrent intermediate-stage HCC.
The advantage of synergistic effect of TACE combined with ablation may explain the reason why TACE–Ablation could achieve better tumour control and survival outcomes than TACE alone (Lencioni 2010; Rossi et al. 2000; Wang et al. 2010). First, TACE could reduce the heat-sink effect of subsequent ablation by obstructing the tumour-feeding flow, thus permitting a lager enough ablation zone of ablation. Second, the hyperthermia in the tumour area induced by ablation could strengthen the effect of chemotherapeutic anticancer drugs injected during the TACE procedure. Third, the digital subtraction angiography (DSA) technique performing during TACE could help to detect more tumours which might be too small to be recognised in imaging scans before treatment. Furthermore, TACE could help to control these micro-metastasis lesions. Through the TACE procedure, tumour number and tumour location were identified, which made the subsequent ablation performed easily and clearly. Therefore, TACE combined with ablation was an effective strategy and should be considered when patients experienced recurrent intermediate-stage HCC.
In treatment of primary intermediate-stage HCC, TACE–Ablation had been compared with TACE alone in several retrospective studies and meta-analysis (Azuma et al. 2016; Wang et al. 2010; Yin et al. 2014; Xu et al. 2013), and the combination group showed better tumour control and overall survival than TACE-alone group. Similarly, our results suggested that TACE–Ablation was also superior to TACE alone with respect to longer overall survival and progression-free survival in patients with recurrent intermediate-stage HCC. Consistent with our study, Yang et al. conducted a retrospective study in 2009 comparing the efficacy of combination therapy of TACE and radiofrequency ablation (RFA) with single treatment in recurrent HCC after hepatectomy and found that TACE combined with RFA was more effective in treating recurrent HCC after hepatectomy compared to single RFA or TACE treatment (Yang et al. 2009). In spite of the similar results, our study conducted a relatively larger cohort than that of Yang’s study (183 patients vs 66 patients) and enrolled the specific patients with recurrent intermediate-stage HCC; while in Yang’s study, patients with early-stage recurrent HCC might confound the results. In addition, our study included more detailed information about the clinical characteristics both of the primary and recurrent HCC, complications during the treatment, and tumour response after treatment. PSM was performed to reduce bias duo to the confounding variables at baseline and there was no significant difference between the two groups in terms of baseline clinical characteristics after PSM. In summary, our results support that TACE combined with ablation is superior to TACE alone in terms of tumour control and survival outcomes in patients with recurrent intermediate-stage HCC.
We assessed the safety of both TACE–Ablation group and TACE-alone group and found that both the major complication rates and the minor complication rates were higher in the TACE–Ablation group than that in the TACE-alone group. The results were in accordance with expectation, after all, the patients in the TACE–Ablation group suffered adverse effects from both TACE and ablation procedure. TACE combined with ablation had shown to be safe in both primary and recurrent HCC patients with low rates of major complication (0–4.7%) (Kagawa et al. 2010; Peng et al. 2012, 2018). In our study, the major complication rate of TACE–Ablation group is 6.9%, which was slightly higher than previous study. One treatment-related death after treatment occurred in the TACE–Ablation group, which contributed the higher major complication rates in our study. We reviewed the death case in detail and found that the infectious shock induced by ablation-related gastrointestinal perforation was the diagnosis of death. In the future, it was especially important to perform artificial ascites or pleural effusion when the liver lesions were adjacent to gastrointestinal tract or diaphragm (Kondo et al. 2006). In general, TACE combined with ablation was relatively safe in treating patients with recurrent intermediate-stage HCC with acceptable complication rates.
There are several limitations in our study. First, our study is a retrospective single-centre experience and our results may be influenced by selected bias. Second, the number of patients enrolled in our study is relatively small, and this limits the robustness of our analyses. Third, although we performed propensity score matching to balance patient demographics, selection bias might not have been completely avoided duo to the retrospective nature of this study. Therefore, more multi-centre study with larger sample size is needed to perform to identify better treatment strategy for recurrent intermediate-stage HCC.
In conclusion, TACE combined with ablation could achieve better OS and PFS than that of TACE alone for patients with recurrent intermediate-stage HCC after hepatectomy. Further prospective randomised controlled clinical trials are needed to validate these findings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Guarantor of the article: YY. Conceptualization: BL and YY; Study design: CW, YLand JQ; Acquisition of data: CW, YY, YZ; Methodology: DZ and YW; Formal analysis and interpretation: CW, WH, YZ, KL and RZ; Writing—original draft preparation: CW and YL; Writing—review and editing: BL and YY; Statistical analysis: CW, JQ, YY and BL. Funding acquisition: YY; Study supervision: BL and YY. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81772598, 81772625 and 81802421), the Guangdong Provincial Natural Science Foundation of China (No. 2017A030311006); and the Guangzhou Science and Technology Program of China (No. 201804020093). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and material
The datasets generated and analysed during the current study are available in the Research Data Deposit public platform (www.researchdata.org.cn).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Approval was obtained from the ethics committee of Sun Yat-sen University Cancer Centre. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chenwei Wang, Yadi Liao and Jiliang Qiu have contributed equally to this work
Binkui Li and Yunfei Yuan have contributed equally to this work.
Contributor Information
Binkui Li, Email: libinkui@mail.sysu.edu.cn.
Yunfei Yuan, Email: yuanyf@mail.sysu.edu.cn.
References
- Ahmed M, Technology Assessment Committee of the Society of Interventional R (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update: supplement to the consensus document. J Vasc Interv Radiol 25:1706–1708. 10.1016/j.jvir.2014.09.005 [DOI] [PubMed] [Google Scholar]
- Austin PC (2011) Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 10:150–161. 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma S et al (2016) Efficacy of additional radiofrequency ablation after transcatheter arterial chemoembolization for intermediate hepatocellular carcinoma. Hepatol Res 46:312–319. 10.1111/hepr.12566 [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Bruix J, Llovet JM (2002) Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology (Baltimore, Md) 35:519–524. 10.1053/jhep.2002.32089 [DOI] [PubMed] [Google Scholar]
- Chao Y et al (2015) The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: Final results of the START trial. Inter J Cancer 136:1458–1467. 10.1002/ijc.29126 [DOI] [PubMed] [Google Scholar]
- Galle PR et al (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69:182–236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- Gordic S et al (2017) Evaluation of HCC response to locoregional therapy: validation of MRI-based response criteria versus explant pathology. J Hepatol 67:1213–1221. 10.1016/j.jhep.2017.07.030 [DOI] [PubMed] [Google Scholar]
- He W et al (2018) Nomogram to predict survival of patients with recurrence of hepatocellular carcinoma after surgery. Clin Gastroenterol Hepatol 16(756–764):e710. 10.1016/j.cgh.2017.12.002 [DOI] [PubMed] [Google Scholar]
- Johnson SR, Tomlinson GA, Hawker GA, Granton JT, Feldman BM (2018) Propensity score methods for bias reduction in observational studies of treatment effect. Rheum Dise Clin N Am 44:203–213. 10.1016/j.rdc.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Kagawa T et al (2010) Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: comparison with surgical resection. Cancer 116:3638–3644. 10.1002/cncr.25142 [DOI] [PubMed] [Google Scholar]
- Kondo Y, Yoshida H, Shiina S, Tateishi R, Teratani T, Omata M (2006) Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg 93:1277–1282. 10.1002/bjs.5374 [DOI] [PubMed] [Google Scholar]
- Lencioni R (2010) Loco-regional treatment of hepatocellular carcinoma. Hepatology (Baltimore, Md) 52:762–773. 10.1002/hep.23725 [DOI] [PubMed] [Google Scholar]
- Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60. 10.1055/s-0030-1247132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W et al (2018) Microwave vs radiofrequency ablation for hepatocellular carcinoma within the Milan criteria: a propensity score analysis. Aliment Pharmacol Ther 48:671–681. 10.1111/apt.14929 [DOI] [PubMed] [Google Scholar]
- Llovet JM et al (2002) Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. The Lancet 359:1734–1739. 10.1016/s0140-6736(02)08649-x [DOI] [PubMed] [Google Scholar]
- Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. The Lancet 362:1907–1917. 10.1016/s0140-6736(03)14964-1 [DOI] [PubMed] [Google Scholar]
- Lo C (2002) Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology (Baltimore, Md) 35:1164–1171. 10.1053/jhep.2002.33156 [DOI] [PubMed] [Google Scholar]
- Marrero JA et al (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md) 68:723–750. 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- Peng Z, Zhang Y, Liang H, Lin X, Guo R, Chen M (2012) Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology 262:689–700. 10.1148/radiol.11110637 [DOI] [PubMed] [Google Scholar]
- Peng Z et al (2018) Combined transcatheter arterial chemoembolization and radiofrequency ablation versus hepatectomy for recurrent hepatocellular carcinoma after initial surgery: a propensity score matching study. Eur Radiol 28:3522–3531. 10.1007/s00330-017-5166-4 [DOI] [PubMed] [Google Scholar]
- Peng Z et al (2019) Microvascular invasion as a predictor of response to treatment with sorafenib and transarterial chemoembolization for recurrent intermediate-stage hepatocellular carcinoma. Radiology 292:237–247. 10.1148/radiol.2019181818 [DOI] [PubMed] [Google Scholar]
- Poon R, Fan S, Lo C, Ng I, Liu C, Lam C, Wong J (2001) Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg 234:63–70. 10.1097/00000658-200107000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM (2006) Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 243:229–235. 10.1097/01.sla.0000197706.21803.a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R (2011) Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 37:212–220. 10.1016/j.ctrv.2010.07.006 [DOI] [PubMed] [Google Scholar]
- Rossi S et al (2000) Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology 217:119–126. 10.1148/radiology.217.1.r00se02119 [DOI] [PubMed] [Google Scholar]
- Sangro B, Inarrairaegui M, Bilbao JI (2012) Radioembolization for hepatocellular carcinoma. J Hepatol 56:464–473. 10.1016/j.jhep.2011.07.012 [DOI] [PubMed] [Google Scholar]
- Shimose S et al (2019) Prognostic Impact of Transcatheter Arterial Chemoembolization (TACE) Combined with radiofrequency ablation in patients with unresectable hepatocellular carcinoma: a comparison to TACE alone using decision-tree analysis after propensity score matching. Hepatol Res. 10.1111/hepr.13348 [DOI] [PubMed] [Google Scholar]
- Team RC (2013) R: a language and environment for statistical computing
- Wang W, Shi J, Xie WF (2010) Transarterial chemoembolization in combination with percutaneous ablation therapy in unresectable hepatocellular carcinoma: a meta-analysis. Liver Int 30:741–749. 10.1111/j.1478-3231.2010.02221.x [DOI] [PubMed] [Google Scholar]
- Xu LF et al (2013) Large primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapy. J Gastroenterol Hepatol 28:456–463. 10.1111/jgh.12088 [DOI] [PubMed] [Google Scholar]
- Yang W et al (2009) Combination therapy of radiofrequency ablation and transarterial chemoembolization in recurrent hepatocellular carcinoma after hepatectomy compared with single treatment. Hepatol Res 39:231–240. 10.1111/j.1872-034X.2008.00451.x [DOI] [PubMed] [Google Scholar]
- Yang Z et al (2019) Lipiodol deposition in portal vein tumour thrombus predicts treatment outcome in HCC patients after transarterial chemoembolisation. Eur Radiol. 10.1007/s00330-019-06157-0 [DOI] [PubMed] [Google Scholar]
- Yin X et al (2014) Transcatheter arterial chemoembolization combined with radiofrequency ablation delays tumor progression and prolongs overall survival in patients with intermediate (BCLC B) hepatocellular carcinoma. BMC Cancer 19(14):849. 10.1186/1471-2407-14-849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Li HL, Guo CY, Luo SX (2018) Comparison of the efficacy and prognostic factors of transarterial chemoembolization plus microwave ablation versus transarterial chemoembolization alone in patients with a large solitary or multinodular hepatocellular carcinomas. Korean J Radiol 19:237–246. 10.3348/kjr.2018.19.2.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available in the Research Data Deposit public platform (www.researchdata.org.cn).