Abstract
Background
Calprotectin is a heterodimer formed by S100A8 and S100A9 proteins which are enhanced during hepatic carcinogenesis and the increased expression of both proteins promotes malignant progression of hepatocellular carcinoma. The potential correlation between ascitic Calprotectin and HCC was not studied.
Methods
100 patients were stratified into a case group which enrolled 50 patients with cirrhotic ascites and documented HCC and a control group consisted of 50 patients with cirrhotic ascites without HCC. They were evaluated by liver function tests, abdominal ultrasound and routine ascitic fluid examination including ascetic Calprotectin and results were validated in another group (n = 100).
Results
Calprotectin level was significantly higher in the HCC group with insignificant difference regarding total cell count, PNLs, ascitic albumin, LDH, CEA and SAAG. It correlated with serum creatinine (r = 0.245, p = 0.014) and number of focal hepatic lesions (r = 0.309, p = 0.002). In the validation group, 28 patients had elevated ascitic Calprotectin of which 21 patients had developed HCC (75%) after a mean period of 3.8 ± 1.54 months. A cut of value 126 ng/ml was accurate to predict HCC in liver cirrhosis with ascites with a sensitivity of 93.3% specificity 94%, AUC 0.950, Youden’s J value = 0.873, p = 0.0001.
Conclusion
Ascitic Calprotectin may offer an easy, affordable marker that can predict the early occurrence of HCC.
Keywords: Ascites, Cirrhosis, Calprotectin, Hepatocellular carcinoma, Prediction, HCV, Focal lesion, Ultrasound, Early, Prevention
Introduction
Hepatocellular carcinoma (HCC) is one of the most common lethal cancers; early detection is of great importance for a better outcome and increased survival. Calprotectin is a heterodimer formed by S100A8 and S100A9 proteins (Lehmann et al. 2014). It constitutes about 60% of the soluble protein content of the neutrophilic cytosol (Striz and Trebichavsky 2004).
It exerts its antimicrobial properties by sequestering manganese and zinc in the presence of calcium, since these transition metals are essential to the survival of micro-organisms thus considered a part of the innate immune system (Clark et al. 2016; Hood and Skaar 2013). The potential correlation between Calprotectin and hepatocellular carcinoma (HCC) was not studied before.
HCC which is the final destination of advanced liver cirrhosis due to a variety of etiological factors primarily HCV, HBV. Experimental data revealed that S100A8 and S100A9 are enhanced during hepatic carcinogenesis and the increased expression of both proteins promotes malignant progression of HCC (De Ponti et al. 2015).
S100A8/A9 are elevated in primary tumors originated from sites which do not normally express these proteins mainly the liver (Németh et al. 2009) and gastric mucosa (Yong and Moon 2007), however, the expression decreases in invasive or anaplastic tumors of the squamous epithelium mainly esophageal tumors (Kong et al. 2004; Khammanivong et al. 2013).
Fecal Calprotectin is commonly used as a laboratory tool in patients with colitis to rule out inflammatory bowel disease and is determined using enzyme-linked immunosorbent assay, its assay in the ascetic fluid is not routinely done, however, previous studies tried to link it to the magnitude of polymorphonuclear neutrophil (PNLs) count and hence to the occurrence of spontaneous bacterial peritonitis (SBP) (Ahmed Abdel-Razik et al. 2016).
Early diagnosis of HCC was based on regular follow-up by abdominal ultrasonography and assessment of alpha-fetoprotein (AFP), a cutoff 20 ng/mL had sensitivity for HCC up to 65%, with a specificity up to 94%, however, the test had limited value in small-sized HCC which does not secrete AFP, also false-positive results were seen in advanced cirrhosis and acute hepatitis (Gupta et al. 2013).
The AFP-L3 isoform had been approved as HCC biomarker; it has a high specificity, but limited by the low sensitivity in early diagnosis of HCC and should be combined with other biomarkers to increase the sensitivity (Marrero et al. 2009).
Other novel postulated biomarkers for early detection of HCC are Des-γ-carboxy-prothrombin which has high diagnostic specificity and can exclude malignant from non-malignant liver conditions and may be of benefit in AFP negative cases (Chen et al. 2018).
Glypican-3 (GPC3) is positively expressed in 60–90% of surgically removed HCC specimens, serum levels correlate with tissue expression, however, the diagnostic accuracy for early detection still unsatisfactory (Jia et al. 2014).
Golgi protein-73 (GP73) is another promising marker with a sensitivity and specificity of 77% and 91%, respectively, and should be combined with other markers to increase the power of prediction (Dai et al. 2015), this is the same for osteopontin (Duarte-Salles et al. 2016) and Dickkopf-1 (Shen et al. 2012), they should be combined with other biomarkers to increase their accuracy, in addition, they are expensive, need special techniques in assessment and their utility in clinical practice still requires validation.
Based on the fact that Expression S100A8/A9 is linked to HCC tumorigenesis, this study was designed to examine the relationship of ascitic fluid Calprotectin a potential, easy performed marker that could predict the occurrence of HCC in high-risk patients with cirrhotic ascites.
Materials and methods
Patients' selection
This prospective, case–control study was conducted at the Hepatology clinic, Department of Internal Medicine, Zagazig University, from January 2016 till December 2019.
Patients were included if they had cirrhotic ascites and high-risk factors for HCC as older age, diabetes, thrombocytopenia, advanced liver cirrhosis due to HCV or HBV (Pang et al. 2015).
Exclusion criteria were patients on regular immunosuppressive drugs; antibiotic prophylaxis for SBP prior to the intervention; autoimmune diseases; any disease accompanied with a suspected increase in Calprotectin as spontaneous bacterial peritonitis; abdominal malignancy as gastric, pancreatic, colorectal, ovarian cancers, cholangiocarcinoma, liver metastasis, peritoneal carcinomatosis, renal failure, sepsis, abdominal operations within 3 months of the study; other causes of ascites as renal and cardiac failure, nutritional and endocrinal as myxedema, alcoholic cirrhosis.
The patients were stratified into a case group that enrolled 50 patients with cirrhotic ascites and documented HCC; a control group consisted of 50 patients with cirrhotic ascites without HCC. They were matched for risk factors as age, sex, diabetes, etiology and severity of the liver disease.
The efficiency of ascetic Calprotectin was assessed in a validation group composed of 100 patients with cirrhotic ascites and high-risk HCC factors as indicated above, which met the same inclusion and exclusion criteria. Patients with increased ascetic Calprotectin were censored and followed up by repeated abdominal ultrasound and AFP every 2 months for a period of 12 months.
Informed consent was signed by patients taking their permission to participate in the study. The study methods were approved by the research ethics board of the Zagazig Faculty of Medicine, Egypt (ZU-IRB-2205/2016). The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki and its later amendments.
All patients underwent a detailed medical history mainly of recent surgery, peptic ulcer disease, clinical examination of vital signs, signs of portal hypertension such as dilated abdominal veins, splenomegaly. Signs of liver cell failure such as jaundice, ascites, lower limb edema, fetor hepaticus, flapping tremor, spider angiomata.
Laboratory evaluation
Liver function tests which had included serum levels of aminotransferases, total bilirubin, albumin, total protein and alkaline phosphatase. Evaluation of bleeding tendency by assessing prothrombin time (PT), partial thromboplastin time (PTT) and international normalized ratio (INR). Renal function tests as serum creatinine and blood urea nitrogen.
According to the protocol, diagnostic paracentesis of ascitic fluid was performed for all patients with ascites and liver cirrhosis under complete aseptic condition. 30 ml of fluid was extracted, tests on ascitic fluid samples included cell count, measurement of albumin, total protein, glucose, lactate, LDH, amylase and carcinoembryonic antigen (CEA); LDH and amylase were compared to the serum level. The serum-ascites albumin gradient (SAAG) was calculated as the difference of albumin in serum and albumin in ascites.
10 ml was inoculated in aerobic and anaerobic blood culture bottles; according to the results of PNLs and cultures, spontaneous bacterial peritonitis, culture-negative neutrocytic ascites, monomicrobial non-neutrocytic bacterascites were excluded.
5 ml sample of ascitic fluid was withdrawn immediately for Calprotectin at the initial presentation of the patients which was detected by an ELISA using immune diagnostic ELISA kit (Immundiagnostik AG, Bensheim, Germany), the measuring range of the test was 0.2–12 ng /ml ascites with an intra- and inter-assay coefficient of 4.5% and 11%, respectively.
The diagnosis of SBP was based on the presence of > 500 neutrophils /µL or > 250 PNLs count in the ascites in the absence of secondary peritonitis (Enomoto et al. 2014).
Abdominal ultrasonography (USG)
The patients were examined after 6 h fast. Criteria of portal hypertension were detected by measuring Portal vein diameter, splenic bipolar diameter, and splenic vein diameter, criteria of liver cirrhosis, severity of ascites, hepatic focal lesions, portal vein thrombosis, peritoneal deposits, and intra-abdominal masses.
Diagnosis of hepatocellular carcinoma (HCC)
Assessment and stratification of patients with HCC was done according to Barcelona Clinic Liver Cancer (BCLC), it assessed the tumor burden, liver function according to CTP score, and patient performance status, it stratified the survival of patients into 0, A, B, C, and D stages (European Association for the Study of The Liver 2012).
Statistical analysis
All statistical analyses were performed using the SPSS version 20 software (SPSS Inc., Chicago, IL, USA). Shapiro–Wilk test was used to test the distribution of data. Parametric data were expressed in mean ± standard deviation, whereas none parametric data were expressed in the form of median (minimum–maximum). Independent samples Student's t test was used to compare between two groups of normally distributed variables, while Mann–Whitney U test was used for non- normally distributed variables. Spearman correlation analysis was done to correlate between numerical data. A p value of less than 0.05 indicated statistical significance.
Analysis of the receiver operator characteristics (ROC) and calculation of the area under the curve (AUC) were used to evaluate ascetic Calprotectin ability to predict the presence of HCC. The sensitivity, specificity, positive and negative likelihood ratios (LR + and LR-), positive and negative predictive values were determined. The accuracy of the test was calculated. The performance of the cut off value was judged by calculation of Youden’s J value; values near 1 indicated good performance of the cut off value (J = Sensitivity + Specificity—1).
Results
Patient characteristics
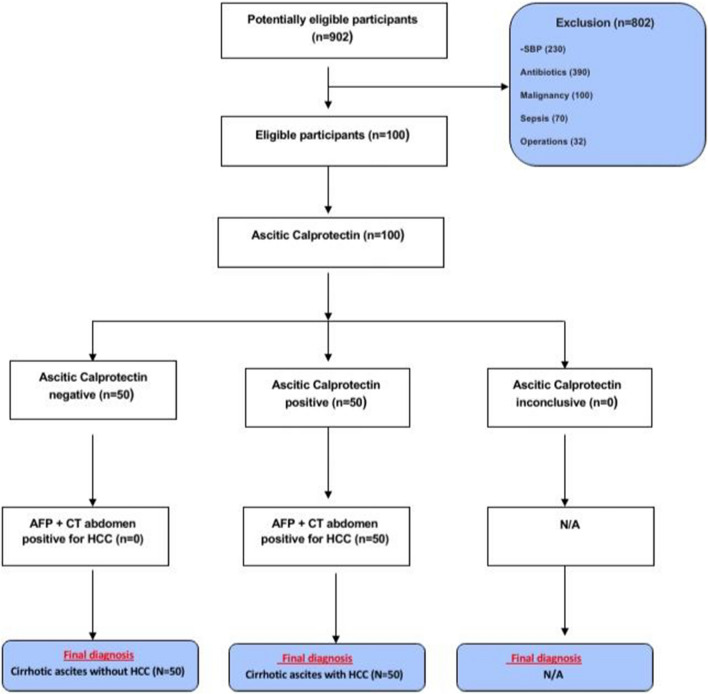
Out of 902 patients with advanced liver disease and ascites, we selected 100 patients with liver cirrhosis and ascites (Fig. 1); a study group with documented HCC (n = 50) who were initially diagnosed with abdominal USG with 2.4 ± 1.2 (1–5) focal lesions, portal vein thrombosis (PVT) was diagnosed in 13 patients (BCLC stage C, D) and confirmed with Triphasic CT abdomen, 23 patients were diabetic on insulin therapy (46%).
Fig. 1.
Study flowchart
Liver cirrhosis was due to HCV (N = 41), HBV (N = 9). SBP and abdominal infections or malignancy were excluded. They were compared with a control group composed of 50 patients with liver cirrhosis and cirrhotic ascites due to HCV (N = 45), HBV (n = 5) after exclusion of HCC. They were matched by age, sex and other inclusion criteria as shown in (Table 1).
Table 1.
Baseline characteristics of the studied patients and controls
| Case (n = 50) | Control (n = 50) | P | |
|---|---|---|---|
| F/M | 14/36 | 11/39 | 0.2 |
| Age | 58.6 ± 4 | 54 ± 4.6 | 0.16 |
| Diabetes (n %) | 23(46%) | 14 (28%) | 0.01 |
| AST IU/L | 76.7 ± 23.9 | 65.7 ± 19.1 | 0.01 |
| ALT IU/L | 64 ± 21 | 53.7 ± 18.4 | 0.48 |
| Total bilirubin mg/dl | 1.8 ± 0.4 | 2 ± 0.6 | 0.89 |
| Albumin gm/dl | 2.8 ± 0.45 | 2.67 ± 0.35 | 0.7 |
| INR | 2.4 ± 0.13 | 1.86 ± 0.5 | 0.06 |
| Creatinine mg/dl | 1.4 ± 0.24 | 1.32 ± 0.3 | 0.09 |
| WBC /mcL | 8.5 ± 2.1 | 7.5 ± 0.7 | 0.1 |
| HG gm/dl | 10.6 ± 0.96 | 11.4 ± 0.7 | 0.1 |
| PLT/uL | 56.3 ± 13 | 90 ± 24 | 0.03 |
| FBS mg/dl | 193.5 ± 31.5 | 109 ± 15.6 | 0.02 |
| No of focal lesions | 2.44 ± 1.2 (1–5) | – | – |
| AFP ug/ml | 833 ± 108 (SE) | 16.3 ± 6.9 | 0.0001 |
| CEA ng/ml | 2.2 ± 1.3 | 2.3 ± 0.9 | 0.09 |
| Ascitic TLC (cell /mm3) | 189 ± 56 | 203 ± 31 | 0.13 |
| Ascitic PNL (cell /mm3) | 63 ± 28 | 96 ± 36 | 0.09 |
| Ascitic protein gm/dl | 2.1 ± 0.17 | 1.7 ± 0.14 | 0.001 |
| Ascitic ALB gm/dl | 0.9 ± 0.3 | 1.01 ± 0.34 | 0.2 |
| LDH U/l | 199 ± 31 | 194.5 ± 19 | 0.7 |
| Glucose | 156 ± 24 | 82 ± 14 | 0.01 |
| SAAG | 1.9 ± 0.5 | 1.7 ± 0.6 | 0. 14 |
| CEA ng/ml | 1.5 ± 0.9 | 1.3 ± 0.8 | 0.1 |
| Calprotectin ng/ml | 294 ± 108 (170–600) | 38 ± 21 (12–90) | 0.001 |
Bold values indicate p < 0.05 is considered significant
Ascitic fluid analysis had revealed a statistically significant difference regarding ascitic total protein and glucose levels being higher in HCC group (p = 0.012, 0.01), Calprotectin level was significantly higher in HCC group with insignificant difference as regards total cell count and PNLs, ascitic albumin, LDH, CEA and SAAG (Table 1).
Ascitic Calprotectin had a significant positive correlation with serum creatinine (r = 0.245, p = 0.014) and number of focal hepatic lesions (r = 0.309, p = 0.002).
Ascitic Calprotectin was validated in 100 patients with cirrhotic ascites after application of inclusion and exclusion criteria. They were composed of 83 males and 17 females with mean age 50.9 ± 5.3 years, 56 patients (56%) were diabetic with a mean HBA1c 9.5 ± 1.2, elevated AST, ALT, total bilirubin and AFP (80.4 ± 21.2 IU/L, 67 ± 15 IU/L, 2.1 ± 0.7 mg/dl, 112.5 ± 180 ug/dl, respectively, mean SAAG 1.4 ± 0.4 and mean ascitic Calprotectin value 110 ± 138 g/ml.
Twenty eight patients showed elevated ascitic Calprotectin (28%). They were followed-up for 1 year by abdominal USG and AFP level every 2 months; the primary endpoint was the identification of HCC by USG confirmed by AFP and Triphasic abdominal CT.
During the follow-up period, 21 patients with elevated levels of ascitic Calprotectin developed HCC (75%) after a mean period of 3.8 ± 1.54 months, 13/21 patients were diabetic (61.9%). The demographic and laboratory data for the HCC subgroup are shown in Table 2; it included 25 patients [21 patients with high Calprotectin and 4 patients from the normal Calprotectin subgroup]. Ascitic Calprotectin was significantly higher in the HCC subgroup (346 ± 100 ng/ml) when compared to the non-HCC subgroup (47 ± 16 ng/ ml) (p = 0.001) (Fig. 2).
Table 2.
Baseline characteristics of the HCC and no-HCC subgroups in the validation group
| HCC (N = 25) | Non-HCC (N = 75) | P | |
|---|---|---|---|
| F/M | 22/3 | 62/13 | 0.02 |
| Age | 51.6 ± 6.2 | 50.3 ± 5.3 | 0.16 |
| Diabetes (n, %) | 16(64%) | 40(53.3%) | 0.01 |
| AST IU/L | 72.6 ± 14 | 80 ± 12.5 | 0.09 |
| ALT IU/L | 70 ± 13 | 66 ± 8.4 | 0.48 |
| Total bilrubin mg/dl | 2.3 ± 0.8 | 2.01 ± 0.7 | 0.89 |
| Albumin gm/dl | 2.5 ± 0.42 | 2.4 ± 0.34 | 0.7 |
| INR | 2.1 ± 0.3 | 1.6 ± 0.4 | 0.06 |
| CTP score | 0.45 ± 11 | 10 ± 0.4 | 0.12 |
| Creatinine mg/dl | 1.6 ± 0.2 | 1.4 ± 0.23 | 0.09 |
| PLT/uL | 71.2 ± 10.3 | 77 ± 11 | 0.034 |
| HBA1C | 9.7 ± 0.9 | 7.2 ± 1.2 | 0.02 |
| AFP ug/ml | 378.6 ± 172 | 13 ± 5.2 | 0.0001 |
| Focal lesions | 1.5 ± 0.8 | – | – |
| Ascitic TLC (cell /mm3) | 239 ± 45 | 220 ± 41 | 0.13 |
| Ascitic PNL (cell /mm3) | 100 ± 18 | 86 ± 21 | 0.09 |
| Ascitic protein gm/dl | 1.9 ± 0.37 | 2.01 ± 0.24 | 0.089 |
| Ascitic ALB gm/dl | 0.93 ± 0.28 | 0.88 ± 0.2 | 0.2 |
| LDH U/l | 169 ± 21 | 188 ± 27 | 0.7 |
| Glucose | 187 ± 19 | 110 ± 23 | 0.01 |
| SAAG | 1.7 ± 0.5 | 1.36 ± 0.5 | 0. 2 |
| Calprotectin ng/ml | 346 ± 100 | 47 ± 16 | 0.001 |
| CEA ng/ml | 1.7 ± 0.56 | 2.02 ± 0.78 | 0.23 |
Bold values indicate p < 0.05 is considered significant
Fig. 2.

Ascitic Calprotectin is significantly higher in patients with cirrhotic ascites complicated with HCC compared to patients without HCC
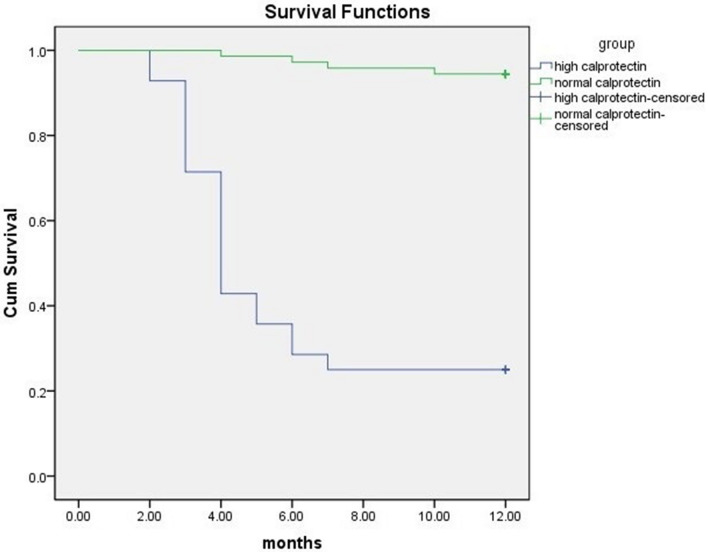
In the subgroup with increased Ascitic Calprotectin who developed HCC, the mean number of focal lesions 1.5 ± 0.8 confirmed by AFP (378.6 ± 172 ug/dl) and Triphasic CT, however, four patients in the subgroup with normal level Calprotectin developed HCC (5.6%) after 7.5 ± 2.1 months as shown in Fig. 3. They had a CTP score class C and were considered as BCLC stage D.
Fig. 3.
Time of occurrence of HCC in a group with elevated ascitic Calprotectin compared to a group with normal value
Based on the ROC that was applied (Fig. 4) to determine the cut-off value of ascitic Calprotectin associated with the development of HCC and its corresponding Youden’s J value, a cut of value 126 ng/ml was accurate to predict HCC in liver cirrhosis with ascites with a sensitivity of 93.3% specificity 94%, AUC 0.950, Youden’s J value = 0.873, p = 0.0001.
Fig. 4.
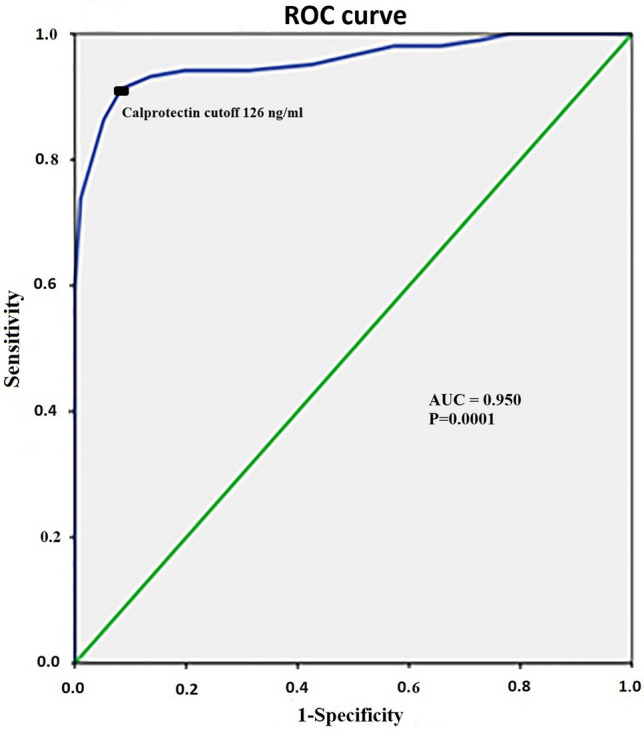
Receiver operating characteristics analysis of the ascitic Calprotectin to identify risk of occurrence of HCC
The calculated Odds ratio was 51, 95% CI 13.6–191.4, P < 0.0001. The positive predictive value for ascetic Calprotectin was 53.8%, 95% CI 38.2–60.1, negative predictive value 97% with accuracy 90.9%, 95% CI 82—95.1.
Discussion
Liver cirrhosis is one of the most important risk factors for the occurrence of HCC, whatever the cause of cirrhosis (Yuen et al. 2009). Therefore, early HCC detection in high-risk patients can help to improve survival and optimize proper management for the best outcome.
In Egypt, HCC is the second most common cancer in males and comes in the sixth order in females (Ziada et al. 2016). Previous studies had documented the increasing role of HCV in the development of HCC in Egypt, accounting for 50% of cases and a decrease in the influence of HBV (El-Zayadi et al. 2005).
Accidental hepatic focal lesion detection by bedside ultrasound examination may approach 22% (Ziada et al. 2016), therefore, HCC was diagnosed in most patients at a time when therapeutic approaches cannot be more helpful or effective.
Calprotectin, a well-known marker of disease activity in inflammatory bowel disease that differentiates it from the functional disorders (Soyfoo et al. 2009; Mao et al. 2012) but its relation to malignancy is rarely mentioned before, it may be elevated locally due to the influx of inflammatory cells into the malignant tissue, also due to its apoptotic effect on malignant cells (Mumolo et al. 2018).
Calprotectin detection in ascitic fluid is not a common investigation; it was evaluated in cirrhotic patients with SBP which was found to be correlated with the magnitude of PNLs count and reliably predicts the occurrence of SBP (Burri et al. 2013), thus it may replace other alternative methods (Fernandes et al. 2016).
The current study evaluated for the first time, the possible use of ascitic Calprotectin as a novel and predictor marker of HCC in high-risk cirrhotic patients with ascites, the level was compared in two groups; a study group with ascites and documented HCC and a control group which enrolled patients with cirrhotic ascites in the absence of HCC; ascetic Calprotectin was significantly higher in patients with HCC (294 ± 108 vs. 38 ± 21 ng/ml, respectively, p = 0.001).
The test was validated in a cirrhotic group with high risk for HCC after exclusion of abdominal infection and malignancy. 28 patients who showed a high level of ascitic Calprotectin were censored and followed-up together with the rest of the patients for 1 year. HCC was diagnosed (21/28, 75%) in the subgroup with high ascitic Calprotectin after 3.8 ± 1.54 months, while four patients in the subgroup with normal ascitic Calprotectin developed HCC (5.6%) after 7.5 ± 2.1 months.
Ascitic Calprotectin could predict HCC at a cut-off value of 126 ng/ml in liver cirrhosis with ascites (p = 0.0001) after exclusion of other causes of ascites in liver cirrhosis. The test was accurate at 90.9% with a positive likelihood ratio of 9.2, odds ratio 51.
A study conducted by Hornann et al. (2003) on 24 patients with documented malignant diseases; (liver metastasis, n = 16, primary hepatocellular carcinoma, n = 4, peritoneal carcinomatosis, n = 4), it had revealed that malignancy was significantly associated with high ascitic Calprotectin concentrations (P < 0.0001), with a cut-off value 190 ug/l, the sensitivity and specificity of high ascites Calprotectin concentrations for malignant disease were 80% and 91%, respectively.
A study had assessed 120 ascitic fluid samples and 8 samples of malignant peritoneal fluid (HCC, pancreatic cancer and renal cell carcinoma) as a control group, it showed that Calprotectin levels in malignant ascites ranged from 47 to 2596 ng/ml with a median of 401 ng/ml and were significantly higher compared to uninfected cirrhotic ascites (p < 0.001) (Lutz et al. 2015). Nonetheless, Calprotectin level is still smaller when compared to the level measured in cases of SBP that may reach thousands of ng/ml and this is consistent with our findings.
HCC is commonly assessed for treatment and prognosis via BCLC staging so, measurement of Calprotectin in the ascitic fluid applies only to stage C, D of BCLC.
The limitation of the current study was being a single-center study, however, the test was evaluated and validated in an adequate number of patients who were followed-up in an adequate period of time, and we recommend to propagate this test as an investigation on the ascitic fluid of cirrhotic patients with ascites with high risk of HCC as a routine test on ascetic fluid; a rise of ascitic Calprotectin in the confirmed absence of SBP or secondary peritonitis should raise the attention towards HCC.
In conclusion, HCC occurrence is discovered accidently in advanced stages in most of the patients when therapeutic measures cannot be offered, so the early prediction in high-risk patients by ascitic Calprotectin can help to provide an early and effective therapy.
Abbreviations
- USG
Abdominal ultrasonography
- CEA
Carcinoembryonic antigen
- HCC
Hepatocellular carcinoma
- ROC
Receiver operator characteristics
- PNLs
Polymorphonuclear neutrophils
- SAAG
Serum-ascites albumin gradient
- SBP
Spontaneous bacterial peritonitis
Author contributions
ASH: research methodology. AH, AAA and MSM: clinical analysis, data collection. ASH: ultrasound evaluation and statistical analysis.
Funding
Self-funded.
Compliance with ethical standards
Conflict of interests
There are no conflicts of interest.
Ethical approval
The study was approved by Zagazig University institutional research committee. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
Guarantor of the article
Amr Shaaban Hanafy.
Footnotes
The original online version of this article was revised due to correction in figures 3 and 4.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/18/2021
A Correction to this paper has been published: 10.1007/s00432-021-03829-7
References
- Ahmed Abdel-Razik A, Mousa N, Elhammady D et al (2016) Ascitic fluid calprotectin and serum procalcitonin as accurate diagnostic markers for spontaneous bacterial peritonitis. Gut Liver 10(4):624–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri E, Schulte F, Muser J et al (2013) Measurement of Calprotectin in ascitic fluid to identify elevated polymorphonuclear cell count. World J Gastroenterol 19(13):2028–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang Y, Li S et al (2018) Direct comparison of five serum biomarkers in early diagnosis of hepatocellular carcinoma. Cancer Manag Res 10:1947–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HL, Jhingran A, Sun Y et al (2016) Zinc and manganese chelation by neutrophil S100A8/A9 (Calprotectin) limits extracellular Aspergillus fumigates Hyphal growth and corneal infection. J Immunol 196(1):336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M, Chen X, Liu X, et al (2015). Diagnostic value of the combination of Golgi protein 73 and alpha-fetoprotein in hepatocellular carcinoma: a meta-analysis Plos One 10(10), Article e0140067. [DOI] [PMC free article] [PubMed]
- De Ponti A, Wiechert L, Schneller D et al (2015) A pro-tumorigenic function of S100A8/A9 in carcinogen-induced hepatocellular carcinoma. Cancer Lett 28 369(2):396–404 [DOI] [PubMed] [Google Scholar]
- Duarte-Salles T, Misra S, Stepien M et al (2016) Circulating osteopontin and prediction of hepatocellular carcinoma development in a large European population. Canc Prev Res 9(9):758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Zayadi AR, Badran HM, Barakat EM et al (2005) Hepatocellular carcinoma in Egypt: a single center study over a decade. World J Gastroenterol 11:5193–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto H, Inoue S, Matsuhisa A, et al (2014). Diagnosis of spontaneous bacterial peritonitis and an in situ hybridization approach to detect an "unidentified" pathogen. Int J Hepatol:634617. [DOI] [PMC free article] [PubMed]
- European Association for the Study of The Liver; European Organization For Research And Treatment Of Cancer (2012) EASL-EORTC Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943 [DOI] [PubMed] [Google Scholar]
- Fernandes SR, Santos P, Fatela N et al (2016) Ascitic Calprotectin is a novel and accurate marker for spontaneous bacterial peritonitis. J Clin Lab Anal 30(6):1139–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Bent S, Kohlwes J (2013) Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C, a systematic review and critical analysis. Ann Intern Med 139(1):46–50 [DOI] [PubMed] [Google Scholar]
- Hood M, Skaar E (2013) Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10(8):525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornann C, Christensen E, Schlichting P et al (2003) Ascites fluid and plasma Calprotectin concentrations in liver disease. Scand J Gastroenterol 38:415–420 [DOI] [PubMed] [Google Scholar]
- Jia X, Liu J, Gao Y et al (2014) Diagnosis accuracy of serum glypican-3 in patients with hepatocellular carcinoma: a systematic review with meta-analysis. Arch Med Res 45(7):580–588 [DOI] [PubMed] [Google Scholar]
- Khammanivong A, Wang C, Sorenson BS et al (2013) S100A8/A9 (Calprotectin) negatively regulates G2/M cell cycle progression and growth of squamous cell carcinoma. PLoS ONE 8(7):e69395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong JP, Ding F, Zhou CN et al (2004) Loss of myeloid-related proteins 8 and myeloid-related proteins 14 expression in human esophageal squamous cell carcinoma correlates with poor differentiation. World J Gastroenterol 15 10(8):1093–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann FS, Burri E, Beglinger C (2014) The role and utility of faecal markers in inflammatory bowel disease. Therapeutic Adv Gastroenterol 8(1):23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz P, Pfarr K, Nischalke HD et al (2015) The ratio of Calprotectin to total protein as a diagnostic and prognostic marker for spontaneous bacterial peritonitis in patients with liver cirrhosis and ascites. Clin Chem Lab Med 53(12):2031–2039 [DOI] [PubMed] [Google Scholar]
- Mao R, Xiao YL, Gao X et al (2012) Fecal Calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis 18:1894–1899 [DOI] [PubMed] [Google Scholar]
- Marrero JA, Feng Z, Wang Y et al (2009) Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 137(1):110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumolo MG, Bertani L, Ceccarelli L et al (2018) From bench to bedside: Fecal Calprotectin in inflammatory bowel diseases clinical setting. World J Gastroenterol 24(33):3681–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh J, Stein I, Haag D et al (2009) S100A8 and S100A9 are novel nuclear factor kappa B target genes during malignant progression of murine and human liver carcinogenesis. Hepatology 50(4):1251–1262 [DOI] [PubMed] [Google Scholar]
- Pang Q, Qu K, Bi JB et al (2015) Thrombocytopenia for prediction of hepatocellular carcinoma recurrence: Systematic review and meta-analysis. World J Gastroenterol 7 21(25):7895–7906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Fan J, Yang XR et al (2012) Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol 13(8):817–826 [DOI] [PubMed] [Google Scholar]
- Soyfoo MS, Roth J, Vogl T et al (2009) Phagocyte-specific S100A8/A9 protein levels during disease exacerbations and infections in systemic lupus erythematosus. J Rheumatol 36:2190–2194 [DOI] [PubMed] [Google Scholar]
- Striz I, Trebichavsky I (2004) Calprotectin—a pleiotropic molecule in acute and chronic inflammation. Physiol Res Acad Sci Bohemoslovaca 53(3):245–253 [PubMed] [Google Scholar]
- Yong HY, Moon A (2007) Roles of calcium-binding proteins S100A8 and S100A9 in invasive phenotype of human gastric cancer cells. Arch Pharm Res 30(1):75–81 [DOI] [PubMed] [Google Scholar]
- Yuen MF, Tanaka Y, Fong DY et al (2009) Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol 50:80–88 [DOI] [PubMed] [Google Scholar]
- Ziada DH, El Sadany S, Soliman H et al (2016) Prevalence of hepatocellular carcinoma in chronic hepatitis C patients in mid delta, Egypt: a single center study egypt. Natl Canc Inst 28(4):257–262 [DOI] [PubMed] [Google Scholar]