Abstract
Purpose
In the present study, we have systematically examined the clinical significance of Nectin-4 (encoded by the PVRL-4 gene), a marker for breast cancer stem cells (CSCs), in cancer metastasis and angiogenesis using a variety of human specimens, including invasive duct carcinoma (IDC) with multiple grades, several types of primary tumors to local and distant relapses, lymph node metastases and circulating tumor cells (CTCs).
Methods
Nectin-4 was overexpressed in more than 92% of samples with 65.2% Nectin-4-positive cells. The level of expression was increased with increasing tumor grade (GI–III) and size (T1–4) of IDC specimens.
Results
More induction of Nectin-4 was noted in relapsed samples from a variety of tumors (colon, tongue, liver, kidney, ovary, buccal mucosa) in comparison to primary tumors, while paired adjacent normal tissues do not express any Nectin-4. A high expression of Nectin-4 along with other representative markers in CTCs and lymph node metastasis was also observed in cancer specimens. An increased level of Nectin-4 along with representative metastatic (CD-44, Sca1, ALDH1, Nanog) and angiogenic (Ang-I, Ang-II, VEGF) markers were noted in metastatic tumors (local and distant) in comparison to primary tumors that were correlated with different grades of tumor progression. In addition, greater expression of Nectin-4 was observed in secondary tumors (distant metastasis, e.g., breast to liver or stomach to gall bladder) in comparison to primary tumors.
Conclusion
Our study demonstrated a significant correlation between Nectin-4 expression and tumor grade as well as stages (p < 0.001), suggesting its association with tumor progression. Nectin-4 was overexpressed at all stages of metastasis and angiogenesis, thus appearing to play a major role in tumor relapse through the PI3K–Akt–NFκβ pathway.
Electronic supplementary material
The online version of this article (10.1007/s00432-019-03055-2) contains supplementary material, which is available to authorized users.
Keywords: Nectin-4, Metastasis, Angiogenesis, Circulating tumor cells, Cancer relapse
Purpose
Metastasis and angiogenesis are the two main obstacles for treatment of cancers. Metastasis is a complex process by which malignant cancer cells spread into other parts of the body and form secondary tumors that become more resistant to chemotherapeutic drugs (Jiang et al. 2015; Melzer et al. 2017). Although surgical resection and adjuvant therapy is capable of curing well-confined primary tumors, metastatic cancer is largely incurable because of its systemic spread and the resistance of disseminated tumor cells to current therapies (Valastyan and Weinberg 2011). One of the major causes of tumor relapse and resistance to therapy can be attributed to the presence of cancer stem cells (CSCs). These are a small population of cells residing within the bulk of tumors. CSCs are involved in the phenotypic epithelial-to-mesenchymal transition (EMT) and mesenchymal-to-epithelial transition (MET), exhibit self-renewal ability, and possess high DNA repair and drug-efflux capabilities (Klonisch et al. 2008; Siddharth et al. 2016; Brabletz 2012; Vitale et al. 2017; Borah et al. 2015; Rosa et al. 2016). Cancer metastasis initiates with invasion of tumor cells through the wall of blood/lymph vessels with subsequent spread to distant sites via formation of numerous new vascular networks, a process known as angiogenesis (Paduch 2016). Tumor-secreted angiogenic factors activate nearby endothelial cells to release certain proteases which degrade the basement membrane and allow endothelial cells to migrate, proliferate and form new vessels (Neve et al. 2014). The angiogenic switch is a critical and important step in the transition of a dormant tumor to a malignant phenotype (Petrovic 2016). Under hypoxic conditions, tumor cells promote the formation of new blood vessels by secreting various pro-angiogenic growth factors and by changing the expression of cell adhesion molecules, such as matrix metalloproteinases (MMPs) that facilitate cancer invasion and migration (Harris 2002; Stivarou and Patsavoudi 2015).
Previously, several markers of metastasis (e.g., CD44, Sca1, ALDH1, CXCR4, Oct 4 and c-Met) (Vaz et al. 2014; Blogowski et al. 2016) and angiogenesis (e.g., VEGF, TGF-β, cxcl2, angiopoietins (Ang-I and Ang-II) and iNOS) have been reported (Rajabi and Mousa 2017). Multiple drugs have been developed to target these markers in various types of cancers (Giordano et al. 2014; Zhao and Adjei 2015; Vasudev and Reynolds 2014; Ronnekleiv-Kelly et al. 2016). There are several Food and Drug Administration (FDA) approved breast cancer drugs (https://www.cancer.gov/about-cancer/treatment/drugs/breast) that are found to benefit patients with advanced tumors. However, the theoretical promise of anti-angiogenic agents has yet to be fully translated into beneficial medical practice. In particular, drugs either alone or in combination targeting metastatic and angiogenic processes in breast tumors are still lacking (Fakhrejahani and Toi 2014). The clinical failure of anti-angiogenic drugs against breast cancer could also be due to as yet undiscovered factors responsible for metastasis and angiogenesis. For example, the failure of Avastin (bevacizumab) to inhibit VEGF-mediated angiogenesis in metastatic breast cancer suggests that other factors must also be playing vital roles for these processes (Kieran et al. 2012). Thus, there is an urgent need for new drugs that can effectively target metastatic and angiogenic breast tumors.
Recently, using an in vitro cell culture system, we and others have shown that Nectin-4 (Uniprot accn# Q96NY8, encoded by the PVRL-4 gene), has a potential role in drug resistance, cancer cell growth, aggressiveness and metastasis (Das et al. 2015; Nishiwada et al. 2015; Zhang et al. 2016; Siddharth et al. 2017). Nectins are a family of four members (Nectin-1 to 4) Ca2+-independent immunoglobulin-like molecules, which contribute to cell–cell adhesion by heterophilic and homophilic interactions (Lattanzio et al. 2014). Nectins are connected to the actin cytoskeleton through F-actin binding protein (afadin), and thereby regulate several cellular activities ranging from movement, polarization, differentiation, during entry of viruses, and involve different cell adhesion molecules and signal transduction pathways (Baselga and Norton 2002). Nectin-4 expression is confined to the embryo and placenta, whereas the other three Nectins are widely expressed in normal adult tissues (Lattanzio et al. 2014; Fabre et al. 2002). Overexpression of Nectin-4 has been reported in several human cancers, including lung, ovarian, and breast (Fabre-Lafay et al. 2007). Further, expression of the soluble form of Nectin-4 can be a potent diagnostic marker for cancers (Athanassiadou et al. 2011; Derycke et al. 2010; Takano et al. 2009). Nectin-4 is also overexpressed in hepatocellular carcinoma (HCC) and can be used as a prognostic marker for HCC (Ma et al. 2016).
In in vitro studies, Nectin-4 has been shown to promote anchorage-independent cell growth by facilitating cell–cell attachment and maintaining transformed properties of breast cancer cells (Pavlova et al. 2013). A higher expression of epithelial markers and lower expression of mesenchymal markers in parental cell lines as compared to the Nectin-4-knock down cells suggests a significant role of Nectin-4 in the EMT/MET pathway (Boylan et al. 2017). In previous studies, a positive correlation between Nectin-4 with Ki-67 and VEGF has been linked with tumor cell proliferation and angiogenesis (Nishiwada et al. 2015). We have also reported that Nectin-4 promotes anchorage-dependent cell proliferation and is responsible for 5-fluorouracil (5-FU) resistance in colorectal cancer cells (Das et al. 2015). In addition, Nectin-4 is a CSC as well as EMT marker that is induced by Wnt/β-catenin signaling via the signaling PI3K/Akt axis (Siddharth et al. 2017). These and other studies used in vitro cell culture model to describe the role of Nectin-4 in cancer progression, invasiveness and metastasis; however, very few reports directly demonstrate the role of Nectin-4 in cancer biology in patients. In this study, we explored the role of Nectin-4 in cancer metastasis and angiogenesis using different types of patient-derived tumor biopsy specimens with different histological grades, benign and invasive, multiple metastasis (lymph node, local and distant metastasis), as well as recurrence conditions.
Materials and methods
Tissue specimens
Surgically resected specimens, both untreated primary breast carcinomas and paired adjacent normal breast tissues, were collected after modified radical mastectomy (MRM) from breast cancer patients enrolled in the outpatient department of Oncosurgery, Acharya Harihar Regional Cancer Centre, Cuttack, Odisha, India, after the approval of Institutional Ethics Committee (Ethical clearance approval Regd. # ECR/297/Inst/OR/2013). The age of the patients ranged from 28 to 85 years with a median range of 56 years. All the patients included in the study had invasive duct carcinoma (IDC) and their clinicopathological parameters are summarized in Table 1. In addition, tumor and adjacent normal tissues were also collected from patients with different types of cancers, such as oral (n = 32), ovary (n = 10), stomach (n = 8), renal (n = 7) and colon (n = 7) immediately after surgery. Tumor differentiation for breast cancer was classified according to the Scarff–Bloom–Richardson grading system (BR-score). The pathological tumor staging was evaluated by sixth edition of the tumor node metastasis (TNM) classification of the International Union Against Cancer. Both loco-regional and distant relapses were determined by radio-imaging and biopsy. In addition, blood samples were collected in EDTA vials from patients (n = 30) with or without metastasis for isolating circulating tumor cells.
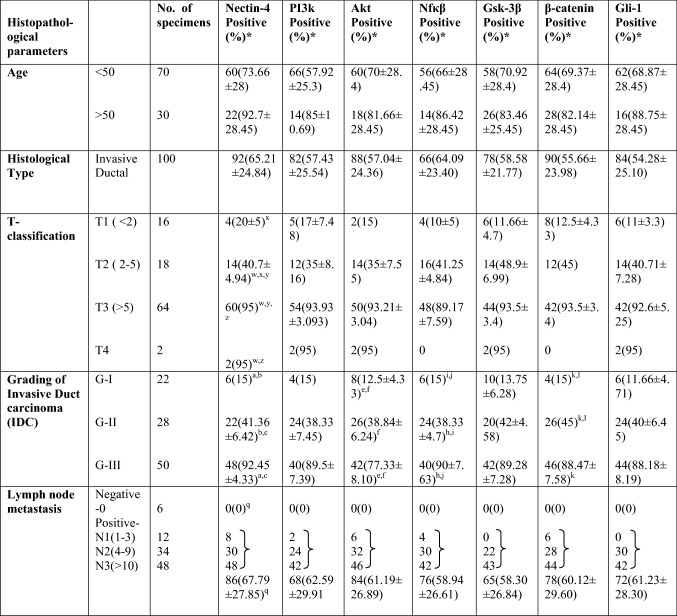
Table 1.
Expressions of different proteins in IDC specimens and their correlation with clinicopathological parameters
*Mean nuclear/cytoplasmic staining (%) ± SD. Statistical analysis were done using one way ANOVA for determining the correlation between clinicopathological parameters and protein expressions. Results were considered significant when p values were as follows: xp < 0.05, b,e,j,lp < 0.005 and a,c,f,h,k,w,y,z,qp < 0.0001
Histopathological study (H&E staining) and immunohistochemical (IHC) analysis
H&E staining and IHC were performed as described in our previous studies (Das et al. 2017). Paraffin-embedded specimens were sectioned at 5 µm thickness, mounted on slides, heated at 60 °C for 30–40 min, dewaxed with xylene and subjected to rehydration by immersing in graded series of alcohols (100%, 90% and 70%, respectively). The sections were dipped into haematoxylin followed by eosin stain and rinsed in water. The sections were dehydrated by immersing in increasing concentrations of alcohol (70%, 90%, and 100%). following xylene and acetone for 2 min each. The images were captured in bright-field microscope at 20× magnification (Leica DM200, USA).
For IHC, rehydrated tissue sections (3 µm thick) were washed in 1X PBS, and then the antigen was retrieved by citric acid buffer (pH 6). Non-specific site blocking and endogenous peroxide activity blocking was done by 5% fetal bovine serum (FBS) and hydrogen peroxide, respectively. Next, sections were incubated with primary antibody overnight at 4 °C. After three washes with 1X PBS, sections were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 30–60 min at room temperature. Sections were washed in 1X PBS and immunoreactivity was visualized using 3,3′-diaminobenzidine (DAB) peroxidase substrate kit (SK-4100, Vector Laboratories, CA, USA) and haematoxylin counter stain. Images were captured at 20X magnification using bright-field microscope (Leica DM2000, USA).
Evaluation of immunostaining
For evaluation of immunostaining, a semiquantitative assessment of both the intensity of staining and the percentage of positively stained cells was used. Total immunoreactivity average score (H-score) was the product of the staining percentage and intensity scores. The average H-score was calculated according to protocol described earlier (Li et al. 2017). A final total average H-score ≤ 3 and ≥ 3 was regarded as low and high expression, respectively. The percentage of stained cells was classified as 0 (0–10%), 1 (11–30%), 2 (31–50%), and 3 (51–100%). Staining intensity was defined as 0 (−/negative), 1 (+/weak), 2 (++/moderate) and 3 (+++/strong). All tissue sections were analyzed by two independent experienced pathologists who were blinded to both the clinicopathological data and results of each other.
Cell culture
Breast cancer (MDA-MB-231, MCF-7, cigarette smoke-transformed (MCF-10A-Tr), colorectal cancer (HCT-116), chemo-resistant (5-FU-R, CIS-R) and chemo-sensitive (H-357) cancer cell lines were maintained according to the protocol described earlier (Das et al. 2015; Satapathy et al. 2015). Briefly, breast cancer cell lines MDA-MB-231, MCF-7, cigarette smoke-transformed (MCF-10A-Tr), and colorectal cancer cell line, HCT-116 were cultured in DMEM supplemented with 10% FBS, 1.5 mM l-glutamine and 1% antibiotics (100 U/ml of penicillin, 10 mg/ml of streptomycin) in a humidified incubator in 5% CO2 at 37 °C. Chemo-resistant cell line, 5-FU-R, sensitive (H-357) and cisplatin-resistant (CIS-R) oral cancer cell lines were maintained according to the protocol described earlier (Das et al. 2015; Sfiligoi et al. 2003). Ficoll-400 (F4375) was obtained from Sigma-Aldrich (St. Louis, USA). Anti-Nectin-4 (#ab57873), anti-CD-44 (#ab23557), anti-Oct4 (#ab109183) and anti-Afadin (#ab90809) antibodies were purchased from Abcam (MA, USA). Anti-Akt (#9272), anti-PI3K (#4292), anti-β-catenin (#9587), anti-c-myc (#9402), anti-cyclin D1 (#2922), anti-p53 (#9282) and anti-Ang-II (#2948) antibodies were purchased from Cell Signaling Technology (MA, USA). Anti-VEGFA (MAA143HU21) and anti-Ang-I (MAA008HU21) antibodies were obtained from Cloud-Clone Corp. (TX, USA). Anti-Nanog (sc-293121), anti-ALDH1 (sc-166362) and anti-GAPDH (sc-25778) antibodies were purchased from Santa Cruz Biotechnology Inc. (CA, USA).
Western blot analysis
Tumor and normal tissue samples were lysed by modified RIPA lysis buffer using a tissue homogenizer. Following lysis, western blot analysis was performed as described in our earlier studies (Satapathy et al. 2015). Briefly, 60 µg of protein was loaded and separated by SDS-PAGE. Proteins were transferred onto PVDF membrane and probed with specific antibodies as per manufacturer’s protocol. Each blot is a representative of three independent experiments. The band intensity of each lane was measured using UVPGelDoc-It®310 and represented by numerical values above proteins band in each panel.
Reverse transcriptase polymerase chain reaction (RT-PCR)
Total cellular RNA was extracted from cell lines, frozen tumors and adjacent non-tumor tissues using TRIzol reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. The cDNA was synthesized from 1 µg of total RNA using the R2D First Strand cDNA Synthesis Kit (GCC Biotech, Kolkata, India). Amplification of a 144-base pair sequence and 266-base pair sequence analogous to Nectin-4 and GAPDH, respectively, was performed with the following primers: Nectin-4 = forward 5′-TGCTCAAGTGCCTGAGTGAA-3′ and reverse 5′-AGACGTAGATGCCGCTGTG-3′ and GAPDH = forward 5′-GAAGGTGAAGGAGTC-3′ and reverse 5′-GAAGATGGTGATGGGATTTC-3′. Messenger RNA (mRNA) expression of Nectin-4 was performed using Hi-G9 taq DNA polymerase. The cycling conditions were as follows: an initial denaturation step of 95 °C for 5 min, followed by 35 amplification cycles involving denaturation at 95 °C for 30 s, annealing at 50 °C for 45 s, extension at 72 °C for 30 s, and final extension at 72 °C for 5 min. Expression of GAPDH is used as loading control to check the equal loading of sample in each lane. The fold-change in the expression of mRNA levels of Nectin-4 is calculated with respect to GAPDH. The amplified product was separated on 1% agarose gel and the intensity of each sample was measured using UVP imaging system (UVP, Cambridge, UK).
Isolation of circulating tumor cells
Circulating tumor cells (CTCs) from patients’ blood were isolated as per the protocol described earlier (Kallergi et al. 2016). Briefly, blood was drawn into EDTA-containing tube and subjected to Ficoll (1.077 g/ml) density gradient centrifugation at 200×g for 20 min at RT (15–25 °C). The upper plasma layer was carefully removed and discarded. The mononuclear/lymphocyte cell layer at the plasma–Ficoll interface was transferred to a new tube, washed twice in 1 X PBS and re-diluted with 1 X PBS and centrifuged at 230×g for 10 min at room temperature. The supernatant was discarded and the cells were resuspended with RBC lysis buffer (NH4Cl, NaHCO3 and EDTA) and incubated for 10 min at room temperature to lyse erythrocytes. Then, diluted with 1 X PBS and centrifuged at 230×g for 10 min. The supernatant was discarded and the pellet was resuspended in 1 X PBS.
Enzyme-linked immunosorbent assay (ELISA)
Expression of Nectin-4 and other proteins were assayed in circulating tumor cells (CTCs) using indirect ELISA. The experiment was performed as per the protocol mentioned earlier (Siddharth et al. 2016). Briefly, the protein antigen was mixed with coupling buffer, coated onto 96-well microplates and incubated overnight at 4 °C. Then, cells in each well were washed with wash buffer followed by blocking with blocking solution (1% BSA in PBST) and incubated at room temperature for 30 min. Cells were washed with PBST and incubated with HRP conjugated secondary antibody for 1 h. After washing with PBST, substrate solution (2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonic acid) was added and the absorbance of the colored product was measured using microplate reader at 405 nm.
Statistical analysis
Correlation between markers and clinicopathological parameters was determined using Chi square analysis. The statistical significance was calculated using GraphPad Prism version 5 Software (La Jolla, CA, USA). The results were represented as a mean ± SD of multiple independent experiments. Analysis of data was done by ANOVA and paired t test wherever applicable. The statistical significance ‘p’ values are mentioned in respective figure legends and in table.
Results
Nectin-4 expression associates with tumor invasiveness
To understand the relationship between Nectin-4 expression and the invasiveness of the tumor, we monitored the expression of Nectin-4 along with representative tumor invasiveness markers in breast tissue from patient samples using IHC analysis. For this study, we collected 100 invasive duct carcinoma (IDC) specimens of different histological grades (Grade I–III; G-I: n-22, G-II: n-28 and G-III: n-50) along with their paired adjacent normal tissues. The detailed clinicopathological parameters of each patient sample and other information (e.g., age, tumor size, grade and lymph node metastasis) are provided in Table 1. The number of carcinoma specimens and the percentage of tumor cells displaying Nectin-4, PI3K, Akt, NFκβ, GSK-3β, β-catenin and Gli-1 expression were significantly associated with the tumor grade (Table 1) (Benvenuto et al. 2016). H&E staining of all IDC samples showed characteristic cancerous nature and marked differences in paired adjacent normal tissues (Fig. 1a). The IHC staining of each slide was evaluated by calculating the average H-score in total number of samples taken according to the procedure described in materials and methods. The average H-score of the relative expression of representative proteins in different grades is plotted in graphs on the right side of each panel of Fig. 1b–h. The expression was observed in all tested markers, such as Nectin-4, PI3K, NFκβ, Akt, GSK-3β, Gli-1 and β-catenin. All samples examined showed positive staining, and a great majority overexpressed the following: Nectin-4 (92%), PI3K (82%), Akt (88%), NFkβ (66%), Gsk-3β (78%), β-catenin (90%) and Gli-1 (84%). It was also noted that the expression of each marker increased with the increasing grade (G-I to G-III) of tumors with negligible expression in their normal counterparts (Fig. 1). Of the 100 IDCs examined, approximately 6/22 (28%) G-I, 22/28 (77%) G-II, and 48/50 (92.8%) G-III, respectively, showed positive Nectin-4 staining. The mean percentage of cells showing Nectin-4 staining was 65.2%, which was correlated with tumor grade. Significant association was observed between Nectin-4 expression and histopathological grading (p < 0.001) in IDCs (Table 1). Similar trends of significant association were also noted for other markers with histological gradings; such as PI3K, Akt, NFκβ, GSK-3β, β-catenin and Gli-1 (p < 0.001) in IDCs as mentioned in Table 1. Thus, expression of proteins associated with tumor aggressiveness positively correlated with grades of tumors where Nectin-4 expression was significantly higher than other proteins (Fig. 1).
Fig. 1.
Expression of representative tumor invasive markers in different grades of invasive duct carcinoma (IDC) specimens. a H&E staining of normal and different grades (I–III) of 100 IDCs. b–h Expressions of different proteins analyzed by IHC of paired adjacent normal tissue and different grade (I–III) of IDCs. The bar graphs on right side of IHC images indicate the average H-score of the staining. Data was the mean ± SD of twenty independent experiments. Statistical significance was determined by paired t test (*p < 0.05), (**p < 0.01), (***p < 0.001). The IHC images were captured at ×20 magnification under bright-field microscope and was one of the representatives of multiple independent experiments (n > 20). Scale bar represents 20 µm
Histopathological evaluation showed that most of the patients exhibited positive Lymph node metastasis except six [lymph node negative (N0)]. Immunostaining revealed positive staining (mean percentage) of cells for Nectin-4 (67.79%), PI3K (62.59%), Akt (61.19%), NFκβ (58.94%), GSK-3β (58.30%), β-catenin (60.12%), and Gli-1 (61.23%) in lymph node-positive samples (Table 1). All the markers presented a significant association with lymph node-positive samples (p < 0.005 or p < 0.001). No association was found between Nectin-4 expression and lymph node negative samples (Table 1).
Further, tumor size was also correlated with Nectin-4 expression as well as other above-mentioned tumor associated markers. For the samples from the few patients in this study with T4 type tumor, all expressed Nectin-4 and the above-mentioned tumor associated markers (p < 0.001). Samples with T3-type tumors revealed Nectin-4 expression in 60/64 (93.75%) of IDCs with 95% of positively stained cells (p < 0.05). T2- and T1-types displayed Nectin-4 expression in 14/18 (77.77%) and 4//16 (25%) of IDCs with a mean cytoplasmic staining (positively stained cells) of 40.7% and 20% (p < 0.005), respectively. Thus, the above data showed that Nectin-4 expression increased not only with increasing grade but also with increasing tumor size (Table 1).
Nectin-4 promotes breast cancer progression mediated through PI3K–Akt signaling in clinical specimens
Using an in vitro cell culture model, we have previously demonstrated that Nectin-4 plays a role in maintenance of self-renewal of cells through Wnt/β-catenin signaling via the PI3K–Akt axis (Siddharth et al. 2017). However, there is no systematic study of Nectin-4 in cancer relapse, invasiveness and angiogenesis directly with clinical samples. To address the issue, we monitored by western blotting the protein expression of Nectin-4 along with other representative cancer markers and their corresponding signaling components in different grades of IDCs and paired adjacent normal tissues of six individual patients, respectively. The expression of PI3K and Akt was increased by 3.8- and 3.0-fold in G-III, followed by 2.5-fold in G-II and 1.5-fold in G-I tumor as compared to paired adjacent normal tissues (p < 0.05) (Fig. 2a.i). The expression of NFκβ and GSK-3β was also increased by more than 4.0-fold in G-III tumor (p < 0.05), followed by 2.5-fold in G-II (p < 0.005), and more than 1.5-fold in G-I tumor, respectively (p < 0.05) (Fig. 2a.i). The expression of β-catenin and Gli-1 was increased by more than threefold in G-III tumor (p < 0.001), followed by a 2.5- and 1.8-fold in G-II, and by only 2.0- and 1.4-fold in G-I tumor (p < 0.05), respectively (Fig. 2a.i). However, the expression of Nectin-4 was seven and fivefold higher in G-III and G-II (p < 0.001 and p < 0.001), respectively, as compared to G-I tumor (p < 0.05). In stark contrast, none of the paired adjacent normal samples examined showed any expression of Nectin-4 (Fig. 2a.i). The fold-change in expression of each protein in all three grades and the fold-change in expression of those proteins in different histological grades (G-I, G-II and G-III) as well as their statistical significance are shown in Supplementary Figures 1 and 2. Thus, these results indicate that increased Nectin-4 expression is correlated to histological grades and is overexpressed in aggressive/poorly differentiated tumors.
Fig. 2.
Nectin-4 is linked with tumor progression. Relative expressions of representative tumor proliferation markers in relapsed specimens were measured by western blotting. a.i Normal tissue and different grades (I–III) tumor specimens. a.ii Normal tissue, primary and relapsed tumor specimens. GAPDH served as loading control. The numerical values above each blot were the relative fold-change with respect to the control measured by densitometer. Blot was the representation of three independent experiments of six different samples taken from six patients. b Overexpression of Nectin-4 mRNA in recurrent tumors: b.i Nectin-4 mRNA levels in drug-sensitive and in resistance (5-FU-R and CIS-R) breast cancer cell lines. b.ii Nectin-4 mRNA levels in different types of cancers with paired adjacent normal tissue, primary and relapsed tumors, respectively. The GAPDH mRNA served as an internal control. Data was expressed as fold-change with either respect to lower Nectin-4 expressing cell lines or tumor tissues and was presented in numerical values above each image. The gel image was the representation of one the replicates of three different independent experiments. Samples were taken from six patients
Next, we measured the expression of all the above-mentioned proteins in normal, primary and local relapsed tumor samples from six individual patient. As shown in Fig. 2a.ii, the expression of PI3K, Akt, NFκβ, GSK-3β, β-catenin and Gli-1 was increased by 3.5- to 6.8-fold (p < 0.05) and 1.2- to 3.0-fold (p < 0.05) in recurrent and primary tumors, respectively, as compared to corresponding paired adjacent normal tissues. The expression level of Nectin-4 was increased by more than 6.8-fold in relapsed as compared to primary tumors and paired adjacent normal tissues (p < 0.001) (Fig. 2a.ii). Thus, these results suggest that Nectin-4-mediated cancer progression may utilize GSK-3β/β-catenin (Wnt–Tcf pathway) and Gli-1 (Hedgehog pathway) signaling cascades for cell-renewal that also requires the PI3K–Akt–NFκβ signaling cascade.
RT-PCR was employed to examine the Nectin-4 gene expression at the mRNA level in different cancer cell lines: breast (MCF-7, MDA-MB-231 and MCF-10AT), oral [H-357 and cisplatin resistance H-357 (CIS-R)], colon [HCT-116 and 5-fluorouracil resistant HCT-116 (5-FU-R)] along with multiple primary and relapsed tumor samples. As expected, there was no Nectin-4 mRNA expression in MDA-MB-231 cells which have lost the Nectin-4 gene. Elevated Nectin-4 mRNA was observed in the cigarette smoke-transformed breast epithelial cell line, MCF-10AT (Fig. 2b.i). About 2.0- and 3.5-fold higher expression of Nectin-4 mRNA was noted in MCF-7 and MCF-10AT cells, respectively, as compared to the control MDA-MB-231 cells (p < 0.05) (Fig. 2b.i). Furthermore, the expression of Nectin-4 mRNA in two drug resistant cell lines, 5-FU-R and CIS-R, was 6.5- and 5.5-fold, respectively, significantly higher than their parental control HCT-116 and H-357 cell lines, respectively (p < 0.05) (Fig. 2b.i).
Additionally, Nectin-4 mRNA levels were assessed in various recurrent tumors along with their primary cancer and normal tissue counterparts, such as breast, tongue, renal, buccal mucosa, colon, ovary and inguinal node of six individual patients. Interestingly, an increase of more than 5.0-fold was detected for Nectin-4 mRNA in all of the recurrent tumors compared to their primary tumors, with no expression in paired adjacent normal tissues (p < 0.05) (Fig. 2b.ii). These results thus imply that Nectin-4 may be responsible for drug resistance and relapse of tumors.
Increased level of Nectin-4 expression is associated with cancer recurrence
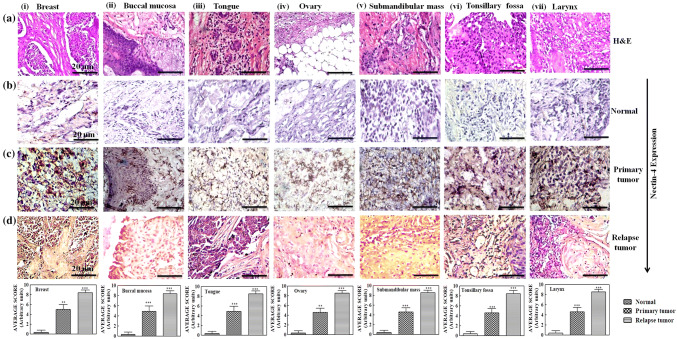
From the above results, it appears that Nectin-4 may have a potential role for breast cancer progression and relapse. Next, we extended our studies to other cancers, including ovary, buccal mucosa, tongue, sub-mandibular mass, tonsillary fossa and larynx. We took three specimens of each patient’s paired adjacent normal tissue, primary, and relapsed tumors of six individual patients. H&E staining clearly distinguished the normal and tumor samples (Fig. 3). Increased Nectin-4 expression was observed in recurrent IDC (p < 0.001) as compared to the primary tumors (p < 0.005) and their paired adjacent normal tissues (Fig. 3). The average H-scoring of Nectin-4 expression in histological sections of different type of cancers is also represented as bar graphs below each panel in Fig. 3. All cancer samples showed elevated Nectin-4 expression, which was approximately threefold higher in all types of relapsed tumors compared to their primary tumors (p < 0.005). However, we did not observe Nectin-4 expression in paired adjacent normal samples of the six relapsed specimens (Fig. 3).
Fig. 3.
Overexpression of Nectin-4 in different recurrent tumors. Columns i–vii show the expression of Nectin-4 in different types of recurrent tumors. H&E staining of different type of recurrent tumors (row ‘a’). IHC expression of Nectin-4 in normal tissue, primary and relapse tumor (rows ‘b–d’), respectively. The bar graph below each column shows the average H-score of IHC slides. Data were the mean ± SD of six different experiments. The images were captured by bright-field microscopy at ×20 magnification. The image shown was the one of the best representative of six different experiments of six specimens taken from each type of cancers. Statistical significance was determined by paired t test (*p < 0.05), (**p < 0.01), (***p < 0.001). Scale bar represents 20 µm
Involvement of Nectin-4 in metastasis and angiogenesis
Though the angiopoetins Ang-I and Ang-II both are expressed at appreciable levels in the majority of breast cancers, Ang-II shows explicit association with tumor aggressiveness. Thus, Ang-I and Ang-II can be effective predictors for recurrence of both node-positive and node-negative breast cancers (Sfiligoi et al. 2003). Despite the fact that lymph node status is considered an important prognostic indicator of cancer metastasis and/or advanced staging of cancer, most instances of lymph node-negative breast cancer still relapse. In this study three samples, matched primary tumor, axillary lymph nodes (ALNs) with metastasis and normal tissue were taken from six IDC patients and immunohistochemical analysis was done (Lattanzio et al. 2014). Our IHC data indicate that the primary tumor and the ALNs express Nectin-4 in addition to the angiogenic elements, Ang-I and Ang-II (Fig. 4a). Significant association was observed between Nectin-4 expression and ALNs (p < 0.005). The expression of Ang-I was lower in primary tumors and ALNs as compared to the expression of Ang-II (p < 0.05) (Fig. 4a). More than 60% and 30–40% of cells showed Ang-II and Ang-I positive cells in ALNs, respectively. Positive association was also noted between ALNs and Ang-II (p < 0.005) as well as with Ang-I (p < 0.05). The primary tumor tissue displayed 50% of Ang-II and 30% of Ang-I positive cells (p < 0.05). The paired adjacent non-tumor tissue showed very little expression of Ang-II and Ang-I. Taken together, since Nectin-4 is expressed in both metastatic and angiogenic tumors, it can be a bonafide marker for metastasis and angiogenesis in patients with IDC, while Ang-I and Ang-II status are likely a marker of angiogenesis only.
Fig. 4.
Role of Nectin-4 in angiogenesis and metastasis. a Expressions of Ang-I and Ang-II in axillary lymph nodes (ALNs) metastasis and breast cancer (a) histopathology of H&E staining of ALNs. Rows b–d show the expression of Nectin-4, Ang-I and Ang-II by IHC analysis in different types of samples. The bar graphs on the right side of IHC data indicate the average H-score of IHC. Data was the mean ± SD of six independent experiments. Statistical significance was determined by paired t test: (*p < 0.05), (**p < 0.01), (***p < 0.001).The images were taken by bright-field microscopy at ×20 magnification. Scale bar represents 20 µm. Samples taken from six patients. b Western blot analysis showing the expressions of Nectin-4, Afadin, NFκβ and Akt in ALNs and cancer samples. GAPDH served as loading control. Blot was the one of the representation of three independent experiments. Samples were taken from six patients. c Expressions of representative metastasis and angiogenesis markers in different local and distant relapse as well as different grades of samples. GAPDH served as the loading control. The numerical values above each blot as determined by densitometry represent fold-change with respect to the control. Blot was the one of representation of three independent experiments. Samples were collected from six patients. d Expression of metastatic markers of circulating tumor cells (CTCs). ELISA showing the expressions of CSC markers, d.i ALDH1, d.ii CD44 and d.iii Nectin-4 with CTCs from patients with different metastatic cancers. Data were the mean of three independent experiments. Data were the mean ± SD of three independent experiments Statistical significance was determined by paired t test: (*p < 0.05), (**p < 0.01), (***p < 0.001)
To explore the role of Nectin-4 in migration and proliferation of cancer cells, the expression of Afadin, NFκβ and Akt was evaluated in paired adjacent normal tissues, primary tumor and ALNs of IDC specimens (n = 6) by western blotting. Afadin binds to Nectins via actin filaments leading to cell–cell adhesion and tight junction formation (Takai and Nakanishi 2003). In these experiments, we observed a more than 3.0-fold decrease of Afadin in ALNs as compared to the primary tumors (p < 0.05) (Fig. 4b). This indicates the loss of cell–cell adhesion junctions, thereby regulates EMT in metastatic tumors that overexpress Nectin-4 (6.0-fold activation as compared to the primary tumor) (p < 0.05) (Fig. 4b). A more than threefold increased expression of the transcription factor NFκβ, and serine/threonine kinase Akt, which plays a critical role in preventing apoptosis and enhancing cell survival, was found in ALNs as compared to their primary tumors (p < 0.05) (Fig. 4b).
Since our previously published in vitro data suggested that Nectin-4 can be a marker for breast CSCs and plays a major role in metastasis (Siddharth et al. 2017), in this study we examined whether this also holds true in clinical samples. For these experiments, we obtained samples from different grades (GI–III), local (ALNs) as well as distant relapse sites from a total of six specimens, to measure the expression of representative metastatic and angiogenic marker proteins. The expression of CSC markers (Sca-I, CD44, Oct-4, Nanog and ALDH-1) and angiogenic markers (VEGFA, Ang-I and Ang-II) in relapsed tumors were significantly greater than the primary tumors and the paired adjacent normal tissues (Fig. 4c.i). The expression of Sca-I, ALDH-1 and Nanog was increased by 4.0-fold and 2.5 in relapsed samples (p < 0.05) as compared to the primary tumors without showing any expression in normal tissues. The expression of CD44 and Oct-4 was increased by 4.2- and 3.2-fold in relapsed tumors (p < 0.05), followed by primary tumors that showed activation by 3.5- and 2.6-fold (p < 0.05), respectively, as compared to the paired adjacent normal tissues. The expression of the above proteins in normal samples was very low or negligible (Fig. 4c.i). VEGFA and angiopoetins (Ang-I and Ang-II) showed associated expression. Elevated expression of Ang-II and VEGFA (4.5- and 3.5-fold, respectively) and low expression of Ang-I was observed in relapsed tumors (p < 0.05) with a very low expression in primary tumors (p < 0.05) (Fig. 4c.i). Interestingly, the expression of Nectin-4 was 5.0-fold higher in relapsed tumors as compared to primary tumors (p < 0.005). The paired adjacent normal tissues showed no expression of Nectin-4 (Fig. 4c.i). Increased expression of metastatic (Sca-I, CD 44, Oct-4, Nanog and ALDH-1) and angiogenic (VEGFA, Ang-I and Ang-II) markers was markedly noticed in the most aggressive grade, G-III (p < 0.005), with decreased expression in G-II (p < 0.05), followed by G-I (p < 0.05) (Fig. 4c.ii). The expression of Nectin-4 was more than 5.0-fold higher in G-III (p < 0.005), followed by threefold in G-II, only 1.0-fold in G-I in IDCs (p < 0.05), and no expression in paired adjacent normal tissues (Fig. 4c.ii).
To further strengthen and ascertain the metastatic and angiogenic potential of Nectin-4 in these pathways, we measured the expression of above proteins in distant relapsed samples (DRS), their primary tumors and normal tissues of by western blotting. Similar to the above data, expression of stemness markers (Sca-I, CD 44, Oct-4, Nanog and ALDH-1) were increased by 3.8-, 4.2-, 3.2-, 4.0- and 4.0-fold, respectively, in DRS (p < 0.05) and elevated up to 2.0-, 1.2-, 2.6-,1.0- and 2.0-fold in primary tumors (p < 0.05) as compared to the paired adjacent normal tissues (Fig. 4c.iii). Angiogenic markers VEGFA and Ang-II were also increased to more than threefold in DRS as compared to their primary tumor or normal tissue (p < 0.05) (Fig. 4c.iii). Interestingly, the expression of Nectin-4 was significantly (5.5-fold) higher in DRS compared to primary tumor samples (p < 0.05) (Fig. 4c.iii). Taken together, these and the above results suggest that Nectin-4 may associate with metastatic and angiogenic pathways in breast cancer progression.
CTCs are the tumor cells released in the bloodstream, circulate through the body, and serve a pivotal role in metastasis. A correlation of EMT and CTCs for the formation of metastasis and angiogenesis has already been documented in a model system (Yu et al. 2013; Aktas et al. 2009). To further understand whether Nectin-4 is also a prominent component of CTCs, we measured the expression of Nectin-4 as well as other established CSC markers, such as CD44 and ALDH-1 by ELISA, in CTCs isolated from blood samples from patients with different primary and metastatic cancers. The study was carried out in at least three different patients with same type of cancer. An approximately 4.5- and 3.8-fold increase in ALDH-1 expression was noted in breast cancers that had metastasized to the liver and ovary, respectively (p < 0.05) (Fig. 4d.i). Further, a 3.0- and 2.5-fold elevation of ALDH1 expression was observed when a pancreatic tumor had metastasized to the liver, and a stomach tumor had metastasized to gall bladder (p < 0.05) (Fig. 4d.i). Similar trends were also found in the expression of CD44. Elevated expression of CD44 was noted in case of a breast tumor metastasis to liver (4.5-fold), a pancreatic tumor metastasis to liver (2.9), a breast tumor metastasis to ovary (3.0) and a stomach tumor metastasis to gall bladder (2.0) (p < 0.05) (Fig. 4d.ii). Interestingly, expression of Nectin-4 was 5.0- and 3.8-fold higher in breast cancers that had metastasized to liver and ovary, respectively, as compared to paired adjacent normal tissue (p < 0.05). There was a 3.5- and 2.5-fold elevation of Nectin-4 expression when a pancreatic tumor had metastasized to liver (p < 0.005) and a stomach tumor had metastasized to gall bladder (p < 0.05), respectively (Fig. 4d.iii). Thus, the expression of Nectin-4 was significantly higher in CTCs of metastatic cancers than the expression of ALDH-1 and CD44.
Using cell line model systems, we have previously shown that Nectin-4 physically interacts with the adaptor protein Afadin and increases cancer cell growth through PI3K–Akt–NFκβ signaling (Siddharth et al. 2017). To determine whether the same pathway is activated in different clinical cancer types with elevated Nectin-4 expression, we measured the expression of the major components of this signaling cascade by western blotting (Supplementary Figure 3). In agreement with our in vitro studies (Siddharth et al. 2017), the elevated/activated status of these major components suggests that Nectin-4 may cause cancer cell proliferation through PI3K–Akt–NFκβ signaling.
Discussion
Cancer cells follow a series of complex, sequential steps during metastasis to distant organs, which are mediated by selected genes of (Jin and Mu 2015): (1) Invasion and migration—metastasis begins when some cancer cells from primary tumors undergo an EMT that is controlled by genes including Twist, Slug, Snail and Zeb1. The loss of E-cadherins and gain of N-cadherins or vimentin, transition from CD44−/CD24+ to CD44+/CD24−, and expression of matrix metalloproteinases (MMPs) are the major phenomena of EMT. (2) Circulation—the metastatic cells transit in blood stream as circulating tumor cells (CTCs) and then attach to distant organs. These cells undergo EMT to MET transition with high heterogeneity in their morphological and phenotypic profile and grow as secondary tumors. (3) Extravasations—the increased expression of Ereg, MMP-1, MMP-2 and Cox-2 genes facilitate disruption of vascular junctions leading cancer cells to invade distant organs. (4) Survival—the disseminated tumor cells (DTCs) colonize at secondary sites by inducing the expression of certain cell survival genes, such as Cscl12 and Igf1. (4) Outgrowth—the surviving cancer cells modify the distant stroma and trigger intrinsic oncogenic signaling pathways for proliferation by triggering the expression of additional genes such as Pthrp, Jagg1, IL-8, IL-11, Cxcl1, Wnt and Notch. The risk of metastasis development increases with lymph node metastasis, large size of primary tumor, and loss of histopathological differentiation (grade), which are well known to be cancer prognostic markers (Page 1991). In the present study, we have for the first time systematically examined the role of the cell–cell adhesion molecule Nectin-4 in clinical samples of different types of cancers at different stages of progression, local and distant metastasis, lymph nodes and CTCs.
Using an in vitro model system, we and others have demonstrated the role of Nectin-4 in cancer cell proliferation, drug resistance and EMT/MET transition (Das et al. 2015; Nishiwada et al. 2015; Siddharth et al. 2017; Boylan et al. 2017). Although there are few studies describing the role of Nectin-4 in cancer invasiveness and metastasis in a specific type of breast cancer patients (Lattanzio et al. 2014; Ma et al. 2016), its mechanistic role in tumor progression, metastasis and angiogenesis with clinical samples has been lacking. Our present study has carefully evaluated Nectin-4 expression in a cohort of breast cancer samples including their primary and relapsed samples (both local relapse and distant relapse).
We used more than 100 IDC specimens of different grades (Grade I–III) with the corresponding relapsed samples to measure Nectin-4 expression along with other representative markers for cell aggressiveness. Nectin-4 was overexpressed in more than 92% of tumor samples. In 65.2% of Nectin-4 positive tumors, the expression was increased with increasing grade (from I to III) of tumor progression. We observed maximum expression of Nectin-4 in relapsed samples, with no expression in paired adjacent normal tissues from the same patient. Nectin-4 expression also positively correlated with tumor grade, tumor size and lymph node status. This percentage of Nectin-4 expression is within the range of previously published studies on breast carcinoma (Fabre-Lafay et al. 2007; Athanassiadou et al. 2011). These findings demonstrate that Nectin-4 overexpression is associated with more aggressive clinical behavior and presents a worse prognosis for patients. Our studies further suggest a link of Nectin-4 expression with the expression of other aggressive (Akt, PI3K, NFκβ and GSK-3β) and CSC representative (β-catenin and HH-Gli-1) markers that were increased with increased tumor grades and in relapsed tumors. Most likely, the role of Nectin-4 in tumor aggressiveness is dependent upon Wnt–Tcf-signaling of CSC self-renewal, which is supported by our earlier in vitro studies (Das et al. 2015).
To further validate the role of Nectin-4 beyond breast cancer progression, we measured Nectin-4 expression in other tumor types with primary and relapsed clinical specimens, such as ovary, tongue, colon and renal. The highest Nectin-4 expression was always observed in relapsed samples, whereas a moderate level in primary and no expression in normal samples support the hypothesis that Nectin-4 contributes to cancer progression. The elevated expression of Nectin-4 in association with the elevated expression of representative metastatic (CD44, Oct-4, Nanog, and ALDH1) and angiogenic (Ang-1, Ang-II, and VEGFA) markers in tumor samples with Grade-I to Grade-III, relapsed as well as distant metastases, suggests that Nectin-4 also contributes to metastasis and angiogenesis. Increased Nectin-4 in association with elevated VEGFA, Ang-I and Ang-II in lymph node samples also supports the idea that Nectin-4 contributes to metastasis and angiogenesis.
The increased Nectin-4 mRNA in several cancer cell lines, including drug resistant 5-FU-R and CIS-R cells, and in relapsed tumor samples when compared to primary tumors, and no expression in paired adjacent normal samples, provides further evidence concerning the contribution of Nectin-4 to tumor progression and metastasis. Next, our findings of elevated Nectin-4 expression, along with other CTC markers from the blood samples from patients with metastases, strongly suggest that Nectin-4 expression is associated with metastasis. Further, observations of the associated overexpression of PI3K–Akt–NFκβ pathway proteins in the wide tumor types examined in this study supports our in vitro data (Das et al. 2015) that Nectin-4 is associated with oncogenic Akt-PI3K-NFκβ signaling during tumor progression and metastasis.
In conclusion, Nectin-4 is predominantly expressed in tumors with the most aggressive histopathological grades and in relapsed tumors. The association of Nectin-4 with aggressive phenotypes and recurrent tumors suggests that Nectin-4 contributes to both metastasis and angiogenesis and does so by modulating key factors of their major signaling pathways. Finally, our findings in CTCs suggest that Nectin-4 contributes to EMT/MET transitions in clinical blood samples. Our data suggests that Nectin-4 is associated with all later and lethal stages of tumor progression including metastasis, angiogenesis, and relapse (Fig. 5). Thus, Nectin-4 expression should be used as a predictive marker for cancer diagnosis and can be a target for development of targeted therapeutics.
Fig. 5.
Schematic representation of metastasis and angiogenesis processes. The metastatic cascade is depicted starting from the primary tumor site to distant sites via (i) local invasion, (ii) intravasation, (iii) circulation, (iv) extravasation, (v) survival, (vi) outgrowth, and finally (vii) angiogenesis
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors thankfully acknowledge a Rajiv Gandhi National fellowship (RGNF) to CS. SM was supported by a fellowship from the Government of Ethiopia, Africa. Authors also acknowledge Science and Engineering Research Board, DST, Govt of India, for the funding of this research work. The authors sincerely thank Mark Zakshevsky, Department of Anatomy and Cell Biology, University of Florida, Gainesville, Florida, for proofreading the manuscript.
Author contributions
CS performed the majority of the experiments. KG provided samples, NR helped to perform pathological studies. DN, RP, SC and SM performed some of the experiments and statistical analysis. CNK conceived the idea, planned the experiments and wrote the MS. SN and MDW contributed to writing the manuscript.
Funding
The work is partially supported by DST-SERB project of CNK.
Compliance with ethical standards
Conflict of interest
All the authors listed have approved the manuscript and declare no conflict of interest.
Ethical approval
Study approval was obtained from Acharya Harihar Regional Cancer Centre, Cuttack, Odisha, India (Ethical clearance approval Regd. # ECR/297/Inst/OR/2013).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S (2009) Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 11:R46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanassiadou AM, Patsouris E, Tsipis A, Gonidi M, Athanassiadou P (2011) The significance of Survivin and Nectin-4 expression in the prognosis of breast carcinoma. Folia Histochem Cytobiol 49:26–33 [DOI] [PubMed] [Google Scholar]
- Baselga J, Norton L (2002) Focus on breast cancer. Cancer Cell 1:319–322 [DOI] [PubMed] [Google Scholar]
- Benvenuto M, Masuelli L, De Smaele E, Fantini M, Mattera R, Cucchi D, Bonanno E, Di Stefano E, Frajese GV, Orlandi A, Screpanti I, Gulino A, Modesti A, Bei R (2016) In vitro and in vivo inhibition of breast cancer cell growth by targeting the hedgehog/GLI pathway with SMO (GDC-0449) or GLI (GANT-61) inhibitors. Oncotarget 7:9250–9270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blogowski W, Bodnarczuk T, Starzynska T (2016) Concise review: pancreatic cancer and bone marrow-derived stem cells. Stem Cells Transl Med 5:938–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borah A, Raveendran S, Rochani A, Maekawa T, Kumar DS (2015) Targeting self-renewal pathways in cancer stem cells: clinical implications for cancer therapy. Oncogenesis 4:e177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan KL, Buchanan PC, Manion RD, Shukla DM, Braumberger K, Bruggemeyer C, Skubitz AP (2017) The expression of Nectin-4 on the surface of ovarian cancer cells alters their ability to adhere, migrate, aggregate, and proliferate. Oncotarget 8:9717–9738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T (2012) EMT and MET in metastasis: where are the cancer stem cells? Cancer Cell 22:699–701 [DOI] [PubMed] [Google Scholar]
- Das D, Satapathy SR, Siddharth S, Nayak A, Kundu CN (2015) NECTIN-4 increased the 5-FU resistance in colon cancer cells by inducing the PI3K-AKT cascade. Cancer Chemother Pharmacol 76:471–479 [DOI] [PubMed] [Google Scholar]
- Das S, Tripathi N, Siddharth S, Nayak A, Nayak D, Sethy C, Bharatam PV, Kundu CN (2017) Etoposide and doxorubicin enhance the sensitivity of triple negative breast cancers through modulation of TRAIL-DR5 axis. Apoptosis 22:1205–1224 [DOI] [PubMed] [Google Scholar]
- Derycke MS, Pambuccian SE, Gilks CB, Kalloger SE, Ghidouche A, Lopez M, Bliss RL, Geller MA, Argenta PA, Harrington KM et al (2010) Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker. Am J Clin Pathol 134:835–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre S, Reymond N, Cocchi F, Menotti L, Dubreuil P, Campadelli-Fiume G, Lopez M (2002) Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted C-C′-C″-D beta-strands of the nectin1 V domain. J Biol Chem 277:27006–27013 [DOI] [PubMed] [Google Scholar]
- Fabre-Lafay S, Monville F, Garrido-Urbani S, Berruyer-Pouyet C, Ginestier C, Reymond N, Finetti P, Sauvan R, Adelaide J, Geneix J et al (2007) Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer 7:73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhrejahani E, Toi M (2014) Antiangiogenesis therapy for breast cancer: an update and perspectives from clinical trials. Jpn J Clin Oncol 44:197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G, Febbraro A, Venditti M, Campidoglio S, Olivieri N, Raieta K, Parcesepe P, Imbriani GC, Remo A, Pancione M (2014) Targeting angiogenesis and tumor microenvironment in metastatic colorectal cancer: role of aflibercept. Gastroenterol Res Pract 2014:526178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL (2002) Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2:38–47 [DOI] [PubMed] [Google Scholar]
- Jiang WG, Sanders AJ, Katoh M, Ungefroren H, Gieseler F, Prince M, Thompson SK, Zollo M, Spano D, Dhawan P (2015) Tissue invasion and metastasis: molecular, biological and clinical perspectives. Semin Cancer Biol 35(Suppl):S244–S275 [DOI] [PubMed] [Google Scholar]
- Jin X, Mu P (2015) Targeting breast cancer metastasis. Breast Cancer (Auckl) 9:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallergi G, Politaki E, Alkahtani S, Stournaras C, Georgoulias V (2016) Evaluation of isolation methods for circulating tumor cells (CTCs). Cell Physiol Biochem 40:411–419 [DOI] [PubMed] [Google Scholar]
- Kieran MW, Kalluri R, Cho YJ (2012) The VEGF pathway in cancer and disease: responses, resistance, and the path forward. Cold Spring Harb Perspect Med 2:a006593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonisch T, Wiechec E, Hombach-Klonisch S, Ande SR, Wesselborg S, Schulze-Osthoff K, Los M (2008) Cancer stem cell markers in common cancers—therapeutic implications. Trends Mol Med 14:450–460 [DOI] [PubMed] [Google Scholar]
- Lattanzio R, Ghasemi R, Brancati F, Sorda RL, Tinari N, Perracchio L, Iacobelli S, Mottolese M, Natali PG, Piantelli M (2014) Membranous Nectin-4 expression is a risk factor for distant relapse of T1-T2, N0 luminal—a early breast cancer. Oncogenesis 3:e118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Zhang JF, Yao SM, Huang H, Zhang S, Zhao M, Huang JA (2017) Decreased expression of speckle-type POZ protein for the prediction of poor prognosis in patients with non-small cell lung cancer. Oncol Lett 14:2743–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Sheng Z, Lv Y, Liu W, Yao Q, Pan T, Xu Z, Zhang C, Xu G (2016) Expression and clinical significance of Nectin-4 in hepatocellular carcinoma. Onco Targets Ther 9:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer C, von der Ohe J, Hass R (2017) Breast carcinoma: from initial tumor cell detachment to settlement at secondary sites. Biomed Res Int 11:8534371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve A, Cantatore FP, Maruotti N, Corrado A, Ribatti D (2014) Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed Res Int 2014:756078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwada S, Sho M, Yasuda S, Shimada K, Yamato I, Akahori T, Kinoshita S, Nagai M, Konishi N, Nakajima Y (2015) Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J Exp Clin Cancer Res 34:30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paduch R (2016) The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol (Dordr) 39:397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page DL (1991) Prognosis and breast cancer. Recognition of lethal and favorable prognostic types. Am J Surg Pathol 15:334–349 [DOI] [PubMed] [Google Scholar]
- Pavlova NN, Pallasch C, Elia AE, Braun CJ, Westbrook TF, Hemann M, Elledge SJ (2013) A role for PVRL4-driven cell-cell interactions in tumorigenesis. Elife 2:e00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic N (2016) Targeting angiogenesis in cancer treatments: where do we stand? J Pharm Pharm Sci 19:226–238 [DOI] [PubMed] [Google Scholar]
- Rajabi M, Mousa SA (2017) The role of angiogenesis in cancer treatment. Biomedicines 5:34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnekleiv-Kelly SM, Burkhart RA, Pawlik TM (2016) Molecular markers of prognosis and therapeutic targets in metastatic colorectal cancer. Surg Oncol 25:190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa R, D’Amato V, De Placido S, Bianco R (2016) Approaches for targeting cancer stem cells drug resistance. Expert Opin Drug Discov 11:1201–1212 [DOI] [PubMed] [Google Scholar]
- Satapathy SR, Siddharth S, Das D, Nayak A, Kundu CN (2015) Enhancement of cytotoxicity and inhibition of angiogenesis in oral cancer stem cells by a hybrid nanoparticle of bioactive quinacrine and silver: implication of base excision repair cascade. Mol Pharm 12:4011–4025 [DOI] [PubMed] [Google Scholar]
- Sfiligoi C, de Luca A, Cascone I, Sorbello V, Fuso L, Ponzone R, Biglia N, Audero E, Arisio R, Bussolino F et al (2003) Angiopoietin-2 expression in breast cancer correlates with lymph node invasion and short survival. Int J Cancer 103:466–474 [DOI] [PubMed] [Google Scholar]
- Siddharth S, Das S, Nayak A, Kundu CN (2016) SURVIVIN as a marker for quiescent-breast cancer stem cells—an intermediate, adherent, pre-requisite phase of breast cancer metastasis. Clin Exp Metastasis 33:661–675 [DOI] [PubMed] [Google Scholar]
- Siddharth S, Goutam K, Das S, Nayak A, Nayak D, Sethy C, Wyatt MD, Kundu CN (2017) Nectin-4 is a breast cancer stem cell marker that induces WNT/beta-catenin signaling via Pi3k/Akt axis. Int J Biochem Cell Biol 89:85–94 [DOI] [PubMed] [Google Scholar]
- Stivarou T, Patsavoudi E (2015) Extracellular molecules involved in cancer cell invasion. Cancers 7:238–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Nakanishi H (2003) Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci 116:17–27 [DOI] [PubMed] [Google Scholar]
- Takano A, Ishikawa N, Nishino R, Masuda K, Yasui W, Inai K, Nishimura H, Ito H, Nakayama H, Miyagi Y et al (2009) Identification of Nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res 69:6694–6703 [DOI] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147:275–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudev NS, Reynolds AR (2014) Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis 17:471–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz AP, Ponnusamy MP, Seshacharyulu P, Batra SK (2014) A concise review on the current understanding of pancreatic cancer stem cells. J Cancer Stem Cell Res. 10.14343/JCSCR.2014.2e1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale I, Manic G, De Maria R, Kroemer G, Galluzzi L (2017) DNA damage in stem cells. Mol Cell 66:306–319 [DOI] [PubMed] [Google Scholar]
- Yu M, Bardia A, Wittner BS, Stott SL, Smas MDT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM et al (2013) Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339:580–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu S, Wang L, Wu Y, Hao J, Wang Z, Lu W, Wang XA, Zhang F, Cao Y et al (2016) A novel PI3K/AKT signaling axis mediates Nectin-4-induced gallbladder cancer cell proliferation, metastasis and tumor growth. Cancer Lett 375:179–189 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Adjei AA (2015) Targeting angiogenesis in cancer therapy: moving beyond vascular endothelial growth factor. Oncologist 20:660–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.