Summary
Background
Chikungunya virus (CHIKV) continues to cause explosive epidemics in Brazil. We investigated its transmission dynamics in Salvador, Brazil, to understand the factors driving its reemergence and spread.
Methods
In this epidemiological study, we analyzed by census tracts the chikungunya cases reported in Salvador during the 2019–2020 epidemics. We used SaTScan software to identify spatiotemporal clusters and assessed how census tract characteristics (socioeconomic, environmental, and prior chikungunya occurrence) influenced chikungunya incidence through a Bayesian spatial model using Integrated Laplace Approximation (INLA).
Findings
Citywide, 19,129 cases (mean age: 40.2, range: 0–112; male: 41.8%, female: 58.0%, non-binary: 0.2%) were reported between 2016 and 2020, with a significant increase in 2019 and 2020 (4549 and 13,071 cases, respectively). We found nine spatiotemporal clusters in 2019 and seven in 2020, with 17.2% (387 of 2252) overlap of census tracts between the two years. The chikungunya incidence by census tract was negatively correlated with income and vegetation but positively correlated with land surface temperature. The census tract level incidence in 2020 exhibited a non-linear correlation with the 2019 incidence; up to a certain level, the 2020 risk increased as the 2019 incidence increased, but when the 2019 incidence was extreme, the 2020 risk was reduced.
Interpretation
These findings suggest that CHIKV transmission is localized, even during what appeared to be a citywide epidemic, creating high-risk pockets within the city. Socioeconomic factors, environmental conditions, and prior chikungunya incidence, probably reflecting herd immunity, all influence case incidence.
Funding
Secretary of Health of Salvador, Federal University of Bahia, Oswaldo Cruz Foundation, National Council for Scientific and Technological Development, Foundation for Research Support of the Bahia State, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES), Clinical and Applied Research Network in Chikungunya, Global Virus Network, Burroughs Wellcome Fund, Wellcome Trust, and the United States National Institutes of Health.
Keywords: Chikungunya virus, Transmission dynamics, Epidemiology, Risk factors, Epidemic, Herd immunity, Spatiotemporal analysis, Incidence
Research in context.
Evidence before this study
Chikungunya epidemics in places with naive populations and abundant mosquito vectors have been characterized by numerous cases over a short period, often in spatially well-defined areas. However, the seroprevalence of antibodies specific against chikungunya virus (CHIKV) remains patchy in the Americas. CHIKV transmission dynamics that could lead to these heterogeneous spread patterns remain understudied in large urban centres. We searched in PubMed and Scielo without language restrictions from database inception up to September 30, 2024, for studies published with the terms “chikungunya spatiotemporal” or “chikungunya transmission dynamics”, or “chikungunya recurrence”. We found chikungunya transmission dynamics studies from Latin America, the Caribbean, Europe, and Asia. However, most studies on CHIKV transmission dynamics in urban areas were at the country or state levels, but not at the municipality or sub-municipality level. Investigations on recurrent CHIKV epidemics in the same urban setting are also limited.
Added value of this study
We report an epidemiological and spatiotemporal study that examined in high spatio-temporal resolution the transmission dynamics of chikungunya recurrence in Salvador, the fifth-most-populated city in Brazil. We show that chikungunya cases during the major epidemics of 2019 and 2020 exhibited spatial and temporal clustering, potentially reflecting the influence of factors that facilitate or impede CHIKV transmission and result in areas with varying levels of population immunity. Furthermore, we found correlations of chikungunya incidence at the census tract (statistical subdivisions of a municipality) level with demographic, socioeconomic, and environmental factors, as well as with the previous level of chikungunya incidence.
Implications of all the available evidence
Our findings suggest that chikungunya epidemics comprise smaller, synchronized or sequential outbreaks, with highly variable spatial distributions due to socioeconomic and ecological factors, as well as the previous level of chikungunya incidence, a proxy for population immunity. These results may contribute to chikungunya surveillance, prevention and control strategies, and immunization programs.
Introduction
Chikungunya virus (CHIKV) is a mosquito-borne alphavirus transmitted to humans by Aedes aegypti and Ae. albopictus, two globally widespread invasive mosquitoes.1 CHIKV infection typically causes an acute febrile illness with severe arthralgia affecting multiple joints, which can persist for months or longer in more than 30% of patients.2 Four CHIKV genotypes have been described: the West African, Asian, Indian Ocean and East/Central/South African (ECSA) lineages.1 These genotypes differ in their geographic distribution, and the diverse immune and pathophysiological responses elicited by them may modulate disease outcomes, resulting in variations in the severity and duration of symptoms.2 The disease can substantially impact the well-being and quality of life of the population affected,3 and can also lead to severe cases, with central nervous system involvement and death.4,5 In November 2023, a vaccine against chikungunya was approved in the United States of America, but immunization programs in the most affected countries may face delays in its deployment.
Since the 1950's CHIKV has been reported to cause small outbreaks in several African countries and later in Asia.1 In 2004, CHIKV reemerged in Kenya, then spread and caused larger epidemics in the Indian Ocean Basin, India and other parts of South Asia, the Indian Ocean Islands, and Southeast Asian countries.1 In late 2013, the Asian lineage of CHIKV was introduced into the Americas through the Caribbean region and then spread to continental American countries.1 In August 2014, a second CHIKV lineage (ECSA, the East-Central-South African) was identified in the Americas in Feira de Santana City, Bahia State, Brazil, probably introduced by a CHIKV-infected traveller returning from Angola.6 Since then, CHIKV has been causing continuous yearly epidemics, accumulating over 3.7 million cases in 50 countries or territories in the Americas up to 2023.4
Chikungunya epidemics have been characterized by numerous cases during a short period following the introduction of the virus into a naive population.4 However, the seroprevalence of specific antibodies against CHIKV remains patchy in the Americas.7 For example, one year after CHIKV was first detected in Managua City, Nicaragua, CHIKV seropositivity was estimated at 13.1% among persons aged ≥15 years,8 but a national survey conducted across the country in the same year found an overall seroprevalence of 32.8% on participants >2 years, ranging from 0.7 to 73% depending on the locality.9 In addition, seropositivity in Haiti after virus introduction reached 78.4%,10 suggesting that the magnitude of CHIKV spread can vary substantially in different settings. Populations with lower seropositivity levels allow chikungunya recurrence, which is defined as an increase in chikungunya incidence after years with lower case numbers.11 Conversely, a population with higher seropositivity can limit the potential for chikungunya recurrence because previous CHIKV infection can promote lifelong immunity and protect against re-infection and human amplification.7 However, the CHIKV transmission dynamics that could lead to these heterogenous CHIKV spread patterns remain understudied in large urban centers.12 Here, we combined epidemiological and spatiotemporal analyses to examine in high-resolution the transmission dynamics and socio-ecological factors of chikungunya recurrence in Salvador, the fifth-most-populated city in Brazil.
Methods
Setting context
The city of Salvador, the capital of the Bahia State, is a coastal metropolis located in the Northeast region of the country and 115 km from Feira de Santana, the city where the first chikungunya case involving the ECSA lineage was reported in Brazil in 2014, resulting in a large outbreak.6 Salvador has tropical weather and a 2.4 million population. The city is endemic to many human arboviral pathogens that undergo a human-amplified transmission cycle, such as Zika (ZIKV) and dengue viruses (DENV).13, 14, 15 Chikungunya cases in Salvador were first detected in early 201513,14 and the first epidemic peaked in August 2015. Still, it was probably overlooked because the social and public health attention was directed to an almost simultaneous, large Zika epidemic.16 A community-based seroprevalence study conducted within two years following the initial chikungunya epidemic in Salvador revealed that 11.8% of the 1776 participants had IgG antibodies against CHIKV.17 This finding indicates that approximately 90% of the Salvador population remained susceptible to CHIKV infection, leaving them vulnerable to future chikungunya epidemics. In the interim between the initial epidemic in 2015 and the recurrence of citywide epidemics in 2019 and 2020, Salvador experienced only limited CHIKV outbreaks in some streets or small areas.18
Study design, variables, and data sources
This spatiotemporal epidemiological study combined multi-data sources from surveillance notifications of chikungunya cases from 01 January 2016 to 31 December 2020 with socioeconomic, demographic and weather data from Salvador. The reported chikungunya cases were confirmed by laboratory or clinical-epidemiological criteria, as defined by the Brazilian Ministry of Health surveillance system.19 Laboratory confirmation criteria included viral isolation, detection of viral RNA by RT-PCR, or detection of IgM antibody during acute or convalescent illness in a patient with compatible clinical symptoms (acute onset fever and arthralgia). Clinical-epidemiological confirmation criteria included the presence of compatible symptoms unexplained by other clinical conditions in a person with an epidemiological link to a laboratory-confirmed case or living/visiting an area of endemic or epidemic CHIKV transmission. The individualized data on reported chikungunya cases were obtained from the National Notifiable Diseases Information System (Sistema de Informacão de Agravos de Notificacão, SINAN) from the Epidemiological Surveillance Division of the Salvador Health Secretary. Because the chikungunya cases detected in Salvador in 2015 were not reported to SINAN, they were not included in this study.
Reported chikungunya cases from the 2-year period of the recurrent chikungunya epidemics in Salvador (01/01/2019–31/12/2020) were georeferenced based on the patient address using the MMQGIS plugin in the QGIS software version 3.12 (QGIS Development Team, 2016) based on OpenStreetMap database (for software references, please refer to the Supplementary Material). Additionally, the case address geolocations were linked to the census tract. The geolocation of 980 chikungunya cases (5.6% of all cases reported between 2019 and 2020) had to be manually revised using Google Maps because the automatic geolocation did not match the expected neighbourhood of the cases. After these revisions, we were able to geolocate the addresses of 83.6% of the cases from 2019 to 2020. The remaining cases had incongruent or uncompleted addresses and were not included in the spatiotemporal analysis.
Census-tract-level data on demographics and socioeconomic characteristics of Salvador's population were obtained from the 2010 census compiled by the Brazilian Institute of Geography and Statistics (the most recent census with available data at the time of the study design).20 The following data were obtained for the 3584 city census tracts: head of the household mean income, proportion of literate household heads, proportion of residents >60 years of age, and proportion of each self-identified race/ethnicity. Also, we examined access to urban infrastructure, which includes the proportion of households with proper sewage disposal, the proportion with access to public water supply, and the proportion with a garbage collection service. Vector control data were unavailable at the census tract level and not included in the analysis.
Topographic variables, such as mean elevation in relation to sea level, land surface temperature (LST), and Soil-adjusted vegetation index (SAVI), were obtained from raster images downloaded from the United States Geological Survey (USGS) Earth Explorer database. The mean LST map was built with nine Landsat 8 satellite images (band 10, dates: March 13, 2016; October 15, 2016; January 6, 2018; May 14, 2018; August 2, 2018; October 6, 2018; December 8, 2018; January 25, 2019; and December 11, 2019). The SAVI map used a Sentinel II satellite image from August 16, 2019. The Sentinel II images were previously radiometrically calibrated to reflectance values at the top of the atmosphere and corrected atmospherically by the dark pixel method with the Semi-Automatic Classification tool in the QGIS software version 3.12.
Ethics statement
All study procedures followed the ethical standards for research involving human beings, defined by the resolution 466/2012 of the Brazilian National Health Council and were approved by the ethics committee of the Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Brazil (approval number CAAE 76294523.5.0000.0040). The ethics committee granted a waiver for the need to obtain consent from participants, as we analyzed an anonymized dataset of reported chikungunya cases in Salvador. The dataset contained no sensitive information, such as names or dates of birth, aside from residential addresses.
Statistical analysis
All analyses were carried out in R environment version 4.3.1 and maps were generated using QGIS software version 3.12. Descriptive analyses included the temporal distribution of the weekly number of chikungunya reported cases in Salvador between 2016 and 2020 and maps with the geolocation of the residence address of the reported cases and census tract incidences in 2019 and 2020. Annual incidences of reported cases of Chikungunya were calculated based on the estimated population of Salvador from 2016 to 2020, provided by the Brazilian Institute of Geography and Statistics.20 To detect clusters of the reported cases during the epidemics of 2019 and 2020, we performed space-time analyses using the SaTScan software (details in Supplementary Material). Also, spatial multivariable regression analyses using Integrated Nested Laplace Approximation (INLA) with a negative binomial distribution were applied to assess correlations between the accumulated risk of chikungunya in 2019 and 2020 per census tract and demographic, socioeconomic, and environmental variables (details in Supplementary Material). A backward selection method was manually applied to the INLA spatial model, comparing performance based on the Deviance Information Criterion (DIC) to select the best model (details in Supplementary Material). The regression analyses also assessed correlations between the observed levels of chikungunya incidence in 2019 and 2020.
Role of the funding source
The study's funders had no role in study design, data collection, data analysis, data interpretation, or writing of this report.
Results
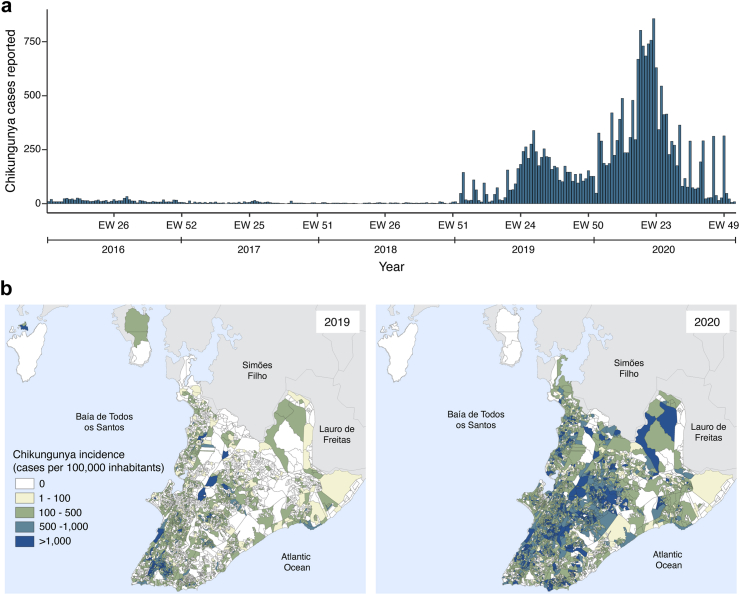
Between January 2016 and December 2020, 19,129 chikungunya cases were reported in Salvador (mean age: 40.2, range: 0–112; male: 41.8%, female: 58.0% and non-binary 0.2%; ethnicity not shown due to data incompleteness) (Fig. 1A). In 2016, 977 cases were reported (36.5 cases per 100,000 inhabitants). In 2017 and 2018, fewer cases were observed (349 and 183 cases; 13.0 and 6.8 cases per 100,000 inhabitants, respectively). In 2019, 4549 cases were reported (170.0 cases per 100,000 inhabitants), while in 2020, 13,071 cases were reported (488.5 cases per 100,000 inhabitants) (Fig. 1B). The incidence in 2019 and 2020 was 2.9 and 12.9 times higher than that of 2016, respectively. The epidemic peaks (>30% of cases per year) were between June and August 2019 and from April to June 2020, with 2210 and 8124 cases, respectively.
Fig. 1.
Spatiotemporal distribution of chikungunya in Salvador between 2016 and 2020. (A) Temporal distribution of the weekly number of chikungunya reported cases in Salvador, Brazil, between 2016 and 2020. (B) Annual incidence of chikungunya reported cases by census tract in 2019 and 2020.
The spatiotemporal analyses for the 2019 and 2020 epidemic waves included 3386 (74.4% of 4549) cases from 2019 to 11,341 (86.8% of 13,071) cases from 2020, which were geolocated in all 3516 census tracts where inhabitants were reported by the census (68 census tracts had no inhabitants reported and were excluded from the census tract level analyses) (Supplementary Figure S1). Based on the space-time permutation model, we identified sixteen significant spatiotemporal clusters, nine during the 2019 epidemic and seven during the 2020 epidemic (Fig. 2).
Fig. 2.
Spatiotemporal distribution of the identified chikungunya cases clusters during (A) 2019 and (B) 2020 in Salvador, Brazil.
The nine 2019 clusters affected 20.6% (739) of the 3584 census tracts, exposing a population of 533,491 inhabitants (Fig. 2A and Table 1). The median number of cases in each of these clusters was 61 (interquartile range [IQR]: 31–268), with a median attack rate of 269 cases per 100,000 inhabitants (IQR: 159–457). Their median duration was 104 days (IQR: 59–146), and six of them started between May and June.
Table 1.
Clusters of chikungunya reported cases detected in Salvador City in 2019 and 2020.
| Cluster identification number and year | Cluster start date | Duration (days) | Populationa | Area (km2) | Number of reported chikungunya casesb | Expected number of chikungunya casesb | Relative riskc | Attack Rate (Cases per 100,000 pop)d |
|---|---|---|---|---|---|---|---|---|
| 2019 | ||||||||
| 1 | 9 Jan | 41 | 19,700 | 0.76 | 53 | 2.88 | 18.71 | 269.04 |
| 2 | 29 May | 104 | 148,784 | 7.74 | 792 | 54.29 | 18.75 | 532.32 |
| 3 | 5 Jun | 41 | 5564 | 0.51 | 91 | 0.81 | 115.13 | 1635.51 |
| 4 | 5 Jun | 139 | 44,582 | 2.56 | 133 | 21.69 | 6.34 | 298.33 |
| 5 | 19 Jun | 174 | 232,878 | 10.50 | 402 | 141.62 | 3.09 | 172.62 |
| 6 | 26 Jun | 104 | 44,924 | 3.73 | 61 | 16.39 | 3.77 | 135.78 |
| 7 | 26 Jun | 111 | 22,121 | 1.24 | 32 | 8.61 | 3.74 | 144.66 |
| 8 | 17 Jul | 153 | 11,800 | 1.57 | 30 | 6.31 | 4.78 | 254.24 |
| 9 | 4 Sep | 76 | 3138 | 0.26 | 12 | 0.84 | 14.34 | 382.41 |
| 2020 | ||||||||
| 1 | 7 Feb | 153 | 265,024 | 15.71 | 1899 | 473.81 | 4.61 | 716.54 |
| 2 | 21 Feb | 132 | 85,731 | 5.80 | 409 | 132.37 | 3.17 | 477.07 |
| 3 | 13 Mar | 69 | 177,492 | 5.84 | 566 | 144.24 | 4.08 | 318.89 |
| 4 | 3 Apr | 76 | 258,225 | 31.25 | 712 | 230.83 | 3.22 | 275.73 |
| 5 | 3 Apr | 83 | 119,061 | 6.48 | 465 | 116.1 | 4.13 | 390.56 |
| 6 | 10 Apr | 76 | 256,023 | 13.22 | 1123 | 228.86 | 5.34 | 438.63 |
| 7 | 1 May | 34 | 265,546 | 41.90 | 367 | 107.9 | 3.48 | 138.21 |
Note: All clusters presented a statistical significance with a p-value <0.0001.
Population of the census tracts where the cluster of chikungunya cases occurred.
Reported and expected number of chikungunya cases: for the set of census tracts where a cluster of chikungunya cases was identified during the cluster period. The expected number of cases was based on the overall incidence of reported chikungunya cases in Salvador City during the cluster identification periods.
Relative risks for the ratio between the number of observed and expected chikungunya cases in the census tracts comprising a cluster.
Estimated attack rates for the set of census tracts where a cluster of chikungunya cases was identified per 100,000 inhabitants.
In 2020, seven clusters occurred, affecting 53.0% (1900) of 3584 city census tracts with an exposed population of 1,427,102 people (Fig. 2B and Table 1). The median number of cases in each cluster from 2020 was 566 (IQR: 409–1123), and the median attack rate was 391 cases per 100,000 inhabitants (IQR: 276–477). Their median duration was 76 days (IQR: 69–132, and six of the seven 2020 clusters started between February and April. Among all census tracts involved in any of the sixteen clusters detected in these two years, 387 (17.2%) were part of a cluster in 2019 and 2020, while 352 (15.6%) were part of a cluster only in 2019 and 1513 (67.2%) only in 2020.
The relative risks between the observed and expected number of cases for the census tracts and period of each cluster in 2019 ranged from 3.7 to 115.1, while in 2020, the relative risks were between 3.2 and 5.3 (Table 1). In addition, cases related to a cluster accounted for 47.4% (1606 of 3386) of geolocated cases in 2019 and 48.9% (5541 of 11,341) in 2020.
The most parsimonious model in INLA included four variables that were significantly associated with the cumulative incidence of chikungunya cases during 2019 and 2020, suggesting that census tracts with higher average income, human population density per km2, and vegetation coverage had lower chikungunya risk, while census tracts with higher temperatures had higher chikungunya risk (Table 2 and Supplementary Table S1). We also analyzed the 2019 and 2020 chikungunya epidemics separately and found consistency in the risk coefficients for most variables when comparing 2019 and 2020 separately with the model for the 2019–2020 cumulative incidence (Table 2). Inconsistencies occurred for the vegetation coverage and altitude, which were significantly correlated with chikungunya risk in 2020, but not in 2019. Notably, we found that the level of chikungunya incidence in 2020 was associated with incidence in 2019, but this association was not linear. Compared to the census tracts with lower incidences in 2019 (less than 500 cases per 100,000 inhabitants), the census tracts with intermediate (500–2999 cases per 100,000 inhabitants) and high chikungunya incidences in 2019 (3000–4499 cases per 100,000 inhabitants) had increased risks in 2020 and these increases were statistically significant (Table 2 and Supplementary Table S1). In contrast, for census tracts with extremely high levels of chikungunya incidence in 2019 (>4500 per 100,000 inhabitants), the 2020 incidence was similar to that observed in census tracts with low incidences in 2019 (Table 2 and Supplementary Table S1).
Table 2.
Multivariable analyses showing sociodemographic and environmental factors associated with chikungunya cases in the census sectors of Salvador, Brazil.
| Census tracts characteristics | 2019–2020a |
2019 |
2020b |
|---|---|---|---|
| Incidence risk ratio (95% confidence interval) | |||
| Head of household mean Income (per thousands of reaisc) | 0.86 (0.83–0.90) | 0.89 (0.84–0.94) | 0.87 (0.83–0.91) |
| Density (Inhabitants per 100 m2) | 0.78 (0.77–0.80) | 0.80 (0.77–0.83) | 0.78 (0.76–0.80) |
| Land Surface Temperature (per 1 °C) | 1.18 (1.12–1.25) | 1.22 (1.12–1.33) | 1.15 (1.08–1.22) |
| Soil Adjusted Vegetation Index (per unit increase) | 0.45 (0.22–0.92) | 1.07 (0.34–3.41) | 0.36 (0.17–0.77) |
| Altitude (per 100 m) | 1.19 (0.90–1.57) | 0.91 (0.59–1.39) | 1.35 (1.00–1.82) |
| Chikungunya incidence in 2019 | |||
| <500 cases per 100,000 pop. | – | – | 1.00 |
| 500–2999 cases per 100,000 pop. | – | – | 1.63 (1.42–1.88) |
| 3000–4499 cases per 100,000 pop. | – | – | 7.89 (4.60–13.54) |
| ≥4500 cases per 100,000 pop. | – | – | 1.20 (0.51–2.86) |
Note: Values with statistical significance are in bold. Statistical significance was determined when the 95% confidence interval did not enclose the value reflecting no effect (incidence risk ratio of 1).
Cumulative incidence between 2019 and 2020.
In the case of the 2020 model, the 2019 incidence was included, categorized into four categories.
The average dollar exchange rate for 2010 (at the time of the census) was 1 real = 0.57 US dollars.
Discussion
In this epidemiological study, to examine the CHIKV recurrent transmission dynamics pattern in a large urban setting we conducted a comprehensive spatiotemporal analysis of chikungunya cases reported in Salvador, Brazil, over two years. Our findings showed that the distribution of cases was spatially heterogeneous in 2019 and 2020 and characterized by significant spatial–temporal clusters. These observations are consistent with those of reported chikungunya cases at municipal and state levels in Brazil11,12 and may reflect the pattern of CHIKV transmission in other urban settings. Noteworthy, a patchy distribution of chikungunya cases has also been identified within urban neighbourhoods,18,21 reinforcing the notion that CHIKV transmission dynamics exhibit a “pocket pattern” across different geographical scales, including neighbourhoods, municipalities, and states. This finding is particularly relevant because it indicates that, even within cities highly affected by CHIKV epidemics, there may be neighbourhoods where viral transmission has been low and which are, therefore, potentially susceptible to outbreaks. Consequently, this could affect the immunization program against chikungunya in the near future.
The incidence of chikungunya in the 2020 epidemic was substantially higher than in the 2019 epidemic. This difference reflects the combination of a larger geographical area affected by the clusters of cases in 2020, which had a population ∼2.7-fold greater than that in the joint areas where the clusters occurred in 2019, combined with the relatively higher average attack rates observed in the clusters of the 2020 epidemic. Paradoxically, the restriction on social interactions and movement of people implemented during the COVID-19 pandemic might have fostered the epidemic in 2020 compared to 2019. As more people stayed home, indoor and peridomestic infection risk may have increased. This is supported by the current dogma that CHIKV transmission occurs primarily in the peridomestic environment with inefficient spread,22 possibly because infected people are typically highly debilitated and bedridden.4,10 On the other hand, the decrease in human mobility around the city may have reduced the spread of the virus to distant areas, restricting the emergence of other clusters of cases. It is also possible that the reduction in vector control actions since all the health-sector efforts were directed at dealing with the COVID-19 pandemic influenced the increase in chikungunya incidence in 2020.
Interestingly, although the 2020 clusters were larger and had a higher attack rate than those of 2019, we identified fewer clusters in 2020 compared to 2019 (7 and 9, respectively). In addition, the median duration of the 2020 clusters was shorter than that of 2019. These suggest that, although the 2020 clusters were more explosive, they were less likely to seed new clusters of cases compared to the previous year. In contrast, they expanded faster, reaching larger areas. Further studies are needed to address the effect of human mobility on CHIKV transmission dynamics in urban settings.
Despite the lower average attack rate of the 2019 clusters compared to 2020, the relative risks between the observed and expected incidences for each cluster tended to be higher in 2019 than in 2020, which is partially explained by the lower citywide incidence in 2019 than in 2020. Yet, one of the 2019 clusters reached a notable relative risk of 115.13. This exceptional increase in case reports was noticed by the Salvador Epidemiological Surveillance, which initiated local investigation and vector control actions to contain the upsurge in cases (Cardoso, Head of the Service, personal communication). Although these efforts may have been too late to prevent additional cases, they may have strengthened case detection and reporting, leading to an overestimation of the relative risk in this cluster compared to other areas that did not receive such investigation.
We also identified a correlation between lower socioeconomic status and higher chikungunya incidence during the 2019 and 2020 epidemics. The link between socioeconomic status and the risk of urban arbovirus infection (e.g., DENV and ZIKV) has been previously documented.15,23 Wealthier areas have better access to vector-control interventions (e.g., improved sanitation infrastructure, use of repellents and air-conditioning, and other vector mitigation strategies), which may not be fully available in lower-income census tracts,23,24 resulting in higher risk of urban arbovirus transmission, as we observed.
We found that census tracts with higher temperatures were associated with a higher risk of chikungunya incidence. This could be attributed to the reduced extrinsic incubation time at higher temperatures, facilitating Ae. aegypti transmission.25,26 Interestingly, the annual temperature range in Salvador remains relatively constant without much fluctuation. The mean LST of the census tracts ranged between 24 and 34.3 °C and we observed a trend of higher incidences during the epidemic period of 2019–2020 in census tracts with a mean surface temperature around 29 °C (Supplementary Figure S2). Also, we observed that the peak of the epidemics in 2019 and 2020 occurred between April and July, which coincides with Salvador's rainy and cooler season when the monthly mean temperature ranges between 26.4 °C in April and 24.2 °C in July. This may reflect the vector ecology of Ae. aegypti (the main vector of CHIKV in Brazil), which uses artificial water containers for its larval development with its reproductive cycle maximized at temperatures of around 25–30 °C, increasing infectivity and transmissibility.27
Lower-density census tracts also exhibited higher chikungunya incidences, in contrast to what was reported during chikungunya outbreaks in Italy.28 This discrepancy may be linked to Salvador's urban verticalization (mainly in wealthier areas), in which areas with high-rise buildings tend to have better sanitation and housing infrastructure that reduces the interaction between vectors and the population while increasing human population density. This effect was observed in Singapore, where dengue incidence and Ae. aegypti pupal density showed to be lower in high-rise housing areas.29 Nevertheless, further studies are warranted to investigate the effect of urban verticalization on urban arbovirus epidemics.
We observed a strikingly non-linear relationship between the census tract incidences in 2019 and 2020. The strong association when the 2019 incidence presented relatively intermediate and high levels, but not extremely high values, may be due to the combination of the presence in these census tracts of socio-economic and environmental factors that favoured CHIKV transmission with a still high proportion of susceptible people. Interestingly, the very low and insignificant association between the extremely high level of incidence in 2019 and the incidence in 2020 may be due to a herd immunity effect, as we can hypothesize that census tracts highly affected by the 2019 CHIKV epidemic may not have enough susceptible population for efficient virus transmission.7 However, the number of census tracts found to be in the highest incidence category in 2019 (>4500 cases per 100,000 inhabitants) was very low (n = 4), limiting our analytical power. Future studies will be needed after periods of chikungunya epidemics to assess the association between different levels of population exposure to the virus and the dynamics of CHIKV transmission in space.
Our study has several limitations. First, the analysis was based on passive surveillance data, which only included reported cases (i.e., patients who sought medical care, were suspected of chikungunya and reported). Therefore, the surveillance system did not capture asymptomatic cases or oligosymptomatic chikungunya patients. Although asymptomatic or inapparent infections were found to account for up to 49% of infections during a chikungunya epidemic in Nicaragua caused by the Asian lineage, the frequency of asymptomatic infection is likely much lower (<20%) for infections caused by the ECSA lineage causing the 2019–2020 epidemics in Salvador.30 Second, laboratory confirmation was obtained for only 17.8% (3413 of 19,165 cases) of chikungunya cases included in the study. This limited diagnostic testing may have resulted in misclassification of cases due to the overlap in clinical presentations between chikungunya and other urban arboviruses circulating in Salvador, such as dengue and Zika, and with COVID-19 cases in 2020. The overload of laboratory services due to the COVID-19 pandemic may also have hampered the diagnostic investigation of patients with suspected chikungunya. Third, 25.6% of cases in 2019 and 13.2% of cases in 2020 were not georeferenced and were consequently excluded from our spatial analyses, potentially introducing selection bias because cases occurring in low-income neighbourhoods are more likely to have non-standardized addresses, hampering geolocation. However, we found that incidence was inversely correlated with the census tract mean income of the heads of households; thus, if this bias occurred, it should have been in the direction of reducing the strength of this correlation. The direction of the effect of not including these cases on the other correlations we evaluated is more difficult to predict. Lastly, clustering analysis was solely based on the census tracts of residence, which is usually, however, the most likely place for arboviral infection.22
In conclusion, our findings provide valuable insights into the spatiotemporal dynamics and associated socio-environmental risk factors of chikungunya epidemics in large urban settings. Notably, the massive chikungunya epidemics in Salvador may be substantially composed of smaller, synchronized or sequential community outbreaks. This implies that prompt identification of the initial areas where case clusters are emerging and timely implementation of targeted vector-control interventions may effectively contain outbreaks. In addition, our results highlight that the distribution of chikungunya cases can be highly heterogeneous in space. Cities affected by chikungunya epidemics may harbour communities with low levels of CHIKV transmission, which may trigger further outbreaks due to the maintenance of pockets of non-exposed individuals. Thus, these factors should be considered in chikungunya surveillance, prevention, and control strategies, including the immunization program.
Contributors
HDA, CWC, GSR and UK conceptualized the study. HDA, CWC, RLS and MP contributed to the acquisition of data. HDA, JC, GMC and RLS contributed to the data analysis. HDA, CWC, JC, WMdS, UK and GSR contributed to data interpretation. HDA, WMdS, and GSR drafted the manuscript. UK, SCW, MGR, WMdS, CWC, and GSR revised the manuscript. HAD and CWC accessed and verified all the data reported in the study. All authors read and approved the final version of the manuscript and had access to all the data in the study.
Data sharing statement
The data supporting the study analyses are available from the corresponding author upon reasonable request. They are not publicly available because information on the address and geolocation of the reported case households could compromise their privacy.
Editor's note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Statement on AI responsible use
During the preparation of this work, the authors used Grammarly in order to improve their writing by correcting grammar and style. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Declaration of interests
GSR serves as a consultant for Valneva, a vaccine company that developed a CHIKV vaccine. Other authors declare no conflict of interest.
Acknowledgements
This study was supported by the Secretary of Health of Salvador, Federal University of Bahia, and Oswaldo Cruz Foundation. GSR was supported by Brazilian National Council for Scientific and Technological Development (grant no. 311365/2021-3), Foundation for Research Support of the State of Bahia (grant no. PET0022/2016), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES), and the Clinical and Applied Research Network in Chikungunya (REPLICK). WMdS was supported by a Global Virus Network fellowship, Burroughs Wellcome Fund (#1022448), and Wellcome Trust–Digital Technology Development award (Climate Sensitive Infectious Disease Modelling; 226075/Z/22/Z). SCW was supported by NIH grants R24 AI120942 and U01AI151801. Authors were not precluded from accessing data in the study, and they accept responsibility to submit for publication.
Footnotes
Disclaimer: This summary is available in Portuguese in the Supplementary Material.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2025.101003.
Appendix A. Supplementary data
References
- 1.Weaver S.C., Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med. 2015;372:1231–1239. doi: 10.1056/NEJMra1406035. [DOI] [PubMed] [Google Scholar]
- 2.Paixão E.S., Rodrigues L.C., Costa M. da CN., et al. Chikungunya chronic disease: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2018;112:301–316. doi: 10.1093/trstmh/try063. [DOI] [PubMed] [Google Scholar]
- 3.Silva M.M.O., Kikuti M., Anjos R.O., et al. Risk of chronic arthralgia and impact of pain on daily activities in a cohort of patients with chikungunya virus infection from Brazil. Int J Infect Dis. 2021;105:608–616. doi: 10.1016/j.ijid.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 4.de Souza W.M., Ribeiro G.S., de Lima S.T.S., et al. Chikungunya: a decade of burden in the Americas. Lancet Reg Health Am. 2024;30 doi: 10.1016/j.lana.2023.100673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerqueira-Silva T., Pescarini J.M., Cardim L.L., et al. Risk of death following chikungunya virus disease in the 100 Million Brazilian Cohort, 2015-18: a matched cohort study and self-controlled case series. Lancet Infect Dis. 2024 doi: 10.1016/S1473-3099(23)00739-9. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira M.G., Andrade A.M.S., Da Costa M.C.N., et al. East/Central/South African genotype chikungunya virus, Brazil, 2014. Emerg Infect Dis. 2015;21:906. doi: 10.3201/eid2105.141727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeiro G.S., Hamer G.L., Diallo M., Kitron U., Ko A.I., Weaver S.C. Influence of herd immunity in the cyclical nature of arboviruses. Curr Opin Virol. 2020;40:1–10. doi: 10.1016/j.coviro.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuan G., Ramirez S., Gresh L., et al. Seroprevalence of anti-chikungunya virus antibodies in children and adults in Managua, Nicaragua, after the first chikungunya epidemic, 2014-2015. PLoS Negl Trop Dis. 2016;10:2014–2015. doi: 10.1371/journal.pntd.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministerio del Poder Ciudadano para la Salud de Nicaragua Seroprevalencia y tasa de ataque clínica por chikungunya en Nicaragua, 2014-2015. Rev Panam Salud Publica. 2017;41 doi: 10.26633/RPSP.2017.59. https://iris.paho.org/handle/10665.2/34103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogier E.W., Moss D.M., Mace K.E., et al. Use of bead-based serologic assay to evaluate chikungunya virus epidemic, Haiti. Emerg Infect Dis. 2018;24:995–1001. doi: 10.3201/eid2406.171447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Souza W.M., de Lima S.T.S., Simões Mello LM., et al. Spatiotemporal dynamics and recurrence of chikungunya virus in Brazil: an epidemiological study. Lancet Microbe. 2023;4:e319–e329. doi: 10.1016/S2666-5247(23)00033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freitas L.P., Schmidt A.M., Cossich W., Cruz O.G., Carvalho M.S. Spatio-temporal modelling of the first Chikungunya epidemic in an intra-urban setting: the role of socioeconomic status, environment and temperature. PLoS Negl Trop Dis. 2021;15 doi: 10.1371/journal.pntd.0009537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardoso C.W., Paploski I.A.D., Kikuti M., et al. Outbreak of exanthematous illness associated with Zika, chikungunya, and dengue viruses, Salvador, Brazil. Emerg Infect Dis. 2015;21:2274–2276. doi: 10.3201/eid2112.151167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva M.M.O., Tauro L.B., Kikuti M., et al. Concomitant transmission of dengue, chikungunya, and Zika viruses in Brazil: clinical and epidemiological findings from surveillance for acute febrile illness. Clin Infect Dis. 2019;69:1353–1359. doi: 10.1093/cid/ciy1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kikuti M., Cunha G.M., Paploski I.A.D., et al. Spatial distribution of dengue in a Brazilian Urban slum setting: role of socioeconomic gradient in disease risk. PLoS Negl Trop Dis. 2015;9:1–18. doi: 10.1371/journal.pntd.0003937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardoso C.W., Kikuti M., Prates A.P.P.B., et al. Unrecognized emergence of chikungunya virus during a Zika virus outbreak in Salvador, Brazil. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anjos R.O., Mugabe V.A., Moreira P.S.S.S., et al. Transmission of chikungunya virus in an urban slum, Brazil. Emerg Infect Dis. 2020;26:1364–1373. doi: 10.3201/eid2607.190846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tauro L.B., Cardoso C.W., Souza R.L., et al. A localized outbreak of Chikungunya virus in Salvador, Bahia, Brazil. Mem Inst Oswaldo Cruz. 2019;114:1–4. doi: 10.1590/0074-02760180597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brasil, Ministério da Saúde. Secretaria de Vigilância. Departamento de Vigilância das DoençasTransmissíveis Chikungunya: manejo clínico. 2017. http://bvsms.saude.gov.br/bvs/publicacoes/chikungunya_manejo_%0Aclinico_1ed.pdf
- 20.IBGE Censo demográfico 2010—resultados do universo. 2010. http://www.ibge.gov.br
- 21.Anjos R.O., Portilho M.M., Jacob-Nascimento L.C., et al. Dynamics of chikungunya virus transmission in the first year after its introduction in Brazil: a cohort study in an urban community. PLoS Negl Trop Dis. 2023;17 doi: 10.1371/JOURNAL.PNTD.0011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salje H., Lessler J., Paul K.K., et al. How social structures, space, and behaviors shape the spread of infectious diseases using chikungunya as a case study. Proc Natl Acad Sci U S A. 2016;113:13420–13425. doi: 10.1073/pnas.1611391113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Power G.M., Vaughan A.M., Qiao L., et al. Socioeconomic risk markers of arthropod-borne virus (arbovirus) infections: a systematic literature review and meta-analysis. BMJ Glob Heal. 2022;7:7735. doi: 10.1136/bmjgh-2021-007735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardosh K.L., Ryan S., Ebi K., Welburn S., Singer B. Addressing vulnerability, building resilience: community-based adaptation to vector-borne diseases in the context of global change. Infect Dis poverty. 2017;6 doi: 10.1186/S40249-017-0375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Souza W.M., Weaver S.C. Effects of climate change and human activities on vector-borne diseases. Nat Rev Microbiol. 2024 doi: 10.1038/S41579-024-01026-0. [DOI] [PubMed] [Google Scholar]
- 26.Bellone R., Failloux A.B. The role of temperature in shaping mosquito-borne viruses transmission. Front Microbiol. 2020;11 doi: 10.3389/FMICB.2020.584846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mordecai E.A., Caldwell J.M., Grossman M.K., et al. Thermal biology of mosquito-borne disease. Ecol Lett. 2019;22:1690–1708. doi: 10.1111/ele.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solimini A., Virgillito C., Manica M., et al. How habitat factors affect an Aedes mosquitoes driven outbreak at temperate latitudes: the case of the Chikungunya virus in Italy. PLoS Negl Trop Dis. 2023;17 doi: 10.1371/JOURNAL.PNTD.0010655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seidahmed O.M.E., Lu D., Chong C.S., Ng L.C., Eltahir E.A.B. Patterns of urban housing shape dengue distribution in Singapore at neighborhood and country scales. GeoHealth. 2018;2:54–67. doi: 10.1002/2017GH000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bustos Carrillo F., Collado D., Sanchez N., et al. Epidemiological evidence for lineage-specific differences in the risk of inapparent chikungunya virus infection. J Virol. 2019;93 doi: 10.1128/JVI.01622-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.