Abstract
Background
According to the prior 2017 review (Rabbitts et al), approximately 20% of children and adolescents develop chronic postsurgical pain (CPSP; ie, pain persisting >3 months after surgery) after major surgeries, which is associated with adverse functional and psychological consequences. A major barrier was that definitions of CPSP applied were highly variable. Since that prior review was conducted (n=4 studies in meta-analysis), numerous relevant studies have been published warranting an update.
Objective
The aims of this current review were to: (1) provide an updated prevalence estimate for pediatric CPSP and (2) examine definitions of pediatric CPSP applied in current research.
Evidence review
Prospective, observational studies examining CPSP using a validated self-report pain intensity measure in children were included. 4884 unique publications were screened with 20 articles meeting inclusion criteria. Risk of bias using Quality in Prognostic Study tool ranged from low to high.
Findings
The pooled prevalence of CPSP among mostly major surgeries was 28.2% (95% CI 21.4% to 36.1%). Subgroup analysis of spinal fusion surgeries identified a prevalence of 31% (95% CI 21.4% to 43.5%). Using Grading of Recommendations, Assessment, Development, and Evaluation, the certainty in prevalence estimates was moderate. Studies used a range of valid pain intensity measures to classify CPSP (eg, Numeric Rating Scale), often without pain interference or quality of life measures.
Conclusions
The overall prevalence of pediatric CPSP is higher than estimated in the prior review, and quality of studies generally improved though with some heterogeneity. Standardizing the measurement of CPSP will facilitate future efforts to combine and compare data across studies.
PROSPERO registration number
CRD42022306340.
Keywords: CHRONIC PAIN; Pediatrics; Pain, Postoperative
Introduction
Pediatric surgeries are often accompanied by acute pain, psychological distress, and functional impairments. Previously it was reported that approximately 20% of children and adolescents undergoing major surgery develop chronic postsurgical pain (CPSP).1 The ICD-11 recently defined CPSP as pain that: develops or increases in intensity after a surgical procedure; persists beyond the healing process (ie, longer than 3 months); is localized to the surgical field or projected to a referred area; and other causes of pain are excluded,2 and specified pain intensity, pain-related distress, and functional interference as overarching elements of pain severity. For children and adolescents experiencing CPSP, it is also associated with psychological consequences (eg, low mood, anxiety, fear of movement),3,6 limited functioning (eg, limitations on activities, lower school attendance),4 and poorer health outcomes.7
Since the publication of the 2017 systematic review describing the prevalence of pediatric CPSP,1 there has been an increase in focus on this area and a growing number of studies published reporting on CPSP across a range of pediatric surgeries.8 In the prior review only four studies identified reporting pediatric CPSP prevalence, thus limited the ability to conduct a meta-analysis. Further, since the time of publication, the design of studies focused on CPSP in children has likely improved, potentially addressing the previous reviews concerns about insufficient sample size of relevant studies, and heterogeneity of available studies in design, results, and definitions of CPSP. An accurate estimate of the prevalence of CPSP is critical to guide the assessment, prevention, and management of CPSP. In addition, recommendations for operationalizing CPSP definitions in research are critically needed. Altogether, this indicates that an update of the 2017 review is warranted.
In adults, extensive literature has documented rates of CPSP from 10% to 50% after varied surgical procedures including, thoracotomy, mastectomy, limb amputation, inguinal hernia repair, coronary artery bypass surgery, and cesarean section. Similar to adults, varied pediatric surgeries (eg, spinal fusion, pectus repair, appendectomy) may also lead to differing rates of CPSP, but to date, no meta-analyses have been conducted examining influence of type of surgery on rates of pediatric CPSP.
The primary aim of this updated systematic review and meta-analysis was to examine, summarize, and update the original 2017 review, synthesizing previously included and newly published studies reporting the prevalence of pediatric CPSP. This will provide a more precise estimate of how many children and adolescents are expected to develop CPSP, what the prevalence is per type of surgery, as well as when the estimates are recorded (ie, 3–5 months after surgery or 6–12 months after surgery). Secondarily, we aimed to summarize the definitions of pediatric CPSP being used in research in this area, to guide efforts toward a standardized definition.
Methods
The review protocol was registered with PROSPERO, the international prospective register of systematic reviews, on March 3, 2022 (CRD42022306340). There were no deviations from the protocol.
Eligibility criteria
The formulated eligibility criteria follow the PICOS (Population, Intervention/Exposure, Comparison, Outcomes, and Study) framework.9 The population included children <19 years and >6 years, as 6 years old is the lowest age at which children can reliably self-report pain.10 Children with neurological disabilities or cancer were excluded due to potential confounding effects (eg, different pain processes). Studies with participants outside the eligible age range were considered for inclusion if the majority of participants were within the eligible age range, or if the data was stratified by age and separately reported the eligible age range. The intervention was surgery with general or regional anesthetic, excluding diagnostic, non-invasive, cancer, or dental procedures. We evaluated those who developed CPSP (comparison was those who developed CPSP vs those who did not). The outcome of interest was: (1) postoperative outcomes 3–12 months after surgery, and (2) presence and severity of pain using a validated self-report pain measure as identified by Birnie et al11 and commonly used postsurgery pain validated measures (ie, Scoliosis Research Society-24 (SRS-24)).12 The studies that we included were: observational studies written in English. Dissertations, abstracts only, guidelines/consensus, protocols, reviews, single case reports, case series with less than 10 participants, retrospective, randomized controlled trials, qualitative, and non-human or cell studies were excluded. The criteria used in this update were the same as Rabbitts et al,1 however, we required a validated pain measure in this update.
Information sources, search strategy, and selection process
The databases Cochrane Database of Systematic Reviews, MEDLINE, EMBASE, and PsycINFO were searched by a librarian starting from the search date used in the previous Rabbitts et al review1 (January 2016) to April 20, 2023. The 2023 search was modified from the 2016 search to include new MeSH terms related to CPSP (online supplemental file 1). All identified articles were assessed through the Joanna Briggs Institute’s System for the Unified Management, Assessment, and Review of Information software. Each article was screened against the eligibility criteria using a 10-step exclusion hierarchical structure. Two reviewers independently screened each title and abstract to assess the eligibility of articles published since the original systematic review from 20171 (VW, GG, SDF, BNR, and JR). Two reviewers then independently screened each full-text for eligibility (VW, SDF, BNR). Given the more rigorous criteria for inclusion in this updated systematic review compared with the previous systematic review from 2017,1 articles included in the previous review were independently re-assessed for eligibility by two reviewers (VW and BNR). Disagreements were resolved by consensus with a third reviewer when necessary (JR). Full-texts were then assessed for additional criteria (ie, multiple publications reporting on the same sample) and excluded by group consensus, consulting the first/senior authors when necessary.
Data collection process and data items
Data were extracted from the included studies by one team member (VW) and completed and reviewed by another (SDF), both working independently.
Outcomes
The primary outcome in this review was pain prevalence 3–12 months after surgery where the presence of pain (eg, any pain, moderate-severe pain) was defined by the individual study using a validated self-report measure of pain (ie, data extracted include a number with pain at follow-up, total number at follow-up, and percentage with pain at follow-up). The assessments of pain reports recalling the totality of the past 6 months were excluded to account for potentially less accurate or biased pain recall scores over time.13 14 Studies that included a pain intensity on a continuum (eg, Numeric Rating Scale (NRS), Visual Analog Scale (VAS)) often used cut-offs to define pain presence (eg, NRS≥4, NRS≥3, VAS≥30 mm) and we recorded the pain prevalence based on the individual study’s definition. We abstracted information on elements of the full ICD-11 definition of pain severity (ie, combination of pain intensity, pain-related distress, and functional interference) as well as of CPSP (ie, other causes of pain excluded) where available.2
Secondary outcomes in this review included psychosocial factors and pain interference 3–12 months after surgery. These outcomes were extracted if they were assessed using validated measurements and if the measures were linked to the primary outcome (eg, CPSP vs no CPSP, moderate-severe CPSP vs no-mild CPSP). Examples of validated measures were Functional Disability Inventory (FDI), Pediatric Quality of Life Inventory (PedsQL), and Douleur Neuropathique 4 (DN4).
Data were also sought for the following variables to describe the study characteristics and/or to conduct the sensitivity analyses: age distribution, sex distribution, race/ethnicity, surgery type, timepoint(s) for the outcome assessments, measure(s) used to assess pain severity, type of pain assessment (eg, NRS), and definition of prevalence of pain at follow-up (including cut-offs).
Study risk of bias assessment
To critically assess risk of bias (RoB) of individual studies, each included article was reviewed and rated independently by two team members (CSP, SDF) using the Quality in Prognostic Study (QUIPS) tool.15 Discrepancies were resolved through discussion and consensus. Risks were assessed in the following four QUIPS categories: (1) study participation, (2) study attrition, (3) outcome measurement, and (4) study confounding. The two remaining QUIPS categories (prognostic factor measurement; statistical analysis; and reporting of predictive models) were not assessed as they were not relevant to this review. For each of the four included QUIPS categories, a study was given a qualitative value of “low,” “moderate,” or “high” RoB as per the QUIPS guidelines. A participation rate of less than 20% was considered a low rate (participation rate=n consented/n approached×100%) and attrition of greater than 30% was considered high attrition (non-completer rate=n not completing the study/n enrolled×100%)16; thus, high risk.
Effect measures and synthesis methods
We limited the articles included in the meta-analysis to those who reported prevalence of CPSP as per our primary outcome definition. If studies reported a percentage only for presence of CPSP, a prevalence rate was calculated based on percentage and sample size at follow-up.
Results were pooled using a random-effects model to account for heterogeneity where there was sufficient data (>2 studies) with the Comprehensive Meta-Analysis (V.4) software. Results were reported with 95% CIs.17 Three prevalence rate estimates were calculated: (1) overall prevalence 3–12 months after surgery (note: if multiple prevalence rates were reported in a study, the rate closest to the 3-month mark was chosen due to the clinical need for intervention at earlier time points), (2) 3–5 months after surgery, and (3) 6–12 months after surgery. These prevalence rates (ie, 3–5 months and 6–12 months) were added post hoc to reflect the early and late prevalence points in the included studies. Statistical heterogeneity was assessed using the I2 statistic. I2 was compared with thresholds specified in the Cochrane Handbook (<40% low; 30%–60% moderate; 50%–90% substantial; 75%–100% considerable).18 Where possible (ie, three or more studies in one group), meta-analysis was conducted for groups of surgery types (eg, scoliosis) where heterogeneity was considerable. The potential impact of publication bias was assessed using Egger’s test and funnel plots. Statistical significance was inferred when p values were below 0.05.
Certainty assessment
The GRADE approach (Grading of Recommendations, Assessment, Development, and Evaluation)19 20 was applied to provide an overall certainty assessment for each meta-analysis beyond the individual study level. GRADE is a comprehensive and transparent process of evaluating the certainty of evidence for effect estimates. This approach is used in interventional systematic reviews and has more recently been extended to non-intervention, prevalence studies.21 We applied GRADE domains of RoB, inconsistency (statistical heterogeneity), indirectness, publication bias, and imprecision. Inconsistency in estimates was based on variance in point estimates, overlap in CIs, and statistical significance of heterogeneity statistics. We considered studies with a small sample size (<100) to have an imprecise prevalence estimate and wide CIs. Publication bias was determined from Egger tests and the funnel plots. Ratings of the five domains for each analysis were decided through consensus of two coders.
Findings
Search results
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the study selection process is depicted in figure 1. The updated database searches yielded 4871 unique publications after duplicates were removed. Additionally, 13 articles from the original systematic review were included in the study selection process as our criteria for included studies was modified from the original systematic review. A total of 185 articles underwent full-text review, with 21 articles (20 studies) included in this updated review (five of which articles were included in the previous systematic review from 2017).1 Eight articles from the original review were excluded because the study did not provide a prevalence rate (n=7)22,28 or the article did not use a validated measure of pain to assess prevalence (n=1).29 The two articles by Pagé et al30 31 were based on the same sample but report on different data and are thus both included and presented together in this review. We included Julien-Marsollier et al,32 however, the Julien-Marsollier et al article expands on their article from 2017 (change of scope and increase in sample size), which we excluded to avoid duplicate samples. 20 studies were therefore selected for inclusion in the review, representing 20 pediatric CPSP estimates.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram. no refers to studies in the Rabbitts et al review.1.
Study characteristics
The study characteristics are presented in table 1. Across included studies, sample sizes ranged from 32 to 1433 participants, age from a mean/median of 12 to 16 years, and sex (% female) from 10% to 86%. The samples consisted of more than 60% females in 75% of the studies and the proportion of participants lost to follow-up ranged from 0% to 79%. The included studies include the following surgery types: spine surgery (n=11), pectus carinatum/excavatum repair (n=2), major spine or chest wall surgery (n=1), appendectomy (n=1), and foot surgery (n=1). Others focus on broader categories such as elective, general, or major musculoskeletal surgery (n=4).
Table 1. Study characteristics for included samples (n=20).
| Study | CPSP definition | N baseline | Age, years (range)† | Sex: N female/N male (% female) | Race/ethnicity | Surgery type | N follow-up | % lost to follow-up |
|---|---|---|---|---|---|---|---|---|
| Bailey et al45 | Measure: NRSScale: 0–10, higher scores indicating more painAssessment type: average pain in past 24 hoursTime of assessment: 3 months, 6 months, 12 monthsCut-off: NRS≥3 | 220 | 14.6 (10–20) | 189/31 (86.0%)§ | NR | Posterior scoliosis correction | 148 (3 months), 155 (6 months), 138 (12 months) | 30–37 |
| Batoz et al42 | Measure: VASScale: 0–100 mm, higher scores indicating more painAssessment type: pain in the operated areaTime of assessment: 3 monthsCut-off: VAS≥30 | 291 | 12.0 (6–18) | 116/175 (39.9%) | NR | Elective surgery (61.8% orthopedics, 13.8% laparotomy/scopic, 8.6% thoracic surgery, 8.6% uro/inguinal surgery) | 258 (3 months) | 13 |
| Beeckman et al46 | Measure: NRSScale: 0–10, higher scores indicating more painAssessment type: average pain in past 24 hoursTime of assessment: 6 monthsCut-off: NRS≥4 | 100 | 15.2 (12–18) | 77/23 (77.0%) | White 99%, Asian 1% | Posterior spinal fusion surgery | 88 (6 months) | 12 |
| Chidambaran et al47 | Measure: NRSScale: 0–10, higher scores indicating more painAssessment type: pain in the previous month or at the time of administration of pain assessmentTime of assessment: 2–3 months and 10–12 monthsCut-off: NRS≥4 over previous month or during pain assessment or (for 10 to 12-month time point) reported pain that affected daily life activities/sleep any time during follow-up beyond a year | 144 | 14.4 (10–18)‡ | 106/38 (73.4%)§ | Caucasian 84.9%, no other race/ethnicity data described | Posterior spine fusion surgery | 127 (2–3 months), 110 (10–12 months) | 12–24 |
| Fraser et al48 | Measure: “Do you currently have any pain? Y/N”Scale: Y/NAssessment type: pain level at time of assessmentTime of assessment: 3 monthsCut-off: “Y” for any pain | 110 | 15.849 | 11/99 (10.0%) | NR | Minimally invasive pectus excavatum repair | 32 (3 months) | 71 |
| Julien-Marsollier et al50 | Measure: NRS and DN4Scale: NRS (0–10, higher scores indicating more pain) and DN4 (0–10, higher scores indicating more pain)Assessment type: NRS (maximal pain at movement) and DN4 (around the wound scar)Time of assessment: 12 monthsCut-off: NRS and DN4≥4 | 36 | 15 (<18 years) | 31/5 (86.1%) | NR | Posterior fixation spinal surgery | 36 (12 months) | 0 |
| Julien-Marsollier et al32 | Measure: NRS and DN4Scale: NRS (0–10, higher scores indicating more pain) and DN4 (0–10, higher scores indicating more pain)Assessment type: NRS (maximal pain at movement) and DN4 (around the wound scar)Time of assessment: 12 monthsCut-off: NRS and DN4≥4 | 69 | Controls: 14.5 (11.5–17.5) Cases (ORA): 15.0 (12.0–18.0) | 56/13 (81.2%) | NR | Posterior fixation spinal surgery | 64 (12 months) | 7 |
| Knudsen et al*35 | Measure: Present Pain Intensity (PPI) of the Short Form McGill Pain Questionnaire (SF-MPQ)Scale: 0–5, higher scores indicating more painAssessment type: NRTime of assessment: 6 monthsCut-off: Mild pain or higher (PPI≥1) | 36 | 16 (13–23)¶ | 3/25 (11.0%) | NR | Surgical pectus carinatum repair | 28 (6 months) | 22 |
| Landman et al51 | Measure: Pain domain of Scoliosis Research Society-22 (SRS-22)Scale: 1–5, higher scores indicating less painAssessment type: pain in the last monthTime of assessment: 12 monthsCut-off: “Mild-to-severe” category | 1433 | NR (8–22) | 1152/244 (80.4%) | NR | Posterior spinal fusion surgery | 295 (12 months) | 79 |
| Lescot et al52 | Measure: NRSScale: 0–10, higher scores indicating more painAssessment type: NRTime of assessment: 12 monthsCut-off: “Mild-to-severe” category | 40 | 13 (10–15)¶ | 32/8 (80.0%) | NR | Calcaneonavicular coalitions or too-long anterior process surgery | 18 (3 months), 16 (12 months) | 55–60 |
| Narayanasamy et al*53 | Measure: NRSScale: 0–10, higher scores indicating more painAssessment type: pain over the previous month or during the time of pain assessmentTime of assessment: 6 months and 12 monthsCut-off: NRS≥4 | 144 | 14.9 (12.06, 17.75)¶ | 93/51 (64.8%)§ | Caucasian 81%, African-American 12%, Other 7%, Non-Hispanic 96%** | Posterior spine fusion surgery | 105 (6 months), 71 (12 months) | 38 |
| Ocay et al54 | Measure: Pain domain of Scoliosis Research Society-30 (SRS-30)Scale: 1–5, higher scores indicating less painAssessment type: pain in the last monthTime of assessment: 6 monthsCut-off: “Mild” and “Moderate-severe” categories | 106 | 15.4 (10–18)‡ | 81/25 (76.4%) | NR | Posterior spinal fusion surgery | 76 (6 months) | 28 |
| Pagé et al31 | Measure: NRSScale: 0–10, higher scores indicating more painAssessment type: subjective experience of pain intensity (I)Time of assessment: 6 months and 12 monthsCut-off: NRS≥4 | 83 | 13.8 (8–18) | 56/27 (67.5%) | Caucasian 64%, no other race/ethnicity described | General surgery (thoracotomy, thoracoabdominal surgery, Nuss/Ravitch procedure, sternotomy, laparotomy, ostomy) or orthopedic surgery (scoliosis, osteotomy, plate insertion tibia/femur, open hip reduction, hip capsulorrhaphy) | 61 (6 months), 59 (12 months) | 27–29 |
| Palabiyik and Demir55 | Measure: NRSScale: 0–10, higher scores indicating more painAssessment type: pain in surgery area (lower right abdomen)Time of assessment: 6 monthsCut-off: NRS≥3 | 158 | 12.8 (8–18) | 61/97 (38.6%) | NR | Appendectomy | 158 (6 months) | 0 |
| Perry et al56 | Measure: VASScale: 0–10, higher scores indicating more painAssessment type: past 7-day recallTime of assessment: ±4–6 monthsCut-off: VAS≥2 | 36 | 14.0 (10–17)‡ | 27/9 (75.0%) | White 58.3%, black 11.1%, Asian 5.6%, other/missing 25% | Corrective spinal fusion surgery | 36 (4–-6 months) | 0 |
| Rabbitts et al38 | Measure: NRSScale: 0–10, higher scores indicating more painAssessment type: daily pain intensity for 7 daysTime of assessment: 4 monthsCut-off: NRS≥4 | 60 | 14.7 (10–18) | 40/20 (66.7%) | White 83.4%, African-American 3.3%, Asian 3.3%, other/not reported 10% | Major spine or chest wall surgery | 54 (4 months), 46 (12 months) | 28 |
| Rabbitts et al34 | Measure: NRS and PedsQLScale: NRS (0–10, higher scores indicating more pain), PedsQL (0–100, lower scores indicating more pain)Assessment type: daily pain intensity for 7 daysTime of assessment: 6 monthsCut-off: (1) greater than minimal pain (pain intensity ≥3) on more than 50% of days during the daily monitoring period and (2) impairment in HRQOL (PedsQL<74.9) | 119 | 14.9 (10.0–18.9) | 75/44 (63.0%) | White 78.2%, African American 4.2%, Asian 3.4%, other/not reported 14.3% | Major musculoskeletal surgery (spinal fusion for idiopathic spinal deformity, Nuss procedure for pectus deformity, or hip or femur osteotomy) | 114 (4 months) | 11 |
| Rosenbloom et al4 | Measure: NRSScale: 0–10, higher scores indicating more painAssessment type: subjective experience of pain intensity (I) Time ofassessment: 6 months and 12 monthsCut-off: NRS≥4 | 265 | 14.1 (8–18) | 155/110 (58.5%) | Caucasian 65.95%, African Canadian 6.03%, South Asian 5.60%, East Asian 4.35%, African Caribbean 1.72%, Hispanic 1.72%, Aboriginal 1.29%, other 11.64% | General surgery (thoracotomy, thoracoabdominal surgery, Nuss/Ravitch pectus repair, sternotomy, laparotomy, laparoscopic-assisted; colectomy, ileostomy, J-pouches) or orthopedic surgery (osteotomy, plate insertion tibial/femur, surgery for scoliosis). 50.2% surgery for scoliosis and 35.5% osteotomy | 214 (6 months), 225 (12 months) | 15–19 |
| Sieberg et al57* | Measure: Pain domain of Scoliosis Research Society-30 (SRS-30)Scale: 1–5, higher scores indicating less painAssessment type: pain in the last monthTime of assessment: 12 monthsCut-off: '“Moderate-severe” category | 260 | 14.4 (8–21)†† | 137/53 (72.3%)§†† | Caucasian 81.7%, Black or African American 8.4%, Asian or Asian American 3.1%, Hispanic 1.6%, other 1.6%†† | Spinal fusion surgery (89.9% posterior, 10.1% anterior)†† | 190 (12 months) | 27 |
| Sieberg et al58 | Measure: NRSScale: 0–10, higher scores indicating more painAssessment type: subjective experience of pain intensity (I)Time of assessment: 4–6 monthsCut-off: NRS>0 | 32 | 13.9 (10–17) | 25/7 (78.1%) | White 87.5%, black 9.375%, Asian 3.125% | Spinal fusion surgery | 27 (4–6 months) | 16 |
Study characteristics presented for the sample completing follow-up assessments (in contrast to baseline presented for the other studies).
Reported as mean (range) or median.
Did not explicitly state range, but stated “Aged (range)” in the Methods section (eg, as an inclusion criterion).
n derived from percentage.
Reported as median (range).
Narayanasamy et al53 report race/ethnicity for n=109 patients with CPSP outcome included in Pediatric Pain Screening Tool (PPST)-CPSP association.
Sieberg et al57 reports descriptive statistics for n=190 patients which completed 12-month follow-up.
CPSP, chronic postsurgical pain; DN4, Douleur Neuropathique 4; NR, not reported; NRS, Numeric Rating Scale; ORA, opioid-reduced anesthesia; PedsQL, Pediatric Quality of Life Inventory; VAS, Visual Analog Scale.
Definitions of CPSP
Studies varied in their definitions of CPSP and the timing of assessment. The most common definition was a score of 4 or more (/10) on the NRS (n=12), which was labeled as “moderate to severe pain.” Three studies reported CPSP as a 3 (or 30 mm) or more on the NRS or VAS and labeled that pain as “moderate to severe.” Three studies identified CPSP as non-zero pain on either the Present Pain Intensity (PPI), SRS, or VAS; and two studies classified CPSP as the presence of pain at 3 or 12 months postsurgery as “yes/no.”
Further, the type of pain assessment varied between the studies and three studies carried out more than one type of pain assessment. Some assessed “current pain” while others assessed recalled pain (eg, over the past 24 hours, over the past 7 days). Few studies (n=5) explicitly indicated the referenced state of participants during the pain assessment (eg, at rest, during movement) or the pain location (eg, surgery area).
We were unable to identify studies referring to the full ICD-11 pain severity definition (ie, combination of pain intensity, pain-related distress, and functional interference33) as most studies did not include in their prevalence rates a measure of quality of life, functional disability, and distress. Rabbitts et al,34 Knudsen et al,35 and Rosenbloom et al,4 however, did report a combination of a pain intensity measure and a measure of functional disability or quality of life (which includes psychosocial and physical domains) in their definitions or CPSP.
Measurement of postsurgical psychosocial factors, distress, and pain interference
A total of eight studies investigated postsurgical quality of life, pain-related disability, and/or pain interference. The measures used for the investigation included the PedsQL (n=5), FDI (n=5), NRS-U (n=2), DN4 (n=1), painDETECT (n=1), and Child Activity Limitations Interview (n=1). These outcome assessments were conducted at one or more of the following timepoints: 2–3 months, 3 months, 6 months, 10–12 months, 12 months.
Risk of bias in studies
The four individual RoB ratings for each included article, and the distribution of RoB ratings for each QUIPS category are shown in figure 2A,B.
Figure 2. Risk of bias ratings for included articles (n=20).

The proportion of articles that received a low RoB rating ranged from 26% to 63%, a moderate RoB rating from 21% to 47%, and a high RoB from 0% to 53% across the QUIPS categories (figure 2B). The QUIPS category in most articles exhibited weaknesses in study attrition (74% of the articles) followed by study participation (63% of the articles; figure 2B). Other examples of weaknesses for studies attributable to higher RoB were (1) inadequate description of or suboptimal study participation, (2) inadequate description of or suboptimal study attrition, (3) missing information about the setting and delivery of surveys/questionnaires, and/or (4) lack of consideration for confounding factors in the study design or analyses.
Prevalence of CPSP
The pooled prevalence of CPSP was 28.2% (95% CI 21.4% to 36.1%) with considerable heterogeneity (I2=92%; figure 3). All 20 studies (n=3742) reported CPSP prevalence, ranging from 10% to 63%. The median prevalence across studies was 26.0% (25th percentile=18.0%, 75th percentile=41.8%).
Figure 3. Forest plot of prevalence rates for included articles (n=20).
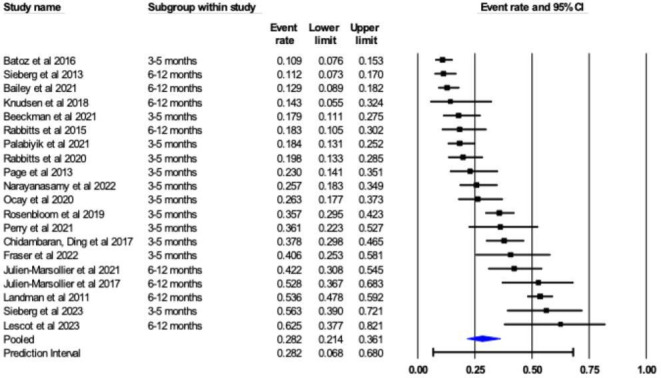
In terms of the CPSP prevalence 3–5 months after surgery, the pooled prevalence was 27% (95% CI 20.2% to 35.4%) with substantial heterogeneity (I2=86%). 11 studies reported prevalence at that time point (n=1482) ranging from 10% to 56%.
The prevalence of CPSP 6–12 months after surgery was reported in 14 studies (n=3054) with a pooled prevalence rate of 29% (95% CI 21.1% to 38.9%) and a range of prevalence rates (11% to 63%). There was considerable heterogeneity (I2=92%).
A subgroup analysis on prevalence of CPSP by surgery type was performed. Sufficient data was only available to conduct a subgroup analysis for spinal fusion surgeries (n=11). The pooled prevalence of CPSP after spinal fusion surgery was 31% (95% CI 21.4% to 43.5%) and ranged from 11% to 56% figure 4. There was considerable heterogeneity (I2=93%).
Postsurgical psychosocial factors, distress, and pain interference
Four studies examined psychosocial factors at follow-up with the CPSP assessment, four studies evaluated distress from CPSP, and nine studies examined functional disability related at follow-up. The NRS-U, FDI, and CALI scores at follow-up were higher for cases (eg, “late recovery” trajectory, CPSP) than controls (eg, “early recovery” trajectory, no CPSP) across the studies.
Certainty of evidence
Using the GRADE approach, certainty for each of the three primary estimates was rated at moderate due to the studies having low consistency resulting from unexplained statistical heterogeneity. Table 2 summarizes the results including quality assessments according to GRADE criteria, pooled prevalence estimates, and certainty in the estimates based on GRADE analysis. There were no substantial concerns with limitations (ie, all studies were prospective with no sampling biases), indirectness (ie, all studies used validated measures to assess CPSP), imprecision (ie, all studies had small CIs, 12 of the 20 articles included samples with 100 or more participants), or publication bias (ie, Egger’s were non-significant, funnel plots were within reasonable ranges).
Table 2. Certainty assessment of pooled estimates using Grading of Recommendations, Assessment, Development, and Evaluation assessment.
| Quality assessment | Summary of findings | Certainty in prevalence estimates | ||||||
|---|---|---|---|---|---|---|---|---|
| Prevalence category (Studies (n)) | Limitations | Inconsistency | Indirectness | Imprecision | Publication Bias | Pooled prevalence (95% CI) | Patients (n) | |
| Overall prevalence (n=20) | No serious limitations | High degree of statistical heterogeneity (I2=91.76%) | No serious indirectness | No serious imprecision | Undetected | 0.28 (0.21 to 0.36) | 3742 | Moderate |
| 3–5 month’s prevalence (n=11) | No serious limitations | High degree of statistical heterogeneity (I2=86.75%) | No serious indirectness | No serious imprecision | Undetected | 0.27 (0.20 to 0.35) | 1482 | Moderate |
| 6–12 month’s prevalence (n=14) | No serious limitations | High degree of statistical heterogeneity (I2=92.23%) | No serious indirectness | No serious imprecision | Undetected | 0.29 (0.21 to 0.39) | 3054 | Moderate |
Discussion
This systematic review summarized results from 20 unique samples and found that the pooled prevalence CPSP was 28% (95% CI 21.4% to 36.1%) with moderate certainty, with a reported range of 10%–63%. This rate is higher than our previous systematic review, which reported a prevalence rate of 20% from four unique samples.1 Despite considerable clinical heterogeneity, the current estimate of 28% received a moderate degree of certainty as per GRADE, indicating that this is a representative assessment of pediatric CPSP. Further, we subanalyzed the data by surgery type and found that the prevalence rate for spinal fusion surgery was 31% (95% CI 21.4% to 43.5%). This is double the recently reported prevalence of CPSP in adult spine surgeries (ie, 14%).36 While the aim of the review was to include all types of surgeries, the majority of studies that met inclusion criteria consisted of major surgeries (eg, spinal fusion, orthopedic surgeries), and thus our results reflect major surgeries.
The rates in CPSP differed by how pain intensity was measured and categorized.37 For example, when looking at how each study assessed pain, maximal pain at movement surrounding the surgical area resulted in relatively high pain estimates. Further, when evaluating non-zero pain intensity, the prevalence of CPSP was high.
This review aimed to include prevalence estimates that reflected the ICD-11 overarching definition of chronic pain as well as the ICD-11 definition of CPSP,2 published after the Rabbitts et al review.1 However, only three studies included measures of functional disability (eg, with the FDI)4 or quality of life (eg, PedsQL)35 38 and two studies assessed pain-related distress.4 30 Studies rarely reported if they assessed pain at the surgical site or if they were measuring pain anywhere in the body after surgery. Further, no studies evaluated the diagnosis of CPSP based on excluding other causes. Therefore, our overall estimate is based on a combination of pain intensity alone using the NRS, PPI, VAS, or SRS, and the presence of pain in combination with the distress and functional interference from pain. No studies included full criteria for CPSP (eg, exclusion of other causes of pain as well as pain from a pre-existing pain problem). The rates of pediatric CPSP may, therefore, be different with these additional parameters included in the definition and may strengthen the GRADE assessment. Notably, Hofer et al (2022)39 defined CPSP in adults with pain intensity alone, and CPSP with pain-related impaired functioning, and found differences in prevalence rates with the different definitions (ie, when including measures of functional impairment, the rates of CPSP were lower).
Since Rabbitts et al1 was published, a number of higher-quality studies were published as indicated through the RoB assessment. However, approximately half of the studies included in this review scored a high RoB due to study attrition and secondarily through low study participation. This indicates that while there have been improvements in study methodology for this area of research, there are still domains where rigor can be increased (table 3).
Table 3. Review of gaps and proposed solutions to variation in pediatric chronic postsurgical pain (CPSP) prevalence rates.
| Gap | Proposed solution |
|---|---|
| Inconsistent measurement and definitions of pain intensity | Establish a priori cut-off scores (eg, >3/10 on a Numeric Rating Scale indicates moderate to severe pain intensity)Use age-appropriate validated measures of pain intensity, prioritizing child self-report |
| Absence of measurement of functional disability or emotional distress, which are necessary criteria for defining chronic postsurgical pain | Include measures of functional disability and emotional distress that are validated in the age range being studied |
| Lack of pain location identified | Report the location of pain relative to surgical site |
| Mixed surgical samples | Consideration of surgery typeLarger samples to allow for subgroup analyses by surgery type |
| Limited diversity in samples | Increasing inclusivity in all stages of research and reporting of sample diversity (eg, race, ethnicity, sex, gender, disability) |
Recommendations
This review highlights the variability in measuring pediatric CPSP. There are differences in: the timing of assessment in the chronic phase of the development of CPSP; the measures used for assessing pain intensity; the assessment of pain intensity measured with movement or at rest; the inclusion of a measure of distress and functional limitations; and the location of the pain relative to the site of surgery. All of the points of variation led to each study developing their own definition of CPSP. However, the ICD-112 provides criteria for defining CPSP and future studies could aim to align their prevalence rate of CPSP to this new guidance. However, two criteria may not be possible to evaluate without physical examination (ie, exclusion of other causes of pain, pre-existing pain condition), therefore, it is likely that prevalence rates will be slightly inflated.
Towards a standardized method of assessing for pediatric CPSP, studies should include components of assessment of pain severity as specified by the ICD-11. This includes developmentally appropriate validated measures of pain intensity (eg, NRS-11, VAS),11 functional impairment (eg, FDI, PedsQL), and psychological distress (eg, NRS-Unpleasantness). Specifically in reference to pain intensity, when identifying prevalence of CPSP studies should determine a priori cut-off scores pain. As expected, studies in this review that used cut-off scores for moderate-severe pain (ie, pain intensity of 4 or more) to indicate CPSP reported lower prevalence rates than those where any pain (ie, non-zero pain intensity) was considered CPSP, however, many studies did not determine these “levels” of pain prior to the outcome being measured. Measures of pain should also reference pain location, an ICD-11 criteria for CPSP (eg, whether it is at the surgical site, referred from the surgical site, or another area of the body).
The results of this systematic review and meta-analysis have significant clinical implications. First, this updated prevalence data on CPSP in pediatric patients can guide clinical service design and policy decision-making. With the rate of CPSP in pediatrics being one in four children undergoing surgery, there is a strong indication of the need for service development and implementation of evidence-based strategies.40 Pediatric transitional pain services, such as the one described by Isaac et al,41 should be prioritized in pediatric surgical pain care. These services involve participation from multiple disciplines, such as pain medicine, psychology, and physiotherapy, which covers the biopsychosocial nature of the development of CPSP. Second, the results from this review highlight that the assessment of CPSP should occur earlier than 6 months postsurgery (ie, at 3 months postsurgery), as most of the children with CPSP at 12 months had CPSP at 3 months postsurgery. There should not be a delay in services provided for CPSP given the serious negative consequences on a child’s functioning and development.
Limitations
This review should be considered in the light of several limitations. First, although the QUIPS is a well-established RoB tool for prospective studies, it is not designed for prevalence rate studies. The tool is designed to fully evaluate the validity and bias for studies in which data is collected by the study team in the future (ie, assessing methodologic factors that interfere with the selection and data collection/analysis process). As such, the review team made a number of modifications and rules about each category so that consistency across studies included in this review could be had. Second, several studies used mixed samples of surgeries,4 31 34 42 thus it is not clear if there is a difference in prevalence rates for different surgeries. In the adult literature, there is substantive variation in the estimates of different surgeries, suggesting this may matter.43 Future studies could use larger samples or focus on one particular type of surgery to reduce clinical heterogeneity. Due to low number of studies doing specific surgeries, we were only able to assess CPSP by surgery type for spine surgery, which had a prevalence rate of 31%. Third, the majority of the samples included in this review lacked diversity or did not report the diversity within their samples, thus impacting generalizability. Future studies should follow established guidelines for collecting, reporting, and analyzing data on race.44
Conclusions
This review updates earlier work on the prevalence of pediatric CPSP, with the previous review including four unique estimates, whereas the current review included 20 unique estimates. Through meta-analysis, the prevalence of pediatric CPSP was estimated with moderate certainty at 28%, which is concerningly higher than previously reported. Based on updated ICD criteria, future studies should include not only pain intensity in their pain prevalence rates, but also measures of functional limitations and psychological distress in a standardized way, with the aim of improving precision in future studies.
Supplementary material
Acknowledgements
This work was done as part of BNR’s post-doctorate at The Hospital for Sick Children, Toronto, Canada. BNR was funded by CIHR Banting Postdoctoral Fellowship, Pain in Child Health Louise and Alan Edwards Postdoctoral Award, and Restracomp. Production of this document has also been made possible through a financial contribution from Health Canada. The views expressed here do not necessarily represent the views of Health Canada. We thank Gurpreet Brar for her contributions with formatting this work. JR was supported National Institutes of Health, National Institutes of Arthritis, Musculoskeletal, and Skin Diseases under award number K24AR080786-02 (PI: JR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Funding: JR was supported by National Institutes of Health National Institutes of Arthritis, Musculoskeletal, and Skin Diseases under award number by K24AR080786-02 (PI: JR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Production of this document has also been made possible through a financial contribution from Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Collaborators: N/A.
References
- 1.Rabbitts JA, Fisher E, Rosenbloom BN, et al. Prevalence and Predictors of Chronic Postsurgical Pain in Children: A Systematic Review and Meta-Analysis. J Pain. 2017;18:605–14. doi: 10.1016/j.jpain.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schug SA, Lavand’homme P, Barke A, et al. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. 2019;160:45–52. doi: 10.1097/j.pain.0000000000001413. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbloom BN, Katz J. Modeling the transition from acute to chronic postsurgical pain in youth: A narrative review of epidemiologic, perioperative, and psychosocial factors. Can J Pain . 2022;6:166–74. doi: 10.1080/24740527.2022.2059754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenbloom BN, Pagé MG, Isaac L, et al. Pediatric Chronic Postsurgical Pain And Functional Disability: A Prospective Study Of Risk Factors Up To One Year After Major Surgery. J Pain Res. 2019;12:3079–98. doi: 10.2147/JPR.S210594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabbitts JA, Aaron RV, Fisher E, et al. Long-Term Pain and Recovery After Major Pediatric Surgery: A Qualitative Study With Teens, Parents, and Perioperative Care Providers. J Pain. 2017;18:778–86. doi: 10.1016/j.jpain.2017.02.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabbitts JA, Fisher E. Postsurgical pain in children: unraveling the interplay between child and parent psychosocial factors. Pain. 2017;158:1847–8. doi: 10.1097/j.pain.0000000000001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabbitts JA, Groenewald CB, Tai GG, et al. Presurgical psychosocial predictors of acute postsurgical pain and quality of life in children undergoing major surgery. J Pain. 2015;16:226–34. doi: 10.1016/j.jpain.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbloom BN, Pavlova M, Katz J. Special issue: Developmental perspectives on the transition of acute to chronic pain after surgery. Can J Pain . 2022;6:46–8. doi: 10.1080/24740527.2022.2090323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Methley AM, Campbell S, Chew-Graham C, et al. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579. doi: 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Baeyer CL, Spagrud LJ, McCormick JC, et al. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. Pain. 2009;143:223–7. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Birnie KA, Hundert AS, Lalloo C, et al. Recommendations for selection of self-report pain intensity measures in children and adolescents: a systematic review and quality assessment of measurement properties. Pain. 2019;160:5–18. doi: 10.1097/j.pain.0000000000001377. [DOI] [PubMed] [Google Scholar]
- 12.Rothenfluh DA, Neubauer G, Klasen J, et al. Analysis of internal construct validity of the SRS-24 questionnaire. Eur Spine J. 2012;21:1590–5. doi: 10.1007/s00586-012-2169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zonneveld LN, McGrath PJ, Reid GJ, et al. Accuracy of children’s pain memories. Pain. 1997;71:297–302. doi: 10.1016/s0304-3959(97)03379-4. [DOI] [PubMed] [Google Scholar]
- 14.Chogle A, Sztainberg M, Bass L, et al. Accuracy of pain recall in children. J Pediatr Gastroenterol Nutr. 2012;55:288–91. doi: 10.1097/MPG.0b013e31824cf08a. [DOI] [PubMed] [Google Scholar]
- 15.Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 16.Babic A, Tokalic R, Amílcar Silva Cunha J, et al. Assessments of attrition bias in Cochrane systematic reviews are highly inconsistent and thus hindering trial comparability. BMC Med Res Methodol. 2019;19:76. doi: 10.1186/s12874-019-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins PT, Greene S. The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions version 5.1.0.www.handbook.cochrane.org Available. [Google Scholar]
- 19.Spencer FA, Iorio A, You J, et al. Uncertainties in baseline risk estimates and confidence in treatment effects. BMJ. 2012;345:e7401. :bmj.e7401. doi: 10.1136/bmj.e7401. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 22.Carreon LY, Sanders JO, Diab M, et al. The Minimum Clinically Important Difference in Scoliosis Research Society-22 Appearance, Activity, and Pain Domains After Surgical Correction of Adolescent Idiopathic Scoliosis. Spine (Phila Pa 1986) 2010;35:2079–83. doi: 10.1097/BRS.0b013e3181c61fd7. [DOI] [PubMed] [Google Scholar]
- 23.Connelly M, Fulmer RD, Prohaska J, et al. Predictors of postoperative pain trajectories in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) . 2014;39:E174–81. doi: 10.1097/BRS.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 24.Lind M, Enderlein D, Nielsen T, et al. Clinical outcome after reconstruction of the medial patellofemoral ligament in paediatric patients with recurrent patella instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:666–71. doi: 10.1007/s00167-014-3439-x. [DOI] [PubMed] [Google Scholar]
- 25.Mariconda M, Andolfi C, Cerbasi S, et al. Effect of surgical correction of adolescent idiopathic scoliosis on the quality of life: a prospective study with a minimum 5-year follow-up. Eur Spine J. 2016;25:3331–40. doi: 10.1007/s00586-016-4510-8. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura A, Morita A, Fukuda A, et al. Functional recovery of the donor knee after autologous osteochondral transplantation for capitellar osteochondritis dissecans. Am J Sports Med. 2011;39:838–42. doi: 10.1177/0363546510388386. [DOI] [PubMed] [Google Scholar]
- 27.Pellegrino LN, Avanzi O. Prospective evaluation of quality of life in adolescent idiopathic scoliosis before and after surgery. J Spinal Disord Tech. 2014;27:409–14. doi: 10.1097/BSD.0b013e3182797a5e. [DOI] [PubMed] [Google Scholar]
- 28.Strömqvist F, Strömqvist B, Jönsson B, et al. Lumbar disc herniation surgery in children: outcome and gender differences. Eur Spine J. 2016;25:657–63. doi: 10.1007/s00586-015-4149-x. [DOI] [PubMed] [Google Scholar]
- 29.Lillehei CW, Masek BJ, Shamberger RC. Prospective study of health-related quality of life and restorative proctocolectomy in children. Dis Colon Rectum. 2010;53:1388–92. doi: 10.1007/DCR.0b013e3181e8efc5. [DOI] [PubMed] [Google Scholar]
- 30.Pagé MG, Huguet A, Katz J. In: Oxford textbook of paediatric pain. McGrath PJ, Stevens BJ, Walker SM, editors. Oxford, UK: Oxford University Press; 2013. Prevention of the development and maintenance of paediatric chronic pain and disability; pp. 39–49. [Google Scholar]
- 31.Pagé MG, Stinson J, Campbell F, et al. Identification of pain-related psychological risk factors for the development and maintenance of pediatric chronic postsurgical pain. J Pain Res. 2013;6:167–80. doi: 10.2147/JPR.S40846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Julien-Marsollier F, Assaker R, Michelet D, et al. Effects of opioid-reduced anesthesia during scoliosis surgery in children: a prospective observational study. Pain Manag. 2021;11:679–87. doi: 10.2217/pmt-2020-0100. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization International statistical classification of diseases and related health problems. [15-May-2024]. https://icd.who.int Available. Accessed.
- 34.Rabbitts JA, Palermo TM, Zhou C, et al. Psychosocial Predictors of Acute and Chronic Pain in Adolescents Undergoing Major Musculoskeletal Surgery. J Pain. 2020;21:1236–46. doi: 10.1016/j.jpain.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knudsen MV, Pilegaard HK, Grosen K. Pain and sensory disturbances following surgical repair of pectus carinatum. J Pediatr Surg. 2018;53:733–9. doi: 10.1016/j.jpedsurg.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Alshammari HS, Alshammari AS, Alshammari SA, et al. Prevalence of Chronic Pain After Spinal Surgery: A Systematic Review and Meta-Analysis. Cureus. 2023;15:e41841. doi: 10.7759/cureus.41841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilron I, Lao N, Carley M, et al. Movement-evoked Pain versus Pain at Rest in Postsurgical Clinical Trials and in Meta-analyses: An Updated Systematic Review. Anesthesiology. 2024;140:442–9. doi: 10.1097/ALN.0000000000004850. [DOI] [PubMed] [Google Scholar]
- 38.Rabbitts JA, Zhou C, Groenewald CB, et al. Trajectories of postsurgical pain in children: risk factors and impact of late pain recovery on long-term health outcomes after major surgery. Pain. 2015;156:2383–9. doi: 10.1097/j.pain.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofer DM, Lehmann T, Zaslansky R, et al. Rethinking the definition of chronic postsurgical pain: composites of patient-reported pain-related outcomes vs pain intensities alone. Pain. 2022;163:2457–65. doi: 10.1097/j.pain.0000000000002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pain SfKi. Organization HS CAN/hso 13200:2023 pediatric pain management. 2023
- 41.Isaac L, Rosenbloom BN, Tyrrell J, et al. Development and expansion of a pediatric transitional pain service to prevent complex chronic pain. Front Pain Res (Lausanne) 2023;4:1173675. doi: 10.3389/fpain.2023.1173675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Batoz H, Semjen F, Bordes-Demolis M, et al. Chronic postsurgical pain in children: prevalence and risk factors. A prospective observational study. Br J Anaesth. 2016;117:489–96. doi: 10.1093/bja/aew260. [DOI] [PubMed] [Google Scholar]
- 43.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9:723–44. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- 44.Hood AM, Booker SQ, Morais CA, et al. Confronting Racism in All Forms of Pain Research: A Shared Commitment for Engagement, Diversity, and Dissemination. J Pain. 2022;23:913–28. doi: 10.1016/j.jpain.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bailey KM, Howard JJ, El-Hawary R, et al. Pain Trajectories Following Adolescent Idiopathic Scoliosis Correction: Analysis of Predictors and Functional Outcomes. JB JS Open Access. 2021;6:e20.00122. doi: 10.2106/JBJS.OA.20.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beeckman M, Hughes S, Van der Kaap-Deeder J, et al. Risk and Resilience Predictors of Recovery After Spinal Fusion Surgery in Adolescents. Clin J Pain. 2021;37:789–802. doi: 10.1097/AJP.0000000000000971. [DOI] [PubMed] [Google Scholar]
- 47.Chidambaran V, Ding L, Moore DL, et al. Predicting the pain continuum after adolescent idiopathic scoliosis surgery: A prospective cohort study. Eur J Pain. 2017;21:1252–65. doi: 10.1002/ejp.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fraser JA, Briggs KB, Svetanoff WJ, et al. Short and long term outcomes of using cryoablation for postoperative pain control in patients after pectus excavatum repair. J Pediatr Surg. 2022;57:1050–5. doi: 10.1016/j.jpedsurg.2022.01.051. [DOI] [PubMed] [Google Scholar]
- 49.Periwal KL, Gupta BK, Panwar RB, et al. Prevalence of rheumatic heart disease in school children in Bikaner: an echocardiographic study. J Assoc Physicians India. 2006;54:279–82. [PubMed] [Google Scholar]
- 50.Julien-Marsollier F, David R, Hilly J, et al. Predictors of chronic neuropathic pain after scoliosis surgery in children. Scand J Pain. 2017;17:339–44. doi: 10.1016/j.sjpain.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Landman Z, Oswald T, Sanders J, et al. Prevalence and Predictors of Pain in Surgical Treatment of Adolescent Idiopathic Scoliosis. Spine (Phila Pa 1986) 2011;36:825–9. doi: 10.1097/BRS.0b013e3181de8c2b. [DOI] [PubMed] [Google Scholar]
- 52.Lescot T, Compagnon R, Accadbled F, et al. Evaluation of surgical outcomes of calcaneonavicular coalition and too-long anterior process in children: A prospective study. Orthop Traumatol Surg Res. 2024;110:103620. doi: 10.1016/j.otsr.2023.103620. [DOI] [PubMed] [Google Scholar]
- 53.Narayanasamy S, Yang F, Ding L, et al. Pediatric Pain Screening Tool: A Simple 9-Item Questionnaire Predicts Functional and Chronic Postsurgical Pain Outcomes After Major Musculoskeletal Surgeries. J Pain. 2022;23:98–111. doi: 10.1016/j.jpain.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ocay DD, Li MMJ, Ingelmo P, et al. Predicting Acute Postoperative Pain Trajectories and Long-Term Outcomes of Adolescents after Spinal Fusion Surgery. Pain Res Manag. 2020;2020:9874739. doi: 10.1155/2020/9874739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palabiyik O, Demir G. Chronic Pain after Open Appendectomy and Its Effects on Quality of Life in Children Aged 8-18 Years. Pain Res Manag. 2021;2021:6643714. doi: 10.1155/2021/6643714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perry M, Sieberg CB, Young EE, et al. The Potential Role of Preoperative Pain, Catastrophizing, and Differential Gene Expression on Pain Outcomes after Pediatric Spinal Fusion. Pain Manag Nurs. 2021;22:44–9. doi: 10.1016/j.pmn.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sieberg CB, Simons LE, Edelstein MR, et al. Pain prevalence and trajectories following pediatric spinal fusion surgery. J Pain. 2013;14:1694–702. doi: 10.1016/j.jpain.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sieberg CB, Lunde CE, Wong C, et al. Pilot Investigation of Somatosensory Functioning and Pain Catastrophizing in Pediatric Spinal Fusion Surgery. Pain Manag Nurs. 2023;24:27–34. doi: 10.1016/j.pmn.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



