Abstract
The COVID-19 pandemic caused a global health crisis that resulted in millions of deaths. Effective vaccines have played central roles in curtailing the pandemic. Here, we developed a down-converting near-infrared IIb (NIR-IIb; 1500 to 1700 nanometers) luminescent, pure NaErF4@NaYF4 rare-earth nanoparticle (pEr) as vaccine carriers. The pEr nanoparticles were coated with three layers of cross-linked biocompatible polymers (pEr-P3; ~55 nanometers) and conjugated to the receptor binding domain (RBD) of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein. Upon subcutaneous injection of the pEr-P3-RBD nanovaccine in mice, in vivo NIR-IIb imaging revealed active vaccine trafficking and migration to lymph nodes through lymphatic vessels. Two doses of the adjuvant-free vaccine elicited long-lasting (>7 months) high titers of serum viral neutralization antibody and anti-RBD immunoglobulin G, along with robust RBD-specific germinal center B cells and T follicular helper cells. We devised in vivo NIR-II molecular imaging of RBD-specific cells in lymph nodes, opening noninvasive assessments of vaccine-elicited immune responses longitudinally.
An NIR-II emitting nanoparticle vaccine for SARS-CoV-2 enables in vivo imaging and long-lasting immune responses.
INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus surfaced in 2019 and rapidly exploded into a global pandemic with a death toll of ~7 million worldwide (1). The deployment of effective vaccines is instrumental in curtailing the pandemic. Thirteen vaccines have received emergency use authorization from the World Health Organization (WHO), including mRNA vaccines and protein subunit vaccines (2–4). The mRNA vaccines have been highly successful but come with some constrains such as cost and ultracold storage conditions (5–8). Subunit vaccines use antigenic segments of the pathogen (e.g., the spike protein) to induce both humoral and cellular immune responses and can offer wider accessibility especially in low- to mid-income countries (9, 10). A disadvantage of SARS-CoV-2 subunit vaccines is lower immunogenicity and requires adjuvants to boost the immune response (11–16). In general, vaccine-induced immunity wanes over time (17–20), requiring frequent booster doses. There remains a need of developing vaccine platforms that are more accessible for production, offer greater efficacy, and have more durable immune response.
Patients infected with SARS-CoV-2 have shown strong antibody responses against the viral spike protein (21–24), a trimeric glycoprotein that facilitates viral entry into host cells (25, 26). The spike protein is the primary target of COVID-19 vaccines and contains S1 and S2 subunits (27–29) that mediate receptor binding and host cell membrane fusion, respectively. The receptor binding domain (RBD) is a 25-kDa portion of the S1 subunit that recognizes the human angiotensin-converting enzyme 2 (ACE2) receptor. When isolated from the rest of the spike protein, RBD retains receptor binding to ACE2 and works as an independent functional domain (30–32). Several studies have explored RBD-based subunit vaccines, including a clinically approved ZF2001 (Zifivax) subunit vaccine adjuvanted with aluminum hydroxide (9, 24, 33). In addition, ferritin-based nanoparticle vaccines SΔC-Fer, containing the spike-protein ectodomain trimer and adjuvants, and the more stable version of SΔC-Fer, named DCFHP, have been developed (17, 34). These studies highlight the potential of RBD-based subunit vaccine against SARS-CoV-2.
Recently, we showed that near-infrared IIb (NIR-IIb; 1500 to 1700 nm) photoluminescent rare-earth Er–based nanoparticles (named “pEr”) conjugated to a model tumor antigen ovalbumin (OVA) and complexed with a CpG-B adjuvant afforded a potent cancer vaccine, eradicating existing OVA-expressing EG.7 tumors and providing prophylactic protection for mice against EG.7 (35). In vivo NIR-IIb fluorescence imaging in the 1500- to 1700-nm range clearly resolved vaccine trafficking and migration to lymph nodes (LNs) in real time. NIR-IIb imaging benefited from suppressed light scattering, diminished autofluorescence, and deep tissue imaging depth, allowing visualization of cells, blood vasculatures, and lymphatic vessels with high spatiotemporal resolution at subcentimeter depths through intact mouse skin and tissues (32–41).
Here, we explored pEr nanoparticle as an in vivo NIR-IIb imageable/trackable vaccine carrier for infectious disease. We formulated a SARS-CoV-2 vaccine by conjugating RBD to pEr nanoparticles encased in three cross-linked hydrophilic, biocompatible polymer layers (pEr-P3; ~55 nm), without using any adjuvant done for other SARS-CoV-2 protein subunit vaccines (11–16, 36–40). We immunized mice with two doses of pEr-P3-RBD [wild type (WT) and Omicron BA.1 variant, respectively] via subcutaneous injection and used NIR-IIb in vivo imaging to observe the migration of pEr-P3-RBD from lymphatic vessels to LNs. Two weeks later, the neutralizing antibody titers against SARS-CoV-2 pseudoviruses were much higher than those reported for infected human patients (21–24) and >140-fold higher than in mice vaccinated by RBD protein–only groups. Antibodies in the sera of mice immunized by pEr-P3-RBD (WT) and pEr-P3-RBD (Omicron BA.1 variant) showed specificity to their respective pseudoviruses but cross-reacted to discernible degrees. Seven months after two doses of vaccination, we found that the neutralizing antibody titers in mice sera against SARS-CoV-2 increased instead of decreasing from the titers measured at 14 days postboosting. The results suggest that the pEr-P3-RBD nanovaccine elicits strong, durable responses of mice against SARS-CoV-2. Ex vivo flow cytometry analysis of immune cells in the LNs of mice after two pEr-P3-RBD vaccine doses observed increased RBD-specific germinal center (GC) B cells, T follicular helper cells (TFH), and immunoglobulin (Ig) class-switched GC B cells, confirming strong immune responses to the pEr-P3-RBD nanovaccine.
In vivo, we probed RBD-specific molecular signals in the LNs of vaccinated mice by subcutaneously injecting an NIR dye-RBD conjugate (IR840-RBD) and performing NIR-II imaging to glean its trafficking to LNs and assessing RBD-specific signals in the LNs longitudinally. We observed much stronger and more prolonged RBD-specific signals in the LNs of mice vaccinated by pEr-P3-RBD than in control mice administered with phosphate-buffered saline (PBS) as a control. The result corroborated the ex vivo flow cytometry data showing higher RBD-specific B cells in LNs, suggesting an effective methodology of noninvasive in vivo NIR-II molecular imaging of LNs for assessing immune responses to vaccination.
RESULTS
Synthesis of pEr nanoparticles for vaccine carriers and in vivo NIR-IIb imaging
We synthesized pEr nanoparticles composed of a pure hexagonal β-phase NaErF4 core (without other rare-earth dopants) with a hexagonal β-phase NaYF4 shell (Fig. 1A and fig. S1; synthesis details in Materials and Methods) (35). Transmission electron microscopy (TEM) imaging showed the pEr nanoparticles with an average diameter of ~26.8 nm (Fig. 1B and fig. S2) with lattice fringes corresponding to the 0.558 nm d spacing of (100) planes of the β-phase NaErF4 core (fig. S2). An ~5-nm NaYF4 shell was resolved (fig. S2) (35, 41, 42). When excited at 975 nm, the pEr nanoparticles exhibited bright down-conversion luminescence in the 1500- to 1700-nm NIR-IIb range (Fig. 1, C and D) (35, 41, 42). The large >500-nm Stokes shift afforded near-zero tissue autofluorescence (43), suppressed light scattering of the long wavelength NIR-IIb light (44) and deep tissue penetration for in vivo fluorescence imaging with high spatiotemporal resolution (35, 41–55).
Fig. 1. NIR-II/SWIR luminescent nanoparticles for the SARS-CoV-2 pEr-P3-RBD nanovaccine.
(A) Schematic of the pEr-P3-RBD (RBD conjugated to ─NH2 groups on the pEr-P3 surface via EDC chemistry). P3 refers to cross-linked three layers of hydrophilic polymers coated on the pEr nanoparticles. (B) TEM image of pEr nanoparticles comprised the NaErF4 core and NaYF4 shell; scale bar, 20 nm. (C) Energy level diagram of the erbium showing the NIR-II down conversion pathway. (D) Absorption (red curve) and emission (blue curve) spectra of pEr nanoparticles in cyclohexane (prior to P3 coating). The dashed vertical line shows the excitation wavelength (975 nm) for pEr nanoparticles. a.u., arbitrary units. (E) DLS spectra of pEr in cyclohexane (gray), hydrophilic pEr-P3 in 1×PBS buffer (blue). The average size for pEr nanoparticles is ~25.5 nm, and that for pEr-P3 nanoparticles is ~55.5 nm.
For biocompatibility, the pEr nanoparticles were transferred into an aqueous solution by coating with three layers of cross-linked hydrophilic polymers (Fig. 1A) [called “P3” coating consisting of branched polyethylene glycol (PEG)–NH2, polyacrylic acid (PAA), and PEG-NH2 layers] on the surfaces of the particles (see Materials and Methods) (35, 41, 45). The hydrodynamic sizes of the pEr-P3 nanoparticles, as measured by dynamic light scattering (DLS), were ~55.5 nm compared to ~25.5 nm in an organic solvent prior to P3 coating (Fig. 1E). The pEr nanoparticles coated with only a single layer of branched PEG-NH2 (pEr-P1) had an average size of 43.7 nm, and those coated with two layers of cross-linked hydrophilic polymers (branched PEG-NH2 and PAA, called pEr-P2) had an average size of 50.7 nm (fig. S3). The zeta potentials of pEr-P1, pEr-P2, and pEr-P3 were measured to be −0.7, −17.1, and 5 mV, respectively (fig. S4), correlating with the surface charges added to each of the successive coating layers. The amino functional groups on the outermost PEG layer were used for conjugation with antigens for vaccine construction (35, 41, 45).
pEr-P3-RBD nanoparticles as a trackable vaccine by in vivo NIR-II imaging
The SARS-CoV-2 RBD proteins (~25 kDa, 10 μg) were conjugated covalently to the ─NH2 groups on the pEr-P3 (0.8 mg) nanoparticles using 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) chemistry to form the pEr-P3-RBD nanovaccine (Fig. 1A; see Materials and Methods). The conjugation efficiency of RBD (WT) on pEr-P3 nanoparticles is 93.6% (fig. S5). We used the WT RBD (32, 56) and Omicron BA.1 type RBD (sequences shown in fig. S6) to prepare two vaccines, pEr-P3-RBD (WT) and pEr-P3-RBD (Omicron), respectively. DLS analysis revealed a small increase in nanoparticle hydrodynamic size after RBD conjugation from the initial ~55.5 nm (pEr-P3) to ~56.5 nm (pEr-P3-RBD) (fig. S2D). The zeta potential of pEr-P3-RBD (WT) was measured to be 4.7 mV (fig. S7), lower than that of pEr-P3 at 5.0 mV. TEM imaging indicated that both the P3 coating process and the conjugation process did not cause any structural damage to the pEr nanoparticles (fig. S8).
The pEr-P3-RBD vaccines were subcutaneously administered into the tail base of 6-week-old female BALB/c mice (Fig. 2A). The pEr nanoparticle was used both as an antigen delivery vehicle and an in vivo NIR-II imaging agent to visualize the vaccine trafficking pathway after subcutaneous injection. By performing wide-field NIR-IIb imaging using an InGaAs camera (under an excitation of ~975 nm while detecting the pEr luminescent emission in the range of 1500 to 1700 nm, as shown in Fig. 2B), we observed a clear increase in the pEr signal in the draining inguinal LN (iLN) (57, 58) of mice over time, reaching a substantial level at ~2 hours postinjection (p.i.) and peaking at ~24 hours p.i. (Fig. 2D). Migration of the pEr-P3-RBD to the axillary LN (aLN) was also observed (Fig. 2C). NIR-IIb signals were detected in the lymphatic vessels connecting the injection site to the iLN, as well as those linking the iLN to the aLN, revealing the trafficking pathway of the vaccine after administration (Fig. 2C). Efficient transport of vaccines to LNs is an important initial step of eliciting immune responses.
Fig. 2. In vivo NIR-IIb imaging/tracking of subcutaneously administrated pEr-P3-RBD vaccine.
(A) Immunization schedule; female BALB/c mice (n = 10) aged 6 weeks were immunized by subcutaneous injection at the mouse tail base with the pEr-P3-RBD nanovaccine on day 0 and day 21. (B) Simplified schematic of the in vivo NIR-II imaging system. CCD, charge-coupled device. (C) NIR-IIb luminescence images (975-nm excitation with a power density of ~50 mW cm−2, 1500- to 1700-nm detection, and the exposure time is 20 ms, continuous wave mode) showing the nanovaccine migrated from the injection site to mice iLNs along the lymphatic vessels and then to the proper aLNs after subcutaneous injection at the tail base of a mouse. Images were recorded at 6 hours after prime injection. h, hours. (D) Averaged pEr-P3-RBD signals in iLNs of mice normalized by background signals (in a region away from the LNs) plotted as a function of time after subcutaneous injection of pEr-P3-RBD vaccines. Error bars represent the SD of five repeated experiments. Data are presented as mean values ± SD.
The pEr NIR emission signals at the injection site of mice decreased and disappeared over a period of ~42 days (Fig. 2D and figs. S9 and S10), indicating sustained release of the vaccine following a rapid release period. Faint pEr vaccine signals started to appear in the liver and spleen at ~6 hours p.i. and then decreased gradually (fig. S9). At 24 hours p.i., pEr vaccine signals started to appear in the feces (fig. S11). Within 2 weeks, ~85% of the injected pEr in the vaccine were excreted through feces, whereas no pEr was detected in the urine, suggesting excretion of the subcutaneously injected nanoparticles through the liver/biliary route, similar to the excretion pathway for intravenously injected P3-coated nanoparticles [mostly eliminated from the body with feces in ~2 weeks (59)]. Note that we conducted histopathological examination of the organ tissues of two groups of mice euthanized at 21 days and 9 months, respectively, after the second dose of vaccination. No notable differences in histopathology were observed between the pEr-P3-RBD–vaccinated groups (n = 8) and the PBS-treated control group (n = 4) (figs. S12 and S13). We also conducted blood biochemistry tests on mice 14 days after the prime dose to assess liver function, kidney function, pancreatic function, cardiac function, and systemic metabolism. No notable differences in blood biochemistry were observed among the pEr-P3-RBD–vaccinated group (n = 4), the RBD-vaccinated group (n = 4), and the PBS-treated control group (n = 4) (figs. S14 to S16). We observed no indications of either short-term or long-term toxicity of pEr-P3-RBD in the vaccinated mice.
Body temperature and serological anti-RBD IgG responses following immunization
We immunized three groups (n = 10 in each group) of mice with pEr-P3-RBD (WT), pEr-P3-RBD (Omicron BA.1), and ~10 μg of pure RBD (WT) protein, respectively, all without any adjuvants. Each dose of the pEr-based nanovaccine contained ~10 μg of RBD antigens conjugated to ~0.8 mg of pEr-P3 nanoparticles. At 24 hours after the prime dose, the body temperatures of pEr-P3-RBD (Omicron)–immunized group (37.3° ± 0.3°C) and pEr-P3-RBD (WT)–immunized group (37.2° ± 0.2°C) were slightly higher than those of the protein-only RBD (WT) group (37.0° ± 0.2°C) (fig. S17A). At 48 hours p.i., the body temperature of the pEr-P3-RBD (Omicron) group was 37.3° ± 0.3°C, and the pEr-P3-RBD (WT) group was similar to the RBD (WT) group (37.1° ± 0.2°C). Three weeks later, we administrated the second dose, and at 24 hours after boosting, both the pEr-P3-RBD (Omicron) and pEr-P3-RBD (WT) groups showed more pronounced increases in body temperature; the pEr-P3-RBD (Omicron) group and the pEr-P3-RBD (WT) group reached 37.5° ± 0.3°C and 37.8° ± 0.3°C, respectively, compared to 37.2° ± 0.3°C for the RBD (WT) only group (fig. S17A). At 48 hours after boosting, mice in the two pEr-vaccinated groups still had slightly elevated body temperatures before recovering to the normal temperature of 37.3° ± 0.3°C at around 72 hours p.i. These results suggested that mice exhibited stronger immune responses to pEr-P3-RBD (Omicron) than to pEr-P3-RBD (WT). In addition, we monitored the body weights of treated mice and noticed a slight decrease after the second dose of the nanovaccine before the mice resuming normal rate of weight gain (fig. S17B).
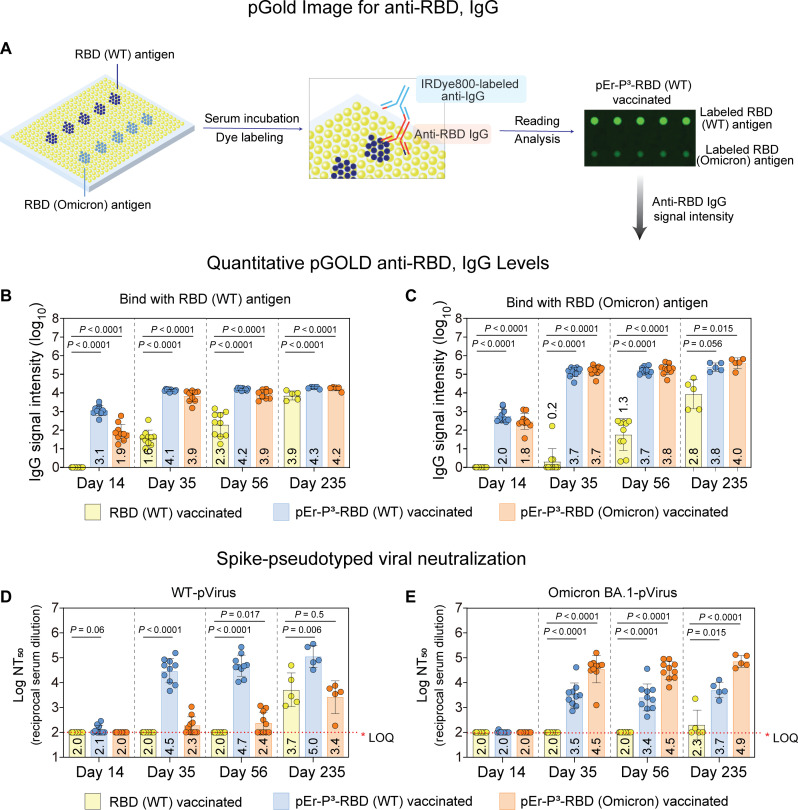
For mice immunized with pEr-P3-RBD (WT), pEr-P3-RBD (Omicron BA.1), and RBD (WT) protein only vaccines (n = 10), we measured serum levels of anti-RBD IgG antibodies 14 days after the primary vaccination and 14 days, 35 days, and 7 months after boosting, using the nanoplasmonic gold (pGOLD) assay (60–62). We microarrayed SARS-CoV-2 RBD (WT) and RBD (Omicron BA.1) antigen spots on the pGOLD platform (60, 62, 63) to capture IgG antibodies in the 10,000-fold diluted blood samples collected from mice and then labeled the captured antibodies with antimouse IgG-IRDye800 (Fig. 3A and fig. S18; see Materials and Methods) (62). The pGOLD assay showed that, 14 days after the prime vaccine dose (Fig. 3, B and C), mice immunized by pEr-P3-RBD (WT) or pEr-P3-RBD (Omicron BA.1) elicited anti-RBD IgG against both WT and Omicron BA.1 RBD antigens, suggesting cross-activity of the antibodies between the two variants. Very low IgG signals were detected in the RBD only vaccinated group. Fourteen and 35 days after boosting (day 35 and day 56), the pEr-P3-RBD (WT) and pEr-P3-RBD (Omicron BA.1)–immunized mice showed high levels of anti-RBD IgG against both WT RBD antigens and Omicron BA.1 type RBD antigens compared to the RBD only group. The high anti-RBD IgG levels persisted over 7 months after full vaccination (day 235) and remained higher than those observed in the RBD only group.
Fig. 3. Humoral antibody responses elicited by the pEr-P3-RBD nanovaccine.
The humoral antibody responses were detected using the pGOLD nanoplasmonic platform and pseudoviral neutralization antibody assay. (A) Note that RBD (WT)/RBD (Omicron) antigens were printed on the surface of pGOLD (62). Anti-RBD in the mouse serum are first captured by the assay and the labeled with anti-mouse IgG-infrared fluorescent dye (IgG-IRDye800). Binding between IgG with antigens is evaluated by reading the fluorescence intensities of IRDye800. (B) Quantitative anti-RBD (WT) IgG levels and (C) anti-RBD (Omicron) IgG levels in the serum of the mice vaccinated with RBD (WT) antigen-only, pEr-P3-RBD (WT), and pEr-P3-RBD (Omicron BA.1) without any adjuvant. The serum was collected on day 14, day 35, day 56, and day 235. Each point represents an individual animal. Error bars represent the SD of 10 (day 14, day 35, and day 56) or 5 (day 235) repeated experiments. Data are presented as mean values ± SD. (D) Log NT50 for the neutralizing antibody levels against the WT SARS-CoV-2 pseudovirus and (E) Omicron BA.1 SARS-CoV-2 pseudovirus in the serum of the mice vaccinated with RBD (WT) antigens, pEr-P3-RBD (WT), and pEr-P3-RBD (Omicron BA.1) without any adjuvants. Each dose of the vaccine contains 10 μg of RBD antigens. Note that, in (D) and (E), each point represents the NT50 titer measured with an individual animal. Error bars represent the SD of 10 (day 14, day 35, and day 56) or 5 (day 235) repeated experiments. The NT50 of 1:100 dilution is set as the limit of quantitation (LOQ).
SARS-CoV-2 neutralizing antibody responses elicited by pEr-P3-RBD immunization
We characterized the serum neutralizing antibody responses of vaccinated mice using a SARS-CoV-2 pseudovirus neutralization assay (Fig. 3, D and E) (17, 64, 65). We measured the end point of 50% reduction of virus expression (NT50) value, which was the serum dilution factor at which the diluted serum was able to neutralize 50% of the virus. All three groups of mice [vaccinated with pEr-P3-RBD (WT), pEr-P3-RBD (Omicron BA.1), and ~10 μg of pure RBD (WT) protein, respectively] displayed a low NT50 value 14 days after a single immunization dose (prime), but 14 days after boosting (day 35), mice immunized with the pEr-P3-RBD (WT) vaccine exhibited strong neutralizing activity against WT SARS-CoV-2 pseudotyped virus (WT-pVirus) with NT50 ~ 3.1 × 104, higher than the neutralizing NT50 levels of <1.9 × 103 in convalescent serum from selected patients with SARS-CoV-2 (66). Sera of these mice also exhibited neutralizing activity against the Omicron BA.1 variant pseudotyped viruses but with a 10-fold lower NT50 titer of ~3.1 × 103. For mice immunized with two doses of pEr-P3-RBD (Omicron BA.1), high serum antibody neutralizing activity against the Omicron BA.1 variant of SARS-CoV-2 pseudotyped viruses with NT50 ~3.1 × 104 was observed, compared to the lower NT50 of ~200 against the WT virus (Fig. 3, D and E). These results showed strong variant-specific neutralizing antibody responses but with noticeable cross-reactivity of viral neutralizing antibodies between the two variants.
Sera from mice immunized with two doses of RBD protein only failed to show detectable neutralizing activities against either the WT or Omicron BA.1 variant of SARS-CoV-2 with NT50 < 100, which was the limit of quantification of the neutralization assay. At day 56, the neutralizing activities in the sera of all three groups showed little change over those measured on day 35.
We monitored the immunized mice 7 months after boosting (day 235, n = 5 per group) and detected increased NT50 value, i.e., higher viral neutralization activities than at day 56 in the sera of mice vaccinated with the pEr-P3-RBD (WT), with NT50 ~105 against the WT virus and ~5 × 103 against the Omicron BA.1 virus. Sera neutralizing antibodies from mice vaccinated with pEr-P3-RBD (Omicron BA.1) exhibited NT50 ~8 × 104 against the Omicron BA.1 virus and NT50 ~5 × 103 against the WT virus. In contrast, sera from mice treated with free WT RBD exhibited NT50 ~5 × 103 against the WT virus and NT50 ~200 against the Omicron BA.1 virus, still much lower than the pEr-P3-RBD–immunized groups. Notably, NIR-IIb imaging observed that pEr-P3-RBD was released from the injection site over ~42 days p.i. (fig. S10), which could be responsible for the sustained immune stimulation to mice, prolonged effectiveness of the vaccine, and increased serum neutralizing antibodies beyond 7 months after full immunization.
GC responses stimulated by pEr-P3-RBD immunization
To further evaluate the immune responses elicited by pEr-P3-RBD vaccines, we analyzed GC-related immune cells after vaccination. The immune response of BALB/c mice skews toward a humoral [T helper 2 (TH2)] response (67). To conservatively test the ability of pEr-P3-RBD nanovaccines to stimulate a GC B cell response, we immunized C57BL/6 mice that skew toward cellular (TH1) responses (67). A similar approach was taken in studies of the BNT162b SARS mRNA vaccine that analyzed humoral responses in BALB/c mice and cellular responses in C57BL/6 (68, 69). We immunized three groups of C57BL/6 mice (n = 16 mice per group) on days 0 and 21 by subcutaneous injection at the tail base with PBS only, WT RBD protein alone, pEr-P3-RBD (10 μg of WT RBD and 0.8 mg of pEr-P3 per mouse). On day 14 after primary immunization, eight mice in each group were euthanized. On day 14 after the booster dose, the remaining mice were euthanized (n = 8), and the iLNs were collected, processed into single-cell suspensions, and stained with a panel of fluorophore-conjugated antibodies (table S1). Flow cytometry was then used to analyze GC B cells (CD19+CD95+GL7+), WT RBD-specific GC B cells (CD19+GL7+CD95+RBD-binding+), class-switched (IgM−) cells within GC B cells (CD19+CD95+GL7+IgM−), memory B cells (MBCs) (CD19+IgM−CD38+), RBD-specific MBCs (CD19+IgM−CD38+RBD-binding+), TFH cells (CD3+CD4+PD1+CXCR5+), and plasma cells (CD44+CD138+) (see fig. S19A for gating strategies).
On day 14 after primary immunization, we found higher percentages of WT RBD-specific GC B cells (fig. S19D) and WT RBD-specific MBCs (fig. S19E) in the iLNs of pEr-P3-RBD (WT)–immunized mice compared to control mice administered with PBS, suggesting that immunization enhanced RBD-specific response in the former. Fourteen days after boosting by pEr-P3-RBD (WT), the percentages further increased (Fig. 4C), consistent with continued expansion of WT RBD-specific GC B cells in the iLN after boosting. We also observed a significant increase in TFH cells in the iLNs of pEr-P3-RBD (WT) vaccinated mice compared to mice treated by PBS control (Fig. 4D; gating strategy in fig. S19A).
Fig. 4. GC B cell and TFH cell responses induced by the pEr-P3-RBD vaccine.
The 6-week-old C57BL/6 mice were immunized by RBD (WT) antigen-only and pEr-P3-RBD (WT) on day 0 and day 21 without any adjuvant, and flow cytometry analysis of iLNs conducted on day 35. (A) GC B cells (CD19+CD95+GL7+), (B) class-switched (IgM−) within GC B cells (CD19+CD95+GL7+IgM−), (C) RBD-specific GC (CD19+GL7+CD95+RBD-binding+), and (D) TFH cell response in iLNs on day 35; n = 8 for each group. One-way analysis of variance (ANOVA) followed by Tukey’s test was applied in (A) to (D). Differences between groups were considered significant for P values < 0.05. Error bars represent the SD of eight repeated experiments. Data are presented as mean values ± SD.
To investigate long-lasting memory responses, we analyzed memory-related immune cell population (70) including RBD-specific MBC and plasma cells (figs. S20 and S21) in iLNs and observed significant increases in the iLNs of pEr-P3-RBD (WT) vaccinated mice compared to control mice administered with PBS. MBCs circulated in the periphery and rapidly differentiated into long-lived plasma or reentered the GC for another round of affinity maturation upon secondary antigen exposure (71).
In vivo NIR-IIb molecular imaging of RBD-specific cells
Next, we explored in vivo molecular imaging and tracking of RBD-specific cells for assessing immune responses of mice to vaccination without euthanizing the mice. We conjugated RBD (WT) to an organic NIR dye IR840 to form an IR840-RBD probe for targeted fluorescent labeling of RBD receptors in the LNs of mice (Fig. 5, A and B; see Materials and Methods for conjugation details). The organic dye was excited at 808 nm and exhibited an emission tail into the >1000-nm NIR-II range (absorption and emission spectra shown in fig. S22), well suited for NIR-II imaging in vivo (35, 52, 53). We first immunized mice (n = 3) with pEr-P3-RBD (WT) vaccines at day 0 and day 21, together with a control group of mice (n = 3) injected with PBS. At day 35, we subcutaneously injected the IR840-RBD complexes at the tail base of mice and imaged the trafficking and distribution of the probes. To differentiate the IR840-RBD probes from pEr signals from the preinjected pEr-P3-RBD (WT) vaccine, we used an 808-nm laser excitation (as opposed to the 975-nm laser for pEr excitation) and detected in the 1000- to 1200-nm range (as opposed to 1500- to 1700-nm range for pEr) for imaging the IR840-RBD probe distribution in vivo. This avoided any fluorescence signal crossing between IR840-RBD probe and pEr-P3-RBD (WT) vaccine (Fig. 5B).
Fig. 5. In vivo NIR-IIb molecular imaging of RBD-specific cells in LNs of mice after immunization.
(A) Immunization schedule; the female BALB/c mice were immunized by subcutaneous (sc) injection at the mouse tail base with the pEr-P3-RBD (WT) nanovaccine on day 0 and day 21 treated with only PBS were used as a control group; n = 3 in each group. The mice were injected the IR840-RBD (WT) at the mouse tail base (subcutaneously) on day 35. (B) Color photograph and multiplexed NIR-II imaging were conducted on pEr-P3, IR840 in PBS buffer. Under 808-nm excitation and 1000- to 1200-nm imaging conditions, only IR840 was detectable whereas pEr-P3 was undetectable. Under 975-nm excitation and 1500- to 1700-nm imaging conditions, only pEr-P3 was detectable whereas IR840 was undetectable. (C) NIR-II luminescence images (808-nm excitation with a power density of ~70 mW cm−2, 1000- to 1200-nm detection, and the exposure time is 10 ms) showing the IR840-RBD (WT) trafficking pathways in mice immunized by pEr-P3-RBD (WT) and (D) treated by only PBS. Images were recorded at different time points as indicated after injection. Representative images from one mouse from each group are shown here. d, days. (E) In vivo IR840-RBD (WT) signal intensity in iLNs and (F) bladder normalized by the background plotted as a function of time after subcutaneous injection of IR840-RBD (WT). Error bars represent the SD of three repeated experiments. Data are presented as mean values ± SD. (G) pEr-P3-RBD (WT) vaccine or only PBS induces RBD-specific cell response in iLNs at day 35. The percentage of RBD-specific cells in live cells was counted by flow cytometry. Error bars represent the SD of eight repeated experiments. Data are presented as mean values ± SD.
At 2 hours after subcutaneous administration of the IR840-RBD probe at mouse tail base, we observed that IR840-RBD probe signals in the iLN of pEr-P3-RBD (WT) vaccinated mice were significantly higher than those in the iLN of PBS-treated control mice and persisted over a much longer time (>10 days) (Fig. 5, C to E). This suggested binding of the IR840-RBD probes to the more abundant RBD-specific cells elicited in the iLN of vaccinated mice than in the control group, consistent with ex vivo flow cytometry data (Fig. 5G). Furthermore, at 2 hours p.i., the bladder signals of IR840-RBD probes in PBS-treated mice were higher than in the pEr-P3-RBD (WT)–vaccinated group (Fig. 5F), suggesting little retention in the LNs and more rapid excretion of the injected IR840-RBD probes (within 24 hours) in the PBS-“immunized” control group. The IR840-RBD complexes were ~26.5 kDa, below the glomerular filtration molecular weight cutoff of 30 to 50 kDa (72). For injected IR840-RBD probes that were not bound and captured by RBD-specific cells in the LNs, the probes were rapidly excreted through the kidney to the bladder. In PBS-immunized mice, the facile excretion (fig. S23) corroborated with high IR840-RBD bladder signals and rapid signal disappearance from the body. In contrast, in pEr-P3-RBD–vaccinated mice, the injected IR840-RBD probes were bound/captured by abundant RBD-specific cells in the LNs, giving strong LN NIR-II fluorescence signals that persisted over a much more extended period of time. These results suggested that through-tissue NIR-II molecular imaging of mouse LNs offered rapid, in vivo noninvasive assessments of immune responses to vaccination (35).
DISCUSSION
Vaccination is central to preventing infectious disease outbreaks, curtailing pandemics and preventing/treating cancer. Developing a vaccine delivery system that allows for simultaneous tracking of vaccine migration and distribution and assessing immune responses in vivo could bring paradigm changing opportunities for vaccine development platforms. Recently, NIR-IIb (1500 to 1700 nm) imaging of a cancer vaccine pEr-P3-OVA-CpG B, using pEr-P3 as both a vaccine carrier and imaging probe allowed in vivo visualization of vaccine trafficking through the lymphatic systems of mice. The strong anticancer immune responses elicited were assessed by noninvasive longitudinal NIR-II molecular imaging of the tumor microenvironment in vivo, revealing abundant antigen-positive CD8+ cytotoxic T lymphocytes induced in the tumor microenvironment (35).
The current work establishes a pEr-P3–based vaccine for SARS-CoV-2, introducing infrared-emitting trackable vaccines to infectious disease. We envisage a NIR-II/SWIR nanovaccine platform, composed of a set of NIR-II emitting nanoparticles and/or organic fluorophores for vaccine carrier and molecular imaging probes. The platform can be used to guide vaccine formulations, visualize vaccine trafficking/migration in vivo, assess immune responses by molecular imaging of immune cells in mouse LNs longitudinally, correlate with vaccine efficacy, and provide rapid feedback to vaccine formulation optimization.
In vivo NIR-IIb fluorescence imaging exploits reduced light scattering and vanished autofluorescence owing to the large Stokes shift of >500 nm with an excitation wavelength of 985 nm and emission wavelength in the range of 1500 to 1700 nm. High-resolution NIR-IIb fluorescence imaging can visualize biological structures and dynamics with single-cell resolution at subcentimeter depths (35, 41–55) through intact mouse skin and tissues. In the current work, we observed pEr-P3-RBD vaccine trafficking through lymphatic networks shortly after subcutaneous administration and homing to the LNs of mice that peaked in ~24 hours. The facile vaccine migration to LNs was a key step to triggering effective immune responses (Fig. 2C). Previously, a control vaccine formulation built on the same pEr nanoparticles with a different phospholipid-PEG coating failed to migrate to LNs, leading to poor vaccination efficacy (35).
The pEr nanoparticles coated with P3 (consisting of branched PEG, PAA, and PEG layers) contain stable hydrophilic layers without falling off in vivo, which is crucial to both biocompatibility/short retention in the body (59) and stable attachment of protein subunit vaccine “cargos” (35). Also, the hydrodynamic size of the pEr-P3-RBD vaccine is ~55 nm (Fig. 1A), in a favorable size range suggested for effective vaccine formulation (73, 74). Overall, the cross-linked stable surface coating, stable antigen immobilization on the pEr nanoparticles, and facile migration of the vaccine complex to the LNs are the key factors responsible for the highly effective vaccination effect and sustained stimulation of the immune system.
Immunization of BALB/c mice with the pEr-P3-RBD afforded persistent vaccine release over ~42 days, as gleaned from the gradual decreases in NIR-IIb vaccine fluorescence at the subcutaneous injection site (fig. S10). This correlated with the long-lasting serological anti-RBD IgG and neutralization antibody responses over >7 months after full vaccination. Without any adjuvants, 14 days after two doses of immunization, the pEr-P3-RBD nanovaccine elicited neutralization antibody titer NT50 even higher than those elicited by several protein subunit vaccines with adjuvants (Table 1).
Table 1. Cross-neutralizing antibody responses induced by SARS-CoV-2 vaccines.
Representative SARS-CoV-2 (WT) vaccines (82–91) that induce antibodies with cross-neutralizing activity against the WT or Omicron BA.1 variant of SARS-CoV-2. LNP, lipid nanoparticle.
| Name | Vaccine format | Adjuvant | Neutralizing and cross-neutralizing activity |
|---|---|---|---|
| pEr-P3-RBD | SARS-CoV-2 RBD–based subunit vaccine | NT50 = 100,000 Omicron BA.1: NT50 = 5000 (two doses) | |
| Fc-RBD (82) | SARS-CoV-2 RBD-Fc–based subunit vaccine | Freund’s incomplete adjuvant | NT50 = 7166 (two doses) |
| VSV-ΔG-spike (83) | Competent recombinant VSV-ΔG-spike | NT50 = 2011 (one dose) | |
| ARCoV mRNA-LNP (84) | mRNA-LNP encoding the RBD | NT50 = 5704 (two doses) | |
| DYAI-100A85 (85) | SARS-CoV-2-spike-RBD subunit vaccine | Aldydrogel 85 | NT50 = 5233 (two doses) |
| pSer-RBD (86) | Phosphoserine peptides modified the RBD | Alum | NT50 = 8000 (two doses) |
| αGalCer-RBD (87) | Self-adjuvanting lipoprotein conjugate αGalCer-RBD subunit vaccine | NT50 = 11,549 (two doses) | |
| SCTV01E (88) | Spike-trimer protein-based tetravalent COVID-19 vaccine | SCT-VA02B | NT50 = 3554 Omicron BA.1: NT50 = 6431 (two doses) |
| ASD254 (89) | Subunit vaccine ASD254, chitosan + RBD + γ-PGA | NT50 = 100,000 (two doses) | |
| mRNA-HB27-LNP (90) | NT50 = 28,056 (one dose) | ||
| RBD mRNA-LNP (91) | LNP-encapsulated mRNA encoding the SARS-CoV-2 RBD | NT50 = 10,000 (one dose) |
GCs are microanatomical structures within secondary lymphoid organs, such as LNs, where antigen-activated B cells undergo somatic hypermutation of the B cell receptor gene, followed by clonal selection, resulting in antibody affinity maturation, proliferation of selected B cells, and cell differentiation into long-lived antibody-producing MBCs and plasma cells (75). Mouse experiments demonstrated that SARS-CoV-2 mRNA vaccines elicit potent GC B cells responses and TFH cells, closely intertwined with an efficient induction of SARS-CoV-2–specific neutralizing antibodies and MBC (76). Data from humans also showed that SARS-CoV-2 vaccination requires GC B cell responses to support development of plasma cells and MBC (77, 78). Booster dosing of humans with mRNA SARS-CoV-2 spike vaccine induced persistence of spike-binding GC B cells and plasma blasts in draining LNs and associated enhancement of humoral immunity (79). The percentage of class-switched MBC increases after the second (booster) vaccine dose (80). Overall, the evidence argues that a robust GC response is a prerequisite for protective immunity. Our ex vivo flow cytometry revealed that pEr-P3-RBD vaccines induced high levels of RBD-specific GC B cells localized in vaccine-draining LNs 14 days after primary immunization of mice, which further increased 2 weeks after the boost vaccine dose (Fig. 4C). Such RBD-specific GC responses strongly correlated with the observation of high neutralizing antibody production (Fig. 3, D and E). We also found that specific GC B cell responses were accompanied by a robust increase in TFH cells after primary immunization of mice (fig. S19), which was further enhanced 2 weeks after boosting (Fig. 3, D and E). These results corroborated with previous findings made with effective vaccines (81).
Last, we devised noninvasive, in vivo molecular imaging of mice LNs for assessing the immune responses to vaccination without having to euthanize the mice (Fig. 5). Upon subcutaneous injection of a fluorophore-labeled RBD probe, we performed longitudinal NIR-II imaging and observed prolonged retention (11 days versus 3 days) of the injected probes in the LNs of mice immunized with the potent pEr-P3-RBD vaccine but not in mice immunized by PBS control (Fig. 5, C to E). The long retentions of RBD-fluorophore probes in LNs were due to specific targeting and binding to the abundant RBD-specific immune cells (e.g., GC B cells) in the LNs elicited by vaccination. In contrast, for control mice injected with PBS, the fluorophore-labeled RBD probes rapidly exited from the LNs without binding to targets and excreted with mice urine (Fig. 5D and fig. S23). In addition, our imaging revealed that the excretion of IR840-RBD in the control mice was through the kidneys, with a short retention time in the LNs due to the lack of binding to antigen-specific immune cells elicited in the LNs, which was in contrast to the long retention time in the case of pEr-P3-RBD–treated mice. This suggested that pEr-P3-RBD can initiate strong immune responses in the LNs of immunized mice. Subcutaneously administrated NIR-II imaging probes can migrate to draining LNs of mice and bound to specific targets, allowing for molecular imaging in the LNs. Previously, similarly administrated anti-CD169–quantum dot a (NIR-IIa emission: 1200 to 1400 nm) and anti-CD3–quantum dot c (NIR-IIc emission: 1700 to 2000 nm) trafficked to mice LNs and afforded specific molecular imaging of CD169+ macrophages in the subcapsular sinus and CD3+ T cells inside mice LNs (51). Overall, in vivo noninvasive NIR-II molecular imaging of mice LNs presents exciting opportunities in assessing immune responses of mice to vaccination and immunotherapy in a noninvasive, longitudinal manner and could aid the development of vaccines and immunotherapeutics.
In this work, we developed a subunit SARS-CoV-2 nanovaccine using NIR-II/SWIR emitting rare-earth nanoparticles as both vaccine carrier/vector and imaging probes in the NIR-IIb 1500- to 1700-nm subwindow. After administration, noninvasive, longitudinal NIR-II imaging gleaned trafficking and migration of the nanovaccine from the injection site to LNs throughout the body via the lymphatic system, eliciting strong, long-lasting immune responses reflected by high serological anti-RBD IgG titer, SARS-CoV-2 pseudovirus neutralization activities, and RBD-specific B cells and TFH cells in mice LNs. The pEr-P3 nanoparticles as effective subunit vaccine carriers are general for both cancer (35) and infectious disease. The nanoparticle-P3 vehicles have stable hydrophilic surface coatings and allow for robust attachment of antigens for efficient vaccine cargo loading and facile trafficking to mice LNs for vaccine delivery. Moreover, we explored in vivo molecular imaging of mouse LNs for assessing immune responses to vaccination in a noninvasive manner. The NIR-II/SWIR nanovaccine platform offers a set of NIR-II emitting nanoparticles for vaccine carrier and molecular imaging probes, opening previously unknown possibilities for vaccine development.
MATERIALS AND METHODS
Materials
1-Octadecene (ODE), oleic acid, poly(maleic anhydride-alt-1-octadecene) (PMH; average molecular weight: 30 to 50 kDa), MES hydrate, 4-(dimethylamino)pyridine (DMAP), PAA (average molecular weight: 1800 Da), EDC, 2-amino-2-(hydroxymethyl)-1,3-propanediol (Tris base), rare-earth(III) acetate hydrate (RE: Er), yttrium trifluoroacetate, and sodium trifluoroacetate were purchased from Sigma-Aldrich. Hexane and chloroform were purchased from Fisher Scientific. Methoxy PEG amine (mPEG-NH2; average molecular weight: 5 kDa) was purchased from Laysan Bio. 8-Arm PEG amine (8Arm-PEG-NH2·HCl; average molecular weight: 40 kDa) was purchased from Advanced Biochemicals. Both SARS-CoV-2 (2019-nCoV) Spike RBD Protein (His Tag) (WT) and SARS-CoV-2 B.1.1.529 (Omicron) Spike RBD Protein (His Tag) were purchased from Sino Biological. IR840 was purchased from AAT Bioquest. All chemicals were used without further purification.
Methods
Synthesis of hexagonal β-phase pEr nanoparticles
The synthesis of hexagonal β-phase NaErF4 core nanocrystals involved a modified version of a previously described procedure (42). In a typical synthesis, erbium(III) acetate hydrate (0.258 g), oleic acid (4.8 ml), and 1-octadecene (12 ml) were combined in a 50-ml flask. The mixture was heated to 150°C under an argon flow for 30 min and then allowed to cool to 50°C. A methanol solution (10 ml) containing ammonium fluoride (4 mmol) and sodium hydroxide (2.5 mmol) was added, and the resulting mixture was stirred for 1 hour. The reaction mixture was heated to 100°C to evaporate the methanol and then gradually heated to 300°C (~10°C/min) under argon. The temperature was maintained at 300°C for 60 min and then increased to 305°C and kept for an additional 19 min. The temperature was then reduced to room temperature to obtain the core NaErF4 nanocrystals. The nanocrystals were precipitated by adding ethanol and collected by centrifugation (1900g, 5 min), before being dispersed in cyclohexane.
To synthesize NaErF4@NaYF4 core@shell nanoparticles, hexagonal β-phase NaYF4 nanocrystals were applied onto the previously synthesized NaErF4 core nanocrystals using an epitaxial growth method. Sodium trifluoroacetate (0.136 g), yttrium trifluoroacetate (0.428 g), oleic acid (6.4 ml), and 1-octadecene (6.5 ml) were mixed with the NaErF4 core nanocrystals in 3 ml of cyclohexane. The mixture was heated to 120°C under vacuum for 30 min to remove the cyclohexane. The temperature was then increased to 300°C and held for 75 min, followed by further elevation to 305°C and sustained for 19 min. After cooling to room temperature, the nanoparticles were purified by adding ethanol and collected by centrifugation (1900g, 5 min), repeated three times. The nanoparticles were then dispersed in 3 ml of cyclohexane. This process was repeated twice to coat an additional shell, resulting in the final pEr nanoparticles.
Surface modification of pEr nanoparticles with P3 coating
To prepare the pEr-P3 product, PMH (80 mg, 30 to 50 kDa) was dissolved in 5 ml of chloroform and mixed with pEr nanoparticles (32 mg) dispersed in cyclohexane. The solution was stirred for 1 hour, and the organic solvent was allowed to evaporate overnight. An aqueous solution of DMAP (80 mg in 6 ml of water) was added to the mixture, which was then sonicated to achieve a well-dispersed pEr@PMH solution. To remove excess PMH and DMAP, the solution was centrifuged at 14,000 rpm for 2 hours, and the resulting sediment was resuspended in 3 ml of MES solution (10 mM, pH = 11). Next, 8Arm-PEG-NH2 (12 mg) dissolved in 3 ml of MES solution and EDC (8 mg) was added, and the solution was shaken for 3 hours. Any excess ─COOH groups derived from PMH were neutralized by adding a Tris base solution (40 mg). After an additional 3 hours of reaction, large aggregates were removed by centrifugation at 4400 rpm for 30 min. The excess PEG and reaction by-products were eliminated by dialyzing the supernatant against water for 12 hours (300 kDa, changing water more than eight times). The solution was then concentrated using a centrifugal filter (100 kDa) and lastly resuspended in 3 ml of MES solution. PAA (4 mg) and EDC (8 mg) dispersed in 3 ml of MES solution were added to the mixture. After a 1-hour reaction, potential large floccules were removed by centrifugation (4400 rpm for 30 min). The excess PAA was eliminated by washing the supernatant through a centrifugal filter (100 kDa), and the resulting product was resuspended in 3 ml of MES solution. Last, mPEG-NH2 (4 mg), 8Arm-PEG-NH2 (0.8 mg), and EDC (8 mg) dissolved in 3 ml of MES solution were added to create the final shell coating (P3 coating) and reacted for 3 hours, resulting in the final pEr-P3 product.
Characterization
NaErF4@NaYF4 (core-shell) nanoparticles were characterized by powder x-ray diffraction (Rigaku MiniFlex 600 benchtop) with Cu Kα radiation. TEM images were taken with an FEI Tecnai G2 F20 X-TWIN Transmission Electron Microscope. DLS measurements were performed on a Brookhaven Instrument Nanobrook Omni. The luminescent properties of pEr nanoparticles were studied using a home-built NIR spectrograph with a spectrometer (Acton SP2300i) equipped with a liquid nitrogen–cooled InGaAs linear array detector (Princeton OMA-V). For pEr nanoparticles, a 975-nm diode laser was used as excitation. Absorption spectra were acquired with a Cary 5000 ultraviolet-visible-NIR spectrophotometer (Varian) with a scan speed of 600 nm/min.
Synthesis of pEr-P3-RBD vaccines
pEr-P3 (in 30 μl of MES solution, containing ~0.8 mg of pEr-P3 nanoparticles), RBD (10 μg), and EDC (0.2 mg) were mixed in 400 μl of MES solution (10 mM, pH = 11) and shaken for 3 hours. The solution was centrifuged for 5 min with a speed of 4400 rpm to remove any large floccules. The supernatant was washed three times by a centrifugal filter (100 kDa) and then dispersed in 50 μl of 1×PBS solution to the former pEr-P3-RBD vaccine.
Synthesis of the IR840-RBD probe
IR840 (AAT Bioquest, 10 nmol), WT RBD (1 μg), and 50 μl of PBS were mixed together. The pH of the solution was adjusted to 9.0 using 1 M phosphate buffer, and the mixture was shaken for 3 hours. The solution was washed four times by a centrifugal filter (10 kDa) and then dispersed in 20 μl of 1×PBS solution to the former IR840-RBD probe.
Conjugation efficiencies of RBD on pEr-P3 nanoparticles
The bicinchoninic acid (BCA) protein assay kit is a commonly used tool for determining the total protein concentration in biological samples. This assay relies on the ability of proteins to reduce copper(II) ions to copper(I) ions, which subsequently react with BCA to produce a purple-colored complex. The intensity of this color, measurable via spectrophotometry at 562 nm, is proportional to the protein concentration. The absorbance at 562 nm corresponds to the peak of the colored complex formed by the reaction between the protein and the BCA reagent.
To assess conjugation efficiencies, we first prepared RBD (WT) solutions with varying concentrations (2, 4, 6, 8, and 10 μg/ml) and measured their absorbance at 562 nm. We constructed a calibration curve (fig. S5), which provided a reference to link absorbance values to protein concentrations.
In our study, we used 4 mg of pEr-P3 nanoparticles and 50 μg of RBD for the conjugation process. After the reaction, the solution was washed through a 100-kDa filter to separate unbound protein, and the resulting filtrate was collected and diluted to a final volume of 5 ml. The filtrate was then mixed with the BCA reagent and incubated at 60°C for 30 min. The absorbance of the filtrate was measured at 562 nm using a NanoDrop spectrophotometer, yielding an absorbance of 0.95. This corresponded to a free protein concentration of 9.36 μg/ml in the filtrate, translating to an overall conjugation efficiency of ~93.6%.
Mouse immunizations
Female BALB/c mice (6 weeks old) were purchased from the Charles River Laboratories. The mice were randomly divided into three groups (n = 5 to 10 per group) and were immunized subcutaneously at the base of the tail with 50 μl of 1×PBS solution of one of the following SARS-COV-2 vaccines: RBD (WT, 10 μg per mouse), pEr-P3-RBD (WT, 10 μg of RBD and 0.8 mg of pEr-P3 per mouse), pEr-P3-RBD (Omicron BA.1, 10 μg of RBD and 0.8 mg of pEr-P3 per mouse). Mice were immunized on day 0 and day 21. All mice were maintained at Stanford University according to the Public Health Service Policy for “Humane Care and Use of Laboratory Animals” following a protocol approved by the Stanford University Administrative Panel on Laboratory Animal Care (APLAC-33709). The mice were bled 14 days after the primary immunization, as well as 14 and 35 days after the booster dose. Serum samples were isolated using Sarstedt serum collection tubes by centrifuging at 10,000g for 5 min and heat inactivated for 30 min at 56°C.
In vivo wide-field NIR-II fluorescence imaging
The NIR-II wide-field fluorescence images were captured using a two-dimensional water-cooled InGaAs camera (Ninox640, Raptor Photonics) operating at −21°C. A 1500-nm long-pass filter (FELH1500, Thorlabs) was used to select the desired wavelength range. Imaging windows of 1500 to 1700 nm were generated as the InGaAs camera’s detection range extended up to ~1700 nm. The pEr nanoparticles were visualized by detecting emission within the 1500- to 1700-nm range, using a 20-ms exposure time and a power density of ~50 mW cm−2.
Mouse handling
All animal experiments were approved by the Stanford Institutional Animal Care and Use Committee (approval number: 11253). All experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Six-week-old BALB/c and C57BL/6 female mice were purchased from Charles River Laboratories. Bedding, nesting material, food, and water were provided by the Stanford Veterinary Service Center (VSC) facility. The mice were housed on a 12-hour light/12-hour dark at ambient temperature (20° to 25°C) and humidity (50 to 65%) in Stanford VSC. The hair was removed with hair removal lotion (Nair, Softening Baby Oil). Mice were randomly selected from cages for all experiments. During in vivo imaging, all mice were anesthetized by a rodent anesthesia machine with O2 gas (2 liters min−1) mixed with 2.5% isoflurane.
Data processing
The luminescent and absorbance spectra raw data were processed in Origin 2021 (OriginLab). For the wide-field NIR-II fluorescence imaging data, the signal intensity of pEr nanoparticles in the LNs was measured across the entire iLN area using ImageJ/Fiji (2021). The background signal was obtained from a randomly selected area without vasculature or lymphatic vessels. This allowed for the calculation of the ratio between the signal intensity in the LNs and the background, providing an accurate quantification and analysis of the pEr nanoparticles’ signal specifically in the LNs.
Nanoplasmonic gold (pGOLD) assay
To investigate the immune response elicited by pEr-P3-RBD (WT), pEr-P3-RBD (Omicron BA.1), and RBD (WT) protein vaccines (n = 10), we assessed serum levels of anti-RBD IgG antibodies at various time points. Two weeks after the primary vaccination, as well as 2 weeks, 5 weeks, and 7 months after the booster immunizations, we used the nanoplasmonic gold (pGOLD) assay (60, 62). The pGOLD platform featured microarrayed antigen spots, including SARS-CoV-2 spike protein S1 subunit, RBD (WT), and RBD (Omicron BA.1). These spots were used to capture IgG antibodies present in the 10,000-fold diluted blood samples collected from the mice. The captured antibodies were then labeled with antimouse IgG-IRDye800.
We used a confocal microscopy scanner (Nirmidas Biotech) to image the pGOLD biochip and then analyzed the IgG antibodies bound to each antigen spot based on the fluorescence intensities of the IRDye800 dye. The median fluorescence intensity (MFI) signal was quantified for each microarray using the MidaScan Software version 2.0.0. To calculate the average MFI, the antigen spots with the highest and lowest MFIs were removed for each channel. This resulted in a single signal intensity that was used to measure antibody detection in each sample.
Production of pseudotyped lentivirus
Six million human embryonic kidney (HEK) 293T cells were seeded with D10 media [Dulbecco’s modified Eagle’s medium (DMEM) + 10% fetal bovine serum (FBS), l-glutamate, penicillin, streptomycin, and 10 mM Hepes] onto a 10-cm dish 1 day prior to transfection. A five-plasmid system (65) was used for viral production. The Spike vector contained the 21–amino acid truncated form of the SARS-CoV-2 Spike sequence from the Wuhan-Hu-1 strain of SARS-CoV-2 or the sequences from variants of concern, Omicron BA.1. The plasmids were added to a final volume of 1 ml of D10 media in the following ratios: 10 μg of pHAGE-Luc2-IRS-ZsGreen, 3.4 μg of FL Spike, 2.2 μg of HDM-Hgpm2, 2.2 μg of HDM-Tat1b, and 2.2 μg of pRC-CMV-Rev1b. Then, 30 μl of Bio T reagent was added to the mixture and incubated for 10 min at room temperature. Subsequently, additional 9 ml of D10 media was added. The transfection mixture was then slowly transferred to the 10-cm dish containing previously seeded HEK293T cells. The culture medium was removed 24 hours after transfection and replaced with a fresh D10 medium. Viral supernatants were harvested 72 hours after transfection by spinning at 300g for 5 min followed by filtering through a 0.45-μm filter. Viral stocks were aliquoted and stored at −80°C until further use.
Neutralization assay
The target cells used for infection in viral neutralization assays were from a HeLa cell line stably overexpressing the SARS-CoV-2 receptor, ACE2, as well as the protease known to process SARS-CoV-2, TMPRSS2. ACE2/TMPRSS2/HeLa cells were plated 1 day before infection at 10,000 cells per well. White-walled, clear-bottom, 96-well plates were used for the assay (Thermo Fisher Scientific). On the day of the assay, dilutions of the serum were made into a sterile D10 medium [DMEM + 10% FBS, l-glutamine, penicillin (100 U/ml), streptomycin (100 U/ml), and 10 mM Hepes] to a final volume of 60 μl. Samples were assayed in technical duplicate in each experiment. All other wells contained only D10 medium. A virus mixture was made containing the virus of interest in D10 medium and polybrene. Virus dilutions into medium were selected such that a suitable signal would be obtained in the virus-only wells. A suitable signal was selected such that the virus-only wells would achieve a luminescence of at least >1,000,000 relative light units. Sixty microliters of this virus mixture was added to each of the serum dilutions to make a final volume of 120 μl in each well. Virus-only wells were made containing 60 μl of D10 medium and 60 μl of virus mixture. Cell-only wells were made containing 120 μl of D10 medium. The diluted serum/virus mixture was incubated for 1 hour at 37°C. Following incubation, the medium was removed from the cells on the plates made day before, replaced with 100 μl of diluted serum/virus dilutions, and incubated at 37°C for ~48 hours. Infectivity readout was performed by measuring luciferase with a microplate reader (Tecan). Normalized values were fitted with a three-parameter nonlinear regression inhibitor curve in GraphPad Prism (v9.2.0) to obtain NT50 values.
Animal handling and vaccine inoculation for flow cytometry studies
Six-week-old C57BL/6 mice were randomly divided into four groups (n = 8 per group) and were immunized subcutaneously at the base of the tail with 50 μl of 1×PBS solution of one of the following SARS-COV-2 vaccines: RBD (WT, 10 μg per mouse), pEr-P3-RBD (WT, 10 μg of RBD and 0.8 mg of pEr-P3 per mouse), pEr-P3-RBD (Omicron BA.1, 10 μg of RBD and 0.8 mg of pEr-P3 per mouse), or PBS as a control.
Cell preparation for flow cytometry analysis
The iLNs and spleen were collected and mechanically dissociated using a syringe rubber head in complete RPMI media supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 1% penicillin/streptomycin, and 25 mM Hepes. The resulting tissue suspension was filtered through a 70-μm cell strainer to obtain a single-cell suspension, followed by ammonium chloride potassium (ACK) lysis (Lonza) to remove red blood cells. The cells were then washed once by centrifugation at 300g for 5 min at 4°C and resuspended in cold PBS with 1% FBS for subsequent staining.
Flow cytometry analysis of GC B cells, MBCs, TFH cells, and plasma cells
Single-cell suspensions were first stained for viability with Ghost Dye Violet 510 (Tonbo Biosciences). Then, cells were washed and incubated with Fc receptor blocking antibody anti-CD16/32 (clone 2.4G2, BD Biosciences) at a ratio of 1:200 for 5 min, followed by addition of biotinylated RBD precoupled to phycoerythrin (PE)–conjugated streptavidin (12-4317-87; eBioscience) prior to staining with fluorochrome-conjugated antibodies in a fluorescence-activated cell sorting (FACS) staining buffer (1x PBS, 3% FBS, 1 mM EDTA, and 0.02% sodium azide): CD19 (clone 1D3/CD19, BioLegend), CD38 (clone 90, BD Biosciences), CD95 (clone Jo2, BD Biosciences), CD138 (clone 281-2, BD Biosciences), CD44 (clone IM7, BioLegend), CD3 (clone 17A2, BioLegend), CXCR4 (clone L276F12; 146511; BioLegend), CD86 (clone GL1; 105043; BioLegend), CD4 (clone GK1.5, BioLegend), CXCR5 (clone L138D7, BioLegend) and PD1 (clone 29F.1A12, BioLegend), and goat anti-mouse IgM (μ-chain specific; 115-001-020; Jackson ImmunoResearch). For IgM and the antigen probe (RBD probe), the staining started with those antibodies/probes separately, followed by staining with the remaining antibodies. Surface staining was carried out for 30 min on ice. After staining, cells were washed and fixed with BD Cytofix (4.2%) (BD Biosciences) for 10 min at room temperature. Stained cells were analyzed using the BD FACS Diva v8.01 software, associated with a BD Symphony flow cytometer at the Stanford Shared FACS Facility. Data were analyzed using the FlowJo software (BD Biosciences).
Acknowledgments
Funding: This work was supported by the National Institutes of Health Pioneer Award (NIH DP1-NS-105737).
Author contributions: Y.J. and H.D. designed the research. Y.J., M.S., and N.A.H. performed the research. Y.J., M.S., N.A.H., A.B., M.Z., F.W., F.R., J.L., G.Z., Y.M., J.Z.A., E.M., and H.D. analyzed the data. Y.J., H.D., M.S., E.M. and N.A.H. wrote the paper.
Competing interests: H.D. is a scientific adviser and cofounder of Nirmidas Biotech Inc., which commercialized the pGOLD assay platform. The authors declare that they have no other competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
The PDF file includes:
Figs. S1 to S23
Table S1
Legend for movie S1
Other Supplementary Material for this manuscript includes the following:
Movie S1
REFERENCES AND NOTES
- 1.World Health Organization, WHO Coronavirus Disease (COVID-19) Dashboard, https://covid19.who.int/ [accessed 27 June 2023].
- 2.World Health Organization, COVID-19 Vaccines with WHO Emergency Use Listing, https://extranet.who.int/prequal/vaccines/covid-19-vaccines-who-emergency-use-listing.
- 3.World Health Organization, COVID-19 vaccine tracker and landscape, https://who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 4.Yap C., Ali A., Prabhakar A., Prabhakar A., Pal A., Lim Y. Y., Kakodkar P., Comprehensive literature review on COVID-19 vaccines and role of SARS-CoV-2 variants in the pandemic. Ther. Adv. Vaccines Immunother. 9, 25151355211059791 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine-Tiefenbrun M., Yelin I., Alapi H., Katz R., Herzel E., Kuint J., Chodick G., Gazit S., Patalon T., Kishony R., Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat. Med. 27, 2108–2110 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Berec L., Šmíd M., Přibylová L., Májek O., Pavlík T., Jarkovský J., Zajíček M., Weiner J., Barusová T., Trnka J., Protection provided by vaccination, booster doses and previous infection against covid-19 infection, hospitalisation or death over time in Czechia. PLOS ONE 17, e0270801 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polack F. P., Thomas S. J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J. L., Pérez Marc G., Moreira E. D., Zerbini C., Bailey R., Swanson K. A., Roychoudhury S., Koury K., Li P., Kalina W. V., Cooper D., Frenck R. W., Hammitt L. L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D. B., Mather S., Dormitzer P. R., Şahin U., Jansen K. U., Gruber W. C., C4591001 Clinical Trial Group , Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baden L. R., El Sahly H. M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S. A., Rouphael N., Creech C. B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B. S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., COVE Study Group , Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang N., Shang J., Jiang S., Du L., Subunit vaccines against emerging pathogenic human coronaviruses. Front. Microbiol. 11, 298 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moyle P. M., Toth I., Modern subunit vaccines: Development, components, and research opportunities. ChemMedChem 8, 360–376 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Mekonnen D., Mengist H. M., Jin T., SARS-CoV-2 subunit vaccine adjuvants and their signaling pathways. Expert Rev. Vaccines 21, 69–81 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng H., Fu Y.-X., Innovative adjuvant augments potency of a SARS-CoV-2 subunit vaccine. Cell Res. 32, 331–332 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y., Huang X., Yuan L., Wang S., Zhang Y., Xiong H., Chen R., Ma J., Qi R., Nie M., Xu J., Zhang Z., Chen L., Wei M., Zhou M., Cai M., Shi Y., Zhang L., Yu H., Hong J., Wang Z., Hong Y., Yue M., Li Z., Chen D., Zheng Q., Li S., Chen Y., Cheng T., Zhang J., Zhang T., Zhu H., Zhao Q., Yuan Q., Guan Y., Xia N., A recombinant spike protein subunit vaccine confers protective immunity against SARS-CoV-2 infection and transmission in hamsters. Sci. Transl. Med. 13, eabg1143 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buntinx E., Brochado L., Borja-Tabora C., Yu C. Y., Alberto E. R., Montellano M. E. B., Carlos J. C., Toloza L. B., Hites M., Siber G., Clemens R., Ambrosino D., Qin H., Chen H. L., Han H. H., Hu B., Li P., Baccarini C., Smolenov I., Immunogenicity of an adjuvanted SARS-CoV-2 trimeric S-protein subunit vaccine (SCB-2019) in SARS-CoV-2-naïve and exposed individuals in a phase 2/3, double-blind, randomized study. Vaccine 41, 1875–1884 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grigoryan L., Lee A., Walls A. C., Lai L., Franco B., Arunachalam P. S., Feng Y., Luo W., Vanderheiden A., Floyd K., Wrenn S., Pettie D., Miranda M. C., Kepl E., Ravichandran R., Sydeman C., Brunette N., Murphy M., Fiala B., Carter L., Coffman R. L., Novack D., Kleanthous H., O’Hagan D. T., van der Most R., McLellan J. S., Suthar M., Veesler D., King N. P., Pulendran B., Adjuvanting a subunit SARS-CoV-2 vaccine with clinically relevant adjuvants induces durable protection in mice. NPJ Vaccines 7, 55 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan H.-X., Juno J. A., Lee W. S., Barber-Axthelm I., Kelly H. G., Wragg K. M., Esterbauer R., Amarasena T., Mordant F. L., Subbarao K., Kent S. J., Wheatley A. K., Immunogenicity of prime-boost protein subunit vaccine strategies against SARS-CoV-2 in mice and macaques. Nat. Commun. 12, 1403 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weidenbacher P. A.-B., Sanyal M., Friedland N., Tang S., Arunachalam P. S., Hu M., Kumru O. S., Morris M. K., Fontenot J., Shirreff L., Do J., Cheng Y.-C., Vasudevan G., Feinberg M. B., Villinger F. J., Hanson C., Joshi S. B., Volkin D. B., Pulendran B., Kim P. S., A ferritin-based COVID-19 nanoparticle vaccine that elicits robust, durable, broad-spectrum neutralizing antisera in non-human primates. Nat. Commun. 14, 2149 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin E. G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., Doolman R., Asraf K., Mendelson E., Ziv A., Rubin C., Freedman L., Kreiss Y., Regev-Yochay G., Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 385, e84 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Q., Ji K., Tian S., Wang F., Huang B., Tong Z., Tan S., Hao J., Wang Q., Tan W., Gao G. F., Yan J., A single-dose mRNA vaccine provides a long-term protection for hACE2 transgenic mice from SARS-CoV-2. Nat. Commun. 12, 776 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandolesi M., Sheward D. J., Hanke L., Ma J., Pushparaj P., Perez Vidakovics L., Kim C., Àdori M., Lenart K., Loré K., Castro Dopico X., Coquet J. M., McInerney G. M., Karlsson Hedestam G. B., Murrell B., SARS-CoV-2 protein subunit vaccination of mice and rhesus macaques elicits potent and durable neutralizing antibody responses. Cell Rep. Med. 2, 100252 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbiani D. F., Gaebler C., Muecksch F., Lorenzi J. C. C., Wang Z., Cho A., Agudelo M., Barnes C. O., Gazumyan A., Finkin S., Hägglöf T., Oliveira T. Y., Viant C., Hurley A., Hoffmann H.-H., Millard K. G., Kost R. G., Cipolla M., Gordon K., Bianchini F., Chen S. T., Ramos V., Patel R., Dizon J., Shimeliovich I., Mendoza P., Hartweger H., Nogueira L., Pack M., Horowitz J., Schmidt F., Weisblum Y., Michailidis E., Ashbrook A. W., Waltari E., Pak J. E., Huey-Tubman K. E., Koranda N., Hoffman P. R., West A. P., Rice C. M., Hatziioannou T., Bjorkman P. J., Bieniasz P. D., Caskey M., Nussenzweig M. C., Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 584, 437–442 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouwer P. J. M., Caniels T. G., van der Straten K., Snitselaar J. L., Aldon Y., Bangaru S., Torres J. L., Okba N. M. A., Claireaux M., Kerster G., Bentlage A. E. H., van Haaren M. M., Guerra D., Burger J. A., Schermer E. E., Verheul K. D., van der Velde N., van der Kooi A., van Schooten J., van Breemen M. J., Bijl T. P. L., Sliepen K., Aartse A., Derking R., Bontjer I., Kootstra N. A., Wiersinga W. J., Vidarsson G., Haagmans B. L., Ward A. B., de Bree G. J., Sanders R. W., van Gils M. J., Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science 369, 643–650 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoury D. S., Cromer D., Reynaldi A., Schlub T. E., Wheatley A. K., Juno J. A., Subbarao K., Kent S. J., Triccas J. A., Davenport M. P., Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 27, 1205–1211 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Premkumar L., Segovia-Chumbez B., Jadi R., Martinez D. R., Raut R., Markmann A. J., Cornaby C., Bartelt L., Weiss S., Park Y., Edwards C. E., Weimer E., Scherer E. M., Rouphael N., Edupuganti S., Weiskopf D., Tse L. V., Hou Y. J., Margolis D., Sette A., Collins M. H., Schmitz J., Baric R. S., de Silva A. M., The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 5, eabc8413 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L., Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 30, 343–355 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wrapp D., Wang N., Corbett K. S., Goldsmith J. A., Hsieh C.-L., Abiona O., Graham B. S., McLellan J. S., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367, 1260–1263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walls A. C., Park Y.-J., Tortorici M. A., Wall A., McGuire A. T., Veesler D., Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181, 281–292.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann M., Kleine-Weber H., Pöhlmann S., A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell 78, 779–784.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y., Yang C., Xu X., Xu W., Liu S., Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 41, 1141–1149 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q., Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367, 1444–1448 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F., Structural basis of receptor recognition by SARS-CoV-2. Nature 581, 221–224 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X., Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581, 215–220 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.-Y., Wang Q., Zhou H., Yan J., Qi J., Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 181, 894–904.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powell A. E., Zhang K., Sanyal M., Tang S., Weidenbacher P. A., Li S., Pham T. D., Pak J. E., Chiu W., Kim P. S., A single immunization with spike-functionalized ferritin vaccines elicits neutralizing antibody responses against SARS-CoV-2 in mice. ACS Cent. Sci. 7, 183–199 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren F., Wang F., Baghdasaryan A., Li Y., Liu H., Hsu R., Wang C., Li J., Zhong Y., Salazar F., Xu C., Jiang Y., Ma Z., Zhu G., Zhao X., Wong K. K., Willis R., Christopher Garcia K., Wu A., Mellins E., Dai H., Shortwave-infrared-light-emitting probes for the in vivo tracking of cancer vaccines and the elicited immune responses. Nat. Biomed. Eng 8, 726–739 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuo T.-Y., Lin M.-Y., Coffman R. L., Campbell J. D., Traquina P., Lin Y.-J., Liu L. T.-C., Cheng J., Wu Y.-C., Wu C.-C., Tang W.-H., Huang C.-G., Tsao K.-C., Chen C., Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 10, 20085 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arunachalam P. S., Feng Y., Ashraf U., Hu M., Walls A. C., Edara V. V., Zarnitsyna V. I., Aye P. P., Golden N., Miranda M. C., Green K. W. M., Threeton B. M., Maness N. J., Beddingfield B. J., Bohm R. P., Scheuermann S. E., Goff K., Dufour J., Russell-Lodrigue K., Kepl E., Fiala B., Wrenn S., Ravichandran R., Ellis D., Carter L., Rogers K., Shirreff L. M., Ferrell D. E., Deb Adhikary N. R., Fontenot J., Hammond H. L., Frieman M., Grifoni A., Sette A., O’Hagan D. T., Van Der Most R., Rappuoli R., Villinger F., Kleanthous H., Rappaport J., Suthar M. S., Veesler D., Wang T. T., King N. P., Pulendran B., Durable protection against the SARS-CoV-2 Omicron variant is induced by an adjuvanted subunit vaccine. Sci. Transl. Med. 14, eabq4130 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalita P., Padhi A. K., Zhang K. Y. J., Tripathi T., Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb. Pathog. 145, 104236 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qamar M. T. U., Shahid F., Aslam S., Ashfaq U. A., Aslam S., Fatima I., Fareed M. M., Zohaib A., Chen L.-L., Reverse vaccinology assisted designing of multiepitope-based subunit vaccine against SARS-CoV-2. Infect. Dis. Poverty 9, 132 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y., Wang L., Cao H., Liu C., SARS-CoV-2 S1 is superior to the RBD as a COVID-19 subunit vaccine antigen. J. Med. Virol. 93, 892–898 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong Y., Ma Z., Wang F., Wang X., Yang Y., Liu Y., Zhao X., Li J., Du H., Zhang M., Cui Q., Zhu S., Sun Q., Wan H., Tian Y., Liu Q., Wang W., Garcia K. C., Dai H., In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles. Nat. Biotechnol. 37, 1322–1331 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson N. J. J., He S., Diao S., Chan E. M., Dai H., Almutairi A., Direct evidence for coupled surface and concentration quenching dynamics in lanthanide-doped nanocrystals. J. Am. Chem. Soc. 139, 3275–3282 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Diao S., Hong G., Antaris A. L., Blackburn J. L., Cheng K., Cheng Z., Dai H., Biological imaging without autofluorescence in the second near-infrared region. Nano Res. 8, 3027–3034 (2015). [Google Scholar]
- 44.Diao S., Blackburn J. L., Hong G., Antaris A. L., Chang J., Wu J. Z., Zhang B., Cheng K., Kuo C. J., Dai H., Fluorescence imaging in vivo at wavelengths beyond 1500 nm. Angew. Chem. Int. Ed. Engl. 54, 14758–14762 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Ma Z., Wang F., Zhong Y., Salazar F., Li J., Zhang M., Ren F., Wu A. M., Dai H., Cross-link-functionalized nanoparticles for rapid excretion in nanotheranostic applications. Angew. Chem. Int. Ed. Engl. 59, 20552–20560 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Zhang M., Yue J., Cui R., Ma Z., Wan H., Wang F., Zhu S., Zhou Y., Kuang Y., Zhong Y., Pang D.-W., Dai H., Bright quantum dots emitting at ~1,600 nm in the NIR-IIb window for deep tissue fluorescence imaging. Proc. Natl. Acad. Sci. U.S.A. 115, 6590–6595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang F., Qu L., Ren F., Baghdasaryan A., Jiang Y., Hsu R., Liang P., Li J., Zhu G., Ma Z., Dai H., High-precision tumor resection down to few-cell level guided by NIR-IIb molecular fluorescence imaging. Proc. Natl. Acad. Sci. U.S.A. 119, e2123111119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma Z., Zhang M., Yue J., Alcazar C., Zhong Y., Doyle T. C., Dai H., Huang N. F., Near-infrared IIb fluorescence imaging of vascular regeneration with dynamic tissue perfusion measurement and high spatial resolution. Adv. Funct. Mater. 28, 1803417 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang F., Ma Z., Zhong Y., Salazar F., Xu C., Ren F., Qu L., Wu A. M., Dai H., In vivo NIR-II structured-illumination light-sheet microscopy. Proc. Natl. Acad. Sci. U.S.A. 118, e2023888118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong G., Antaris A. L., Dai H., Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017). [Google Scholar]
- 51.Wang F., Ren F., Ma Z., Qu L., Gourgues R., Xu C., Baghdasaryan A., Li J., Zadeh I. E., Los J. W. N., Fognini A., Qin-Dregely J., Dai H., In vivo non-invasive confocal fluorescence imaging beyond 1,700 nm using superconducting nanowire single-photon detectors. Nat. Nanotechnol. 17, 653–660 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang F., Wan H., Ma Z., Zhong Y., Sun Q., Tian Y., Qu L., Du H., Zhang M., Li L., Ma H., Luo J., Liang Y., Li W. J., Hong G., Liu L., Dai H., Light-sheet microscopy in the near-infrared II window. Nat. Methods 16, 545–552 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong Y., Dai H., A mini-review on rare-earth down-conversion nanoparticles for NIR-II imaging of biological systems. Nano Res. 13, 1281–1294 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan H., Du H., Wang F., Dai H., Molecular imaging in the second near-infrared window. Adv. Funct. Mater. 29, 1900566 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu S., Yang Q., Antaris A. L., Yue J., Ma Z., Wang H., Huang W., Wan H., Wang J., Diao S., Zhang B., Li X., Zhong Y., Yu K., Hong G., Luo J., Liang Y., Dai H., Molecular imaging of biological systems with a clickable dye in the broad 800- to 1,700-nm near-infrared window. Proc. Natl. Acad. Sci. U.S.A. 114, 962–967 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S., Zhou Y., Du L., Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 17, 613–620 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shao L., Mori S., Yagishita Y., Okuno T., Hatakeyama Y., Sato T., Kodama T., Lymphatic mapping of mice with systemic lymphoproliferative disorder: Usefulness as an inter-lymph node metastasis model of cancer. J. Immunol. Methods 389, 69–78 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Kikuchi R., Sukhbaatar A., Sakamoto M., Mori S., Kodama T., A model system for studying superselective radiotherapy of lymph node metastasis in mice with swollen lymph nodes. Clin. Transl. Radiat. Oncol. 20, 53–57 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma Z., Wang F., Zhong Y., Salazar F., Li J., Zhang M., Ren F., Wu A. M., Dai H., Cross-link-functionalized nanoparticles for rapid excretion in nanotheranostic applications. Angew. Chem. Weinheim Bergstr. Ger. 132, 20733–20741 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.K. P. Bliden, T. Liu, D. Sreedhar, J. Kost, J. Hsiung, S. Zhao, D. Shan, A. Usman, N. Walia,A. Cho, C. Jerjian, U. S. Tantry, P. A. Gurbel, M. Tang, H. Dai, Evolution of anti-SARS-CoV-2 IgG antibody and IgG avidity post Pfizer and Moderna mRNA vaccinations. medRxiv 2021.06.28.21259338 [Preprint] (2021). 10.1101/2021.06.28.21259338. [DOI]
- 61.T. Liu, J. Hsiung, S. Zhao, J. Kost, D. Sreedhar, K. Olson, D. Keare, J. Roche, C. V. Hanson,C. Press, J. Boggs, J. P. Rodriguez-Soto, J. G. Montoya, M. Tang, H. Dai, High-accuracy multiplexed SARS-CoV-2 antibody assay with avidity and saliva capability on a nano-plasmonic platform. bioRxiv 2020.06.16.155580 [Preprint] (2020). 10.1101/2020.06.16.155580. [DOI]
- 62.Liu T., Hsiung J., Zhao S., Kost J., Sreedhar D., Hanson C. V., Olson K., Keare D., Chang S. T., Bliden K. P., Gurbel P. A., Tantry U. S., Roche J., Press C., Boggs J., Rodriguez-Soto J. P., Montoya J. G., Tang M., Dai H., Quantification of antibody avidities and accurate detection of SARS-CoV-2 antibodies in serum and saliva on plasmonic substrates. Nat. Biomed. Eng. 4, 1188–1196 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Tabakman S. M., Lau L., Robinson J. T., Price J., Sherlock S. P., Wang H., Zhang B., Chen Z., Tangsombatvisit S., Jarrell J. A., Utz P. J., Dai H., Plasmonic substrates for multiplexed protein microarrays with femtomolar sensitivity and broad dynamic range. Nat. Commun. 2, 466 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rogers T. F., Zhao F., Huang D., Beutler N., Burns A., He W.-T., Limbo O., Smith C., Song G., Woehl J., Yang L., Abbott R. K., Callaghan S., Garcia E., Hurtado J., Parren M., Peng L., Ramirez S., Ricketts J., Ricciardi M. J., Rawlings S. A., Wu N. C., Yuan M., Smith D. M., Nemazee D., Teijaro J. R., Voss J. E., Wilson I. A., Andrabi R., Briney B., Landais E., Sok D., Jardine J. G., Burton D. R., Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 369, 956–963 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crawford K. H. D., Eguia R., Dingens A. S., Loes A. N., Malone K. D., Wolf C. R., Chu H. Y., Tortorici M. A., Veesler D., Murphy M., Pettie D., King N. P., Balazs A. B., Bloom J. D., Protocol and reagents for pseudotyping lentiviral particles with SARS-CoV-2 spike protein for neutralization assays. Viruses 12, 513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ni L., Ye F., Cheng M.-L., Feng Y., Deng Y.-Q., Zhao H., Wei P., Ge J., Gou M., Li X., Sun L., Cao T., Wang P., Zhou C., Zhang R., Liang P., Guo H., Wang X., Qin C.-F., Chen F., Dong C., Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity 52, 971–977.e3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yagi J., Arimura Y., Takatori H., Nakajima H., Iwamoto I., Uchiyama T., Genetic background influences Th cell differentiation by controlling the capacity for IL-2-induced IL-4 production by naive CD4+ T cells. Int. Immunol. 18, 1681–1690 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Vogel A. B., Kanevsky I., Che Y., Swanson K. A., Muik A., Vormehr M., Kranz L. M., Walzer K. C., Hein S., Güler A., Loschko J., Maddur M. S., Ota-Setlik A., Tompkins K., Cole J., Lui B. G., Ziegenhals T., Plaschke A., Eisel D., Dany S. C., Fesser S., Erbar S., Bates F., Schneider D., Jesionek B., Sänger B., Wallisch A.-K., Feuchter Y., Junginger H., Krumm S. A., Heinen A. P., Adams-Quack P., Schlereth J., Schille S., Kröner C., de la Caridad Güimil Garcia R., Hiller T., Fischer L., Sellers R. S., Choudhary S., Gonzalez O., Vascotto F., Gutman M. R., Fontenot J. A., Hall-Ursone S., Brasky K., Griffor M. C., Han S., Su A. A. H., Lees J. A., Nedoma N. L., Mashalidis E. H., Sahasrabudhe P. V., Tan C. Y., Pavliakova D., Singh G., Fontes-Garfias C., Pride M., Scully I. L., Ciolino T., Obregon J., Gazi M., Carrion R. Jr., Alfson K. J., Kalina W. V., Kaushal D., Shi P.-Y., Klamp T., Rosenbaum C., Kuhn A. N., Türeci Ö., Dormitzer P. R., Jansen K. U., Sahin U., BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 592, 283–289 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Li C., Lee A., Grigoryan L., Arunachalam P. S., Scott M. K. D., Trisal M., Wimmers F., Sanyal M., Weidenbacher P. A., Feng Y., Adamska J. Z., Valore E., Wang Y., Verma R., Reis N., Dunham D., O’Hara R., Park H., Luo W., Gitlin A. D., Kim P., Khatri P., Nadeau K. C., Pulendran B., Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 23, 543–555 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurosaki T., Kometani K., Ise W., Memory B cells. Nat. Rev. Immunol. 15, 149–159 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Reusch L., Angeletti D., Memory B-cell diversity: From early generation to tissue residency and reactivation. Eur. J. Immunol. 53, e2250085 (2023). [DOI] [PubMed] [Google Scholar]
- 72.Graham R. C., Karnovsky M. J., Glomerular permeability. Ultrastructural cytochemical studies using peroxidases as protein tracers. J. Exp. Med. 124, 1123–1134 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bachmann M. F., Jennings G. T., Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 10, 787–796 (2010). [DOI] [PubMed] [Google Scholar]
- 74.Reddy S. T., van der Vlies A. J., Simeoni E., Angeli V., Randolph G. J., O’Neil C. P., Lee L. K., Swartz M. A., Hubbell J. A., Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 25, 1159–1164 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Mesin L., Ersching J., Victora G. D., Germinal center B cell dynamics. Immunity 45, 471–482 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lederer K., Castaño D., Gómez Atria D., Oguin T. H., Wang S., Manzoni T. B., Muramatsu H., Hogan M. J., Amanat F., Cherubin P., Lundgreen K. A., Tam Y. K., Fan S. H. Y., Eisenlohr L. C., Maillard I., Weissman D., Bates P., Krammer F., Sempowski G. D., Pardi N., Locci M., SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity 53, 1281–1295.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lederer K., Bettini E., Parvathaneni K., Painter M. M., Agarwal D., Lundgreen K. A., Weirick M., Muralidharan K., Castaño D., Goel R. R., Xu X., Drapeau E. M., Gouma S., Ort J. T., Awofolaju M., Greenplate A. R., Le Coz C., Romberg N., Trofe-Clark J., Malat G., Jones L., Rosen M., Weiskopf D., Sette A., Besharatian B., Kaminiski M., Hensley S. E., Bates P., Wherry E. J., Naji A., Bhoj V., Locci M., Germinal center responses to SARS-CoV-2 mRNA vaccines in healthy and immunocompromised individuals. Cell 185, 1008–1024.e15 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laidlaw B. J., Ellebedy A. H., The germinal centre B cell response to SARS-CoV-2. Nat. Rev. Immunol. 22, 7–18 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turner J. S., O’Halloran J. A., Kalaidina E., Kim W., Schmitz A. J., Zhou J. Q., Lei T., Thapa M., Chen R. E., Case J. B., Amanat F., Rauseo A. M., Haile A., Xie X., Klebert M. K., Suessen T., Middleton W. D., Shi P.-Y., Krammer F., Teefey S. A., Diamond M. S., Presti R. M., Ellebedy A. H., SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 596, 109–113 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goel R. R., Apostolidis S. A., Painter M. M., Mathew D., Pattekar A., Kuthuru O., Gouma S., Hicks P., Meng W., Rosenfeld A. M., Dysinger S., Lundgreen K. A., Kuri-Cervantes L., Adamski S., Hicks A., Korte S., Oldridge D. A., Baxter A. E., Giles J. R., Weirick M. E., McAllister C. M., Dougherty J., Long S., D’Andrea K., Hamilton J. T., Betts M. R., Luning Prak E. T., Bates P., Hensley S. E., Greenplate A. R., Wherry E. J., Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals after mRNA vaccination. Sci. Immunol. 6, eabi6950 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cavazzoni C. B., Hanson B. L., Podestà M. A., Bechu E. D., Clement R. L., Zhang H., Daccache J., Reyes-Robles T., Hett E. C., Vora K. A., Fadeyi O. O., Oslund R. C., Hazuda D. J., Sage P. T., Follicular T cells optimize the germinal center response to SARS-CoV-2 protein vaccination in mice. Cell Rep. 38, 110399 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Z., Xu W., Xia S., Gu C., Wang X., Wang Q., Zhou J., Wu Y., Cai X., Qu D., Ying T., Xie Y., Lu L., Yuan Z., Jiang S., RBD-Fc-based COVID-19 vaccine candidate induces highly potent SARS-CoV-2 neutralizing antibody response. Signal Transduct. Target. Ther. 5, 282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yahalom-Ronen Y., Tamir H., Melamed S., Politi B., Shifman O., Achdout H., Vitner E. B., Israeli O., Milrot E., Stein D., Cohen-Gihon I., Lazar S., Gutman H., Glinert I., Cherry L., Vagima Y., Lazar S., Weiss S., Ben-Shmuel A., Avraham R., Puni R., Lupu E., Bar-David E., Sittner A., Erez N., Zichel R., Mamroud E., Mazor O., Levy H., Laskar O., Yitzhaki S., Shapira S. C., Zvi A., Beth-Din A., Paran N., Israely T., A single dose of recombinant VSV-∆G-spike vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 11, 6402 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang N.-N., Li X.-F., Deng Y.-Q., Zhao H., Huang Y.-J., Yang G., Huang W.-J., Gao P., Zhou C., Zhang R.-R., Guo Y., Sun S.-H., Fan H., Zu S.-L., Chen Q., He Q., Cao T.-S., Huang X.-Y., Qiu H.-Y., Nie J.-H., Jiang Y., Yan H.-Y., Ye Q., Zhong X., Xue X.-L., Zha Z.-Y., Zhou D., Yang X., Wang Y.-C., Ying B., Qin C.-F., A thermostable mRNA vaccine against COVID-19. Cell 182, 1271–1283.e16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nechooshtan R., Ehrlich S., Vitikainen M., Makovitzki A., Dor E., Marcus H., Hefetz I., Pitel S., Wiebe M., Huuskonen A., Cherry L., Lupu E., Sapir Y., Holtzman T., Aftalion M., Gur D., Tamir H., Yahalom-Ronen Y., Ramot Y., Kronfeld N., Zarling D., Vallerga A., Tchelet R., Nyska A., Saloheimo M., Emalfarb M., Ophir Y., Thermophilic filamentous fungus C1-Cell-Cloned SARS-CoV-2-Spike-RBD-Subunit-Vaccine adjuvanted with Aldydrogel®85 protects K18-hACE2 mice against lethal virus challenge. Vaccine 10, 2119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodrigues K. A., Rodriguez-Aponte S. A., Dalvie N. C., Lee J. H., Abraham W., Carnathan D. G., Jimenez L. E., Ngo J. T., Chang J. Y. H., Zhang Z., Yu J., Chang A., Nakao C., Goodwin B., Naranjo C. A., Zhang L., Silva M., Barouch D. H., Silvestri G., Crotty S., Love J. C., Irvine D. J., Phosphate-mediated coanchoring of RBD immunogens and molecular adjuvants to alum potentiates humoral immunity against SARS-CoV-2. Sci. Adv. 7, eabj6538 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang J., Wen Y., Zhou S.-H., Zhang H.-W., Peng X.-Q., Zhang R.-Y., Yin X.-G., Qiu H., Gong R., Yang G.-F., Guo J., Self-adjuvanting lipoprotein conjugate αGalCer-RBD induces potent immunity against SARS-CoV-2 and its variants of concern. J. Med. Chem. 65, 2558–2570 (2022). [DOI] [PubMed] [Google Scholar]
- 88.Wang R., Huang H., Yu C., Sun C., Ma J., Kong D., Lin Y., Zhao D., Zhou S., Lu J., Cao S., Zhang Y., Luo C., Li X., Wang Y., Xie L., A spike-trimer protein-based tetravalent COVID-19 vaccine elicits enhanced breadth of neutralization against SARS-CoV-2 Omicron subvariants and other variants. Sci. China Life Sci. 66, 1818–1830 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee I.-J., Lan Y.-H., Wu P.-Y., Wu Y.-W., Chen Y.-H., Tseng S.-C., Kuo T.-J., Sun C.-P., Jan J.-T., Ma H.-H., Liao C.-C., Liang J.-J., Ko H.-Y., Chang C.-S., Liu W.-C., Ko Y.-A., Chen Y.-H., Sie Z.-L., Tsung S.-I., Lin Y.-L., Wang I.-H., Tao M.-H., A receptor-binding domain-based nanoparticle vaccine elicits durable neutralizing antibody responses against SARS-CoV-2 and variants of concern. Emerg. Microbes Infect. 12, 2149353 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deng Y.-Q., Zhang N.-N., Zhang Y.-F., Zhong X., Xu S., Qiu H.-Y., Wang T.-C., Zhao H., Zhou C., Zu S.-L., Chen Q., Cao T.-S., Ye Q., Chi H., Duan X.-H., Lin D.-D., Zhang X.-J., Xie L.-Z., Gao Y.-W., Ying B., Qin C.-F., Lipid nanoparticle-encapsulated mRNA antibody provides long-term protection against SARS-CoV-2 in mice and hamsters. Cell Res. 32, 375–382 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tai W., Zhang X., Drelich A., Shi J., Hsu J. C., Luchsinger L., Hillyer C. D., Tseng C.-T. K., Jiang S., Du L., A novel receptor-binding domain (RBD)-based mRNA vaccine against SARS-CoV-2. Cell Res. 30, 932–935 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S23
Table S1
Legend for movie S1
Movie S1