Abstract
The mechanism for neurological deficits from carbon monoxide (CO) poisoning is unclear. In a series of 150 patients with CO poisoning, we found marked elevations of blood-borne inflammatory filamentous (F-) actin–coated microparticles (MPs), neutrophil activation, and a 90% reduction in the normal level of plasma gelsolin (pGSN), a protein capable of lysing F-actin–coated MPs. This led to studies in a murine model where the same events occur and cause neuroinflammation with cognitive dysfunction. All events are recapitulated when F-actin MPs are injected intravenously, which establishes a blood-to-brain-to-blood inflammatory cycle that persists for weeks. All changes, including cognitive dysfunction, can be abrogated by an injection of human recombinant pGSN within 2 weeks after CO poisoning. These findings demonstrate that CO-induced neurological injury has an inflammatory etiology. Because of MP-mediated communications between the brain and systemic circulation, CO-induced cognitive deficits may be reversible with a pharmaceutical intervention.
Inflammatory microparticles cause inflammation and functional deficits after CO poisoning that are mitigated by plasma gelsolin.
INTRODUCTION
Carbon monoxide (CO) is a common worldwide poison estimated to affect ~137 people per million annually, with the highest incidence in some developing countries (1–3). There is no active surveillance program for CO poisoning in the United States. A recent estimate based on emergency department (ED) visits for unintentional CO poisoning suggested that this occurs in 56.5 people per million, approximately 5593/year, although an older analysis that included intentional and fire-related CO exposures estimated a prevalence of nearly 10-fold higher (4, 5). Deaths due to CO typically occur before reaching medical care, but morbidity occurs in ~25 to 50% of survivors from severe CO poisoning despite medical management (4, 6, 7). CO-induced neurological sequelae such as cognitive, psychological, vestibular, and motor impairments cost more than $1 billion annually for direct hospital care and lost earnings (8).
Identifying a pharmaceutical agent that could diminish adverse effects of CO poisoning would have a major clinical impact as there is no antidote. An agent capable of binding CO in blood is under investigation, but currently the only interventions are normal pressure and hyperbaric oxygen (9). Both can diminish the body burden of CO, and hyperbaric oxygen has several additional benefits (7). However, only ~6% of US hospitals have hyperbaric chambers; thus, availability is limited (10).
The etiology for neurological deficits that arise from days to weeks after CO poisoning remains unclear, as the risk correlates poorly with hypoxic stress assessed by blood carboxyhemoglobin (COHb) levels (1). Patients with neurological sequelae show gradations of demyelination and cerebral white matter changes as shown by magnetic resonance imaging, along with elevations of myelin basic protein (MBP) in cerebrospinal fluid (11–14).
Neutrophil activation occurs in patients with acute CO poisoning (15). In animal models, neutrophil activation drives cerebral microvascular oxidative stress at the blood-brain barrier (BBB), causing alterations in MBP that trigger an adaptive immunological response and functional neurological deficits (15–17). These events are abrogated by neutropenia, antioxidants, or inhibiting neutrophil adherence. Adding to this sequence, recent studies indicate that neuropathology is initiated by CO-induced activation of microglia and astrocytes (18). Inflammatory microparticles (MPs) generated within the central nervous system (CNS) expressing the microglial transmembrane protein-119 (TMEM) or astrocyte glial fibrillary acidic protein (GFAP) co-expressing thrombospondin-1 (TSP) travel via the glymphatic system to the deep cervical lymph nodes (DCLNs) and then to the bloodstream. There, the TSP-expressing MPs activate neutrophils to generate a second generation of MPs causing systemic inflammation that perturbs the BBB and sustains a neuroinflammatory cycle (18).
Recent findings with the murine CO poisoning model closely parallel a brain-to-blood MP-mediated inflammatory cascade shown in a murine model of decompression sickness (DCS) (19). A pharmaceutical agent, recombinant human plasma gelsolin (rhu-pGSN), was shown to mitigate vascular injuries in the DCS model where blood-borne MPs that express filamentous (F-) actin are generated by neutrophils and exacerbate neuroinflammation (20, 21). Once produced, F-actin MPs can establish neutrophil auto-activation through a three-receptor complex including CD36, Toll-like receptor 4 (TLR4), and the receptor for advanced glycation end products (RAGE) (21). Among the consequences of F-actin MP production is consumption of pGSN, a cytoplasmic actin-binding protein with a variety of anti-inflammatory actions including lysis of F-actin–expressing MPs (20–22). These interactions consume pGSN so that plasma levels fall (20, 22). More generally, clinical and animal studies have consistently shown that pGSN is depleted by inflammatory conditions (20, 21, 23).
We investigated whether diminished pGSN levels and F-actin–expressing MPs occurred in patients with CO poisoning (24). Early findings prompted studies investigating the role of F-actin MPs and benefits of infusing rhu-pGSN in the murine CO poisoning model.
RESULTS
Clinical parameters associated with CO poisoning
We evaluated 150 patients who sustained acute CO poisoning between November 2015 and August 2023 seen in EDs at the University of Maryland Medical Center in Baltimore or Wonju Severance Christian Hospital, South Korea. Table 1 lists some assessments of clinical status, laboratory measurements, and interventions when patients were first seen in the ED and whether they had sustained transient unconsciousness, as some data suggest that this event portends higher risk of neurological complications (25–27). Blood samples separate from those used for clinical management were obtained for measurements of MPs, neutrophil activation, and pGSN levels. As shown in Table 2, virtually all values were significantly different from a group of 150 healthy controls that were age and sex matched to the CO group, indicating the presence of inflammatory MPs, neutrophil activation, and a ~90% decline in pGSN in those sustaining CO poisoning.
Table 1. Results for all patients with CO poisoning and those with 1 month follow-up.
Clinical findings are shown for patients who sustained CO poisoning in the first column. Those with follow-up results at 1 month as Global Deterioration Scale (GDS) are shown in the second with a split between those with a good outcome (GDS = 1) and neurological sequelae (GDS > 1). The rows show the %COHb level, blood lactate, and troponin when first seen in the ED, number (and %) of those who sustained a loss of consciousness and who were intubated in the ED, first Glasgow Coma Score (GCS) on ED arrival, estimated duration of CO exposure with the number (n) of patients where an estimate could be made, number of patients who received hyperbaric oxygen treatment (HBO2), and GDS score at 1 month follow-up. Values are mean ± SD for continuous variables and count (proportion) for categorical variables. Statistical comparison was made between good outcome and neurological sequelae groups.
| All patients (n = 150) | GDS follow-up (n = 98) | ||
|---|---|---|---|
| Good (n = 65) | Neuro-Seq (n = 33) | ||
| COHb% | 19.8 ± 11.6 | 19.2 ± 10.9 | 21.3 ± 12.6 |
| Lactate (mM) | 3.8 ± 5.0 | 4.0 ± 6.5 | 4.0 ± 3.2 |
| Troponin (ng/ml) | 1111 ± 3628 | 286 ± 772 | 3120 ± 6328* |
| LOC, n (%) | 65 (43%) | 29 (43%) | 16 (50%) |
| Intubated, n (%) | 30 (20%) | 12 (17%) | 11 (34%)* |
| Acute GCS | 13.1 ± 3.7 | 13.0 ± 3.5 | 12.2 ± 4.3 |
| CO exposure (hours) | 9.6 ± 23.1 (n = 78) | 14.0 ± 34.3 (n = 30) | 4.7 ± 4.7 (n = 13) |
| HBO treatment | 133 (89%) | 59 (91%) | 29 (88%) |
| GDS (1 month) | 1.9 ± 1.7 (n = 97) | 1.0 ± 0.0 | 3.8 ± 1.8* |
*P < 0.05 (t test).
Table 2. Laboratory findings for controls, all patients with CO poisoning, and those with 1 month follow-up.
Comparisons among the control group, all patients with CO exposure, patients with follow-up results at 1 month and GDS = 1, and those where 1-month GDS was >1. Rows demonstrate age, % male, plasma GSN, flow cytometry results including MPs/μl, % of MPs expressing various protein markers specific to different cells including neutrophils (CD66b), endothelium (CD146), platelets (CD41a), and neutrophils (PMN) % expressing protein values above a minimum threshold for CD18, MPO, pGSN, and CD41a [indicative of platelet-neutrophil interaction as described previously (15)]. Data are mean ± SD. Statistical comparisons were made between control and all 150 patients with CO poisoning and values with P < 0.05 (t test) indicated by +, and between patients with good outcome versus neurological sequelae with P < 0.05 indicated by *. NS, not significant.
| Control (n = 150) | All CO (n = 150) | CO-good (n = 65) | CO-DNS (n = 33) | |
|---|---|---|---|---|
| Age (years) | 45.3 ± 16.3 | 47.9 ± 17.2 NS | 44.4 ± 15.0 | 54.4 ± 19.4* |
| % Male | 65.3 ± 47.8 | 65.3 ± 47.8 NS | 63.2 ± 48.6 | 50.0 ± 50.8 |
| pGSN (μg/ml) | 178.1 ± 161.5 | 11.6 ± 11.7+ | 13.4 ± 16.4 | 11.6 ± 6.8 |
| MPs/μl | 1100 ± 447 | 1423 ± 489+ | 1485 ± 560 | 1431 ± 435 |
| % CD66b | 12.6 ± 5.8 | 16.6 ± 7.3+ | 17.2 ± 0.9 | 16.3 ± 6.7 |
| % CD146 | 26.4 ± 10.6 | 26.7 ± 8.8+ | 27.0 ± 9.8 | 24.3 ± 7.5 |
| % TSP | 11.3 ± 4.5 | 13.6 ± 6.1+ | 13.6 ± 5.8 | 14.0 ± 6.9 |
| % CD41 | 6.8 ± 2.4 | 7.6 ± 7.6 NS | 7.8 ± 7.8 | 7.0 ± 2.1 |
| % TMEM | 29.4 ± 4.5 | 31.5 ± 4.9+ | 32.5 ± 4.7 | 30.5 ± 6.4 |
| % F-actin | 17.1 ± 2.0 | 20.3 ± 9.0 | 20.1 ± 4.5 | 20.3 ± 5.6 |
| PMN % CD18 | 4.2 ± 5.2 | 7.2 ± 4.7+ | 7.2 ± 5.0 | 6.6 ± 3.2 |
| PMN % MPO | 4.2 ± 3.8 | 8.4 ± 4.7+ | 9.8 ± 5.0 | 9.0 ± 4.4 |
| PMN % CD41 | 5.9 ± 4.2 | 9.6 ± 4.4+ | 9.7 ± 3.8 | 10.2 ± 5.3 |
| PMN % pGSN | 5.0 ± 2.0 | 4.5 ± 2.9 NS | 5.2 ± 3.3 | 4.6 ± 2.8 |
Neurological and cognitive outcomes of patients were assessed using the Global Deterioration Scale (GDS) (score range: 1 to 7; see Materials and Methods) at 1 month after exposure (28). Scoring was based on assessing whether there was a deterioration from prepoisoning status; thus, neurological deficits present before CO exposure did not factor into outcome determination. Follow-up, either when patients visited hospital clinics or via telephone interviews with patients or their caregivers, was achieved in 98 patients. Clinical assessments (Table 1) and laboratory results (Table 2) are shown for all patients with CO poisoning and separate columns identify results for the 98 with follow-up where those with an excellent outcome (GDS = 1, no cognitive decline) were separated from those with neurological sequelae, scores greater than 1 (n = 33). Patient age, serum troponin level, and the need for endotracheal intubation were significantly different between patients with GDS scores of 1 versus greater than 1, as shown in Table 1. Loss of consciousness (LOC) in a patient’s history did not have a significant effect on risk for development of neurological sequelae, whether they had been endotracheally intubated or not. There were no significant differences for laboratory values shown in Table 2 among the patients with CO poisoning based on sex or race, nor were there relationships to GDS scores greater than 1. No significant differences were found when comparing patients enrolled in South Korea (n = 68) versus those enrolled in the United States. There were also no significant differences among measured parameters when comparing those with GDS scores of 2 or 3 versus 4 through 7. Similarly, the sources of CO were not significantly different between those who recovered versus those suffering from neurological sequelae. For both groups, burning wood or charcoal, as for example in a hibachi, was the leading source (51% for both groups) and gasoline engine exhaust was the second most common source (12% in the sequelae group and 16% among those who fully recovered).
As shown in Table 1, 89% of patients underwent HBO2 treatment. There are challenges to estimating delays between emergency scene management and the start of HBO2. We used the total time that patients received ambient pressure supplemental O2 via reservoir nonrebreather face mask to estimate the delay, as this intervention was invariably begun at the scene of CO exposure. This information was available for 82 patients where time to HBO2 was estimated as 4.3 ± 2.2 hours. Within this group, follow-up was achieved for 50 patients where the delay to HBO2 was 4.0 ± 2.2 hours for those with GDS = 1 (n = 32) and 4.3 ± 2.5 (n = 18; NS) hours among those who had neurological sequelae. Concurrent with these studies, the murine model was used to assess possible roles of MPs and of pGSN in CO poisoning.
Murine CO exposure causes elevations of MPs and neutrophil activation
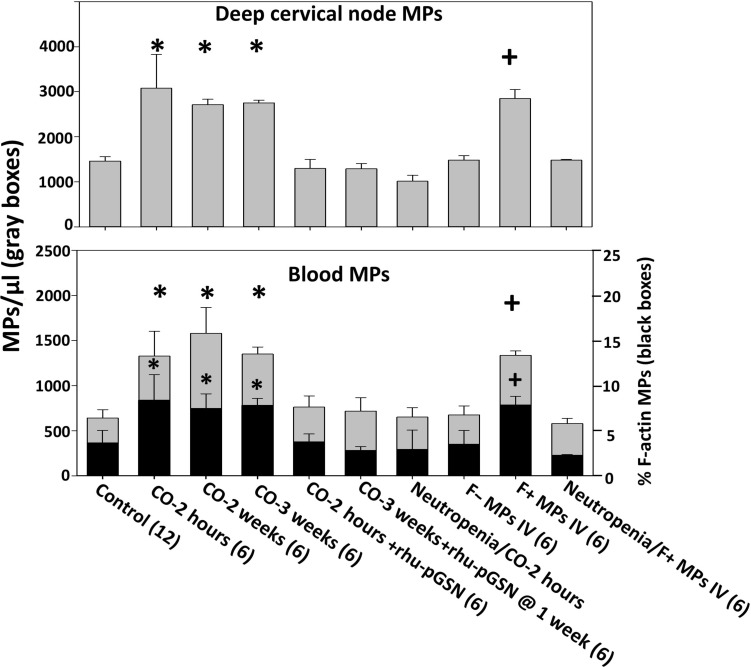
Mice exposed to CO for 1 hour, as described in Materials and Methods, exhibited elevations of MPs in the DCLN that drain brain glymphatics and blood, and values were similar whether studied at 2 hours, 2 weeks, or 3 weeks after CO exposure (Fig. 1). For these studies and all described below, nearly equal numbers of male and female mice were used and no significant differences based on sex were identified. F-actin–expressing MPs were scarce in DCLN from control mice [2.4 ± 2.3% (mean ± SD), n = 16] and not significantly different following CO exposure (2.3 ± 1.6, n = 6). In contrast, F-actin–expressing MPs were markedly higher in the blood of CO-exposed mice compared to control mice, and elevations persisted for 3 weeks (Fig. 1, black bars).
Fig. 1. Deep cervical lymph node and blood microparticles (MPs).
The total number of MPs in deep cervical nodes and blood (gray boxes) and % of blood MPs expressing F-actin (black boxes) are shown. The data represent mean ± SD (n for each group shown along the abscissa), where *P < 0.001 vs. control (ANOVA) and +P < 0.001 for those injected with F-positive MPs versus F-negative MPs (t test). Subgroups of MPs expressing protein markers are shown in table S1 (A and B). Groups include control; mice exposed to CO and euthanized 2 hours later, 2 weeks later, and 3 weeks later; mice exposed to CO and immediately injected with a sterile solution of rhu-pGSN (38.4 mg/ml) at a dose of 27 mg/kg intravenously and euthanized 2 hours later; mice exposed to CO, injected with rhu-pGSN immediately after the CO exposure, and euthanized 3 weeks later; mice rendered neutropenic, exposed to CO, and euthanized 2 hours later; naïve mice injected with 60,000 MPs in 200 μl of sterile PBS that were F-actin negative (F− MPs) or F-actin positive (F+ MPs); and neutropenic mice injected with F-actin–positive MPs.
Several proteins were probed on the surface of MPs to provide insight into the cell sources of these particles (tables S1 and S2). MPs from DCLN were probed for GFAP, TMEM, TSP, MBP and neuronal pentraxin receptor (NPR). Blood-borne MPs were evaluated for these markers in addition to the neutrophil Ly6G protein, CD41a—a platelet surface protein, and a protein combination consistent with MPs derived from endothelial cells (CD31+ and CD41-dim) (table S1, A and B). As with prior studies, the total number of MPs subgroups exceeds 100%, thus indicating that MPs share surface proteins. A role for neutrophils in CO responses was again shown because MPs were significantly lower in neutropenic CO-exposed mice (Fig. 1) (15–17).
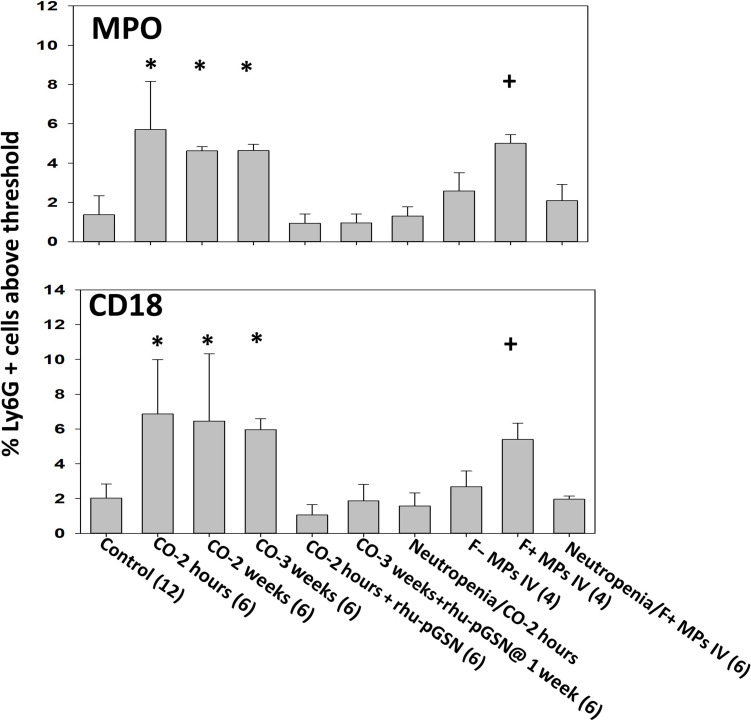
Neutrophil activation was assessed by flow cytometry as an increase in surface expression of CD18 (a component of β2 integrins) and myeloperoxidase (MPO), indicative of degranulation. Higher percentages of cells above a threshold were observed in CO-exposed mice for up to 3 weeks (Fig. 2). We also assessed the presence of pGSN on neutrophils, motivated by some published observations, but found only nominal changes following CO exposure (20). A similar pattern in neutrophils from patients with CO poisoning is shown in Table 2.
Fig. 2. Neutrophil activation.
Neutrophil activation was assessed by flow cytometry as the % of Ly6G positive cells expressing myeloperoxidase (MPO) and CD18 on the membrane surface above a threshold concentration present on control cells. Mouse groups and data are as described in Fig. 1. *P < 0.001 vs. control (ANOVA) and +P < 0.001 for those injected with F-positive MPs versus F-negative MPs (t test).
F-actin–positive MPs trigger blood and DCLN changes
Naïve mice were intravenously injected with either 60,000 F-actin MPs or F-actin–negative MPs isolated from the blood of CO-exposed mice. Only injections of F-actin MPs increased MPs in blood and DCLN above control levels in recipient mice and caused neutrophil activation that persisted for 3 weeks (Figs. 1 and 2). Neutropenic mice did not exhibit these changes. Studies with MPs isolated from control mice, where F-actin MPs are rare, were not conducted as these injections do not trigger MP elevations or neutrophil activation (18).
pGSN decreases with CO exposure
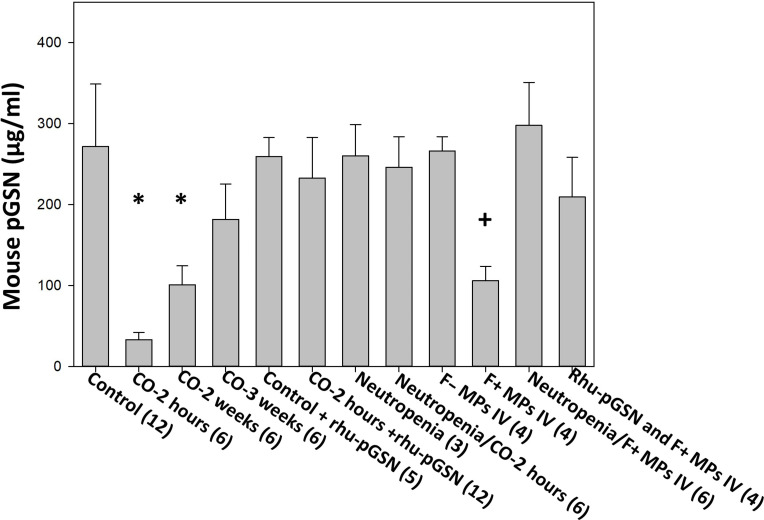
The levels of pGSN in mice exposed to CO were significantly decreased when studied at 2 hours, 2 weeks, or 3 weeks after CO exposure (Fig. 3). A similar decrease was also found when mice were injected with F-actin MPs from CO-exposed mice, but not with MPs that did not express F-actin. Further, neutropenic mice did not exhibit decreases in pGSN when exposed to CO or when injected with F-actin MPs.
Fig. 3. Plasma gelsolin values.
The data indicators and mouse groups are as described in Fig. 1. Additional groups in the figure include control mice injected with rhu-pGSN at 27 mg/kg intravenously and euthanized 2 hours later, neutropenic mice not exposed to CO, and mice injected with 60,000 F-actin MPs in 200 μl of sterile PBS where the MPs were first incubated for 15 min with rhu-pGSN to cause particle lysis (20). The rhu-pGSN dose was the same as in other groups (27 mg/kg mouse) so that for an average 20-g mouse, the F-actin MPs had been incubated with rhu-pGSN at a concentration of ~2.7 μg/μl. *P < 0.001 vs. control (ANOVA) and +P < 0.001 for those injected with F-positive MPs versus F-negative MPs (t test).
Rhu-pGSN abrogates changes induced by CO and by F-actin MPs
Infusion of rhu-pGSN immediately following CO exposure attenuated MP elevations of DCLN and blood as well as neutrophil activation (Figs. 1 and 2). We also found that rhu-pGSN could be infused up to 2 weeks after CO exposure, and when mice were euthanized 1 week later, elevations of MPs in DCLN and blood, and neutrophil activation were not present. Prior studies have shown that rhu-pGSN lyses murine F-actin MPs (20). To evaluate whether a soluble substance versus intact MPs may be causing the observed effects, F-actin MP suspensions were prepared but first incubated for 15 min with rhu-pGSN added before the solution was injected into naïve mice. This injection caused no significant elevations of DCLN or blood MPs, neutrophil activation, or loss of pGSN (MP data are shown in tables S1 and S2; pGSN data are shown in Fig. 3). Notably, injections of rhu-pGSN into control mice did not alter the pGSN level. This was anticipated because the enzyme-linked immunosorbent assay does not detect human pGSN.
Vascular integrity disturbances
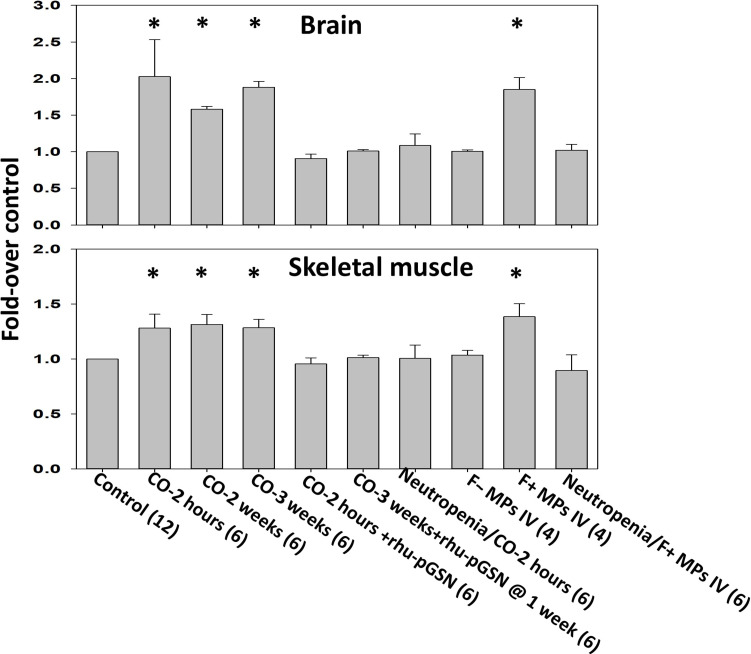
To assess vascular damage, mice were perfused with rhodamine-labeled lysine-fixable dextran (2 × 106 Da) and efflux was assessed as previously described (18). Efflux within the brain and skeletal muscle vasculature was found in CO-exposed mice for 3 weeks, and in naïve mice injected with F-actin MPs obtained from CO-exposed mice (Fig. 4). Infusion of rhu-pGSN immediately after CO exposure prevented vascular leakage. A similar effect was observed in mice injected at 2 weeks and euthanized 1 week later. Injections of F-actin–negative MPs did not perturb vasculature, nor was a vascular leak detected in neutropenic mice injected with F-actin MPs or those exposed to CO (Fig. 4).
Fig. 4. Murine vascular leakage of 2 × 106 Da rhodamine-labeled dextran.
Extravasation of dextran in brain and leg skeletal muscle was evaluated as described in Materials and Methods. Mouse groups are as described in Fig. 1. Data are fold difference in rhodamine-dextran/mg tissue protein (mean ± SD) versus the values in control mice processed concurrently with each experimental group. *P < 0.001 vs. control (ANOVA) and +P < 0.001 for those injected with F-positive MPs versus F-negative MPs (t test).
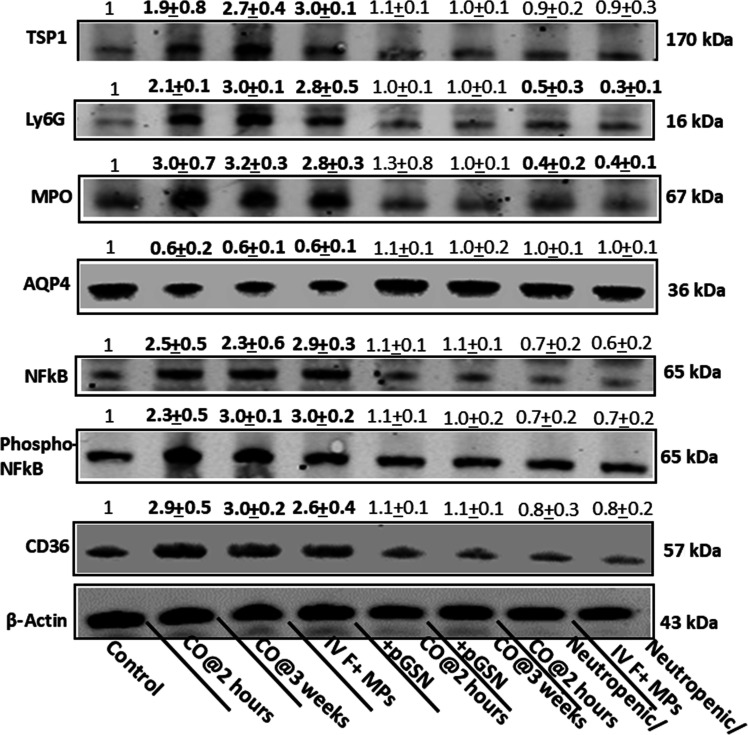
Neuroinflammation post-CO
Endothelium-enriched brain homogenates were probed by Western blot to assess inflammatory changes (Fig. 5) (18). For 3 weeks after CO exposure, brains exhibited significantly higher expression of NF-κB, phosphorylated (p65) NF-κB, TSP, and CD36, and loss of aquaporin-4 (AQP4). Neutrophil sequestration was evidenced by increases in MPO and Ly6G. Elevations were similar in mice injected with F-actin MPs. Infusion of rhu-pGSN immediately after CO exposure prevented inflammatory changes, and this was also observed in mice injected at 2 weeks and euthanized 1 week later. CO-exposed neutropenic mice and neutropenic mice injected with F-actin MPs also failed to demonstrate these changes.
Fig. 5. Neuroinflammation assessed by Western blot.
A representative Western blot of brain homogenates is shown for mouse groups as described in Fig. 1 (not shown are data for 2 weeks post-CO and mice injected with F-actin–negative MPs as values for these mice were not significantly different from control) where numbers above each band indicate mean ± SD (n = 6 mouse brains per lane) band densities relative to β-actin of control samples from replicate studies. Bold numbers indicate values statistically significant from control (P ≤ 0.01 ANOVA). Blots were probed for NF-κB, p65-phosphorylated NF-κB (pNF-κB), myeloperoxidase (MPO), thrombospondin-1 (TSP), Ly6G, and CD36 proteins.
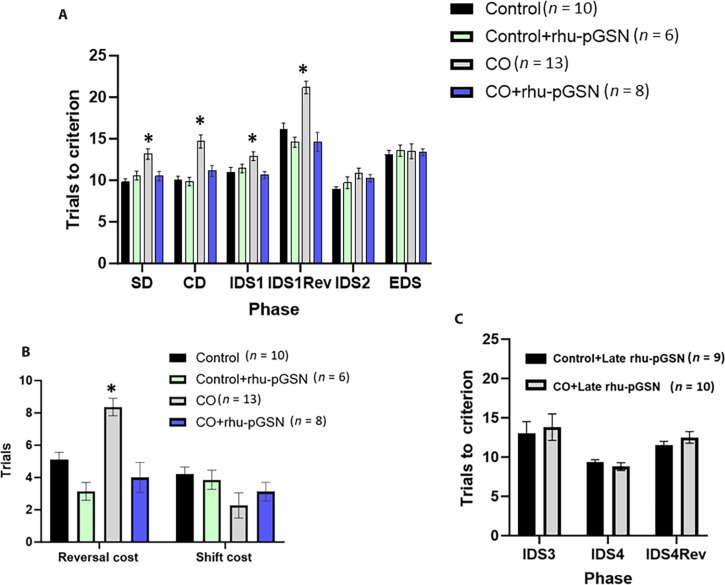
Cognitive deficits in mice after CO exposure
Executive function impairment is among the most common complications sustained by CO poisoning survivors (4, 6, 7). Comparable functioning in animals can be assessed using an attention set shifting and reversal task (29). Scores for each phase, as described in Materials and Methods, are shown in Fig. 6A. Acclimation of mice into testing conditions began 3 days after CO exposure, and testing was completed by 3 weeks after CO exposure. As shown, CO-exposed mice exhibited statistically significant differences from control mice on each of the first four phases. The figure caption describes the results from repeated-measures analysis of variance (ANOVA) on the trials to criterion required by mice to complete each phase, where statistically significant effects of phase were found, as well as the phase × exposure interaction, and phase × treatment interaction. The main effects of CO exposure, rhu-pGSN treatment, and the interaction were significant (P < 0.001).
Fig. 6. Attentional set shift scores for control and CO-exposed mice.
(A) Trials to criterion for progressive tasks. Where indicated, rhu-pGSN injection occurred immediately after CO exposure and control mice injected at the same time. The CO group demonstrated differences on each of the first four phases, the smallest value F(3,33) = 4.09, P < 0.01 (ANOVA). The CO mice significantly differed from control mice on each of these phases, P < 0.05 (*). The trials to criterion to complete each phase was significant, F(5,165) = 53.55, P < 0.001 (RM-ANOVA) as a phase × exposure interaction, F(5,165) = 2.91, P < 0.015, and phase × treatment interaction, F(5,165) = 5.71, P < 0.001. The main effects of CO, F(1,33) = 22.89, P < 0.001, rhu-pGSN treatment, F(1,33) = 15.53, P < 0.001, and the interaction, F(1,33) = 19.10, P < 0.001, were significant. (B) Reversal and extradimensional shift cost scores. No significant differences were detected on the extradimensional shift cost, but CO-exposed mice were significantly less likely to demonstrate an attentional set formation as confirmed by the χ2 statistic. Values are mean ± SD, * indicates P < 0.05 versus control. (C) Effect of late rhu-pGSN administration. After tasks were performed as in (B), mice were injected with rhu-pGSN so that during the third week, repetitive IDS testing (IDS 3 and 4) and reversal tasks were assessed. No significant differences between CO-exposed and control mice were found.
Modification in the predictive stimulus modality, or the so-called extradimensional “shift” (EDS), was calculated as EDS1 − IDS2. This task requires recruitment of dissociable neural circuits and is thought to reflect ventromedial prefrontal cortex and striatum function. The number of mice in each group that required at least two more trials to solve the EDS was considered to have formed an attentional set. Analysis of the likelihood that each mouse in each group formed a set was conducted using χ2 statistic (Fig. 6B). The CO-exposed group was the only one that differed significantly from control (P = 0.04), suggesting that this group used a rudimentary problem-solving strategy throughout the task.
To assess the effect of late rhu-pGSN administration at 2 weeks after CO exposure, after the first set of studies, some CO-exposed mice and a control group were injected and tested over the ensuing week, completing the second testing within 3 weeks after CO exposure. Repetitive intradimensional shift (IDS) testing (IDS 3 and 4) and IDS reversal (Fig. 6C) in the CO group were not significantly different from the control.
Separate groups of mice were tested on the attention set shift and reversal task after injections of F-actin–negative (n = 4) or –positive MPs (n = 6). Mice treated with F-actin MPs performed poorly compared to those treated with negative MPs. Repeated-measures ANOVA confirmed a significant effect of F-actin MPs, F(1,8) = 21.99, P < 0.002. One-way ANOVA on each phase confirmed that these mice performed significantly worse on the CD and reversal phases of the task compared to those injected with F-actin–negative MPs (all P < 0.02).
Ex vivo studies of murine and human MPs and neutrophils
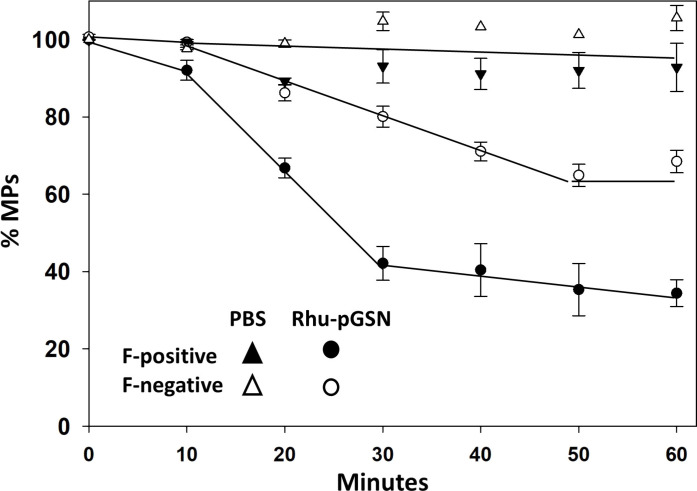
The impact of rhu-pGSN on human MPs lysis was evaluated using samples obtained from four patients with CO poisoning (Fig. 7). Each sample was separated into F-actin–expressing and F-actin–negative MPs. As shown, rhu-pGSN caused notably greater lysis of F-actin–expressing particles.
Fig. 7. Lysis of human MPs by rhu-pGSN.
Suspensions of MPs from four patients with CO poisoning were first separated into those expressing F-actin (F-positive) and those not expressing F-actin (F-negative). Suspension of 1200 MPs in 250 μl of PBS was then incubated with 19 μg of rhu-pGSN (76 μg/ml) or PBS and samples were taken at intervals for particle counting. Data show mean ± SD MPs/μl at up to 1 hour of incubation.
Additional evidence was sought for neutrophil activation in both mice and patients with CO poisoning. Lysates of neutrophils were analyzed from four patients with CO poisoning and three samples from CO-exposed mice and compared to healthy controls. Western blots of CO-exposed murine neutrophils and those from patients with CO poisoning exhibited between a 1.5- and 2.4-fold increased content of NF-κB, p65-phosphorylated NF-κB, Src kinase, and phosphorylated Src kinase (fig. S1). Immunoprecipitation of neutrophil lysates was also performed to look for protein linkages to the nitric oxide synthase-1 adaptor protein (NOS1AP), a membrane protein that we previously reported to be involved when neutrophils are activated by F-actin MPs (20, 21). Figure S2 demonstrates that neutrophils from patients with CO poisoning and CO-exposed mice exhibited between a 1.5- and 3.7-fold greater linkage of the three receptors involved with F-actin MPs binding, RAGE, CD36, and TLR4. Other proteins linked to NOS1AP, including nitric oxide synthase-2 (NOS2), Src kinase, ASC (adaptor molecule apoptosis-associated speck-like protein containing a CARD—a component of the NLRP3 inflammasome), and cytoskeletal components p130CAS and filamin, were increased by 1.3- to 3.7-fold. All changes in CO-exposed cells were significant (P < 0.02, ANOVA).
DISCUSSION
Patients suffering from CO poisoning exhibit a systemic inflammatory response involving a wide variety of MPs, neutrophil activation, and an alarming loss of pGSN. However, the only findings that set those who sustained neurological morbidity apart from those who recovered were being an average of a decade older, requiring endotracheal intubation, and manifesting evidence of cardiac injury with an elevated troponin. It is notable that there were no significant differences in character of the CO-induced responses such as changes in Glasgow Coma Scale, incidence of unconsciousness, and COHb levels. We also could not discern differences in duration of CO exposure, although that parameter is exceedingly difficult to accurately measure. The fact that nearly 90% of patients received HBO2 treatment may have diminished the incidence of poor outcomes (GDS ≥ 4, 17% of the follow-up group). Nonetheless, these findings suggest that cardiovascular compromise heightens the risk for neurological morbidity. This is consistent with animal models, as transient cardiac compromise was shown to play a major role in perivascular neutrophil adherence to the brain vasculature and CO-induced oxidative stress (30, 31).
Results indicate that the same inflammatory changes observed in patients directly contribute to the development of neurological morbidity in the murine model. The cyclic process previously described, involving CNS-derived MPs and circulating F-actin MPs, is sustained for at least 3 weeks (18). The fact that injections of F-actin MPs can initiate the same systemic and CNS inflammatory changes and cognitive deterioration as CO exposure clearly focuses attention on these particles. The murine model results indicate that elevations of cervical lymph node and blood MPs occur in response to CO poisoning and F-actin–expressing blood MPs, which are produced when neutrophils are stimulated by MPs, initiating a brain vascular leak, perivascular neutrophil sequestration (assessed as Western blot elevations of neutrophil-specific Ly6G and MPO), and neuroinflammation (assessed as Western blot elevations of the p65 subunit of NF-κB, serine 536 phosphorylated NF-κB and TSP), with increases of node and blood MPs (20, 21). Additional changes in brain include loss of astrocyte AQP4, which could perturb glymphatic flow (32). Elevations of CD36, a class B pattern recognition receptor, could augment MPs targeting the brain because phosphatidylserine is a CD36 ligand that is heavily expressed on MP membrane. CD36 is found predominantly on microvascular endothelial cells in healthy brain and increases on astrocytes and microglia over a span of hours following oxidative stress or an ischemic insult (33–35).
Injections of rhu-pGSN can arrest the inflammatory process and protect against the development of neurological deterioration in mice. The reduction of pGSN in CO-exposed mice persists for 2 weeks (Fig. 3) and administering rhu-pGSN even this late can abrogate MP elevations, neutrophil activation, vascular leak, neuroinflammation, and cognitive deficits. Thus, the reductions in pGSN observed in both patients and the murine model contribute to the ongoing injuries. Our data provide only a static view on the pGSN value in patients with CO poisoning. The magnitude of decrease is correlated with poor outcome in a variety of disorders, but its rate of consumption or persistent depression may distinguish those who sustain neurological morbidity from CO poisoning (20–23). The fact that rhu-pGSN injections 2 weeks after CO exposure can abrogate the inflammatory cycle in mice underscores the self-sustaining nature of MP-mediated inflammation and indicates that neurological deficits are reversible. Whether the same holds true in humans obviously needs further study.
On a more fundamental basis, F-actin MPs may serve as a common inflammatory mediator. We have shown their role in such varied disorders as DCS and vascular complication from diabetes mellitus (19, 21, 22, 36). In vitro studies suggest that the F-actin shell offers rigidity to the particles such that the phosphatidylserine ubiquitously present on the surface of MPs is rendered immunogenic. We found the same biochemical evidence for neutrophil activation, including protein linkages to NOS1AP, in patients with CO poisoning and in the murine model, which again suggests that the mechanism of activation is the same. MPs and other extracellular vesicles generated systemically can exacerbate traumatic and other causes of brain injury, and reciprocally, CNS-derived MPs cause peripheral vascular disorders once liberated to the bloodstream (37–39). Further work is needed in examining a more general role for extracellular vesicles in CNS pathophysiology.
There are myriad anti-inflammatory effects of pGSN, any of which could be contributing to the benefits observed with rhu-pGSN infusions in CO poisoning (23). Regarding effects on neutrophils, we were surprised to find no significant differences in pGSN presence on the cell surface of control versus patients with CO poisoning, or on murine neutrophils. We interpret this as evidence that rhu-pGSN administration is not having a direct anti-inflammatory effect on neutrophils and its therapeutic benefit with CO poisoning is related to F-actin MPs lysis. MPs carry a cargo of inflammatory cytokines and other mediators of cell signaling. Although the presence of these agents would persist following MPs lysis, their uptake and local concentration would be different.
Limitations to our study include its lack of information on pGSN levels and F-actin MPs in patients with CO poisoning once they are removed from the contaminated environment. In future studies, follow-up analysis may demonstrate whether the difference between recovery and development of neurological sequelae is based on loss rate of the pGSN in blood and eradication of F-actin MPs. Along similar lines, clinical utilization of rhu-pGSN might be investigated as a treatment for CO-induced neurological sequelae. Another limitation to our clinical observations is the loss to follow-up of ~35% of patients with CO poisoning. This obviously could impact our assessments of risk factors as well as the incidence of neurological complications.
MATERIALS AND METHODS
Experimental design
The study began with evaluating the presence of MPs and neutrophil activation in healthy individuals (controls) and those who had sustained CO poisoning. Subsequent studies with our published murine model of CO poisoning were conducted to investigate the role of F-actin MPs in CO-induced inflammatory responses.
Patient study
All procedures were completed in accordance with the Declaration of Helsinki and approved by the Institutional Review Boards (University of Maryland—HP-00064816, Yonsei University Wonju College of Medicine—CR320058) to obtain blood for laboratory analysis from patients undergoing ED evaluation for suspected acute CO poisoning. Informed consent from all patients was obtained after the nature and possible consequences of the studies were explained. Acute CO poisoning was diagnosed according to patient history and elevated COHb level. Clinical management of patients was performed independently of the blood sample collections. All patients received 100% O2 by tight-fitting nonrebreather face mask during prehospital medical care and while undergoing evaluations in the ED. They received at least one treatment with hyperbaric oxygen (HBO2) as soon as feasible and if they had experienced any LOC; manifested abnormal neurological signs or cognitive dysfunction, cardiovascular dysfunction, elevated cardiac enzymes, and ischemic electrocardiogram changes; or had a COHb ≥25%. HBO2 treatments were performed at either 2.8 or 3.0 atmospheres absolute (ATA) for 60 to 120 min. There were 13 patients who were not treated with HBO2: 9 patients in the good outcome group and 4 patients in the group with neurological sequelae (13% of each group).
Neurological and cognitive outcomes of patients were assessed at 1-month after CO exposure using the GDS with a score range of 1 to 7. Most assessments were made during patient visits to the outpatient department. For patients in poor condition or unable to visit the outpatient department, guardians were interviewed by telephone to evaluate their conditions. GDS scores were classified to reflect favorable (one to three points) and poor (four to seven points) outcomes as in a prior publication (40). Blood samples obtained by the clinical team during the initial ED visit were collected in Cyto-chex BCT tubes (Streck Inc., Omaha, NE), numerically coded, and analyzed between 3 and 10 days later. Samples from South Korea were shipped via express mail to the senior author’s laboratory. Prior work has shown that MPs and neutrophil characteristics remain unchanged when samples are processed within 3 weeks from the time of acquisition and refrigeration is not required (41).
Materials
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Recombinant human gelsolin was provided by BioAegis Therapeutics (North Brunswick, NJ). The antibodies and flow cytometry reagents used are as follows: annexin V–FITC (BD, catalog no. 556419), anti-β-actin (Sigma-Aldrich, St. Louis, MO, catalog no. A2066), anti-biotin (Sigma-Aldrich, catalog no. A0185), anti-CD66b, anti-Ly6G eFluor450 (eBioscience, San Diego, CA, catalog no. 48-5931-82), anti-AQP4 (Abcam, catalog no. ab81355), anti-Ly6G eFluor450 (eBioscience, San Diego, CA, catalog no. 48-5931-82), anti-mouse MPO-PE (Hycult Biotech, Plymouth Meeting, PA, catalog no. HM1051PE-100), anti-CD31 BV510 (BD, catalog no. 563089), anti-CD41 PerCP Cy5.5 (BioLegend, San Diego, CA, catalog no. 133918), anti-GFAP Brilliant Violet 421 (BioLegend, San Diego, CA catalog no. 644719), anti-transmembrane protein (TMEM) 119-APC (Abcam, catalog no. ab225494), anti-NPR AF-790 (Santa Cruz Biotechnology, Dallas, TX, catalog no. sc-390081), anti-MBP PerCP (Novus Biologicals, Centennial, CO, catalog no. NBP2-22121PCP), anti–TSP-1 (Santa Cruz, catalog no. 393504 and 393504FITC), anti-Filamin antibody (Abcam, catalog no. ab76289), anti-human MPO (Hycult Biotech, Plymouth Meeting, PA, catalog no. HM1051PE-100), anti-NOS1AP (Abcam, catalog no. ab190686), anti-NOS2 (Santa Cruz, catalog no. sc-7271), anti-p130CAS antibody (Invitrogen, catalog no. PA5-83601), anti-p65 subunit of NF-κB (Abcam, catalog no. ab32536), anti-phosphorylated at serine-536 (Phospho-Ser536) p65 NF-κB (Cell Signaling, Danvers, MA, catalog no. 3031), anti-phospho SrcK antibody (Invitrogen, catalog no. PA5-97364), anti-RAGE (Santa Cruz, catalog no. sc-365154), anti-SrcK antibody (Santa Cruz, catalog no. sc-5266), and anti-TLR4 antibody (Invitrogen, catalog no. 48-2300). Antibodies for flow cytometry and Western blots were used specifically for those applications as documented by the manufacturers and at the recommended concentrations. Verification that the anti-actin antibody recognizes β-actin was demonstrated by Western blot and mass spectroscopy in a prior publication (20). Positive staining in flow cytometry was determined following the fluorescence-minus-one control test.
Animals
Experiments were performed using young, adult (10 to 12 weeks old) male and female mice, with approximately equal numbers in all experimental groups. All studies were approved by the University of Maryland Institutional Animal Care and Use Committee under protocol no. 1221011. C57BL/6J mice (Mus musculus) were purchased from the Jackson Laboratory (Bar Harbor, ME). Their care was in accordance with institutional guidelines and they were housed in the university animal facility with a 12/12-hour light-dark cycle. Housing and all experiments were conducted at temperatures of 22 to 24°C and 40 to 70% humidity. Mice received water ad libitum and were fed Laboratory Rodent Diet 5001 (PMI Nutritional Inc., Brentwood, MO). Mice were either left to breathe room air (control) or subjected to a 1-hour exposure to CO [1000 parts per million (ppm) for 40 min followed by 3000 ppm for 20 min; achieving a COHb of ~54%], some were injected with a sterile solution of rhu-pGSN (38.4 mg/ml) at a dose of 27 mg/kg intravenously, and then studied and euthanized as described in the text. Randomization of mice for experimentation was performed by first collecting all mice to be used in a day into a single plastic cage and then randomly selecting an individual mouse for use as the daily control or in an experimental group. Studies spanned 14 months, with acclimatized mice purchased in groups of 6 to 12 at biweekly intervals and used according to a block design, where individual blocks represented mice selected as control or experimental groups. Data were scored and analyzed in a blinded manner such that the scorer did not know an animal’s group assignment. All mice involved in this project were included in the data analysis; none were excluded.
Cervical lymph node MP acquisition and analysis
The cervical lymph nodes were collected from mice and processed as previously described (42). Two to six nodes were taken from each mouse, from which MPs were isolated and analyzed. Detailed methods along with representative box plots illustrating the enumeration strategy have been published (42).
Blood MP acquisition and processing
Blood-borne MPs were isolated and prepared for analysis by flow cytometry as previously described (42). Briefly, heparinized blood was centrifuged for 5 min at 1500g. EDTA was then added to the supernatant to achieve a concentration of 12.5 mM to prevent MP aggregation. This suspension was centrifuged at 15,000g for 30 min, and the supernatant was used for analysis. Separation of F-actin–positive MPs was achieved by incubating MP suspensions with phalloidin-biotin conjugates followed by streptavidin conjugated to 1-μm-diameter MagVigen magnetic nanoparticles (Nvigen Inc., Sunnyvale, CA). This suspension was incubated for 12 hours before separation using a magnet. The washing and magnetic bead separation procedures followed the manufacturer’s recommended protocols. Suspensions of MPs remaining after magnetic extraction were centrifuged at 100,000g for 1 hour to pellet particles for analysis.
Isolation of neutrophils
Blood from healthy human volunteers and mice was processed as previously described (20, 43). Samples for Western blots were prepared following published procedures (44). NOS1AP immunoprecipitation was carried out by incubating cell lysates with anti-NOS1AP IgG, which was then extracted using magnetic protein A/G nanoparticles (Nvigen Inc., Sunnyvale, CA) as described in prior publications (44). After Western blotting, protein band densities were quantified based on band pixel counts and normalized to the NOS1AP band in each lysate. Representative blots showing pixel counts for the blots were presented in a prior publication (21).
MPs for reinjection
MPs donor mice were exposed to the CO protocol and euthanized 2 hours later. Blood-borne MPs were isolated (as described above), and blood MPs were separated based on F-actin expression using phalloidin-conjugated magnetic beads as previously described. Blood MPs, after the initial 15,000g supernatant was obtained, were parceled among centrifuge tubes at a ratio of 250 μl + 4 ml of phosphate-buffered saline (PBS) and centrifuged at 100,000g for 60 min (typically three to four tubes per experiment were used). Supernatant (4 ml) was then carefully removed and the pellet containing the MPs was resuspended in sterile PBS. The MPs were counted and diluted to ensure that 60,000 MPs in 200 μl of PBS were intravenously injected via a tail vein into naïve mice. Recipient mice were then subjected to tests and euthanasia as described in the text. The choice of using injections of 60,000 F-actin–positive or –negative MPs per mouse came from evaluating the range of MPs in the blood of CO-exposed versus control mice (data reflected in Fig. 1). Control mice had 14 to 40 F-actin–positive MPs/μl of blood (mean of 23 ± 10); CO-exposed mice had 80 to 220/μl (109 ± 46). As the average mouse blood volume was ~1 ml, the aim was to increase the number of F-actin–positive MPs to ~80/μl.
Cognitive testing
The attentional set shifting and reversal tests were based on the methods described by Bissonette and Powell (29). Starting 3 days after CO exposure, mice were trained to dig in one of two small bowls for a small food reward. Once stable digging was established, mice progressed through a series of discriminations and reversal phases. Mice began with a simple discrimination in which the only difference between the bowls was the odor (e.g., garlic versus coffee). Once mice achieved a criterion of eight consecutive correct choices, the next phase began. This was followed by a compound discrimination (CD) phase in which two bowls differed in both the odor and digging media (small beads versus small pieces of paper), with odor continuing to be the predictive stimulus dimension for the food reward. A third phase, IDS, followed the CD, in which the stimuli changed, but odor remained the predictive stimulus. Once the response criterion was achieved, the contingency was reversed for the reversal phase (R). A second IDS was conducted (IDS), and lastly, an EDS was conducted, in which the digging media became the predictive dimension. If mice were using the stimulus dimension of odor to solve previous phases, they would require more trials to solve the EDS. Mice were tested over three to five consecutive days, depending on performance. Demonstration of an attentional set formation was defined as requiring at least two more trials to solve the EDS relative to the preceding IDS (IDS2).
MP analysis
All reagents and solutions used for MP isolation and analysis were filtered with a 0.1-μm filter (EMD Millipore, Billerica, MA). MPs were analyzed as described previously (22, 43). In brief, flow cytometry was performed with an eight-color, triple-laser MACSQuant Analyzer (Miltenyi Biotec Corp., Auburn, CA) using MACSQuantify software version 2.13.3 for data analysis. MACSQuant was calibrated every other day using calibration beads (Miltenyi Biotec Corp., Auburn, CA). Forward and side scatter were set at logarithmic gain. Photomultiplier tube voltage and triggers were optimized to detect submicrometer particles. Microbeads of three different diameters, 0.3 μm (Sigma-Aldrich, St. Louis, MO), 1.0 μm, and 3.0 μm (Spherotech Inc., Lake Forest, IL), were used for the initial settings and before each experiment as an internal control. Samples were suspended in annexin binding buffer solution (1:10 v/v in distilled water; BD Pharmingen, San Jose, CA), with the previously listed antibodies. Examples of blood-borne and cervical particles analysis have been published previously (42). All reagents and solutions used for MP analysis were sterile and filtered through a 0.1-μm filter. MPs were defined as annexin V–positive particles with diameters ranging from 0.3 to 1 μm diameter. The concentration of MPs in the sample tubes was determined by the MACSQuant Analyzer according to the exact volume of solution from which MPs were analyzed.
Western blotting
After the behavioral assessment, the animals were euthanized and their brains were harvested and stored at −80°C. Protein samples were prepared using homogenates of brains perfused with positively charged colloidal silica in a manner to enrich samples for endothelium (43). The total protein concentrations of the samples were determined by the Pierce bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific), and the samples were aliquoted and stored at −80°C until further use. Sample proteins (20 μg) were resolved using 4 to 12% Mini-PROTEAN TGX Precast Protein Gels (Bio-Rad) and the separated proteins were transferred onto nitrocellulose membranes (Bio-Rad) using the Trans-Blot Turbo Blotting System. Membranes were blocked with Intercept (TBS) Blocking Buffer (Li-Cor) for 1 hour at room temperature followed by overnight incubation with the primary antibodies AQP (1:1000), MPO (1:1000), CD36 (1:1000), TSP1 (1:1000), Ly6G (1:1000), NF-κB (1:1000), pNF-κB (1:1000), and β-actin (1:1000) at 4°C. The membranes were then rinsed with PBST (three washings for 10 min each), incubated with the secondary antibodies (IRDye 680RD or 800CW anti-mouse or anti-rabbit IgG, 1:3000) for 1 hour at room temperature, and washed with PBST (three washings for 10 min). The bands were visualized by an Odyssey infrared imaging system (Li-Cor, Lincoln, NE).
Statistical analysis
Results are expressed as the mean ± SD for four or more independent experiments with analysis using SigmaStat (Jandel Scientific, San Jose, CA). Data normality was assessed using the Shapiro-Wilk test. Details for the statistical analysis for each assay are provided in figure legends. Briefly, for two group comparisons, a two-tailed, unpaired Student’s t test was used. For multiple group comparisons, a one-way ANOVA followed by Newman-Keuls post hoc test was used. For all studies, a result was deemed statistically significant if P < 0.05.
Acknowledgments
We would like to acknowledge the assistance of the physicians and nurses in our emergency departments for their enthusiastic cooperation with obtaining the blood samples for this study.
Funding: This work was supported by grant N00014-22-1-2818 from the US Office of Naval Research (S.R.T.) and grant R01-NS-122855 from the National Institute of Neurological Disorders and Stroke (S.R.T.).
Author contributions: Conceptualization: A.K.A., K.S., Y.S.C., A.R.B., Y.Li., V.M.B., Y.Le., and S.R.T. Methodology: A.K.A., K.S., A.R.B., Y.S.C., Y.Li., and S.R.T. Investigation: A.K.A., K.S., J.W., Y.S.C., V.M.B., A.R.B., Z.I., A.D., Y.Le., and S.R.T. Visualization: A.K.A., J.W., A.R.B., and S.R.T. Supervision: A.K.A., K.S., and S.R.T. Funding acquisition: S.R.T. Resources: A.K.A., Y.S.C., V.M.B., A.R.B., Y.Le., and S.R.T. Project administration: A.K.A., K.S., A.R.B., V.M.B., Y.Le., and S.R.T. Data curation: A.K.A., K.S., V.M.B., and S.R.T. Formal analysis: A.K.A., J.W., V.M.B., A.R.B., A.D., Y.Li., and S.R.T. Validation: A.K.A., K.S., A.R.B., V.M.B., and S.R.T. Writing—original draft: A.K.A., K.S., J.W., A.R.B., and S.R.T. Writing—review and editing: A.K.A., K.S., J.W., Y.S.C., Y.Li., V.M.B., A.R.B., Z.I., A.D., Y.Le., and S.R.T.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 and S2
Tables S1 and S2
REFERENCES AND NOTES
- 1.Raub J. A., Mathieu-Nolf M., Hampson N. B., Thom S. R., Carbon monoxide poisoning—A public health perspective. Toxicology 145, 1–14 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Mattiuzzi C., Lippi G., Worldwide epidemiology of carbon monoxide poisoning. Hum. Exp. Toxicol. 39, 387–392 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) , Carbon monoxide-related deaths—United States, 1999-2004. MMWR Morb. Mortal. Wkly Rep. 56, 1309–1312 (2007). [PubMed] [Google Scholar]
- 4.Shin M., Bronstein A. C., Glidden E., Malone M., Chang A., Law R., Boehmer T. K., Strosnider H., Yip F., Morbidity and mortality of unintentional carbon monoxide poisoning: United States 2005 to 2018. Ann. Emerg. Med. 81, 309–317 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hampson N. B., Weaver L. K., Carbon monoxide poisoning: A new incidence for an old disease. Undersea Hyperb. Med. 34, 163–168 (2007). [PubMed] [Google Scholar]
- 6.Stearns D., Sircar K., National unintentional carbon monoxide poisoning estimates using hospitalization and emergency department data. Am. J. Emerg. Med. 37, 421–426 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hampson N. B., Piantadosi C. A., Thom S. R., Weaver L. K., Practice recommendations in the diagnosis, management and prevention of carbon monoxide poisoning. Am. J. Respir. Crit. Care Med. 186, 1095–1101 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Hampson N. B., Cost of accidental carbon monoxide poisoning: A preventable expense. Prev. Med. Rep. 3, 21–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azarov I., Wang L., Rose J. J., Xu Q., Huang X. N., Belanger A., Wang Y., Guo L., Liu C., Ucer K. B., McTiernan C. F., O'Donnell C. P., Shiva S., Tejero J., Kim-Shapiro D. B., Gladwin M. T., Five-coordinate H64Q neuroglobin as a ligand-trap antidote for carbon monoxide poisoning. Sci. Transl. Med. 8, 368ra173 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampson N. B., Little C. E., Hyperbaric treatment of patients with carbon monoxide poisoning in the United States. Undersea Hyperb. Med. 32, 21–26 (2005). [PubMed] [Google Scholar]
- 11.Beppu T., Fujiwara S., Nishimoto H., Koeda A., Narumi A., Mori K., Ogasawara K., Sasaki M., Fractional anisotropy in the centrum semiovale as a quantitative indicator of cerebral white matter damage in the subacute phase in patients with carbon monoxide poisoning: Correlation with the concentration of myelin basic protein in cerebrospinal fluid. J. Neurol. 259, 1698–1705 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ide T., Kamijo Y., The early elevation of interleukin 6 concentration in cerebrospinal fluid and delayed encephalopathy of carbon monoxide poisoning. Am. J. Emerg. Med. 27, 992–996 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Kuroda H., Fujihara K., Mugikura S., Takahashi S., Kushimoto S., Aoki M., Altered white matter metabolism in delayed neurologic sequelae after carbon monoxide poisoning: A proton magnetic resonance spectroscopic study. J. Neurol. Sci. 360, 161–169 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Kamijo Y., Soma K., Ide T., Recurrent myelin basic protein elevation in cerebrospinal fluid as a predictive marker of delayed encephalopathy after carbon monoxide poisoning. Am. J. Emerg. Med. 25, 483–485 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Thom S. R., Bhopale V. M., Han S. T., Clark J. M., Hardy K. R., Intravascular neutrophil activation due to carbon monoxide poisoning. Am. J. Respir. Crit. Care Med. 174, 1239–1248 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han S. T., Bhopale V. M., Thom S. R., Xanthine oxidoreductase and neurological sequelae of carbon monoxide poisoning. Toxicol. Lett. 170, 111–115 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thom S. R., Bhopale V. M., Fisher D., Zhang J., Gimotty P., Delayed neuropathology after carbon monoxide poisoning is immune-mediated. Proc. Natl. Acad. Sci. U.S.A. 101, 13660–13665 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruhela D., Bhopale V. M., Kalakonda S., Thom S. R., Astrocyte-derived microparticles initiate a neuroinflammatory cycle due to carbon monoxide poisoning. Brain Behav. Immun. Health 18, 100398 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thom S. R., Bhopale V. M., Bhat A. R., Arya A. K., Ruhela D., Qiao G., Li X., Tang S., Xu S., Neuroinflammation with increased glymphatic flow in a murine model of decompression sickness. J. Neurophysiol. 129, 662–671 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhopale V. M., Ruhela D., Brett K. D., Nugent N. Z., Fraser N. K., Levinson S. L., DiNubile M. J., Thom S. R., Plasma gelsolin modulates the production and fate of IL-1β-containing microparticles following high-pressure exposure and decompression. J. Appl. Physiol. 130, 1604–1613 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thom S. R., Bhopale V. M., Arya A. K., Ruhela D., Bhat A. R., Mitra N., Hoffstad O., Malay D. S., Mirza Z. K., Landis J. C., Lev-Tov H. A., Kirsner R. S., Hsia R.-C., Levinson S. L., DiNubile M. J., Margolis D. J., Blood-borne microparticles are an inflammatory stimulus in type 2 diabetes mellitus. ImmunoHorizons 7, 71–80 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arya A. K., Balestra C., Bhopale V. M., Tuominen L. J., Räisänen-Sokolowski A., Dugrenot E., L'Her E., Bhat A. R., Thom S. R., Elevations of extracellular vesicles and inflammatory biomarkers in closed circuit SCUBA divers. Int. J. Mol. Sci. 24, 5469 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piktel E., Levental I., Durnás B., Janmey P., Bucki R., Plasma gelsolin: Indicator of inflammation and its potential as a diagnostic tool and therapeutic target. Int. J. Mol. Sci. 19, 2516 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thom S. R., Bhopale V. M., Milavonava T. M., Hardy K. R., Logue C. J., Lambert D. S., Troxel A. B., Ballard K., Eisinger D., Plasma biomarkers in carbon monoxide poisoning. Clin. Toxicol. 48, 47–56 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi S., Delayed neurologic sequelae in carbon monoxide intoxication. Arch. Neurol. 40, 433–435 (1983). [DOI] [PubMed] [Google Scholar]
- 26.Smith J. S., Brandon S., Morbidity from acute carbon monoxide poisoning at three-year follow-up. Br. Med. J. 1, 318–321 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weaver L., Valentine K. J., Hopkins R. O., Carbon monoxide poisoning: Risk factors for cognitive sequelae and the role of hyperbaric oxygen. Am. J. Respir. Crit. Care Med. 176, 491–497 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Reisberg B., Ferris S. H., de Leon M. J., Crook T., The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 139, 1136–1139 (1982). [DOI] [PubMed] [Google Scholar]
- 29.Bissonette G. B., Powell E. M., Reversal learning and attentional set-shifting in mice. Neuropharmacology 62, 1168–1174 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thom S. R., Carbon monoxide-mediated brain lipid peroxidation in the rat. J. Appl. Physiol. 68, 997–1003 (1990). [DOI] [PubMed] [Google Scholar]
- 31.Thom S. R., Dehydrogenase conversion to oxidase and lipid peroxidation in brain after carbon monoxide poisoning. J. Appl. Physiol. 73, 1584–1589 (1992). [DOI] [PubMed] [Google Scholar]
- 32.Morgensen F. L., Delle C., Nedergaard M., The glymphatic system (En)during inflammation. Int. J. Mol. Sci. 22, 7491 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Bonilla L., Racchumi G., Murphy M., Anrather J., Iadecola C., Endothelial CD36 contributes to postischemic brain injury by promoting neutrophil activation via CSF3. J. Neurosci. 35, 14783–14793 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y., Zhang J., Cui W., Silverstein R. L., CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J. Exp. Med. 219, e20211314 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunz A., Abe T., Hochrainer K., Shimamura M., Anrather J., Racchumi G., Zhou P., Iadecola C., Nuclear factor-kappaB activation and postischemic inflammation are suppressed in CD36-null mice after middle cerebral artery occlusion. J. Neurosci. 28, 1649–1658 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thom S. R., Bennett M., Banham N. D., Chin W., Blake D. F., Rosen A., Pollock N. W., Madden D., Barak O., Marroni A., Balestra C., Germonpre P., Pieri M., Cialoni D., Le P.-N. J., Logue C., Lambert D. S., Hardy K. R., Sward D., Yang M., Bhopale V., Dujic Z., Association of microparticles and neutrophil activation with decompression sickness. J. Appl. Physiol. 119, 427–434 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Zhao Z., Zhou Y., Tian Y., Li M., Dong J.-f., Zhang J., Cellular microparticles and pathophysiology of traumatic brain injury. Protein Cell 8, 801–810 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang M., Kosterin P., Salzberg B. M., Milovanova T. N., Bhopale V. M., Thom S. R., Microparticles generated by decompression stress cause central nervous system injury manifested as neurohypophysial terminal action potential broadening. J. Appl. Physiol. 115, 1481–1486 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar A., Stoica B. A., Loane D. J., Yang M., Abulwerdi G., Khan N., Kumar A., Thom S. R., Faden A. I., Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J. Neuroinflammation 14, 47 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S. J., Thom S. R., Kim H., Hwang S. O., Lee Y., Park E. J., Lee S. J., Cha Y. S., Effects of adjunctive therapeutic hypothermia combined with hyperbaric oxygen therapy in acute severe carbon monoxide poisoning. Crit. Care Med. 48, e706–e714 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Thom S. R., Milovanova T. N., Bogush M., Bhopale V. M., Yang M., Bushmann K., Pollock N. W., Ljubkovic M., Denoble P., Dujic Z., Microparticle production, neutrophil activation and intravascular bubbles following open-water SCUBA diving. J. Appl. Physiol. 112, 1268–1278 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Ruhela D., Bhopale V. M., Yang M., Yu K., Weintraub E., Greenblatt A., Thom S. R., Blood-borne and brain-derived microparticles in morphine-induced anti-nociceptive tolerance. Brain Behav. Immun. 87, 465–472 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Thom S. R., Yang M., Bhopale V. M., Huang S., Milovanova T. N., Microparticles initiate decompression-induced neutrophil activation and subsequent vascular injuries. J. Appl. Physiol. 110, 340–351 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Thom S. R., Bhopale V. M., Yu K., Huang W., Kane M. A., Margolis D. J., Neutrophil microparticle production and inflammasome activation by hyperglycemia due to cytoskeletal instability. J. Biol. Chem. 292, 18312–18324 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 and S2
Tables S1 and S2