Abstract
The presence of alkali metal cations in the electrolyte substantially affects the reactivity and selectivity of electrochemical carbon dioxide (CO2) reduction (CO2R). This study examines the role of cations in CO2R on single-crystal and polycrystalline Au under controlled mass-transport conditions. It establishes that CO2 adsorption is the rate-determining step regardless of cation type or surface structure. Density functional theory calculations show that electron transfer occurs to a solvated CO2-cation complex. A more positive potential of zero charge enhances CO2R activity only on Au with similar surface coordination. The symmetry factor (β) of the rate-determining step varies with surface structure and cation identity, with density functional theory calculations indicating β’s sensitivity to surface and double-layer structures. These findings emphasize the importance of both surface and double-layer structures in understanding cation effects on CO2R.
Alkali metal cations’ influence on CO2 electroreduction was investigated using Au electrodes with varying surface structures.
INTRODUCTION
The electrochemical carbon dioxide (CO2) reduction (CO2R) process driven by renewable electricity is a promising technique to convert relatively inert CO2 gas into useful chemicals and fuels (1–3). Since the pioneering work of Akira and Hori, it is known that alkali metal cations in the electrolyte alter the reaction activity and product selectivity of CO2R (4). CO2R activity has been observed to decrease in the order of Cs+ > K+ > Na+ > Li+, with up to two orders of magnitude difference in CO2R activity between Li+ and Cs+ on various coinage metals under identical applied potential (5–7). Several hypotheses have been proposed for the role of cations, including interfacial pH buffering (8, 9), changes in interfacial electrical field (5, 10), electrostatic interaction with reaction intermediates (7, 11), chemical interaction with adsorbed intermediates through specific adsorption (12, 13), reorganization of interfacial water (14, 15), non–electric field effect (16, 17), and cation-coupled electron transfer (18, 19). However, these effects are often intertwined and challenging to quantify experimentally (20, 21), making a comprehensive molecular-level understanding of alkali metal cation effects on CO2R a subject of ongoing debate.
Koper and co-workers reported notable surface structure sensitivity of cations during CO reduction on Cu single-crystal electrodes, where the onset potential for ethylene formation depends on both the cation identity and surface structure on Cu(hkl) (22). Other studies on CO2R to CO on Au have predominantly focused on polycrystalline surfaces. Systematic investigations using well-defined single-crystal electrodes could help distinguish cation effects from surface structure effects in CO2R. In addition to cation interactions, the activity and selectivity of CO2R on different catalysts also depend on the mass transport conditions (23, 24). For example, Goyal et al. demonstrated that the faradic efficiency of CO2 to CO on Au increased by nearly 20% with improved mass transport (25).
Here, experiments were conducted to meticulously control the surface structure, mass transport conditions, and electrolyte solution purity to investigate how cations influence the catalysis of CO2R on Au surfaces. Three single-crystal Au electrodes, Au(100), Au(110), and Au(111), along with two polycrystalline Au (pcAu) electrodes prepared using different surface cleaning methods [electrochemically polished Au (EP-pcAu) and flame-annealed Au (FA-pcAu)], were studied in CO2-saturated LiHCO3, NaHCO3, KHCO3, RbHCO3, and CsHCO3 electrolytes using a rotating ring-disk electrode (RRDE) assembly. The Au ring electrode served as a real-time detector for quantifying CO generated from the Au disk (25, 26). The study revealed that alkali metal cations exhibit surface structure–dependent effects. For pcAu, the observed trends with cations varied substantially depending on the surface preparation methods (e.g., flame annealed versus electrochemically polished). Overall, the promotional effects of cations vary across different single-crystal and polycrystalline surface structures. Furthermore, cations were found to influence the potential of zero charge (PZC) values, and a positive correlation between the CO2R activity and PZC was observed only on Au surfaces with comparable surface coordination numbers.
Combining Tafel analysis and kinetic isotope effect (KIE) experiments, this study demonstrates that the rate-determining step (RDS) is a CO2 adsorption step coupled with electron transfer, irrespective of the cation identity and surface structure. The symmetry factor (β) of the RDS was found to vary with the cation identity and the surface structure, indicating that cations play a crucial role in the electrokinetics of CO2R. Density functional theory (DFT) calculations of the symmetry factors suggest that electron transfer occurs to a solvated CO2-cation complex, providing insights into the cation-dependent electrokinetics observed during CO2R.
RESULTS
Electrochemical characterization of the Au catalyst surface
Cation dependence on CO2R reaction was obtained on well-characterized single-crystal and pcAu electrodes. To verify the distribution of surface facet on each Au electrode, we recorded cyclic voltammograms (CVs) of Au oxidation immediately after CO2R experiments (Fig. 1). Typical voltammetric features such as a broad double-layer region (from 0 to 1.3 VRHE) and distinct gold oxide peaks serve as characteristic fingerprints of a clean Au(hkl)/electrolyte interface (27). Additional voltammograms, depicted in fig. S1, confirm that thermally induced surface reconstruction of Au (28) persists during active CO2 electrolysis, evidenced by discernible lifting peaks within the double-layer region even after the CO2R experiment. For simplicity, reconstructed Au surfaces like Au(100)-hex, Au(110)-(1 × 2), and Au(111)-(√3 × 22) are abbreviated as Au(100), Au(110), and Au(111), respectively, with their corresponding surface coordination numbers (CNs) of 9, 7, and 9, respectively. The correlation between Au oxide peaks and their surface structures is well documented on pcAu electrodes (29, 30), where peak potentials for Au(100), Au(110), and Au(111) planes are ~1.42, 1.45, and 1.58 VRHE (31), respectively. These Au oxidation peaks are used here to qualitatively infer the distribution of surface facets on pcAu.
Fig. 1. Cyclic voltammetry characterization of different Au electrodes.
CVs of Au(100), Au(110), Au(111) single-crystal pcAu, EP-pcAu, and FA-pcAu recorded at 50 mV s−1 in Ar-saturated 0.1 M H2SO4 with a rotation rate of 1600 rpm.
The same pcAu electrode exhibits varying surface facet distributions depending on the surface treatment method. Electrochemical polishing (EP-pcAu) commonly exposes predominantly Au(100)-like surface features, while flame annealing (FA-pcAu) results in mainly Au(111) facets (30). These voltammetric features offer a straightforward qualitative comparison between pcAu surfaces, although quantifying the exact facet distribution is complex. The surface facet distribution of the pcAu electrode appears consistent before and after CO2R, as depicted in fig. S2. Subsequent sections of this study report that pcAu electrodes prepared with different surface treatments exhibit distinct intrinsic CO2R activities and respond differently to cation effects.
Cation dependence of CO2R
The partial current densities of CO generated during CO2R on various Au electrodes in 0.1 M MHCO3 (M = Li, Na, K, Rb, and Cs) electrolytes are illustrated in Fig. 2. Across different Au catalysts, no consistent cation trend is observed when comparing CO2R activity at the same applied potential. Instead, the promotional effect of cations varies depending on the surface structure and the potential window. For instance, on Au(100), CO2R activity decreases in the order of Cs+ > Rb+ > K+ > Na+ > Li+ at −0.5 VRHE (Fig. 2A). Conversely, on Au(111), relative CO2R activity shows Cs+ ≈ Rb+ ≈ K+ > Na+ > Li+ (Fig. 2C), where Cs+, Rb+, and K+ are statistically similar. A FA-pcAu catalyst, rich in Au(111) features, exhibits a different cation trend with Rb+ > K+ > Cs+ > Na+ > Li+ (Fig. 2E) at −0.5 VRHE. This unexpected variation suggests that the pcAu surface cannot be simply considered as a linear combination of the contributions from Au(100), Au(110), and Au(111) surfaces. High-index surface facets, defect sites, and grain boundaries likely play crucial roles in shaping the observed cation trends on pcAu surfaces. These findings indicate that the impact of cations on interfacial pH buffering (5) or mean electric double-layer field (32) cannot solely account for the observed cation effects at this potential. If these factors were dominant, a consistent cation trend (Cs+ > Rb+ > K+ > Na+ > Li+) would be expected regardless of the electrode surface structure. Therefore, alternative mechanisms must be considered to explain the structure-sensitive cation effects observed.
Fig. 2. Partial current densities of CO measured by RRDE voltammetry on different Au electrodes in 0.1 M alkali metal bicarbonate electrolytes.
Partial current densities of CO measured on (A) Au(100), (B) Au(110), (C) Au(111), (D) EP-pcAu, and (E) FA-pcAu during CO2R at 15 mV s−1 and 1600 rpm in CO2-saturated 0.1 M bicarbonate electrolytes (pH = 6.8, T = 25 ± 1°C). The current densities represent the average values, and the error bars (SDs) are calculated on the basis of three independent experiments.
Correlating PZC with CO2R activity
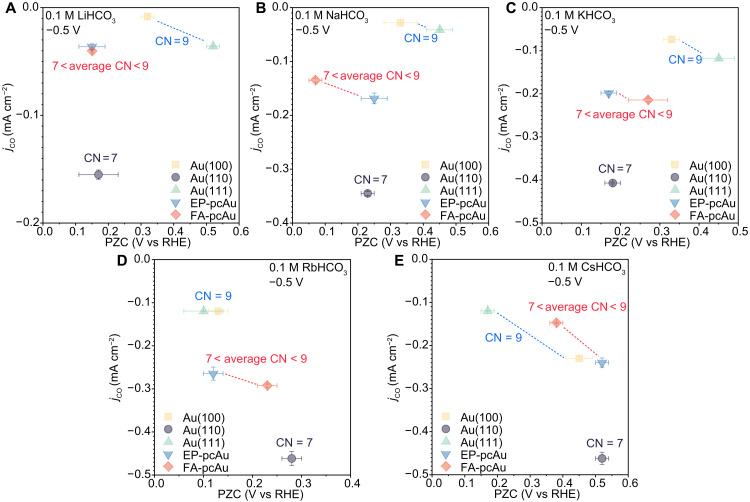
To explore the potential origins of the observed cation effects, we plotted the partial current densities of CO at −0.5 VRHE (~−0.9 VSHE) against PZC, as depicted in Fig. 3. The PZC values were determined according to the methods outlined in Materials and Methods. Previous experimental and multiscale modeling studies (23, 33–35) suggest that CO2R activity on Au is primarily governed by the reductive adsorption of CO2 within a potential range of −0.7 to −1.0 VSHE. Our own investigations in this study (see Supplementary Note 1) corroborate this argument, where surface charge density has been proposed as a descriptor for the CO2 adsorption step. Specifically, a more positive PZC results in higher negative surface charge density at the same cathodic potential, thereby favoring CO2 adsorption (5, 34). According to this hypothesis, if cation effects are correlated with surface charge density, the corresponding variations in the measured PZC values would be observed.
Fig. 3. Partial current densities of CO plotted as a function of PZC at −0.5 VRHE on the same Au electrode.
Partial current densities of CO measured on (A) Au(100), (B) Au(110), (C) Au(111), (D) EP-pcAu, and (E) FA-pcAu in CO2-saturated 0.1 M bicarbonate electrolytes (pH = 6.8, T = 25 ± 1°C). Error bars (SDs) are calculated on the basis of three independent experiments.
Cations have been observed to influence the PZC on Au surfaces (fig. S11). However, further investigation reveals that there is no clear correlation between the PZC value and the partial current density of CO (indicative of CO2R activity) when varying the cation. For example, on Au(111), similar CO2R activities are observed in K+-, Rb+-, and Cs+-containing electrolytes despite a notable 0.35 V difference in PZC between Rb+ and K+ electrolytes (Fig. 3C). In addition, electrolytes containing Rb+ generally exhibit higher CO2R activities compared to those with Na+ and Li+ on the same Au electrode, even though the corresponding PZC values in Rb+ can vary widely, being either more positive or negative compared to the other ions. When comparing partial CO current densities as a function of PZC for the same cation across different catalytic surfaces (Fig. 4), Au(100) shows higher CO2R activities compared to Au(111) despite having a more positive PZC in the Cs+-containing electrolyte. Conversely, Au(110) displays much higher CO2R activities with a less positive PZC (Fig. 4, A to C). This discrepancy can be attributed to the unique characteristics of the Au(110) surface, which is known to expose highly active undercoordinated sites (CN = 7) compared to the relatively flat Au(100) and Au(111) terrace sites (CN = 9) (36). A positive correlation between the PZC value and CO2R activity is observed only between electrode surfaces with similar surface CNs, such as Au(100) and Au(111) terrace sites (CN = 9). This suggests that while there may be some relationship between PZC (or surface charge) and activity in specific cases, such correlations cannot be generalized across a broader range of catalysts. In conclusion, the observed structure-sensitive cation effects on CO2R activity cannot be solely explained by variations in PZC or surface charge density, highlighting the complex interplay of surface structure, coordination environment, and electrochemical activity in determining catalytic performance.
Fig. 4. Partial current densities of CO plotted as a function of PZC at −0.5 VRHE in the same electrolyte.
Partial current densities of CO measured in CO2-saturated 0.1 M (A) LiHCO3, (B) NaHCO3, (C) KHCO3, (D) RbHCO3, and (E) CsHCO3 electrolytes (pH = 6.8, T = 25 ± 1°C). The Au electrodes with comparable surface CN are linked with dashed lines for a better comparison. Error bars (SDs) are calculated on the basis of three independent experiments.
Factors beyond PZC or surface charge density, such as the interaction between cations and adsorbed CO2 intermediates, may play a more important role in influencing CO2R activity. Cations can selectively stabilize adsorbed CO2 or its formation transition state, leading to varied catalytic outcomes. For example, previous ab initio molecular dynamics simulations (11) suggest that Cs+ may more effectively stabilize adsorbed CO2 compared to Li+ on the same Au surface. This stronger electrostatic interactions between partially dehydrated Cs+-*CO2 complexes could enhance the overall CO2R activity. While the direct experimental measurement of these electrostatic interactions between hydrated cations and adsorbed CO2 is challenging, empirical observations can provide insights into such interactions before resorting to more detailed theoretical modeling with DFT. Therefore, experimental evidence is crucial in delineating these interactions, laying the groundwork for subsequent DFT calculations to comprehensively model and understand the mechanism driving cation-dependent CO2R reaction activity.
Critical role of alkali metal cations in the electrokinetics of CO2R
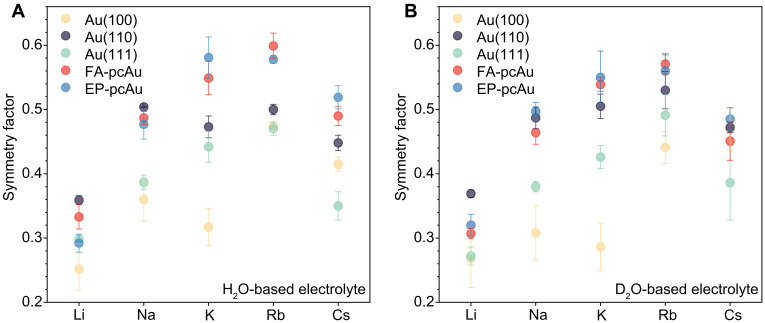
Tafel analysis of CO2R under purely kinetic control on various Au electrodes in both H2O- and D2O-based electrolytes was performed, and the results are presented as symmetry factor (β) in Fig. 5 (see representative results in fig. S16). Note that only the transfer coefficient (α) can be derived from Tafel analysis for a multielectron transfer process, while β refers to a single-electron transfer step. However, measured α equals to β of the first step in this study because the first step is the RDS of CO2R on Au (see more details in Supplementary Note 1). A volcano-type relation was observed between the symmetry factor and the alkali cation across all Au electrodes in both H2O- and D2O-based electrolytes. This finding suggests that β values for CO2R are linked to the cations involved in the CO2 adsorption step (RDS). Specifically, the β value increases in the order of Li+ < Na+ < K+ < Rb+ and then decreases from Rb+ to Cs+. This trend holds consistently across the different Au electrodes, with Rb+ yielding the highest β values among all alkali metal cations on all Au surfaces except EP-pcAu. The similarity in β values observed between H2O- and D2O-based electrolytes further supports the notion that these effects are associated with the cation’s influence during the CO2 adsorption process where no proton transfer is involved.
Fig. 5. Values of symmetry factor (β) derived from Tafel plots of CO2R to CO as a function of cation identity.
Values of β obtained in 0.1 M (A) H2O-based and (B) D2O-based bicarbonate electrolytes (pH = 6.8, T = 25 ± 1°C). Error bars (SDs) are calculated on the basis of three independent experiments.
The variation in β values with the surface structure adds another layer of complexity to understanding the cation effect. There is no overarching trend observed across different surface structures. For instance, Au(111) exhibits a higher β value compared to Au(100) in the K+ electrolyte, whereas the lowest β value is observed on Au(111) in the Cs+ electrolyte. This variability underscores the significance of considering electrode surface structures when assessing cation effects on CO2R. The distinct β values suggest that the interaction between cations and the CO2 adsorption process is influenced by the specific crystallographic orientation of the electrode surface. Therefore, the surface structure plays a crucial role in determining how cations modulate the kinetics and efficiency of CO2R on Au electrodes.
The CO2 adsorption process involving electron transfer can be represented by the equation
| (1) |
where * denotes an available surface site and *CO2− represents an adsorbed CO2. Assuming that Butler-Volmer kinetics applies to this step in the absence of mass transport effects, the partial current density of CO () can be described by
| (2) |
where kθ is the standard rate constant, is the activity of CO2 near the electrode surface, θ* is the fraction of available surface sites, η is the overpotential, β is the symmetry factor (related to the electron transfer kinetics), F is the Faraday constant, R is the gas constant, and T is the temperature. In Eq. 2, it is assumed that the pre-exponential factor does not vary substantially with the cation on the same Au surface within the investigated potential window (variation less than one order of magnitude; see representative exchange current densities in table S10). When changing cations across the same Au surface structure at the same applied potential, the partial current density of CO is expected to increase as the β value increases. This correlation aligns with the observed volcano-type relation of β versus cation identity, where Rb+-containing electrolytes generally exhibit higher partial current densities of CO. The data also highlight the limitations of using a commonly simplified β value of 0.5 in kinetic modeling for CO2R, as it can lead to substantial uncertainties in estimating reaction activity and selectivity. Therefore, it is recommended to determine β values from experiments or additional theoretical modeling (37, 38) to provide a more accurate assessment of cation effects in CO2R. These β values serve as sensitive indicators of how cations influence CO2R kinetics, particularly when PZC values remain relatively unchanged. The subsequent section delves into the molecular origins of β for the CO2 adsorption reaction and its dependence on electrical double-layer properties with DFT. This approach aims to provide deeper insights into the mechanisms underlying cation effects in CO2 electroreduction.
Calculating the symmetry factor
DFT calculations were used to determine the electrokinetic symmetry factor β, focusing on the influence of cations and electrochemical double-layer (EDL) properties. Specifically, the process of CO2 adsorption and electron transfer to form *CO2− was investigated, which is proposed to be the RDS of CO2R on Au electrodes (as discussed in Supplementary Note 1). In these calculations, EDL considerations were incorporated using a compartmentalized EDL theory model, as described in prior research (39). This model helps account for the activation barrier of the RDS in relation to the properties of the EDL, enhancing the understanding of how cations affect the kinetics of CO2 electroreduction.
The symmetry factor (β) is calculated by taking the first derivative of the activation barrier with reference to the applied potential (U)
| (3) |
Details of the derivations and calculation of the symmetry factor are discussed in Supplementary Note 2. The value of β is determined by properties of the EDL interface (ε and d) and the change in electronic properties of the surface-bound species (μ and α) between the initial and activated states. The dipole moment and polarizability changes (calculated along the surface normal direction) are calculated between the initial state [bare Au(111) surface or surface with (solvated) cation preadsorbed depending on the reaction path considered] and the transition state complex [including the adsorbing CO2 along with (solvated) cations depending on the reaction path]. Note that the |e| term appears in Eq. 3, where a + 1 cation (proton, alkali cation) is moved from the bulk electrolyte reference to the surface during the reaction being studied. In this case, the value of β is 1 if the interfacial dipole and polarizability changes [Δμ, Δ(αμ), and Δα] are 0 during the reaction process.
Equation 3 quantifies how the symmetry factor is sensitive to both the electronic character of the transition state and the approximation of the electrode-electrolyte interfacial properties (ε and d for the Helmholtz model used herein). Discussion of dominant variables when calculating β is discussed in Supplementary Note 2. Calculated values of β are strongly dependent on the magnitude of the dipole moment change and approximated EDL width. Extension of this approach for reaction models with explicit consideration of the alkali cations and H2O is denoted in Supplementary Note 2.1. A dielectric constant of 2 was used to represent the EDL as suggested by prior works (40, 41).
Reaction path models
The reaction path of electroreduction of CO2 to form a chemisorbed CO2δ− species (*CO2−) is modeled on the Au(111), Au(100), and Au(110) surfaces. A series of local models of this reaction is considered, first neglecting any interaction of CO2 and the surface with water or alkali cations and then including explicit water molecules and alkali cations. As discussed below, a reaction pathway where (explicitly solvated) CO2 adsorbs without direct cation interaction is inconsistent with the experimentally observed β value, whereas electron transfer to a solvated CO2-cation complex is a plausible reaction model of CO2R because of consistency with the experimentally observed values of β.
Formation of *CO2− with explicit cations and H2O
Supplementary Note 3 details that a simplistic model of the *CO2− transition state lacks sufficient charge transfer from the metal to form a large enough magnitude of the dipole moment and therefore does not align with the experimentally observed values of β. Incorporating explicit cations and H2O into the DFT model for *CO2− formation increases the dipole moment change along the reaction path by stabilizing additional charge transfer and/or placing that charge further from the metal surface. Reaction paths for the formation of *CO2− with K+ and H2O explicitly considered were first studied on the Au(111) surface. Figure 6 illustrates different transition state models of *CO2− interacting with an explicit alkali metal cation (K+) and explicit H2O. Calculated symmetry factors are compared with βexp values obtained from experiments using 0.1 M KHCO3 on the Au(111) surface.
Fig. 6. DFT modeling of formation of *CO2− with explicit cations and H2O.
(A) Profile of βDFT with reference to EDL width from different transition state models of *CO2− formation with explicit K* and H2O. The dash horizontal black line represents βexp = 0.44 for the 0.1 M KHCO3 electrolyte on the Au(111) surface. The blue solid line is the profile of βDFT for the simple *CO2− model as seen in Fig. 7. The red solid and dashed lines represent the profiles of βDFT for the coadsorbed cation models, respectively, for *CO2− + *K(H2O) and *CO2− + *K(H2O)5. The purple solid and dashed lines represent the profiles of βDFT for the coordinated cation model, respectively, for *CO2-K and *CO2-K-H2O. A dielectric constant of 2 was chosen when calculating βDFT. (B) Optimized geometries of *CO2− with explicit H2O and K* are shown. The structure of *CO2− is derived from constrained geometries on the basis of COOH*. Atom colors are as follows: yellow, Au; blue, K; gray, C; red, O. Each activated complex label is color coded according to the lines in fig. S6A.
A sufficient dipole moment change upon the formation of the activated complex is needed to reconcile the experimentally observed symmetry factor values. The dipole moment change required for βDFT = βexp = 0.44, at an EDL width of 2.9 Å, is Δμ = −0.81 e− Å (Eq. 3, with a cation involved) or Δμ = 0.67 e− Å (eq. S11, without a cation involved). The simple model for *CO2− (Δμ = −0.48 e− Å) discussed in Supplementary Note 3 is shown by the blue line in Fig. 6A. *CO2− without explicit cations or H2O molecules fails to provide sufficient dipole moment changes, thereby deviating from βexp.
In considering explicit K+ and H2O, decisions are made regarding the placement and number of ions and solvent molecules within the DFT model. In this work, we considered two cation-assisted transition state models: (i) a coadsorbed model [*CO2− + *K(H2O)n] and (ii) a coordinated model [*CO2K(H2O)n]. Optimized geometries of these cation-assisted models are shown in Fig. 6B. The profile of β with reference to the EDL width for the coadsorption of a cation with its first hydration shell [K(H2O)n*] was considered and represented by the red solid and dashed line. Explicit H2O considerations are necessary when modeling *CO2− with a coadsorbed K* since geometry optimization of the bent *CO2− is unsuccessful without explicit H2O (fig. S24). Variations in the predicted dipole moment changes (resulting βDFT) are observed with the number and placement of explicit H2O molecules, as shown in table S6. However, regardless of the number of H2O molecules considered, the coadsorbed model is inconsistent with experimentally observed values of β. For βDFT = βexp = 0.44, unphysical EDL widths of 1.7 and 2.1 Å would be required for the respective transition state models of *CO2− and K(H2O)5* and *CO2− and K(H2O)*. This discrepancy arises as a consequence of the absolute magnitude of the dipole moment changes for the coadsorbed *CO2− and K(H2O)n* models of the transition state, which is not sufficiently large enough, thus requiring unphysical values of the EDL width to agree with βexp = 0.44.
Both the simple *CO2− and coadsorbed cation models have an insufficient dipole moment change to agree with the experimentally observed symmetry factors, requiring the positive countercharge to be ~2 Å from the surface. This indicates that the cation must be close enough to interact and potentially coordinate with the surface-bound CO2−. Subsequent investigation explored the plausible transition states through a cation-coordinated model between *CO2− and K+. The optimized geometries of the cation-coordinated models of the transition states are shown in Fig. 6B as *CO2K and *CO2K(H2O). βDFT values of the cation-coordinated model, with and without an explicit H2O, are shown as the green dashed and solid lines in Fig. 6A. With the addition of one explicit H2O molecule, the profile of βDFT with reference to EDL width is similar to the transition state model without any explicit H2O, where the differences arise from the polarizability change and not the dipole moment change.
Comparing with βexp, the βDFT of the cation-coordinated models agrees with βexp = 0.44 at an EDL width of 5.9 Å. The cation-coordinated model agrees with βexp at physical EDL widths because of the larger dipole moment exhibited from the transition state (Δμ = −1.61 e− Å for *KCO2). This suggests that the coordination between the explicit cation and *CO2− within the DFT model is necessary for the DFT model to capture the symmetry factors observed experimentally. Tables S7 and S8 also show that the cation-coordinated model produces sufficiently large dipole moment changes across different cations (Li+, Na+, Rb+, and Cs+) on the Au(111) surface. These DFT results suggest that the transition state of *CO2− is attained with explicit and direct interaction with alkali cations, where electron transfer occurs to this solvated CO2-cation complex during CO2R. This finding is consistent with observations by Koper and co-workers who noted that alkali cations were required in solution to facilitate CO2R (11) on Au through a coordinative mechanism.
The local DFT model used to examine the electron-accepting complex during CO2R does not necessarily capture the full range of states that may occur within a dynamic EDL. Sufficient sampling of a substantial number of ions and H2O molecules (>100) in a dynamic model of an electrochemical interface would require prohibitively long ab initio dynamic simulations (39, 42, 43). As a result, proposing a transition state that is stabilized by long-range interactions from multiple countercharge ions (electrostatic adsorption) or a larger network of H2O molecules is not feasible within a computationally tractable DFT periodic cell. Local static models, such as those used here, introduce uncertainty on the placement of ions and explicit H2O. Classical molecular dynamics can support the length and timescales of the electrode-electrolyte interface and provide reasonable sampling configurations of the electrolyte (42, 44, 45). However, molecular dynamics does not offer a straightforward approach for examining reactions involving electron transfer. Although these challenges prevent certainty that the models used here represent the exact reaction path, our calculated βDFT values across local transition state models can offer insights into plausible mechanisms in comparison with experimental results. The cation-coordinated model emerges as the most reasonable model of the transition state during the formation of *CO2−. The sensitivity of the DFT model is shown through the various placements of the cation within the DFT model, which dictates the magnitude of the dipole moment changes and, ultimately, the symmetry factors.
Trends across cations
Cation-coordinated transition states were systematically evaluated across different alkali cations and low-index Au facets [(111), (100), and (110)], with the goal correlating DFT-predicted symmetry factors with experimental trends. However, achieving consistent trends requires understanding how properties of the EDL (dielectric constant and effective countercharge distribution) vary with both the cation type and facet orientation. Figure 7 attempts such a comparison under the assumption that these EDL properties remain uniform, with values set calibrated to the DFT and experimental values observed for K/Au(111). In Fig. 7, it becomes evident that the trends across alkali cations and Au facets do not align between DFT predictions and experimental observations. This discrepancy may stem from challenges in accurately modeling the local interface or from assuming uniformity in the EDL properties across varying cations and facets. Further discussion of the potential origins of the disagreement between DFT and experiment is discussed in Supplementary Note 6. This disagreement between DFT and experiment underscores the unresolved mechanistic basis of the experimental trends. However, it strongly indicates that these trends arise from variations in EDL properties—specifically the effective dielectric response within the EDL region and the distribution of countercharges—that are dependent on both the alkali cation and Au facet under investigation. Elucidation of the double-layer properties is a fundamental challenge in electrocatalysis. Many DFT-based approaches have been developed to include features of the double layer, although they come with varying approximations and associated benefits or disadvantages in analyzing electrocatalysis phenomena. We refer the interested reader to the following book chapters or method development papers that provide review and perspective on these approaches, including considerations directly for CO2R (39, 46–48).
Fig. 7. Comparison between DFT-predicted symmetry factor and experimental symmetry factor.
(A) DFT-predicted symmetry factors across different alkali metal cations and surface facets of Au. The EDL width is assumed constant across alkali metal cations on the Au(111) surface. A Helmholtz EDL width of 5.9 Å was used, fitted to give βDFT = βexp for K+ on Au(111). A cation-coordinated model represents the transition state for *CO2−. (B) Experimentally derived symmetry factors across different alkali metal cations and surface facets of Au derived from Fig. 5.
DISCUSSION
A comprehensive understanding of cation effects on CO2R is critical for developing a highly efficient electrode-electrolyte interface for building practical CO2R electrolyzers. To this end, CO2R was investigated on five model Au electrodes under well-defined mass-transport conditions in 0.1 M bicarbonate electrolytes (LiHCO3, NaHCO3, KHCO3, RbHCO3, and CsHCO3). Different cation trends were observed across the different Au electrodes, strongly suggesting that cation effects are structure sensitive and that the electrode surface structure plays an important role in cation effects on CO2R.
Although a more positive PZC favors higher CO2R activity on Au surface sites with comparable coordination numbers, PZC is a limited descriptor. Our Tafel analysis and KIE experiments suggest that the CO2 adsorption step, coupled with a concomitant electron transfer step, is most likely the RDS during CO2R on Au. Both the surface structure and cation identity can alter the symmetry factor of the RDS, with a volcano-type relation observed on almost all Au electrodes across different cations, where Rb+ gives the largest β values. DFT calculations further confirm that electron transfer occurs to a solvated CO2-cation complex in the RDS of CO2R. The symmetry factor of the RDS is sensitive to both the surface structure and EDL structure. Therefore, the observed structure-sensitive cation effects result from the interplay between the EDL structure and the electrode surface structure during CO2R. Both the electrode surface structure and EDL structure must be considered to explain cation effects, presenting a challenge for the electronic structure and atomistic modeling of CO2R.
MATERIALS AND METHODS
Chemicals
The electrolyte solutions were prepared from H2SO4 (99.999% purity, Sigma-Aldrich), Li2CO3 (99.999%, Thermo Scientific Chemicals), Na2CO3 (99.999%, Thermo Scientific Chemicals), K2CO3 (99.997%, Thermo Scientific Chemicals), Rb2CO3 (99.975%, Thermo Scientific Chemicals), Cs2CO3 (99.994%, Thermo Scientific Chemicals), K3Fe(CN)6 (99%, Sigma-Aldrich), D2O (99.9 atom % D, Sigma-Aldrich), Chelex-100 (sodium form, 50 to 100 mesh, Sigma-Aldrich), and ultrapure water (Millipore Milli-Q, 18.2 megohm·cm, total organic carbon of <3 parts per billion). Ar (99.998%, Linde), CO (99.99%, Linde), CO2 (99.999%, Linde), and H2 (99.995%, Linde) were used for purging the electrolyte solutions.
Single-crystal electrode preparation
Gold single-crystal disk electrodes (99.999%; diameter, 5 mm; thickness, 4 mm) were purchased from Princeton Scientific. All single-crystal electrodes were cut with a nominal orientation accuracy smaller than 0.1° and the polishing roughness smaller than 10 nm. Before each independent measurement, the electrodes were flame annealed to red heat for ~10 s with a propane torch (note that the room light should be turned off for a better observation of the temperature color) and cooled down to room temperature in an argon stream (~30 min). Then, the polished single-crystal surface was attached to a droplet of Milli-Q water and carefully transferred to a clean polytetrafluoroethylene mounting block to assemble the RRDE tip.
EP-pcAu disk and ring electrode preparation
Before each experiment, the gold disk (99.99%; diameter, 5 mm; thickness, 4 mm; Pine Instruments) and ring electrodes (geometric surface area ≈ 0.110 cm2; 99.99%, Pine Instruments) were mechanically polished on Buehler polishing cloth with decreasing sizes of 1-, 0.25-, and 0.1-μm diamond suspensions (Electron Microscopy Sciences). Next, the RRDE tip was sonicated three times in acetone and ultrapure water (3 min each) to remove any impurities attached to the RRDE tip. Then, the RRDE tip was transferred to the electrochemical polishing cell, and the gold disk electrode and gold ring electrode were short circuited and cycled between 0.05 and 1.75 VRHE for 150 cycles at 1 V/s in 0.1 M argon-saturated H2SO4. A representative CV of the Au ring electrode is shown in fig. S2.
FA-pcAu disk electrode preparation
Before each experiment, the gold disk (99.99%; diameter, 5 mm; thickness, 4 mm; Pine Instruments) was mechanically polished on Buehler polishing cloth with decreasing sizes of 1-, 0.25-, and 0.1-μm diamond suspensions (Electron Microscopy Sciences). Next, the RRDE tip was sonicated three times in acetone and ultrapure water (3 min each) to remove any impurities attached to the RRDE tip. Then, the gold disk was disassembled from the RRDE tip, flame annealed to red heat for ~10 s with a propane torch, and cooled down to room temperature in an argon stream. Then, the gold disk surface was attached with a droplet of Milli-Q water and carefully transferred to a polytetrafluoroethylene mounting block to assemble the RRDE tip.
Purification of the H2O-based electrolyte
To prepare prepurified electrolyte solutions with ultralow content of polyvalent metallic impurities, M2CO3(M = Na, K, Rb, and Cs; trace metal basis) and disposable polystyrene spoons (VWR) were used to prepare a 0.05 M M2CO3 solution with ultrapure water in a clean polystyrene plastic bottle to avoid any possible trace metal contamination from the glassware, then ~30 g of Chelex-100 per mole of M2CO3 was added to the solution, and then this mixture was stirred at 700 rpm for 24 hours. Any glassware should be avoided during the long-term (≥24 hours) prepurification process because of the possible leaking of Si4+ from glass corrosion in alkaline M2CO3 solution. Next, insoluble Chelex-100 resin particles were removed from the electrolyte solution by vacuum filtration with the help of a polyethersulfone membrane (pore size, 0.45 μm; VWR). The filtered solution volume was adjusted using a 1-liter polymethylpentene plastic volumetric flask (BRAND), and the ultrapure electrolyte solution was stored in a clean polystyrene bottle.
In the case of 0.05 M Li2CO3, the pre-electrolysis method was adopted instead of Chelex-100 because Chelex-introduced Na+ may support non-negligible CO2R activity. Briefly, the 0.05 M Li2CO3 electrolyte was pre-electrolyzed at a constant reducing current of −10 mA for ~24 hours with a stirring rate of 700 rpm in a Nafion membrane–separated (Nafion 117, Sigma-Aldrich) two-compartment cell. The gold foil (2.5 cm by 2.5 cm, 99.99%, Thermo Scientific Chemicals) and a graphite rod (99.9995%, Thermo Scientific Chemicals) were used as working and counter electrodes, respectively. Gold wires (99.999%, Sigma-Aldrich) were used to build the electrical contact between the electrodes and the potentiostat. Before finishing the pre-electrolysis process, the working electrode was taken out from the solution under the potentiostat control to avoid possible oxidation of deposited metal impurities at open-circuit voltage.
Before each independent experiment, 100 ml of 0.05 M carbonate solution was bubbled through CO2 for 1 to 2 hours with a flow rate of 120 SCCM (standard cubic centimeters per minute) using a mass flow controller (Sierra Instruments), and the pH of the solution was verified to be around 6.8 by using a calibrated pH meter (XL20, Thermo Fisher Scientific), which indicates the complete conversion from a 0.05 M carbonate solution to a 0.1 M CO2-saturated bicarbonate solution. The electrolyte was checked using electrochemical characterizations described in a previous work (49).
Purification of the D2O-based electrolyte
It has been reported that even commercial D2O with the highest purity cannot be used as received for accurate KIE measurement (50). To purify the D2O-based electrolyte, the commercial D2O solvent was illuminated for 6 hours under ultraviolet light using a dual-wavelength ultraviolet lamp (185 nm/254 nm, 25 W) to remove organic carbon contamination. Then, the same procedures as purifying the H2O-based electrolyte were followed to remove potential polyvalent metallic impurities from the D2O-based electrolyte.
General electrochemical measurements
All the electrochemical measurements were carried out in homemade standard three-electrode glass cells (volume ≈ 180 ml). The counter electrode was a high-purity graphite rod (99.9995%, Thermo Scientific Chemicals) separated from the working compartment with a porous glass frit. Either a homemade reversible hydrogen electrode (RHE) or a leak-free Ag/AgCl reference electrode (3.4 M KCl, Innovative Instruments) was used as the reference electrode, which was separated from the working compartment through a homemade Luggin capillary. All the glassware was rinsed and sonicated before each independent experiment with diluted Piranha solution and boiling ultrapure water three times (3 min each), and we note that sonication is necessary because contaminants accumulated in glass frits are hard to be removed completely by rinsing only. For all measurements, 85% ohmic drop compensation was performed using a CHI 760D bipotentiostat (CH Instruments), and the remaining 15% drop was compensated manually after the experiment. The ohmic drop of the system was determined by carrying out potentiostatic electrochemical impedance spectroscopy (PEIS) at 0.10 VRHE (ΔV = 10 mV, from 100 kHz to 1 Hz). The ohmic resistance of the cell (Ru) was obtained by extrapolation of the fitted modified Randles equivalent circuit with a constant phase element as shown in fig. S10. The measured potentials were converted to the RHE scale by ERHE = EAg/AgCl + 0.210 V + 0.059 V × pH.
PZC determination
To determine the values of PZC, additional PEIS measurements (ΔV = 10 mV, from 100 kHz to 1 Hz) were carried out in CO2-saturated 1 mM MHCO3 (pH = 4.8; M = Li, Na, K, Rb, and Cs) electrolytes with a rotation rate of 1600 rpm using a Gamry reference 600+ potentiostat. The double-layer capacitance was determined by fitting the PEIS data with a modified Randles equivalent circuit as shown in fig. S10. Briefly, Cdl was directly derived from a constant phase element term [ZCPE = Cdl′−1(jω)−n], and we assume that Cdl′ represents the true double-layer capacitance when the n value is larger than 0.90, which indicates that the double layer presents pure capacitive behavior without showing notable frequency dispersion behavior. As shown in fig. S11, the potential of global double-layer capacitance minimum in the 1 mM bicarbonate electrolyte corresponds to the PZC. Tabulated PZC values are shown in table S1.
RRDE experiments
To perform CO2R study using an RRDE setup, the disk and ring electrodes were prepared as mentioned before. The measurements were conducted in a 0.1 M CO2-saturated MHCO3 electrolyte solution (pH = 6.8, T = 25 ± 1°C). The disk electrodes were immersed in the electrolyte under potential control at 0.10 VRHE, while the ring electrode was cycled between 0.05 and 1.75 VRHE for five cycles at 50 mV s−1. Then, the ring electrode potential was set to 0.98 VRHE and the disk electrode was scanned between 0.10 and 0 VRHE at 15 mV s−1 (or 5 mV s−1 for obtaining Tafel plots) for 10 cycles to allow the ring current background to reach a steady-state value; the disk was scanned to more negative potentials at 15 mV s−1 to conduct CO2R, while the ring potential was set to 0.98 VRHE once a stable ring background current was recorded. To derive Tafel slopes from CO2R polarization curves measured from the ring currents, a lower scan rate of 5 mV s−1 was used to achieve a better signal-to-noise ratio and the potential window between −0.35 and −0.44 VRHE was chosen as the fitting region of the Tafel plot for the RDS. The collection efficiency was measured immediately after the RRDE experiment and voltammetric surface characterization. The reported results were averaged on the basis of three independent experimental measurements, in which freshly prepared electrolytes and electrodes were used. At potentials more negative than −0.50 VRHE with a scan rate of 15 mV s−1, the RRDE tip suffered from a bubble attachment issue because of a higher total current density, which was especially evident in Rb+- and Cs+-containing electrolytes; thus, the most negative potential was chosen to be −0.50 VRHE.
To further ensure the validity of the RRDE voltammetry method in studying the electrokinetics of CO2R, scan-rate dependent voltammetry and chronoamperometry of CO2R experiments were conducted as shown in figs. S5 and S6, respectively. There is no detectable major difference in current densities upon changing scan rates (15 to 1 mV s−1) or changing from cyclic voltammetry to chronoamperometry; thus, it is safe to assume that our measured currents approach the steady state.
Collection efficiency determination
After each RRDE experiment, the apparent collection efficiency () was determined in a separate cell containing 0.1 M Ar-saturated NaHCO3 with 10 mM K3Fe(CN)6. The disk was cycled between 0.30 and 1.20 VRHE, and the ring potential was set to 1.20 VRHE; representative results are shown in fig. S4. The collection efficiency was calculated according to Eq. 4
| (4) |
Electrochemical surface area determination
After CO2R experiment, characterization cyclic voltammetry of the disk electrode was obtained between 0.05 and 1.75 VRHE at a scan rate of 50 mV s−1. The electrochemically active surface area of the pcAu electrode was determined by calculating the total charge from integrating the reduction peak in the characterization CV and dividing it by the specific charge, which corresponds to the reduction of one monolayer of gold monoxide (386 μC cm−2) (51).
RRDE data processing
The partial current density of CO evolution is calculated from the background-subtracted ring current (), the apparent collection efficiency (), and the electrochemically active surface area of the disk ()
| (5) |
Then, the faradic efficiency for CO formation can be calculated as
| (6) |
where is the experimentally obtained total current on the disk.
Symmetry factor (β) value determination
The Tafel slopes were determined from a straight-line region in the Tafel plots, and the fitted Tafel slopes are considered to be valid only when they are independent of enhanced mass transport rate (variation, <5%), which indicates that the CO2R activity is under purely kinetic control. Typical Tafel plots are shown in figs. S16 and S17. The valid Tafel slopes were converted to symmetry factor values as below
| (7) |
and the measurements were repeated three times independently to ensure that the derived values are statistically meaningful.
Electronic structure calculations
The Vienna Ab Initio Simulation Package was used for all electronic structure calculations in this work (52–55). The projector augmented wave method was used to approximate the core-valence electrons (56, 57). The Perdew, Burke, and Ernzerhof exchange correlation functional within the generalized gradient approximation was used for all calculations (58–60). An energy cutoff value of 450 eV was used to truncate the plane wave basis set. The ionic convergence limit was set to 0.05 eV Å−1 (EDIFFG), while the electronic convergence limit was set to 10−5 eV (EDIFF). A Fermi smearing width of 0.2 (SIGMA) was used for surface-bound species on the basis of the Methfessel-Paxton scheme (61). Additional s semi-core valence electron states are treated for all alkali metal cations.
Model construction
The Au(111), Au(110), and Au(100) surface facets were modeled as five-layer 3 by 3 periodic surface slabs. The top two layers were relaxed to undergo surface reconstruction, while the bottom three layers were constrained to represent the bulk material. A 15-Å vacuum region was applied across all surface facets. Dipole moments within the surface model were calculated by applying a dipole correction in the normal direction of the periodic surface (IDIPOL = 3; LDIPOL = .TRUE.). A Monkhorst-Pack method was used to sample the Brillouin zone (62, 63). A 5 by 5 by 1 k-point mesh grid was used for the Au(100) surface, a 7 by 7 by 1 k-point mesh grid for the Au(110) surface, and a 11 by 11 by 1 k-point mesh grid for the Au(111) surface.
Acknowledgments
We acknowledge A. Lutton and K. D. Aztergo for assistance with inductively coupled plasma mass spectrometry measurements at the Trace Element Research Laboratory in OSU.
Funding: This work was supported by the National Science Foundation (NSF CHE 1455162) and in part by the Ohio State University Department of Chemistry and Biochemistry. Computational efforts were supported by the National Science Foundation (NSF-CBET 1939464).
Author contributions: Conceptualization: Z.C., A.J.-W.W., M.J.J., and A.C.C. Methodology, investigation, and visualization: Z.C., A.J.-W.W., M.J.J., and A.C.C. Supervision: M.J.J. and A.C.C. Writing—original draft: Z.C., A.J.-W.W., M.J.J., and A.C.C. Writing—review and editing: Z.C., A.J.-W.W., M.J.J., and A.C.C.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Notes 1 to 6
Figs. S1 to S36
Tables S1 to S11
References
REFERENCES AND NOTES
- 1.Nitopi S., Bertheussen E., Scott S. B., Liu X., Engstfeld A. K., Horch S., Seger B., Stephens I. E. L., Chan K., Hahn C., Nørskov J. K., Jaramillo T. F., Chorkendorff I., Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Jones J.-P., Prakash G. K. S., Olah G. A., Electrochemical CO2 reduction: Recent advances and current trends. Isr. J. Chem. 54, 1451–1466 (2014). [Google Scholar]
- 3.Stephens I. E. L., Chan K., Bagger A., Boettcher S. W., Bonin J., Boutin E., Buckley A. K., Buonsanti R., Cave E. R., Chang X., Chee S. W., da Silva A. H. M., de Luna P., Einsle O., Endrődi B., Escudero-Escribano M., de Araujo J. V. F., Figueiredo M. C., Hahn C., Hansen K. U., Haussener S., Hunegnaw S., Huo Z., Hwang Y. J., Janáky C., Jayathilake B. S., Jiao F., Jovanov Z. P., Karimi P., Koper M. T. M., Kuhl K. P., Lee W. H., Liang Z., Liu X., Ma S., Ma M., Oh H.-S., Robert M., Cuenya B. R., Rossmeisl J., Roy C., Ryan M. P., Sargent E. H., Sebastián-Pascual P., Seger B., Steier L., Strasser P., Varela A. S., Vos R. E., Wang X., Xu B., Yadegari H., Zhou Y., 2022 roadmap on low temperature electrochemical CO2 reduction. J. Phys. Energy 4, 042003 (2022). [Google Scholar]
- 4.Akira M., Yoshio H., Product selectivity affected by cationic species in electrochemical reduction of CO2 and CO at a Cu electrode. Bull. Chem. Soc. Jpn. 64, 123–127 (1991). [Google Scholar]
- 5.Ringe S., Clark E. L., Resasco J., Walton A., Seger B., Bell A. T., Chan K., Understanding cation effects in electrochemical CO2 reduction. Energy Environ. Sci. 12, 3001–3014 (2019). [Google Scholar]
- 6.Thorson M. R., Siil K. I., Kenis P. J. A., Effect of cations on the electrochemical conversion of CO2 to CO. J. Electrochem. Soc. 160, F69–F74 (2013). [Google Scholar]
- 7.Resasco J., Chen L. D., Clark E., Tsai C., Hahn C., Jaramillo T. F., Chan K., Bell A. T., Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 139, 11277–11287 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Ayemoba O., Cuesta A., Spectroscopic evidence of size-dependent buffering of interfacial pH by cation hydrolysis during CO2 electroreduction. ACS Appl. Mater. Interfaces 9, 27377–27382 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Singh M. R., Kwon Y., Lum Y., Ager J. W. III, Bell A. T., Hydrolysis of electrolyte cations enhances the electrochemical reduction of CO2 over Ag and Cu. J. Am. Chem. Soc. 138, 13006–13012 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Hussain G., Pérez-Martínez L., Le J.-B., Papasizza M., Cabello G., Cheng J., Cuesta A., How cations determine the interfacial potential profile: Relevance for the CO2 reduction reaction. Electrochim. Acta 327, 135055 (2019). [Google Scholar]
- 11.Monteiro M. C. O., Dattila F., Hagedoorn B., García-Muelas R., López N., Koper M. T. M., Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 4, 654–662 (2021). [Google Scholar]
- 12.Ovalle V. J., Hsu Y.-S., Agrawal N., Janik M. J., Waegele M. M., Correlating hydration free energy and specific adsorption of alkali metal cations during CO2 electroreduction on Au. Nat. Catal. 5, 624–632 (2022). [Google Scholar]
- 13.Chen X., McCrum I. T., Schwarz K. A., Janik M. J., Koper M. T. M., Co-adsorption of cations as the cause of the apparent pH dependence of hydrogen adsorption on a stepped platinum single-crystal electrode. Angew. Chem. Int. Ed. 56, 15025–15029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z.-Q., Banerjee S., Thoi V. S., Shoji Hall A., Reorganization of interfacial water by an amphiphilic cationic surfactant promotes CO2 reduction. J. Phys. Chem. Lett. 11, 5457–5463 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Li J., Li X., Gunathunge C. M., Waegele M. M., Hydrogen bonding steers the product selectivity of electrocatalytic CO reduction. Proc. Natl. Acad. Sci. U.S.A. 116, 9220–9229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malkani A. S., Li J., Oliveira N. J., He M., Chang X., Xu B., Lu Q., Understanding the electric and nonelectric field components of the cation effect on the electrochemical CO reduction reaction. Sci. Adv. 6, eabd2569 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Q., Wallentine S. K., Deng G.-H., Rebstock J. A., Baker L. R., The solvation-induced onsager reaction field rather than the double-layer field controls CO2 reduction on gold. JACS Au 2, 472–482 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin S.-J., Choi H., Ringe S., Won D. H., Oh H.-S., Kim D. H., Lee T., Nam D.-H., Kim H., Choi C. H., A unifying mechanism for cation effect modulating C1 and C2 productions from CO2 electroreduction. Nat. Commun. 13, 5482 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin X., Vegge T., Hansen H. A., Cation-coordinated inner-sphere CO2 electroreduction at Au–water interfaces. J. Am. Chem. Soc. 145, 1897–1905 (2023). [DOI] [PubMed] [Google Scholar]
- 20.Waegele M. M., Gunathunge C. M., Li J., Li X., How cations affect the electric double layer and the rates and selectivity of electrocatalytic processes. J. Chem. Phys. 151, 160902 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Malkani A. S., Anibal J., Xu B., Cation effect on interfacial CO2 concentration in the electrochemical CO2 reduction reaction. ACS Catalysis 10, 14871–14876 (2020). [Google Scholar]
- 22.Pérez-Gallent E., Marcandalli G., Figueiredo M. C., Calle-Vallejo F., Koper M. T. M., Structure- and potential-dependent cation effects on CO reduction at copper single-crystal electrodes. J. Am. Chem. Soc. 139, 16412–16419 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang B. A., Ozel T., Elias J. S., Costentin C., Nocera D. G., Interplay of homogeneous reactions, mass transport, and kinetics in determining selectivity of the reduction of CO2 on gold electrodes. ACS Cent. Sci. 5, 1097–1105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corpus K. R. M., Bui J. C., Limaye A. M., Pant L. M., Manthiram K., Weber A. Z., Bell A. T., Coupling covariance matrix adaptation with continuum modeling for determination of kinetic parameters associated with electrochemical CO2 reduction. Joule 7, 1289–1307 (2023). [Google Scholar]
- 25.Goyal A., Marcandalli G., Mints V. A., Koper M. T. M., Competition between CO2 reduction and hydrogen evolution on a gold electrode under well-defined mass transport conditions. J. Am. Chem. Soc. 142, 4154–4161 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui Z., Wong A. J.-W., Janik M. J., Co A. C., Negative reaction order for CO during CO2 electroreduction on Au. J. Am. Chem. Soc. 146, 23872–23880 (2024). [DOI] [PubMed] [Google Scholar]
- 27.Hamelin A., Cyclic voltammetry at gold single-crystal surfaces. Part 1. Behaviour at low-index faces. J. Electroanal. Chem. 407, 1–11 (1996). [Google Scholar]
- 28.A. Dakkouri, D. Kolb, Reconstruction of Gold Surfaces (Marcel Dekker, 1999).
- 29.Jeyabharathi C., Ahrens P., Hasse U., Scholz F., Identification of low-index crystal planes of polycrystalline gold on the basis of electrochemical oxide layer formation. J. Solid State Electrochem. 20, 3025–3031 (2016). [Google Scholar]
- 30.Sun S.-G., Cai W.-B., Wan L.-J., Osawa M., Infrared absorption enhancement for CO adsorbed on Au films in perchloric acid solutions and effects of surface structure studied by cyclic voltammetry, scanning tunneling microscopy, and surface-enhanced IR spectroscopy. J. Phys. Chem. B. 103, 2460–2466 (1999). [Google Scholar]
- 31.Štrbac S., Adžć R. R., Hamelin A., Oxide formation on gold single crystal stepped surfaces. J. Electroanal. Chem. Interf. Electrochem. 249, 291–310 (1988). [Google Scholar]
- 32.Rebstock J. A., Zhu Q., Baker L. R., Exploring the influence of interfacial solvation on electrochemical CO2 reduction using plasmon-enhanced vibrational sum frequency generation spectroscopy. ChemCatChem 16, e202301301 (2024). [Google Scholar]
- 33.Dong Q., Zhang X., He D., Lang C., Wang D., Role of H2O in CO2 electrochemical reduction as studied in a water-in-salt system. ACS Cent. Sci. 5, 1461–1467 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ringe S., Morales-Guio C. G., Chen L. D., Fields M., Jaramillo T. F., Hahn C., Chan K., Double layer charging driven carbon dioxide adsorption limits the rate of electrochemical carbon dioxide reduction on Gold. Nat. Commun. 11, 33 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng W., Zhang P., Seger B., Gong J., Unraveling the rate-limiting step of two-electron transfer electrochemical reduction of carbon dioxide. Nat. Commun. 13, 803 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mezzavilla S., Horch S., Stephens I. E. L., Seger B., Chorkendorff I., Structure sensitivity in the electrocatalytic reduction of CO2 with gold catalysts. Angew. Chem. Int. Ed. 58, 3774–3778 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Akhade S. A., Bernstein N. J., Esopi M. R., Regula M. J., Janik M. J., A simple method to approximate electrode potential-dependent activation energies using density functional theory. Catal. Today 288, 63–73 (2017). [Google Scholar]
- 38.Nie X., Esopi M. R., Janik M. J., Asthagiri A., Selectivity of CO2 reduction on copper electrodes: The role of the kinetics of elementary steps. Angew. Chem. Int. Ed. 52, 2459–2462 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Agrawal N., Wong A. J.-W., Maheshwari S., Janik M. J., An efficient approach to compartmentalize double layer effects on kinetics of interfacial proton-electron transfer reactions. J. Catal. 430, 115360 (2024). [Google Scholar]
- 40.Fumagalli L., Esfandiar A., Fabregas R., Hu S., Ares P., Janardanan A., Yang Q., Radha B., Taniguchi T., Watanabe K., Gomila G., Novoselov K. S., Geim A. K., Anomalously low dielectric constant of confined water. Science 360, 1339–1342 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Tran B., Zhou Y., Janik M. J., Milner S. T., Negative dielectric constant of water at a metal interface. Phys. Rev. Lett. 131, 248001 (2023). [DOI] [PubMed] [Google Scholar]
- 42.Sundararaman R., Vigil-Fowler D., Schwarz K., Improving the accuracy of atomistic simulations of the electrochemical interface. Chem. Rev. 122, 10651–10674 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz K., Sundararaman R., The electrochemical interface in first-principles calculations. Surf. Sci. Rep. 75, 100492 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shandilya A., Schwarz K., Sundararaman R., Interfacial water asymmetry at ideal electrochemical interfaces. J. Chem. Phys. 156, 014705 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Karnes J. J., Weitzner S. E., Akhade S. A., Baker S. E., Duoss E. B., Varley J. B., A hybrid quantum–classical study of ion adsorption at the copper electrode. J. Phys. Chem. C 126, 12413–12423 (2022). [Google Scholar]
- 46.K.-Y. Yeh, M. J. Janik, in Computational Catalysis, A. Asthagiri, M. J. Janik, Eds. (The Royal Society of Chemistry, 2013), pp. 116–156. [Google Scholar]
- 47.S. Maheshwari, Y. Li, N. Agrawal, M. J. Janik, in Advances in Catalysis, C. Song, Ed. (Academic Press, 2018), vol. 63, pp. 117–167. [Google Scholar]
- 48.Wong A. J.-W., Tran B., Agrawal N., Goldsmith B. R., Janik M. J., Sensitivity analysis of electrochemical double layer approximations on electrokinetic predictions: Case study for CO reduction on copper. J. Phys. Chem. C 128, 10837–10847 (2024). [Google Scholar]
- 49.Cui Z., Marx M. A., Tegomoh M. N., Co A. C., A guide to evaluate electrolyte purity for CO2 reduction studies. ACS Energy Lett. 8, 5201–5205 (2023). [Google Scholar]
- 50.Yang Y., Agarwal R. G., Hutchison P., Rizo R., Soudackov A. V., Lu X., Herrero E., Feliu J. M., Hammes-Schiffer S., Mayer J. M., Abruña H. D., Inverse kinetic isotope effects in the oxygen reduction reaction at platinum single crystals. Nat. Chem. 15, 271–277 (2023). [DOI] [PubMed] [Google Scholar]
- 51.Rand D. A. J., Woods R., The nature of adsorbed oxygen on rhodium, palladium and gold electrodes. J. Electroanal. Chem. Interf. Electrochem. 31, 29–38 (1971). [Google Scholar]
- 52.Kresse G., Hafner J., Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993). [DOI] [PubMed] [Google Scholar]
- 53.Kresse G., Hafner J., Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994). [DOI] [PubMed] [Google Scholar]
- 54.Kresse G., Furthmüller J., Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996). [Google Scholar]
- 55.Kresse G., Furthmüller J., Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev. B 54, 11169–11186 (1996). [DOI] [PubMed] [Google Scholar]
- 56.Kresse G., Joubert D., From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999). [Google Scholar]
- 57.Blöchl P. E., Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994). [DOI] [PubMed] [Google Scholar]
- 58.Perdew J. P., Burke K., Ernzerhof M., Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- 59.Perdew J. P., Burke K., Ernzerhof M., Generalized gradient approximation made simple [Phys. Rev. Lett. 77, 3865 (1996)]. Phys. Rev. Lett. 78, 1396–1396 (1997). [DOI] [PubMed] [Google Scholar]
- 60.Perdew J. P., Chevary J. A., Vosko S. H., Jackson K. A., Pederson M. R., Singh D. J., Fiolhais C., Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46, 6671–6687 (1992). [DOI] [PubMed] [Google Scholar]
- 61.Methfessel M., Paxton A. T., High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 40, 3616–3621 (1989). [DOI] [PubMed] [Google Scholar]
- 62.Monkhorst H. J., Pack J. D., Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976). [Google Scholar]
- 63.Pack J. D., Monkhorst H. J., “Special points for Brillouin-zone integrations”—A reply. Phys. Rev. B 16, 1748–1749 (1977). [Google Scholar]
- 64.Devi N., Williams C. K., Chaturvedi A., Jiang J. J., Homogeneous electrocatalytic CO2 reduction using a porphyrin complex with flexible triazole units in the second coordination sphere. ACS Appl. Energy Mater. 4, 3604–3611 (2021). [Google Scholar]
- 65.Todoroki N., Tei H., Tsurumaki H., Miyakawa T., Inoue T., Wadayama T., Surface atomic arrangement dependence of electrochemical CO2 reduction on gold: Online electrochemical mass spectrometric study on low-index Au(hkl) surfaces. ACS Catal. 9, 1383–1388 (2019). [Google Scholar]
- 66.Marcandalli G., Villalba M., Koper M. T. M., The importance of acid–base equilibria in bicarbonate electrolytes for CO2 electrochemical reduction and CO reoxidation studied on Au(hkl) electrodes. Langmuir 37, 5707–5716 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wuttig A., Yaguchi M., Motobayashi K., Osawa M., Surendranath Y., Inhibited proton transfer enhances Au-catalyzed CO2-to-fuels selectivity. Proc. Natl. Acad. Sci. U.S.A. 113, E4585–E4593 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Y. Hori, in Modern Aspects of Electrochemistry, C. G. Vayenas, R. E. White, M. E. Gamboa-Aldeco, Eds. (Springer New York, 2008), pp. 89–189. [Google Scholar]
- 69.Dunwell M., Luc W., Yan Y., Jiao F., Xu B., Understanding surface-mediated electrochemical reactions: CO2 reduction and beyond. ACS Catal. 8, 8121–8129 (2018). [Google Scholar]
- 70.Rostamikia G., Janik M. J., Borohydride oxidation over Au(111): A first-principles mechanistic study relevant to direct borohydride fuel cells. J. Electrochem. Soc. 156, B86–B92 (2009). [Google Scholar]
- 71.Schwarz H. A., Dodson R. W., Reduction potentials of CO2- and the alcohol radicals. J. Phys. Chem. 93, 409–414 (1989). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Notes 1 to 6
Figs. S1 to S36
Tables S1 to S11
References