Congenital syphilis, first described by Gaspar Torella in 1497, results primarily from the transplacental passage of Treponema pallidum and continues to cause major negative consequences worldwide.1 After a steady decline in U.S. cases of primary and secondary syphilis after 1990, which led to hopes of its elimination, rates hit a nadir in 2001. Unfortunately, the rates have subsequently increased among men and women of reproductive age, as well as infants, and in 2021, the rate of congenital syphilis in the United States was the highest it has been in nearly 30 years.2
EPIDEMIOLOGY
Syphilis, like many sexually transmitted infections (STIs), disproportionately affects populations with limited access to health care.2–5 The annual U.S. rates of primary and secondary syphilis among Native American and Alaskan Native persons, Native Hawaiian and Pacific Islanders, and Black women, as compared with White women, have increased over the past 5 years by factors of 8, 4, and 3.5, respectively.2,3,5 Along with the rise in cases of syphilis among persons of reproductive age,2 cases of congenital syphilis increased by 754.8% from 2012 to 2021; currently, 1 in every 1300 live births is affected.2 Since these data reflect only identified and reported cases of syphilis, they probably represent an underestimate of the incidence.2,6,7 Cases of syphilis in late infancy and early childhood among international adoptees or refugees have also been increasing.8 Escalating rates of congenital syphilis predated the coronavirus disease 2019 (Covid-19) pandemic but increased further as public health programs shifted to respond to Covid-19.2,5,9–13 The effect of untreated syphilis on maternal and neonatal health outcomes is profound, with mother-to-child transmission of syphilis estimated to cost $3.6 million in disability-adjusted life-years and $309 million in medical costs globally.14–16
PATHOGENESIS
Congenital syphilis usually results from transplacental passage of T. pallidum to the fetus during disseminated maternal infection. Less frequently, neonatal infection occurs through exposure to syphilitic genital lesions at the time of delivery.17–25 T. pallidum has a small genome with limited outer membrane protein expression, which renders the organism essentially undetectable by the fetal immune system after exposure, leading to persistent fetal infection.20–22 The risk to the fetus of congenital infection is 50 to 70% in pregnancies complicated by early syphilis but decreases to 15% if maternal syphilis was contracted more than a year before the pregnancy.2,17–26 Transmission may occur at any time during pregnancy, and the risk of transmission is thought to increase with the duration of gestational exposure. However, the estimation of risk may be confounded by infection that predated pregnancy.17–25 Limited data suggest that fetal infection occurs through placental infection, followed by amniotic fluid infection, and ultimately, hematologic dysfunction, leading to nonimmune hydrops, though the true sequence remains unknown.17
MATERNAL SCREENING
Serologic testing at the first prenatal visit is mandated by most states in the United States and is recommended by health authorities worldwide.27–29 The STI guidelines of the Centers for Disease Control and Prevention (CDC) recommend repeat screening during pregnancy at 28 weeks’ gestation and at delivery for women who live in high-prevalence areas; women who are considered to be at increased risk for syphilis because of sex with multiple partners, sex in conjunction with drug use, or transactional sex; women receiving late or no prenatal care; and women who are incarcerated (or have an incarcerated partner) or have unstable housing.27 Although there is no consensus definition of high prevalence, 95% of states reported at least one case of congenital syphilis in 2021.2 A comprehensive social and sexual history is crucial for assessment of individual risk. In a large review of gestational syphilis, however, nearly half of patients did not report any risk factors,30 indicating that screening based solely on behavioral risk factors may miss a substantial proportion of syphilis infections.26,31–34
To better capture structural risk factors for syphilis such as community and sexual networks, predictive models that incorporate early syphilis rates among men, violent crime rates, race or ethnic group, urbanicity, population size, and the presence or absence of a neighboring county with early syphilis have been developed.35,36 These risk scores have varying accuracy in identifying U.S. counties with an elevated risk of syphilis, including congenital syphilis. Further work is needed to translate these findings into practical use.
CLINICAL PRESENTATION OF SYPHILIS DURING PREGNANCY
The clinical presentation of syphilis does not differ between pregnant and nonpregnant women. Primary syphilis is characterized by one or more indurated chancres at the site of inoculation, which are usually painless and occur within 3 weeks after exposure, although the chancres can have an atypical appearance and may be painful.27 After the primary lesion has resolved, a macular rash often appears during the secondary phase. The rash frequently, but not always, involves the palms and soles, and it may be desquamative. These diverse characteristics make the diagnosis challenging. A syphilitic rash should be distinguished from other rashes that can occur during pregnancy such as atopic eruption of pregnancy or pemphigoid gestationis. Clinical manifestations in primary and secondary syphilis may overlap, particularly in persons with human immunodeficiency virus (HIV) infection.27,37–40 The nonspecific symptoms of primary and secondary syphilis may result in a delayed diagnosis during pregnancy.17,24 Other clinical findings of secondary syphilis can include lymphadenopathy, alopecia, condyloma lata, and oral mucosal patches27 (Fig. 1 and 2).
Figure 1. Clinical Manifestations of Primary and Secondary Syphilis.
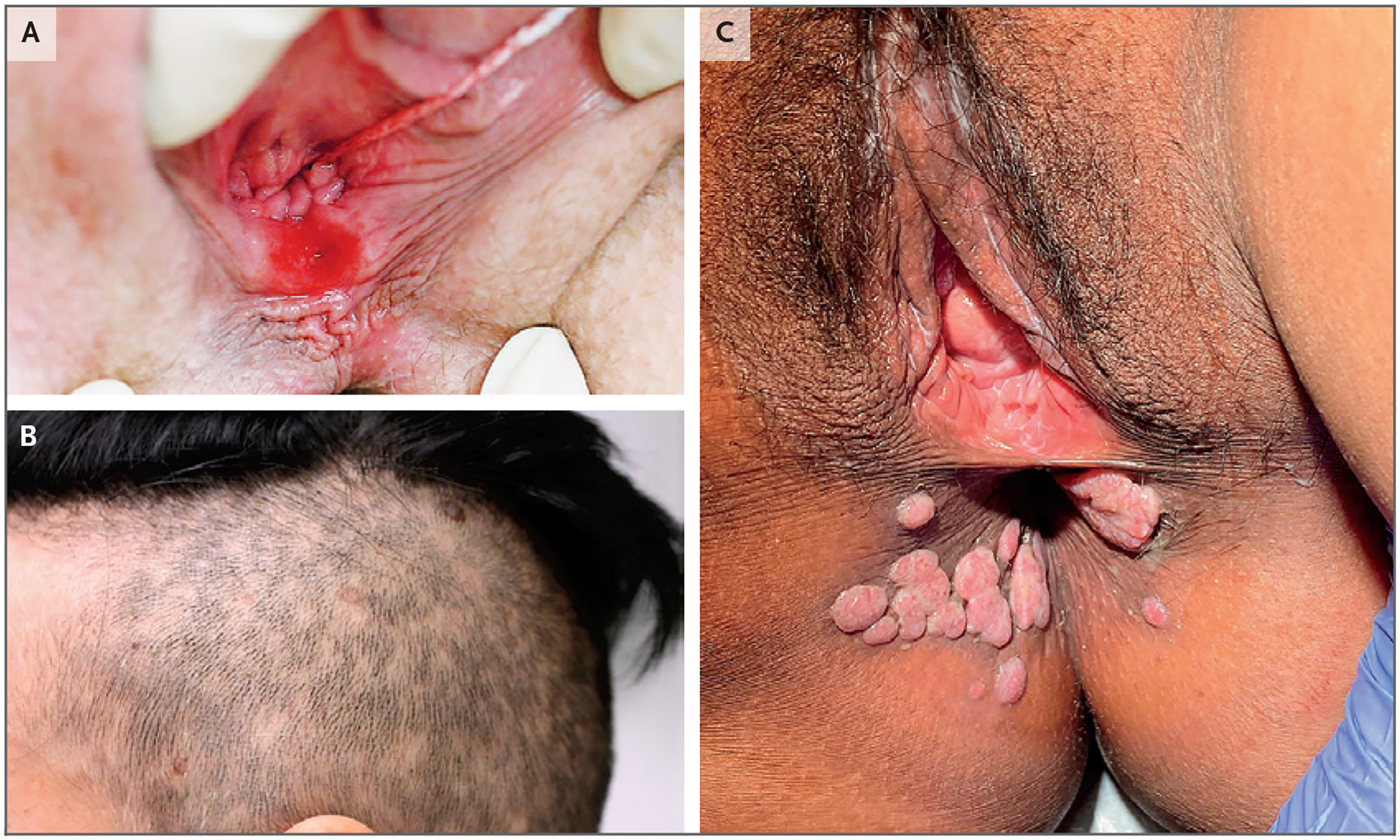
Panel A shows a chancre in primary syphilis. Panels B and C show alopecia and condyloma lata, respectively, in secondary syphilis.
Figure 2. Oral and Cutaneous Manifestations of Secondary Syphilis.
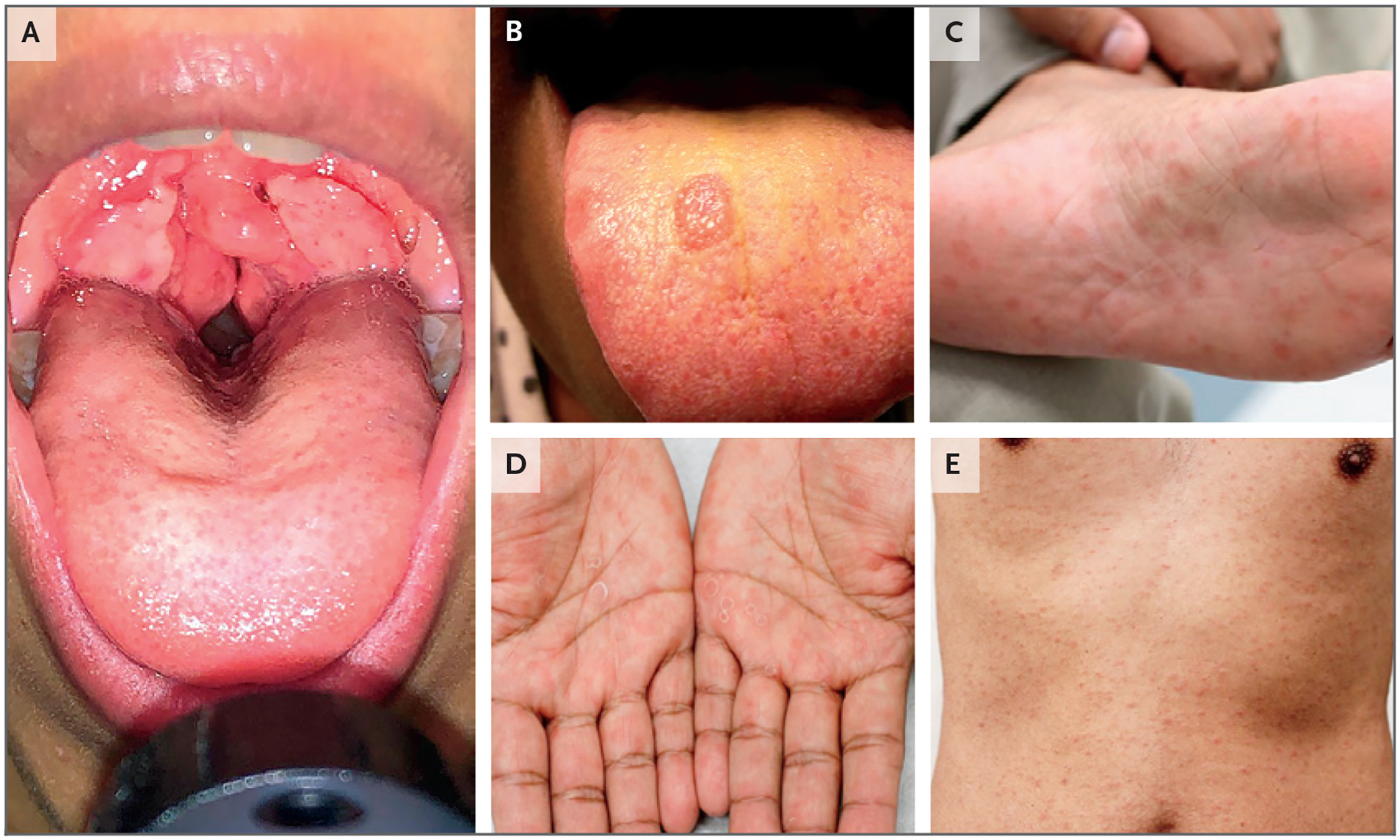
Panel A shows oral condyloma lata, Panel B shows a mucosal patch on the tongue, and Panels C, D, and E show plantar, palmar, and truncal rashes, respectively.
Resolution of the signs and symptoms of syphilis defines latent infection, which is detected only by serologic testing. Latent infection acquired within the preceding year is referred to as early latent syphilis, and accurate classification requires an interview with the patient, physical examination, a review of serologic test results and the treatment history, and information about the infection status of the patient’s sexual partner (or partners).27 Up to a quarter of untreated persons have recurrent secondary syphilis within the first year after infection, and pathognomonic clinical findings may be absent.24,27 Syphilis remains latent in approximately 70% of untreated persons but progresses to tertiary syphilis in the remaining 30%.27
DIAGNOSIS OF SYPHILIS DURING PREGNANCY
Dark-field microscopy, direct fluorescence antibody testing, and immunohistochemical or silver staining can be used to evaluate the lesions of early syphilis through direct detection of T. pallidum, though most clinical settings are not equipped to perform these tests, and direct fluorescence antibody testing is not currently available in the United States. Nucleic acid amplification tests (NAATs) are available, and the results can be used for a clinical diagnosis if they are validated by the standards established in the Clinical Laboratory Improvement Amendments. However, no assay that directly detects T. pallidum has been approved by the Food and Drug Administration (FDA) for commercial use in the United States.27
Serologic diagnosis of syphilis is the same in pregnant and nonpregnant persons and can be made with the use of either the traditional or reverse sequence algorithm (see Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).27 The traditional syphilis screening algorithm begins with a nontreponemal immunoassay (e.g., a rapid plasma reagin test or Venereal Disease Research Laboratory [VDRL] test), followed by a treponemal immunoassay (e.g., the T. pallidum particle agglutination [TP-PA] assay) for confirmation. In the reverse sequence algorithm, an automated treponemal immunoassay is used first, typically a treponemal enzyme immunoassay or chemiluminescence assay, which then requires a quantitative nontreponemal test for confirmation. A second treponemal-specific test with different antigens from those used in the original treponemal-specific test (e.g., the TP-PA assay) should be performed when the reverse sequence algorithm generates discordant results.27 There are more than 18 different types of treponemal tests available for use in the United States, and the choice of algorithm to be used at a given clinical practice or institution should be based on available laboratory resources, test volume, and patient populations served.27 A patient with reactive results on both treponemal and nontreponemal tests should be considered to have active infection unless prior treatment resulting in a decrease by a factor of 4 from the pretreatment nontreponemal titer is appropriately documented and there is no concern about reinfection according to the timing described in the CDC STI guidelines.27
Irrespective of the testing algorithm used, quantitative nontreponemal values are used to monitor disease activity and the response to therapy in pregnant women, which is the approach used in nonpregnant persons.27 Because effective syphilis treatment during pregnancy is time-sensitive, nontreponemal titers are assessed at 8 weeks after treatment (vs. ≥6 months after treatment in nonpregnant persons) and at delivery, unless there is concern about reinfection or treatment has failed.27 A sustained increase by a factor of 4 from pretreatment titers may represent treatment failure or reinfection and requires retreatment of the patient.27 The guidelines for syphilis management after delivery are the same as those for nonpregnant persons.27
TREATMENT OF SYPHILIS DURING PREGNANCY
The only antimicrobial agent proven to be both safe and efficacious for the treatment of syphilis during pregnancy is parenteral benzathine penicillin G, administered intramuscularly according to the clinical stage, an approach that does not differ during pregnancy (Table S1).27 If the patient has a history of penicillin allergy, evaluation with skin testing or an oral penicillin challenge is encouraged during pregnancy.27,41 If these tests are not feasible or if the patient has a confirmed IgE-mediated allergy, inpatient desensitization should be performed.41–46 A history of a severe hypersensitivity syndrome, such as the Stevens–Johnson syndrome, warrants consultation with an allergist.27
Nonpenicillin antimicrobial agents have not been shown to have bactericidal activity against T. pallidum and are not recommended for the treatment of syphilis during pregnancy.27,46–49 If alternative regimens are used during pregnancy, the newborn should be evaluated and potentially treated for congenital infection.27 The Jarisch–Herxheimer reaction occurs in up to 40% of women treated for syphilis during pregnancy and is characterized by cramping, pyrexia, and myalgias.27,44,45 Continuous fetal heart rate monitoring for 12 to 24 hours after treatment should be considered to confirm fetal well-being if the mother presents with symptoms that are consistent with the Jarisch–Herxheimer reaction.17–24
Although no randomized, controlled trials have compared dosing regimens in pregnant women, prospective cohort studies have shown more than 95% efficacy when a single dose of 2.4 million units of benzathine penicillin G is used in early infection, with little increase in efficacy when higher or multiple doses are used.17–22 For late latent infection or infection of unknown duration, three doses of 2.4 million units of benzathine penicillin G, administered weekly, are recommended. If the interval between doses exceeds 9 days, treatment is considered to be inadequate and should be reinitiated.27 Careful staging of syphilis ensures appropriate use of antimicrobial agents, eliminating the unnecessary administration of additional doses of benzathine penicillin G, along with stringent follow-up, when early syphilis is mischaracterized as late latent syphilis or syphilis of unknown duration.
A decrease by a factor of 4 in maternal nontreponemal titers may indicate a maternal treatment response but does not confirm the absence of neonatal infection. When treatment is started in the third trimester, there may not be time for nontreponemal titers to fall by a factor of 4 before delivery, complicating confirmation of a maternal cure and requiring continued surveillance for the exposed dyad.50–52 Local health departments and the National Network of STD Clinical Prevention Training Centers (www.nnptc.org) can be invaluable resources in assisting clinicians with decisions about the management of syphilis.
DIAGNOSIS AND MANAGEMENT OF FETAL INFECTION
The sensitivity of dark-field microscopy for visualizing T. pallidum in samples from amniocentesis ranges from 42 to 86%,20,53 but dark-field microscopy remains impractical because of its limited availability.20 Direct fluorescence antibody testing for T. pallidum is not available in the United States. Nucleic acid amplification testing has a sensitivity ranging from 75 to 100%.53–57 Given the limited sensitivities of dark-field microscopy (when available) and nucleic acid amplification testing, ultrasonography is the most commonly used method to examine a fetus for evidence of congenital syphilis.24,51,52 Ultrasonographic evidence of intrauterine infection, which can be detected after 18 weeks of gestation, when the fetus is able to mount an immune response to T. pallidum infection, includes fetal hepatomegaly (in 80% of cases); anemia, as indicated by the peak systolic velocity of the middle cerebral artery (33%) (Fig. S2); placentomegaly (27%) (Fig. 3A); polyhydramnios (12%); and nonimmune hydrops (10%).24,51,52,58–64 However, the absence of ultrasonographic abnormalities does not rule out congenital infection. The prevalence of congenital syphilis at delivery ranges from 12 to 15% among at-risk fetuses with no ultrasonographic findings of congenital infection.50,51
Figure 3. Sonographic, Radiographic, and Cutaneous Manifestations of Congenital Syphilis.
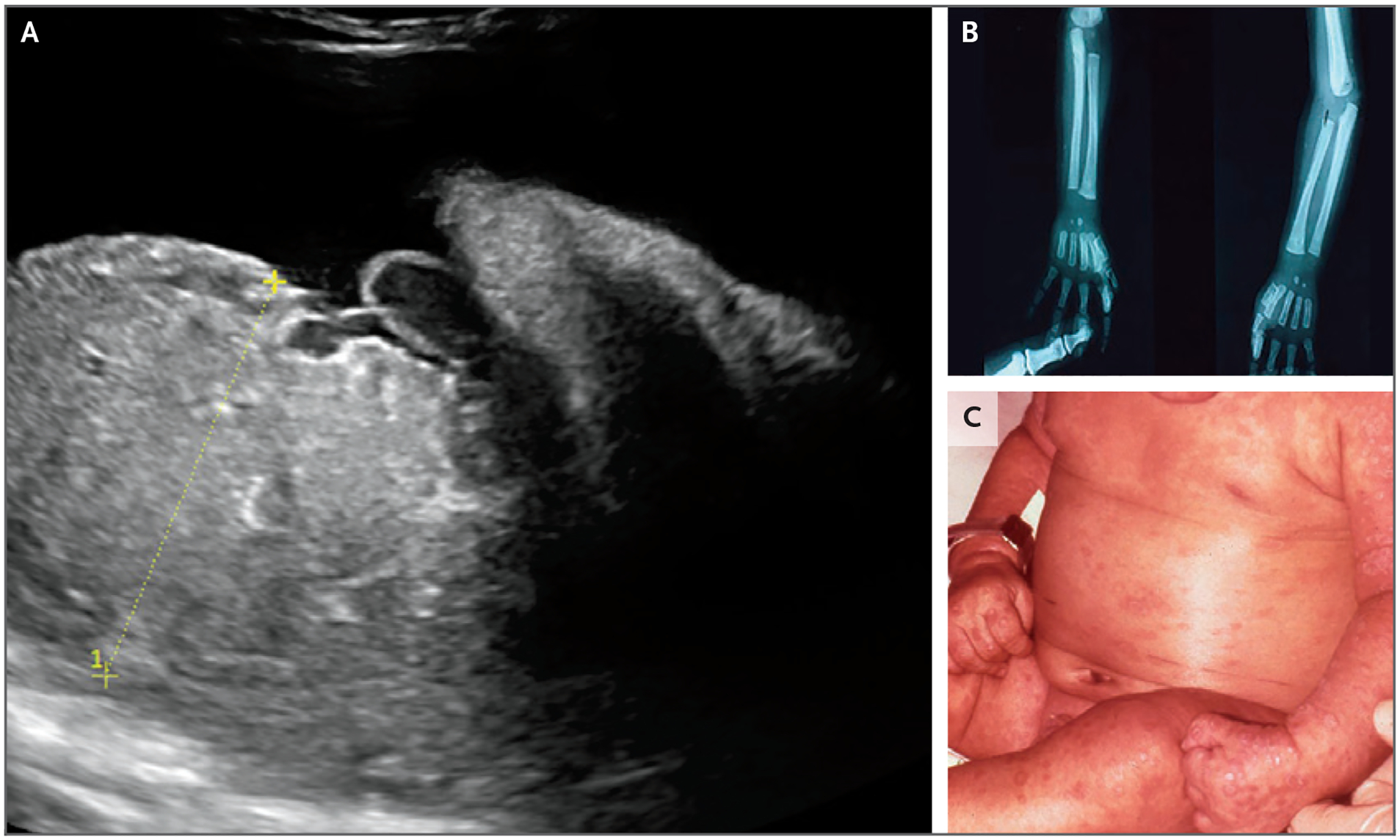
Panel A shows ultrasonographic evidence of placentomegaly (>95% for gestational age). Panels B and C show radial and ulnar periostitis and maculopapular rash, respectively, in early congenital syphilis.
Fetal anemia and related hydrops generally resolve within 3 weeks after treatment of maternal syphilis, with subsequent normalization of fetal liver and placental measurements, which may take up to 15 weeks after treatment (Fig. S3).51 Early detection and appropriate treatment before the third trimester allow ultrasonographic abnormalities to resolve completely in most fetuses.50,51 Accordingly, ultrasonography should precede antepartum treatment, since abnormal findings, especially nonimmune hydrops, indicate a risk of obstetrical complications.17,27,50,51 More than 80% of fetuses with hydrops are delivered preterm because of concern about fetal status. Exposure to T. pallidum by-products from maternal treatment may play a contributory role.17,50,51 Fetal ultrasonographic abnormalities should be monitored serially to inform the pediatric care team about potential complications at birth.
The stage of syphilis and nontreponemal titers in the mother remain crude surrogates for the infection risk in fetuses without ultrasonographic markers of infection. One study showed that up to 16% of newborns with congenital syphilis were born to treated women who had an appropriate decline by a factor of 4 in nontreponemal titers before delivery.50 However, in some studies, congenital syphilis was not observed if the interval between adequate treatment and delivery was more than 28 days.34,52 Given these data, early maternal treatment should be emphasized.26 Unfortunately, some newborns have congenital syphilis irrespective of the adequacy of maternal treatment, underscoring the need for improved diagnostics for neonatal infection.2,68–71
NEONATAL EVALUATION AND MANAGEMENT
As with prenatal diagnosis of syphilis, neonatal diagnosis can be made by a variety of methods, many of which do not have FDA approval and are not widely available.27,54–57,65 Immunohistochemical evidence of placental infection, which has 67 to 82% sensitivity and 58% specificity, can aid in establishing the diagnosis.72 Since maternal IgM does not cross the placenta, the neonatal IgM level may have a diagnostic role, though the performance characteristics of current assays are unknown.73,74 Since maternal treponemal antibodies can be passively transferred to the fetus in the absence of active neonatal infection, evaluation of this marker is not helpful.27,66–71,73,74 Nontreponemal IgA and IgG antibodies are more useful for neonatal diagnosis, though sensitivity is reduced because they also passively cross the placenta.73 The diagnostic criteria include nontreponemal titers in the neonate that are four times the titers in the mother, but the absence of such results does not rule out the diagnosis of congenital syphilis.27,69–71,75 Most newborns with congenital syphilis have nontreponemal titers that are reduced by a factor of 2 to 4, as compared with maternal nontreponemal titers.27,69–71,75
Given the paucity of diagnostic tests for the newborn, treatment decisions are typically based on a combination of factors, including identification of syphilis in the mother, assessment of the adequacy of maternal treatment, the interval between the initiation of maternal treatment and delivery (with an interval of >30 days considered to be adequate), comparison of maternal and neonatal nontreponemal serologic titers at delivery, and the presence or absence of clinical, laboratory, and radiographic evidence of syphilis in the neonate.27
Infants born to mothers with syphilis should undergo nontreponemal testing and clinical evaluation for signs of congenital syphilis. Newborns with syphilis may be preterm or small for gestational age.21–32 Women with syphilis have a higher rate of preterm birth than women with other genital tract infections, even with adjustment for confounding factors.76 Symptomatic newborns have anemia and thrombocytopenia (in 37% of cases), hepatobiliary dysfunction (33 to 100%), bullous or mucocutaneous lesions (40%), and osteochondritis or periostitis (75%) (Fig. 4).71,77–79 Lesions in the long bones that have a moth-eaten appearance because of demineralization are pathognomonic for congenital syphilis.77 Syphilitic rhinitis (“snuffles”) and fever are also common.77–79 Newborns meeting the criteria for confirmed or highly probable syphilis should undergo evaluation for neurosyphilis and receive treatment with weight-based, intravenous aqueous crystalline penicillin G (Table S2), even if ampicillin and gentamicin have already been administered for suspected neonatal sepsis.27
Figure 4. Clinical Signs of Early Congenital Syphilis.
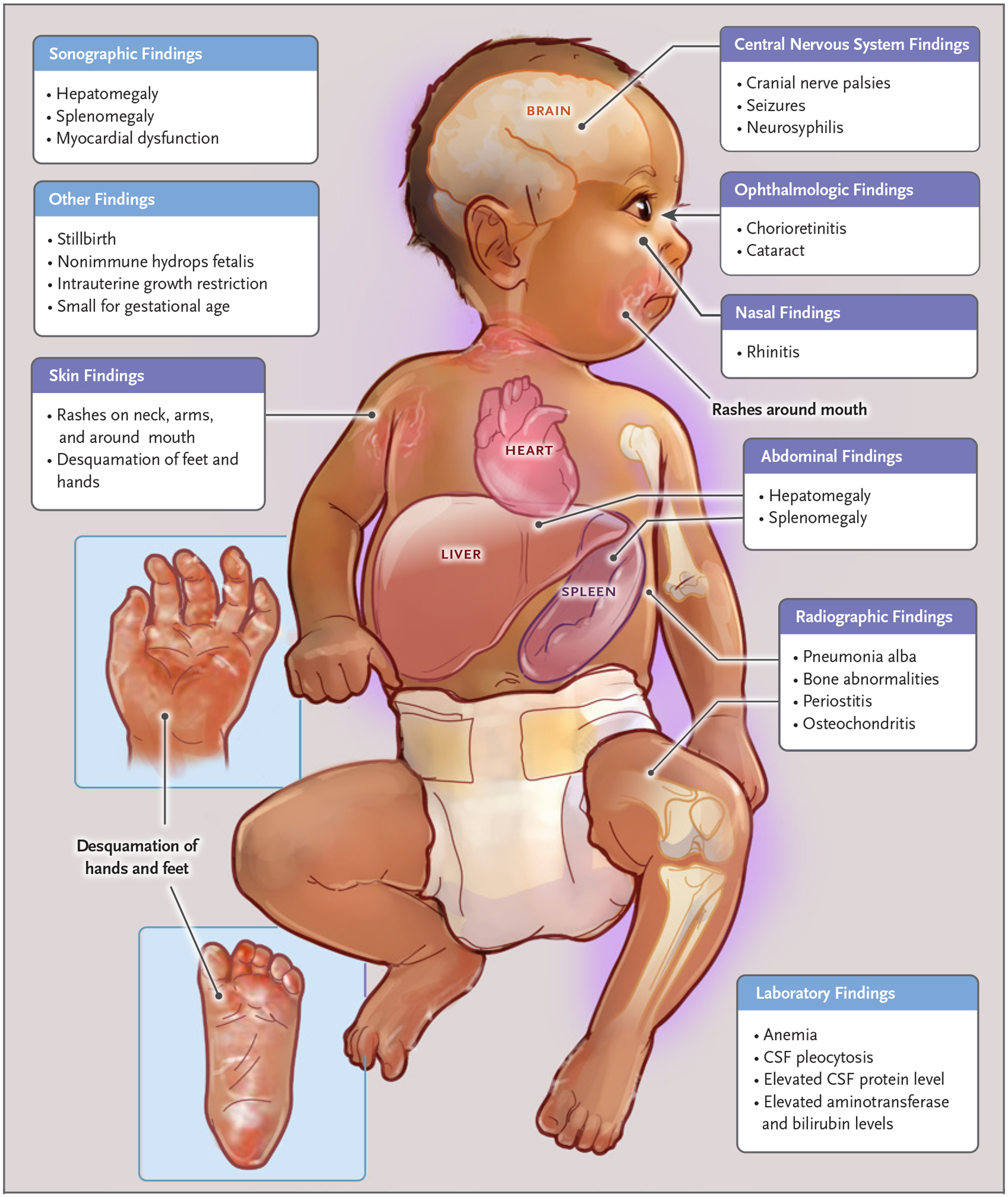
Clinical findings of early congenital syphilis include neurosyphilis, syphilitic rhinitis, anemia, hepatobiliary dysfunction, cutaneous lesions or rash, and osteochondritis or periostitis. Shown are clinical signs in children under 2 years of age.
Up to 60% of symptomatic newborns have neurosyphilis, which is manifested as seizures, ophthalmologic abnormalities, cranial nerve palsies, or cerebral infarcts in 5 to 40% of affected newborns.71,77–80 Cerebrospinal fluid (CSF) test results are difficult to interpret, since nontreponemal antibodies may be passively transferred from plasma into the CSF. The sensitivity of a CSF VDRL test is approximately 50%, with 90% specificity.71,77–80 The long-term neurodevelopmental sequelae of neurosyphilis are largely unknown. The lack of research in this field is a major impediment to the development of targeted interventions in early childhood that can better serve affected children. Prominent clinical and radiographic features of early congenital syphilis are shown in Figure 3B and 3C and Figure 5.
Figure 5. Clinical Manifestations of Congenital Syphilis.
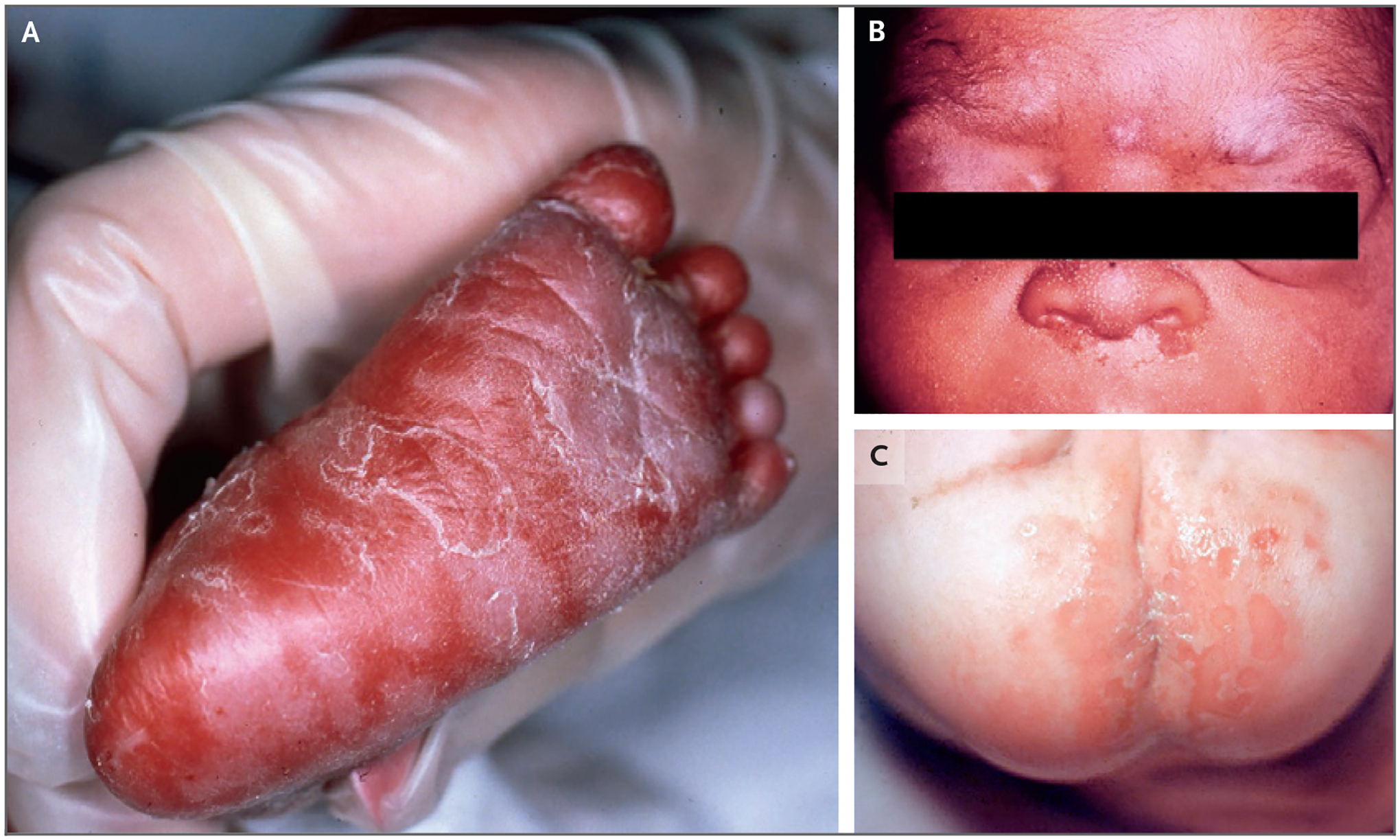
Panel A shows neonatal plantar rash, Panel B shows snuffles, and Panel C shows erosive lesions involving the buttocks.
Late manifestations of congenital syphilis, which may occur after 2 years of age, include bony abnormalities in the midline face and lower extremities, Hutchinson’s teeth (notched, diastematic teeth), ocular abnormalities, and sensorineural hearing loss.78–80 Clinical suspicion of syphilis infection at any age should prompt a full evaluation by a pediatric infectious disease specialist.26
One of the key challenges to diagnosing and adequately treating congenital syphilis at birth is the high rate of asymptomatic cases.27,68,78–80 Asymptomatic newborns have no clinical or laboratory evidence of infection, but manifestations of disease may occur days to months later in the absence of treatment.27,78–80 Between 2014 and 2018, 55.5% of live-born infants in the United States who had congenital syphilis were reported to be asymptomatic.70 In 2% of these infants, the diagnosis was established after the neonatal period. Conversely, a substantial percentage of at-risk, asymptomatic newborns do not acquire syphilis and are treated conservatively on the basis of the current CDC treatment guidelines.27 The CDC surveillance case definition for congenital syphilis was established to standardize reporting and is not meant for clinical diagnosis.
All neonates with reactive nontreponemal tests should be monitored serologically every 2 to 3 months to ensure normalization of the tests results. Passively transferred maternal nontreponemal antibodies can be present in an infant until 15 months of age. In most uninfected newborns, however, nontreponemal titers will be normal by 6 months of age.27 If elevated titers persist after 6 months, the infant should undergo evaluation and treatment as described above.27 In a treated newborn, elevated or persistent nontreponemal tests after 6 to 12 months of age should prompt reevaluation, including CSF analysis and treatment if persistent infection is suspected.27 The rate of loss to follow-up is as high as 65% in some U.S. regions, increasing the risk of a missed or delayed diagnosis and neonatal complications.4,81 Programs supporting maternal and neonatal adherence to longitudinal follow-up are needed in order to reduce morbidity. Patient-centered care, medication delivery, and mobile testing modeled after perinatal HIV programs may be of benefit.
CHALLENGES AND MISSED OPPORTUNITIES
Elimination of perinatal syphilis is possible with timely diagnosis and treatment during pregnancy.27 On the basis of surveillance data, the most common missed opportunities for the prevention of congenital syphilis are delayed prenatal care or none (in 42% of perinatal syphilis cases), inadequate maternal treatment (31%), late identification of maternal seroconversion during pregnancy (14%), and prenatal care without syphilis testing (8%)2 (Fig. S4). According to preliminary surveillance data from the CDC, 3761 cases of congenital syphilis have been reported in 2022, which represents an increase in cases over the past decade, and 88% of these cases resulted from a lack of timely testing, inadequate treatment, or both in infected pregnant persons.82 Missed opportunities vary by U.S. region, underscoring the need to tailor prevention to the local setting.2,4,31–36 Given the limited usefulness of using individual risk behavior on the part of pregnant persons and their partners to predict syphilis, approaches should be refined to specifically identify the patient and contextual characteristics that make syphilis screening during the third trimester or at delivery cost-effective.
New diagnostic approaches that are capable of detecting T. pallidum infection in the newborn are needed. Direct T. pallidum detection at birth may result in more timely treatment. Numerous T. pallidum surface or subsurface lipoprotein targets have been evaluated for use in diagnostic research. Polymerase-chain-reaction assays targeting POLA, TPP47, or both and NAATs targeting 16S and 23S rRNA, TMPC, and TMPA are currently being investigated for diagnosis, including through testing of specimens obtained noninvasively, such as anal, vaginal, or oral mucosal swabs.54–57,83,84 Timely diagnosis at birth would substantially reduce the burden of follow-up that disproportionately disadvantages patients with socioeconomic barriers such as transportation, child care, or insurance costs.4,26,30–36
If benzathine penicillin G is not available because of a critical shortage, which is currently the case in the United States, administration of the drug should be prioritized for pregnant persons and newborns.27 Oral doxycycline is recommended as an alternative therapy except during pregnancy, including in the postpartum period. Although short-term administration of doxycycline during breast-feeding is not contraindicated, adherence may be compromised because of hesitancy related to the potential adverse effects among breast-feeding infants, such as enamel staining and skeletal defects.85,86 Penicillin G procaine, a prior treatment option for neonates with possible, confirmed, or highly probable congenital syphilis is no longer available, because it has been discontinued by the manufacturer.27 An ongoing study is evaluating alternative regimens for the treatment of syphilis during pregnancy, including high-dose amoxicillin (ClinicalTrials.gov number, NCT05309928). Other trials have evaluated treatment with macrolides, tetracyclines, and cephalosporins in nonpregnant adults. However, there is a paucity of research dedicated to the evaluation of alternative therapies for congenital syphilis and syphilis during pregnancy.47–49
Persistent challenges to the elimination of congenital syphilis include structural health care barriers that may be compounded by mental illness and substance use.4,38–41 Fear of legal or punitive actions resulting in loss of child custody perpetuates barriers to prenatal care among women who struggle with substance use disorders.38–41 Despite increased efforts to improve syphilis testing and treatment, strategies to improve treatment of sexual partners are failing.87,88 A study in Uganda that used phone calls and text messages to notify partners of pregnant women with syphilis showed a low rate of partner treatment (<20%). Study participants expressed an unwillingness to receive syphilis treatment because of fear of injections and loss of dignity.87 Treatment of male partners is an important contributor to breaking the chain of maternal infection and reinfection that contributes to congenital syphilis.
The risk factors associated with lack of an appropriate syphilis diagnosis and follow-up for mother–infant dyads have been the focus of CDC-supported and other federal, state, and city intervention efforts.89–91 New initiatives include implementation of opt-out screening at the time of pregnancy testing, including in the emergency department (ED) and at urgent care centers, and rapid syphilis testing with presumptive treatment if the result of an isolated treponemal-specific test is positive or if the physical examination and symptoms are suggestive of syphilis.27 Identification and immediate treatment through the ED may also increase health equity for those disproportionately affected by STIs, who often lack access to health care.27,73,89
FUTURE NEEDS
As the congenital syphilis epidemic continues unabated, novel approaches are needed. It is important to provide tailored local interventions that address the social and structural factors associated with disparities in prenatal care and STI detection, an approach that is consistent with the Sexually Transmitted Infections National Strategic Plan.90 New federal funding and partnerships through the CDC and the National Institutes of Health have been established to support local efforts to eliminate congenital syphilis.89–91 Standardized approaches to screening, new and improved diagnostic and therapeutic options, and other interventions addressing congenital syphilis that are embedded within a health equity framework are likely to improve the diagnosis and treatment of congenital syphilis, which in turn should substantially reduce the morbidity and mortality associated with this largely preventable disease.
Supplementary Material
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Obladen M Curse on two generations: a history of congenital syphilis. Neonatology 2013; 103: 274–80. [DOI] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention. Sexually transmitted disease surveillance 2021. (https://www.cdc.gov/std/statistics/2021/default.htm).
- 3.Martin EG, Ansari B, Rosenberg ES, et al. Variation in patterns of racial and ethnic disparities in primary and secondary syphilis diagnosis rates among heterosexually active women by region and age group in the United States. Sex Transm Dis 2022; 49: 330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coselli J, Rac M, Wilson D, Wang C, Sibai BM, Stafford IA. Risk factors associated with gaps in post-treatment surveillance for perinatal syphilis. Am J Obstet Gynecol 2022; 226: Suppl: S673–S675. [Google Scholar]
- 5.Stafford IA, Coselli JO, Wilson DF, Wang CY, Sibai BM. Comparison of sexually transmitted infections and adverse perinatal outcomes in underserved pregnant patients before vs during the COVID-19 pandemic in Texas. JAMA Netw Open 2022; 5(2): e220568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin J, Yang T, Xiao S, Tan H, Feng T, Fu H. Reported estimates of adverse pregnancy outcomes among women with and without syphilis: a systematic review and meta-analysis. PLoS One 2014; 9(7): e102203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindstrand A, Bergström S, Bugalho A, Zanconato G, Helgesson AM, Hederstedt B. Prevalence of syphilis infection in Mozambican women with second trimester miscarriage and women attending antenatal care in second trimester. Genitourin Med 1993; 69: 431–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phares CR, Liu Y, Wang Z, et al. Disease surveillance among U.S.-Bound immigrants and refugees — Electronic Disease Notification system, United States, 2014–2019. MMWR Surveill Summ 2022; 71(SS-2): 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright SS, Kreisel KM, Hitt JC, Pagaoa MA, Weinstock HS, Thorpe PG. Impact of the COVID-19 pandemic on Centers for Disease Control and Prevention-funded sexually transmitted disease programs. Sex Transm Dis 2022; 49(4): e61–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanford KA, Almirol E, Schneider J, Hazra A. Rising syphilis rates during the COVID-19 pandemic. Sex Transm Dis 2021; 48(6): e81–e83. [DOI] [PubMed] [Google Scholar]
- 11.National Coalition of STD Directors. COVID-19 & the state of the STD field: phase II. August 2020. (https://www.ncsddc.org/resource/covid-19-the-state-of-the-std-field-phase-ii/).
- 12.Stockman JK, Wood BA, Anderson KM. Racial and ethnic differences in COVID-19 outcomes, stressors, fear, and prevention behaviors among US women: Web-based cross-sectional study. J Med Internet Res 2021; 23(7): e26296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogunbodede OT, Zablotska-Manos I, Lewis DA. Potential and demonstrated impacts of the COVID-19 pandemic on sexually transmissible infections. Curr Opin Infect Dis 2021; 34: 56–61. [DOI] [PubMed] [Google Scholar]
- 14.Cheah IGS. Economic assessment of neonatal intensive care. Transl Pediatr 2019; 8: 246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bureau of Labor Statistics. Consumer Price Index (CPI) — all urban consumers, medical care services (ID CUSR0000S-AM2), 2009–2017. (https://www.bls.gov/data).
- 16.Zou Y, Liao Y, Liu F, et al. The annual economic burden of syphilis: an estimation of direct, productivity, and intangible costs for syphilis in guangdong initiative for comprehensive control of syphilis sites. Sex Transm Dis 2017; 44: 671–7. [DOI] [PubMed] [Google Scholar]
- 17.Hollier LM, Harstad TW, Sanchez PJ, Twickler DM, Wendel GD Jr. Fetal syphilis: clinical and laboratory characteristics. Obstet Gynecol 2001; 97: 947–53. [DOI] [PubMed] [Google Scholar]
- 18.Nathan L, Bohman VR, Sanchez PJ, Leos NK, Twickler DM, Wendel GD Jr. In utero infection with Treponema pallidum in early pregnancy. Prenat Diagn 1997; 17: 119–23. [DOI] [PubMed] [Google Scholar]
- 19.Alexander JM, Sheffield JS, Sanchez PJ, Mayfield J, Wendel GD Jr. Efficacy of treatment for syphilis in pregnancy. Obstet Gynecol 1999; 93: 5–8. [DOI] [PubMed] [Google Scholar]
- 20.Wendel GD Jr, Sánchez PJ, Peters MT, Harstad TW, Potter LL, Norgard MV. Identification of Treponema pallidum in amniotic fluid and fetal blood from pregnancies complicated by congenital syphilis. Obstet Gynecol 1991; 78: 890–5. [PubMed] [Google Scholar]
- 21.Wendel GD Jr, Sheffield JS, Hollier LM, Hill JB, Ramsey PS, Sánchez PJ. Treatment of syphilis in pregnancy and prevention of congenital syphilis. Clin Infect Dis 2002; 35: Suppl 2: S200–S209. [DOI] [PubMed] [Google Scholar]
- 22.Nathan L, Bawdon RE, Sidawi JE, Stettler RW, McIntire DM, Wendel GD Jr. Penicillin levels following the administration of benzathine penicillin G in pregnancy. Obstet Gynecol 1993; 82: 338–42. [PubMed] [Google Scholar]
- 23.Wicher V, Wicher K. Pathogenesis of maternal-fetal syphilis revisited. Clin Infect Dis 2001; 33: 354–63. [DOI] [PubMed] [Google Scholar]
- 24.Rac MWF, Stafford IA, Eppes CS. Congenital syphilis: a contemporary update on an ancient disease. Prenat Diagn 2020; 40: 1703–14. [DOI] [PubMed] [Google Scholar]
- 25.Michelow IC, Wendel GD Jr, Norgard MV, et al. Central nervous system infection in congenital syphilis. N Engl J Med 2002; 346: 1792–8. [DOI] [PubMed] [Google Scholar]
- 26.Thornton C, Chaisson LH, Bleasdale SC. Characteristics of pregnant women with syphilis and factors associated with congenital syphilis at a Chicago hospital. Open Forum Infect Dis 2022; 9: ofac169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Sexually transmitted infections (STI) treatment guidelines, 2021. (https://www.cdc.gov/std/treatment-guidelines/default.htm).
- 28.US Preventive Services Task Force. Screening for syphilis infection in pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA 2018; 320: 911–7. [DOI] [PubMed] [Google Scholar]
- 29.Guidelines for perinatal care. 8th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2017. [Google Scholar]
- 30.Trivedi S, Williams C, Torrone E, Kidd S. National trends and reported risk factors among pregnant women with syphilis in the United States, 2012–2016. Obstet Gynecol 2019; 133: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthias JM, Rahman MM, Newman DR, Peterman TA. Effectiveness of prenatal screening and treatment to prevent congenital syphilis, Louisiana and Florida, 2013–2014. Sex Transm Dis 2017; 44: 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuda A, Katz AR, Park IU, et al. Congenital syphilis: a case report demonstrating missed opportunities for screening and inadequate treatment despite multiple health care encounters during pregnancy. Sex Transm Dis 2021; 48(9): e124–e125. [DOI] [PubMed] [Google Scholar]
- 33.Drame FN, Urban MA, Inscho RR, et al. Best practices implementation: congenital syphilis prevention efforts in Monroe County, New York, 2018. Sex Transm Dis 2022; 49: 310–2. [DOI] [PubMed] [Google Scholar]
- 34.Lin JS, Eder ML, Bean SI. Screening for syphilis infection in pregnant women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018; 320: 918–25. [DOI] [PubMed] [Google Scholar]
- 35.Cuffe KM, Kang JDY, Dorji T, et al. Identification of US counties at elevated risk for congenital syphilis using predictive modeling and a risk scoring system. Sex Transm Dis 2020; 47: 290–5. [DOI] [PubMed] [Google Scholar]
- 36.Kimball A, Torrone E, Miele K, et al. Missed opportunities for prevention of congenital syphilis — United States, 2018. MMWR Morb Mortal Wkly Rep 2020; 69: 661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen SA, Hambie EA, Pettit DE, Perryman MW, Kraus SJ. Specificity, sensitivity, and reproducibility among the fluorescent treponemal antibody-absorption test, the microhemagglutination assay for Treponema pallidum antibodies, and the hemagglutination treponemal test for syphilis. J Clin Microbiol 1981; 14: 441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park IU, Fakile YF, Chow JM, et al. Performance of treponemal tests for the diagnosis of syphilis. Clin Infect Dis 2019; 68: 913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med 2020; 382: 845–54. [DOI] [PubMed] [Google Scholar]
- 40.Donders GG, Desmyter J, Hooft P, Dewet GH. Apparent failure of one injection of benzathine penicillin G for syphilis during pregnancy in human immunodeficiency virus-seronegative African women. Sex Transm Dis 1997; 24: 94–101. [DOI] [PubMed] [Google Scholar]
- 41.Desravines N, Waldron J, Venkatesh KK, Kwan M, Boggess KA. Outpatient penicillin allergy testing in pregnant women who report an allergy. Obstet Gynecol 2021; 137: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wendel GD Jr, Stark BJ, Jamison RB, Molina RD, Sullivan TJ. Penicillin allergy and desensitization in serious infections during pregnancy. N Engl J Med 1985; 312: 1229–32. [DOI] [PubMed] [Google Scholar]
- 43.Pham MN, Ho H-E, Desai M. Penicillin desensitization: treatment of syphilis in pregnancy in penicillin-allergic patients. Ann Allergy Asthma Immunol 2017; 118: 537–41. [DOI] [PubMed] [Google Scholar]
- 44.Farmer TW. Jarisch-Herxheimer reaction in early syphilis treated with crystalline penicillin G. J Am Med Assoc 1948; 138: 480–5. [DOI] [PubMed] [Google Scholar]
- 45.Bowen JH, Cole HN, Driver JR, et al. Herxheimer reactions in penicillin treatment of syphilis in pregnancy. Arch Derm Syphilol 1948; 58: 735–9. [Google Scholar]
- 46.Cross JB, McCain JR, Heyman A. The use of crystalline penicillin G in the treatment of syphilis in pregnancy. Am J Obstet Gynecol 1949; 57: 461–5. [DOI] [PubMed] [Google Scholar]
- 47.Hook EW III, Behets F, Van Damme K, et al. A phase III equivalence trial of azithromycin versus benzathine penicillin for treatment of early syphilis. J Infect Dis 2010; 201: 1729–35. [DOI] [PubMed] [Google Scholar]
- 48.Stafylis C, Keith K, Mehta S, et al. Clinical efficacy of cefixime for the treatment of early syphilis. Clin Infect Dis 2021; 73: 907–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zengarini C, Carpanese MA, Vara G, Conni A, Piraccini BM, Gaspari V. Analysis of serological treatment response to doxycycline versus benzathine penicillin in syphilis infections, a retrospective single-center study. Dermatol Ther 2022; 35(8): e15586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rac MWF, Bryant SN, Cantey JB, McIntire DD, Wendel GD Jr, Sheffield JS. Maternal titers after adequate syphilotherapy during pregnancy. Clin Infect Dis 2015; 60: 686–90. [DOI] [PubMed] [Google Scholar]
- 51.Rac MWF, Bryant SN, McIntire DD, et al. Progression of ultrasound findings of fetal syphilis after maternal treatment. Am J Obstet Gynecol 2014; 211(4): 426.e1–426.e6. [DOI] [PubMed] [Google Scholar]
- 52.Sheffield JS, Sánchez PJ, Morris G, et al. Congenital syphilis after maternal treatment for syphilis during pregnancy. Am J Obstet Gynecol 2002; 186: 569–73. [DOI] [PubMed] [Google Scholar]
- 53.Cooper JM, Sánchez PJ. Congenital syphilis. Semin Perinatol 2018; 42: 176–84. [DOI] [PubMed] [Google Scholar]
- 54.Pillay A, Liu H, Chen CY, et al. Molecular subtyping of Treponema pallidum subspecies pallidum. Sex Transm Dis 1998; 25: 408–14. [DOI] [PubMed] [Google Scholar]
- 55.Grimprel E, Sanchez PJ, Wendel GD, et al. Use of polymerase chain reaction and rabbit infectivity testing to detect Treponema pallidum in amniotic fluid, fetal and neonatal sera, and cerebrospinal fluid. J Clin Microbiol 1991; 29: 1711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Dai X, Ren Z, Lin H, Cao W, Ye X. A novel nested real-time polymerase chain reaction for Treponema pallidum DNA in syphilis biospecimens. Sex Transm Dis 2019; 46: 41–6. [DOI] [PubMed] [Google Scholar]
- 57.Grange PA, Gressier L, Dion PL, et al. Evaluation of a PCR test for detection of treponema pallidum in swabs and blood. J Clin Microbiol 2012; 50: 546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mari G, Deter RL, Carpenter RL, et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. N Engl J Med 2000; 342: 9–14. [DOI] [PubMed] [Google Scholar]
- 59.Duncan JR, Schenone MH, Argoti PS, Mari G. Middle cerebral artery peak systolic velocity in perinatal cytomegalovirus infection. J Clin Ultrasound 2019; 47: 372–5. [DOI] [PubMed] [Google Scholar]
- 60.Hernandez-Andrade E, Scheier M, Dezerega V, Carmo A, Nicolaides KH. Fetal middle cerebral artery peak systolic velocity in the investigation of non-immune hydrops. Ultrasound Obstet Gynecol 2004; 23: 442–5. [DOI] [PubMed] [Google Scholar]
- 61.Delle Chiaie L, Buck G, Grab D, Terinde R. Prediction of fetal anemia with Doppler measurement of the middle cerebral artery peak systolic velocity in pregnancies complicated by maternal blood group alloimmunization or parvovirus B19 infection. Ultrasound Obstet Gynecol 2001; 18: 232–6. [DOI] [PubMed] [Google Scholar]
- 62.Society for Maternal-Fetal Medicine (SMFM), Norton ME, Chauhan SP, Dashe JS. Society for maternal-fetal medicine (SMFM) clinical guideline #7: nonimmune hydrops fetalis. Am J Obstet Gynecol 2015; 212: 127–39. [DOI] [PubMed] [Google Scholar]
- 63.Hoddick WK, Mahony BS, Callen PW, Filly RA. Placental thickness. J Ultrasound Med 1985; 4: 479–82. [DOI] [PubMed] [Google Scholar]
- 64.Vintzileos AM, Neckles S, Campbell WA, Andreoli JW Jr, Kaplan BM, Nochimson DJ. Fetal liver ultrasound measurements during normal pregnancy. Obstet Gynecol 1985; 66: 477–80. [PubMed] [Google Scholar]
- 65.Stoll BJ, Lee FK, Larsen S, et al. Clinical and serologic evaluation of neonates for congenital syphilis: a continuing diagnostic dilemma. J Infect Dis 1993; 167: 1093–9. [DOI] [PubMed] [Google Scholar]
- 66.Rawstron SA, Bromberg K. Comparison of maternal and newborn serologic tests for syphilis. Am J Dis Child 1991; 145: 1383–8. [DOI] [PubMed] [Google Scholar]
- 67.Mascola L, Pelosi R, Blount JH, Alexander CE, Cates W Jr. Congenital syphilis revisited. Am J Dis Child 1985; 139: 575–80. [DOI] [PubMed] [Google Scholar]
- 68.Wozniak PS, Cantey JB, Zeray F, et al. Congenital syphilis in neonates with non-reactive nontreponemal test results. J Perinatol 2017; 37: 1112–6. [DOI] [PubMed] [Google Scholar]
- 69.Dorfman DH, Glaser JH. Congenital syphilis presenting in infants after the newborn period. N Engl J Med 1990; 323: 1299–302. [DOI] [PubMed] [Google Scholar]
- 70.Kimball A, Bowen VB, Miele K, et al. Congenital syphilis diagnosed beyond the neonatal period in the United States: 2014–2018. Pediatrics 2021; 148(3): e2020049080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sánchez PJ. Congenital syphilis. Adv Pediatr Infect Dis 1992; 7: 161–80. [PubMed] [Google Scholar]
- 72.Sheffield JS, Sánchez PJ, Wendel GD Jr, et al. Placental histopathology of congenital syphilis. Obstet Gynecol 2002; 100: 126–33. [DOI] [PubMed] [Google Scholar]
- 73.Satyaputra F, Hendry S, Braddick M, Sivabalan P, Norton R. The laboratory diagnosis of syphilis. J Clin Microbiol 2021; 59(10): e0010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herremans M, Notermans DW, Mommers M, Kortbeek LM. Comparison of a treponema pallidum IgM immunoblot with a 19S fluorescent treponemal antibody absorption test for the diagnosis of congenital syphilis. Diagn Microbiol Infect Dis 2007; 59: 61–6. [DOI] [PubMed] [Google Scholar]
- 75.Miller JL, Meyer PG, Parrott NA, Hill JH. A study of the biologic falsely positive reactions for syphilis in children. J Pediatr 1960; 57: 548–52. [DOI] [PubMed] [Google Scholar]
- 76.Gao R, Liu B, Yang W, et al. Association of maternal sexually transmitted infections with risk of preterm birth in the United States. JAMA Netw Open 2021; 4(11): e2133413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rasool MN, Govender S. The skeletal manifestations of congenital syphilis: a review of 197 cases. J Bone Joint Surg Br 1989; 71: 752–5. [DOI] [PubMed] [Google Scholar]
- 78.David M, Hcini N, Mandelbrot L, Sibiude J, Picone O. Fetal and neonatal abnormalities due to congenital syphilis: a literature review. Prenat Diagn 2022; 42: 643–55. [DOI] [PubMed] [Google Scholar]
- 79.Medoro AK, Sánchez PJ. Syphilis in neonates and infants. Clin Perinatol 2021; 48: 293–309. [DOI] [PubMed] [Google Scholar]
- 80.Ropper AH. Neurosyphilis. N Engl J Med 2019; 381: 1358–63. [DOI] [PubMed] [Google Scholar]
- 81.Stafford IA, Berra A, Minard CG, et al. Challenges in the contemporary management of syphilis among pregnant women in New Orleans, LA. Infect Dis Obstet Gynecol 2019; 2019: 2613962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McDonald R, O’Callaghan K, Torrone E, et al. Vital signs: missed opportunities for preventing congenital syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep 2023; 72: 1269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Getman D, Lin M, Barakat N, et al. Analytical performance characteristics of a new transcription-mediated amplification assay for Treponema pallidum. J Clin Microbiol 2021; 59(8): e0051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zondag HCA, van Dam AP, Bosch J, et al. Timely diagnosis of incubating syphilis infections using Treponema pallidum Transcription Mediated Amplification assay. Clin Infect Dis 2023; 77: 1717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doxycycline use by pregnant and lactating women. Food and Drug Administration, 2017. (https://www.fda.gov/drugs/bioterrorism-and-drug-preparedness/doxycycline-use-pregnant-and-lactating-women#top). [Google Scholar]
- 86.Hale T. Medications and mothers milk. 9th ed. Amarillo, TX: Pharmasoft Publishing, 2000. [Google Scholar]
- 87.Parkes-Ratanshi R, Mbazira Kimeze J, Nakku-Joloba E, et al. Low male partner attendance after syphilis screening in pregnant women leads to worse birth outcomes: the Syphilis Treatment of Partners (STOP) randomised control trial. Sex Health 2020; 17: 214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakku-Joloba E, Kiguli J, Kayemba CN, et al. Perspectives on male partner notification and treatment for syphilis among antenatal women and their partners in Kampala and Wakiso districts, Uganda. BMC Infect Dis 2019;19: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Office of Health Equity. Community-based approaches to reducing STDs: community engagement toolkit. Atlanta: Centers for Disease Control and Prevention, 2019. (https://www.cdc.gov/std/health-disparities/cars-toolkit-2020.pdf). [Google Scholar]
- 90.Sexually transmitted infections national strategic plan for the United States: 2021–2025. Washington, DC: Department of Health and Human Services, 2020. (https://www.hhs.gov/sites/default/files/STI-National-Strategic-Plan-2021-2025.pdf). [Google Scholar]
- 91.Preventing congenital syphilis in the U.S. Centers for Disease Control and Prevention, 2019. (https://www.cdc.gov/std/stats18/310702A_Congential_Syphilis_FS_Final-508.pdf)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


