Abstract
Bronchopleural fistula (BPF) is a rare yet severe complication following lobectomy, with no standardized treatment protocol established. We present a case of a 65-year-old male with chronic obstructive pulmonary disease who developed multiple BPFs postresection of the right lower lobe due to lung cancer and with tumor recurrence and metastasis. We employed a comprehensive management strategy comprising rigorous infection control, meticulous chest drainage, antitumor treatment, and targeted deployment of bronchial stents. This approach not only resolved the BPFs but also achieved a complete response of the lung cancer, extending progression-free survival to over 3 years.
Keywords: Bronchopleural fistula, endoscopic intervention, lung cancer, tracheal bronchial stents
Bronchopleural fistula (BPF) is characterized as an aberrant connection between the bronchial tree and the pleural cavity. Although it is a relatively rare complication following lobectomy and pneumonectomy, BPF represents a considerable clinical challenge due to its associated high mortality rate. This case exemplifies the effectiveness of a multidisciplinary approach in managing multiple BPFs, employing various types of bronchial stents. This approach could potentially serve as a treatment model for similar cases, demonstrating the value of diverse, coordinated interventions.
Case Report
We report a 65-year-old male, a long-term smoker with chronic obstructive pulmonary disease (COPD), who underwent a right lower lobe (RLL) resection for a mass detected in the RLL [Figure 1a]. Due to the unavailability of positron emission tomography-computed tomography (CT) scanning equipment, alternative diagnostic modalities were employed, including enhanced CT scans of the chest and abdomen, brain magnetic resonance imaging (MRI) with contrast, and bone scintigraphy. Enhanced chest CT revealed a solid nodule approximately 6 mm in diameter in the RLL, whereas enhanced brain MRI and bone scintigraphy showed no evidence of distant metastatic lesions. A robot-assisted thoracoscopic right lower lobectomy was performed under combined intravenous anesthesia with tracheal intubation on November 24, 2020. Lymph nodes at stations 2, 4, 7, 10, 11, and 12 were meticulously dissected, excluding those at station 9. Postoperative pathology revealed invasive adenocarcinoma [Figure 2a], invading the visceral pleura without involvement of the bronchial margins. Lymph node involvement was identified in groups 11R, 7, and 4R [Figure 2b]. Immunohistochemical analysis was performed to assess PD-L1 (22C3) expression using surgically excised cancer tissue, resulting in a tumor proportion score of <1%. Concurrently, next-generation sequencing was utilized to screen for driver mutations in lung cancer-associated genes, which yielded negative results. The postoperative diagnosis was right lung adenocarcinoma (pT2aN2M0, stage IIIa). On December 15, 2020, the patient began exhibiting symptoms of BPF, including persistent cough, sputum production, fever, and chest tightness. Subsequent diagnostic investigations confirmed a BPF at the right main bronchus (RMB) [Figure 1b and Figure 3a], accompanied by a concurrent lung infection and hydropneumothorax. The initial management consisted of chest drainage and administering broad-spectrum antibiotics.
Figure 1.
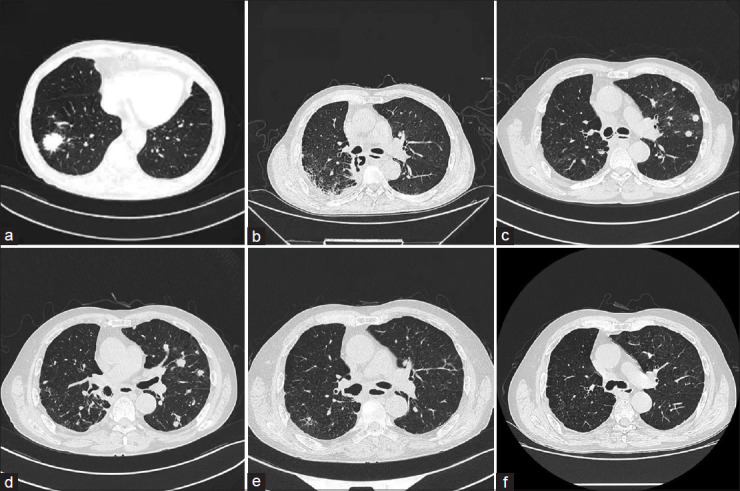
Computed tomography images. (a) A solitary round pulmonary mass detected in right lower lobe, with dimensions of approximately 3.3 cm in length and 2.8 cm in width, exhibited well-defined margins and a homogeneous density. (b) A fistula in the right main bronchus 20 days postsurgery. (c) Tumor recurrence and metastasis. (d) Tumor progression. (e) Partial response. (f) CR. RLL: Right lower lobe; RMB: Right main bronchus; PR: Partial response; CR: Complete response
Figure 2.
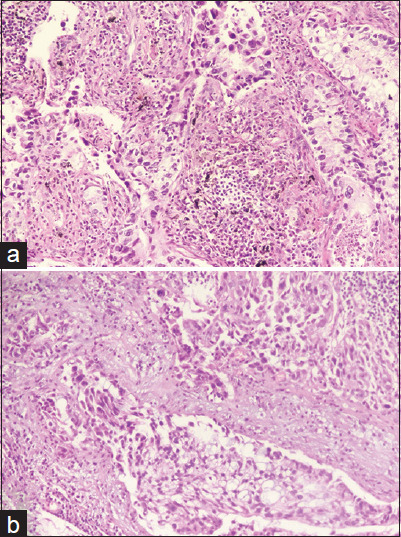
Pathological images of invasive lung adenocarcinoma. (a) Invasive adenocarcinoma in the right lower lobe, comprising approximately 60% alveolar and 40% solid patterns. (b) Station 4R lymph node
Figure 3.

Images under endobronchial view. (a) A fistula in the right main bronchus (RMB). (b) Deployment of silicone stent in RMB. (c) Fistula in RLL. (d) Deployment of metal stents. (e) Metal stents removed. (f) RML and closed fistula in RLL. RMB: Right main bronchus; RLL: Right lower lobe; RML: Right middle lobe
To facilitate fistula healing and manage infection, a custom Y-shaped silicone stent (DUMON® Novatech, France) was inserted on December 31, 2020, under general anesthesia during a rigid bronchoscopy procedure [Figure 3b]. This intervention effectively alleviated symptoms initially. However, the patient’s condition deteriorated 3 days later, necessitating a subsequent bronchoscopic assessment. A new fistula was discovered at the anastomosis site of the RLL resection [Figure 3c]. Given the patient’s poor overall health and ongoing infection, taking into account a comprehensive assessment of the clinical situation and the patient’s preferences, a decision was made to temporarily pursue conservative treatment, including persistent chest drainage and intensified infection control measures. These strategies resulted in decreased pleural effusion and relief from coughing. The patient had severe thoracic infection, and compromised physical condition precluded the use of adjuvant chemotherapy postoperatively.
A follow-up chest CT on April 20, 2021, revealed tumor recurrence and metastasis [Figure 1c], accompanied by an increase in serum CEA levels from normal to 20.3 ng/mL, prompting the initiation of oral anlotinib due to the patient’s intolerance to chemotherapy. On May 20, 2021, the silicone stent was removed, showing complete healing of the fistula at RMB, though the fistula at RLL had enlarged. To address this, a combination of self-expandable covered metallic stents (Micro-Tech, China) was employed [Figure 3d]. The intervention began with the placement of a Y-shaped stent, with branches extending into the right up lobe (RUL) and bronchus intermediate (BI), followed by an I-shaped stent positioned such that its distal end extended into the right middle lobe (RML) and its proximal end sat within the Y-stent in the intermediate bronchus. The distal end of the I-shaped stent was left uncovered to prevent displacement. The decision to deploy a self-expandable covered metal Y-stent in both the RUL and the BI, rather than a single I-shaped metal stent in the RML bronchus, was driven by anatomical challenges and past surgical outcomes. The angled relationship between the bronchi could lead an I-shaped stent to irritate the bronchial wall, risking excessive granulation, wall damage, or fistula formation with the potential for massive bleeding. In addition, despite the healing of the initial fistula in the RML, the risk of recurrence necessitated robust protection, achieved through a combination of Y-shaped and I-shaped stents, which not only prevents severe complications but also minimizes the chance of scarring and stenosis observed in our similar past cases. This strategy successfully sealed the fistula, and the chest drainage tube remained in place. Subsequent chest CT on May 21, 2021, demonstrated an increase in metastatic lesions [Figure 1d], accompanied by a further rise in the patient’s serum CEA levels to 30.4 ng/mL, indicating a poor response to anlotinib. Following improvements in the patient’s physical condition and control of chest infection, a multidisciplinary discussion led to the initiation of a quadruple therapy regimen for cancer treatment. This regimen retained anlotinib and introduced pemetrexed and platinum-based chemotherapy, along with camrelizumab immunotherapy.
Three months following treatment, notable improvements were documented [Figure 1e], including a significant reduction in pulmonary metastases and resolved pleural effusion, and normalization of the patient’s serum CEA levels, which facilitated the removal of the chest tube. On August 23, 2021, the metal stents were extracted following confirmation of the complete healing of the fistula [Figure 3e]. The patient’s overall health markedly improved, evidenced by a weight gain of 15 kg. After completing six cycles of chemotherapy, immunotherapy, and anlotinib, a complete response (CR) was achieved. Maintenance therapy consisting of camrelizumab and anlotinib was administered, with bimonthly chest CT scans showing stable disease. On May 10, 2023, both immunotherapy and anlotinib were discontinued as the patient sustained CR [Figures 1f and 3f]. The patient continues to undergo regular reexaminations every 2–3 months and has maintained progression-free survival for over 3 years. A flow chart detailing the entire diagnostic and treatment process is depicted in Figure 4.
Figure 4.
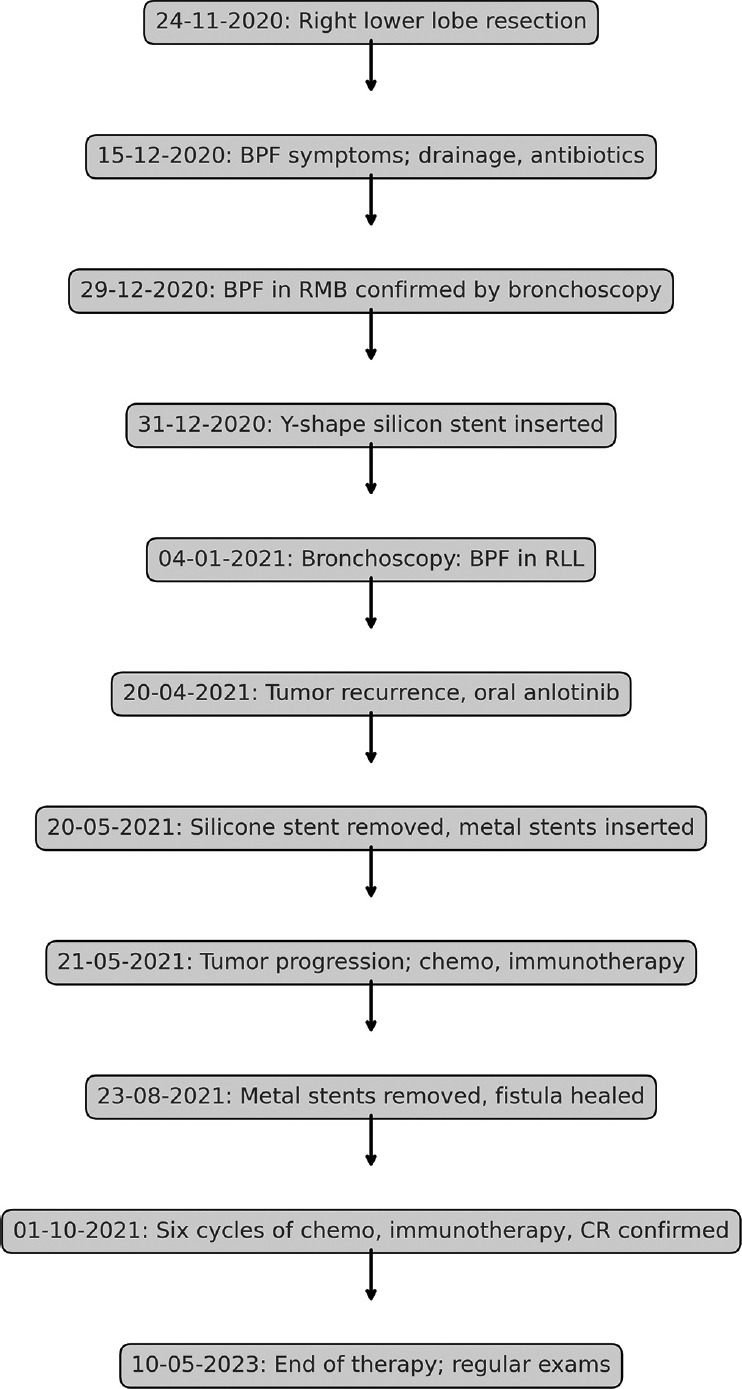
Flowchart of the diagnostic and treatment process
Discussion
BPF is a rare yet life-threatening complication, with an incidence ranging from 0.5% to 20% across various procedures and centers and mortality rates as high as 70%.[1] Identified risk factors include male gender, low body mass index, reduced vital capacity, extensive resections, and specific surgical techniques.[2]
The occurrence of a BPF in the RMB following resection of RLL is atypical, as this site is not commonly associated with such complications post-RLL surgeries. In this case, the development of a BPF in RMB following surgery can be attributed to a combination of factors: anatomical predispositions from extensive smoking and COPD, which altered lung architecture; iatrogenic injury during meticulous lymph node dissection around the RMB that may have damaged the bronchial wall; and postoperative stresses such as infections and persistent coughing, all contributing to the fistula’s formation.
Standardized treatment protocols for BPF remain undefined.[3,4] However, the advent and proliferation of endoscopic technologies have ushered in a new era of bronchoscopic interventions, offering less invasive alternatives that are customized to patient-specific requirements.[5,6] In particular, the use of endoscopic treatments for BPF is increasingly preferred over surgical approaches in certain clinical scenarios.[7,8] Moreover, the introduction of tracheobronchial stents has transformed the management of BPF, providing an effective means to seal fistulas without necessitating additional invasive procedures.[9]
This case highlights the essential role of prompt and precise medical management, which includes the use of broad-spectrum antibiotics coupled with targeted antitumor therapies. The complexity of managing BPF in patients with lung cancer necessitates a delicate balance between controlling tumor progression and minimizing exacerbation of the fistula. This report illustrates the efficacy of employing diverse stent types within a holistic management strategy, underscoring the potential for innovative techniques to enhance clinical outcomes in similar future cases. Nevertheless, the application of multiple stent combinations demands extensive clinical expertise and exemplary teamwork. Accurate customization and careful selection of stent specifications are critical to maximizing therapeutic effectiveness and minimizing complications, which can be particularly challenging. Preoperative measurement and planning using imaging software such as Mimics were instrumental in customizing stents that were critical for ensuring the intervention was both effective and minimally invasive, providing a tailored solution to the unique anatomical challenges of this case.
In conclusion, effective management of BPF requires prompt diagnosis and a customized therapeutic regimen. This case report demonstrates the successful integration of personalized endoscopic techniques with systemic therapies, resulting in sustained remission in complex BPF instances. It accentuates the potential of innovative strategies in the management of comparable clinical situations and underscores the significance of adaptable methodologies within the swiftly advancing field of medical science.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Conflicts of interest
There are no conflicts of interest.
Funding Statement
This study was supported by Yantai Science and Technology Development Plan (No. 2020YD004).
References
- 1.Grotberg JC, Hyzy RC, De Cardenas J, Co IN. Bronchopleural fistula in the mechanically ventilated patient: A concise review. Crit Care Med. 2021;49:292–301. doi: 10.1097/CCM.0000000000004771. [DOI] [PubMed] [Google Scholar]
- 2.Matsunaga T, Suzuki K, Hattori A, Fukui M, Takamochi K. Risk factors for bronchopleural fistula based on surgical procedure and sex in 4794 consecutive patients undergoing anatomical pulmonary resection. Surg Today. 2024;54:617–26. doi: 10.1007/s00595-023-02761-2. [DOI] [PubMed] [Google Scholar]
- 3.Cusumano G, Alifano M, Lococo F. Endoscopic and surgical treatment for bronchopleural fistula after major lung resection: An enduring challenge. J Thorac Dis. 2019;11:S1351–6. doi: 10.21037/jtd.2019.03.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woldemariam ST, Molla IB, Merine SK, Yilma DG. Prevalence and treatment outcome of bronchopleural fistula: A multi-center study in Ethiopia. J Cardiothorac Surg. 2023;18:227. doi: 10.1186/s13019-023-02325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Date N, Komatsu T, Fujinaga T. A novel technique for endobronchial watanabe spigot placement for bronchopleural fistula: A traction method. Eur J Cardiothorac Surg. 2021;60:1237–8. doi: 10.1093/ejcts/ezab332. [DOI] [PubMed] [Google Scholar]
- 6.Ho E, Srivastava R, Hegde P. Bronchopleural fistula closure with amplatzer device: Our case and reviewing a decade of experience. J Bronchology Interv Pulmonol. 2020;27:e41–5. doi: 10.1097/LBR.0000000000000637. [DOI] [PubMed] [Google Scholar]
- 7.Jin L, Li Y. Bronchoscopic interventions for bronchopleural fistulas. Ther Adv Respir Dis. 2023;17:17534666231164541. doi: 10.1177/17534666231164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motus IY, Bazhenov AV, Basyrov RT, Tsvirenko AS. Endoscopic closure of a bronchopleural fistula after pneumonectomy with the amplatzer occluder: A step forward? Interact Cardiovasc Thorac Surg. 2020;30:249–54. doi: 10.1093/icvts/ivz241. [DOI] [PubMed] [Google Scholar]
- 9.Zeng J, Wu X, Chen Z, Zhang M, Ke M. Modified silicone stent for the treatment of post-surgical bronchopleural fistula: A clinical observation of 17 cases. BMC Pulm Med. 2021;21:10. doi: 10.1186/s12890-020-01372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


