INTRODUCTION
Pill esophagitis is a condition characterized by injury and inflammation to the esophageal mucosa, resulting from ingestion of certain medications.1–4 Common presenting symptoms include odynophagia, dysphagia, and chest pain, with potential associated complications including esophageal ulceration or strictures, and rarely gastrointestinal bleeding, perforation, intramural hematoma, and mediastinitis.5 Pill esophagitis may occur due to drug-related factors (ie, direct mucosal irritation, chemical injury) or patient-related factors (ie, esophageal dysmotility, decreased saliva production, taking medications without water, or laying down immediately afterward).6–10 Pill esophagitis is an uncommon condition with an estimated incidence of 3.9 per 100,000 individuals per year.7 Multiple medications have been reported in the literature to be associated with pill esophagitis including various antibiotics, nonsteroidal anti-inflammatory drugs, bisphosphonates, ascorbic acid, potassium chloride, ferrous sulfate, warfarin, and chemotherapeutic agents.1–6 Isotretinoin is an oral medication used to treat moderate-to-severe acne vulgaris, with only a few case reports showing its association with pill esophagitis. Here, we present a case of a 28-year-old man with a medical history of acne vulgaris treated with isotretinoin who presented with postprandial odynophagia and was found to have pill esophagitis.
CASE REPORT
A 28-year-old man presented to the emergency department with a 24-hour history of sudden onset, postprandial odynophagia that began 3-4 hours after dinner. The patient had taken the isotretinoin pill immediately after finishing the meal. The pain with swallowing was initially present only when consuming solids, but within hours, began to also occur with intake of liquids. The patient's medical history was significant only for acne vulgaris for which he was taking isotretinoin 40 mg by mouth in the evenings after dinner for the past 5 months. He was otherwise healthy and had no prior episodes of dysphagia, odynophagia, chest pain, heartburn, or regurgitation. He did not have underlying gastroesophageal reflux disease, autoimmune diseases, asthma, seasonal allergies, alcohol or tobacco use, and had not undergone previous surgeries or endoscopy. The patient inconsistently took vitamin D 5,000 IU weekly over the past 6 months (last dose 5 days before presentation), but otherwise, denied any other medication or supplement use. The patient denied previous family history of gastrointestinal or autoimmune disorders.
At presentation, the patient was hemodynamically stable. Physical examination revealed an alert, healthy appearing man in mild distress, but able to tolerate secretions. Oral examination revealed no white exudates, plaques, or lesions. Laboratory tests including complete blood count and basic metabolic panel, and electrocardiogram were unremarkable. Chest X-ray was negative for any acute process. The patient was administered a gastrointestinal cocktail consisting of aluminum hydroxide and viscous lidocaine with no symptom relief.
Given persistent symptoms, the gastroenterology team was consulted and urgent esophagogastroduodenoscopy was performed. Esophagogastroduodenoscopy revealed 2 superficial nonbleeding esophageal ulcers in the proximal esophagus with the largest lesion measuring 7 mm (Figures 1 and 2). The esophagus otherwise appeared normal without rings, furrows, exudates, edema, stenosis, or erosive esophagitis. Patchy moderate inflammation characterized by congestion and erythema was noted in the stomach. There were no gross lesions in the duodenal bulb. Biopsies were obtained from the edges of the esophageal ulcer and demonstrated focal and mild active esophagitis with separate fragments of necroinflammatory exudate, suggestive of pill-induced mucosal injury given clinical history.
Figure 1.

Proximal esophageal ulcer. Arrows point to the nonbleeding esophageal ulcers found on esophagogastroduodenoscopy.
Figure 2.
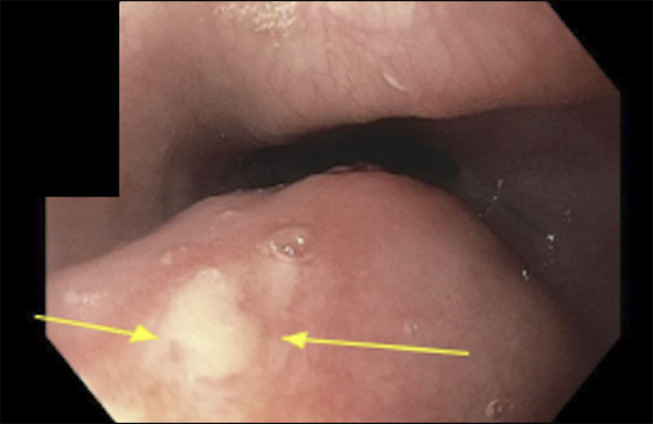
Proximal esophageal ulcer. Arrows point to the nonbleeding esophageal ulcers found on esophagogastroduodenoscopy.
The patient was diagnosed with isotretinoin-induced pill esophagitis given compatible clinical history, and endoscopy and pathology findings. He was instructed to stop isotretinoin and start once daily pantoprazole until follow-up with outpatient dermatology and gastroenterology. The patient noted some improvement in symptoms over the next 2 days and experienced complete symptom resolution 1 week after stopping isotretinoin. At 1-month follow-up, the patient was instructed to stop pantoprazole and remain off isotretinoin.
DISCUSSION
The differential diagnosis for acute onset odynophagia and dysphagia is broad and includes infectious esophagitis, such as Candida esophagitis, herpes simplex virus esophagitis, cytomegalovirus esophagitis, pill esophagitis, and erosive esophagitis. In this case, the patient's endoscopic and histological findings, as well as clinical presentation raised suspicion for pill esophagitis secondary to isotretinoin. The patient did not have an underlying immunosuppressive condition, and pathology was negative for cytomegalovirus and herpes simplex virus. Endoscopy did not demonstrate white plaques, furrows, rings, exudates, edema, or stenosis making Candida esophagitis, eosinophilic esophagitis, and mechanical obstruction unlikely. The proximal location of the ulcerations without distal esophageal inflammation and lack of gastroesophageal reflux symptoms made erosive esophagitis less likely. Before attributing symptoms to pill esophagitis entirely, providers should pause and consider other etiologies before making this diagnosis. In this case, the Naranjo adverse drug effects scale (score of 6) and WHO-UMC causality category system are suggestive of probable/likely adverse drug effect.11,12 Similar to our case, in 2 other case reports, patients were diagnosed with isotretinoin-induced pill esophagitis once ruling out other etiologies.13,14 In both cases, symptoms resolved following discontinuation of isotretinoin and administration of a proton-pump inhibitor.
As outlined above, pill esophagitis often results from direct mechanical and chemical irritation.6 Risk factors of pill esophagitis include patient positioning and medication size, which can be mitigated by administering the medication whole with at least 8 ounces of water and staying upright for at least 30 minutes after swallowing the medication. Isotretinoin manufacturers note esophageal irritation may occur when the medication is not swallowed whole with a glass of water.15–17 In general, increased medication and esophageal mucosa contact time can increase the risk of pill esophagitis. In our case, the patient's nightly administration of isotretinoin may have predisposed him to pill esophagitis due to increased contact time, and this may have been mitigated if the medication was taken with a glass of water and the patient remained upright for at least 30 minutes afterwards. Our unusual case highlights that providers should consider a diagnosis of pill esophagitis in patients presenting with acute odynophagia and taking a potential culprit medication.
DISCLOSURES
Author contributions: G. Hourany: contributed to conception and design, contributed to acquisition, analysis, and interpretation, drafted manuscript, critically revised manuscript, gave final approval. JS Samaan: contributed to conception and design, contributed to acquisition, analysis, and interpretation, drafted manuscript, critically revised manuscript, gave final approval. AK Kamboj: contributed to conception and design, contributed to acquisition, analysis, and interpretation, drafted manuscript, critically revised manuscript, gave final approval. F. Ibarra: contributed to conception and design, contributed to acquisition, analysis, and interpretation, drafted manuscript, critically revised manuscript, gave final approval, agrees to be accountable for all aspects of work ensuring integrity and accuracy. F. Ibarra is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Jamil S. Samaan, Email: Jamil.samaan@cshs.org.
Amrit K. Kamboj, Email: Amrit.Kamboj@cshs.org.
Francisco Ibarra, Jr, Email: fibarra@chsu.edu.
REFERENCES
- 1.Dağ MS, Öztürk ZA, Akın I, Tutar E, Çıkman Ö, Gülşen MT. Drug-induced esophageal ulcers: Case series and the review of the literature. Turk J Gastroenterol. 2014;25(2):180–4. [DOI] [PubMed] [Google Scholar]
- 2.Kim SH, Jeong JB, Kim JW, et al. Clinical and endoscopic characteristics of drug-induced esophagitis. World J Gastroenterol. 2014;20(31):10994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zografos GN, Georgiadou D, Thomas D, Kaltsas G, Digalakis M. Drug-induced esophagitis. Dis Esophagus. 2009;22(8):633–7. [DOI] [PubMed] [Google Scholar]
- 4.Grossi L, Ciccaglione AF, Marzio L. Esophagitis and its causes: Who is “guilty” when acid is found “not guilty.” World J Gastroenterol. 2017;23(17):3011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kikendall JW. Pill esophagitis. J Clin Gastroenterol. 1999;28(4):298–305. [DOI] [PubMed] [Google Scholar]
- 6.Jaspersen D. Drug-induced oesophageal disorders: Pathogenesis, incidence, prevention and management. Drug Saf. 2000;22(3):237–49. [DOI] [PubMed] [Google Scholar]
- 7.Pemmada V, Shetty A, Murali M, et al. Pill esophagitis: Clinical and endoscopic profile. J Dig Endosc. 2023;14(04):197–202. [Google Scholar]
- 8.Hey H, Jørgensen F, Sørensen K, Hasselbalch H, Wamberg T. Oesophageal transit of six commonly used tablets and capsules. Br Med J (Clin Res Ed). 1982;285(6356):1717–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pouderoux P, Shi G, Tatum RP, Kahrilas PJ. Esophageal solid bolus transit: Studies using concurrent videofluoroscopy and manometry. Am J Gastroenterol. 1999;94(6):1457–63. [DOI] [PubMed] [Google Scholar]
- 10.Dent J, Dodds WJ, Friedman RH, et al. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest. 1980;65(2):256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45. [DOI] [PubMed] [Google Scholar]
- 12.The Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment (https://www.who.int/docs/default-source/medicines/pharmacovigilance/whocausality-assessment.pdf). Accessed September 15, 2024.
- 13.Weinberg E, Wan D. Isotretinoin-induced esophagitis in an immunocompetent patient: 579. Am J Gastroenterol. 2012;107:S240. [Google Scholar]
- 14.Amichai B, Grunwald MH, Odes SH, Zirkin H. Acute esophagitis caused by isotretinoin. Int J Dermatol. 1996;35(7):528–9. [DOI] [PubMed] [Google Scholar]
- 15.Absorica [package insert]. Galephar Pharmaceutical Research, Inc: Humacao, PR: (https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021951s013lbl.pdf#page=32) (2019). [Google Scholar]
- 16.Accutane [package insert]. Roche Laboratories, Inc: Nutley, NJ: (https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018662s061MedGuide.pdf) (2010). [Google Scholar]
- 17.Sotret [package insert]. Ranbaxy Laboratories, Inc: Jacksonville, FL: (https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/Sotret.pdf) (2006). [Google Scholar]


