SUMMARY
Historically, the rat has been the preferred animal model for behavioral studies. Limitations in genome modification have, however, caused a lag in their use compared to the bevy of available transgenic mice. Here, we have developed several transgenic tools, including viral vectors and transgenic rats, for targeted genome modification in specific adult rat neurons using CRISPR-Cas9 technology. Starting from wild-type rats, knockout of tyrosine hydroxylase was achieved with adeno-associated viral (AAV) vectors expressing Cas9 or guide RNAs (gRNAs). We subsequently created an AAV vector for Cre-dependent gRNA expression as well as three new transgenic rat lines to specifically target CRISPR-Cas9 components to dopaminergic neurons. One rat represents the first knockin rat model made by germline gene targeting in spermatogonial stem cells. The rats described herein serve as a versatile platform for making cell-specific and sequence-specific genome modifications in the adult brain and potentially other Cre-expressing tissues of the rat.
Keywords: CRISPR-Cas9, AAV, transgenic rat, MANF, genome editing, brain, lsl-Cas9, spermatogonial stem cells
Graphical Abstract

INTRODUCTION
Since its debut in the mid-2000s, the application of CRISPR- Cas9 technology has revolutionized the field of genome editing. Originally identified as an adaptive defense mechanism in bacteria (Barrangou et al., 2007; Gasiunas et al., 2012; Jinek et al., 2012) and subsequently engineered for the modification of genes in eukaryotes (Mali et al., 2013; Cong et al., 2013; Jinek et al., 2013; Hou et al., 2013; Cho et al., 2013), the Cas9 nuclease identifies target DNA molecules for cleavage using complimentary short guide RNAs (gRNA) (Zhang et al., 2014; Sander and Joung, 2014). Crucial for target DNA recognition and nuclease activity is a protospacer adjacent motif (PAM) on the nontarget strand at the 30 end of the target sequence. Once recognized, the DNA is cleaved 3 nucleotides (nt) upstream of the PAM sequence by the endonuclease domains of Cas9 (Jinek et al., 2012), creating a sequence-specific double-strand break that is subsequently resolved by host-cell processes for nonhomologous end joining (NHEJ) or homology-directed repair (reviewed by Doudna and Charpentier, 2014; Sander and Joung, 2014). The typical result of a CRISPR-mediated lesion is a mutated DNA sequence con- taining an insertion or deletion (indel) mutation.
The components of the CRISPR-Cas9 system have been delivered to rodent embryos by transfection or direct injection to facilitate the creation of novel loss-of-function alleles and transgenic knockins for both mice (Yang et al., 2013; Wang et al., 2013; Shen et al., 2013; Li et al., 2013a) and rats (Ma et al., 2014; Chapman et al., 2015; Li et al., 2013a, 2013b). Importantly, these components can be packaged into lipid nanoparticles (Finn et al., 2018) or genetically encoded into viral vectors (Suzuki et al., 2016; Shao et al., 2018; Swiech et al., 2015), allowing for the introduction of sequence-specific modifications into the genome of early postnatal and adult rodents using systemic or local injection. The ability to temporally control genome modification of somatic cells circumvents developmental adaptations or diminished survival caused by some germline mutations. Using CRISPR-Cas9 technology, it is now possible to alter the genome by sequence-specific knockout, knockin, or correction of one or several target genes at any time during the lifespan of an animal.
The ability of CRISPR-Cas9 to introduce genetic modifications in neurons of adult mice has previously been demonstrated (Suzuki et al., 2016; Nishiyama et al., 2017; Swiech et al., 2015; Platt et al., 2014). Although CRISPR-Cas9 has been used to make germline mutations in rats (Ma et al., 2014; Chapman et al., 2015; Li et al., 2013b; Pradhan and Majumdar, 2016; Remy et al., 2017), manipulations of the genome of specific neurons in the rat brain have not been described. Rats are the animal model of choice for behavioral research for historical, technical, and developmental reasons (larger size, more elaborate cognitive and social behavior, etc.; reviewed by Ellenbroek and Youn, 2016; Homberg et al., 2017). The use of rats is particularly prominent within the field of substance abuse disorders and addiction research, where complex drug-abuse-related operant behaviors have been successfully modeled (Parker et al., 2014). Another field that has heavily relied upon rat models for preclinical testing of therapeutic strategies is Parkinson’s disease, a disorder characterized by degeneration of the nigrostriatal dopaminergic pathway leading to motor disabilities (Duty and Jenner, 2011; Creed and Goldberg, 2018). Both Parkinson’s disease and substance abuse disorders are linked to alterations in the function of midbrain dopaminergic neurons. In the lateral midbrain, the dopaminergic neurons of the substantia nigra (SN) send projections to the dorsal striatum, where they are involved in control of movements, whereas more medially located dopaminergic neurons of the ventral tegmental area (VTA) innervate the ventral striatum (nucleus accumbens) and prefrontal cortex to participate in motivation and reward modalities. Therefore, the ability to alter the genetic composition of the midbrain dopaminergic neurons during various stages of disease progression can shed light on the function of genes in the manifestation of relevant pathologies, which in turn could enable the development of novel therapies.
RESULTS
Genome Modification in Rat Midbrain by Co-transduction with Cas9 and gRNA or Nickase and Grna
Adult female pregnant and male Sprague-Dawley rats (purchased from the BioLASCO, Taipei, Taiwan) were used in this study. Experimental procedures followed the guidelines of the ‘Principles of Laboratory Care’ (National Institutes of Health Publication No. 86–23, 1996) and were approved by the National Health Research Institutes (Taiwan) Animal Care and Use Com- mittee. (+)Methamphetamine HCl, dantrolene, MK801, Glu and nifedipine were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
To first demonstrate viral-vector-mediated genome modification in the adult rat brain, we targeted the rat tyrosine hydroxylase (Th) gene. The Th gene is expressed in the dopaminergic neurons of the midbrain and has a distinct, bilateral pattern of immunoreactivity on brain sections. We constructed gRNAs that bind within the first exon of the Th gene or Rosa26 locus (control) and validated them by co-transfection with a Cas9 expression plasmid into rat PC12 cells (Figure S1). These gRNA expression cassettes were then cloned into an AAV vector (serotype 1) that expresses an EGFP reporter (“AAV Th gRNAs” and “AAV control gRNAs”). We first tested the AAV vectors in rat primary cortical neurons and found evidence of mutations at the Th locus at
1 week after co-transduction with AAV Th gRNAs and AAV Cas9. Using tracking of indels by decomposition (TIDE) analysis, we identified different types of mutations at the Th locus (Figure S2). We then analyzed WT Long-Evans (LE) rats for tyrosine hydroxylase (TH) protein knockout following the co-delivery of AAV Cas9 and AAV Th gRNAs to the midbrain (Table S1). Compared to the contralateral hemisphere that received AAV Cas9 and AAV control gRNAs, densitometry analysis revealed a significant reduction (~40%) in TH immunoreactivity in the SN at 2, 3, and 6 weeks post-transduction with AAV Cas9 and AAV Th gRNAs (Figure 2A, top, and Figure 2B). The reduction of TH immunoreactivity was specific to the co-delivery of Cas9 and gRNA, as no decrease in TH immunoreactivity was observed in WT animals injected with gRNA alone (Figure 2B). While the reduction in TH immunoreactivity in the midbrain leveled off at the earliest time point (2 weeks), we observed a time-dependent decrease in the density of TH-positive fibers in the striatum, progressing from an ~25% to 53% reduction between 2 and 6 weeks post-transduction (Figure 2A, bottom, and Figure 2C).
Figure 2.
Co-delivery of AAV Th gRNA and AAV Cas9 or AAV Nickase into WT Rats Leads to Decreased TH Immunoreactivity (A and D) Representative images of the midbrain (MB; top) and striatum (bottom) from WT rats 6 weeks following a co-injection of (A) AAV Cas9 or (D) AAV Cas9 nickase with either AAV control gRNAs (right side [R]) or AAV Th gRNAs (left side [L]) into the MB. GFP fluorescence, as a marker of gRNA delivery, and TH immunoreactivity, as a marker of TH knockout efficacy, were assessed. (B and E) Quantification of TH immunoreactivity in the SN shown as Th gRNA-injected compared to control gRNA-injected side with or without a co-injection of AAV Cas9. Both datasets were normalized to DAPI following 2-, 4-, or 6-week incubations. Each data point represents a coronal section (3–4 sections/animal), and each color represents a distinct animal (n = 3–5/group). (B) One-way ANOVA, F3,10 = 34.8, p < 0.0001; a versus b: p < 0.0001, Tukey’s multiple comparisons test. (E) One-way ANOVA, F3,33 = 10.59, p < 0.0001; a versus b: p < 0.01; a versus c: p < 0.05, a versus d: p = 0.226, b and c versus d: p < 0.001, Tukey’s multiple comparisons test. (C and F) Quantification of TH immunoreactivity in the striatum, as described for (B and E) (3–4 coronal sections/animal from 3–5 animals/group). (C) One-way ANOVA, F3,10 = 52.72, p < 0.0001; a versus b: p < 0.05 (4 wk); a versus b: p < 0.01 (6 wk); a versus c: p < 0.001; b versus c: p < 0.0001 (4 wk); b versus c: p < 0.0001 (6 wk), Tukey’s multiple comparison test. (F) One-way ANOVA, F3,37 = 31.20, p < 0.0001; a versus b and c, p < 0.0001; a versus d, p = 0.986; b versus d, p = 0.0001; c versus d, p < 0.0001, Tukey’s multiple comparisons test. Scale bars, 500 mm. The same group of animals injected with control gRNA only are used for reference in the midbrain (B and E) and the striatum (C and F).
Modifications within the Cas9 catalytic sites have generated variants that retain the ability for gRNA-directed DNA binding but produce a break or “nick” on only one strand of the DNA double helix (Gasiunas et al., 2012; Ran et al., 2013). Taken on its own, a nick in the DNA backbone created in this way is not very mutagenic; however, these nickases (or Cas9n variants) can still create indels when combined with a mix of two gRNAs that target proximal sites on opposite strands (Ran et al., 2013). Since this technique uses two gRNAs, it expands the target sequence necessary to create an indel to 40 nt and should therefore lead to fewer off-target effects. To test the capability of nickase to induce genetic alterations in the adult rat brain, we designed an AAV vector expressing nickase under the MeCP2 promoter and delivered it to the rat midbrain by co-injection with an AAV expressing a pair of “nickase-compatible” gRNAs targeting the Th gene. The co-injection of AAV nickase and AAV Th gRNAs resulted in a significant loss of TH immunoreactivity in the ipsilateral midbrain and striatum compared to the contralateral hemisphere that received AAV nickase and AAV control gRNAs (Figures 2D–2F). However, using this approach, the loss of TH appeared to exhibit a slower onset compared to that achieved by AAV Cas9 delivery. Significant reductions in TH immunoreactivity were observed 4 and 6 weeks post-transduction, resulting in an average 30% and 47% loss of TH immunoreactivity in the SN (Figure 2E) and striatum (Figure 2F), respectively.
Characterization of DAT-iCre Rats
Although the selection of transgene promoter, viral vector tropism, and method of vector delivery provide some control over transgene expression, the intracranial delivery of viral vectors does not generally allow for targeting of specific neuronal populations. Therefore, to selectively alter the genetic composition of dopaminergic neurons, we created a transgenic rat line expressing iCre under the control of the rat Dat (Slc6a3) promoter. A bacterial artificial chromosome (BAC) containing the Dat gene was recombineered to express iCre and randomly integrated into the LE rat genome. Six founder rats were identified, three of which had germline-trans- missible transgenes. Analysis by droplet digital PCR determined that lines 1, 5, and 6 contained two, eight, and one copies of the transgene per haploid genome, respectively. Line 1, which had two copies per haploid genome, exhibited an occasional loss of a copy within the litters produced in the first five generations. In situ RNA hybridization in the SN/VTA of DAT-iCre line 6 transgenic rats showed nearly complete colocalization of Dat and Cre signal (Figure 3A), with Dat and Cre colocalized in 96.5% ± 2.1% of the cells (total of 9 sections from 3 rats, mean ± SD). Dat-reactive cells that showed no Cre expression represented 1.3% ± 0.8% of the population counted, and 2.4% ± 2.5% cells were Cre positive and Dat negative. Expression of Cre in dopaminergic neurons was also verified with immunohistochemistry using antibodies for TH and Cre (Figure 3B). The signal from Cre mRNA and protein was absent in the midbrain of WT animals (Figures 3A and 3B).
Figure 3.
Characterization of DAT-iCre Rats (A and B) Representative images of (A) in situ RNA hybridization and (B) immunohistochemical staining of the midbrain in WT and DAT-iCre transgenic rats.(A) Green, Cre; red, dopamine transporter (Dat); blue, DAPI staining. (B) Green, Cre; red, tyrosine hydroxylase (TH). Scale bars, 20 mm.
A series of experiments were performed to examine whether the presence of the transgene affected dopaminergic neuron properties. Expression of iCre in rat dopaminergic neurons did not change the midbrain or striatal expression of TH for all lines tested (Figures 3C and 3D). Also, the protein levels of striatal DAT from a membrane preparation were constant across all DAT-iCre transgenic lines and WT rats (Figure 3E). Moreover, the DAT-iCre rats showed normal internalization of DAT (Figure 3F) and excitatory amino acid transporter 3 (EAAT3) (Figure 3G) in response to amphetamine treatment. For DAT protein assays (Figures 3E–3G), line 5 showed the highest variability but also had the highest number of transgene copies. Dopaminergic neurons from DAT-iCre line 6 rats had electrophysiological properties similar to WT rats, displaying a tonic firing pattern characteristic if dopaminergic neurons (Figure 3H), with a firing rate (Figure 3I), input resistance (Figure 3J), and holding current (Figure 3K) that did not differ from WT rats. Fast-scan cyclic voltammetry in dorsal striatal slices obtained from DAT-iCre line 6 rats revealed no significant differences in dopamine release or uptake following a single pulse electrical stimulation relative to WT controls (Figures 3M and 3N). In addition, cocaine (5 mM), a known antagonist of DAT, acutely inhibited dopamine uptake to the same extent in both genotypes (Figures 3L and 3M), further confirming that the presence of the transgene does not alter DAT function.
To test the specificity of Cre-mediated recombination in DAT-iCre line 6 rats, both WT and DAT-iCre rats received intranigral injections of a viral vector expressing a Cre-dependent color-changing reporter, AAV Nuc-flox-(mCherry)-EGFP (Table S1). This vector produces nuclear-localized “Nuc” mCherry by default but switches to producing nuclear-localized GFP in the presence of Cre recombinase. Two weeks after the injection, DAT-iCre rats showed expression of EGFP in dopaminergic neurons (TH-reactive cells), indicating that Cre-dependent recombination of the transgene had resulted in a deletion of the mCherry coding region (Figure 3O). The signal from EGFP was not detected in WT rats or in non-dopaminergic cells (TH negative) in DAT-iCre rats. When we injected the same virus into additional regions (the prefrontal cortex, hypothalamus, and striatum), we observed the strongest expression in the midbrain; however, noticeable staining was also observed in the hypothalamus (Figure S3). DAT-iCre line 6 rats were also crossed with LE Dio-mCherry transgenic rats, and the expression of Cre and mCherry was vali- dated with immunohistochemistry (Figure S4). Both Cre and mCherry showed robust expression in the midbrain. Similar to the Cre-reporter virus results, some GFP-positive cells were detected in the hypothalamus of the rats, although we did not observe Cre expression in this area as we did in the midbrain (Figures 3 and S4). The expression of Dat mRNA in the rat hypothalamus has been previously described (Meister and Elde, 1993). Based on our characterization of the different DAT-iCre lines, we proceeded with line 6 as the “DAT-iCre” line.
Delivery of Cre-Dependent gRNA to DAT-iCre Rats Causes Disruption of TH
To achieve genome modification that is restricted to dopaminergic neurons, we modified a Cre-dependent expression system (Tiscornia et al., 2004) for gRNA expression. The foundation of this system is a modified murine U6 promoter that contains a loxP element just upstream of the transcription start site. By coupling this to the U6 gene’s transcription termination sequence and another loxP element, we created a floxed (“flanked by loxP”) transcription terminator referred to as a loxstop-lox (LSL), which prevents the transcription of a downstream gRNA in the absence of Cre activity (Figure S5). To demonstrate Cre-dependent gRNA expression, rat PC12 cells were co-transfected with a mix of plasmids expressing Cre-independent Th gRNA, Cre-dependent Th gRNA, Cas9, and either iCre or Flpo, a recombinase that does not act on lox sites. Using the T7E1 assay, evidence of Th gene mutagenesis was only observed in the presence of Cre (Figure S5). We produced an AAV vector with this Cre-dependent gRNA to rat Th (AAV LSL-Th gRNA) and co-injected it with our AAV Cas9 vector unilaterally into the midbrain of the DAT-iCre rats (Table S1). Six weeks post-transduction, we observed an ~50% decrease of TH immunoreactivity in the SN of the injected hemisphere (Figures 4A and 4B) and a concomitant 44% reduction of TH staining in the ipsilateral striatum (Figures 4C and 4D). WT LE rats injected with the same viral vectors also showed an ~20% reduction of TH immunoreactivity in the ipsilateral hemisphere, possibly due to injection-induced tissue damage or GFP overexpression. However, the loss of TH immunoreactivity in the nigrostriatal pathway of DAT-iCre rats was significantly greater than that observed in WT rats (Figures 4B and 4D). A similar trend in TH protein loss was observed using western blot analysis of midbrain tissue punches from AAV-injected WT and DAT-iCre animals (Figure S6).
Figure 4.
Unilateral Injection of LSL-gRNA in Rats Expressing Cre in Dopaminergic Neurons Enables Region- and Cell-Type-Specific Th Editing (A and C) Representative images demonstrate unilateral loss of TH immunoreactivity in the (A) midbrain and (C) striatum of DAT-iCre rats (bottom), but not WT animals (top), 6 weeks following co-delivery of AAV Cas9 and AAV LSL-Th gRNA to the left (L) SN. GFP fluorescence represents transduction by AAV. (B) Quantification of optical density of TH immunoreactivity relative to DAPI in the ipsilateral compared to the contralateral SN of animals described in (A). Each point represents one analyzed coronal section (n = 3–4 sections/animal), and each color represents a distinct animal (n = 5–6/group, unpaired t test, t(9) = 4.166, **p = 0.0024). (D) Quantification of optical density of TH immunoreactivity relative to DAPI in the ipsilateral compared to the contralateral striatum of animals described in (C). Each point represents one analyzed coronal section (n = 3–4 sections/animal), and each color represents a distinct animal (n = 5–6/group, unpaired t test, t(11) = 2.896, *p = 0.0177). Scale bars, 500 mm
Generation of Transgenic LSL-Cas9 Transgenic Rats for Cre-Dependent Expression of Cas9
We created a transgenic rat to express a FLAG-tagged Cas9 in a Cre-dependent manner. A CAG promoter-driven LSL-Cas9 construct was targeted to the rat Rosa26 locus. PCR genotyping and sequencing confirmed appropriate targeting to the Rosa26 locus. To verify that the LSL-Cas9 transgenic rats express Cas9 following Cre-dependent recombination, rats received intracranial injections of AAV iRFP-iCre or AAV iRFP-Flpo to express either Cre recombinase or Flpo recombinase (as control) in the midbrain (Table S1). Four weeks post-transduction, we observed FLAG immunoreactivity in the midbrain following transduction by AAV iRFP-Cre, but not by AAV iRFP-Flpo (Figure 5A), indicating Cre-dependent recombination and expression of FLAG-tagged Cas9. Injections of the viral vectors resulted in comparable levels of iRFP fluorescence, demonstrating similar viral delivery of both recombinases. No FLAG immunoreactivity was observed when either virus was injected into WT animals.
Figure 5.
Characterization and Use of an LSL-Cas9 Transgenic Rat for Cre-Dependent Knockout of TH (A) Representative confocal images of colocalization of iCre recombinase and the FLAG-tagged Cas9 transgene in LSL-Cas9 rats. FLAG immunoreactivity is not observed following delivery of Flpo, a non-Cre recombinase. iRFP fluorescence indicates comparable delivery of Flpo- and iCre-encoding viruses. (B) Representative confocal images of unilateral TH loss in the SN of LSL-Cas9 rats 4 weeks after a midbrain injection of AAV iCre and AAV control gRNAs (right side, top) or AAV Th gRNAs (left side, bottom). Comparable EGFP fluorescence in control and Th gRNAs-injected hemispheres indicates comparable viral delivery between conditions. (C) A TH-immunostained striatal section from a rat injected as in (B) (L, left side; R, right side). (D) Quantification of optical density of TH immunoreactivity in the SN and striatum of animals described in (B) and (C). Each data point represents one analyzed coronal section (n = 3–4 sections/animal), and each color represents a different animal (n = 4). Scale bars represent 50 mm (A), 100 mm (B), and 500 mm (C).
Co-delivery of Cre Recombinase and gRNAs Alters Gene Expression in the Midbrain of LSL-Cas9 Rats
The midbrain of LSL-Cas9 transgenic rats was injected with a combination of AAV iCre and AAV Th gRNAs or AAV control gRNAs (Table S1). Four weeks later, the hemisphere that received Th gRNAs displayed a 45% reduction in nigral TH immunoreactivity compared to the control gRNAs-injected hemisphere (Figures 5B and 5D). Similarly, TH immunoreactivity in the ipsilateral striatum was decreased to ~60% of the contralateral side (Figures 5C and 5D). Comparable viral transduction between the hemispheres was observed based on detection of EGFP fluorescence that was co-expressed with each gRNA vector (Figures 5B and 5C).
Mesencephalic astrocyte-derived neurotrophic factor (MANF) is a protein expressed in the rodent midbrain (Lindholm et al., 2008) and has been reported to have therapeutic effects on dopaminergic neurons in rodent models of Parkinson’s disease (Voutilainen et al., 2009). The ability to modify genes linked to alterations in disease pathology could provide important insight into the functions of these genes and reveal new opportunities for treatment development. We therefore produced an AAV vector with gRNAs that would specifically target the Manf gene and allow for downregulation of MANF expression (Figure 6A). This AAV Manf gRNAs vector was tested in vitro by co-transduction with AAV Cas9 into rat primary cortical neurons. Resolvase treatment of the Manf PCR product revealed cleaved fragments (Figure 6B) consistent with the introduction of indel mutations verified by subsequent cloning and sequencing of the PCR DNA (Figure 6C). In addition, primary neurons transduced with AAV Cas9 and AAV Manf gRNA showed a robust decrease in Manf mRNA (Figure 6D) and protein levels (Figure 6E) 1 week post-transduction.
Figure 6.
Developing an Assay for Knockout of MANF In Vivo (A) A schematic of the gRNA-binding sites and the PCR assay used to amplify the 893 nt flanking the second exon of rat Manf. (B–E) Rat primary cortical neurons were transduced with AAV Cas9 and AAV Manf gRNAs or AAV control gRNAs and harvested 1 week later for determination of mutagenesis and knockout. (B) Co-transduction with AAV Cas9 and AAV Manf gRNAs resulted in resolvase-induced cleavage of the PCR product (arrows). (C) An alignment of seven independently isolated clones of the PCR fragment shows precise +A insertions among the alleles. Knockout of Manf was verified with (D) real- time qRT-PCR and (E) Wes analyses of Manf mRNA and protein levels, respectively. (D) Manf mRNA levels were normalized to the geometric mean of reference genes and presented as 2-ddCq ± upper and lower limits (n = 3, unpaired t test using dCq values, t(4) = 20.27, ****p < 0.0001). (E) The MANF protein band density was normalized to actin and presented as density relative to control gRNA (mean ± SE, n = 3, unpaired t test, t(4) = 4.999, **p = 0.0075). The arrow in the cropped blot points at the ~25-kDa MANF band. (F) Representative images of unilateral loss of MANF immunoreactivity in the SN of LSL-Cas9 rats four weeks after co-injection of AAV iCre and AAV control gRNAs or AAV Manf gRNAs. GFP fluorescence represents delivery of gRNA. Scale bar 100 mm.(G) Quantification of the optical density of MANF immunoreactivity in the SN of LSL-Cas9 or WT animals injected as described in (F). Each data point represents one analyzed coronal section (n = 3–4/animal), and each color represents data from a distinct animal (n = 4/group, unpaired t test, t(6) = 5.437, **p = 0.0016).
Following in vitro validation of the MANF knockout assay, the LSL-Cas9 transgenic rats received an injection into the SN with a mix of AAV iCre and AAV Manf gRNA or AAV control gRNA (Table S1). Four weeks post-transduction, the ipsilateral SN that received MANF gRNA displayed an average 35% reduction of MANF immunoreactivity relative to the contralateral region injected with control gRNA (Figures 6F and 6G). Detection of EGFP fluorescence was used as a reference to ensure similar viral delivery of EGFP-tagged gRNAs between hemispheres (Figure 6F).
Genetic Editing of Midbrain Neurons in an LSL-Cas9- Nickase Rat Created by CRISPR-Cas9 Modifications of Spermatogonial Stem Cells
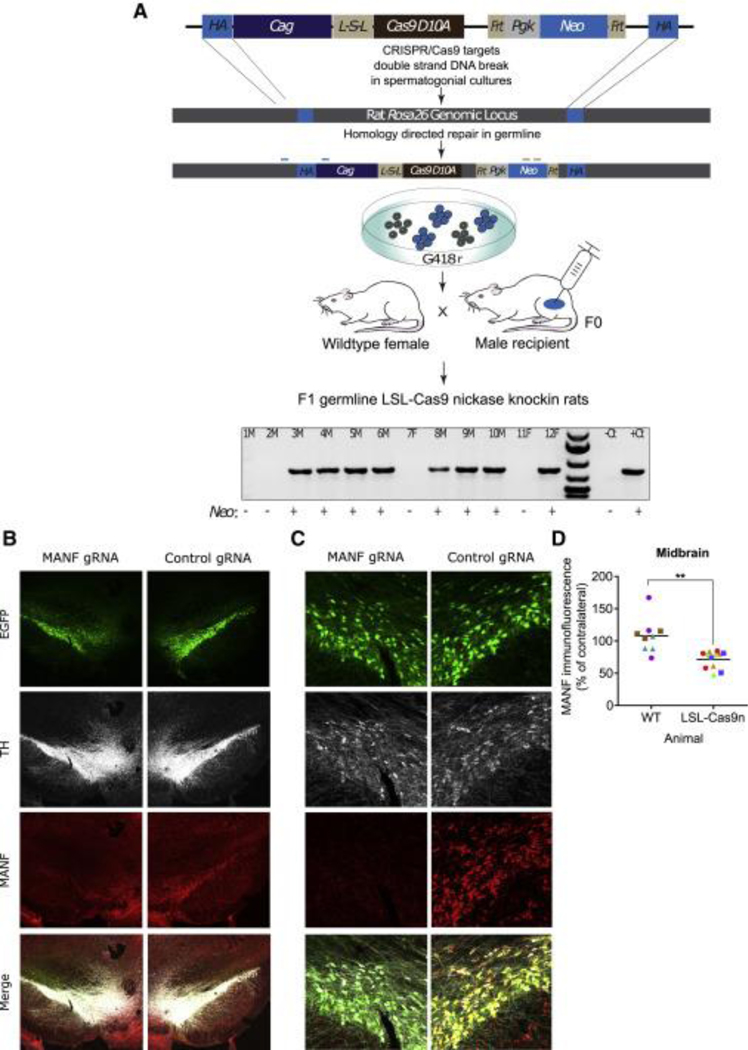
CRISPR-Cas9-mediated gene targeting in donor spermatogonial stem cell cultures via the NHEJ DNA repair pathway was previously shown to provide an efficient approach for knockout rat production (Chapman et al., 2015). Here, we demonstrate highly efficient CRISPR-Cas9-mediated homology-directed repair (HDR) in rat spermatogonial stem cell cultures by targeted insertion of an ~11.9 kb Cre-dependent, LSL-nickase (SpCas9(D10A)) transgene into the Rosa26 genomic locus (Figure 7A). The Rosa26 locus is commonly used as a “safe harbor” site to support robust, ubiquitous transgene expression in rats and mice (Kisseberth et al., 1999; Remy et al., 2014; Kobayashi et al., 2012; Tsuchida et al., 2016). Like in LSL-Cas9 rats, the LSL-nickase gene targeting construct was designed to mediate transgene expression upon removal of the floxed transcription terminator signals by Cre recombinase (Figure 7A). The LSL-nickase targeting construct also contained an internal Frt-Pgk- Neo-Frt selection cassette in between the Rosa26 locus homology arms to facilitate genetic selection for targeted spermatogonial stem cells in culture (Figure 7A).
Figure 7.
Production of LSL-Cas9 Nickase Knockin Rats by HDR in Spermatogonial Stem Cells (A) Transgenic LSL-nickase rats were produced using donor rat spermatogonia harboring an ~11.9 kb, CAG promoter-driven floxed-stop FLAG-tagged SpCas9(D10A) transgene that was precisely targeted to the rat Rosa26 germline locus by CRISPR-Cas9-mediated HDR. Targeted spermatogonia were genetically selected for in G418-containing culture medium and then injected into recipient rat testes (F0, founder males) to produce spermatozoa that transmitted the targeted transgene to F1 germline mutant progeny (LSL-nickase hemizygotes). Genotyping of F1 progeny derived from a recipient male transplanted with spermatogonia genetically modified with the LSL-nickase targeting construct demonstrated the expected 797-bp PCR amplicon using primers that hybridize to rRosa26 locus and CAG transgene, respectively; - and + at the bottom of the gel represent genotyping results from primers that hybridize to the neomycin (Neo) selection cassette; F, female F1 pup DNA; M, male F1 pup DNA; WT (-Ct) and transgenic (+Ct) rat DNA from F1 progeny were generated from an earlier litter. (B and C) Representative midbrain images at (B) 23 and (C) 203 magnification indicating unilateral loss of MANF immunoreactivity in transgenic LSL- Cas9 nickase rats 4 weeks following co-injection of AAV iCre and AAV Manf gRNAs or AAV control gRNAs into the SN. GFP fluorescence indicates viral transduction. (D) Quantification of the optical density of MANF immunoreactivity in the SN of animals described in (B) and (C). Each data point represents one coronal section (n = 3/animal), and each color repre- sents data from a distinct animal (n = 3–4/group, unpaired t test, t(5) = 5.553, **p = 0.0026).
Spermatogonial cultures stably modified with the LSL-nickase targeting construct were selected in G418-containing medium following co-transfection with a pX459 plasmid expressing Cas9 and Rosa26_A gRNA (neon electroporation). Selected spermatogonia were then transplanted into busulfan-treated, male-sterile recipient rats (Ivics et al., 2011). Recipient-founder males were paired with WT female Sprague-Dawley rats at ~60 days post-transplantation for breeding and the first transgenic F1 mutant progeny were produced d107 post-transplantation. Genomic PCR analyses identified 20 of 34 total pups (n = 5 litters) as germline mutants harboring the correctly targeted LSL-nickase construct in their Rosa26 locus (Figure 7A). Thus, rat spermatogonial lines are amenable to genetic modification by CRISPR-Cas9-mediated HDR using relatively large circular plasmids as donor templates.
The resulting LSL-nickase rat was backcrossed with WT LE for three or four generations, and Cre-dependent nickase activity was tested by injecting AAV Manf gRNAs or control gRNAs along with AAV iCre into the midbrain (Table S1). Four weeks later, the rats showed unilateral loss of MANF immunoreactivity in the hemisphere injected with AAV Manf gRNAs (Figures 7B–7D), suggesting that Cre-induced recombination and expression of rat-derived nickase can be used to drive modifications of endogenous genes in rat dopaminergic neurons. Delivery of gRNA and Cre to the midbrain of WT rats did not affect MANF immunoreactivity of the cells (Figure 7D).
Generation of LSL-Cas9 3 DAT-iCre Double-Transgenic Rats to Allow for Dopaminergic Neuron-Specific Modification of Target Genes
Immunohistochemical validation of the transgene expression in the rats showed that the FLAG-tagged Cas9 transgene colocalized with TH in the SN of double-positive animals (Figure 8A). Cell quantification from three animals (two sections per animal) revealed 96% ± 0.02% FLAG+TH+ cells, 4.00% ± 0.02% FLAG-TH+ cells, and 0.69% ± 0.44% FLAG+TH-cells in the SN (mean ± SE).
Figure 8.
Characterization and Use of the LSL-Cas9 3 DAT-iCre Double-Transgenic Rat for Knockout of MANF in the Midbrain Dopaminergic Neurons (A) Representative confocal images of TH and FLAG-tagged Cas9 colocalization in the SN of LSL-Cas9 3 DAT-iCre double-transgenic rats. (B) Representative midbrain images depicting unilateral loss of MANF immunoreactivity (white arrowheads) in TH+ dopaminergic cells in LSL-Cas9 3 DAT-iCre rats 4 weeks following injection of AAV control or AAV Manf gRNAs into the SN. (C) High-magnification confocal images acquired 4 weeks after AAV injection demonstrating selective loss of MANF immunoreactivity in TH+ cells that received Manf gRNAs (right), but not in those that received control gRNAs (left). GFP fluorescence indicates viral transduction. Open arrowheads signal to GFP+TH- cells in which MANF immunoreactivity is maintained in both control and experimental conditions. Closed arrowheads signal to GFP+TH+ cells in which MANF immunoreactivity is lost in the hemisphere receiving Manf gRNAs, but not control.
Next, we injected the midbrain of the double transgenic LSL-Cas9 X DAT-iCre rats with either AAV Manf gRNAs or AAV control gRNAs. The animals were perfused after a 4-week recovery period, and their brains were sectioned for fluorescence microscopy. Using EGFP fluorescence as a marker for transduction and TH immunoreactivity as a surrogate marker for dopaminergic neurons (GFP+TH+), we observed that the subpopulation of MANF-immunoreactive cells (Figures 8B and 8C, closed arrowheads) was reduced from ~96% on the side receiving AAV control RNAs to 3% on the side receiving AAV Manf gRNA (Figure 8D). Conversely, when scoring the transduced non-dopaminergic cells (GFP+TH-) in the same sections, the subpopulations of MANF-immunoreactive cells (Figure 8C, open arrowheads) were relatively similar (90% ± 4% versus 79% ± 6%) (Figure 8D). This supports that the reduction in MANF immunoreactivity is Cre dependent and that in this model, Cas9 activity is restricted to dopaminergic cell types
DISCUSSION
The development of the CRISPR-Cas9 system has provided unprecedented capabilities for genome editing in mammals. Here, we describe a novel combination of AAV vectors and transgenic rats that enable the cell-type-specific genome modification of neurons in the adult rat brain. Our methods include the use of transgenic rats expressing a Cre-dependent Cas9 (LSL-Cas9) or Cas9 nickase (LSL-Cas9 nickase) that can be combined with a virally delivered Cre approach or crossed with Cre-driver rat lines, such as the DAT-iCre rat that we describe here. Once either of these Cas9 transgenes has been switched “ON” due to Cre recombinase activity, the subsequent delivery of gRNAs specific to the user’s gene(s) of interest (e.g., Manf or Th as shown here) produces a loss of detectable protein expression where Cas9 is expressed.
While this work is focused on dopaminergic neurons (as spec- ified by our DAT-iCre driver rat), the Cre-dependent LSL-Cas9 and LSL-nickase rats and the Cre-dependent gRNA viral vectors represent a versatile set of tools that should be readily applicable to other neuronal (or non-neuronal) cell types using alternative methods of Cre delivery to restrict CRISPR activity to the tissue and cell type of interest. For example, a growing repertoire of Cre-driver rats specifying, e.g., GABAergic (Sharpe et al., 2017), D1 receptor, D2 receptor, and parvalbumin (Pvalb)-expressing neurons is already available at the Rat Resource and Research Center or commercially. With the recent development of rAAV2-retro (Tervo et al., 2016), an AAV serotype capable of retrograde transduction, it is now feasible to apply CRISPR-based modifications with projection-specific control.
Here, we demonstrate successful CRISPR-Cas9-mediated gene targeting directly in the rat germline by HDR within cultures of fully functional spermatogonial stem cells. Direct germline gene targeting in spermatogonial stem cells streamlines the production of custom model organisms, reduces resource expenditure, and eliminates birth of unutilized mosaic and/or chimeric animals (Chapman et al., 2015). The efficiency of LSL-Cas9 nick- ase rat production by HDR at the Brown Norway Rosa26 locus indicated that 50%–100% of genetically selected stem sper- matogonia harbored monoallelic and/or biallelic targeted insertions (i.e., ~59% donor germline transmission of tgLSL-Cas9 nickase). Here, we utilized the inbred Brown Norway rat genome database (Gibbs et al., 2004) to facilitate production of gene targeting constructs with homology arms and gRNAs compatible with Brown Norway rat spermatogonial lines. Genetic tractability of spermatogonial stem cell lines derived from Brown Norway lines and potentially those from commonly employed inbred and outbred rat strains will greatly expand the toolbox for genetically engineering rat models needed to advance research within a broad spectrum of biomedical fields, particularly the neurosciences.
When utilizing the Cas9 editing approach, the location of the double-stranded DNA break caused by Cas9 is predictable and specific, but the novel sequence that results from cellular repair mechanisms typically varies among cells (Cong et al., 2013). It is important that users of this technology keep in mind that indels may occur on one or both alleles of the target sequence and the resulting indels in a single cell may differ from one another and that populations of treated cells may contain a mosaic of different genotypes even when treated with identical gRNAs. Our TIDE analysis of mutations from primary neurons showed that several types of indels were detected at either the Th or Rosa26 locus. Ideally, two-thirds of these lesions should result in translational frameshift and cause a truncation in the encoded polypeptide. However, there is evidence that the frequency and spectrum of indel sequences is affected by regions of “microhomology” proximal to the break site, and bioinformatic tools are now available to predict the likelihood of frameshifts (Bae et al., 2014). When optimizing the position of the indel in relation to the start and stop codons of the gene of interest, it is preferable to be closer to the start codon, since an early frameshift omits more of the original polypeptide than a frameshift that occurs later and therefore is more likely to result in the loss of enzyme activity and/or antibody recognition. Ultimately, the conclusion that can be drawn from experiments employing these tools will be defined by the phenotypic and genotypic changes that can be demonstrated with the available tools and methods for assaying the target gene(s) of in- terest. The use of a control gRNA (e.g., Rosa26) is highly recommended to help control for phenotypic alterations due to delivery of the CRISPR-Cas9 system.
In the current study, we used AAV vectors to deliver gRNAs (singly or as nickase-compatible pairs) to each of the target genes. We observed effective knockout of the target proteins by assessing immunoreactivity for TH and MANF in the rat midbrain. Following injection of AAV iCre with an AAV gRNA to Th or a control gRNA into the midbrain of LSL-Cas9 rats, we observed a decrease in TH immunoreactivity only in the hemisphere that received Th gRNA. The loss of TH did not appear to reflect the loss of dopaminergic neurons, since GFP expression from the gRNA vectors was comparable in the striatum and midbrain between the two hemispheres. In LSL-Cas9 rats crossed with DAT-iCre rats, we used gRNAs to target Manf, a gene that is expressed in both dopaminergic and non-dopaminergic neurons in the midbrain. Using this approach, we observed a selective loss of MANF only in the midbrain dopaminergic neurons and only of the hemisphere that received MANF gRNAs. An EGFP reporter expressed by the AAV RNAs confirmed that both dopaminergic and non-dopaminergic neurons were transduced in this paradigm, demonstrating that the selective knockdown of MANF in TH+ cells is not simply due to a biased tropism of the virus. Taken together, these data indicate that cell-specific reduction in MANF protein, or any protein, is possible using the double-transgenic line and single AAV gRNA injection.
We examined changes in protein expression for our targeted genes at 2, 4, and 6 weeks post-delivery of the different components of the CRISPR-Cas9 system in WT rats. Although both delivery of Cas9 and Cas9 nickase were effective in reducing expression of the target protein, the duration required to detect significant knockout varied depending on the method utilized. When considering an approach to CRISPR-Cas9 genome editing in the rat brain, numerous factors may contribute to the time required to produce peak loss of the target. Such factors include the half-life of the target protein, titer of virus, volume of virus, and viral promoter and should be evaluated empirically for novel gene targets. We therefore advise pilot studies to determine the optimal conditions needed to achieve adequate loss of target protein immunoreactivity and, more importantly, protein activity.
In this study, we also describe for the first time a DAT-iCre rat produced on the LE background. In contrast to a previously described DAT-Cre rat using an IRES-Cre targeted to the endogenous rat Dat locus of Sprague-Dawley background (Liu et al., 2016), we randomly integrated a BAC expressing iCre from the rat DAT promoter into the genome of LE embryos. In DAT-Cre mice, the insertion of an IRES-Cre element has been shown to affect DAT activity in the striatum (O’Neill et al., 2017). Here, using a variety of techniques, we show that the expression of iCre in our DAT-iCre rat is restricted to dopaminergic neurons in the midbrain and does not impair the electrophysiological properties of dopamine neurons, evoked-dopamine release and uptake in striatum, or endogenous TH and DAT protein expression. We initially obtained six founder lines, three of which did not produce progeny carrying the transgene (i.e., no germline transmission). The remaining three lines varied in copy number per haploid genome (one, two, and eight copies). Of note, line 1 had two copies and occasionally lost a copy within litters, resulting in different recombination kinetics. This serves as a cautionary note when using BAC transgenics with more than one copy per haploid genome. Because the transgene’s locus of insertion was random and has not been determined, all results presented herein were obtained by breeding hemizygous rats to WT rats or LSL-Cas9 rats.
In conclusion, the viral vectors and transgenic rats described in the present study can be combined to enable both regional and cell-specific genomic editing in the adult rat brain. Our results suggest that these tools can be combined with other existing Cre-driver rats and viral vectors to study loss of gene function in cells of the rat nervous system as well as other tissues. With the described ability to introduce mutations in dopaminergic neurons, it may also be possible to provide repair templates to tag or create specific mutations using viral vector delivery (Nishiyama et al., 2017; Ishizu et al., 2017), thereby testing gene func- tion in models of substance abuse or creating disease models of Parkinson’s disease (e.g., LRKK2, synuclein, PINK, and DJ-1).
STAR+METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Brandon K. Harvey (bharvey@mail.nih.gov).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
All animal experiments were conducted on adult rats (males and females) in accordance with the National Institute of Health (NIH) guidelines for animal research. All experiments were approved by the Animal Care and Use Committees (ACUC) of the Intramural Research Programs at the National Institute on Drug Abuse and the National Institute of Mental Health. The rats were group-housed in a 12 h light-dark cycle with ad libitum access to rodent chow and water. The assignment of animals to experimental groups was done based on genotyping results, with each animal serving as its own internal control (unilateral intracranial injections of experimental gRNA versus control gRNA).
DAT-iCre transgenic rat
For the DAT-iCre rats, a bacterial artificial chromosome (BAC) containing the rat Dat gene (CH230–275E16) was obtained from the Children’s Hospital Oakland Research Institute (CHORI), and recombineered to replace the start codon of Dat with cassette containing iCre (improved Cre recombinase) and the polyadenylation signal from the gene for bovine growth hormone (Warming et al., 2005). This BAC was injected into the pronuclei of LE rat zygotes. These were transferred to pseudopregnant Sprague-Dawley females as single cell zygotes on the day of injection or allowed to develop to 2-cell embryos the next day before transfer. The resulting line, LE- Tg(Slc6a3-iCre)6Ottc, is registered at the Rat Genome Database (RGD: 9588578) and deposited at the Rat Resource and Research Center (RRRC #758; University of Missouri, Columbia MO, USA). Animals were bred with WT LE rats from Charles River Laboratories for 10 generations before crossing with LSL-Cas9 rats for experiments. This line has one copy of the transgene per haploid genome as determined by droplet digital PCR.
LSL-Cas9 transgenic rat
The LSL-Cas9 donor plasmid was constructed using the coding region for FLAG-tagged NLS-tagged SpCas9, which was amplified from pX458 (Addgene 48138) and inserted downstream of the CAG promoter and a series of polyadenylation signals flanked by loxP sites (floxed stop). The entire expression cassette was flanked on each side by homologous arms (1 kb each) corresponding to sequences on each side of the area targeted by the Rosa26 gRNAs (Figure S3). For the generation of LSL-Cas9 rats, this donor plasmid was mixed with one pair of nickase-compatible gRNAs identified in the rat Rosa26 locus (Table S2), synthesized and transcribed using Guide-IT synthesis kit (Clontech, Mountain View, CA, USA), and injected into the pronuclei of LE rat zygotes along with mRNA encoding Cas9 nickase (Tri-Link, San Diego, CA, USA). Injected embryos were incubated overnight. Those that developed to the 2-cell stage were transferred to pseudopregnant Sprague-Dawley females and brought to term. The tissue from pups was genotyped by PCR and sequence confirmed. The resulting transgenic rat was designated “LE-(ROSA)26 em1(CAG-Cas9)Ottc” and registered with the Rat Genome Database (#13208224) and deposited at the Rat Resource and Research Center (RRRC#833). Herein, LE-(ROSA)26 em1(CAG-Cas9)Ottc rats are referred to as “LSL-Cas9” rats. Animals were bred with WT LE rats from Charles River Laboratories for 4–5 generations then heterozygous animals were crossed to generate homozygous animals for experiments.
LSL-Cas9 x DAT-iCre transgenic rat
DAT-iCre rats were backcrossed to WT LE rats at least 10 generations before crossing the LSL-Cas9 rats that had been crossed at least 4 generations with WT LE.
DIO-mCherry transgenic reporter rat
The coding region of mCherry was amplified with linkered oligos and Addgene #26975 as a template. This insert was recombined into the backbone (pAAV EF1a DIO EYFP, Addgene 27056, digested with NheI and AscI restriction enzymes) using In-Fusion cloning mix (Clontech) to produce pAAV EF1a DIO mCherry (Addgene #47626). The pAAV EF1a DIO mCherry plasmid was digested with MluI and RsrII, gel purified away from the plasmid backbone, and microinjected into fertilized oocytes harvested from a LE rat by the NIMH Transgenic Core. Surviving pups were screened for the integrated transgene by PCR genotyping. This line (LE-Tg(DIO-mCherry) 2Ottc) has 0.5–0.7 copies of the transgene per copy of Ggt1 (indicating 1 copy per haploid genome) as determined by droplet digital PCR. The resulting transgenic rat was designated “LE-Tg(DIO-mCherry)2Ottc” and registered with the Rat Genome Database (#8693598) and deposited at the Rat Resource and Research Center (RRRC#687). Herein, “LE-Tg(DIO-mCherry)2Ottc” rats are referred to as “DIO-mCherry” rats. Animals were bred with WT LE rats from Charles River Laboratories for 4–5 generations, then heterozygous animals were crossed with DAT-iCre animals for detection of mCherry expression.
CRISPR-Cas9-mediated knockin of LSL-Cas9 nickase using rat spermatogonial stem cells
The LSL-Cas9n donor plasmid was constructed using the coding region for FLAG-tagged NLS-tagged SpCas9(D10A), which was amplified from pX461 (Addgene 48140) and inserted downstream of the CAG promoter and a series of polyadenylation signals flanked by loxP sites (floxed stop) and upstream of an FRT-flanked neomycin selection marker (FRT-PGK-NeoR-FRT). The entire expression cassette was flanked on each side by homologous arms corresponding to sequences spanning 191 bp upstream and 604 bp downstream of the area targeted by the Rosa26 gRNAs (Figure S3). LSL-Cas9 nickase rats were generated at UT Southwestern Medical Center’s Mutant Rat Resource by spermatogonia-mediated gene transfer (Chapman et al., 2015) using sterile-testis complementation (Richardson et al., 2009). Brown Norway rat (Charles River) spermatogonial cultures were co-transfected with the LSL-Cas9n donor plasmid (20 mg) and a plasmid expressing the Rosa26_A gRNA along with FLAG-tagged Cas9–2A-GFP (20 mg) using a Neon transfection apparatus (~4 3 106 total spermatogonia transfected in 850 mL Resuspension Buffer R; 10X electroporations in 100 mL tips with 2 pulses at 1100 V, 20 m) and then selected in a G418-containing medium (70 mg/mL G418 d2-d8 post-electroporation) on feeder layers of DR4 mouse embryonic fibroblasts, prepared as previously described (Chapman et al., 2015). Selected G418-resistant spermatogonial culture was transplanted into a single testis of busulfan-treated, male-sterile Brown Norway recipient rats (Ivics et al., 2011). Transplanted recipient rats (n = 3) were paired with wild-type female Sprague Dawley (Envigo) rats at ~60 days post-transplantation for breeding to generate F1 hybrid Brown Norway: Sprague Dawley germline mutant progeny. The resulting transgenic rat was backcrossed on to LE rats and designated “LE.Cg-(ROSA)26 em1(CAG-Cas9*D10A)Ottc,” registered with the Rat Genome Database (#13602097), and deposited at the Rat Resource and Research Center (RRRC#834). Herein, “LE.Cg- (ROSA)26 em1(CAG-Cas9*D10A)Ottc” rats are referred to as “LSL-Cas9 nickase” rats.
Cell culture
Primary cortical neurons were isolated from E15–16 Sprague-Dawley rats (mix of males and females) as described previously (Howard et al., 2008) and plated on PEI-coated 96-well plates. Cells were maintained at 37°C with 5.5% carbon dioxide in neurobasal media (Thermo Fisher Scientific) supplemented with 2% B-27 (Thermo Fisher Scientific) and 0.5 mM L-glutamine (Sigma-Aldrich) with a 50% media exchange every fortnight. On 6 days-in-vitro (DIV6), cells were transduced with 5 mL AAV Cas9 (0.6–1.0×1012 vg/mL) and AAV Manf gRNA or AAV control gRNA (1.7–1.9×1012 vg/mL). On DIV15, cells were harvested for RNA isolation or protein analysis. Each sample consists of cells from three wells pooled together.
PC-12 cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher Scientific) supplemented with 10% heat-inactivated horse serum, 5% fetal bovine serum (FBS), and 1% penicillin/streptomycin at 37oC with 5.5% carbon dioxide. The cell line has been confirmed to be rat using PCR analysis for various genes, and photomicrographs show similar morphology to images available on ATCC website (https://www.attc.org) for PC-12 cells. Cells plated onto a 24-well plate underwent transfection procedures with a total of 0.5 mg plasmid DNA/well (triple-transfection with plasmids encoding Cas9, gRNA, and Cre or Flpo in a ratio of 2:1:1, respectively) using Lipofectamine 3000 reagent (1.5 mL/well; Thermo Fisher Scientific). Two days post-transfection, cells were re-plated onto a 24-well plate, and one day later, the cells were exposed to a 48-h puromycin (2 mg/mL) selection procedure. The selected cells were maintained in selection-free media for four days before collection of genomic DNA.
METHOD DETAILS
Viral vector construction
The AAV vector encoding SpCas9 (pX551) was a gift from Feng Zhang (Addgene plasmid # 60957; Swiech et al., 2015) and the corresponding AAV is referred to as “AAV Cas9” herein. This plasmid was modified to encode the D10A nickase variant by PCR with mutagenic primers and ligation-independent cloning to produce pAAV MeCP2 HA-SpCas9n(D10A), an AAV packaging vector (Addgene 112719), referred to herein as “AAV nickase.”
Guide RNA seed sequences composing an “SpCas9 nickase”-compatible pair were selected for rat Rosa26, Th, and Manf using http://zlab.bio/guide-design-resources (Table S2), synthesized as an oligonucleotide duplex and ligated into the BbsI restriction sites of pmU6-gRNA and phU6-gRNA (gifts from Charles Gersbach, Addgene 53187 and 53188; Kabadi et al., 2014). These mU6 and hU6 expression cassettes were then inserted into pAAV EF1a eGFP (Addgene 60058; Savolainen et al., 2014) using ligation-independent cloning (InFusion, Takara) to produce Addgene 113156, 113155, and 116157.
The modification of the mouse U6 promoter to include a transcription terminator flanked by loxP elements was previously described (Tiscornia et al., 2004). It was adapted for expressing gRNAs in AAV vectors by synthesizing the mU6* and the floxed mU6 terminator as a gBLOCK (Integrated DNA Technologies, Coralville, IA) and using it to replace the mU6 promoter in pmU6-gRNA (Addgene 53187), to produce Addgene 113160. The addition of the TH4042 A gRNA sequence followed by the transfer of the entire expression cassette into the AAV backbone was performed using the method described above, to produce pAAV (flox-stop) Th gRNA A EF1a eGFP (Addgene 113158).
The nuclear-red-to-green Cre-dependent switching vector, pAAV EF1a Nuc-flox(mCherry)-eGFP (Addgene 112677) was constructed by amplifying the coding region of mCherry from Addgene 47636 with linkers encoding a nuclear localization signal followed by a loxP site. This amplicon was recombined into pAAV EF1a flox(tagRFP)-eGFP upstream of loxP-eGFP using ligation-independent cloning.
The AAV vectors expressing the iRFP713 reporter along with iCre or Flpo recombinases (Addgene 112683 and 112684) were constructed by using the coding region for iRFP713 tagged with a nuclear localization signal to replace the eGFP cassette in pAAV CMV- IE eGFP-2A-iCre and pAAV CMV-IE eGFP-2A-FLPo.
The AAV vector expressing iCre (pAAV EF1a-Cre; Addgene 89760) was constructed by using the coding region for iCre (Shimshek et al., 2002) to replace the V5-synuclein region in Addgene 60057.
All constructs were sequence verified. All gRNA-containing vectors co-express GFP under the EF1alpha promoter. Schematics of the vectors produced in this study and deposited at Addgene are shown in Figure S3.
Production of AAV vectors
All AAV vectors were produced using triple transfection method as previously described (Howard and Harvey, 2017). All vectors were produced using serotype 1 capsid proteins and titered by droplet digital PCR.
Intracranial injections
Adult LE rats (> 8 weeks) were anesthetized with intraperitoneal injections of ketamine (80 mg/kg) and xylazine (8 mg/kg) and placed into a stereotaxic frame (Stoelting Co., Wood Dale, IL). The rats received intracranial AAV injections into the SN at the following coordinates: AP-5.8, ML ± 1.8, DV-7.4 mm from the bregma. A total of 1.0 mL of AAV was injected into each hemisphere at a rate of 0.5 mL/min using a Nanofil 10 mL syringe with a 33G blunt needle and coupled to a UMP4 microinjector pump (World Precision In- struments, Sarasota, FL). Following injection, the needle was lifted 0.5 mm, and kept in place for an additional 4 min to reduce backflow of the AAV. Detailed information on viral injections and animals is provided in Table S1.
Immunohistochemistry
Rats were deeply anesthetized with isoflurane and transcardially perfused with heparinized phosphate-buffered saline (PBS) followed by a 4% paraformaldehyde (PFA) solution. The brains were post-fixed in 4% PFA for 2 h, and then incubated in 18% and 30% sucrose before being flash-frozen. Using a freezing microtome, 30 mm cryosections were collected and washed for 30 min in PBS. Following blocking in 4% goat serum and 0.3% Triton X-100 for 1 h at room temperature (RT), the sections were incubated in primary antibody in blocking solution (1:2000 mouse anti-TH, Millipore, Burlington, MA, #MAB318, RRID:AB_2201528; 1:2000 rabbit anti-MANF, custom-made by Yenzym Antibodies, South San Francisco, CA (Henderson et al., 2013); 1:1000 rabbit anti-Cre-recombinase, BioLegend, San Diego, CA, #908001, RRID:AB_2565079; or 1:1000 mouse anti-FLAG, Sigma-Aldrich, St. Louis, MO, #F1804, RRID:AB_262044) overnight at 4oC. The next day, sections were washed in PBS for 30 min and then incubated in secondary antibody in blocking solution at RT (1:500 dilution, all from Thermo Fisher Scientific; goat anti-mouse Alexa Fluor (AF) 568, #A-11004, RRID:AB_141371; goat anti-rabbit AF680, #A-21109, RRID:AB_2535758, goat anti-mouse AF680, #A-21058, RRID:AB_2535724; goat anti-rabbit AF568, #A-11011, RRID:AB_143157; or goat anti-rabbit AF488, #A-11034, RRID:AB_2576217). Next, sections were washed for 30 min in PBS, followed by a 10-min wash in DAPI (1:3000, Thermo Fisher Scientific). Sections were mounted onto microscope slides with Mowiol mounting media (Sigma-Aldrich).
Image analysis and cell counting
Microscopy
Confocal images were acquired using a Nikon system (Nikon Eclipse E800 microscope body, Nikon C2 Confocal microscope system, NIS-Elements imaging software version 4.40; Nikon Instruments Inc., Melville, NY). Low-magnification images were taken using an Olympus MVX10 microscope (Tokyo, Japan) attached to a DP80 camera.
Densitometry Analysis
Densitometry analysis of midbrain and striatal coronal sections was performed in Fiji/ImageJ (Schindelin et al., 2012) (version 2, NIH). After background subtraction, a rectangular region of interest (ROI) was drawn around the SN of the control (contralateral) hemisphere (received control gRNA or other injection control), using eGFP fluorescence (if available) as a guide to determine size and placement. The same ROI was used for the ipsilateral SN (received Th or Manf gRNA). The integrated density of TH or MANF immunofluorescence was normalized to DAPI, and all ipsilateral results are presented as percentage of contralateral side.
Cell counting
Sections from the midbrain of LSL-Cas9 X DAT-iCre rats were co-immunolabelled for FLAG and TH, or MANF and TH. The SN was imaged at 20x magnification using confocal microscopy. Immunopositive cells were manually identified and counted using Fiji/ImageJ software.
RNA in situ hybridization (RNAscope)
Three DAT-iCre transgenic rats and one WT animal were euthanized with isoflurane and their brains were removed and immediately frozen in dry ice. Brains were cut into 12 mm sections, directly mounted onto Superfrost Plus slides (Thermo Fisher Scientific, Waltham, MA) and stored at −80oC until further processing. RNA in situ hybridization of Dat and iCre mRNA was performed according to the User Manual for Fresh Frozen Tissue using RNAscope Multiplex Fluorescent Reagent Kit (Advanced Cell Diagnostics, Newark, CA). Briefly, mounted brain sections were fixed in 10% neutral buffered formalin (Thermo Fisher Scientific) for 20 min on ice, followed by two rinses in distilled water and then dehydrated in 50%, 70% and 100% ethanol. Slides were kept at −20oC in 100% ethanol overnight. The next day, the slides were dried at RT for 10 min, and then incubated with pre-treatment IV solution at RT for 20 min. After rinsing in PBS, target probes specific for rat Dat and iCre mRNA were applied to the brain sections and incubated at 40oC for 2 h in the HybEZ oven (Advanced Cell Diagnostics). The RNAscope probes used were Rn-Slc6a3-C1, that targets region of 827–1913 NM_012694.2 (#319621), and a custom-made target probe for iCre-C2 (#423321). Sections were then incubated with preamplifier and amplifier probes by applying AMP1 at 40oC for 30 min, AMP2 at 40oC for 15 min and AMP3 at 40°C for 30 min, followed by incubation in AMP4 ALtB 40oC for 15 min. Finally, the sections were stained with DAPI for 30 s to visualize nuclei. Images from RNA hybridized sections were acquired using Nikon inverted microscope with a 20x (N.A 0.8) objective. For quantification of labeled cells, Dat-positive and Cre-positive cells were counted in three sections from three DAT-iCre rats.
Heteroduplex digestion and TIDE analysis
CRISPR-mediated indels were detected by heteroduplex digestion assay with either T7E1 (New England Biolabs) or Resolvase (Takara). Genomic DNA isolation was performed using Macherey-Nagel Tissue Spin columns according to the manufacturer’s instructions. CRISPR-mediated mutagenesis was assayed by amplifying the region surrounding the site complementary to the gRNA(s) using Q5 polymerase (New England Biolabs) and digesting the product with T7E1 or Resolvase. A list of genotyping primers is provided in Table S3.
CRISPR-mediated mosaicism in cell populations was observed using the TIDE analysis. Individual wells of 96-well plate containing primary cortical neurons isolated from Sprague Dawley rats were transduced with AAV Cas9 and either rAAV2-retro Th gRNAs or rAAV2-retro Rosa26 gRNAs. One week later, the genomic DNA was isolated by proteinase K digestion and subjected to TIDE analysis (Brinkman et al., 2014) at the Th and Rosa26 loci. Briefly, the target locus was amplified by PCR using Q5 polymerase using gene-specific primers, then subjected to Sanger sequencing using a nested primer (Table S3). The resulting chromatograms were analyzed by TIDE (TIDE version 2.0.1, code developed by Brinkman et al., 2014, and hosted online at https://tide.deskgen.com/ by Deskgen) using the sequence of the gRNA closest to the sequencing primer with a maximum indel size of 20 basepairs.
RNA isolation and quantitative reverse transcription PCR (RT-qPCR)
RNA was isolated using the Nucleospin RNA kit (Takara Bio, Mountain View, CA), including an on-column DNase treatment. Using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), 0.5 mg RNA was reverse transcribed into cDNA. The cDNA was further diluted 1:20 in water, and 5 mL was loaded as technical duplicates onto white-walled 96-well PCR plates (Bio-Rad), together with a 15 mL master mix consisting of TaqMan Universal PCR Mix (Thermo Fisher Scientific), and 450 nM primers and 100 nM FAM- or HEX- labeled probes (in-house designed, Integrated DNA Technologies) specific for rat mesencephalic astrocyte-derived neurotrophic factor (Manf), RNA polymerase II (Polr2a), and ubiquitin-conjugating enzyme 2i (Ube2i). The following sequences were used for detection of transcripts: Manf forward, cggttgtgctactacattgga; Manf reverse, gggccagaggcttcgatac; Manf probe, ccacagatgatgccgccac caag; Polr2a forward, tagtcctacctactccccaacttc; Polr2a reverse, agtagccaggagaagtgggag; Polr2a probe, actcgcccaccagtcccacc tact; Ube2i forward, gccaccactgtttcatccaaa; Ube2i reverse, gccgccagtccttgtcttc; Ube2i probe, cgtgtatccttctggcacagtgtgc. Real-time qPCR was performed with C1000 Thermal Cycler CFX96 Real-Time System (Bio-Rad) using following template: pre-incubation 12 min (50°C for 4 min, 95°C for 5 min), amplification with 50 repeats (94°C for 20 s, 60°C) for 1 min). Results were quantified using the Bio-Rad CFX Manager software with Cq values determined using the single threshold mode. The average Cq values for Manf were normalized to the geometric mean of the Cq for the reference genes Ube2i and Polr2a (delta Cq), and results are presented as relative expression compared to control group using the 2-ddCq value.
Western blot
For TH western blot, 8- to 10-weeks-old DAT-iCre line 1, 5, 6 transgenic and WT male rats (3 rats from each line) were anesthetized and the brains were removed. The midbrain and striatum regions were collected from single slides with a rat brain slicer matrix (ZIVIC Ins, Pittsburgh, PA) on ice. Tissues were lysed in a modified RIPA buffer containing 50 mM Tris-HCl (pH 7.4), 0.25% sodium deox- ycholate, 150 mM NaCl, 1 mM EDTA, 1% NP-40, and protease inhibitors (Sigma-Aldrich), homogenized 10 s (Polytron PT1200 ho- mogenizer, Kinematica, Luzern, Switzerland), and then centrifuged at 11,000 rpm for 15 min at 4oC. After quantification of protein concentration in the supernatant with a DC assay (Bio-Rad), 10 mg total protein was separated on 4%–12% NuPAGE gels using MES running buffer (Thermo Fisher Scientific). Proteins were transferred to 0.20-mm PDVF membranes (Thermo Fisher Scientific) and immunoblotted with rabbit anti-TH (1:1000; Millipore #AB152, RRID: AB_390204) and mouse anti-actin (1:1000, Abcam, Cambridge, UK, #ab3280, RRID:AB_303668) antibodies in blocking reagent (LI-COR Biosciences, Lincoln, NE). The protein bands were detected with an Odyssey scanner (LI-COR) using IR700- and IR800-labeled secondary antibodies (1:2000, Rockland Immunochemicals, Gilbertsville, PA).
Protein analysis using Wes
10-weeks-old DAT-iCre line 6 transgenic and WT rats (7 rats from each line) unilaterally injected with AAV Cas9 and AAV LSL-Th gRNA into the midbrain were analyzed for TH protein expression through capillary western blot (Wes, Protein simple, San Jose, CA). Six weeks after injection, the animals were anesthetized and the brains were removed. The left and right midbrain regions were collected by taking a 2 mm diameter punch of the tissue. Tissues were lysed in a modified RIPA buffer (described above), sonicated 10 s at 10 A (Ultrasonic Processor GE 70, Cole Parmer, Vernon Hills, IL), and then centrifuged at 11,000 rpm for 15 min at 4oC. After quantification of protein concentration in the supernatant using a DC assay (Bio-Rad), 0.2 mg of total protein was transferred to 12–230 kDa Separation Modules (Protein Simple) for separation and detection using mouse anti-TH (1:50; Millipore #MAB318, RRID: AB_2201528) and mouse anti-actin (1:50; Cell Signal Technology mAb 3700S, RRID: AB_2242334) primary antibodies, and the Anti-Mouse Detection Module (Protein Simple,). The relative amount of TH protein was determined using the areas under peaks from the chemiluminescence chromatograms provided by the Compass for SW software (version 4.0.0, Protein Simple). TH values were normalized to actin before relating the injected left side to the non-injected right side.
For protein detection in primary cortical neuron cultures, cells were lysed in RIPA buffer (above) and 1 mg protein was loaded onto WES 25-well plates for separation of 12–230 kDa proteins (ProteinSimple). MANF and actin proteins were detected using rabbit anti-MANF (YenZym) and mouse anti-actin (Abcam #ab3280) as primary antibodies, and HRP-conjugated anti-rabbit and anti-mouse secondary antibodies according to the instructions in the WES separation kit (ProteinSimple).
Biotinylation and DAT internalization
Coronal midbrain sections were isolated from male WT or DAT-iCre transgenic rats (line 1, 5, and 6). Midbrain sections (1 mm) were hemisected, and one half was treated with vehicle control and the other with 10 mM amphetamine for 30 min at 37°C in oxygenated artificial cerebral spinal fluid (aCSF, in mM: 126 NaCl, 3.5 KCl, 1.3 MgCl2, 2 CaCl2, 1.2 NaH2PO4, 25 NaHCO3, 10 glucose). The tissue was then rapidly chilled to halt subsequent protein trafficking and all membrane proteins were biotinylated with 2 mg/mL of a cell-impermeable biotinylation reagent, sulfosuccinimidyl 2-(biotinamido) methyl-1,3-dithiopropionate (Pierce). Lysates were made of these preparations and biotinylated proteins were isolated with Neutravidin beads (Pierce) and separated on an SDS gel and probed with antibodies directed at either DAT (Millipore, MAB369) or EAAT3 (EAAC11-A, Alpha Diagnostics, San Antonio, TX).
Electrophysiology
Animals were deeply anesthetized with isoflurane and transcardially perfused with an ice-cold modified aCSF (m-aCSF) containing (in mM) 93 NMDG, 93 HCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 Glucose, 5 Na-ascorbate, 2 Thiourea, 3 Na-pyruvate, 10 MgSO4, 0.5 CaCl2. Brains were rapidly removed, and horizontal midbrain slices (200–220 mm) were made using a vibratome (Leica VT-1000S). Slices were then incubated in aCSF containing (mM) 92 NaCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 Glucose, 5 Na-ascorbate, 2 Thiourea, 3 Na-pyruvate, 2 MgSO4, 0.5 CaCl2 (32–34oC) for 15–30 min. Following this, the holding chamber was kept at room temperature for the duration of the experiment. Slices were transferred to a recording chamber and superfused (2–3 mL/min) with aCSF containing (in mM) 126 NaCl, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, 21.4 NaHCO3, 11.1 glucose main- tained at 32–34oC. All solutions were continually oxygenated (95% oxygen, 5% carbon dioxide). Glass pipettes (tip resistance 2–4 MU) were used for whole cell recordings and were filled with an intracellular solution containing (mM) 115 K-gluconate, 20 KCl, 1.5 MgCl2, 0.025 EGTA, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP, 10 Na2-phosphocreatine (pH 7.2–7.3, ~290 mOsm/kg).
Cells were identified using an upright microscope with differential interference optics (Olympus BX61WI) equipped with a scanning confocal system (Olympus Fluoview F1000). Images were acquired using Olympus Fluoview software (version 3.0).
Electrophysiology data were acquired using an Axopatch 200 B amplifier (Molecular Devices, San Jose, CA) in either voltage clamp or current clamp mode. Data were filtered at 5 kHz and digitized at 10 kHz using a National Instruments USB-6221 digitizer (Austin, TX). WinWCP software (University of Strathclyde, Glasgow, UK) was used to collect electrophysiological data. Recordings of spontaneous cell firing rates were performed using zero-current mode. During whole cell voltage clamp recordings, neurons were maintained at a holding potential of −60 mV. Holding currents reported represent the amount of current injected to maintain the cell at −60 mV. Input resistance was calculated using −10 mV steps during voltage clamp recordings.
Voltammetry
Slice preparation
Rats were anesthetized with isoflurane and the brains rapidly removed and placed in ice-cold m-aCSF. Coronal hemisections (280 mm) containing the striatum were cut using a vibratome (Leica VT1000S). Slices were incubated in standard oxygenated aCSF at 34–35°C for 10–15 min, then allowed to stabilize at room temperature for > 30 min prior to initiating recordings. During recordings, slices were continuously superfused with aCSF using a peristaltic pump (Cole-Parmer Instruments, Vernon Hills, IL) at a rate of 2 mL/min, and maintained at 30–32°C.
Voltammetric recordings
Carbon fibers (7 mm diameter) were vacuum-aspirated into borosilicate pipette glass. Pipettes were pulled using a conventional patch-pipette puller, and the ends of the carbon fiber were cut to allow ~25–30 mm exposed length protruding from the pipette tip. Pipettes were back filled with a 4 M potassium acetate/150 mM KCl solution and connected to a standard patch pipette holder/head stage assembly. A patch clamp amplifier (HEKA EVA-8, Digitimer, Ft. Lauderdale, FL) was used to deliver voltage and measure current from the head stage. Voltammetric scan and stimulation timing protocols were performed using PCI-based A/D boards (National Instruments) and LabView-based software (TarHeel CV, University of North Carolina, Chapel Hill, NC). Scans consisted of sweeps from −0.4 to 1.3 V and back to −0.4 V, at a rate of 400 V/s, and were obtained at 50 Hz. A 5 s control period preceded each electrically-evoked response and was used to obtain a background current that was digitally subtracted from the current obtained during the peak of the response. All signals used in analyses matched the expected voltammetric profile for dopaminergic neurons.
Electrically-evoked signals in brain slices
Under stereoscopic magnification, carbon fibers were lowered to a depth of ~100 mm in the dorsolateral striatum. A bipolar stimulating electrode was positioned ~75–100 from the carbon fiber. A single, constant current pulse (10–180 mA, 1 ms duration) was delivered every 90 s to elicit dopamine release. A pre-drug input-output curve was first generated by varying the stimulus intensity. Following construction of the input-output curve, 3–4 baseline signals were obtained at maximal stimulus intensity. A cocaine (5 mM) solution was then added into the recording chamber for 10–15 min, until a stable drug response was observed. Signals were analyzed using a non-linear regression analysis that distinguishes dopamine release from DAT-mediated uptake (Hoffman et al., 2016).
QUANTIFICATION AND STATISTICAL ANALYSIS
Experimental parameters for all in vivo work can be found in Table S1. All results are shown as mean ± standard error (SE) unless otherwise stated in figure legends or Results. Statistical analysis was performed using Prism software (GraphPad, San Diego, CA). For comparison of two experimental groups, Student’s t test was applied. One-way ANOVA and Tukey’s test were used to compare the outcome from more than two treatment groups. For repeated voltammetry measures, a two-way ANOVA together with Holm-Sidak’s test were used to test for differences between WT and DAT-iCre rats. A p value < 0.05 was considered significant. Data points identified as statistical outliers with single Grubbs’ test were removed from the dataset.
Supplementary Material
Figure 1.
Experimental Approaches for Induction of CRISPR-Cas9-Mediated Knockout of Genes in the Rat Midbrain Targeted gene disruption in the adult rat midbrain was achieved using four different approaches. (A) WT rats received co-injections of AAV vectors expressing Cas9 or gRNAs specific to rat tyrosine hydroxylase (Th) gene. (B) The midbrain of transgenic rats (DAT-iCre) expressing Cre in their dopaminergic (DAT-ex- pressing) neurons was targeted with Cas9 and Cre-dependent Th gRNA to modify the Th gene only in dopaminergic neurons. (C) Co-injections of AAV iCre and AAV gRNAs for Th or Manf were performed to modify the Th or Manf genes in transgenic rats expressing Cre- dependent Cas9 or nickase. (D) A cross of the DAT-iCre and LSL-Cas9 rats generated rats with Cas9 expression limited to dopaminergic neurons, allowing for sequence-specific alterations of the genome in dopaminergic neurons with an injection of a single AAV carrying gRNAs.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
|
| ||
| Mouse anti-tyrosine hydroxylase | Millipore | CAT#MAB318; RRID: AB_2201528 |
| Rabbit anti-MANF | YenZym | N/A |
| Rabbit anti-Cre-recombinase | BioLegend | CAT#908001; RRID: AB_2565079 |
| Mouse anti-FLAG | Sigma-Aldrich | CAT#F1804; RRID: AB_262044 |
| Rabbit anti-tyrosine hydroxylase | Millipore | CAT#AB152; RRID: AB_390204 |
| Mouse anti-actin | Abcam | CAT#ab3280; RRID: AB_303668 |
| Mouse anti-actin | Cell Signal Technology | CAT#mab3700S; RRID: AB_2242334 |
| Goat anti-mouse secondary | Protein Simple | CAT#042-205 |
| Goat anti-rabbit Alexa Fluor 568 | Invitrogen | CAT#A11011; RRID: AB_143157 |
| Goat anti-rabbit Alexa Fluor 568 | Invitrogen | CAT#A11036; RRID: AB_10563566 |
| Goat anti-mouse Alexa Fluor 568 | Invitrogen | CAT#A11004; RRID: AB_2534072 |
| Goat anti-mouse Alexa Fluor 488 | Invitrogen | CAT#A11029; RRID: AB_2534088 |
| Goat anti-mouse Alexa Fluor 680 | Invitrogen | CAT#A21058; RRID: AB_2535724 |
|
| ||
| Bacterial and Virus Strains | ||
|
| ||
| NEB Stable competent cells | New England Biolabs | CAT#C3040I |
| Stbl3 competent cells | Thermo Fisher Scientific | CAT#C737303 |
| AAV-Cas9 | This paper | NIDA IRP CORE FACILITY, AAV1, pOTTC1077, Addgene 60957 |
| AAV-TH gRNAs | This paper | NIDA IRP CORE FACILITY, AAV1, pOTTC1088 |
| AAV-nickase | This paper | NIDA IRP CORE FACILITY, AAV1, pOTTC1124 |
| AAV-Nuc-flox(mCherry)-eGFP | This paper | NIDA IRP CORE FACILITY, AAV1, pOTTC1032 |
| AAV-LSL-TH gRNA | This paper | NIDA IRP CORE FACILITY, AAV1, pOTTC1179 |
| AAV-iRFP-Flpo | This paper | NIDA IRP CORE FACILITY, AAV1, pOTTC1440 |
| AAV-iRFP-iCre | This paper | NIDA IRP CORE FACILITY, AAV1, pOTTC1442 |
| AAV-iCre | This paper | NIDA IRP CORE FACILITY, AAV1, pOTTC556 |
| AAV-MANF gRNAs | This paper | NIDA IRP CORE FACILITY, AAV1, pOTTC1388 |
| AAV-control gRNAs | This paper | NIDA IRP CORE FACILITY, AAV1, pOTTC1270 |
|
| ||
| Biological Samples | ||
|
| ||
| Rat brain tissue | This study | N/A |
|
| ||
| Critical Commercial Assays | ||
|
| ||
| Anti-mouse detection module | Protein Simple | CAT#DM-002 |
| 12-230 kDA Separation Module, 25 capillary cartridges | Protein Simple | CAT#SM-W002 |
| T7 Endonuclease I | New England Biolabs | CAT#M0302S |
| Resolvase (Guide-it Mutation Detection Kit) | Takara | CAT#631448 |
| EZ Standard Pack 1 | Protein Simple | PS-ST01EZ-8 |
|
| ||
| Experimental Models: Cell Lines | ||
|
| ||
| PC-12 cells | ATCC | ATCC CRL-1721; RRID: CVCL_0481 |
| HEK293 (used for AAV production) | Dr. Xiao Xiao (UNC) | N/A |
|
| ||
| Experimental Models: Organisms/Strains | ||
|
| ||
| DAT-iCre on Long Evans background | This paper | LE-Tg(Slc6a3-iCre)1Ottc; RGD ID: 9588572; RRRC ID: 730 |
| DIO-mCherry on Long Evans background | This paper | LE-Tg(DIO-mCherry)2Ottc; RGD ID: 8693598; RRRC ID: R687 |
| LSL-Cas9 on Long Evans background | This paper | LE-(ROSA)26 em1(CAG-Cas9)Ottc; RGD ID: 1320822; RRRC ID: 833 |
| LSL-nickase on Long Evans background | This paper | LE.Cg-(ROSA)26 em1(CAG-Cas9*D10A)Ottc; RGD ID: 13602097; RRRC ID: 834 |
|
| ||
| Oligonucleotides | ||
|
| ||
| Manf for:cggttgtgctactacattgga | IDT | N/A |
| rev: gggccagaggcttcgatac | ||
| probe: ccacagatgatgccgccaccaag | ||
| Polr2a for: tagtcctacctactccccaacttc | IDT | N/A |
| rev: agtagccaggagaagtgggag | ||
| probe: actcgcccaccagtcccacctact | ||
| Ube2i for: gccaccactgtttcatccaaa | IDT | N/A |
| rev: gccgccagtccttgtcttc | ||
| probe: cgtgtatccttctggcacagtgtgc | ||
| TH Fwd: GAGAT GGCTACCACTAGCTCGAG | IDT | N/A |
| TH Rev: GAGCCTGAGACAGGGTGATCC | IDT | N/A |
| Rosa26 Fwd: GGGATTCCTCCTTGAGTTGTGGC | IDT | N/A |
| Rosa26 Rev: GGAGGAGATATTCATCTGTAAACCATT AACAGG | IDT | N/A |
| MANF Fwd: GCACTTAGGGGGTTCAGTGTATTC | IDT | N/A |
| MANF Rev: CTACAAAAGCTGATGCTTCACCAGG | IDT | N/A |
| TH seq F: CCAAAGGTTATAGTTCTAACATGAGCCCTTAG | IDT | N/A |
| Rosa 26 seq R: AGCTACAGCCTCGATTTGTGGTG | IDT | N/A |
| DAT-iCre Fwd: CGCACAAGCTGGGAGCTAATGTGAA | IDT | N/A |
| DAT-iCre Rev: CTTCCAGGTGTGTTCAGAGAAG | IDT | N/A |
| LSL-Cas9 50 junction Fwd: GGCTCCTCAGAGAGCCTCG | IDT | N/A |
| LSL-Cas9 50 junction Rev: AGTTATGTAACGCGGAACTC CATATATGG | IDT | N/A |
| LSL-Cas9 30 junction Fwd: CTGTGCCTTCTAGTTGCCAGCC | IDT | N/A |
| LSL-Cas9 30 junction Rev: TTCTGCATTCCAGAAGGAACTA ACTTTTATAGAG | IDT | N/A |
| LSL-Nickase 50 junction Fwd: GCTCAGAAAACTGGCCTTTG | IDT | N/A |
| LSL-Nickase 30 junction Fwd: GAGGCGCTCACAGGTTCC | IDT | N/A |
|
| ||
| Recombinant DNA | ||
|
| ||
| PX551 | Swiech et al., 2015 | Addgene plasmid # 60957 |
| pAAV MeCP2 HA-SpCas9n(D10A) | This paper | Addgene plasmid # 112719 |
| pAAV TH gRNA A+B pair EF1a EGFP | This paper | Addgene plasmid # 113155 |
| pAAV Rosa26 gRNA A+B EF1a EGFP | This paper | Addgene plasmid # 113156 |
| pAAV MANF gRNA A+B EF1a EGFP | This paper | Addgene plasmid # 113157 |
| pAAV (flox-stop) TH gRNA A EF1a eGFP | This paper | Addgene plasmid # 113158 |
| pAAV CMV-IE Nuc-iRFP-2A-iCre | This paper | Addgene plasmid # 112683 |
| pAAV CMV-IE Nuc-iRFP-2A-Flpo | This paper | Addgene plasmid # 112684 |
| pAAV EF1a iCre | This paper | Addgene plasmid # 89760 |
| pAAV EF1a Nuc-flox(mCherry)-EGFP | This paper | Addgene plasmid # 112677 |
| pX458 with rat Rosa26 gRNA A | This paper | Addgene plasmid # 113161 |
| prRosa26v1 LSL FLAG-SpCas9n NeoR | This paper | Addgene plasmid # 113162 |
| prRosa26v2 LSL FLAG-SpCas9 | This paper | Addgene plasmid # 113163 |
| pmU6(loxP-STOP-loxP) BbsI gRNA | This paper | Addgene plasmid # 113160 |
ACKNOWLEDGEMENTS
This study was supported by the Intramural Programs of the National Institute on Drug Abuse and National Institute of Mental Health. M.A., A.D., and J.E.A. were supported by the Academy of Finland. M.A. and A.D. were supported by 3iRegeneration Tekes. J.E.A. was supported by the University of Helsinki Doctoral Program in Drug Research. Transgenic LSL-Cas9 nickase rat production was supported by the UT Southwestern Mutant Rat Resource in Dallas. The NIDA Histology Core and Electrophysiology Core (EVEC) provided technical support. Dr. Francois Vautier oversaw the breeding of the transgenic rats. Doug Howard produced the AAV vectors. Reinis Svarcbahs assisted with the molecular biology in generating vectors. Dr. Deon Harvey made comments on the manuscript. Coronal brain schematics in figures are based on Swanson (2018).
Footnotes
DECLARATION OF INTERESTS
F.K.H. serves as a project consultant for GenomeDesigns Laboratory, LLC (GDL), but GDL had no involvement with this study.
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures and three tables and can be found with this article online at https://doi.org/10.1016/j.neuron.2019.01.035.
REFERENCES
- Bae S, Kweon J, Kim HS, and Kim JS (2014). Microhomology-based choice of Cas9 nuclease target sites. Nat. Methods 11, 705–706. [DOI] [PubMed] [Google Scholar]
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, and Horvath P. (2007). CRISPR provides acquired resis- tance against viruses in prokaryotes. Science 315, 1709–1712. [DOI] [PubMed] [Google Scholar]
- Brinkman EK, Chen T, Amendola M, and van Steensel B. (2014). Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 42, e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KM, Medrano GA, Jaichander P, Chaudhary J, Waits AE, Nobrega MA, Hotaling JM, Ober C, and Hamra FK (2015). Targeted germline modifications in rats using CRISPR/Cas9 and spermatogonial stem cells. Cell Rep. 10, 1828–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SW, Kim S, Kim JM, and Kim JS (2013). Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 230–232. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, and Zhang F. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed RB, and Goldberg MS (2018). New developments in genetic rat models of Parkinson’s disease. Mov. Disord. 33, 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna JA, and Charpentier E. (2014). Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096. [DOI] [PubMed] [Google Scholar]
- Duty S, and Jenner P. (2011). Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 164, 1357–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek B, and Youn J. (2016). Rodent models in neuroscience research: is it a rat race? Dis. Model. Mech. 9, 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn JD, Smith AR, Patel MC, Shaw L, Youniss MR, van Heteren J, Dirstine T, Ciullo C, Lescarbeau R, Seitzer J, et al. (2018). A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 22, 2227–2235. [DOI] [PubMed] [Google Scholar]
- Gasiunas G, Barrangou R, Horvath P, and Siksnys V. (2012). Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. USA 109, E2579–E2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RA, Weinstock GM, Metzker ML, Muzny DM, Sodergren EJ,Scherer S, Scott G, Steffen D, Worley KC, Burch PE, et al. ; Rat Genome Sequencing Project Consortium (2004). Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 428, 493–521. [DOI] [PubMed] [Google Scholar]
- Henderson MJ, Richie CT, Airavaara M, Wang Y, and Harvey BK (2013). Mesencephalic astrocyte-derived neurotrophic factor (MANF) secretion and cell surface binding are modulated by KDEL receptors. J. Biol. Chem. 288, 4209–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Spivak CE, and Lupica CR (2016). Enhanced dopamine release by dopamine transport inhibitors described by a restricted diffusion model and fast-scan cyclic voltammetry. ACS Chem. Neurosci. 7, 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Wöhr M, and Alenina N. (2017). Comeback of the rat in biomedical research. ACS Chem. Neurosci. 8, 900–903. [DOI] [PubMed] [Google Scholar]
- Hou Z, Zhang Y, Propson NE, Howden SE, Chu LF, Sontheimer EJ, and Thomson JA (2013). Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 110, 15644–15649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DB, and Harvey BK (2017). Assaying the stability and inactivation of AAV serotype 1 vectors. Hum. Gene Ther. Methods 28, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DB, Powers K, Wang Y, and Harvey BK (2008). Tropism and toxicity of adeno-associated viral vector serotypes 1, 2, 5, 6, 7, 8, and 9 in rat neurons and glia in vitro. Virology 372, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizu T, Higo S, Masumura Y, Kohama Y, Shiba M, Higo T, Shibamoto M, Nakagawa A, Morimoto S, Takashima S, et al. (2017). Targeted genome replacement via homology-directed repair in non-dividing cardiomyocytes. Sci. Rep. 7, 9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivics Z, Izsvá k Z, Medrano G, Chapman KM, and Hamra FK (2011). Sleeping Beauty transposon mutagenesis in rat spermatogonial stem cells. Nat. Protoc. 6, 1521–1535. [DOI] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, and Charpentier E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, East A, Cheng A, Lin S, Ma E, and Doudna J. (2013). RNA-programmed genome editing in human cells. eLife 2, e00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabadi AM, Ousterout DG, Hilton IB, and Gersbach CA (2014). Multiplex CRISPR/Cas9-based genome engineering from a single lentiviral vector. Nucleic Acids Res. 42, e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseberth WC, Brettingen NT, Lohse JK, and Sandgren EP (1999). Ubiquitous expression of marker transgenes in mice and rats. Dev. Biol. 214, 128–138. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kato-Itoh M, Yamaguchi T, Tamura C, Sanbo M, Hirabayashi M, and Nakauchi H. (2012). Identification of rat Rosa26 locus enables generation of knock-in rat lines ubiquitously expressing tdTomato. Stem Cells Dev. 21, 2981–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Qiu Z, Shao Y, Chen Y, Guan Y, Liu M, Li Y, Gao N, Wang L, Lu X, et al. (2013a). Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat. Biotechnol. 31, 681–683. [DOI] [PubMed] [Google Scholar]
- Li W, Teng F, Li T, and Zhou Q. (2013b). Simultaneous generation and germline transmission of multiple gene mutations in rat using CRISPR-Cas systems. Nat. Biotechnol. 31, 684–686. [DOI] [PubMed] [Google Scholar]
- Lindholm P, Peränen J, Andressoo JO, Kalkkinen N, Kokaia Z, Lindvall O, Timmusk T, and Saarma M. (2008). MANF is widely expressed in mammalian tissues and differently regulated after ischemic and epileptic insults in rodent brain. Mol. Cell. Neurosci. 39, 356–371. [DOI] [PubMed] [Google Scholar]
- Liu Z, Brown A, Fisher D, Wu Y, Warren J, and Cui X. (2016). Tissue specific expression of Cre in rat tyrosine hydroxylase and dopamine active transporter-positive neurons. PLoS ONE 11, e0149379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Shen B, Zhang X, Lu Y, Chen W, Ma J, Huang X, and Zhang L. (2014). Heritable multiplex genetic engineering in rats using CRISPR/Cas9. PLoS ONE 9, e89413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, and Church GM (2013). RNA-guided human genome engineering via Cas9. Science 339, 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister B, and Elde R. (1993). Dopamine transporter mRNA in neurons of the rat hypothalamus. Neuroendocrinology 58, 388–395. [DOI] [PubMed] [Google Scholar]
- Nishiyama J, Mikuni T, and Yasuda R. (2017). Virus-mediated genome editing via homology-directed repair in mitotic and postmitotic cells in mammalian brain. Neuron 96, 755–768.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill B, Patel JC, and Rice ME (2017). Characterization of optically and electrically evoked dopamine release in striatal slices from digenic knock-in mice with DAT-driven expression of channelrhodopsin. ACS Chem. Neurosci. 8, 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CC, Chen H, Flagel SB, Geurts AM, Richards JB, Robinson TE, Solberg Woods LC, and Palmer AA (2014). Rats are the smart choice: rationale for a renewed focus on rats in behavioral genetics. Neuropharmacology 76, 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, et al. (2014). CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 159, 440–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan BS, and Majumdar SS (2016). An efficient method for generation of transgenic rats avoiding embryo manipulation. Mol. Ther. Nucleic Acids 5, e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, and Zhang F. (2013). Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy S, Tesson L, Menoret S, Usal C, De Cian A, Thepenier V, Thinard R, Baron D, Charpentier M, Renaud JB, et al. (2014). Efficient gene targeting by homology-directed repair in rat zygotes using TALE nucleases. Genome Res. 24, 1371–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy S, Chenouard V, Tesson L, Usal C, Ménoret S, Brusselle L, Heslan JM, Nguyen TH, Bellien J, Merot J, et al. (2017). Generation of gene-edited rats by delivery of CRISPR/Cas9 protein and donor DNA into intact zygotes using electroporation. Sci. Rep. 7, 16554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson TE, Chapman KM, Tenenhaus Dann C, Hammer RE, and Hamra FK (2009). Sterile testis complementation with spermatogonial lines restores fertility to DAZL-deficient rats and maximizes donor germline transmission. PLoS ONE 4, e6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander JD, and Joung JK (2014). CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen MH, Richie CT, Harvey BK, Männistö PT, Maguire-Zeiss KA, and Myöhänen TT (2014). The beneficial effect of a prolyl oligopeptidase inhibitor, KYP-2047, on alpha-synuclein clearance and autophagy in A30P transgenic mouse. Neurobiol. Dis. 68, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Wang L, Guo N, Wang S, Yang L, Li Y, Wang M, Yin S, Han H, Zeng L, et al. (2018). Cas9-nickase-mediated genome editing corrects hereditary tyrosinemia in rats. J. Biol. Chem. 293, 6883–6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe MJ, Marchant NJ, Whitaker LR, Richie CT, Zhang YJ, Campbell EJ, Koivula PP, Necarsulmer JC, Mejias-Aponte C, Morales M, et al. (2017). Lateral hypothalamic GABAergic neurons encode reward predictions that are relayed to the ventral tegmental area to regulate learning. Curr. Biol. 27, 2089–2100.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Zhang J, Wu H, Wang J, Ma K, Li Z, Zhang X, Zhang P, and Huang X. (2013). Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 23, 720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshek DR, Kim J, Hu€bner MR, Spergel DJ, Buchholz F, Casanova E, Stewart AF, Seeburg PH, and Sprengel R. (2002). Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis 32, 19–26. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Tsunekawa Y, Hernandez-Benitez R, Wu J, Zhu J, Kim EJ, Hatanaka F, Yamamoto M, Araoka T, Li Z, et al. (2016). In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integra- tion. Nature 540, 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW (2018). Brain maps 4.0-structure of the rat brain: An open access atlas with global nervous system nomenclature ontology and flatmaps. J. Comp. Neurol. 526, 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M, and Zhang F. (2015). In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat. Biotechnol. 33, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo DG, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, et al. (2016). A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92, 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiscornia G, Tergaonkar V, Galimi F, and Verma IM (2004). CRE recombinase-inducible RNA interference mediated by lentiviral vectors. Proc. Natl. Acad. Sci. USA 101, 7347–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida J, Matsusaka T, Ohtsuka M, Miura H, Okuno Y, Asanuma K, Nakagawa T, Yanagita M, and Mori K. (2016). Establishment of nephrin reporter mice and use for chemical screening. PLoS ONE 11, e0157497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutilainen MH, Báck S, Pörsti E, Toppinen L, Lindgren L, Lindholm P, Peränen J, Saarma M, and Tuominen RK (2009). Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J. Neurosci. 29, 9651–9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, and Jaenisch R. (2013). One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warming S, Costantino N, Court DL, Jenkins NA, and Copeland NG (2005). Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33, e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, and Jaenisch R. (2013). One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell 154, 1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wen Y, and Guo X. (2014). CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum. Mol. Genet. 23 (R1), R40–R46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.