INTRODUCTION
Yeasts of the genus Malassezia are unique among the fungal kingdom as the only species to form part of the normal human cutaneous commensal flora. In addition, Malassezia species are able to cause several cutaneous diseases, systemic disease in suitably predisposed humans, and dermatitis in a wide range of animals. Thus, they exist at the very interface between commensal and pathogen and, as such, their interaction with the human immune system is of great interest.
History
The study of the genus Malassezia has been dogged by controversy since it was first described in 1846 by Eichstedt (118). Dissent has occurred over when the organism was first grown, the optimal culture medium, the relationship between the different morphological and colonial variants of the organism, the genus to which it should be assigned and with what name, and the role it plays in a variety of cutaneous diseases.
Despite being described in 1846, the first successful isolation of the organism is generally accepted to be by Panja in 1927 (324), although several previous authors claimed to have grown the organism in vitro (83, 114, 233, 282). The difficulty in culturing the organism was explained by Benham in 1939, when she observed the need for a “fatty substance” in the growth medium (45). Once this lipid requirement had been established, it paved the way for the formulation of various culture media that could reliably recover and maintain the organism (135, 245, 288, 325, 444, 445), enabling work on the taxonomy, physiology, and biochemistry of the genus to be undertaken.
Taxonomy
The taxonomy and nomenclature of Malassezia species has been confused and chaotic until very recently. Malassezia species are dimorphic, existing in both yeast and mycelial phases, and this confounded much of the early work on the organism, since many people believed that the yeast and mycelial forms were distinct organisms, reflected by their inclusion in two separate genera: Pityrosporum for the yeast form and Malassezia for the mycelial form. Additionally, the yeast cell shape is variable and several groups considered the two yeast cell shapes to be separate species: Pityrosporum orbiculare, having round cells, and Pityrosporum ovale, having oval cells.
Eichstedt (118) was the first to describe the fungus associated with lesions of pityriasis versicolor (PV) in 1846, but no name was given to it until 1853, when Robin designated it “Microsporon furfur” (367). Since then it has also been placed in the genera Cryptococcus (363), Saccharomyces (56), Pityrosporum (381), Dermatophyton (114), and Monilia (452). Sabouraud was the first person to suggest that the yeast and mycelial forms might be related (381), but it was not until 1927 that Panja classified them within the same genus (324). The first official taxonomic classification placed them in the genus Pityrosporum and defined two species—P. ovale and P. pachydermatis, associated with animals (260). Gordon then added another species, P. orbiculare, differentiating this species on the basis of its round cell shape (159). By 1970, three species were recognized, P. ovale, P. orbiculare, and P. pachydermatis, and although it was accepted that there was a relationship between the yeast form and the mycelial form, conversion between them had never been demonstrated and so the two distinct genera were maintained (407). This situation was finally resolved in 1977, when three independent groups succeeding in inducing the yeast to produce hyphae in vitro (115, 303, 383). Using a variety of culture conditions, they produced hyphae that were indistinguishable from those on strains seen on patients suffering from PV. It was also observed that both the round and oval yeast forms could produce hyphae, and this led to the suggestion that the round and oval yeast forms and hyphae were simply stages in the life cycle of a single organism (383). The ability to induce the yeast to form mycelial elements paved the way for the unification of the two genera in 1986, with the acceptance of the species names Malassezia furfur (Robin) Baillon (including P. orbiculare, P. ovale, and M. furfur) and Malassezia pachydermatis (including P. pachydermatis) (79). Despite this, many workers maintained the use of the names P. ovale and P. orbiculare and continued to differentiate strains on the basis of cellular and colonial morphologies (128, 288, 402). In 1990, Simmons and Gueho defined another species, M. sympodialis, on the basis of its lower G+C content (54% compared with 66% for M. furfur) and the presence of sympodial budding (402). Cunningham et al. differentiated three serovars of M. furfur, A, B, and C, which had culture and morphological differences that corresponded to serological differences determined by cell surface antigens (101).
Thus, in the early 1990s the taxonomy of the genus Malassezia was still chaotic, with different groups tending to favor their own classification scheme, resulting in an inability to compare work carried out by different groups. This chaos was finally resolved with a seminal publication in 1995 by Guillot and Gueho (170). They assembled 104 isolates of Malassezia species encompassing all the different classifications favoured by different groups and carried out sequencing of the large-subunit rRNA and nuclear DNA complimentarity studies. On the basis of their results, they defined, and later named, seven species of Malassezia: M. furfur, M. sympodialis, M. obtusa, M. globosa, M. restricta, M. slooffiae, and M. pachydermatis (167). These currently accepted species and their corresponding names in other classifications are shown in Table 1. Several subsequent molecular studies have confirmed this classification and taxonomic grouping (173, 207, 267). The characteristics of the different species are summarized in Table 2.
TABLE 1.
Classifications of Malassezia, according to different authorsa
| Pre-1986 classification | Classification according to:
|
|||
|---|---|---|---|---|
| Midgley, 1989 (288) | Simmons and Gueho, 1990 (402) | Cunningham et al., 1990 (101) | Gueho et al., 1996 (167) | |
| Pityrosporum orbiculare | P. orbiculare | M. furfur serovar B | M. globosa | |
| Pityrosporum ovale | P. ovale form 1 | M. slooffiae | ||
| P. ovale form 2 | M. obtusa | |||
| P. ovale form 3 | M. sympodialis | M. furfur serovar A | M. sympodialis | |
| Malassezia furfur | M. furfur | M. furfur | ||
| M. furfur serovar C | M. restricta | |||
| Pityrosporum pachydermatis/P. canis | P. pachydermatis | M. pachydermatis | M. pachydermatis | |
Names on the same line of the table are taken as being synonyms. The seven species in the right-hand column of the table are the most recent and currently accepted classification.
TABLE 2.
Characteristics of the seven species of Malasseziaa
| Characteristic or testb
|
Result for:
|
||||||
|---|---|---|---|---|---|---|---|
| M. furfur | M. sympodialis | M. pachydermatis | M. globosa | M. slooffiae | M. restricta | M. obtusa | |
| Colony morphology and texture | Umbonate, usually smooth, soft, friable | Flat, smooth, shiny, soft | Pale convex, smooth, soft, friable | Rough, course, brittle | Finely folded, brittle | Dull, smooth, hard and brittle | Smooth, flat, sticky |
| Colony color | Cream | Cream to buff | Cream | Cream to buff | Cream to buff | Cream | Cream |
| Cell shape and size | Elongated, oval or spherical, 6 μm | Ovoid, globose, 2.5–5 μm long | Cylindrical, 2.5–4.0 μm long | Spherical, 6–8 μm in diameter | Cylindrical, 1.5–3.5 μm long | Spherical, oval, 2–4 μm | Cylindrical, 4–6 μm |
| Budding pattern | Broad bud base | Some sympodial budding | Broad bud base, pronounced bud scar | Narrow bud base | Broad bud base | Narrow bud base | Broad bud base |
| G+C content (%) | 66.4 | 62.2 | 55.6 | 53.5 | 68.7 | 59.9 | 60.7 |
| Catalase reaction | + | + | v | + | + | − | + |
| DBB reaction | + | + | + | + | + | + | + |
| Urease reaction | + | + | + | + | + | + | + |
| Growth at 37°C | Good | Good | Good | Poor | Good | Poor | Poor |
| Max growth temp (°C) | 40–41 | 40–41 | 40–41 | 38 | 40–41 | 38 | 38 |
| Use as lipid source | |||||||
| Tween 20 | + | − | + | − | + | − | − |
| Tween 40 or 60 | + | + | + | − | + | − | − |
| Tween 80 | + | + | + | − | − | − | − |
| Cremophor EL | v | − | v | − | − | − | − |
| Ability to split esculin | − | + | v | − | − | − | + |
Because of the reclassification of the genus Malassezia and the definition of four new species, a great deal of the work which has already been done will have to be repeated. Although some studies used well-characterized strains that were deposited in culture collections and so can now be reclassified into the new species, many used clinical strains that were not stored, so it is not known how they relate to the new species. Therefore, much of the work reviewed here still cannot be interpreted in the context of the currently accepted species. A further problem noted recently is that there may not always be a direct correlation between the new species and strains classified by previous methods. Saadatzadeh et al. (379) found that strains of Malassezia classified as M. furfur serovar A, which should correspond to M. sympodialis, did not always do so. Therefore, the assumption that M. furfur serovars A, B, and C are synonymous with M. sympodialis, M. globosa, and M. restricta, respectively, may not always be true, and unless the specific strains used in previous studies have been tested and reclassified into the new taxonomic divisions, a direct relationship between strains from old and new classifications cannot be assumed. This problem is further highlighted by the strains deposited in culture collections. Strain 42132 from the American Type Culture collection was originally deposited as P. orbiculare (synonymous with M. globosa) but has been used by many workers as M. furfur, so it remains unclear in which of the new species this organism should be placed. Widespread acceptance and usage of the new classification and use of the new species names is desirable if this situation is to be resolved.
Structure, Physiology, and Biochemistry
Malassezia is able to exist in both yeast and mycelial forms, with the yeast being most commonly associated with normal skin. The yeast form also predominates in culture, although hyphae may be seen with some species (167, 288). Several groups have succeeded in inducing mycelial formation in vitro using a variety of media (115, 303, 383), although not all isolates of Malassezia are able to undergo this transformation (379).
Malassezia species undergo asexual reproduction by monopolar, enteroblastic budding from a characteristic broad base. The mother and daughter cell are divided by a septum, and the daughter cell separates by fission, leaving a bud scar or collarette through which successive daughter cells will emerge (7).
The cell wall of the genus Malassezia is poorly characterized. It is very thick in comparison with other yeasts (about 0.12 μm) and constitutes 26 to 37% of the cell volume (214). The major components of the cell wall are sugars (∼70%), protein (∼10%), and lipids (15 to 20%), with small amounts of nitrogen and sulfur (181, 439). Several workers suggested that the cell wall consisted of two layers (39, 67, 213) with indentations on the inner layer, while other workers have found multiple layers within the wall (401, 426). The most recent work on the cell wall has confirmed the presence of multiple layers, although two main layers were noted, which may explain the previous reports (293). This study also demonstrated the presence of an outer lamellar layer around the cell wall, which had previously been mentioned by other workers (234, 472) but never investigated. The lamellar layer was “membrane-like” with an electron-transparent middle enclosed by two electron-dense lines. The structure of the layer varied with different lipid sources in the medium and stained with Nile blue sulfate, suggesting that it contained lipid. The lamellar layer may play a role in adhesion of the organism to both human skin and indwelling catheters (293). The cytoplasmic membrane adheres closely to the inner surface of the cell wall and follows the indentations present (39, 293, 426, 468).
The number and shape of the mitochondria in each cell may vary (39), differing between the round and oval cell shapes (214). The nucleus has a well-defined limiting membrane (39) surrounded by a granular homogenous nucleoplasm. Vacuoles present in the cell contained lipid and varied in size according to the age of the cell (39).
The physiology of Malassezia species is poorly understood because problems with reliably culturing and maintaining the organism have hindered progress in this area. As early as 1939, Benham noted that Malassezia was unable to ferment sugars (45). The organism can use lipid as the sole source of carbon (301), does not require vitamins, trace elements, or electrolytes (279), and preferentially uses methionine as the sole sulfur source, but it can also use cystine or cysteine (71). It is able to use many amino acids, as well as ammonium salts, as nitrogen sources (279). Although the organism is normally grown in vitro under aerobic conditions, it is also able to grow under microaerophilic and anaerobic conditions (133).
The growth requirement of Malassezia for lipid was first noted in 1939 but was not studied in detail until Shifrine and Marr demonstrated the inability of the organism to form long-chain fatty acids due to a block in de novo synthesis of myristic acid, requiring the addition of preformed fatty acids (395). Subsequent work showed that the addition of most fatty acids with a carbon chain length greater than 10 supported growth and that it did not matter whether odd- or even-numbered carbon chain lengths were used (469). The lipid source used during growth affects the fatty acid composition of the organism, suggesting that the fatty acids are not used as energy sources but, rather, are incorporated directly into cellular lipids without being further metabolized (80). Wilde and Stewart further found that the lipids present on normal human scalps were able to fulfil the lipid requirement of the organism (469).
Malassezia species elaborate a range of enzymes and metabolites. They have lipolytic activity both in vitro (301, 459) and in vivo (85, 272), indicating the production of a lipase. The lipase is located in the cell wall and/or membrane sites in the cytoplasm (85, 349). Ran et al. (349) found that the pH optimum was 5.0 and that lipase production was greatest during the logarithmic phase of growth, perhaps demonstrating its importance in the hydrolysis of lipids for cell growth. In contrast, Plotkin et al. (339) found the pH optimum to be 7.5 but also found that lipase activity was greatest during active cell growth and correlated with substrate concentration. They concluded that there were at least three separate lipases in Malassezia which were essential for cell growth. Mayser et al. (277) studied the lipolytic activity of Malassezia on fatty acid esters and found that it had only minor substrate specificity, with the degree of hydrolysis being determined by the alcohol moiety. In vitro, Malassezia species also produce a phospholipase (360). This phospholipase activity is able to cause the release of arachidonic acid from HEp-2 cell lines (338). Since arachidonic acid metabolites are involved in inflammation in the skin (164), this has been suggested as a mechanism by which Malassezia species may trigger inflammation. Malassezia species produce an enzyme with lipoxygenase activity, as demonstrated by its ability to oxidize free and esterified unsaturated fatty acids, squalene, and cholesterol (304). The resultant production of lipoperoxides may damage cell membranes and consequently interfere with cellular activity—a mechanism that has been proposed to cause the alterations in skin pigmentation associated with PV (108). Cultures of Malassezia produce a characteristic “fruity” smell, first described by Van Abbe (445). Gas chromatographic-mass spectrometric analysis of the gas from the culture headspace of Malassezia grown in lipid containing medium showed it to consist of volatile gamma lactones. This characteristic was unique to Malassezia and was suggested as a possible way to differentiate this genus from others (240). Another metabolite produced is azelaic acid, a C9 dicarboxylic acid. It is produced when Malassezia is grown in the presence of oleic acid and is a competitive inhibitor of tyrosinase, an enzyme involved in the production of melanin (302). In addition to having antibacterial (190, 242) and antifungal (65) activity, azelaic acid inhibits the proliferation of several tumor cell lines (331) and decreases the production of reactive oxygen species in neutrophils by inhibiting cell metabolism (9).
SEROLOGICAL AND ANTIGENIC STUDIES OF MALASSEZIA
Serological Studies of Malassezia Species
Due to the taxonomic confusion which existed with this organism for many years, several groups used serology to determine whether there were any antigenic relationships between yeasts of different cell shapes and the yeast and mycelial phases. Sternberg and Keddie (418) made use of sera from patients with PV to examine the cross-reaction between M. furfur and P. orbiculare. Using tape strips of M. furfur from PV lesions and smears of P. orbiculare from cultures, they obtained fluorescence of “equal brilliance” when the serum was applied to the cells. From this, they concluded that the yeast and hyphal forms had common antigens. In 1979, further antigenic comparison of P. ovale and P. orbiculare was carried out (432). Rabbit antiserum to P. orbiculare was tested against soluble antigenic extracts prepared from both P. ovale and P. orbiculare in the Ouchterlony diffusion test. The reaction between the antiserum and the antigens yielded three bands for P. orbiculare and two bands for P. ovale. The two bands with P. ovale were lines of identity to two of the bands to P. orbiculare, suggesting that there were at least two common antigens. The anti-P. orbiculare antiserum was then labelled with fluorescein isothiocyanate and used in immunofluorescence studies against P. ovale and P. orbiculare, where identical staining was observed. When M. furfur from lesions was tested, staining was also positive and could be removed by absorbtion of the antiserum with cells of either P. ovale or P. orbiculare. Thus, antigenic identity was suggested for P. ovale, P. orbiculare, and M. furfur. A similar study was carried out by a second group of workers, examining the antigens of 18 P. ovale strains (463). Antisera were raised against three strains of P. ovale and tested against antigenic preparations from P. ovale and P. orbiculare by immunodiffusion. Two lines of identity were again noted for the two organisms. Chemical analysis of the antigenic preparations revealed no protein but large amounts of carbohydrate. Purified immunoglobulin G (IgG) fractions of antisera to P. ovale and P. orbiculare were also shown to react with cultured P. ovale and P. orbiculare and cells from PV lesions in a study by Faergemann et al. (138).
Quantitative immunoelectrophoretic techniques in one study revealed up to 63 antigenic components in Malassezia but failed to reveal any significant antigenic differences between P. ovale and P. orbiculare (72). Thus, by 1984, several studies had confirmed the antigenic identity of P. ovale, P. orbiculare, and M. furfur, lending support to the idea that they were all stages in the life cycle of the same organism. However, Midgley (288, 289) used immunoelectrophoresis and an enzyme-linked immunosorbent assay (ELISA) to study various morphological variants and found some antigens specific for the different species and forms that she defined. Takahashi et al. (428) also found three group-specific soluble antigens, using double diffusion, in three variants of Malassezia that were differentiated on the basis of cell shape and metabolic differences.
In 1986, the weight of evidence led to the unification of the different morphological forms within the species M. furfur (79). However, the ability to grow very distinct colony variants from the same site on human skin led workers to define three serovars of M. furfur (101). The serovars, designated A, B, and C, were distinguished on the basis of growth characteristics, colony morphology, and specific surface antigens. Production of antisera to the three colonial forms and absorbtion with homologous and heterologous strains indicated the presence of both serovar-specific and common surface antigens. Recent molecular work describing the six lipophilic species of Malassezia confirms that stable variants do exist within the genus and that they are sufficiently different to be classified as separate species. The finding that the three serovars of Malassezia and the variants defined by Midgley represent distinct species confirmed the validity of their differentiation.
The capacity of Malassezia species to stimulate the immune system is well documented, but its antigenicity in comparison to other organisms has not been well studied. Sohnle and Collins-Lech (410) examined four antigenic extracts from Malassezia and Candida albicans and compared their ability to stimulate the immune system using the lymphocyte transformation (LT) assay or skin tests. They found that 20 to 100 times more extracted protein from Malassezia than C. albicans was required to stimulate the cellular immune response in the assays. The protein content of the preparations was similar for the two organisms, and they suggested that Malassezia was less antigenic than C. albicans. This limited antigenicity was proposed as a reason for the lack of inflammation seen in PV.
Recently, work has been undertaken to analyze the antigens present on the mycelial phase of Malassezia (380). The mycelial phases of two strains were induced, and antisera were raised to yeast-mycelium mixtures. Absorbtions with homologous and heterologous yeast and mycelial cells were carried out to obtain antiserum specific to the mycelial phase. Antigens common to both the yeast and mycelium were demonstrated, but all the antigens on the mycelium were present on the yeast. Thus, mycelium-specific antigens were not found. The serovar-specific antigens present on the yeast cells were not present on the mycelia, and so the mycelia did not have any phase-specific antigens, at least not on the cell surface.
Analysis of the Antigens Present in Malassezia
Although many early studies attempted to examine serological relationships in Malassezia, it is only more recently that detailed studies of the antigenic composition of the organism have been carried out. Sera from patients with Malassezia-associated diseases have been used to perform immunoblots on antigenic preparations of Malassezia that have been electrophoresed to separate out antigens by molecular mass. In this way, the molecular masses of over 80 antigens of minor or major importance have been defined in Malassezia (192, 200, 204, 256, 312, 385). Major antigens are defined as those where more than 50% of patients’ sera bind in immunoblots, although the number of sera tested and the patients from which they was collected varied from study to study. The antigens of Malassezia are shown in Table 3.
TABLE 3.
Antigens found in Malassezia
| Antigen type | Mol mass (kDa) | % of patients’ sera bindinga | Disease | % of healthy subjects’ sera binding | Reference | Source of antigen | Other informationg |
|---|---|---|---|---|---|---|---|
| Major | 9 | 50 | AD | 0 | 200 | P. ovalee | Large nonprotein moiety |
| 73 | AD | 0 | 256 | P. ovale CBS 7854 | |||
| 13 | 73 | AD | 0 | 204 | P. orbiculare ATCC 42132 | ||
| 14.2 | 85 | AD | 0 | 192 | P. orbiculare ATCC 42132 | ||
| 15b | 85 | AD | 0 | 200 | P. ovalee | Large nonprotein moiety | |
| 85 | AD | 0 | 192 | P. orbiculare ATCC 42132 | |||
| 15.5 | 53 | AD | 0 | 192 | P. orbiculare ATCC 42132 | ||
| 17 | 59 | AD | 0 | 204 | P. orbiculare ATCC 42132 | ||
| 19 | 66 | AD | 0 | 192 | P. orbiculare ATCC 42132 | ||
| 20b | 70 | AD | NTc | 480 | M. furfur TIMM 2782 | Mal f 3; homology to peroxisomal membrane proteins of Candida and Aspergillus | |
| 21b | 72 | AD | NT | 480 | M. furfur TIMM 2782 | Mal f 2; homology to peroxisomal membrane proteins of Candida and Aspergillus | |
| 23b | 63 | AD | 0 | 385 | P. ovalee | ||
| 25b | 65 | AD | 4 | 200 | P. ovalee | ||
| 28 | 58 | AD | 0 | 204 | P. orbiculare ATCC 42132 | ||
| 62 | AD | 0 | 192 | P. orbiculare ATCC 42132 | |||
| 35 | 83 | AD | 0 | 320 | M. furfur TIMM 2782 | Mal f 4 | |
| 90 | SD | 20 | 292 | P. orbiculare | |||
| 37b | 70 | AD | 0 | 192 | P. orbiculare ATCC 42132 | Mal f 1 (388); membrane or secreted cell wall protein | |
| NSd | AD | 0 | 388 | M. furfur ATCC 42132 | |||
| 40b | 58 | AD | 0 | 192 | P. orbiculare ATCC 42132 | ||
| 46 | 85 | AD | 0 | 192 | P. orbiculare ATCC 42132 | ||
| 70 | ADf | 0 | 230 | M. globosa NUM 6006 | Glycoprotein (230, 489) | ||
| 52–54 | 75 | AD | 0 | 192 | P. orbiculare ATCC 42132 | ||
| 58 | 53 | AD | 0 | 192 | P. orbiculare ATCC 42132 | ||
| 65 | 67 | SD | 25 | 399 | M. furfur | ||
| 15 | PV | ||||||
| 66 | 100 | “Atopic” | 0 | 292 | P. orbiculare | ||
| 67 | 86 | AD | 0 | 204 | P. orbiculare ATCC 42132 | Protein (488). Expressed only on cell surface (487) | |
| 71 | AD | 0 | 192 | P. orbiculare ATCC 42132 | |||
| 70b | 100 | SD | 25 | 399 | M. furfur | ||
| 84 | PV | ||||||
| 76b | 70 | AD | 0 | 204 | P. orbiculare ATCC 42132 | ||
| 77 | 75 | AD | 0 | 192 | P. orbiculare ATCC 42132 | ||
| 82 | 75 | AD | 0 | 192 | P. orbiculare ATCC 42132 | ||
| 84b | 53 | SD | 0 | 399 | M. furfur | ||
| 86 | 78 | AD | 0 | 204 | P. orbiculare ATCC 42132 | ||
| 96 | 65 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 110b | 60 | AD | 0 | 312 | P. ovalee | ||
| 120b | 73 | Psoriasisf | 0 | 417 | P. ovale | ||
| >200 | 60 | AD | 0 | 312 | P. ovalee | ||
| Mannan | 77 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| Minor | 6 | <10 | AD | 0 | 256 | P. ovale CBS 7854 | |
| 10 | 25 | AD | 0 | 385 | P. ovalee | ||
| 13 | <10 | PV | 0 | 204 | P. orbiculare ATCC 42132 | ||
| <<14 | 47 | AD | 0 | 312 | P. ovalee | ||
| <14 | 27 | AD | 0 | 312 | P. ovalee | ||
| 14 | 16 | AD | 0 | 256 | P. ovale CBS 7854 | Polysaccharide cell wall component (488) | |
| 24 | AD | 0 | 351 | M. furfur ATCC 42132 | Mal f 9 | ||
| 15 | 16 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 30 | AD | 0 | 200 | P. ovalee | Large, nonprotein moiety | ||
| 16 | 25 | AD | 0 | 200 | P. ovalee | ||
| 16.2 | 40 | AD | 0 | 351 | M. furfur ATCC 42132 | Mal f 7 | |
| 17.2 | 48 | AD | 0 | 254 | M. furfur ATCC 42132 | Mal f 6; has sequence homology to cyclophilin from S. pombe | |
| 18 | <50 | AD | 0 | 312 | P. ovalee | ||
| 18.2 | 48 | AD | 0 | 254 | M. furfur ATCC 42132 | Mal f 5; protein | |
| 19.2 | 40 | AD | 0 | 351 | M. furfur ATCC 42132 | Mal f 8 | |
| 20 | 45 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 21 | 43 | AD | 0 | 204 | P. orbiculare ATCC 42132 | ||
| 13 | AD | 0 | 385 | P. ovalee | |||
| 22 | 45 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 23 | <10 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 10 | AD | 0 | 200 | P. ovalee | |||
| 24 | 23 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 48 | AD | 0 | 192 | P. orbiculare ATCC 42132 | |||
| 25 | 20 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 15 | SD | 0 | 399 | M. furfur | |||
| <10 | PV | 0 | 399 | M. furfur | |||
| 26 | 38 | AD | 0 | 385 | P. ovalee | ||
| 38 | AD | 0 | 192 | P. orbiculare ATCC 42132 | |||
| 27 | 42 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| <50 | AD | 0 | 312 | P. ovalee | |||
| 29 | 18 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 30 | 25 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 20 | AD | 4 | 200 | P. ovalee | |||
| 31 | 15 | AD | 0 | 192 | P. orbiculare ATCC 42132 | ||
| 15 | AD | 0 | 200 | P. ovalee | |||
| 32 | <10 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 33 | 10 | AD | 8 | 200 | P. ovalee | ||
| <50 | AD | 0 | 312 | P. ovalee | |||
| <10 | PV | 0 | 399 | M. furfur | |||
| 34 | 38 | AD | 0 | 385 | P. ovalee | ||
| 23 | AD | 0 | 256 | P. ovale CBS 7854 | |||
| 10 | AD | 0 | 200 | P. ovalee | |||
| 35b | 48 | AD | 0 | 192 | P. orbiculare ATCC 42132 | ||
| 35 | SD | 0 | 399 | M. furfur | |||
| <10 | PV | 0 | 399 | M. furfur | |||
| 36 | 10 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 37 | 10 | AD | 0 | 256 | P. ovale CBS 7854 | Protein (488) | |
| 38 | 46 | AD | 0 | 312 | P. ovalee | ||
| 40 | SD | 0 | 399 | M. furfur | |||
| 39 | <10 | AD | 0 | 200 | P. ovalee | ||
| 40 | 42 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 41 | <10 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 15 | SD | 0 | 399 | M. furfur | |||
| 42 | 38 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 43 | 18 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 35 | SD | 0 | 39 | M. furfur | |||
| <10 | PV | ||||||
| 45 | 18 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 20 | AD | 4 | 200 | P. ovalee | |||
| 50 | 38 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 52 | 30 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 54 | 14 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 55 | 15 | AD | 8 | 200 | P. ovalee | ||
| 56 | 10 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| <10 | PV | 0 | 399 | M. furfur | |||
| 60 | 10 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 64 | 16 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 68 | <50 | AD | 0 | 312 | P. ovalee | ||
| 70 | 15 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 71 | <10 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 72 | 40 | AD | 8 | 200 | P. ovalee | ||
| 73 | 42 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 10 | SD | 0 | 399 | M. furfur | |||
| <10 | PV | 0 | |||||
| 76 | <10 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 83 | 35 | AD | 0 | 200 | P. ovalee | ||
| 84 | 15 | PV | 0 | 399 | M. furfur | ||
| 87 | 43 | AD | 0 | 192 | P. orbiculare ATCC 42132 | ||
| 90 | <10 | PV | 0 | 204 | P. orbiculare ATCC 42132 | ||
| 94 | 30 | AD | 0 | 204 | P. orbiculare ATCC 42132 | ||
| 20 | SD | 25 | 399 | M. furfur | |||
| 15 | PV | ||||||
| 97 | 10 | SD | 0 | 417 | P. ovale | ||
| 100 | 47 | Psoriasis | 0 | 417 | P. ovale | ||
| 110 | 10 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 115 | 35 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 120 | 20 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 125 | 10 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 130 | 10 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| <50 | AD | 0 | 312 | P. ovalee | |||
| 140 | 27 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| 150 | 25 | AD | 0 | 256 | P. ovale CBS 7854 | ||
| <50 | AD | 0 | 312 | P. ovalee |
Where exact figures were not stated in the reference, an attempt has been made from the data presented to quantify the percentage of patients’ sera binding.
Also cited as a minor antigen by other workers.
NT, Not tested.
NS, not stated.
Commercial antigen preparation made by ALK, Copenhagen, Denmark.
No reactivity with sera from patients with PV or SD.
Information on the same line in the table relates to the reference cited on that line. Where only one reference is cited for multiple lines, the information all relates to that reference.
Of the multitude of antigens described, a limited number have been further studied and characterized. In 1997, the first major antigen of Malassezia, Mal f 1, was sequenced and expressed (388). The cDNA, of 1,176 bp, coded for a protein with a calculated molecular mass of 36 kDa, with a 22-amino-acid leader peptide. The antigen was thought to correspond to the 37-kDa protein found by various groups using immunoblotting (192, 486). The cDNA sequence data showed no similarity to other known sequences. However, the presence of a hydrophobic region at the N terminus may indicate that the protein is a membrane or secreted cell wall protein (388). The finding of a 37-kDa antigen on the cell surface, but not within cells of Malassezia (487), would support this conclusion. The reactivity of recombinant Mal f 1 was compared with the native protein in immunoblotting and radioallergosorbent tests (RAST) (489). Although there were minor variations depending on the protein expression system used, the recombinant Mal f 1 retained its reactivity in both assays, suggesting that it contained most of the epitopes present in the native protein.
The next two antigens to be characterized, Mal f 2 and Mal f 3, were found to have masses of 21 and 20 kDa, respectively, under reducing conditions (480). Under nonreducing conditions, the masses were 42 and 40 kDa, respectively, suggesting that the antigens were dimers of a single protein, linked by disulfide bonds. There was 51% sequence homology between Mal f 2 and Mal f 3, and they had homology to peroxisomal membrane proteins of Candida boidinii and an allergen of Aspergillus fumigatus. There was no sequence homology to Mal f 1.
Mal f 4, identified by two-dimensional polyacrylamide gel electrophoresis (PAGE) and immunoblotting, was cloned and sequenced (320). The 315-amino-acid protein had a molecular mass of 35 kDa and showed 57% sequence homology to mitochondrial malate dehydrogenase from Saccharomyces cerevisiae. Sera from atopic dermatitis (AD) patients reacted with Mal f 4 in 83% of cases, indicating that it is a major antigen.
Mal f 5 has a molecular mass of 18.2 kDa and 57 and 58% sequence homology to Mal f 2 and Mal f 3, respectively (254). Mal f 6, with a mass of 17.2 kDa, has 82% sequence homology to cyclophilin from Schizosaccharomyces pombe. Both recombinant proteins were able to bind sera from patients with AD. Three other proteins, with masses of 21.3, 14.4, and 9.7 kDa, were also cloned and expressed. They did not have sequence homology to any known protein and may be incomplete cDNA clones, although the recombinant proteins from them were reactive in immunoblots. Subsequent work, using full-length cDNA clones, resulted in the description of three more antigens, Mal f 7, Mal f 8, and Mal f 9 (351). Mal f 7 encoded a protein of 141 amino acids (16.2 k Da), Mal f 8 encoded a protein of 179 amino acids (19.2 k Da), and Mal f 9 encoded a protein of 126 amino acids (14.0 k Da). None of the proteins had sequence homology to any known proteins, and the recombinant proteins reacted with sera from patients with AD in immunoblots.
An antigen recently characterized from M. globosa with a molecular mass of 46 kDa was found to be a major antigen, reacting with 69% of sera from patients with AD (230). The antigen reacted with concanavalin A in lectin blots, indicating that it was a glycoprotein. A 67-kDa antigen was also noted, that was probably a protein.
From the work reviewed above, several conclusions are apparent. First, different groups have defined a variety of antigens, ranging from low- to high-molecular-mass proteins and also high-molecular-mass carbohydrates. Second, many of the antigens have similar masses and may be identical, differing simply in the accuracy with which the mass could be assigned. While some workers have found bands of certain masses difficult to distinguish (204), others have stated that bands could be distinguished in 1-kDa intervals, especially in antigens of <50 kDa (256). Third, as already mentioned, many workers have used immunoblotting to define antigens. This is generally effective at demonstrating the presence of proteins but may be less so at demonstrating carbohydrates, either because they do not enter the sodium dodecyl sulfate (SDS) gel or because they produce diffusely stained bands. Hence, while many protein antigens have been described, with the consequent assumption that proteins are the most important antigens (200), other workers have disputed this finding. Several investigators found that mannan or other high-molecular-mass polysaccharides are also important antigens (111–113, 256, 385). A seminal paper by Zargari et al. (486) may be key to understanding the relative importance of protein and carbohydrate antigens in Malassezia. These investigators made a variety of preparations of Malassezia, using different culture media, strains, incubation temperatures, durations of incubation, and extraction procedures. These preparations were separated on SDS gels and blotted with four sera from AD patients and two monoclonal antibodies, one specific to a 37-kDa antigen and the other specific to a 67-kDa antigen of Malassezia. The protein and carbohydrate content of the preparations were determined. Several important conclusions were drawn. First, the use of solid or liquid culture media to grow the organisms had little effect on the antigens present. The most important variable was the duration of culture. The number of protein antigens decreased with increased length of culture, from the presence of a wide range of proteins at 48 h to cultures in which most proteins were lost, after 4 days or more. A 37-kDa protein antigen was found to be maintained the longest. In contrast, the carbohydrate antigens were found to remain at relatively constant levels over the 21-day culture period. Therefore, the length of culture will have a significant effect on the antigens present in Malassezia and may alter the apparent importance of the protein and carbohydrate antigens found in various studies. Another important finding is that the stability of antigens varies (258). Solutions of antigens, to be used for skin testing, were stored at different temperatures and under different conditions, and their antigenic composition was then tested by immunoblotting. The major antigens studied were the 9-, 20-, and 96-kDa proteins and mannan. Most of the IgE binding components were labile at room temperature or higher temperatures and were degraded after 1 month of storage. Addition of 50% glycerol, a widely used stabilizer, had little effect on their longevity. However, storage at 4°C preserved most of the antigens, some of them for up to 1 year.
Malassezia therefore appears to be an antigenically complex organism, which alters the antigens expressed throughout its growth cycle. In addition, different strains possess diverse antigens and different methods of extraction release different antigens (200). However, in conclusion, both protein and carbohydrate antigens are likely to be important in Malassezia, although the proportion of each may vary during the growth cycle. The protein antigens are likely to be cell wall or cytoplasmic components that can easily be detected in immunoblotting and are present in the early phase of growth. The carbohydrate antigens, probably mannans or mannoproteins, are less easy to detect by immunoblotting and are maintained throughout the growth cycle. The identification and characterization of nine defined antigens of Malassezia are important steps in our understanding of the antigenic composition of this yeast.
NONSPECIFIC IMMUNITY AND IMMUNOMODULATION BY MALASSEZIA
Activation of the Complement Cascade
The complement system, consisting of over 30 proteins, is important in both specific and nonspecific immunity. Activation can occur via two pathways, the classical (mediated by immune complexes) and alternative (mediated by yeast or bacterial cells) pathways, and can result in lysis of certain bacteria and viruses, opsonization, and inflammation (33). The importance of complement in the opsonisation and phagocytosis of many fungi, including C. albicans (414), Aspergillus (232), and Cryptococcus (107), has been demonstrated. Several groups have reported the ability of Malassezia to activate the complement system, via either the alternative pathway (43, 412, 422) or the classical pathway (422). The extent of activation of the alternative pathway was cell concentration and time dependent, reaching a plateau after 30 min (422). None of the investigators determined the molecule responsible for triggering the alternative pathway, but β-glucan in the cell wall may be involved (422). Activation of the classical pathway was also found to be cell concentration and time dependent, with greater activation by heat-killed cells than living cells (422). This ability to activate complement has been suggested as a mechanism responsible for the inflammation associated with seborrheic dermatitis (SD). The proteins involved in the initial steps of the alternative complement pathway are known to be present in the skin (117), and complement-mediated inflammation is associated with many dermatoses, including bullous pemphigoid (198), acne vulgaris (390), and psoriasis (410). Immunohistochemical studies of SD have found that deposits of C3 are present in the lesions, localized solely around the collections of Malassezia cells, and are absent from uninvolved skin (334). Therefore, complement may be involved in the inflammation associated with SD, but it has not been documented in lesions of PV (152).
Phagocytosis of Malassezia
Phagocytosis of microorganisms is an important nonspecific immune mechanism for their removal. Its importance in protection against fungal infections is highlighted by the increased susceptibility of neutropenic patients to many mycoses (370).
There is limited information available about phagocytic uptake and killing of Malassezia. In vitro, neutrophils take up Malassezia in a complement-dependent process, which plateaus after 40 min (358). After 2 h of internalization, only 5% of the cells are killed, but this increases to 23% if the yeasts are pretreated with ketoconazole. The ability of neutrophils to kill Malassezia seems limited. In contrast, 30 to 50% of C. albicans yeast cells (86) and up to 80% of the cells of other fungal genera (299) are killed by neutrophils. The mechanisms by which Malassezia may resist or prevent phagocytic killing are discussed below.
The receptors involved in phagocyte-yeast cell binding have been characterized in a human monocytic cell line as the mannose receptor, β-glucan receptor, and complement receptor type 3 via the alternative complement pathway (423). Uptake of heat-killed Malassezia yeast cells was more efficient than that of live Malassezia yeast cells, although the reason for this is unknown. Recently, it has been reported that when a monocytic cell line, THP1, was stimulated with either live or heat-killed Malassezia, the production of interleukin-8 (IL-8) was increased, while stimulation of a granulocytic cells line, HL-60, resulted in increased levels of both IL-8 and IL-1α (424). Opsonized and live Malassezia yeast cells were more stimulatory than were nonopsonised or heat-killed Malassezia yeast cells. The effects of IL-1α include the activation of lymphocytes, chemotaxis and activation of neutrophils and induction of inflammation (33). IL-8 also induces chemotaxis and activation of neutrophils and T cells. Therefore, the interaction of Malassezia with phagocytic cells may serve to amplify the inflammatory response and encourage further recruitment of phagocytic cells.
Within the skin, Langerhans’ cells are able to take up antigen and then present it to T cells, providing a link between nonspecific immunity and the specific immune response. Using monocyte-derived dendritic cells, Buentke et al. (73) recently demonstrated that whole cells of Malassezia, mannan, and an allergen, r Mal f 5, were taken up more effectively by immature dendritic cells than by mature cells. Significantly more uptake occurred at 37°C than at 4°C, indicating an active process, and IgE-mediated uptake was excluded. The uptake of the whole cells and mannan was inhibited by methyl-α-d-mannopyranoside, indicating involvement of the mannose receptor, while uptake of the nonglycosylated r Mal f5 was by pinocytosis.
Immunomodulation by Malassezia
One of the first studies to demonstrate the ability of Malassezia to modulate the immune system was carried out by Takahashi et al. (429). Different amounts of live or heat-killed suspensions of Malassezia were injected intraperitoneally into mice on various days before they were challenged intraperitoneally with Salmonella enterica serovar Typhimurium. In all cases, pretreatment with even small amounts of Malassezia resulted in some resistance to infection, but maximal benefit was observed if the mice received Malassezia 4 days before the challenge with serovar Typhimurium. Injection of Malassezia resulted in increased numbers and bactericidal activity of intraperitoneal macrophages, indicating that macrophage upregulation was the protective mechanism against subsequent bacterial challenge. The protection was comparable to that induced by Propionibacterium acnes, a known stimulator of the reticuloendothelial system. Two years later, the same group studied the ability of Malassezia to protect mice against challenge with tumor cell lines (430). Pretreatment with Malassezia significantly enhanced survival of the mice when they were challenged with a tumor cell line. The protection was due to stimulation of the macrophages to produce oxygen intermediates. Therefore, these studies demonstrate that Malassezia is able to upregulate phagocytic cells and thus provide enhanced protection against bacterial and tumor cell challenge in animals.
In contrast to these findings, a study by Walters et al. (458) demonstrated that Malassezia was also able to downregulate the immune system. Various preparations (formalized whole cells, culture supernatant, and a cellular fraction) of Malassezia serovar B (synonym, M. globosa) were coincubated with keratinocytes or peripheral blood mononuclear cells (PBMC), and release of IL-1 was determined at various time points. The levels of IL-1β released by PBMC coincubated with formalized whole cells were significantly lower than those of the negative control. The authors suggested that this depression of IL-1β release might contribute to the lack of inflammation seen in diseases such as PV and help Malassezia to evade detection by the immune system.
A subsequent study (217) examined the effect of the three serovars of Malassezia on the production of IL-1β, IL-6 and tumor necrosis factor alpha (TNF-α) by PBMC. PBMC were isolated from four healthy volunteers and cocultured with formalized whole cells of the Malassezia serovars at different ratios (yeast cell-to-PBMC ratio = 1:1, 10:1, and 20:1). The levels of IL-1β, IL-6 and TNF-α were measured in the culture supernatants at 0, 24, and 48 h. PBMC were also cocultured with viable Malassezia cells at a ratio of 20 yeast cells to 1 PBMC, and cytokine levels were determined at 0 and 24 h. In general, although exponential-phase formalized Malassezia cells at some yeast-to-PBMC ratios stimulated increased cytokine production over background levels, stationary-phase cells caused no change or significantly depressed cytokine production at every ratio tested. Viable yeast cells of all three serovars of Malassezia also significantly depressed IL-1β, IL-6 and TNF-α production.
A recent study examined the mechanisms by which this depression of cytokine production by PBMC might be mediated (216). Stationary-phase Malassezia cells were treated with solvents to remove some of the lipid present in the cell wall and the capsule-like layer around the cells. Untreated and solvent-treated cells were then cocultured with PBMC at a ratio of 20 yeasts to 1 PBMC, and cytokine levels in the culture supernatants were determined after 24 h. PBMC cocultured with solvent-treated Malassezia produced amounts of IL-1β, IL-6 and TNF-α that were similar to or significantly greater than constitutive levels. Thus, the removal of lipid from Malassezia ablated its ability to suppress cytokine production by PBMC, and so the lipid in the cell wall and capsular-like layer may be responsible for the lack of inflammation associated with Malassezia in its commensal state.
This ability of Malassezia species to downregulate the production of proinflammatory cytokines is in marked contrast to the effects of most other organisms. Gram-negative bacteria are known to cause overproduction of TNF-α, IL-1, and IL-6, both in vitro and in vivo. This is primarily the cause of septic shock in gram-negative infections and is mediated by lipopolysaccharide (442). Cell wall preparations of gram-positive bacteria, consisting mainly of teichoic acid and peptidoglycan, have also been shown to induce the synthesis of TNF-α and IL-6 by monocytes (187). C. albicans induces TNF-α and IL-6 production by human monocytes (201) and arachidonic acid release from alveolar macrophages (84). Viable C. albicans cells preferentially induce TNF-α, while heat-killed cells preferentially induce IL-1 (201). Aspergillus fumigatus hyphae and conidia also induce the production of IL-1 and TNF-α from mouse macrophages, but in contrast to C. albicans, viable and nonviable organisms do not differ in their effects (433). From this, it can be seen that the effects of Malassezia on cytokine production by mononulear cells appear to be atypical in comparison to most organisms. Malassezia, however, does have similarities to another pathogenic fungus, Cryptococcus neoformans. The effects of C. neoformans on cytokine production by phagocytes are variable, depending on the presence of the polysaccharide capsule. Acapsular mutants induce significant levels of TNF-α and IL-1 (449), while the presence of the polysaccharide capsule downregulates this effect (96). In many ways, this parallels the effects documented with Malassezia, with the lamellar layer downregulating the production of proinflammatory cytokines in much the same way as the capsule of C. neoformans does.
As discussed above, phagocytic killing of Malassezia is a very inefficient process, with only 5% of internalized cells killed after 2 h (358). One possible reason for this limited killing ability of phagocytes may be the production of azelaic acid by Malassezia. Akamatsu et al. (9) examined the effects of azelaic acid on chemotaxis, phagocytosis, and production of reactive oxygen species by neutrophils. They found that chemotaxis and phagocytosis were not affected by azelaic acid, but that the production of O2− and OH· was decreased in a dose-dependent manner and H2O2 production was also reduced. The effects were due to inhibition of cellular metabolism. Azelaic acid has also been shown to scavenge oxygen radicals (146). At present it is not known if azelaic acid is produced in vivo, but it is interesting to speculate that if it is, it may well be involved in protecting the organism from the oxidative killing mechanisms utilized by phagocytes.
A recent preliminary report has also suggested that the lipids associated with the cell wall of Malassezia may be antiphagocytic and involved in protection against killing by neutrophils (H. R. Ashbee, Z. L. Alvarado-Alvarez, Z. Whitehead, and E. G. V. Evans, Proc. Fourth Congr. Eur. Confed. Med. Mycol., abstr. 5, 1998). Yeast cells that had been treated with solvents had significantly greater uptake by neutrophils, leading to an increase in nitroblue tetrazolium reduction, a measure of oxygen radical release. Therefore, the lipid-rich layer around Malassezia may have parallels with the capsule of C. neoformans, which is known to protect the organism against phagocytosis (231). Although Malassezia cells on normal skin are unlikely to come into contact with professional phagocytic cells, neutrophils are present in the inflammatory infiltrates of SD (334) and macrophages are present within the infiltrate of PV (66). Therefore, Malassezia may be exposed to phagocytic cells at certain times.
The apparently contradictory ability of Malassezia to either upregulate or suppress the immune response directed against it has only recently been studied in detail. Understanding how this immunomodulation occurs may well be key to understanding how species of Malassezia occur both as commensals and as pathogens.
COMMENSALISM
Distribution of Malassezia Species on Normal Skin
Malassezia species are members of the normal human cutaneous commensal flora and can be isolated from the sebaceous-rich areas of the skin, particularly the chest, back, and head regions (246, 364). Many studies have examined carriage rates in different populations and different age groups. However, early studies often found low carriage rates due to the limitations in sampling techniques and culture media (264, 415, 435). An extensive study of the distribution of Malassezia species at various sites on adults was carried out by Leeming et al. (246), using an optimized culture medium (245) and a sampling method known to recover 98% of the surface skin flora (470). They examined clinically normal skin at 20 different sites over the entire body surface. Malassezia species were recovered from every subject from the chest, midline back, scalp, ear, and upper inner thigh. The highest mean population densities occurred on the chest, ear, upper back, forehead, and cheeks. Some differences in carriage rates were noted between females and males, with higher population densities from the lower trunk and upper thigh of males. Subsequent studies, using the same medium, have largely confirmed these findings (37, 229). Bergbrant and Faergemann (48) found that the density of Malassezia species on the skin decreased with increasing age, which was probably due to a reduction in the level of lipid on the skin. Therefore, 30-year-old subjects had significantly greater numbers of Malassezia species than did any other age group from 40 to 80 years old. M. pachydermatis is occasionally isolated from human skin, but its presence is transitory and it is not a human commensal (37).
Several recent studies have examined the distribution of the newly defined species of Malassezia on healthy adult human skin. The findings, summarized in Table 4, vary significantly among studies, and there are two possible explanations for this. First, there are genuine differences in the distribution of species on the skin of individuals in different countries, and this has been previously suggested (290). However, even the two studies carried out in Spain show very different results. A second explanation is that the use of swabbing, a nonquantitative and relatively insensitive method, is simply not able to produce the quantitative data needed to determine which species predominate at the different sites studied. Quantitative data on the distribution of the new species on human skin is therefore still awaited.
TABLE 4.
Studies examining the distribution of the new species of Malassezia on healthy adult human skin
| Reference | No. of subjects | Sampling method | Presence on:
|
Country | |||
|---|---|---|---|---|---|---|---|
| Back | Chest | Scalp | Forehead and/or face | ||||
| 31 | 38 | Swabbing | M. globosa (62%), M. sympodialis (38%) | M. globosa (71%), M. sympodialis (16%), M. restricta (6.5%), Nontypeable (6.5%) | M. globosa (33%), M. sympodialis (13%), M. restricta (48%), Nontypeable (6%) | Spain | |
| 94 | 43 | Swabbing | M. sympodialis (71%) Culture negative (29%) | M. sympodialis (5%), M. globosa (7%), culture negative (88%) | Spain | ||
| 300 | 35 | Swabbing | M. globosa (51%), M. sympodialis (26%), culture negative (23%)a | M. globosa (6%), M. furfur (3%), M. sympodialis (3%), M. restricta (3%), culture negative (66%) | M. globosa (9%), M. furfur (6%), M. sympodialis (3%), M. restricta (3%), culture negative (63%) | Japan | |
| 290 | 32 | Not stated | M. globosa (50%), M. sympodialis (34.4%), M. restricta (28.1%), M. slooffiae (12.5%), M. furfur (3.1%) | United Kingdom | |||
These percentages are for the trunk, which includes both the back and the chest.
Colonization rates in children are the subject of some controversy. As with adults, the colonization rates reported are partly a reflection of the sensitivity of the sampling method and culture medium used. Particularly in young children or newborns, swabs may be the only practical sampling method available since more disruptive techniques are unethical. One study of 60 healthy children, who ranged from 2 months to 14 years of age, yielded no positive specimens for Malassezia (4). This contrasts with other studies that have found carriage rates of 74% on the scalp (313), 93% on the back (155), and 87% on the forehead (47) of healthy children. In general, carriage of Malassezia appears to increase around puberty, correlating with the increase in sebaceous gland activity seen at this time (100).
The increasing recognition of Malassezia as a cause of catheter-related fungaemia in premature neonates has provided the impetus to study colonization rates in premature and full-term neonates. The colonization rates recorded range from 37% (344) to 100% (247) in hospitalized neonates. Factors such as young gestational age (8, 29, 44, 344), low birth weight (8, 344), and extended periods of hospitalization (8, 29, 44, 344) may predispose to colonisation in this group. To date, however, no systematic survey of colonization rates has been undertaken in healthy newborns, for whom the picture remains unclear.
Skin Immune System
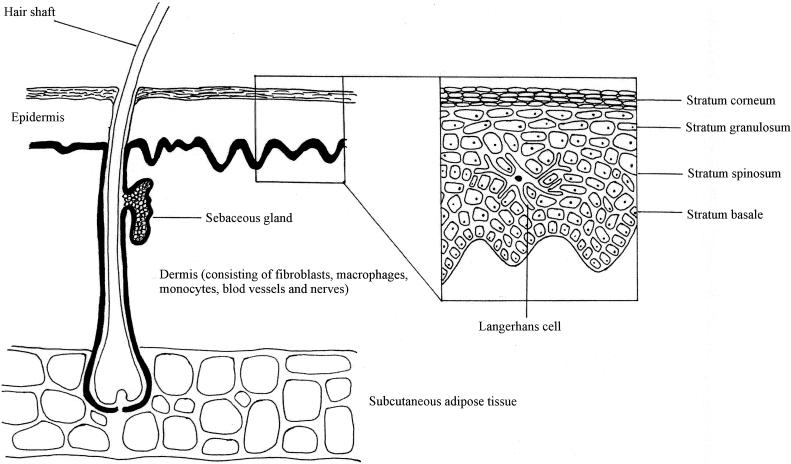
Malassezia is a cutaneous commensal, and thus its first point of contact with the immune system is likely to be via the skin immune system. The skin is the largest organ of the body and serves as the interface between the human host and the environment (60). Its structure and the cells which occur in each layer are detailed in Fig. 1. The skin is unusual because it is constantly exposed to a huge range of antigens, both from commensal and transient populations of microorganisms and also from those derived from the wider environment. Although for many years it was believed to be an inert barrier, the skin is now known to be a complex organ and functions as part of the immune system, playing a role in both nonspecific and specific immune responses.
FIG. 1.
Schematic diagram of the skin structure and the cells within it.
Nonspecific immune responses.
The skin has several facets which function as part of the nonspecific immune response. First, it acts as a physical barrier to infection. Intact skin is relatively resistant to most microorganisms, and it is generally only when breaches occur that they gain entry (427). Second, the presence of a commensal flora on the skin is an important nonspecific immune defense. In addition to Malassezia species, normal skin will have a resident population of other organisms, principally bacteria, including staphylococci and propionibacteria (246). These will compete for nutrients and space, limiting the population size of each group and also competing out pathogens that may attempt to colonize the skin. Finally, shedding of the cells of the epidermis occurs constantly, and the rate of shedding is increased during inflammation (52). This shedding causes the loss of the microorganisms that are colonizing or infecting these cells and prevents invasion into the deeper layers of the skin.
In addition to the barrier function of the skin and its commensal flora, phagocytic cells are important in the nonspecific cutaneous immune response. In skin diseases where significant inflammation occurs, neutrophils occur within the lesions and may lead to an accumulation of mononuclear cells in the dermis (455). Phagocytic cells may then attack organisms by using either oxidative or nonoxidative mechanisms, leading to their removal (454). Several organisms, including dermatophytes, Candida species, and propionibacteria, activate the alternative pathway of the complement cascade, causing the production of molecules with chemotactic activity for neutrophils (106, 353, 462). In this way, neutrophils may be recruited into the skin by the presence of microorganisms.
Other factors involved in the nonspecific immune system of the skin are the lipids found on adult scalps and adult hair, which are fungistatic for certain dermatophytes (54), fungicidal proteins present in the epidermis (210), and the inhibitory effect of unsaturated transferrin on various fungi (23, 222).
Specific immune responses.
In addition to these nonspecific immune functions, the skin is involved in specific immune responses. The skin immune system consists of both cellular and humoral components. The cellular skin immune system includes keratinocytes, Langerhans’ cells, mononuclear cells, mast cells, endothelial cells and T lymphocytes, while humoral components include complement proteins, IgG and IgA, and various cytokines.
The way in which antigens from the skin surface, including those from commensal organisms and superficial pathogens, elicit a specific immune response has been the subject of intense research, and a clearer picture of the mechanisms involved has now emerged (60–63, 396, 419, 420). Langerhans’ cells are bone marrow-derived dendritic cells that form a network within the epidermis and are capable of presenting antigen. Immature Langerhans’ cells express low levels of major histocompatibility complex class II (MHC II) and are capable only of presenting antigen to primed T lymphocytes. Under the influence of TNF-α and granulocyte-macrophage colony-stimulating factor released by keratinocytes, Langerhans’ cells that have processed antigen undergo maturation and migrate to the draining lymph nodes. Here they become potent immunostimulatory cells and prime antigen-specific T lymphocytes. Vascular endothelial cells, lining blood vessels present in that area of the skin, begin to express intercellular adhesion molecule 1, due to the release of IL-1 and TNF-α by keratinocytes. The primed T lymphocytes are then able to adhere to the endothelial cells and migrate out of the veins, via diapedesis, into the surrounding dermal tissue. Once in the dermis, the T lymphocytes secrete gamma interferon, which causes further expression of intercellular adhesion molecule 1 on the endothelial cells and increased MHC II expression on keratinocytes and Langerhans’ cells. Macrophages are drawn into the skin and function as antigen-presenting cells to primed T lymphocytes, so amplifying the response.
One group of cells that are noticeably absent from the skin are B lymphocytes. Despite this, it is known that immunoglobulins specific to the commensal flora are produced in normal individuals (30, 100, 194) and that IgG, IgM, IgE, and secretory IgA are present in human sweat (150, 196, 322), providing a readily available route to gain access to the organisms on the skin. Metze et al. (283) used immunohistochemical techniques to demonstrate that commensal organisms (Malassezia species, Corynebacterium, and cocci) present on the skin surface of normal individuals were coated with immunoglobulins. Thus, antibodies are produced against the commensal flora and are able to reach and attach to these organisms on the skin.
Humoral Immune Responses to Malassezia in Normal Individuals
Immunoglobulins specific to the yeast phase of Malassezia can readily be detected in normal individuals with no history of skin disease, and several groups have studied this humoral response in healthy individuals. Throughout the following sections, Malassezia is used as a generic name, because in many cases it is difficult to identify which species were used in the studies cited.
In 1983, the serum antibody titers to Malassezia, C. albicans, and Trichophyton rubrum were determined in 21 young subjects (aged 23 to 44 years) and 20 elderly subjects (aged 70 to 88 years), none of whom had a “significant” history of superficial fungal infections (413). A tube ELISA was used to determine the titers of IgG, IgA, and IgM and found that all three classes of immunoglobulins to all three organisms were present in both the young and elderly groups. Comparison of the data for young and elderly subjects revealed that the responses were very similar, except for Malassezia, where the levels of IgM were significantly lower in the elderly subjects (P < 0.01).
Faergemann examined titers of antibodies in normal subjects, measured using an indirect-immunofluorescence (IIF) technique (123). Sera were included from 21 adults and 36 children aged 6 months to 15 years. The titer in adults was significantly higher than that found in the children (P < 0.01), possibly due to the sparsity of Malassezia on the skin of the children.
Levels of Malassezia-specific IgG were determined by IIF in the sera of normal subjects ranging from 29 to 81 years of age (48). The titers decreased with increasing age (P = 0.002), with the highest titer being found in 29- to 31-year-old subjects and lower titers being found in older subjects. This paralleled the decreasing population densities of Malassezia on the skin, and so the authors suggested that antibody titers reflected the level of skin colonization.
The sensitivities of IIF and ELISA were compared for their ability to detect IgG specific to Malassezia (203). Sera from 10 healthy adults and five healthy 6-month-old children were assayed using both methods. Titers in the ELISA were much higher than the corresponding titers in the IIF technique, demonstrating that ELISA was the more sensitive method. Additionally, titers of IgG specific to Malassezia were substantially lower in the 6-month-old children than in the adults, although no statistical analysis was carried out on the results.
Cunningham et al. also used ELISA to determine antibody titres to Malassezia serovars A, B, and C in normal individuals of various ages (100). Sera were obtained from 50 nonatopic females with no history of dermatoses. Five age groups were included: 2 to 3 years, 7 to 10 years, 20 to 24 years, 33 to 40 years, and 60 to 64 years, with 10 individuals in each. Titers of IgG and IgM were determined for all the subjects, and titers of IgA were determined for 36 subjects. IgM was present in the sera of 2- to 3-year-old children at levels comparable to those in adults. The titers of IgM were similar for all age groups, except the 60- to 64-year-old group, where they were significantly lower (P < 0.05). IgG titers did not differ significantly between age groups. IgA was not detectable in 18 sera, and its levels were low in all groups, with no differences between age groups or serovars.
The most recent study to define humoral immunity to Malassezia included 868 serum samples from subjects ranging from 0 to 80 years of age (139). However, the subjects were included “independently of the presence or absence of signs of disease attributable to the fungus,” so the results may not be representative of healthy individuals. Antibodies to Malassezia, detected by immunoelectrophoresis, were present in 31% of the samples, with none in children younger than 11 years and the highest prevalence in the 31- to 40-year-old group. No statistical analysis was performed and so it is not known whether the differences were statistically significant. These results contrast with the findings of other groups, who have detected immunoglobulins in children, and may be a reflection of the relative insensitivity of immunoelectrophoresis.
Despite the variety of methods and different antigen preparations used in these studies, some consensus has emerged from the results. The majority of individuals have some antibodies to Malassezia, even from a relatively young age, although a few studies perhaps did not detect them because of the methods used. Antigen is presented to the immune system over a sufficient period to initiate both naive (IgM) and anamnestic (IgG) responses. Levels of IgA are generally low, suggesting that mucosal sensitisation by Malassezia is not an important route.
In the commensal state, Malassezia usually occurs as yeast cells, although mycelium may also be seen (288). Because of this and because of the difficulty in producing the mycelial phase, no investigators have determined the humoral response to mycelium. Recently, this issue was addressed by Saadatzadeh (380), who induced the mycelial phase and used whole mycelial antigens in IIF. Titers of total immunoglobulins, IgM, IgG, IgG subclasses, and IgA were determined in sera from 12 normal healthy adults. All the classes of immunoglobulins were detected, with the highest titers being found for IgG. Appreciable levels of IgM, IgA, and the IgG subclasses were also found. Thus, although the relatively insensitive method of IIF was used, significant levels of humoral immunity to the mycelial phase of Malassezia could be detected in normal individuals. Despite the limited amount of mycelium on normal skin, the immune system recognizes and responds to mycelial antigens. This may be due to the presence of common antigens, shared either with the yeast phase of Malassezia or with other commensal organisms.
Cellular Immune Responses to Malassezia in Normal Individuals
Cellular immunity is known to be of major importance in the host defense against fungal infection (82). The higher incidence of Malassezia-associated dermatoses in patients with cellular immunodeficiencies suggests that cellular immunity is also important in maintaining the organism as a commensal. The incidence of PV is known to be increased in renal transplant recipients (228, 391) and patients receiving steroids (57); folliculitis is seen in bone marrow transplant recipients (74), and the incidence of SD is very high in patients with AIDS (92, 119, 141, 275, 298, 408, 451). Despite this, only one small study has characterized the cellular immune response to Malassezia at various ages. Cunningham (99) studied nine individuals, ranging from 8 to 57 years of age (8 to 10 years, n = 3; 21 to 24 years, n = 3; 50 to 57 years, n = 3), and measured the cellular immune response to whole yeast cells of Malassezia serovars A, B, and C using the leukocyte migration inhibition (LMI) and LT assays. In the LMI assay, she found that there were positive migration inhibition responses in each age group and that the responses did not differ significantly between the age groups. The responses in the LT assay were determined for six individuals, in the age range from 21 to 57 years, and did not differ between age groups; however, the responses to serovars B and C were generally higher than those to serovar A. This small study has limited significance, but it does demonstrate that stimulation of cellular immunity by Malassezia occurs in normal individuals.
In an attempt to gain a clearer picture of cellular immunity to Malassezia in normal individuals, data from control subjects in various studies have been collated in Table 5. From this it can be seen that Malassezia elicits significant cellular immunity in normal individuals. The similarity in responses seen in the different age groups studied (from 8 to 61 years of age) suggests that the levels of cellular immunity remain fairly constant throughout life, although its persistence in individuals older than 61 years has not been studied. It may be that, as occurs with humoral immunity, the level of cellular immunity falls in elderly individuals.
TABLE 5.
Data on cellular immune responses to Malassezia in normal individuals used as control subjects
| Reference | No. of subjects | Age range (yr) | Method | Antigen preparation | Results |
|---|---|---|---|---|---|
| 99 | 3 | 8–10 | LMI | Whole yeast cell of Malassezia serovars A, B, and C | Positive in all age groups; no difference in responses between age groups; responses generally higher to serovars B and CL |
| 3 | 21–24 | LT | |||
| 3 | 50–57 | ||||
| 409 | 15 | 22–40 | LMI | Ether extraction, physical disruption, dialysis, and lyophilization of P. ovale and P. orbiculare | Mean LT response, 21–23a; Mean LMI response, 31.6%b |
| LT | |||||
| 410 | 32 | 20–42 | LT | 1. As above | 31 of 32 positive; mean LT response, 26.5a |
| 2. Ether extraction, dialysis and lyophilization | |||||
| 3. Freeze-thaw, centrifugation | |||||
| 4. Sonication, centrifugation | |||||
| 28 | 20 | 23–45 | LMI | Whole yeast cell of Malassezia serovars A, B, and C | LT, 3 of 20 positive; LMI, 8 of 19 positive; responses generally higher to serovars B and C |
| LT | |||||
| 46 | 15 | 23–61 | LT | Freeze-pressed M. furfur | 15, mean response of ≈31; 12, mean response of ≈27 |
| 12 | 19–49 | ||||
| 328 | 16 | Mean, 56 | LT | 1. Whole cells | Stimulation index obtained with the five antigens: 1, 0–30; 2, 0–50; 3, 0–5; 4, 0–7; 5, 0–12 |
| 2. Bead beater, centrifuged: cytoplasmic | |||||
| 3. Bead beater, ground pellet: cell wall | |||||
| 4. Sonicated, centrifuged: sonicate | |||||
| 5. Commercial preparationc |
Significant response, >4.
Significant response, >20%.
Commercial antigen preparation made by ALK, Copenhagen, Denmark.
The cellular immune response to the mycelial phase of Malassezia in normal individuals has recently been investigated (378). Cellular immunity was measured using the LT and LMI assays in 12 healthy volunteers. None of the subjects gave a positive response in the LT assay, while up to 10 of them responded in the LMI assay, depending on the Malassezia strain used. The level of response was described by the authors as “minimal,” and this may be a reflection of the limited number of mycelial elements on normal human skin.
The presence of Malassezia on the skin as a commensal and the measurable humoral and cellular immune responses to Malassezia in healthy individuals with no history of skin disease present unusual problems when studying patients with Malassezia-associated diseases. To provide convincing evidence that Malassezia may be involved in the disease, the immune response in patients must be significantly different from that in healthy controls. However, the response seen in healthy controls will vary according to their age and possibly the amount of Malassezia that they carry on their skin. Therefore, it is essential in any study of the microbiology or immunology of Malassezia that controls be carefully selected to match both the age and sex of the study population. Comparison of results between groups not matched in this way is meaningless.
DISEASES ASSOCIATED WITH MALASSEZIA
In several early papers there was debate about whether Malassezia was really able to cause disease (273, 369). It is now thought, however, that it is the etiological agent of both cutaneous and systemic diseases. M. pachydermatis is not commonly associated with diseases in humans, although it has been reported to cause canaliculitis (372), wound infection (160), and systemic disease (88, 168, 241, 286, 465) in premature neonates. It is, however, an important pathogen of animals, causing dermatitis and otitis externa in a wide range of animals (169). Since the immune response in humans to this organism have not been studied, it will not be discussed further.
Pityriasis Versicolor
Malassezia is known to be the etiological agent of PV (synonym, tinea versicolor). Under conditions which have yet to be fully elucidated, it undergoes conversion from the yeast to the mycelial form, which is then able to invade the stratum corneum, penetrating both between and through the corneocytes (58, 81, 291, 295, 441). Recent work, however, has found that not all isolates of Malassezia are able to undergo this yeast-mycelium transformation (379). PV is a mild, chronic condition, usually affecting the upper trunk (129); it is characterized by scaly hypo- or hyperpigmented lesions with minimal pruritus (270). The condition occurs mainly between adolescence and middle age, when the sebaceous glands are more active (134), although it has also been reported in children (11, 93, 284, 479) and the elderly (109, 285). Predisposing factors include a “genetic susceptibility” (77, 175), illness or malnutrition (77), increased plasma cortisol level (57, 77, 237), and high ambient temperature and humidity (127). The incidence in temperate climates is around 1% (134, 183), but incidences as high as 40 to 60% have been reported in tropical climates (209). The lesions may be more extensive in tropical climates (288), and the microscopic appearance of the organism from the lesion may be different from that seen in temperate climates. In temperate regions, microscopically the appearance is classically of clusters of yeasts with hyphae that may be branched (“spaghetti and meatballs”), while in tropical regions, oval or cylindrical yeasts with filaments may be seen (288, 291). PV has a chronic, relapsing nature, necessitating frequent retreatment or prophylaxis (130).
The mycology of PV has been extensively studied. The association of the mycelial form of Malassezia with PV lesions was made as early as 1871 (308), when Neumann described hyphae from lesions. Moore successfully cultured Malassezia from PV lesions in 1938 (296), and Gordon described the round yeast form associated with the lesions of PV and normal skin (159). In 1969, Roberts (365) examined 25 patients with PV at presentation and included a further 62 patients retrospectively. Lesions were scraped in 25 patients, and Malassezia was found in all of them by microscopy; however, the proportion of yeast and mycelium varied. For the 27 patients for whom samples were available, 25 samples were culture positive on malt agar overlaid with olive oil. P. orbiculare was isolated in all 25 cultures; in 6 of them it coexisted with P. ovale. Roberts found filaments in some of the samples from clinically normal skin of patients and suggested that filament production had to occur on a “massive scale” to produce clinical lesions of PV.
The following year, McGinley et al. (280) studied 31 patients with PV to assess the proportion of mycelium and yeasts present on normal and lesional skin. The mean count of mycelial elements on lesions was 295,300 cm−2, compared to 155,900 cm−2 yeast forms, i.e. a 2.1:1 ratio. On normal skin this pattern was reversed, with 18,900 yeasts cm−2 and 5,800 mycelia cm−2, a ratio of 1:0.21. In addition, McGinley et al. found that the corneocyte count on lesions was three times higher than that on normal skin, and this provided increased space for the organisms to colonize. They concluded that Malassezia was the causative organism and that the mycelial form of the organism was “instrumental in creating the lesion” of PV.
In 1979, Faergemann and Bernander (132) collected samples from 30 patients with PV, using curettes to scrape both lesional and normal skin. They recovered P. orbiculare from the lesions of all patients and from normal skin of 24 patients but did not isolate P. ovale from any sample. They suggested that P. ovale might not be common in the Swedish population where the study was carried out.
Ashbee et al. (27) carried out a study of the microbiology of PV and took samples from sites on the trunk and head of 10 patients. They measured the total amount of Malassezia and the amount of each serovar (A, B, and C) at each site in an attempt to determine if any particular serovar was associated with the lesions of the disease. They found that although serovar A predominated on the trunk, this was the same in both patients and controls and did not reflect association with the disease. No one serovar predominated on the head sites. Overall, there was no difference in the total population density of Malassezia or the distribution of serovars on lesional skin compared to control skin at the same sites. These findings appear to conflict with those of McGinley et al., but whereas McGinley et al. recorded total counts by microscopy, the Ashbee study used viable counts on culture; hence, the results are not directly comparable.
Since the differentiation of the new species of Malassezia, several groups have published studies examining the mycology of PV (94, 95, 172, 300). The results of these studies are summarized in Table 6. Overall, M. globosa has been isolated from between 25 and 97% of the patients, and some of the authors have suggested that this species may be implicated in the pathogenesis of PV. However, because these studies used qualitative sampling methods, they are not able to produce the quantitative data needed to determine which species, if any, predominates in PV.
TABLE 6.
Studies examining the distribution of the new species of Malassezia on the skin of patients with PV
| Reference | No. of subjects | Site sampled | Sampling method | Species recovered (% of patients) |
|---|---|---|---|---|
| 94 | 100 | Not stated | Scraping | M. globosa (55%), M. sympodialis (11%), M. restricta (2%), M. globosa + M. sympodialis (23%), M. globosa + M. slooffiae (8%), M. globosa + M. restricta (1%) |
| 300 | 22 | Trunk | Swabbing | M. globosa (55%), M. furfur (5%), M. sympodialis (9%), M. pachydermatis (1%), M. slooffiae (5%), negative culture (14%), unknown/contamination (9%) |
| 95 | 96 | Not stated | Scraping | M. globosa (60.4%), M. sympodialis (3.1%), M. globosa + M. sympodialis (29.2%), M. globosa + M. slooffiae (7.3%) |
| 172 | 111 | Not stated | Scraping | M. sympodialis (59.5%), M. globosa (25.2%), M. furfur (10.8%) |
Another aspect of PV, which has received a great deal of interest, is the alteration in skin pigmentation associated with the lesions. Several theories have been proposed to explain hypopigmentation, including the filtering of UV light by the growth of the organism in the skin (14), a block in the transfer of melanosomes to keratinocytes (14, 89, 121, 153, 208), and the inhibition of melanin production by azelaic acid (180, 302) or by lipoxygenase (304). Other groups have suggested that the hyperpigmentation of lesions was due to inflammation (116, 153), increased skin thickness, or larger numbers of organisms in the skin (153). Despite these studies, the mechanisms by which pigmentation is altered remain largely unresolved.
Seborrheic Dermatitis and Dandruff
The relationship between SD and dandruff is controversial. Several authors believe that the two conditions are distinct clinical entities (224, 377), while others believe that dandruff is the mildest, noninflammatory form of SD (104, 398). The involvement of Malassezia in the condition(s) has also been extensively debated (126, 253, 281, 321, 382, 398).
SD presents clinically as scaling and inflammation on the areas of the body rich in sebaceous glands, such as the face, scalp, and upper trunk (166), whereas dandruff is a noninflammatory scaling condition confined to the scalp (252). Lesions of SD occur primarily on the eyebrows, nasolabial folds, cheeks, and sternal and interscapular region (337). In the normal population, the incidence of SD is around 1 to 3% (155, 352), but in patients who are human immunodeficiency virus positive or have AIDS-related complex or AIDS, the incidence is much higher, ranging from 30 to 83% (92, 119, 141, 275, 408), although the incidence of all opportunistic infections has decreased since the widespread use of highly active antiretroviral therapy (323). SD also has a higher than expected incidence in patients with PV (134), Parkinson’s disease (55), spinal injuries (471), or depression (266) or those receiving PUVA treatment (434). In normal individuals, SD tends to start after puberty and become chronic with frequent flares, often precipitated by stress (166). In AIDS patients, the condition may be much more severe (165) and refractory to therapy (408) than in persons who do not have AIDS.
For the purposes of this review, dandruff and SD will be considered a continuum of the same condition, unless they were specifically differentiated in the literature cited.
It was Malassez in 1874 who first associated Malassezia with scaling scalp conditions (268), and the debate about its role in SD and dandruff has continued unabated since then. Innumerable studies have sought to resolve the issue, focusing either on the microbiology of the condition or on the therapeutic efficacy of various antifungal preparations. In the early 1950s, both Martin-Scott (273) and Spoor et al. (415) expressed reservations about the association of Malassezia with SD, since it was found on normal scalps as well as in patients with SD. Van Der Wyk and Hechemy (446) studied the effect on dandruff of inhibiting either the yeast or bacterial populations on the scalp and found that reduction of the yeast population correlated with a decrease in dandruff while inhibition of the bacterial population did not. Two years later, a further study by the same group (161) used tetracycline and nystatin to suppress the normal scalp flora and noted a decrease in dandruff production of over 60%. When a nystatin-resistant strain of Malassezia was reintroduced onto the scalp, dandruff production increased by 88%.
The population densities of Malassezia were assessed on the scalps of normal subjects, patients with dandruff, and patients with SD in another study (281). Malassezia made up 46% of the microbial flora in normal subjects, 74% of the flora in patients with dandruff, and 83% of the flora in patients with SD. Although Malassezia was more numerous and made up a larger proportion of the flora in patients with dandruff or SD, the authors felt that the “data presented here cannot be constructed to argue for or against an etiologic role for bacteria or yeast-like organisms in dandruff.” However, further experiments published by the same group the following year found that suppression of the Malassezia population on the scalp, either alone or in combination with suppression of the bacterial flora, had no effect on dandruff production (253). They concluded that dandruff was merely an excessive desquamation of the scalp and was totally unrelated to the microbial flora. Despite this, several subsequent studies have supported the role of Malassezia in SD and dandruff, demonstrating parallel decreases in the number of organisms and the severity of the condition (185, 333, 382). A recent study used shave biopsy specimens to examine the density of Malassezia in SD lesions and normal skin and found higher densities in SD lesions (334). The authors suggested that because Malassezia was present within the layers of the stratum corneum as well as on the skin surface, the full thickness of skin squames must be examined to gain a true idea of fungal load and that surface culture methods may underestimate the population densities. Furthermore, they felt that this might explain why many studies were unable to detect differences in population densities of Malassezia between patients with SD and healthy controls.
A study in 1993 examined the distribution of M. furfur serovars A, B, and C on the skin of patients with SD to determine whether any particular serovar was associated with the lesions (27). In addition to finding that the total population density of M. furfur was not higher on lesional skin than nonlesional or healthy control skin, the investigators found that no one serovar was associated with the disease. More recently, two studies have examined the distribution of the new species of Malassezia on the skin of patients with SD. In the first study (94), scrapings were taken from lesions from 75 patients and cultures were performed. In 36 patients a single species was isolated; in 35 patients two species were isolated; and in 4 patients three species were isolated. M. restricta was isolated from 43% of patients, and M. globosa was isolated from 34%. The other study (300) included 42 patients, taking swabs from both lesional and nonlesional sites. The investigators found a similarly complex situation, with M. furfur, M. globosa, and M. sympodialis isolated from 21, 21, and 6% of the lesions, respectively, and 31% of lesions yielding no organisms. As with studies of PV, the use of nonquantitative methods will not provide definitive answers to whether a particular species is associated with SD.
Studies of the clinical efficacy of various drugs, in general, add considerable weight to the role of Malassezia in SD and dandruff. Although there are a few dissenting studies (5, 64, 98), by far the majority ascribe successful therapy to an anti-Malassezia mode of action (22, 104, 126, 148, 162, 197, 211, 271, 321, 330, 332, 362, 392, 393, 406). Although simple overgrowth of Malassezia is unlikely to be the cause of SD and dandruff, the balance of evidence suggests that the organism is very important in their etiology and not merely an opportunist colonizing the increased skin surface area.
Malassezia Folliculitis
Malassezia folliculitis consists of pruritic papules and pustules that occur mainly on the trunk and upper arms (125). It was first reported by Weary et al. (460) in the setting of antibiotic therapy and has also been seen in association with pregnancy (188), leukaemia (483), bone marrow transplantation (74), AIDS (142), Down’s syndrome (212), Hodgkin’s disease (184), diabetes (20, 340) and kidney (450) and heart (357) transplantation. Because folliculitis is frequently seen in immunocompromised patients, the importance of differentiating it from lesions of systemic fungal infection has been stressed (225). Folliculitis appears to be more common in tropical countries (1, 199), probably due to the heat and humidity. Although Malassezia has frequently been isolated from folliculitis lesions (137, 149, 212, 318), this may not be significant, since it can also be isolated from normal pilosebaceous follicles (243) and noninflamed open comedones in acne vulgaris (193). Other organisms commonly found in normal follicles include propionibacteria and staphylococci (243). Few studies of Malassezia folliculitis have attempted to culture the follicular contents, but one that did also found propionibacteria and staphylococci present within affected follicles (212). Potter et al. (340) postulated that the release of free fatty acids by the action of lipase from Malassezia might cause the irritation and inflammation associated with the folliculitis. However, this theory was discredited when subsequent studies demonstrated that physiological concentrations of free fatty acids were insufficient to elicit inflammation (347). Follicular occlusion has been suggested as the primary event in this condition, with overgrowth of Malassezia as a secondary event (189). While antifungal therapy has proved efficacious (1, 20, 149), the exact role of Malassezia in this condition remains to be fully elucidated.
Atopic Dermatitis
AD (synonym, atopic eczema) is a common chronic inflammatory skin disease, whose etiology is still unknown (473). Initial symptoms of AD or subsequent exacerbations may be triggered by emotional stress, infections, mechanical or chemical irritants, sweating, or allergens. The allergens implicated include food allergens; aeroallergens, such as pollens and house dust mite (255); and allergens from cutaneous commensals (315) or pathogens (312). Because the barrier function of the skin is impaired in AD (466) and intense pruritus resulting in excoriation is frequently present (312), antigens from organisms present on the skin are highly likely to come into contact with the immune system and elicit a response. Patients with AD generally have raised levels of total IgE, especially in severe disease (255). While IgE may be important in respiratory manifestations of atopy (allergic rhinitis and bronchial asthma), its role in AD is less clear.
The first study to implicate Malassezia in head and neck dermatitis, a particular type of AD, was published by Clemmensen and Hjorth (91). They had previously noted that patients with itchy, eczematous lesions on the face and neck gave positive skin prick reactions to Malassezia. The lesions occurred mainly in young, postpubertal females, and the investigators selected 20 such patients to participate in a double-blind crossover trial comparing the effects of ketoconazole with placebo. They found a significantly greater efficacy of ketoconazole in subjects who had lesions on the head and neck but not in those with more generalized lesions. A large retrospective study of 741 atopic patients confirmed that the largest number of positive skin prick tests (SPT) to Malassezia were seen in patients with head and neck dermatitis (453).
A study of colonization levels in young subjects with AD demonstrated that carriage of Malassezia did not differ significantly between AD patients and healthy controls (69). However, this study did not specifically select patients with head and neck dermatitis, the group in whom Malassezia may be more important.
Treatment with ketoconazole was found to be beneficial in a study of 20 patients with generalized AD (35). In a further study of 60 patients with AD of the head and neck, administration of a topical antifungal along with hydrocortisone was compared with administration of hydrocortisone alone (68). Both patient groups experienced significant improvement in their AD, which did not differ between groups, although the population densities of Malassezia decreased significantly only in the group receiving antifungal.
Other Superficial Diseases
Malassezia has been associated with a wide range of other superficial diseases, including acne vulgaris (21, 206), dacryocystitis (474), seborrhoeic blepharitis (440), neonatal pustulosis (19, 309, 336, 350), confluent and reticulated papillomatosis (136, 215, 223, 236, 366, 481, 482), onychomycosis (97, 400), nodular hair infection (262), and psoriasis (120, 124, 140, 373, 374, 376). In many of these reports, the isolation of Malassezia was taken as proof of its involvement in the disease, an assumption that may not be correct due to its presence on the skin as a commensal.
Malassezia has also been implicated in some more deep-seated infections including mastitis (53), sinusitis (319), septic arthritis (478), and malignant otitis externa (87).
Systemic Diseases
Peritonitis.
The first time that Malassezia was associated with a deep-seated infection was in 1979, when it was isolated from a patient undergoing continuous ambulatory peritoneal dialysis who developed peritonitis (451). After several episodes of apparently “sterile” peritonitis, lipid supplementation of cultures of peritoneal dialysate fluid grew Malassezia. Since this initial report, there have been three further reports of peritonitis due to Malassezia in continuous ambulatory peritoneal dialysis patients (143, 156, 205).
Catheter-related fungemia.
Catheter-related fungemia due to Malassezia has generally been described in neonates who are premature with a variety of underlying conditions, receiving parenteral nutrition (PN) through central venous catheters. The first report of the condition was in a premature neonate who developed pulmonary vasculitis while receiving intravenous lipid emulsion for PN (354). The child developed pneumonia, but an open lung biopsy failed to reveal the cause and the organism was cultured only after the child’s death. Previously, it had been demonstrated that intravenous administration of fat emulsions lead to the deposition of lipid deposits within the pulmonary vasculature (40, 102, 151, 251). At autopsy, Malassezia was found within the lipid deposits in the intima and vessel wall of the pulmonary artery and the surrounding lung tissue. The deposits fulfilled the organism’s requirement for lipid and caused its localization in the lung. Since this initial report, there have been a large number of further cases of fungemia due to Malassezia in neonates (15, 32, 34, 88, 105, 191, 220, 241, 261, 269, 286, 311, 341, 342, 355, 359, 394, 405, 421, 464, 465). To date, only M. furfur and M. pachydermatis have been reported to cause systemic disease. However, since many of the cases were published before the new taxonomy was adopted, it may be that other species are also involved but were not recognized at the time.
Malassezia fungemia has also been found in immunocompromised or immunosuppressed children and adults, most of whom were receiving PN (38, 70, 154, 179, 274, 287, 345, 387, 397). In many cases, antifungal therapy was unsuccessful in eradicating the Malassezia and the central venous catheter had to be removed. Although the isolates involved may be sensitive in vitro to the antifungal administered (345), the organisms may be embedded in a mesh of fibrin on the catheter surface, which protects them by rendering them inaccessible to the antifungal (346). Malassezia fungemia has been increasingly recognized over the last 20 years, but many blood culture systems do not effectively support its growth in vitro (263, 305, 356), and so the incidence may be even higher than current reports suggest.
IMMUNOLOGICAL RESPONSES TO MALASSEZIA IN DISEASE
The reasons why one individual develops a disease caused by Malassezia, but in other people it remains as a commensal are largely unknown. Many studies have examined the immunological responses in these patients and compared them to the responses in healthy individuals to determine whether patients had particular immunological predisposing factors. Despite studies of extensive numbers of patients, no clear immunological predisposing factor has emerged for most diseases.
There are many unanswered questions about the immunology of these diseases. What is the antigenic composition of Malassezia on the skin? How closely do the antigenic preparations used in assays match those present in vivo? Are in vitro immunological assays a true representation of the immune response in the skin during disease? For many of these questions, we still do not know the answers. Reviewing the literature, it is obvious that a wide range of antigenic preparations have been used by different groups (Table 7) as well as different assay methods (Table 7) to determine both humoral and cellular immune responses. Compounding this variability is the use of strains of Malassezia defined by different classifications. In view of these inconsistencies between studies, the surprise is that any answers have emerged rather than that some questions remain unanswered
TABLE 7.
Antigenic preparations and methods used for determination of cellular and humoral immunity to Malassezia species
| Antigenic preparation or method | Reference(s) |
|---|---|
| Preparations | |
| Tape strips of PV lesions | 418 |
| Whole cells | 12, 25, 26, 28, 48–51, 99, 100, 103, 123, 137, 152, 176, 203,219, 328, 476 |
| Physically disrupted cells | 46, 50, 103, 152,307, 314, 328, 409, 411, 413, 417, 436–438 |
| Cytoplasmic antigens | 36, 178, 292, 328 |
| Exoantigens | 139, 399, 485 |
| Type of immune response and method | |
| Humoral | |
| IIF | 12, 48–51, 103, 123, 137, 152, 176,203, 219, 418, 476 |
| Precipitation | 103, 139, 292 |
| Hemagglutination | 152 |
| Dot blot | 178 |
| ELISA | 25,26, 50, 100, 203, 328, 399, 413 |
| Western blot | 292, 328, 399,417 |
| Flow cytometry | 50 |
| RAST | 485, 314 |
| Cellular | |
| LMI | 28, 99, 378, 409, 411 |
| LT | 28, 36, 46, 99, 307, 328, 371, 378, 409–411, 436–438,477 |
Lack of standardization of antigenic preparations in immunologic studies has long been a major limitation in Malassezia research, and the use of defined antigens in future work may finally allow the importance of Malassezia in various diseases to be defined.
Pityriasis Versicolor
Humoral immune responses.
Studies examining the humoral immune response to Malassezia in PV are summarised in Table 8. One of the earliest studies to examine the host response in patients with PV was carried out by Sternberg and Keddie in 1961 (418). They found that 10 normal subjects had what they considered low titers, as did 4 of the 6 PV patients. However, two of the PV patients with extensive disease had higher titers and were noted to have a larger mass of both yeast and hyphae, but the authors were uncertain of the significance of this finding. The small number of patients studied and the relative insensitivity of the method used limit the conclusions that can be drawn from these data.
TABLE 8.
Studies examining the humoral immune response to Malassezia in patients with PV and controls
| Reference | Subjects | Method | Antigenic preparation and strain used | Results |
|---|---|---|---|---|
| 418 | 6 PV, 10 controls | IIF | Tape strips from PV lesions | 2 of 6 PV patients had higher agglutinating-antibody titers, the rest and the controls had low titers |
| 103 | 14 PV, 14 controls | Clinical isolate of P. orbiculare | ||
| IIF | Whole cells | Higher mean titer in patients than controls (P < 0.05) | ||
| Precipitin | Ether extracted, physically disrupted, dialyzed and filter sterilized | No antibodies detected in either patients or controls | ||
| 152 | 40 PV, 31 controls | P. orbiculare, unknown origin | ||
| Hemagglutination | Physically disrupted, shaken in phosphate buffered saline and filtered (soluble antigen) | No differences in titers measured by IIF or hemagglutination between patients and controls | ||
| IIF | Whole cells | |||
| 123 | 30 PV, 22 controls | IIF | Whole cells of P. orbiculare ATCC 42132 | No difference in titers between patients and controls |
| 476 | 30 PV, 30 controls | IIF | Whole cells of P. orbiculare of unknown origin | Titers of IgG and IgM higher in patients than controls, due to higher titers in a subgroup of patients with erythematous lesions (P < 0.005) |
| 178 | 6 PV, 6 controls | Dot blot | P. ovale ATCC 14521 mechanically disrupted and shaken in phosphate-buffered saline (soluble antigen) | No differences in titers of IgG or IgM between patients and controls |
| 25 | 10 PV, 10 controls | ELISA | Whole cells of M. furfur serovars A, B, and C | No significant differences in titers of Igs, IgM, IgG, IgG1–IgG4, IgA, or IgE between patients and controls |
| 26 | ||||
| 399 | 20 PV, 10 controls | ELISA | M. furfur clinical isolates (oval, round and elliptical forms), dialyzed culture medium (exoantigens) | Higher titers of IgG and IgM in patients; no differences in titers of IgA between patients and controls |
| 380 | 12 PV, 12 controls | IIF | Mycelia from strains Hook (M. furfur), GM 216 (M. obtusa), and 2.PV.WY (M. sympodialis), all serovar A | Significantly higher titers of IgM and IgG in patients than controls, but no difference in titers of Igs, IgA, or IgG1–IgG4 between patients and controls |
The next study of humoral immunity in PV was carried out by Da Mert et al. (103). They found that all the patients and controls had antibodies to Malassezia but that patients had significantly higher titers than controls did (P < 0.05). The titers did not correlate with disease activity. The authors concluded that the elevated antibody titers were due to overt infection and not just colonization with the organism. Since no data on the respective ages of the patients and controls or the population densities of Malassezia on their skin were included, such conclusions may not be valid.
The following year, Furukawa et al. (152) published conflicting results, finding no differences in antibody titers between patients with PV and healthy controls. In patients, the titers did not correlate with the duration or distribution of lesions or the presence of pruritus. In patients with high titers, the quantity of Malassezia in stained specimens did not differ from that in patients with low titers.
Another study that was unable to demonstrate differences in antibody titers between PV patients and controls (123) also found that antibody titers did not correlate with either the extent or duration of the lesions. In contrast to Da Mert et al., it was concluded that antibodies to Malassezia were dependent on colonization with the organism and hence could not be used as indicators of disease.
The first study to examine the levels of different isotypes of immunoglobulins was reported by Wu and Chen (476). They found that the mean total IgG and total IgM levels in patients were reportedly significantly higher than the levels in controls (P < 0.005), but the method of statistical analysis was not stated. Titers of IgG and IgM specific to Malassezia were significantly higher in patients than in the control group. The authors subdivided the patients with PV into three groups according to the lesions present, namely, erythematous, pigmented, or hypopigmented. When the data for antibody titers were reanalyzed for these groups, it was found that the group with erythematous lesions had very elevated titers of specific IgG and IgM, which accounted for the overall difference in PV patients. Wu and Chen concluded that the presence of antibodies might explain the containment of the organism within the epidermis.
Hashimoto et al. (178) were unable to find differences in antibody titers of IgG or IgM between six patients with extensive, recurrent PV and healthy controls. They used a dot blot assay (186) with antigen bound to nitrocellulose paper and detected with a labelled second antibody.
Ashbee et al. used an ELISA method to determine antibody titers in patients with PV and controls (25, 26). Despite using a more sensitive technique than those used in previous studies, the authors did not document any significant differences in the titers of any of the classes of immunoglobulins between patients and controls. Levels of the four IgG subclasses were similar, contrasting with the normal proportions of IgG1 through IgG4 in serum, which were 65, 25, 6, and 4%, respectively (326). IgG4 may be a marker of chronic antigen exposure, occurring as a late antibody response to high levels of antigen (389), and so both patients and normals are chronically exposed to the antigens of Malassezia. The presence of IgG1 and IgG3, elicited in response to protein antigens and of IgG2 elicited in response to carbohydrate antigens indicated that Malassezia presents both protein and carbohydrate antigens to the immune system. The finding of Malassezia-specific IgE contrasts with other reports that have suggested that such antibodies are present only in atopic individuals, particularly those with AD (35, 41, 69, 467).
The most recent study to examine antibody responses in PV patients also used an ELISA and Western blot technique to examine sera from PV patients and healthy controls (399). Levels of IgG and IgM antibodies were significantly higher in patients (P < 0.05), but levels of IgA did not differ between patients and controls. Sera from patients with PV, when reacted in Western blots, recognized 12 components of the exoantigen preparation, ranging from 25 to 94 kDa, with the immunodominant 70-kDa molecule being recognized by 84% of patient sera.
All the studies mentioned so far have made use of yeast cells of Malassezia as the antigens. However, it is widely accepted that in PV Malassezia undergoes conversion from the yeast to the mycelial phase, and therefore the immune response directed against the mycelial phase is likely to be important. A study recently has examined the humoral immune response to the mycelial phase of Malassezia in patients with PV and in controls (380). Both patients and controls had measurable antibody titers, and for IgG and IgM, the mean antibody titer in patients was significantly higher than in controls. The authors suggested that a greater load of mycelia or prolonged exposure to the mycelia in patients would explain these results, and hence they were a consequence of disease rather than the cause.
Cellular immune responses.
The diversity of results seen in studies of humoral immunity is mirrored in the studies of cellular immunity, summarized in Table 9. The first two studies of cellular responses in PV patients resulted in the widely stated dogma that PV patients have a cell-mediated immune deficiency specific to Malassezia. In the first study, Sohnle and Collins-Lech (409) used the LMI assay and LT assay. The responses in the LT assay were not significantly different between the two groups, but in the LMI assay the response of patients was significantly lower than that of controls (P < 0.001). In a second study, the same authors again used the LT and LMI assays to measure cellular responses (411), but in contrast to the previous study, they documented “diminished lymphocyte transformation responses” in patients compared to controls. Detailed analysis of their data demonstrates that on day 6 of the LT response there was indeed a significant difference in the responses between patients and controls. However, the authors also stated that the peak response in the LT assay did not occur until day 9, at which time there was no significant difference in the responses between the two groups. This lack of difference in peak responses does not support their conclusion that patients have a cell-mediated immune deficiency. It may be that the rate with which the lymphocytes from patients and controls transformed was different, but they reached the same peak levels. Sohnle and Collins-Lech believed that in patients with PV there were fewer lymphocytes sensitised to Malassezia than in controls. However, since the peak responses were not different between PV patients and controls, this conclusion seems unlikely.
TABLE 9.
Studies examining the cellular immune response to Malassezia in patients with PV and controls
| Reference | Subjects | Method | Antigenic preparation and strain used | Results |
|---|---|---|---|---|
| 409 | 12 PV, 15 controls | LT | Ether extraction, physical disruption, dialysis and lyophilization of P. ovale and P. orbiculare (clinical isolates) | No differences in responses between patients and controls |
| LMI | Responses to P. orbiculare significantly lower in patients than controls (P < 0.001) | |||
| 411 | 18 PV, 42 controls | LT | Ether extraction, physical disruption, dialysis, and lyophilization of P. ovale and P. orbiculare (clinical isolates) | Lower responses to P. orbiculare in patients than controls on day 6 but not day 9 (peak response) |
| 477 | 31 PV, 30 controls | LT | “Crude extract” of P. orbiculare | Higher “lymphocyte responsiveness” in patients than controls |
| 28 | 10 PV, 10 controls | LMI | Whole cells of M. furfur serovars A, B, and C | No differences between patients and controls |
| LT | More patients than controls responded to serovar B (P < 0.05) | |||
| 46 | 12 PV, 12 controls | LT | Freeze-pressed cells of M. furfur (ATCC 42132) | Stimulation significantly lower in patients than controls (P < 0.001) |
| 378 | 12 PV, 12 controls | LMI | Mycelia from strains Hook (M. furfur) | No difference in responses between patients and controls |
| LT | GM 216 (M. obtusa), and 2.PV.WY (M. sympodialis), all serovar A | Patients had higher response to all three antigens than did controls (P < 0.05) |
In contrast to these findings, other workers have not documented any deficiency in cellular immunity to Malassezia in patients with PV. One study found no difference in the lymphocyte proliferation response to mitogens between patients and controls and an increased response to Malassezia in patients (477). Ashbee et al. used the LMI and LT assays to determine cellular immunity to the three serovars of Malassezia (28). More patients than controls responded to serovar B (P < 0.05) in the LMI assay, but there were no differences between the responses of patients and controls for serovars A and C or in the LT assay. Ashbee et al. (28) suggested two possible explanations for the difference between their results and those of Sohnle and Collins-Lech (409, 411). Firstly, when Sohnle and Collins-Lech compared peak responses, there were no significant differences between patients and controls in their results (411). Second, serovar A was found to be the least stimulatory in the LT assays, and Ashbee et al. suggested that use of such a strain by Sohnle and Collins-Lech could lead to the impression of a generalized cellular immune deficiency if no other strains were tested. Therefore, Ashbee et al. found no evidence of a cellular immune deficiency in patients with PV.
Recently, Bergbrant et al. (46) reported significantly lower LT responses to Malassezia in patients with PV than in controls (P < 0.001), but the responses to mitogens did not differ between the two groups.
The cellular immune responses to the mycelial antigens of Malassezia were measured in a recent study (378). In the LT assay, overall, patients had significantly greater responses to antigens than controls (P < 0.05), but in the LMI assay, there were no significant differences in the responses between patients and controls. The generally low response to the mycelial phase of Malassezia was suggested as a possible reason for the chronic nature of PV.
In conclusion, no consensus has emerged with regard to humoral immune responses in PV patients, with some studies reporting higher antibody levels in patients and others finding no differences. Despite early studies suggesting a cellular immune deficiency to Malassezia in patients with PV, other groups have not found this; indeed, several have found increased reactivity to Malassezia in patients.
Lesional infiltrates.
The studies examining both the cellular and humoral immune responses in PV have all quantified responses in peripheral venous blood. Obviously, taking peripheral blood and testing the responses in vitro in an artificially defined system is far different from the response which may occur in the skin during disease. Further information about the likely immune response in the lesions may be obtained by examining the inflammatory infiltrate in lesions. Although PV is generally associated with minimal inflammation, an infiltrate is seen in the lesions. The first study to characterize the infiltrate found increased numbers of T lymphocytes in the lesions, consisting mainly of Th cells with few Tc cells (386). The number of Langerhans’ cell in the dermis was increased in some lesions. Monocyte numbers were also increased in the dermis in some lesions, and monocytes were present in the epidermis in other lesions. Subsequent studies have confirmed the increased number of Langerhans’ cells and the predominantly Th lymphocytic infiltrate (66, 178). However, while one study did not find any monocytes present (178), the other noted a macrophage density of up to 40% (66). The close apposition of Langerhans’ cells to fungal elements in the epidermis may be tangible evidence of antigen presentation by these cells. Most of the infiltrating cells were activated, and expression of HLA-DR was seen in 64% of the cells in the dermis (66). The same pattern of infiltrate has been observed in bacterial, fungal, and noninfectious dermatoses (66).
Seborrheic Dermatitis and Dandruff
Humoral immune responses.
As with PV, there have been many studies examining the humoral immune response to Malassezia in patients with SD, and similarly divergent results have been reported (Table 10).
TABLE 10.
Studies examining the humoral immune responses to Malassezia of patients with SD and dandruff and of controls
| Reference | Subjects | Methoda | Antigenic preparation and strain used | Results |
|---|---|---|---|---|
| 12 | 24 dandruff, 24 hair loss, 10 controls | IIF | Whole cells of P. ovale and P. orbiculare | Highest titers seen in patients with largest amount of dandruff; similar titers for both organisms |
| 292 | 40 SD, 40 controls | CIE | Cytoplasmic antigens from several isolates of unknown origin | 73% of SD patients had antibodies compared to 25% of controls (P < 0.001) |
| ELISA | Cytoplasmic antigens of P. orbiculare, unknown origin | Mean level of antibodies higher in patients due to IgG but not IgM | ||
| 49 | 30 SD, 60 controls | IIF | P. ovale (ATCC 42132) whole cells | No differences in titers of IgG between patients and controls |
| 50 | 10 SD, 10 controls | IIF, FACS, ELISA | P. ovale (ATCC 42132) (whole cells, protein extract, and carbohydrate extract) | The only method able to demonstrate differences in titers between patients and controls was ELISA with the protein antigen (titers lower in patients than controls, P < 0.02) |
| 51 | 30 SD | IIF | P. ovale (ATCC 42132) whole cells | No differences in levels of IgG in patients compared to historical controls |
| 219 | 19 SD, 19 controls | IIF | P. ovale (ATCC 42132) whole cells | No differences in titers of IgG between patients and controls |
| 25 | 10 SD, 10 controls | ELISA | Whole cells of M. furfur serovars A, B, and C | No differences in levels of Igs, IgM, IgG, IgG1–IgG4, or IgA between patients and controls |
| 399 | 25 SD, 10 controls | ELISA | M. furfur clinical isolates (oval, round, and elliptical forms), dialyzed culture medium (exoantigens) | Titers of IgG higher in patients than in controls; titers of IgM higher in patients than controls (to oval form only); no differences in titers of IgA between patients and controls |
| 328 | 19 SD, 19 controls | ELISA | M. furfur L251 and GM 812 (whole cells and cytoplasmic preparation) | No differences in titers of IgG or IgM to either antigen between patients and controls |
CIE, Countercurrent immunoelectrophoresis; FACS, fluorescence activated cell sorter analysis.
The earliest study characterizing antibody titers was carried out by Alexander in 1968 (12) to attempt to determine whether hair loss was related to dandruff. She found that the highest antibody titers correlated with the largest amounts of dandruff but that changes in the severity of the dandruff did not immediately result in changes in titers. Antibody levels did not appear to correlate with hair loss. Statistical analysis of the results was not performed, and so it is unknown if the differences were statistically significant.
It was another 20 years before the next study was carried out, and its results supported the findings of Alexander. More patients with SD had antibodies to Malassezia than did controls (P < 0.001) (292), and the higher levels of antibodies in patients was due to increased IgG, but not IgM, levels. Interestingly, when Western blot analyses were performed on the sera, the main band recognized was a 35-kDa band by sera from the SD patients, while sera from four AD patients recognised a 66-kDa band. However, more recently, a 37-kDa antigen has been described in immunoblots using sera from AD patients, which may be the same as the 35-kDa antigen recognized in this study.
Many groups have found no differences in IgG titers to Malassezia between patients with SD and controls despite studying larger numbers of subjects (49, 51, 176, 219).
The effect of different methods and different antigen preparations on the results of antibody determinations was examined by Bergbrant et al. (50). Comparing combinations of various methods and antigens, the only combination able to find a significant difference in antibody titers between SD patients and controls was an ELISA using a cell wall protein antigen. Unusually, this combination found lower levels of antibodies in patients than in controls (P < 0.02)
Another study of humoral immunity in SD patients carried out by Ashbee et al. (25), found no differences in antibody titers between patients and controls.
Silva et al. (399) demonstrated higher titers of IgG antibody in SD patients than in controls, but the titers of IgA did not differ between groups. Western blots using the sera from the SD patients showed that the immunodominant antigens were 65, 70, and 84 kDa, recognized by 100, 67, and 53% of the sera, respectively. Only 25% of the control sera recognized the 65- and 70-kDa antigens. Therefore, Silva et al. suggested that the 65-, 70-, and 84-kDa components were markers for SD. This differs from the findings of Midgley and Hay (292), in which a 35-kDa antigen was the main one recognized by sera from SD patients.
The most recent study, in 1998, found no significant differences in titers of IgG or IgM antibody between patients and controls (328). Western blots using the cytoplasmic antigen demonstrated recognition of bands ranging from around 30 kDa to over 116 kDa. However, the pattern of bands was similar for both patients and controls, and so no specific protein or group of proteins was a marker of disease, in contrast to the findings of Silva et al. (399).
Neuber et al. (307) studied immunoglobulin production by lymphocytes from patients and controls, either de novo or in vitro, in response to Malassezia. Levels of spontaneously produced IgA and IgG were not different between patients and controls, but levels of IgM were significantly higher in patients (P < 0.05). Production of IgA and IgM was significantly higher in patients after stimulation with Malassezia, but only at one of four antigen concentrations used; at the other concentrations no differences were observed.
In general, most studies have not demonstrated significant differences in antibody levels between patients and controls, although one found lower levels in patients and two found increased levels.
Cellular immune responses.
Relatively few studies have examined cellular immunity in SD. One mechanism postulated to be involved in the pathogenesis of SD is contact sensitization to the antigens of Malassezia. Application of killed Malassezia cells resulted in lesions similar to SD in an early study (297) and scaling of rabbit skin (375). Therefore, Nicholls et al. (310) carried out patch testing on 11 patients with SD by using various concentrations of cell wall and cytoplasmic antigens from Malassezia. They tested untreated skin and skin that had been repeatedly stripped with adhesive tape to remove loosely adherent skin squames and so improve the sensitivity of the test. However, only one patient had an irritant reaction to the antigens. Patch testing of 19 SD patients in another study also yielded no positive result (219), suggesting that contact sensitization to Malassezia is not important in SD.
Another mechanism of hypersensitivity to Malassezia, type I or IgE mediated, has also been studied in SD. Skin prick tests were performed on 19 SD patients with a commercially available protein extract of Malassezia (219). Histamine release in response to the same antigen was also measured in 15 of the SD patients. However, none of the patients gave positive responses in either test. Lymphocyte subpopulations were determined, and a low Th/Ts ratio was noted in 13 of the SD patients, due to an increase in the suppressor T-cell population. Further studies by the same investigators, however, reported normal or high Th/Ts ratios in 30 patients with SD (51). Lymphocyte stimulation with the mitogens phytohemagglutinin and concconavalin A was low in 13 patients but the investigators did not test any Malassezia antigens in the assay. Only four studies have determined cellular immune responses specific to Malassezia in patients with SD (Table 11). Ashbee et al. found that significantly more patients than controls responded to serovars B and C in the LT assay and more patients than controls responded to serovar C in the LMI assay (28). Therefore, patients with SD were generally more responsive to Malassezia than controls were.
TABLE 11.
Studies examining the cellular immune responses to Malassezia of patients with SD and controls
| Reference | Subjects | Method | Antigenic preparation and strain used | Results |
|---|---|---|---|---|
| 28 | 10 SD, 10 controls | LT | Whole cells of M. furfur serovars A, B, and C | More patients responded to serovars B and C than did controls (P < 0.025) |
| LMI | More patients responded to serovar C than did controls (P < 0.025) | |||
| 307 | 10 SD, 10 controls | LT | P. ovale (DSM 6170), sonicated and filtered extract | Mononuclear cells from patients not stimulated by antigen, while mononuclear cells from controls were stimulated |
| 328 | 16 SD, 16 controls | LT | M. furfur L251 and GM 812 (whole cells, cytoplasmic preparation, cell wall preparation, sonicated preparation, and ALK commercial preparation) | No differences in the responses between patients and controls |
| 46 | 15 SD, 15 controls | LT | Freeze-pressed cells of M. furfur (ATCC 42132) | No differences in the responses between patients and controls |
In contrast, Neuber et al. (307) used a sonicated extract of Malassezia and found that stimulation of lymphocytes from healthy controls occurred at two concentrations of the antigen, but the lymphocytes from SD patients were not stimulated. Stimulation of PBMC with Malassezia resulted in significantly higher levels of IL-2 and gamma interferon (IFN-γ) in controls than in patients. However, levels of IL-10 were higher in patients than in controls, both before and after stimulation with Malassezia. They suggested that since IL-10 inhibits monocyte/macrophage-dependent T-cell proliferation and cytokine production, this might explain the reduced response to Malassezia that they documented. However, the stimulation indices reported were all less than 3, much lower than those from other studies. No data was included for mitogen responses for comparison, and it is possible that an inhibitory substance was present in the assay and caused the low stimulation indices.
Parry and Sharpe (328) carried out lymphocyte proliferation assays, and a wide range of responses were noted, but there were no significant differences in responses between patients and controls.
The most recent study of cellular immunity in SD also did not show any difference in response between patients and controls to Malassezia or mitogens (46).
In summary, most studies of cellular immunity in SD have demonstrated either increased reactivity to Malassezia in patients or no differences between patients and controls.
Lesional infiltrates.
An early histological study of SD demonstrated the presence of lymphocytes within lesions (335), and this was confirmed later when they were identified as mainly Th CD4-positive cells (51). Langerhans’ cell numbers were not increased in lesional skin, and HLA-DR expression on keratinocytes was noted only infrequently (51). An extensive study recently found the infiltrate to consist of lymphocytes, macrophages, monocytes, and Langerhans’ cells, with a few granulocytes (131). Within the lesions, expression of NK1 and CD16 were increased, suggesting a nonimmunogenic irritant reaction.
Malassezia Folliculitis
Humoral and cellular immune responses.
The role of the genus Malassezia in folliculitis is a matter of some debate, and there has been only one study characterizing the host immune response (137). It included 32 patients with folliculitis and 25 controls and determined their reactivity to Malassezia in the SPT and titers of Malassezia-specific IgG. The SPT was negative in 27 patients and 22 controls, indicating that a type I hypersensitivity to Malassezia is not responsible for the wheal-and-flare-type reaction seen in Malassezia folliculitis. Titers of IgG to Malassezia were significantly higher in the patients than the controls (P < 0.01). The authors suggested that the presence of Malassezia deep within the follicle would lead to better antibody production in comparison to their normal site on the stratum corneum; however, Malassezia is known to be present in normal follicles, so this explanation is not convincing.
To date, no studies have characterised the cellular immune responses of patients with folliculitis to Malassezia or mitogens.
Lesional infiltrates.
The infiltrate present in Malassezia folliculitis has been extensively studied, and many cell types have been reported. In most cases where the follicle remains intact, the infiltrate has mainly been reported to consist of lymphocytes (59, 74, 131, 149, 158, 460, 483). However, in cases where the follicle had ruptured, the infiltrate was more extensive and varied, consisting of lymphocytes (340, 403), neutrophils (142, 177, 199, 340, 403, 450, 483), macrophages (340, 403, 483), eosinophils (142, 340), or plasma cells (340). Therefore, the extent of the infiltrate seems to be a reflection of whether the follicle had ruptured. This is similar to acne vulgaris, where the initial infiltrate is lymphocytic (316) but after the follicle ruptures a more extensive infiltrate occurs due to the release of follicular contents, which are known to be inflammatory (193, 244, 461). A study recently found an increase in NK1- and CD16-positive cells, which was interpreted as indicating an irritant reaction (131).
Atopic Dermatitis
Of all the conditions with which Malassezia has been associated, AD is perhaps the one currently receiving the most attention and the area of research producing the most interesting results.
Many factors may be involved in the development of AD, including the presence of specific genes within the individual’s genetic material (147); exposure to various environmental (144, 443), food (448), or microbial (2) allergens; and immunological disturbances within the skin. The pivotal role of the immune system is demonstrated by the acquisition of AD by a previously nonatopic individual after a bone marrow transplant (6). The immunological disturbances in patients with AD alter as the disease progresses, but the relative balance between Th1 cells (producing IL-2 and IFN-γ) and Th2 cells (producing IL-4, IL-5, and IL-13) appears to be critical. In the acute phase of AD, the Th2 response predominates, but as the lesions become more chronic, a switch to the Th1 response occurs, probably triggered by the increased levels of IL-12, resulting from lesional infiltration by eosinophils and macrophages. The immunological disturbances present in AD and the clinical manifestations that result are detailed in Table 12. Additionally, increased spontaneous histamine release by basophils, decreased cytotoxic T-cell numbers and function (250), and immediate-type hypersensitivity to a wide range of allergens have all been documented in AD (249).
TABLE 12.
Immunological disturbances in AD and their resultant clinical manifestationsa
| Cell type | Mediatorb | Direct effectb | Clinical manifestation |
|---|---|---|---|
| Th2 | IL-13 | IgE isotype switching | ↑ Serum IgE levels |
| ↑ VCAM expression | Migration of mononuclear cells and eosinophils to lesions | ||
| IL-4 | IgE isotype switching | ↑ Serum IgE levels | |
| Inhibits IFN-γ production | Polarizes response towards Th2 | ||
| Inhibits differentiation of Th1 cells | |||
| Induces low-affinity IgE (CD23) receptor expression on macrophages | IgE binding to macrophages, via CD23, induces secretion of leukotrienes, IL-1, and TNF-α and so contributes to inflammation | ||
| IL-5 | Enhances differentiation, migration, and survival of eosinophils | Migration of eosinophils into lesions | |
| Monocytes | ↑ IL-10, ↑ PGE2 | Inhibit IFN-γ production | Polarize response toward Th2 |
| Mast cells | IL-4 | Drives Th to Th2 | Polarizes response toward Th2 |
| Langerhans’ cells | High- and low-affinity (CD23) IgE receptors | Binding of allergen and presentation to T cells | Contributes to inflammation in lesions |
| Keratinocyte | IL-1, TNF-α and IL-4 | Induction of adhesion molecules within the skin | Migration of lymphocytes, macrophages, and eosinophils into lesions, sustaining inflammation |
| GM-CSF and TNF-α | ↑ Number and antigen-presenting ability of dendritic cells in skin | Contributes to the chronicity of lesions | |
| GM-CSF | Enhances survival and function of monocytes, lymphocytes, and eosinophils | ||
| Eosinophils and macrophages | IL12 | Switch toward Th1 response | Lesions become lichenified and develop epidermal hyperplasia and dermal fibrosis |
Humoral immune responses.
Many groups have measured titers of IgE specific to Malassezia in patients with AD. One early study compared titers of IgE in 34 patients with AD and 10 atopic patients with no eczema but with asthma and/or rhinitis (485). Using RAST, the investigators showed that 65% of the 22 AD patients had an elevated level of IgE to Malassezia, compared to none of the atopic controls (P < 0.001). Examining sera from 131 atopic children, Nordvall and Johansson found that 26 (19.8%) had IgE detected by a freeze-dried and sonicated Malassezia antigen in RAST (314). Levels of specific IgE were low or moderate and were found significantly more frequently in children with current eczema (P < 0.0001). Several further publications by the same groups, using the same antigenic preparation and method, have reported similar results. IgE titers were highest in patients with eczema, either alone or in combination with other atopic manifestations (asthma and rhinitis), compared to those in patients with rhinitis alone (69, 315) or healthy controls (35). Levels of Malassezia-specific IgE were quantified in 15 AD patients, and in 13 of them the level was above the “atopic” cutoff of 0.35 kU/liter (437). For patients, the median amount was 8.3 kU/liter, but all controls had levels of <0.35 kU/liter.
Studies examining patterns of IgE reactivity in AD patients have found that significant cross-reactivity occurs between allergens. Patients with AD who are sensitized to fungi often have severe disease and react to a wide range of allergens (192). Cross-reactivity between yeasts has been documented (306), and IgE to Malassezia has been shown to cross-react with C. albicans (111, 113, 192, 256, 385, 488), molds (314), Saccharomyces (200) and house dust mites (112, 200). The degree to which IgE to Malassezia and C. albicans cross-reacts and the molecule which elicits the cross-reactivity have been the subject of some debate. Several investigators have found significant cross-reactivity between high-molecular-mass glycoproteins or polysaccharides, probably mannans or mannoproteins (110, 111, 113, 256, 385). Because IgE to C. albicans was rarely found in the absence of IgE to Malassezia and because the avidity of IgE to Malassezia was greater than that of IgE to C. albicans, Doekes et al. suggested that anti-C. albicans IgE was due to cross-reacting IgE elicited by polysaccharides present in Malassezia that was present on the patient’s skin (113). Therefore, IgE was never specific to C. albicans but was solely due to cross-reaction. Although some other groups have also found cross-reacting IgE to mannans (256, 385), Zargari et al. have found limited cross-reactivity between Malassezia and C. albicans. They produced monoclonal antibodies specific to two protein allergens in Malassezia and found no cross-reactivity to C. albicans (487, 488), although a third monoclonal antibody to a “non-protein cell wall component” did show some cross-reactivity (488). A subsequent study confirmed the limited cross-reactivity when the investigators found that protein allergens in C. albicans elicited IgE that was specific to the organism (192). Therefore, they were able to demonstrate IgE specific to C. albicans and refute that idea that all IgE was due to cross-reaction with Malassezia.
A recent study made purified mannan and crude extracts from Malassezia, S. cerevisiae, and C. albicans and crude extracts of Rhodotorula rubra and Cryptococcus albidus to study the cross-reactivity of IgE and IgG from patients with AD (257). They found cross-reacting IgE and IgG between the yeast mannans and concluded that mannan contained most of the epitopes eliciting cross-reacting antibodies, whereas there was little cross-reactivity between proteins.
Kroger et al. (235) studied the effect of a sonicated preparation of Malassezia on IgE, IL-2, IL-4, IL-10, and IFN-γ production by PBMC in vitro. They included PBMC from eight patients with AD, five of whom had IgE specific to Malassezia measured by RAST, and five healthy controls. Stimulation with Malassezia resulted in increased levels of IgE in RAST-positive AD patients, but these became significantly increased only if IL-4 was present during the stimulation. Levels of IL-2 and IFN-γ were significantly higher when PBMC from healthy controls were stimulated, while IL-4 and IL-10 levels in PBMC from RAST-positive AD patients were significantly higher. Kroger et al. concluded that this pattern of IgE and cytokine release might be due to Th2 Malassezia-specific cells in patients who were RAST positive. Therefore, in these patients, Malassezia might act a continuous allergic stimulus by stimulating these Th2 Malassezia-specific cells and IgE production.
To date, most groups have found that levels of Malassezia-specific IgE do not correlate with the severity of disease, although they have been reported to correlate with levels of total IgE (35, 69, 467, 485) or levels of IgE specific to C. albicans (314, 467). Therefore, although levels of IgE specific to Malassezia may be elevated in patients with AD, since around 85% of AD patients have increased total levels of IgE in serum (248), this finding may be a consequence of their general atopic state and not proof of a role for Malassezia in AD.
Hypersensitivity to Malassezia.
Two tests that have been widely used clinically to indicate hypersensitivity to allergens are the SPT and patch test. In the SPT, which tests for type I or IgE-mediated hypersensitivity, a drop of dilute antigen is placed on the skin and a needle is used to puncture the skin through the antigen. However, the reliability of this test has been called into question in AD, where patients may react to many allergens without clinical relevance (91, 265). In patch testing, which tests for type IV or delayed-type hypersensitivity, the suspected allergen is applied to the skin under a nonabsorbent adhesive patch and left for 48 h. After this time (or before if burning or itching develops), the patch is removed and the presence of redness and/or swelling noted. The sites are further examined 72 and 96 h after application of the patch, since reactions may not develop until after the removal of the patch. Patients with AD often have decreased delayed-type hypersensitivity (265), in contrast to their increased type I hypersensitivity. A third test is the histamine release assay, in which allergen-specific IgE on basophils is detected by measuring histamine release from a subject’s white blood cells, again testing for type I hypersensitivity. Again, AD patients have increased spontaneous basophil histamine release and hence increased reactivity in this test. All of these tests have been utilized by investigators attempting to define the role of Malassezia in AD.
The first investigators to implicate Malassezia in AD did so on finding that AD patients, particularly those with lesions localized to the head and neck, had a greater response to Malassezia than to histamine in the SPT (91). These preliminary findings were expanded when the same group studied 741 patients with various atopic symptoms, carrying out SPT with an aqueous extract of Malassezia (453). Of the patients with head and neck lesions, 28% reacted to Malassezia, compared with only 6% of patients with generalized AD and 0 to 2% of patients with other manifestations of atopy or urticaria. Other studies that have used the SPT to determine sensitivity to Malassezia have reported similar findings, with increased sensitivity in patients with generalized AD (69, 371, 485) or those with lesions localized to the head and neck (219, 221, 312, 425). While the findings have been broadly similar, discrepancies in the methods exist. Many studies used a commercially available antigen preparation (69, 219, 221, 312, 371, 485), while others used in-house antigens (219, 371, 485). The definitions of positive response also vary. Most have been measured in relation to a histamine control, but the concentrations of histamine used included 1 mg/ml (91), 3 mg/ml (219) and 10 mg/ml (69, 221, 312). Consequently, direct comparisons between results may not be valid. SPT results were found to correlate with levels of Malassezia-specific IgE (69, 221), with histamine release assay results (219), or with total IgE levels (69), but not with the severity of disease (221).
Type I hypersensitivity can also be detected in vitro using the histamine release test. Three studies have used this assay and recorded positive responses to Malassezia in at least 70% of patients with head and neck dermatitis (219, 312, 425) and up to 50% of patients with generalized AD (219, 425). Up to 11% of healthy controls also gave positive responses in one study (49), but none of them were positive in the two other studies (312, 425).
Patch tests have been used to detect hypersensitivity in AD patients, although they have been used less frequently than SPTs. One large study included 118 patients with AD and 40 healthy controls, in addition to patients with various other dermatoses (371). Prior to patch testing with a sonicated extract of Malassezia, skin was scarified with a needle to overcome its barrier function and allow the penetration of antigens. Patch tests, read at 72 h, were positive in 64% of AD patients but only 2.5% of controls. Interestingly, and in contrast to other studies, 53% of patients with PV and 9% of patients with SD were also patch test positive. In another study, including 33 patients with head and neck dermatitis and 22 with generalized AD, much lower patch test positivity to Malassezia was recorded (219). After 72 h, four patients with head and neck lesions (12%) and three patients with generalized AD (14%) were patch test positive but none of the 19 healthy controls were positive. The most recent study also found that 53% of AD patients were patch test positive to Malassezia after 48 h but that none of the controls or patients with SD were (437). Patch test results have been found to correlate with levels of Malassezia-specific IgE (437) but not with SPT results (371), total IgE levels, or disease severity (437).
Therefore, no study using SPTs, patch tests, or histamine release to test reactivity against Malassezia have been able to correlate the results with the severity of AD in patients. Because AD patients may be generally more reactive to a range of allergens, the significance of the preceding results is difficult to assess. Some of the studies did not test antigens other than Malassezia antigens (91, 453), and so these results may not be specific to Malassezia. However, some groups have tested other common allergens in parallel to Malassezia, including C. albicans, Cladosporium herbarum, Aspergillus fumigatus, Penicillium notatum, and Dermatophagoides pteronyssinus (219, 221, 312, 371), and found that generally more patients responded to Malassezia than to the other allergens. In addition, reactivity to Malassezia did not always coincide with reactivity to the other allergens, suggesting that in many patients the response to Malassezia is specific and not simply a reflection of their generalized atopic state.
Cellular immune responses.
Cellular immune responses to Malassezia in AD patients have been measured using lymphocyte proliferation assays. The first study, by Rokugo et al. (371), included an unknown number of patients and controls and demonstrated significantly higher stimulation indices in AD patients than in controls (P < 0.005). Three other studies used a freeze-dried sonicated extract of Malassezia to stimulate PBMC from patients with AD and healthy controls (436–438). Comparison of the data from the first two studies suggests that nine of the patients and five of the controls were common to both studies. The stimulation indices were significantly higher in AD patients than in controls (P < 0.05) (436, 438). In the most recent study, including 15 patients, only 4 patients had significant stimulation indices and the responses in the control group were not measured (437). The responses in the AD group were not significantly different from those in a group of eight patients with SD.
Therefore, although lymphocyte proliferation responses in patients with AD in response to Malassezia do seem to be higher than those in healthy controls, only limited numbers of subjects have been studied and so firm conclusions cannot yet be drawn. While many studies have examined cellular immune responses to Malassezia in peripheral blood lymphocytes, very few have studied responses by lesional T lymphocytes. Tengvall Linder et al. (438) derived T-lymphocyte clones from the skin and peripheral blood of a single patient with AD. Th lymphocytes are thought to be important in the pathogenesis of AD, and previous studies have shown that allergen-specific Th lymphocytes are mainly of the Th2 type (producing IL-4 and IL-5) (250, 447). The T-lymphocyte clones produced from lesional skin were mainly Th lymphocytes (CD4+). Cytokine production was measured from freshly isolated PBMC and the T-lymphocyte clones after stimulation nonspecifically with a mitogen or with Malassezia. Mitogenic stimulation of PBMC produced no measurable cytokines, while stimulation with Malassezia resulted in low levels of IL-4, IL-5 and IFN-γ. Mitogenic stimulation of T-lymphocyte clones produced a range of cytokine responses, characterized as Th0, Th1, and Th2. When the T-lymphocyte clones were stimulated with Malassezia, increased production of IL-5 was found but the levels of IL-4 and IFN-γ were not increased. Therefore, most T-lymphocyte clones from lesional skin had a Th2 or Th2/Th0 cytokine profile, and so the continuous stimulation of T lymphocytes in the skin by Malassezia may be involved in maintaining inflammation in AD. In a subsequent study by the same group (436), cytokine production by PBMC and T-lymphocyte clones from 6 healthy controls and 11 AD patients was compared. Stimulation of PBMC with Malassezia resulted in significantly higher levels of IL-5 in AD patients than in controls but no differences in the levels of IL-4 or IFN-γ. When T-lymphocyte clones were stimulated with Malassezia, there were no differences in the production of IL-4, IL-5, or IFN-γ between the two groups. The most recent study of T-lymphocyte clones generated from patients with AD examined T-lymphocyte receptor usage to determine whether Malassezia might act as a superantigen (202). T-lymphocyte usage did not vary between healthy controls and T-lymphocyte clones from AD patients. The T-lymphocyte receptor usage pattern suggested oligoclonal T-lymphocyte expansion in response to many antigens and so did not support the hypothesis of superantigenic activity by Malassezia.
Studies of AD patients have uniformly demonstrated increased immunological reactivity to Malassezia compared to that in controls. This may be as a result of the impaired barrier function of the patients’ skin, but whatever the reason, the response to Malassezia is likely to contribute to the maintenance of the lesions in AD patients.
Lesional infiltrates.
The infiltrate present in AD lesions varies depending on whether they are acute or chronic. In acute lesions, the epidermis contains few T lymphocytes while the dermis contains a marked perivascular T-lymphocytic infiltrate (mainly activated memory T cells) with few monocytes or macrophages. In contrast, chronic lesions are characterised by dermal infiltrates consisting mainly of macrophages and an increased number of mast cells and eosinophils (250).
Patch tests with Malassezia on the skin of patients with AD have demonstrated a correlation between the patch test score and the size of the dermal T-lymphocyte infiltrate at 72 h (437). The infiltrate consisted mainly of Th lymphocytes (CD4+). There were significantly more eosinophils at patch test sites in AD patients and increased HLA-DR and ICAM-1 expression at 72 h in patch test-positive AD patients than in patch test-negative patients and healthy controls. Therefore, the application of Malassezia to the skin of AD patients elicited an infiltrate that was characteristic of the disease. Another study also noted the presence of a large T-lymphocyte infiltrate (mainly Th lymphocytes) with significant ICAM-1 expression (438).
Other Superficial Diseases
Malassezia has been associated with a wide range of other superficial diseases, and in many of them, therapy with antifungal agents has proved beneficial (21, 124, 136, 140, 215). However, few studies have examined the immune response of patients to Malassezia.
Acne vulgaris.
One study of 18 patients with severe acne vulgaris and 18 age- and sex-matched controls examined humoral immunity to Malassezia (195). It reported no differences in the titers of IgG and IgM specific to Malassezia between patients and controls and concluded that if Malassezia contributed to the inflammation present in acne lesions, the humoral immune response was not involved.
Psoriasis.
Several studies have examined the responses of psoriasis patients to Malassezia. One of the early studies (375) noted that if dense suspensions of Malassezia were applied to the shaved skin of rabbits, lesions similar to psoriasis resulted. The lesions persisted as long as Malassezia continued to be applied but otherwise resolved within 3 to 4 days. Further work by the same group used patch testing with Malassezia to study the response in 10 patients with psoriasis and 10 controls (259). All of the patients developed psoriatic lesions when challenged with Malassezia, and biopsy specimens showed features consistent with psoriasis. Therefore, Malassezia was able to elicit psoriasiform lesions in both animals and humans.
Additional support for the role of Malassezia in psoriasis comes from successful use of ketoconazole in patients (374). Although ketoconazole may act by a direct antifungal mode of action, it has also been shown to suppress Malassezia-induced proliferation of lymphocytes from psoriatic patients (13), thus reducing the response to antigenic stimulation in lesions.
The first study to characterize the humoral immune response to Malassezia in patients with psoriasis was carried out by Squiquera et al. (417). This study included 15 patients, of whom 8 had active disease, and 10 healthy controls. SDS-PAGE of Malassezia extracts was performed, and the test sera were immunoblotted. The sera from psoriatic patients bound to a 120-kDa band (11 of 15) and a 100-kDa band (7 of 15), but none of the control sera recognized these bands. Consequently, the authors suggested that antibodies to the 100- and 120-kDa proteins were specific serologic markers for psoriasis. These antibodies were subsequently shown to recognize the N-acetylglucosamine terminals of glycoproteins present in Malassezia (276). However, both of these proteins are recognized by sera from patients with AD (256, 312), and so they are not specific markers for psoriasis.
Neutrophils form part of the inflammatory infiltrate in the dermis of psoriatic lesions (90). A study examining the chemotaxis of neutrophils from psoriatic patients and controls demonstrated that Malassezia induced significantly more chemotaxis in neutrophils from psoriatics than other groups (75). The effect was specific, since Staphylococcus epidermidis did not affect chemotaxis, and was due to a protein. Psoriatic lesions often develop at sites of trauma (the Koebner phenomenon [294]), and the increased chemotactic response of neutrophils to Malassezia was suggested to play a role in this event.
Only one study has examined the cellular immune responses to Malassezia in patients with psoriasis (36). All the psoriatic patients included (n = 13) had lesions on the scalp, with disease durations ranging from 2 months to 20 years. Proliferation of PBMC was seen in response to Malassezia in all of the patients tested. However, since no healthy controls were included in the study, it is not possible to determine whether the responses differed from those of normal individuals. The response of PBMC was due to CD4+ T lymphocytes and required antigen presentation by HLA-DR+ cells, and so it was not mitogenic stimulation. T-lymphocyte lines were established from psoriatic and nonpsoriatic patients and were stimulated with Malassezia. Similar patterns of response were obtained whether the patient had psoriasis or not, and the authors concluded that Malassezia-specific T lymphocytes were not involved in psoriasis.
Consequently, the role that Malassezia plays in psoriasis is, as yet, undetermined. Although it may contribute to the inflammation associated with the disease, via complement activation and neutrophil recruitment, convincing evidence that it is of prime importance in the pathogenesis of the disease is still lacking.
Overall, it is difficult to rationalize and explain the wide range of results documented for humoral and cellular immune responses to Malassezia in patients with PV, SD, folliculitis, AD, and other superficial diseases. The use of different techniques, different antigenic preparations, and organisms from different classifications makes comparison of results unreliable and may partly explain the disparity in the findings. However, many of these limitations can now be overcome. The characterization of several antigens from Malassezia and the ability to produce them in vitro should provide a reliable source of defined antigen for use in future immunological studies. The unification of different classifications of Malassezia into one scheme should removed the uncertainty about whether similar strains or species are being studied. Lastly, the finding that, at least in AD, there is significant cross-reactivity between mannans of different yeasts may encourage the use of protein antigens in immunological assays used to define the response specific to Malassezia. Although our understanding of the immune response to Malassezia in superficial diseases has advanced significantly over the last 10 years, there are still many interesting questions awaiting an answer.
Systemic Diseases
Malassezia has widely been reported to cause sometimes fatal fungemia in premature neonates and, less frequently, in immunocompromised adults. Most of the neonates affected, in addition to being premature, had a serious underlying disease, had central or peripheral catheters inserted, and received parenteral nutrition containing lipid emulsions. No studies have examined the immunological responses of these infants to Malassezia. This is mainly due to the ethical considerations of removing blood from the neonates for research studies, when many of them are seriously ill and become hypovolemic as a result of essential blood tests.
The immune system of neonates is immature in many ways, and in children born prematurely, this is further compounded. The immunological deficiencies that occur in neonates are summarized in Table 13, and it can be seen that most components of the immune system are functionally impaired, numerically reduced, or absent in premature neonates, rendering them susceptible to a wide range of infections.
TABLE 13.
Immunological deficiencies in neonates that contribute to their predisposition to systemic Malassezia infections
| Immune mechanism | Defect | Reference(s) |
|---|---|---|
| Physical barriers | Skin thin and easily damaged | 122 |
| Skin further disrupted by use of monitoring devices and catheters | ||
| Lack of, or limited, commensal cutaneous flora | 218 | |
| Phagocytosis | Neutrophils functionally impaired | 76 |
| Neutrophil killing impaired in presence of other diseases, including respiratory distress syndrome and meconium aspiration pneumonia | 475 | |
| Neutrophils from premature neonates | ||
| contain less myeloperoxidase | 361 | |
| produce less reactive oxygen species | 16 | |
| have reduced adherence and chemotaxis | 42 | |
| Complement proteins | Levels lower in premature neonates | 317 |
| Reduced opsonic activity due to decreased levels | ||
| Antibody production | Lack of maternal IgG transfer to premature neonates | |
| Inability to produce IgM and IgA | ||
| Shortened IgG half-life | 239 | |
| Cellular immunity | T cells from neonates have | |
| reduced mitogenic responses | 78 | |
| low expression of HLA-DR antigens | 484 | |
| decreased IFN-γ production | 456 | |
| Cellular cytotoxicity impaired | 3, 163 |
The use of central and peripheral venous catheters is commonplace in modern medicine. They provide reliable vascular access for administration of fluids, electrolytes, drugs, nutritional support, and hemodynamic monitoring. An unfortunate consequence is that they also provide a portal of entry through which pathogens may enter. Organisms causing catheter-related infections include coagulase-negative stapylococci, Candida species, and other organisms of low virulence (384). These organisms are “sticky” and so are able to adhere to the plastic of the catheters and colonize them (226, 346). Some are also able to form biofilms, which help them attach more firmly to the catheters and also protect them against both immunological attack and the action of antimicrobial drugs (157). The fact that many of the organisms causing catheter-related infections occur on the skin is not coincidental. The cutaneous commensal flora of the patient or health care workers may be the source of the infecting organism (88, 404). Once colonized, the catheters may serve as a reservoir of infection, constantly reseeding the blood. Therefore, even if the immune system is able to clear the organisms from the blood, the infection may not be resolved until the catheter is removed (348).
Malassezia is able to adhere to central venous catheters (220, 346), possibly within a fibrinous mesh (269). The lipid-rich layer around Malassezia (293) may be important in adhesion in this setting. In most cases, removal of the catheter or cessation of infusion of lipid is all that is required to treat catheter-related Malassezia infections (261, 341). Indeed, attempts to sterilize catheters have been ineffective (345) and persistence of organisms in the absence of lipid infusions (105) indicates that the organisms adhering to the catheter may embolize and maintain the infection, unless the catheter is removed.
The use of lipid infusions for parenteral nutrition is therefore a main predisposing factor common to the patients with systemic Malassezia infections (105, 154, 394, 421). Neonates unable to feed orally due to immaturity or disease often receive nutritional support in the form of intravenous feeding. Lipid emulsions form an integral part of the parenteral nutrition, providing essential fatty acids and a high calorie/fluid volume ratio (182). However, as well as the problem of infection due to the presence of the central venous catheter delivering the parenteral nutrition, the lipid itself may be detrimental to the neonate. It has long been known that lipid substances, such as oleic acid, cause a blockade of the reticuloendothelial system in laboratory animals (416). In humans, linoleic acid, present in some of the lipid emulsions, suppresses the generation of reactive oxygen species and decreases phagocytosis by neutrophils in vitro (10, 145). Additionally, it is interesting to speculate that the high concentrations of lipid in parenteral nutrition might cause thickening or increased adhesiveness of the lipid-rich layer around the yeast, thereby facilitating the colonization of catheters by the organism.
The use of lipid emulsions in neonates has been associated with a variety of complications. Reduced clearance of lipid in premature and acutely ill neonates (17, 174), increased risk of kernicterus in jaundiced neonates (18), potentially increased risk of coronary vascular disease (238), altered prostaglandin synthesis and turnover (151), and deposition of lipid within macrophages leading to altered immunity (227, 329) have all been reported. Additionally, deposition of lipid occurs in the lungs of infants receiving lipid emulsions (40, 102, 251), which may exacerbate preexisting respiratory problems. Therefore, although parenteral nutrition may be essential in providing adequate nutrition for some neonates, its deleterious effects on the immune and other systems further predisposes the infants to infections. Because Malassezia requires an exogenous source of lipid to grow, the use of lipid emulsions provides it with some of the nutrients it requires. Growth of Malassezia has been demonstrated in lipid emulsions (105, 343), explaining the finding that it localizes in the lipid deposits within the lungs. Two methods have been proposed to reduce the risk of Malassezia infections during parenteral nutrition in neonates. First, the use of a filter within the catheter has been shown to retain any Malassezia within the lipid emulsion for up to 48 h (368). However, this will be beneficial only if contaminated infusate is the source of the infection. The other method suggested is the use of lipid emulsions containing medium chain triglycerides, which inhibit the growth of some species of Malassezia (327). While these measures may help prevent some infections with Malassezia, the effects of the lipid emulsions on the immune system will continue to predispose neonates to infection.
CONCLUSIONS
In conclusion, the genus Malassezia is an immunological paradox. In some circumstances, it acts as an adjuvant, activates the complement cascade, and elicits both cellular and humoral immune responses in healthy individuals. In contrast, it also seems to have the ability not only to evade the immune system but actually to suppress the response directed against it. Central to this immunosuppressive phenotype appears to be the high levels of lipids in its cell wall. A tempting but perhaps oversimplistic explanation is that in its commensal role, high levels of lipid downregulate the immune system, but that in its “pathogenic” role, the lipid content of the cell wall may be reduced, allowing greater recognition by the immune system. However, immunological studies of patients with PV or SD do not support this theory. It is known that the structure of the layer varies with different lipid sources (293) and that fatty acids are incorporated directly into cellular lipids without further metabolism (80). Therefore, at least in theory, there is a mechanism by which the lipid present on the skin surface may directly affect Malassezia, resulting in either an immunosuppressive or immunostimulatory phenotype.
One fact which has emerged from recent studies is the importance of nonspecific immunity to Malassezia. Previous studies concentrating on specific cellular and humoral immune responses have largely been disappointing in defining differences in immunological responses between healthy individuals and patients with Malassezia-associated dermatoses. Perhaps now is the time to go back to basics and begin where the immune response does, with nonspecific immunity, in our quest to understand the immunology of this fascinating organism in both health and disease.
Acknowledgments
We thank Eileen Ingham for helpful discussions.
REFERENCES
- 1.Abdel-Razek, M., G. Fadaly, M. Abdel-Raheim, and F. Al-Morsy. 1995. Pityrosporum (Malassezia) folliculitis in Saudi Arabia—diagnosis and therapeutic trials. Clin. Exp. Dermatol. 20:406–409. [DOI] [PubMed] [Google Scholar]
- 2.Abeck, D., and M. Mempel. 1998. Staphylococcus aureus colonisation in atopic dermatitis and its therapeutic implications. Br. J. Dermatol. 139(Suppl. 53):13–16. [DOI] [PubMed] [Google Scholar]
- 3.Abo, T., M. D. Cooper, and C. M. Balch. 1982. Postnatal expression of natural killer cell populations in humans identified by the monoclonal HNK-1 antibody. J. Exp. Med. 155:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham, Z., A. Berderly, and E. Lefler. 1987. Pityrosporum orbiculare in children. Mycoses 30:581–583. [DOI] [PubMed] [Google Scholar]
- 5.Ackerman, A. B., and A. M. Kligman. 1969. Some observations on dandruff J. Soc. Cosmet. Chem. 20:81–101. [Google Scholar]
- 6.Agosti, J. M., J. D. Sprenger, L. G. Lum, R. P. Witherspoon, L. D. Fisher, R. Storb, and W. R. Henderson, Jr. 1988. Transfer of allergen-specific IgE mediated hypersensitivity with allogeneic bone marrow transplantation. N. Engl. J. Med. 319:1623–1628. [DOI] [PubMed] [Google Scholar]
- 7.Ahearn, D. G., and R. B. Simmons. 1998. Malassezia Baillon, p.782–784. In C. P. Kurtzman and J. W. Fell (ed.), The yeasts—a taxonomic study, 4th ed. Elsevier, Amsterdam, The Netherlands.
- 8.Ahtonen, P., O.-P. Lehtonen, P. Kero, E. Tunnela, and V. Havu. 1990. Malassezia furfur colonisation of neonates in an intensive care unit. Mycoses 33:543–547. [DOI] [PubMed] [Google Scholar]
- 9.Akamatsu, H., J. Komura, Y. Asada, Y. Miyachi, and Y. Niwa. 1991. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch. Dermatol. Res. 283:162–166. [DOI] [PubMed] [Google Scholar]
- 10.Akamatsu, H., J. Komura, Y. Miyachi, Y. Asada, and Y. Niwa. 1990. Suppressive effects of linoleic acid on neutrophil oxygen metabolism and phagocytosis. J. Investig. Dermatol. 95:271–274. [DOI] [PubMed] [Google Scholar]
- 11.Akpata, L. E., H. C. Gugnani, and S. J. Utsalo. 1990. Pityriasis versicolor in schoolchildren in Cross River State of Nigeria. Mycoses 33:549–551. [DOI] [PubMed] [Google Scholar]
- 12.Alexander, S. 1968. Loss of hair and dandruff. Br. J. Dermatol. 79:549–552. [DOI] [PubMed] [Google Scholar]
- 13.Alford, R. H., C. G. Vire, B. B. Cartwright, and L. E. King, Jr. 1986. Ketoconazole’s inhibition of fungal antigen-induced thymidine uptake by lymphocytes from patients with psoriasis. Am. J. Med. Sci. 291:75–80. [DOI] [PubMed] [Google Scholar]
- 14.Allen, H. B., C. R. Charles, and B. L. Johnson. 1976. Hyperpigmented tinea versicolor. Arch. Dermatol. 112:1110–1112. [PubMed] [Google Scholar]
- 15.Alpert, G., L. M. Bell, and J. M. Campos. 1987. Malassezia furfur fungaemia in infancy. Clin. Pediatr. 26:528–531. [DOI] [PubMed] [Google Scholar]
- 16.Ambruso, D. R., K. M. Altenburger, and R. B. Johnston. 1979. Defective oxidative metabolism in newborn neutrophils: discrepancy between superoxide anion and hydroxyl radical generation. Pediatrics 64:S722–S725. [PubMed] [Google Scholar]
- 17.Andrew, G., G. Chan, and D. Schiff. 1976. Lipid metabolism in the neonate. I. The effects of Intralipid infusion on plasma triglicerides and free fatty acid concentrations in the neonate. J. Pediatr. 88:273–278. [PubMed] [Google Scholar]
- 18.Andrew, G., G. Chan, and D. Schiff. 1976. Lipid metabolism in the neonate. II. The effect of Intralipid on bilirubin binding in vitro and in vivo. J. Pediatr. 88:279–284. [PubMed] [Google Scholar]
- 19.Aractingi, S., S. Cadrand, P. Reygagne, and D. Wallach. 1991. Non follicular pustules induced by Malassezia furfur in a newborn. Ann. Dermatol. Venereol. 118:856–858. [PubMed] [Google Scholar]
- 20.Archer-Dubon, C., M. E. Icaza-Chivez, R. Orozco-Topete, E. Reyes, R. Baez-Martinez, and S. Ponce de Leon. 1999. An epidemic outbreak of Malassezia folliculitis in three adult patients in an intensive care unit: a previously unrecognized nosocomial infection. Int. J. Dermatol. 38:453–456. [DOI] [PubMed] [Google Scholar]
- 21.Aron-Brunetiere, R., and A. Avram. 1978. Pityrosporum ovale and acne vulgaris. Mykosen Suppl. 1:150–154. [PubMed] [Google Scholar]
- 22.Aron-Brunetiere, R., D. Dompmartin-Pernot, and E. Drouhet. 1977. Treatment of pityriasis capitis (dandruff) with econazole nitrate. Acta Dermato-Venereol. 57:77–80. [PubMed] [Google Scholar]
- 23.Artis, W. M., E. Patrisky, F. Rastinejard, and R. L. Duncan. 1983. Fungistatic mechanisms of human transferin for Rhizopus oryzae and Trichophyton mentagrophytes: alternative to simple iron depletion. Infect. Immun. 41:1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reference deleted.
- 25.Ashbee, H. R., A. Fruin, K. T. Holland, W. J. Cunliffe, and E. Ingham. 1994. Humoral immunity to Malassezia furfur serovars A, B and C in patients with pityriasis versicolor, seborrhoeic dermatitis and controls. Exp. Dermatol. 3:227–233. [DOI] [PubMed] [Google Scholar]
- 26.Ashbee, H. R., J. Gunning, K. T. Holland, W. J. Cunliffe, and E. Ingham. 1995. Titres of IgE specific to Malassezia furfur serovars A, B and C in patients with pityriasis versicolor and controls. J. Investig. Dermatol. 105:492. [Google Scholar]
- 27.Ashbee, H. R., E. Ingham, K. T. Holland, and W. J. Cunliffe. 1993. The carriage of Malassezia furfur serovars A, B and C in patients with pityriasis versicolor, seborrhoeic dermatitis and controls. Br. J. Dermatol. 129:533–540. [DOI] [PubMed] [Google Scholar]
- 28.Ashbee, H. R., E. Ingham, K. T. Holland, and W. J. Cunliffe. 1994. Cell-mediated immune responses to Malassezia furfur serovars A, B and C in patients with pityriasis versicolor, seborrhoeic dermatitis and controls. Exp. Dermatol. 3:106–112. [DOI] [PubMed] [Google Scholar]
- 29.Ashbee, H. R., A. K. Leck, J. W. L. Puntis, W. J. Parsons, and E. G. V. Evans. Colonisation of the skin of newborns and infants by Malassezia—implications for the acquisition of intravascular catheter related fungaemia Infect. Control Hosp. Epidemiol., in press.
- 30.Ashbee, H. R., S. R. Muir, W. J. Cunliffe, and E. Ingham. 1997. IgG subclasses specific to Staphylococcus epidermidis and Propionibacterium acnes in patients with acne vulgaris. Br. J. Dermatol. 136:730–733. [PubMed] [Google Scholar]
- 31.Aspiroz, C., L.-A. Moreno, A. Rezusta, and C. Rubio. 1999. Differentiation of three biotypes of Malassezia species on normal human skin. Correspondence with M. globosa, M. sympodialis and M. restricta. Mycopathologia 145:69–74. [DOI] [PubMed] [Google Scholar]
- 32.Athar, M. A., and L. Stafford. 1993. Malassezia furfur fungemia: a case report. Can. J. Infect. Control 8:63–64. [PubMed] [Google Scholar]
- 33.Austyn, J. M., and K. J. Wood (ed.). 1993. Principles of cellular and molecular immunology. Oxford University Press, Oxford, United Kingdom.
- 34.Azimi, P. H., K. Levernier, L. M. Lefrak, A. M. Petru, T. Barrett, H. Schenck, A. S. Sandhu, G. Duritz, and M. Valesco. 1988. Malassezia furfur: a cause of occlusion of percutaneous central venous catheters in infants in the intensive care nursery. Pediatr. Infect. Dis. J. 7:100–103. [PubMed] [Google Scholar]
- 35.Back, O., A. Scheynius, and S. G. O. Johansson. 1995. Ketoconazole in atopic dermatitis: therapeutic response is correlated with decrease in serum IgE. Arch. Dermatol. Res. 287:448–451. [DOI] [PubMed] [Google Scholar]
- 36.Baker, B. S., A. Powles, J. J. Garioch, C. Hardman, and L. Fry. 1998. Differential T-cell reactivity to the round and oval forms of Pityrosporum in the skin of patients with psoriasis. Br. J. Dermatol. 136:319–325. [PubMed] [Google Scholar]
- 37.Bandhaya, M. 1993. The distribution of Malassezia furfur and Malassezia pachydermatis on normal human skin. S. E. Asian J. Trop. Med. Public Health 24:343–346. [PubMed] [Google Scholar]
- 38.Barber, G. R., A. E. Brown, T. E. Kiehn, F. F. Edwards, and D. Armstrong. 1993. Catheter-related Malassezia furfur fungemia in immunocompromised patients. Am. J. Med. 95:365–370. [DOI] [PubMed] [Google Scholar]
- 39.Barfatani, M., R. J. Munn, and D. A. Schjeide. 1964. An ultrastructural study of Pityrosporum orbiculare. J. Investig. Dermatol. 43:231–233. [PubMed] [Google Scholar]
- 40.Barson, A. J., M. L. Chiswick, and C. M. Doig. 1978. Fat embolism in infancy after intravenous fat infusions. Arch. Dis. Child. 53:218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bayrou, O., C. Pecquet, C. Artigou, S. Laperche, N. Abuaf, D. A. Levy, and F. Leynadier. 1994. Atopic dermatitis of the head and neck: correlation between IgE and anti-Pityrosporum orbiculare and severity of disease. J. Allergol. Clin. Immunol. 93:221. [Google Scholar]
- 42.Bektas, S., B. Goetze, and C. P. Speer. 1990. Decreased adherence, chemotaxis and phagocytic activities of neutrophils from preterm neonates. Acta Paediatr. Scand. 79:1031–1038. [DOI] [PubMed] [Google Scholar]
- 43.Belew, P. W., E. W. Rosenberg, and B. R. Jennings. 1980. Activation of the alternative pathway of complement by Malassezia ovalis (Pityrosporum ovale) Mycopathologia 70:187–191. [DOI] [PubMed] [Google Scholar]
- 44.Bell, L. M., G. Alpert, P. H. Slight, and J. M. Campos. 1988. Malassezia furfur skin colonization in infancy. Infect. Control Hosp. Epidemiol. 9:151–153. [DOI] [PubMed] [Google Scholar]
- 45.Benham, R. W. 1939. The cultural characteristics of Pityrosporum ovale—a lipophilic fungus. J. Investig. Dermatol. 2:187–203. [Google Scholar]
- 46.Bergbrant, I. M., B. Andersson, and J. Faergemann. 1999. Cell-mediated immunity Malassezia furfur in patients with seborrhoeic dermatitis and pityriasis versicolor. Clin. Exp. Dermatol. 24:402–406. [DOI] [PubMed] [Google Scholar]
- 47.Bergbrant, I. M., and A. Broberg. 1994. Pityrosporum ovale culture from the forehead of healthy children. Acta Dermato-Venereol. 74:260–261. [DOI] [PubMed] [Google Scholar]
- 48.Bergbrant, I. M., and J. Faergemann. 1988. Variations of Pityrosporum orbiculare in middle-aged and elderly individuals. Acta Dermato-Venereol. 68:537–540. [PubMed] [Google Scholar]
- 49.Bergbrant, I. M., and J. Faergemann. 1989. Seborrhoeic dermatitis and Pityrosporum ovale: a cultural and immunological study. Acta Dermato-Venereol. 69:332–335. [PubMed] [Google Scholar]
- 50.Bergbrant, I. M., S. Johansson, D. Robbins, K. Bengtsson, J. Faergemann, A. Scheynius, and T. Soderstrom. 1991. The evaluation of various methods and antigens for the detection of antibodies against Pityrosporum ovale in patients with seborrhoeic dermatitis. Clin. Exp. Dermatol. 16:339–343. [DOI] [PubMed] [Google Scholar]
- 51.Bergbrant, I. M., S. Johansson, D. Robbins, A. Scheynius, J. Faergemann, and T. Soderstrom. 1991. An immunological study in patients with seborrhoeic dermatitis. Clin. Exp. Dermatol. 16:331–338. [DOI] [PubMed] [Google Scholar]
- 52.Berk, S. H., N. S. Penneys, and G. Weinstein. 1976. Epidermal activity in annular dermatophytosis. Arch. Dermatol. 112:485–488. [PubMed] [Google Scholar]
- 53.Bertini, B., E. S. Kuttin, and A. M. Beemer. 1975. Cytopathology of nipple discharge due to Pityrosporum orbiculare and cocci in an elderly woman Acta Cytol. 19:38–42. [PubMed] [Google Scholar]
- 54.Bibel, D. J., R. Aly, S. Shah, and H. R. Shinefield. 1993. Sphingosines: antimicrobial barriers of the skin. Acta Dermato-Venereol. 73:407–411. [DOI] [PubMed] [Google Scholar]
- 55.Binder, R. L., and F. J. Jonelis. 1983. Seborrhoeic dermatitis in neuroleptic-induced parkinsonism. Arch. Dermatol. 119:473–475. [PubMed] [Google Scholar]
- 56.Bizzozero, J. 1884. Ueber die Mikrophyton der normalen Oberhaut des Menschen. Arch. Pathol. Anat. Physiol. 98:441–459. [Google Scholar]
- 57.Boardman, C. R., and F. D. Malkinson. 1962. Tinea versicolor in steroid-treated patients. Arch. Dermatol. 85:84–92. [DOI] [PubMed] [Google Scholar]
- 58.Borgers, M., G. Cauwenbergh, M-A. Van De Ven, A. Del Palacio Hernanz, and H. Degreef. 1987. Pityriasis versicolor and P. ovale. Morphologenetic and ultrastructural considerations. Int. J. Dermatol. 26:586–589. [DOI] [PubMed] [Google Scholar]
- 59.Borton, L. K., and R. A. Schwartz. 1981. Pityrosporum folliculitis: a common acneiform condition of middle age. Ariz. Med. 38:598–601. [PubMed] [Google Scholar]
- 60.Bos, J. D. 1997. The skin as an organ of immunity. Clin. Exp. Immunol. 107(Suppl. 1):3–5. [PubMed] [Google Scholar]
- 61.Bos, J. D., and M. L. Kapsenberg. 1989. The skin immune system: its cellular constituents and their interactions. Immunol. Today 7:235–240. [DOI] [PubMed] [Google Scholar]
- 62.Bos, J. D., and M. L. Kapsenberg. 1993. The skin immune system: progress in cutaneous biology. Immunol. Today 14:75–78. [DOI] [PubMed] [Google Scholar]
- 63.Bos, J. D., I. Zonneveld, P. K. Das, S. R. Krieg, C. M. Van Der Loos, and M. L. Kapsenberg. 1987. The skin immune system (SIS): distribution and immunophenotype of lymphocyte subpopulations in normal human skin. J. Investig. Dermatol. 88:569–573. [DOI] [PubMed] [Google Scholar]
- 64.Boyle, J., J. L. Burton, and J. Faergemann. 1986. Use of topical lithium succinate for seborrhoeic dermatitis. Br. Med. J. 292:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brasch, J., and E. Christophers. 1993. Azelaic acid has antimycotic properties in vitro. Dermatology 186:55–58. [DOI] [PubMed] [Google Scholar]
- 66.Brasch, J., H. Martens, and W. Sterry. 1993. Langerhans cell accumulation in chronic tinea pedis and pityriasis versicolor. Clin. Exp. Dermatol. 18:329–332. [DOI] [PubMed] [Google Scholar]
- 67.Breathnach, A. S., B. Gross, and M. Martin. 1976. Freeze fracture replication of cultured Pityrosporum orbiculare. Sabouraudia 14:105–113. [PubMed] [Google Scholar]
- 68.Broberg, A., and J. Faergemann. 1995. Topical antimycotic treatment of atopic dermatitis in head/neck area. A double-blind randomised study. Acta Dermato-Venereol. 75:46–49. [DOI] [PubMed] [Google Scholar]
- 69.Broberg, A., J. Faergemann, S. Johansson, S. G. O. Johansson, I. L. Strannegard, and E. Svejgaard. 1992. Pityrosporum ovale and atopic dermatitis in children and young adults. Acta Dermato-Venereol. 72:187–192. [PubMed] [Google Scholar]
- 70.Brooks, R., and L. Brown. 1987. Systemic infection with Malassezia furfur in an adult receiving long-term hyperalimentation therapy. J. Infect. Dis. 156:410–411. [DOI] [PubMed] [Google Scholar]
- 71.Brotherton, J. 1967. The sulphur metabolism of P. ovale and its inhibition by selenium compounds. J. Gen. Microbiol. 49:393–400. [DOI] [PubMed] [Google Scholar]
- 72.Bruneau, S. M., and R. M. F. Guinet. 1984. Quantitative immunoelectrophoretic study of genus Pityrosporum sabouraud. Mykosen 27:123–136. [DOI] [PubMed] [Google Scholar]
- 73.Buentke, E., A. Zargari, C. Heffler, J. Avila-Carino, J. Savolainen, and A. Scheynius. 2000. Uptake of the yeast Malassezia furfur and its allergenic components by human immature CD1a+ dendritic cells. Clin. Exp. Allergy 30:1759–1770 [DOI] [PubMed] [Google Scholar]
- 74.Buffill, J. A., L. G. Lum, J. G. Caya, C. R. Chitambar, P. S. Ritch, T. Anderson, and R. C. Ash. 1988. Pityrosporum folliculitis after bone marrow transplantation. Ann. Intern. Med. 108:560–563. [DOI] [PubMed] [Google Scholar]
- 75.Bunse, T., and G. Mahrle. 1996. Soluble Pityrosporum-derived chemoattractant for polymorphonuclear leukocytes of psoriatic patients. Acta Dermato-Venereol. 76:10–12. [DOI] [PubMed] [Google Scholar]
- 76.Burgio, G. R., A. G. Ugazio, and L. D. Notarangelo. 1990. Immunology of the Neonate. Curr. Opin. Immunol. 2:770–777. [DOI] [PubMed] [Google Scholar]
- 77.Burke, R. C. 1961. Tinea versicolor: susceptibility factors and experimental infections in human beings. J. Investig. Dermatol. 36:389–402. [DOI] [PubMed] [Google Scholar]
- 78.Bussel, J. B., S. Cunningham-Rundles, E. F. LaGamma, and M. Shellabarger. 1988. Analysis of lymphocyte proliferative response subpopulations in very low birth weight infants and during the first eight weeks of life. Pediatr. Res. 23:457–462. [DOI] [PubMed] [Google Scholar]
- 79.Cannon, P. F. 1986. International Commission on the taxonomy of fungi (ICTF): name changes in fungi of microbiological, industrial and medical importance. Microbiol. Sci. 3:285–287. [PubMed] [Google Scholar]
- 80.Caprilli, F., R. Mercantini, M. Nazzaro-Porro, S. Passi, and A. Tonolo. 1973. Studies of the genus Pityrosporum in submerged culture. Mycopathol. Mycol. Appl. 51:171–189. [DOI] [PubMed] [Google Scholar]
- 81.Carteaud, J. P., M. D. Muir, and P. R. Grant.. 1972. Pityriasis versicolor: emissive and cathodoluminescence examination in the scanning electron microscope. Sabouraudia 10:143–146. [DOI] [PubMed] [Google Scholar]
- 82.Casadevall, A., A. Cassone, F. Bistoni, J. E. Cutler, W. Magliani, J. W. Murphy, L. Polonelli, and L. Romani. 1998. Antibody and/or cell mediated immunity, protective mechanisms in fungal disease—an ongoing dilemma or an unnecessary dispute? Med. Mycol. 36(Suppl. 1):95–105. [PubMed] [Google Scholar]
- 83.Castellani, A. 1925. Notes on three new yeast-like organisms and a new bacilus, with remarks on the clinical conditions from which they have been isolated: furunculosis blastomycetia, macroglossia blastomycetia, stomatitis cryptobacillus. J. Trop. Med. Hyg. 28:217–223. [Google Scholar]
- 84.Castro, M., N. V. C. Ralston, T. I. Morgenthaler, M. S. Rohrbach, and A. H. Limper. 1994. Candida albicans stimulates arachidonic acid liberation from alveolar macrophages through alpha mannan and beta-glucan cell wall components. Infect. Immun. 62:3138–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Catterall, M. D., M. E. Ward, and P. Jacobs. 1978. A reappraisal of the role of Pityrosporum orbiculare in pityriasis versicolor and the significance of extracellular lipase. J. Investig. Dermatol. 71:398–401. [DOI] [PubMed] [Google Scholar]
- 86.Cech, P., and R. I. Lehrer. 1984. Heterogeneity of human neutrophil phagolysosymes: functional consequences for candidacidal activity. Blood 64:147–151. [PubMed] [Google Scholar]
- 87.Chai, F. C., K. A. Auret, K. Christiansen, P. W. Yuen, and D. Gardam. 2000. Malignant otitis externa caused by Malassezia sympodialis. Head Neck 22:87–89. [DOI] [PubMed] [Google Scholar]
- 88.Chang, H. J., H. L. Miller, N. Watkins, M. J. Arduino, D. A. Ashford, G. Midgley, S. M. Aguero, R. Pinto-Powell, C. F. von Reyn, W. Edwards, M. M. McNeil, and W. R. Jarvis. 1998. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N. Engl. J. Med. 338:706–711. [DOI] [PubMed] [Google Scholar]
- 89.Charles, C. R., D. J. Sire, B. L. Johnson, and J. G. Beidler. 1972. Hypopigmentation in tinea versicolor: a histochemical and electronmicroscopic study. Int. J. Dermatol. 12:48–58. [DOI] [PubMed] [Google Scholar]
- 90.Christophers, E., and U. Mrowietz. 1995. The inflammatory infiltrate in psoriasis. Clin. Dermatol. 13:131–135. [DOI] [PubMed] [Google Scholar]
- 91.Clemmensen, O. J., and N. Hjorth. 1983. Treatment of dermatitis of the head and neck with ketoconazole in patients with type I sensitivity to Pityrosporum orbiculare. Semin. Dermatol. 2:26–29. [Google Scholar]
- 92.Coldiron, B. M., and P. R. Bergstresser. 1989. Prevalence and clinical spectrum of skin disease inpatients infected with HIV. Arch. Dermatol. 125:357–361. [PubMed] [Google Scholar]
- 93.Congly, H. 1984. Pityriasis versicolor in a 3 month old baby. Can. Med. Assoc. J. 130:844–845. [PMC free article] [PubMed] [Google Scholar]
- 94.Crespo Erchiga, V., A. Ojeda Martos, A. Vera Casaño, A. Crespo Erchiga, F. Sanchez Fajardo, and E. Guého. 1999. Mycology of pityriasis versicolor. J. Mycol. Med. 9:143–148. [DOI] [PubMed] [Google Scholar]
- 95.Crespo Erchiga, V., A. Ojeda Martos, A. Vera Casaño, A. Crespo Erchiga, and F. Sanchez Fajardo. 2000. Malassezia globosa as the causative agent of pityriasis versicolor. Br. J. Dermatol. 143:799–803. [DOI] [PubMed] [Google Scholar]
- 96.Cross, C. E., and G. J. Bancroft. 1995. Ingestion of acapsular Cryptococcus neoformans occurs via mannose and beta-glucan receptors, resulting in cytokine production and increased phagocytosis of the encapsulated form. Infect. Immun. 63:2604–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Crozier, W. J., and K. A. Wise. 1993. Onychomycosis due to Pityrosporum. Aust. J. Dermatol. 34:109–112. [DOI] [PubMed] [Google Scholar]
- 98.Cuelenare, C., J. De Bersaques, and A. Kint. 1992. Use of topical lithium succinate in the treatment of seborrhoeic dermatitis. Dermatology 184:194–197. [DOI] [PubMed] [Google Scholar]
- 99.Cunningham, A. C. 1991. An investigation of the immune status of man to Malassezia spp. Ph.D. thesis. University of Leeds, Leeds, United Kingdom.
- 100.Cunningham, A. C., E. Ingham, and G. Gowland. 1992. Humoral responses to Malassezia furfur serovars A, B and C in normal individuals of various ages. Br. J. Dermatol. 127:476–481. [DOI] [PubMed] [Google Scholar]
- 101.Cunningham, A. C., J. P. Leeming, E. Ingham, and G. Gowland. 1990. Differentiation of three serovars of Malassezia furfur. J. Appl. Bacteriol. 68:439–446. [DOI] [PubMed] [Google Scholar]
- 102.Dahms, B. B., and T. C. Halpin Jr. 1980. Pulmonary arterial lipid deposit in newborn infants receiving intravenous lipid infusion. J. Pediatr. 97:800–805. [DOI] [PubMed] [Google Scholar]
- 103.Da Mert, G. J., C. H. Kirkpatrick, and P. G. Sohnle. 1980. Comparison of antibody responses in chronic mucocutaneous candidiasis and tinea versicolor. Int. Arch. Allergy Appl. Immunol. 63:69–76. [DOI] [PubMed] [Google Scholar]
- 104.Danby, F. W., W. S. Maddin, L. J. Margesson, and D. Rosenthal. 1993. A randomized, double-blind, placebo-controlled trial of ketoconazole 2% shampoo versus selenium sulfide 2.5% shampoo in the treatment of moderate to severe dandruff. J. Am. Acad. Dermatol. 29:1008–1012. [DOI] [PubMed] [Google Scholar]
- 105.Dankner, W. H., S. A. Spector, R. J. Fiere, and C. E. Davis. 1987. Malassezia fungaemia in neonates and adults: complication of hyperalimentation. Rev. Infect. Dis. 9:743–753. [DOI] [PubMed] [Google Scholar]
- 106.Davies, R. R., and F. Zaini. 1984. Trichophyton rubrum and the chemotaxis of polymorphonuclear leucocytes. Sabouraudia 22:65–71. [PubMed] [Google Scholar]
- 107.Davies, S. F., D. P. Clifford, J. R. Hoidal, and J. E. Repine. 1982. Opsonic requirements for the uptake of Cryptococcus neoformans by human polymorphonuclear leukocytes and monocytes. J. Infect. Dis. 145:870–874. [DOI] [PubMed] [Google Scholar]
- 108.De Luca, C., M. Picardo, A. Breathnach, and S. Passi. 1996. Lipoperoxidase activity of Pityrosporum: charactersiation of by-products and possible role in pityriasis versicolor. Exp. Dermatol. 5:49–56. [DOI] [PubMed] [Google Scholar]
- 109.Disilverio, A., M. Mosca, G. Brandozzi, and M. Gatti. 1989. Pityriasis versicolor in the aged—a clinical investigation and epidemiological survey in 190 elderly hospitalised patients. Mycopathologia 105:187–190. [DOI] [PubMed] [Google Scholar]
- 110.Doekes, G. 1994. Biochemical characteristics of Pityrosporum allergens. Allergy 49:198–199. [DOI] [PubMed] [Google Scholar]
- 111.Doekes, G., M. J. H. Kaal, and A. G. Van Ieperen-Van Dijk. 1993. Allergens of Pityrosporum ovale and Candida albicans. II. Physiochemical characterization. Allergy 48:401–408. [DOI] [PubMed] [Google Scholar]
- 112.Doekes, G., and A. G. Van Ieperen-Van Dijk. 1993. Pityrosporum ovale allergens in conventional house dust mite extracts? J. Allergy Clin. Immunol. 91:1099–1100. [DOI] [PubMed] [Google Scholar]
- 113.Doekes, G., and A. G. Van Ieperen-Van Dijk. 1993. Allergens of Pityrosporum ovale and Candida albicans. I. Cross-reactivity of IgE-binding components. Allergy 48:394–400. [DOI] [PubMed] [Google Scholar]
- 114.Dold, H. 1910. On the so called Bottle Bacillus (Dermatophyton Malassez). Parasitology III:279–287. [Google Scholar]
- 115.Dorn, M., and K. Roehnert. 1977. Dimorphism of Pityrosporum orbiculare in a defined culture medium. J. Investig. Dermatol. 69:244–248. [DOI] [PubMed] [Google Scholar]
- 116.Dotz, W. I., D. M. Henrikson, and G. S. M. Yu. 1985. Tinea versicolor: a light and electron microscopic study of hyperpigmented skin. J. Am. Acad. Dermatol. 12:37–44. [DOI] [PubMed] [Google Scholar]
- 117.Dovezenski, N., R. Billetta, and I. Gigli. 1992. Expression and localization of proteins of the complement system in human skin. J. Clin. Investig. 90:2000–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eichstedt, E. 1846. Pilzbildung in der Pityriasis versicolor. Frorip Neue Notizen aus dem Gebeite der Naturkunde Heilkinde 39:270. [Google Scholar]
- 119.Eisenstat, B. A., and G. P. Wormser. 1984. Seborrhoeic dermatitis and butterfly rash in AIDS. N. Engl. J. Med. 311:189. [DOI] [PubMed] [Google Scholar]
- 120.Elewski, B. 1990. Does Pityrosporum ovale have a role in psoriasis ? Arch. Dermatol. 126:1111–1112. [PubMed] [Google Scholar]
- 121.El-Gothamy, Z., A. Abdel-Fattah, and A. F. Gholy. 1975. Tinea versicolor hypopigmentation: histochemical and therapeutic studies. Int. J. Dermatol. 14:510–515. [DOI] [PubMed] [Google Scholar]
- 122.Evans, N. J., and N. Rutter. 1986. Development of the epidermis in the newborn. Biol. Neonat. 49:74–80. [DOI] [PubMed] [Google Scholar]
- 123.Faergemann, J. 1983. Antibodies to Pityrosporum orbiculare in patients with tinea versicolor and controls of various ages. J. Investig. Dermatol. 80:133–135. [DOI] [PubMed] [Google Scholar]
- 124.Faergemann, J. 1985. Treatment of sebopsoriasis with itraconazole. Mykosen 28:612–618. [DOI] [PubMed] [Google Scholar]
- 125.Faergemann, J. 1985. Lipophilic yeasts in skin disease. Semin. Dermatol. 4:173–184. [Google Scholar]
- 126.Faergemann, J. 1986. Seborrhoeic dermatitis and Pityrosporum orbiculare: treatment of seborrhoeic dermatitis of the scalp with miconazole-hydrocortisone (Daktacort), miconazole and hydrocortisone. Br. J. Dermatol. 114:695–700. [DOI] [PubMed] [Google Scholar]
- 127.Faergemann, J. 1989. Epidemiology and ecology of pityriasis versicolor. Curr. Top. Med. Microbiol. 3:153–167. [DOI] [PubMed] [Google Scholar]
- 128.Faergemann, J. 1993. Pityrosporum ovale and skin diseases. Keio J. Med. 42:91–94. [PubMed] [Google Scholar]
- 129.Faergemann, J. 1993. Pityriasis versicolor. Semin. Dermatol. 12:276–279. [PubMed] [Google Scholar]
- 130.Faergemann, J. 2000. Management of seborrhoeic dermatitis and pityriasis versicolor. Am. J. Clin. Dermatol. 1:75–80. [DOI] [PubMed] [Google Scholar]
- 131.Faergemann, J., I-M. Bergbrant, M. Dohse, A. Scott, and G. Westgate. 2001. Seborrhoeic dermatitis and Pityrosporum (Malassezia) folliculitis: characterisation of inflammatory cells and mediators in the skin by immunohistochemistry. Br. J. Dermatol. 144:549–556. [DOI] [PubMed] [Google Scholar]
- 132.Faergemann, J., and S. Bernander. 1979. Tinea versicolor and Pityrosporum orbiculare: a mycological investigation. Sabouraudia 17:171–179. [PubMed] [Google Scholar]
- 133.Faergemann, J., and S. Bernander. 1981. Microaerophilic and anaerobic growth of Pityrosporum species. Sabouraudia 19:117–121. [PubMed] [Google Scholar]
- 134.Faergemann, J., and T. Fredriksson. 1979. Tinea versicolor with regard to seborrhoeic dermatitis. Arch. Dermatol. 115:966–968. [PubMed] [Google Scholar]
- 135.Faergemann, J., and T. Fredriksson. 1980. Age incidence of Pityrosporum orbiculare on human skin Acta Dermato-Venereol. 60:531–533. [PubMed] [Google Scholar]
- 136.Faergemann, J., T. Fredriksson, and G. Narthors-Windahl. 1980. One case of confluent and reticulate papillomatosis (Gougerot-Carteaud). Acta Dermato-Venereol. 60:269–271. [PubMed] [Google Scholar]
- 137.Faergemann, J., S. Johansson, O. Back, and A. Scheynius. 1986. An immunological and cultural study of Pityrosporum folliculitis. J. Am. Acad. Dermatol. 14:429–433. [DOI] [PubMed] [Google Scholar]
- 138.Faergemann, J., U. Tjernlund, A. Scheynius, and S. Bernander. 1982. Antigenic similarities and differences in genus Pityrosporum. J. Investig. Dermatol. 78:28–31. [DOI] [PubMed] [Google Scholar]
- 139.Faggi, E., G. Pini, E. Campisi, and G. Gargani. 1998. Anti-Malassezia furfur antibodies in the population. Mycoses 41:273–275. [DOI] [PubMed] [Google Scholar]
- 140.Farr, P. M., L. B. Krause, J. M. Marks, and S. Shuster. 1985. Response of scalp psoriasis to oral ketoconazole. Lancet ii:921–922. [DOI] [PubMed] [Google Scholar]
- 141.Farthing, C. F., R. C. D. Staughton, and C. M. E. Rowland Payne. 1985. Skin disease in homosexual patients with AIDS and lesser forms for human T cell leukaemia virus (HTLV III) disease. Clin. Exp. Dermatol. 10:3–12. [DOI] [PubMed] [Google Scholar]
- 142.Ferrandiz, C., M. Ribera, J. C. Barranco, B. Clotet, and J. C. Lorenzo. 1992. Eosinophilic pustular folliculitis in patients with acquired immunodeficiency syndrome. Int. J. Dermatol. 31:193–195. [DOI] [PubMed] [Google Scholar]
- 143.Fine, A., D. Churchill, H. Gault, and P. Furdy. 1983. Pityrosporum pachydermatis peritonitis in a CAPD patient on long-term intraperitoneal antibiotics. Periton. Dial. Bull. 3:108–109. [Google Scholar]
- 144.Fischer, B., N. Yawalkar, K. A. Brander, N. J. Pichler, and A. Helbling. 1999. Coprinus comatus (Shaggy cap) is a potential source of aeroallergen that may provoke atopic dermatitis. J. Allergy Clin. Immunol. 104:836–841. [DOI] [PubMed] [Google Scholar]
- 145.Fischer, G. W., K. W. Hunter, S. R. Wilson, and A. D. Mease. 1979. Inhibitory effect of intralipid on reticuloendothelial function and neutrophil bactericidal activity. Pediatr. Res. 13:494. [Google Scholar]
- 146.Fitton, A., and K. L. Goa. 1991. Azelaic acid. A review of its pharmacological properties and therapeutic efficacy in acne and hyperpigmentary skin disorders. Drugs 41:780–798. [DOI] [PubMed] [Google Scholar]
- 147.Folster Holst, R., H. W. Moises, L. Yang, W. Fritsch, J. Weissenbach, and E. Christophers. 1998. Linkage between atopy and IgE high affinity receptor gene at 11q13 in atopic dermatitis families. Hum. Genet. 102:236–239. [DOI] [PubMed] [Google Scholar]
- 148.Ford, G. P., P. M. Farr, F. A. Ive, and S. Shuster. 1984. The response of seborrhoeic dermatitis to ketoconazole. Br. J. Dermatol. 111:603–607. [DOI] [PubMed] [Google Scholar]
- 149.Ford, G. P., F. A. Ive, and G. Midgley. 1982. Pityrosporum folliculitis and ketoconazole. Br. J. Dermatol. 107:691–695. [DOI] [PubMed] [Google Scholar]
- 150.Forstrom, L., M. E. Goldstyne, and R. K. Winkelmann. 1975. IgE in human eccrine sweat. J. Investig. Dermatol. 64:156–157. [DOI] [PubMed] [Google Scholar]
- 151.Friedman, Z., K. H. Marks, M. J. Maisels, R. Thorson, and R. Naeye. 1978. Effect of parenteral fat emulsions on the pulmonary and reticuloendothelial systems in the newborn infant. Pediatrics 61:694–698. [PubMed] [Google Scholar]
- 152.Furukawa, F., K. Danno, S. Imamura, and Y. Soh. 1981. Histological and serological studies of P. orbiculare in cases of pityriasis versicolor. J. Dermatol. 8:27–30. [DOI] [PubMed] [Google Scholar]
- 153.Galadari, I. 1998. Pityriasis versicolor—Is it infectious or not? Gulf J. Dermatol. 5:33–35. [Google Scholar]
- 154.Garcia, C. R., B. L. Johnston, G. Corvi, L. J. Walker, and W. L. George. 1987. Intravenous catheter-associated Malassezia furfur fungemia. Am. J. Med. 83:790–792. [DOI] [PubMed] [Google Scholar]
- 155.Garcia, R. L. 1976. Skin disorders in air force recruits. J. Assoc. Mil. Dermatol. 2:61. [Google Scholar]
- 156.Gidding, H., L. Hawes, and B. Dwyer. 1989. The isolation of M. furfur from an episode of peritonitis. Med. J. Aust. 151:603. [DOI] [PubMed] [Google Scholar]
- 157.Girgawy, E., and I. Raad. 1999. Catheter-related infections, p.73–80. In M. H. Wilcox (ed.), Infection highlights. Health Press, Oxford, United Kingdom.
- 158.Goodfield, M. J., and E. M. Saihan. 1988. Failure of isotretinoin therapy in Pityrosporum folliculitis. J. Am. Acad. Dermatol. 18:143–144. [DOI] [PubMed] [Google Scholar]
- 159.Gordon, M. A. 1951. Lipophilic yeast organism associated with tinea versicolor. J. Investig. Dermatol. 17:267–272. [DOI] [PubMed] [Google Scholar]
- 160.Gordon, M. A. 1979. Malassezia Pityrosporum pachydermatis (Weidman) Dodge 1935. Sabouraudia 17:305–309. [DOI] [PubMed] [Google Scholar]
- 161.Gosse, R. M., and R. W. Vanderwyk. 1969. The relationship of a nystatin-resistant strain of P. ovale to dandruff. J. Soc. Cosmet. Chem. 20:603–613. [Google Scholar]
- 162.Gould, D. J., P. S. Mortimer, C. Proby, M. G. Davis, P. J. W. Kersey, R. Lindskov, A. Oxholm, A. M. M. Strong, E. Hamill, K. Kenicer, C. Green, J. J. Cream, R. J. Clayton, J. D. Wilkinson, A. Davis, B. R. Allen, R. Marks, L. Lever, M. Y. Moss, P. F. Morse, S. I. Wright, D. F. Horrobin, and J. C. M. Stewart. 1992. Double blind placebo controlled trial of lithium succinate ointment in the treatment of seborrhoeic dermatitis. J. Am. Acad. Dermatol. 26:452–457. [DOI] [PubMed] [Google Scholar]
- 163.Granberg, C., and T. Hirvonen. 1980. Cell mediated lympholysis by fetal and neonatal lymphocytes in sheep and man. Cell. Immunol. 51:13–22. [DOI] [PubMed] [Google Scholar]
- 164.Greaves, M. W., and R. D. R. Camp. 1988. Prostaglandins, leukotrienes, phospholipase, platelet activating factor and cytokines: an integrated approach to inflammation of human skin. Arch. Dermatol. Res. 280:S33–S41. [PubMed] [Google Scholar]
- 165.Grossier, D., E. J. Bottone, and M. Lebwohl. 1989. Association of Pityrosporum orbiculare (Malassezia furfur) with seborrhoeic dermatitis in patients with AIDS. J. Am. Acad. Dermatol. 20:770–774. [DOI] [PubMed] [Google Scholar]
- 166.Gueho, E., T. Boekhout, H. R. Ashbee, J. Guillot, A. Van Belkurn, and J. Faergemann. 1998. The role of Malassezia species in the ecology of human skin and as pathogens. Med. Mycol. 36(Suppl. 1):220–229. [PubMed] [Google Scholar]
- 167.Gueho, E., G. Midgley, and J. Guillot. 1996. The genus Malassezia with description of four new species. Antonie Leeuwenhoek 69:337–355. [DOI] [PubMed] [Google Scholar]
- 168.Gueho, E., R. B. Simmons, W. R. Pruitt, S. A. Meyer, and D. G. Ahearn. 1987. Association of Malassezia pachydermatis with systemic infections of humans. J. Clin. Microbiol. 25:1789–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Guillot, J., and R. Bond. 1999. Malassezia pachydermatis: a review. Med. Mycol. 37:295–306. [DOI] [PubMed] [Google Scholar]
- 170.Guillot, J., and E. Gueho. 1995. The diversity of Malassezia yeasts confirmed by rRNA sequence and nuclear DNA comparisons. Antonie Leeuwenhoek 67:297–314. [DOI] [PubMed] [Google Scholar]
- 171.Guillot, J., E. Gueho, M. Lesourd, G. Midgley, G. Chevrier, and B. Dupont. 1996. Identification of Malassezia species. A practical approach. J. Mycol. Med. 6:103–110. [Google Scholar]
- 172.Gupta, A. K., Y. Kohli, J. Faergemann, and R. C. Summerbell. 2001. Epidemiology of Malassezia yeasts associated with pityriasis versicolor in Ontario, Canada. Med. Mycol. 39:199–206. [DOI] [PubMed] [Google Scholar]
- 173.Gupta, A. K., Y. Kohli, and R. C. Summerbell. 2000. Molecular differentiation of seven Malassezia species. J. Clin. Microbiol. 38:1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gustafson, A., I. Kjellmer, R. Olegard, and L. Victorin. 1972. Nutrition in low birth weight infants. I. Intravenous injection of fat emulsion. Acta Paediatr. Scand. 61:149–158. [DOI] [PubMed] [Google Scholar]
- 175.Hafez, M., and S. El-Shamy. 1985. Genetic susceptibility in pityriasis versicolor. Dermatologica 171:86–88. [DOI] [PubMed] [Google Scholar]
- 176.Hakansson, C., J. Faergemann, and G. B. Lowhagen. 1988. Studies on the lipophilic yeast P. ovale in HIV+ and HIV− homosexual men. Acta Dermato-Venereol. 68:422–426. [PubMed] [Google Scholar]
- 177.Hanna, J. M., W. T. Johnson, and H. W. Wyre. 1983. Malassezia (Pityrosporum) folliculitis occurring with granuloma annulare and alopecia areata. Arch. Dermatol. 119:869–871. [PubMed] [Google Scholar]
- 178.Hashimoto, K., Y. Taniguchi, M. R. Simon, P. W. Noah, E. W. Rosenberg, and L. B. Savoy. 1989. Immunological aspects of superficial fungus infections. Jpn. J. Med. Mycol. 30:81–91. [Google Scholar]
- 179.Hassall, E., T. Ulich, and M. E. Ament. 1983. Pulmonary embolus and Malassezia pulmonary infection related to urokinase therapy. J. Pediatr. 102:722–725. [DOI] [PubMed] [Google Scholar]
- 180.Hattori, M., H. Ogawa, K. Takamori, P. Gritiyaranson, and R. Kotarajaras. 1984. De-(hypo)pigmentation mechanisms of the affected area of pityriasis versicolor. J. Dermatol. 11:63–66. [DOI] [PubMed] [Google Scholar]
- 181.Hechemy, K. E., and R. W. Vanderwyk. 1968. Isolation, purification and chemical analysis of P. ovale cell wall. Bacteriol. Proc. 68:42. [Google Scholar]
- 182.Helbock, H. J., and B. J. Ames. 1995. Use of intravenous lipids in neonates. J. Pediatr. 126:747–748. [DOI] [PubMed] [Google Scholar]
- 183.Hellgren, L., and J. Vincent. 1983. The incidence of tinea versicolor in central Sweden. J. Med. Microbiol. 16:501–502. [DOI] [PubMed] [Google Scholar]
- 184.Helm, K. F., and D. P. Lookingbill. 1993. Pityrosporum folliculitis and severe pruritis in two patients with Hodgkin’s disease. Arch. Dermatol. 129:380–381. [PubMed] [Google Scholar]
- 185.Heng, M. C., C. L. Henderson, D. C. Barker, and G. Haberfelde. 1990. Correlation of Pityrosporum ovale density with clinical severity of seborrhoeic dermatitis as assessed by a simplified technique. J. Am. Acad. Dermatol. 23:82–87. [DOI] [PubMed] [Google Scholar]
- 186.Herbrink, P., F. J. Van Bussel, and S. O. Warnaar. 1982. The antigen spot test (AST): a highly sensitive assay for the detection of antibodies. J. Immunol. Methods 48:293–298 [DOI] [PubMed] [Google Scholar]
- 187.Heumann, D., C. Barras, A. Severin, M. P. Glaser, and A. Tomasz. 1994. Gram positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect. Immun. 62:2715–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Heymann, W. R., and D. J. Wolf. 1986. Malassezia (Pityrosporum) folliculitis occurring during pregnancy. Int. J. Dermatol. 25:49–51. [DOI] [PubMed] [Google Scholar]
- 189.Hill, M. K., M. J. D. Goodfield, F. G. Rodgers, J. L. Crowley, and E. M. Saihan. 1990. Skin surface electron microscopy in Pityrosporum folliculitis—the role of follicular occlusion in disease and the response to oral ketoconazole. Arch. Dermatol. 126:1071–1074. [DOI] [PubMed] [Google Scholar]
- 190.Holland, K. T., and R. A. Bojar. 1989. The effect of azelaic acid on cutaneous bacteria. J. Dermatol. Treat. 1:17–19. [Google Scholar]
- 191.Hruszkewycz, V., P. C. Holtrop, D. G. Batton, R. S. Morden, P. Gibson, and J. D. Band. 1991. Complications associated with central venous catheters inserted in critically ill neonates. Infect. Control Hosp. Epidemiol. 12:544–548. [DOI] [PubMed] [Google Scholar]
- 192.Huang, X., S. G. O. Johansson, A. Zargari, and S. L. Nordvall. 1995. Allergen cross reactivity between Pityrosporum orbiculare and Candida albicans. Allergy 50:648–656. [DOI] [PubMed] [Google Scholar]
- 193.Ingham, E., E. A. Eady, C. E. Goodwin, J. H. Cove, and W. J. Cunliffe. 1992. Pro-inflammatory levels of interleukin-1α-like bioactivity are present in the majority of open comedones in acne vulgaris. J. Investig. Dermatol. 98:895–901. [DOI] [PubMed] [Google Scholar]
- 194.Ingham, E., G. Gowland, R. M. Ward, K. T. Holland, and W. J. Cunliffe. 1987. Antibodies to Propionibacterium acnes and P. acnes exocellular enzymes in the normal population at various ages and in patients with acne vulgaris. Br. J. Dermatol. 116:805–812. [DOI] [PubMed] [Google Scholar]
- 195.Ingham, E., J. P. Leeming, G. Gowland, and W. J. Cunliffe. 1987. Humoral immunity to Pityrosporum in acne patients and controls Br. J. Dermatol. 116:457. [Google Scholar]
- 196.Ishiguro, Y., K. Kato, and T. Ito. 1981. Sensitive solid phase enzyme immunoassay for human IgA, secretory IgA and secretory component. Chin. Chim. Acta 116:237–243. [DOI] [PubMed] [Google Scholar]
- 197.Ive, F. A. 1991. An overview of experience with ketoconazole shampoo Br. J. Clin. Pract. 45:279–284. [PubMed] [Google Scholar]
- 198.Iwatsuki, K., H. Tagami, and M. Yamada. 1983. Induction of leukocyte adherence at the basement membrane zone with subsequent activation of their metabolic pathway by pemphigoid antibodies and complement. Acta Dermato-Venereol. 63:290–295. [PubMed] [Google Scholar]
- 199.Jacinto-Jamora, S., J. Tamesis, and M. L. Katigbak. 1991. Pityrosporum folliculitis in the Philippines—diagnosis, prevalence and management. J. Am. Acad. Dermatol. 24:693–696. [DOI] [PubMed] [Google Scholar]
- 200.Jensen-Jarolim, E., L. K. Poulsen, H. With, M. Kieffer, V. Ottevanger, and P. S. Skov. 1992. Atopic dermatitis of the face, scalp and neck: type I reaction to the yeast Pityrosporum ovale? J. Allergy Clin. Immunol. 89:44–51. [DOI] [PubMed] [Google Scholar]
- 201.Jeremias, J., A. Kalo-Klein, and S. S. Witkin. 1991. Individual differences in tumor necrosis factor and interleukin-1 production induced by viable and heat-killed Candida albicans. J. Med. Vet. Mycol. 29:157–163. [DOI] [PubMed] [Google Scholar]
- 202.Johansson, C., M. Jeddi-Tehrani, J. Grunewald, M. Tengvall Linder, A. Bengtsson, G. Hallden, and A. Scheynius. 1999. Peripheral blood T-cell receptor beta-chain V-repertoire in atopic dermatitis patients after in vitro exposure to Pityrosporum orbiculare extracts. Scand. J. Immunol. 49:293–301. [DOI] [PubMed] [Google Scholar]
- 203.Johansson, S., and J. Faergemann. 1990. Enzyme linked immunosorbent assay for detection of antibodies against Pityrosporum orbiculare. J. Med. Vet. Mycol. 28:257–260. [DOI] [PubMed] [Google Scholar]
- 204.Johansson, S., and K. Karlstrom. 1991. IgE binding components in Pityrosporum orbiculare identified by an immunoblotting technique. Acta Dermato-Venereol. 71:11–16. [PubMed] [Google Scholar]
- 205.Johnson, A. S., E. Bailey, P. A. Wright, and L. Solomon. 1996. Malassezia furfur: a possible cause of culture negative CAPD-peritonitis. Periton. Dial. Int. 16:187–188. [PubMed] [Google Scholar]
- 206.Kang, S. H., and H. U. Kim. 1999. The isolation of Malassezia yeasts in the comedones of acne vulgaris. Korean J. Med. Mycol. 4:33–39. [Google Scholar]
- 207.Kano, R., T. Aizawa, Y. Nakamura, S. Watanabe, and A. Hasegawa. 1999. Chitin synthase 2 gene sequence of Malassezia species. Microbiol. Immunol. 43:813–815. [DOI] [PubMed] [Google Scholar]
- 208.Karaoui, R., M. Bou-Resli, N. S. Al-Zaid, and A. Mousa. 1981. Tinea versicolor: ultrastructural studies on hypopigmented and hyperpigmented skin. Dermatologica 162:69–85. [DOI] [PubMed] [Google Scholar]
- 209.Karaoui, R., M. Bou-Resli, N. S. Al-Zaid, A. Mousa, and M. Selim. 1980. Clinical and epidemiological studies of tinea versicolor in Kuwait. Mykosen 23:351–367. [DOI] [PubMed] [Google Scholar]
- 210.Kashima, M., H. Takahashi, M. Shimozuma, W. L. Epstein, and K. Fukuyama. 1989. Candidacidal activities of proteins partially purified from rat epidermis. Infect. Immun. 57:186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Katsambas, A., C. Anttoniou, E. Frangouli, G. Avgerinou, D. Michailidis, and J. Stratigos. 1989. A double-blind trial of treatment of seborrhoeic dermatitis with 2% ketoconazole cream compared with 1% hydrocortisone cream. Br. J. Dermatol. 121:353–357. [DOI] [PubMed] [Google Scholar]
- 212.Kavanagh, G. M., J. P. Leeming, G. M. Marshman, N. J. Reynolds, and J. L. Burton. 1993. Folliculitis in Down’s syndrome. Br. J. Dermatol. 129:696–699. [DOI] [PubMed] [Google Scholar]
- 213.Keddie, F. M. 1966. Electron microscopy of M. furfur in tinea versicolor. Sabouraudia 5:134–137. [PubMed] [Google Scholar]
- 214.Keddie, F. M., and L. Barajas. 1972. Quantitative ultrastructural variations between P. ovale and P. orbiculare based on serial section electron microscopy. Int. J. Dermatol. 11:40–48. [DOI] [PubMed] [Google Scholar]
- 215.Kellet, J. K., and R. H. Macdonald. 1985. Confluent and reticulate papillomatosis. Arch. Dermatol. 121:587–588. [PubMed] [Google Scholar]
- 216.Kesavan, S., K. T. Holland, and E. Ingham. 2000. The effect of lipid extraction on the immunomodulatory activity of Malassezia species in vitro. Med. Mycol. 38:239–247. [DOI] [PubMed] [Google Scholar]
- 217.Kesavan, S., C. E. Walters, K. T. Holland, and E. Ingham. 1998. The effects of Malassezia on pro-inflammatory cytokine production by human peripheral blood mononuclear cells in vitro. Med. Mycol. 36:97–106. [PubMed] [Google Scholar]
- 218.Keyworth, N., M. R. Millar, and K. T. Holland. 1992. Development of cutaneous microflora in premature neonates. Arch. Dis. Child. 67:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kieffer, M., I. M. Bergbrant, J. Faergemann, G. B. E. Jemec, V. Ottevanger, P. S. Skov, and E. Svejgaard. 1990. Immune responses to Pityrosporum ovale in adult patients with atopic and seborrhoeic dermatitis. J. Am. Acad. Dermatol. 22:739–742. [DOI] [PubMed] [Google Scholar]
- 220.Kim, E. H., R. S. Cohen, P. Ramachandran, and G. F. Glasscock. 1993. Adhesion of percutaneously inserted Silastic central venous lines to the vein wall associated with Malassezia furfur infection. J. Parenter. Enteral Nutr. 17:458–460. [DOI] [PubMed] [Google Scholar]
- 221.Kim, T. Y., I. G. Jang, Y. M. Park, H. O. Kim, and C. W. Kim. 1999. Head and neck dermatitis: the role of Malassezia furfur, topical steroid use and environmental factors in its causation. Clin. Exp. Dermatol. 24:226–231. [DOI] [PubMed] [Google Scholar]
- 222.King, R. D., H. A. Khan, J. C. Foye, J. H. Greenberg, and J. E. Jones. 1975. Transferin, iron and dermatophytes. I. Serum dermatophyte inhibitory component definitely identified as unsaturated transferin. J. Lab. Clin. Med. 86:204–212. [PubMed] [Google Scholar]
- 223.Kirby, J. D., and P. F. Borrie. 1975. Confluent and reticulate papillomatosis Proc. R. Soc. Med. 68:532–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kligman, A. M., K. J. McGinley, and J. J. Leyden. 1976. The nature of dandruff. J. Soc. Cosmet. Chem. 27:111–139. [Google Scholar]
- 225.Klotz, S. A., D. J. Drutz, M. Huppert, and J. E. Johnson. 1982. Pityrosporum folliculitis: its potential for confusion with skin lesions of systemic candidiasis. Arch. Intern. Med. 142:2126–2129. [PubMed] [Google Scholar]
- 226.Klotz, S. A., D. J. Drutz, and J. E. Zajic. 1985. Factors governing adherence of Candida species to plastic surfaces. Infect. Immun. 50:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Koga, Y., V. L. Swanson, and D. M. Hays. 1975. Hepatic ‘intravenous fat pigment’ in infants and children receiving lipid emulsion. J. Pediatr. Surg. 10:641–648. [DOI] [PubMed] [Google Scholar]
- 228.Koranda, F. C., E. A. Dehmel, G. Kahn, and I. Penn. 1974. Cutaneous complications in immunosuppressed renal homograft recipients. JAMA 229:419–424. [PubMed] [Google Scholar]
- 229.Korting, H. C., S. Loferer, and N. Hamm. 1991. The detergent scrub method for quantitative determination of Malassezia furfur on chest and back: comparative evaluation of three different media. Mycoses 34:267–271. [DOI] [PubMed] [Google Scholar]
- 230.Koyama, T., T. Kanbe, A. Ishiguro, A. Kikuchi, and Y. Tomita. 2000. Isolation and characterisation of a major antigenic component of Malassezia globosa to IgE antibodies in sera of patients with atopic dermatitis. Microbiol. Immunol. 44:373–379. [DOI] [PubMed] [Google Scholar]
- 231.Kozel, T. R., G. S. Pfrommer, A. S. Guerlain, B. A. Highison, and G. J. Highison. 1988. Role of the capsule in phagocytosis of Cryptococcus neoformans. Rev. Infect. Dis. 10:436–439. [DOI] [PubMed] [Google Scholar]
- 232.Kozel, T. R., M. A. Wilson, T. P. Farrell, and S. M. Levitz. 1989. Activation of C3 and binding to Aspergillus fumigatus conidia and hyphae. Infect. Immun. 57:3412–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kraus, A. 1913. Uber des Wesen des Sogenannten Unnaschen Flaschen-Bazillus. Arch. Dermatol. Syphilol. 116:723–726. [Google Scholar]
- 234.Kreger-van Rij, N. J. W., and M. Veenhuis. 1970. An electron microscope study of the yeast Pityrosporum ovale. Arch. Microbiol. 71:123–131. [DOI] [PubMed] [Google Scholar]
- 235.Kroger, S., K. Neuber, E. Gruseck, J. Ring, and D. Abeck. 1995. Pityrosporum ovale extracts increase interleukin-4, interleukin-10 and IgE synthesis in patients with atopic eczema. Acta Dermato-Venereol. 75:357–360. [DOI] [PubMed] [Google Scholar]
- 236.Kumar, A. S., and R. K. Pandhi. 1984. Syndrome of Cárteaud and Gougerot—a case report. Mykosen 27:313–315. [DOI] [PubMed] [Google Scholar]
- 237.Kumar, B., I. Kaur, R. Sialy, S. Kaur, and R. J. Dash. 1985. Elevated levels of plasma cortisol in pityriasis versicolor. Ind. J. Venereol. Leprol. 51:205–207. [PubMed] [Google Scholar]
- 238.Kwiterovick, P. O. 1974. Neonatal screening for hyperlipidaemia. Pediatrics 53:455–457. [PubMed] [Google Scholar]
- 239.Kyllonen, K. S., D. W. Clapp, R. M. Kliegman, J. E. Baley, N. Shenker, A. A. Fanaroff, and M. Berger. 1989. Dosage of intravenously administered immune globulin and dosing intervals required to maintain target levels of immunoglobulin G in low birth weight infants. J. Pediatr. 115:1013–1016. [DOI] [PubMed] [Google Scholar]
- 240.Labows, J. N., K. J. McGinley, J. J. Leyden, and G. F. Webster. 1979. Characteristic gamma lactone odour production of the genus Pityrosporum. Appl. Environ. Microbiol. 38:412–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Larocco, M., A. Dorenbaum, A. Robinson, and L. K. Pickering. 1988. Recovery of Malassezia pachydermatis from eight infants in a neonatal intensive care nursery: clinical and laboratory features. Pediatr. Infect. Dis. J. 7:398–401. [DOI] [PubMed] [Google Scholar]
- 242.Leeming, J. P., K. T. Holland, and R. A. Bojar. 1986. The in vitro antimicrobial effect of azelaic acid. Br. J. Dermatol. 115:551–556. [DOI] [PubMed] [Google Scholar]
- 243.Leeming, J. P., K. T. Holland, and W. J. Cunliffe. 1984. The microbial ecology of pilosebaceous units isolated from human skin. J. Gen. Microbiol. 130:803–807. [DOI] [PubMed] [Google Scholar]
- 244.Leeming, J. P., E. Ingham, and W. J. Cunliffe. 1988. The microbial content and complement C3 cleaving capacity of comedones in acne vulgaris. Acta Dermato-Venereol. 68:468–473. [PubMed] [Google Scholar]
- 245.Leeming, J. P., and F. H. Notman. 1987. Improved methods for isolation and enumeration of Malassezia furfur from human skin. J. Clin. Microbiol. 25:2017–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Leeming, J. P., F. H. Notman, and K. T. Holland. 1989. The distribution and ecology of Malassezia furfur and cutaneous bacteria on human skin. J. Appl. Bacteriol. 67:47–52. [DOI] [PubMed] [Google Scholar]
- 247.Leeming, J. P., T. M. Sutton, and P. J. Fleming. 1995. Neonatal skin as a reservoir of Malassezia species. Pediatr. Infect. Dis. J. 14:719–720. [PubMed] [Google Scholar]
- 248.Leung, D. Y. M. 1995. Atopic dermatitis: the skin as a window into the pathogenesis of chronic allergic diseases. J. Allergy Clin. Immunol. 96:302–318. [DOI] [PubMed] [Google Scholar]
- 249.Leung, D. Y. M. 1997. Atopic dermatitis: immunobiology and treatment with immune modulators. Clin. Exp. Immunol. 107(Suppl. 1):25–30 [PubMed] [Google Scholar]
- 250.Leung, D. Y. M. 2000. Atopic dermatitis: new insights and opportunities for therapeutic intervention. J. Allergy Clin. Immunol. 105:860–876. [DOI] [PubMed] [Google Scholar]
- 251.Levene, M. I., J. S. Wigglesworth, and R. Desai. 1980. Pulmonary fat accumulation after intralipid infusion in the preterm infant. Lancet i:815–818. [DOI] [PubMed] [Google Scholar]
- 252.Leyden, J. J., and A. M. Kligman. 1979. Dandruff — cause and treatment. Cosmet. Toiletries 94:23–28. [Google Scholar]
- 253.Leyden, J. J., K. J. McGinley, and A. M. Kligman. 1976. Role of microorganisms in dandruff. Arch. Dermatol. 112:333–338. [PubMed] [Google Scholar]
- 254.Lindborg, M., C. G. M. Magnusson, A. Zargari, M. Schmidt, A. Scheynius, R. Crameri, and P. Whitley. 1999. Selective cloning of allergens from the skin colonizing yeast Malassezia furfur by phage surface display. J. Investig. Dermatol. 113:156–161. [DOI] [PubMed] [Google Scholar]
- 255.Lindgren, L., C. F. Wahlgren, S. G. O. Johansson, I. Wiklund, and S. L. Nordvall. 1995. Occurrence and clinical features of sensitization to Pityrosporum orbiculare and other allergens in children with atopic dermatitis. Acta Dermato-Venereol. 75:300–304. [DOI] [PubMed] [Google Scholar]
- 256.Lintu, P., J. Savolainen, and K. Kalimo. 1997. IgE antibodies to protein and mannan antigens of Pityrosporum ovale in atopic dermatitis. Clin. Exp. Allergy 27:87–95. [PubMed] [Google Scholar]
- 257.Lintu, P., J. Savolainen, K. Kalimo, O. Kortekangas-Savolainen, M. Nermes, and E. O. Terho. 1999. Cross reacting IgE and IgG antibodies to Pityrosporum ovale mannan and other yeasts in atopic dermatitis. Allergy 54:1067–1073. [DOI] [PubMed] [Google Scholar]
- 258.Lintu, P., J. Savolainen, K. Kalimo, and E. O. Terho. 1998. Stability of Pityrosporum ovale allergens during storage. Clin. Exp. Allergy 28:486–490. [DOI] [PubMed] [Google Scholar]
- 259.Lober, C. W., P. W. Belew, E. W. Rosenberg, and G. Bale. 1982. Patch test with killed sonicated microflora in patients with psoriasis. Arch. Dermatol. 118:322–325. [PubMed] [Google Scholar]
- 260.Lodder, J., and N. J. W. Kreger-Van Rij. 1952. Pityrosporum, p.440–445, In C. P. Kurtzman and Jack W. Fell (ed.), The yeasts: a taxonomic study. North-Holland Publishing Co., Amsterdam, The Netherlands.
- 261.Long, J. G., and H. L. Keyserling. 1985. Catheter-related infection in infants due to an unusual lipophilic yeast—Malassezia furfur. Pediatrics 76:896–900. [PubMed] [Google Scholar]
- 262.Lopes, J. O., S. H. Alves, J. P. Benevenga, and C. S. Encarnacao. 1994. Nodular infection of the hair caused by Malassezia furfur. Mycopathologia 125:149–152. [DOI] [PubMed] [Google Scholar]
- 263.Lyons, R., and G. Woods. 1995. Comparison of the BacT/Alert and Isolator blood culture systems for recovery of fungi. Am. J. Clin. Pathol. 103:660–662. [DOI] [PubMed] [Google Scholar]
- 264.Mackee, G. M., and G. M. Lewis. 1938. Dandruff and seborrhoea. I. Flora of “normal” and diseased scalps. J. Investig. Dermatol. 1:131–139. [Google Scholar]
- 265.Mackie, R. M. 1991. Clinical dermatology. An illustrated textbook, 3rd ed. Oxford University Press, Oxford, United Kingdom.
- 266.Maietta, G., P. Fornaro, F. Rongioletti, and A. Rebora. 1990. Patients with mood depression have a high prevalence of seborrhoeic dermatitis. Acta Dermato-Venereol. 70:432–434. [PubMed] [Google Scholar]
- 267.Makimura, K., Y. Tamura, M. Kudo, K. Uchida, H. Saito, and H. Yamaguchi. 2000. Species identification and strain typing of Malassezia species stock strains and clinical isolates based on the DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J. Med. Microbiol. 49:29–35. [DOI] [PubMed] [Google Scholar]
- 268.Malassez, R. 1874. Note sur les champignon du pityriasis simplex. Arch. Physiol. 1:451. [Google Scholar]
- 269.Marcon, M. J., and D. A. Powell. 1987. Epidemiology, diagnosis and management of Malassezia furfur systemic infection. Diagn. Microbiol. Infect. Dis. 7:161–175. [DOI] [PubMed] [Google Scholar]
- 270.Marcon, M. J., and D. A. Powell. 1992. Human infections due to Malassezia spp. Clin. Microbiol. Rev. 5:101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Marks, R. M., A. D. Pearse, and A. P. Walker. 1985. The effects of a shampoo containing zinc pyrithione on the control of dandruff. Br. J. Dermatol. 112:415–422. [DOI] [PubMed] [Google Scholar]
- 272.Marples, R. R., D. T. Downing, and A. M. Kligman. 1972. Influence of Pityrosporum species in the generation of free fatty acids in human surface lipid. J. Investig. Dermatol. 58:155–159. [DOI] [PubMed] [Google Scholar]
- 273.Martin-Scott, J. 1952. The Pityrosporum ovale Br. J. Dermatol. 64:257–273. [DOI] [PubMed] [Google Scholar]
- 274.Masure, O., C. Leostic, M. L. Abalain, C. Chastel, I. Yakoub-Agha, C. Berthou, and J. Briere. 1991. Malassezia furfur septicaemia in a child with leukaemia. J. Infect. 23:335–336. [DOI] [PubMed] [Google Scholar]
- 275.Mathes, B. M., and M. C. Douglass. 1985. Seborrhoeic dermatitis in patients with acquired immunodeficiency syndrome. J. Am. Acad. Dermatol. 13:947–951. [DOI] [PubMed] [Google Scholar]
- 276.Mathov, I., L. Plotkin, C. Abatangelo, R. Galimberti, and L. Squiquera. 1996. Antibodies from patients with psoriasis recognize N-acetylglucosamine terminals in glycoproteins from Pityrosporum ovale. Clin. Exp. Immunol. 105:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Mayser, P., D. Fuhrer, R. Schmidt, and K. Grunder. 1995. Hydrolysis of fatty acid esters by Malassezia furfur: different utilization depending on alcohol moiety. Acta Dermato-Venereol. 75:105–109. [DOI] [PubMed] [Google Scholar]
- 278.Mayser, P., P. Haze, C. Papavassilis, M. Pickel, K. Gruender, and E. Gueho. 1997. Differentiation of Malassezia species: selectivity of Cremophor EL, castor oil and ricinoleic acid for M. furfur. Br. J. Dermatol. 137:208–213. [DOI] [PubMed] [Google Scholar]
- 279.Mayser, P., A. Imkampe, M. Winkeler, and C. Papvassilis. 1998. Growth requirements and nitrogen metabolism of Malassezia furfur. Arch. Dermatol. Res. 290:277–282. [DOI] [PubMed] [Google Scholar]
- 280.McGinley, K. J., L. R. Lantis, and R. R. Marples. 1970. Microbiology of tinea versicolor. Arch. Dermatol. 102:168–171. [PubMed] [Google Scholar]
- 281.McGinley, K. J., J. J. Leyden, R. R. Marples, and A. M. Kligman. 1975. Quantitiative microbiology of the scalp in non-dandruff, dandruff and seborrhoeic dermatitis. J. Investig. Dermatol. 64:401–405. [DOI] [PubMed] [Google Scholar]
- 282.Meirowsky, E. 1911. Uber des Wesen der Unnaschen Flaschenbazillen und uber den flaschen Ban einiger Hautpilze. Arch. Dermatol. Syphilol. 108:129–140. [Google Scholar]
- 283.Metze, D., A. Kersten, W. Jurecka, and W. Gebhart. 1991. Immunoglobulins coat microorganisms of skin surface: a comparative immunohistochemical and ultrastructural study of cutaneous and oral microbial symbionts. J. Investig. Dermatol. 96:439–445. [DOI] [PubMed] [Google Scholar]
- 284.Michalowski, R., and H. Rodziewicz. 1963. Pityriasis versicolor in children. Br. J. Dermatol. 72:397–400. [DOI] [PubMed] [Google Scholar]
- 285.Michalowski, R., and H. Rodziewicz. 1965. Pityriasis versicolor in the aged. Br. J. Dermatol. 77:388–390. [DOI] [PubMed] [Google Scholar]
- 286.Mickelsen, P. A., M. C. Viano-Paulson, D. A. Stevens, and P. S. Diaz. 1988. Clinical and microbiological features of infection with Malassezia pachydermatis in high risk infants. J. Infect. Dis. 157:1163–1168. [DOI] [PubMed] [Google Scholar]
- 287.Middleton, C., and R. M. Lowenthal. 1987. Malassezia furfur fungaemia as a treatable cause of obscure fever in leukaemia patient receiving parenteral nutrition. Aust. N. Z. J. Med. 17:603–605. [DOI] [PubMed] [Google Scholar]
- 288.Midgley, G. 1989. The diversity of Pityrosporum (Malassezia) yeasts in vivo and in vitro. Mycopathologia 106:143–155. [DOI] [PubMed] [Google Scholar]
- 289.Midgley, G. 1993. Morphological variation in Malassezia and its significance in pityriasis versicolor, p267–277. In H. Vanden Bossche (ed.), Dimorphic fungi in biology and medicine, Plenum Press, New York, N.Y.
- 290.Midgley, G. 2000. The lipophilic yeasts: state of the art and prospects. Med. Mycol. 38(Suppl. i):9–16. [PubMed] [Google Scholar]
- 291.Midgley, G., E. Gueho, and J. Guillot. 1998. Disease caused by Malassezia species, p.201–211. In L. Ajello and R. J. Hay (ed.), Topley and Wilson’s Microbiology and microbial infections, vol. 4. Arnold, London, United Kingdom.12649037
- 292.Midgley, G., and R. J. Hay. 1988. Serological responses to Pityrosporum (Malassezia) in seborrhoeic dermatitis demonstrated by ELISA and Western blotting. Bull. Soc. Fr. Mycol. Med. 17:267–276. [Google Scholar]
- 293.Mittag, H. 1995. Fine structural investigations of Malassezia furfur. II. The envelope of the yeast cells. Mycoses 38:13–21. [DOI] [PubMed] [Google Scholar]
- 294.Mohla, G., and R. T. Brodell. 1999. Koebner phenomenon in psoriasis. A common response to skin trauma. Postgrad. Med. 106:39–40. [DOI] [PubMed] [Google Scholar]
- 295.Montes, L. F. 1970. Systemic abnormalities and intracellular site of infections in the stratum corneum. JAMA 213:1469–1472. [PubMed] [Google Scholar]
- 296.Moore, M. 1938. Cultivation of M. furfur, etiological agent of pityriasis versicolor. Mycopathologia 1:53–61. [Google Scholar]
- 297.Moore, M., R. L. Kile, and M. F. Engman. 1936. Pityrosporum ovale (Bottle Bacillus of Unna, Spore of Malassez): cultivation and possible role in seborrhoeic dermatitis. Arch. Dematol. Syphilol. 33:457–472. [Google Scholar]
- 298.Muhlemann, M. F., M. G. Anderson, and F. Paradinas. 1986. Early warning skin signs in AIDS and persistent generalised lymphadenopathy. Br. J. Dermatol. 114:419–424. [DOI] [PubMed] [Google Scholar]
- 299.Murphy, J. W. 1991. Mechanisms of natural resistance to pathogenic fungi. Annu. Rev. Microbiol. 45:509–538. [DOI] [PubMed] [Google Scholar]
- 300.Nakabayashi, A., Y. Sei, and J. Guillot. 2000. Identification of Malassezia species isolated from patients with seborrhoeic dermatitis, atopic dermatitis, pityriasis versicolor and normal subjects. Med. Mycol. 38:337–341. [DOI] [PubMed] [Google Scholar]
- 301.Nazzaro-Porro, M., F. Caprilli, P. Nazzaro, and G. Morpurgo. 1976. Growth requirements and lipid metabolism of Pityrosporum orbiculare. J. Investig. Dermatol. 66:178–182. [DOI] [PubMed] [Google Scholar]
- 302.Nazzaro-Porro, M., and S. Passi. 1978. Identification of tyrosinase inhibitors in cultures of Pityrosporum. J. Investig. Dermatol. 71:205–208. [DOI] [PubMed] [Google Scholar]
- 303.Nazzaro-Porro, M., S. Passi, and F. Caprilli. 1977. Induction of hyphae in cultures in Pityrosporum by cholesterol and cholesterol esters. J. Investig. Dermatol. 69:531–534. [DOI] [PubMed] [Google Scholar]
- 304.Nazzaro-Porro, M., S. Passi, M. Picardo, R. Mercantini, and A. S. Breathnach. 1986. Lipoxygenase activity of Pityrosporum in vitro and in vivo. J. Investig. Dermatol. 87:108–112. [DOI] [PubMed] [Google Scholar]
- 305.Nelson, S. C., Y. C. W. Yau, S. E. Richardson, and A. G. Matlow. 1995. Improved detection of Malassezia species in lipid-supplemented Peds Plus blood culture bottles. J. Clin. Microbiol. 33:1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Nermes, M., J. Savolainen, and O. Kortekangus-Savolainen. 1995. Nitrocellulose-RAST analysis of allergenic cross-reactivity of Candida albicans and Saccharomyces cerevisiae mannans. Int. Arch. Allergy Immunol. 106:118–123. [DOI] [PubMed] [Google Scholar]
- 307.Neuber, K., S. Kroger, E. Gruseck, D. Abeck, and J. Ring. 1996. Effects of Pityrosporum ovale on proliferation, immunoglobulin (IgA, G, M) synthesis and cytokine (IL2, IL10, IFNgamma) production of peripheral blood mononuclear cells from patients with seborrhoeic dermatitis. Arch. Dermatol. Res. 288:532–536. [PubMed] [Google Scholar]
- 308.Neumann, I. 1871. Textbook of skin diseases. R. Hardwick, London. United Kingdom.
- 309.Niamba, P., F. X. Weill, J. Sarlangue, C. Labreze, B. Couprie, and A. Taieb. 1998. Is common neonatal cephalic pustulosis (neonatal acne) triggered by Malassezia sympodialis ? Arch. Dermatol. 134:995–998. [DOI] [PubMed] [Google Scholar]
- 310.Nicholls, D. S., G. Midgley, and R. J. Hay. 1990. Patch testing against Pityrosporum antigens. Clin. Exp. Dermatol. 15:75–78. [DOI] [PubMed] [Google Scholar]
- 311.Nicholls, J. M., K. Y. Yuen, and H. Saing. 1993. Malassezia furfur infection in a neonate. Br. J. Hosp. Med. 49:425–427. [PubMed] [Google Scholar]
- 312.Nissen, D., L. J. Petersen, R. Esch, E. Svejgaard, P. S. Skov, L. K. Poulsen, and H. Nolte. 1998. IgE sensitization to cellular and culture filtrates of fungal extracts in patients with atopic dermatitis. Ann. Allergy Asthma Immunol. 81:247–255. [DOI] [PubMed] [Google Scholar]
- 313.Noble, W. C., and G. Midgley. 1978. Scalp carriage of Pityrosporum species: the effect of physiological maturity, sex and race. Sabouraudia 16:229–232. [DOI] [PubMed] [Google Scholar]
- 314.Nordvall, S. L., and S. Johansson. 1990. IgE antibodies to Pityrosporum orbiculare in children with atopic diseases. Acta. Paediatr. Scand. 79:343–349. [DOI] [PubMed] [Google Scholar]
- 315.Nordvall, S. L., L. Lindgren, S. G. O. Johansson, S. Johansson, and B. Petrini. 1992. IgE antibodies to Pityrosporum orbiculare and Staphylococcus aureus in patients with very high serum total IgE. Clin. Exp. Allergy 22:756–761. [DOI] [PubMed] [Google Scholar]
- 316.Norris, J. F. B., and W. J. Cunliffe. 1988. A histological and immunocytochemical study of early acne lesions. Br. J. Dermatol. 118:651–659. [DOI] [PubMed] [Google Scholar]
- 317.Noterangelo, L. D., C. Chirico, A. Chiara, A. Colombo, G. Rondini, A. Plebani, A. Martin, and A. G. Ugazio. 1984. Activity of classical and altenative pathways of complement in preterm and small for gestational age infants. Pediatr. Res. 18:281–285. [DOI] [PubMed] [Google Scholar]
- 318.Nyirjesy, P., J. M. Nixon, C. A. Jordan, and H. R. Buckley. 1994. Malassezia furfur folliculitis of the vulva: olive oil solves the mystery. Obstet. Gynaecol. 84:710–711. [PubMed] [Google Scholar]
- 319.Oberie, A. D., S. M. Fowler, and W. D. Grafton. 1981. Pityrosporum isolate from upper respiratory tract. Am. J. Clin. Pathol. 76:112–116. [DOI] [PubMed] [Google Scholar]
- 320.Onishi, Y., M. Kuroda, H. Yasueda, A. Saito, E. Sono-Koyama, S. Tunasawa, T. Hashida-Okado, T. Yagihara, K. Uchida, H. Yamaguchi, K. Akiyama, I. Kato, and K. Takesako. 1999. Two-dimensional electrophoresis of Malassezia allergens for atopic dermatitis and isolation of Mal f4 homologs with mitochondrial malate dehydrogenase. Eur. J. Biochem. 261:148–154. [DOI] [PubMed] [Google Scholar]
- 321.Ortonne, J. P., J. P. Lacour, A. Vitetta, and Y. Le Fichoux. 1992. Comparative study of ketoconzole 2% foaming gel and betamethasone dipropionate 0.05% lotion in the treatment of seborrhoeic dermatitis in adults. Dermatology 184:275–280. [DOI] [PubMed] [Google Scholar]
- 322.Page, C. O., and J. S. Remington. 1967. Immunologic studies in normal human sweat. J. Lab. Clin. Med. 69:634–50. [PubMed] [Google Scholar]
- 323.Palella, F. J., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, and S. D. Holmberg. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853–860. [DOI] [PubMed] [Google Scholar]
- 324.Panja, G. 1927. The Malassezia of the skin, their cultivation, morphology and species. Trans. 7th Congr. Far East. Assoc. Trop. Med. 2:442–456. [Google Scholar]
- 325.Panja, G. 1946. A new oil for enhancement of growth of the Malassezia and subsequent study of serological reactions and pathogenicity. Indian Med. Gaz. 81:305–306. [PMC free article] [PubMed] [Google Scholar]
- 326.Papadea, C., and I. J. Check. 1989. Human immunoglobulin G and immunoglobulin G subclasses: biochemical, genetic and clinical aspects. Crit. Rev. Clin. Lab. Sci. 27:27–58. [DOI] [PubMed] [Google Scholar]
- 327.Papavassilis, C., K. K. Mach, and P. A. Mayser. 1999. Medium-chain triglycerides inhibit growth of Malassezia: implications for prevention of systemic infection. Crit. Care Med. 27:1781–1786. [DOI] [PubMed] [Google Scholar]
- 328.Parry, M. E., and G. R. Sharpe. 1998. Seborrhoeic dermatitis is not caused by an altered immune response to Malassezia yeast. Br. J. Dermatol. 139:254–263. [DOI] [PubMed] [Google Scholar]
- 329.Passwell, J. H., R. David, D. Katznelson, and B. E. Cohen. 1976. Pigment deposition in the reticulo-endothelial system after fat emulsion infusion. Arch. Dis. Child. 51:366–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Peter, R. U., and U. Richarz-Barthauer. 1995. Successful treatment and prophylaxis of scalp seborrhoeic dermatitis and dandruff with 2% ketoconazole shampoo: results of a multicentre, double-blind placebo-controlled trial. Br. J. Dermatol. 132:441–445. [DOI] [PubMed] [Google Scholar]
- 331.Picardo, M., S. Passi, M. C. Sirianna, M. Fiorilli, G. D. Russo, E. Cortesi, G. Barile, A. S. Breathnach, and M. S. Nazzaro-Porro. 1985. Activity of azelaic acid on cultures of lymphoma- and leukemia-derived cells lines, normal resting cells and stimulated lymphocytes and 3T3 fibroblasts. Biochem. Pharmacol. 34:1653–1658. [DOI] [PubMed] [Google Scholar]
- 332.Pierard, G. E., C. Pierard-Franchimont, J. Van Cutsem, A. Rurangirwa, M. L. Hoppenbrouwers, and P. Schrooten. 1991. Ketoconazole 2% emulsion in the treatment of seborrhoeic dermatitis. Int. J. Dermatol. 30:806–809. [DOI] [PubMed] [Google Scholar]
- 333.Pierard-Franchimont, C., J. E. Arrese, G. Durupt, G. Ries, G. Cauwenbergh, and G. E. Pierard. 1998. Correlation between Malassezia spp. load and dandruff severity. J. Mycol. Med. 8:83–86. [Google Scholar]
- 334.Pierard-Franchimont, C., J. E. Arrese, and G. E. Pierard. 1995. Immunohistochemical aspects of the link between Malassezia ovalis and seborrhoeic dermatitis. J. Eur. Acad. Dermatol. Venereol. 4:14–19. [Google Scholar]
- 335.Pinkus, H., and A. H. Mehregan. 1966. The primary histologic lesion of seborrhoeic dermatitis and psoriasis. J. Investig. Dermatol. 46:109–116. [DOI] [PubMed] [Google Scholar]
- 336.Plantin, P., H. Cartier, F. Geffroy, and L. Broussine. 1995. Neonatal pustulosis due to Malassezia furfur. Arch. Pediatr. 2:1016. [DOI] [PubMed] [Google Scholar]
- 337.Plewig, G. 1986. Seborhoeic dermatitis, p.375–381. In A. Rook (ed.), Textbook of dermatology, vol. 1, 4th ed. Blackwell Scientific Publishers, Oxford, United Kingdom.
- 338.Plotkin, L. I., I. Mathov, L. Squiquera, and J. Leoni. 1998. Arachidonic acid released from epithelial cells by Malassezia furfur phospholipase A(2): a potential pathophysiologic mechanism. Mycologia 90:163–169. [Google Scholar]
- 339.Plotkin, L. I., L. Squiquera, I. Mathov, R. Galimberti, and J. Leoni. 1996. Characterization of the lipase activity of Malassezia furfur. J. Med. Vet. Mycol. 34:43–48. [DOI] [PubMed] [Google Scholar]
- 340.Potter, B. S., C. F. Burgoon, and W. C. Johnson. 1973. Pityrosporum folliculitis. Arch. Dermatol. 107:388–391. [DOI] [PubMed] [Google Scholar]
- 341.Powell, D. A., J. Aungst, S. Snedden, N. Hansen, and M. Brady. 1984. Broviac catheter-related M. furfur sepsis in 5 infants receiving IV fat emulsions. J. Pediatr. 105:987–990. [DOI] [PubMed] [Google Scholar]
- 342.Powell, D. A., and M. T. Brady. 1985. Malassezia furfur sepsis in neonates—reply. J. Pediatr. 107:644.3930682 [Google Scholar]
- 343.Powell, D. A., D. E. Durrell, and M. J. Marcon. 1986. Growth of Malassezia furfur in parenteral fat emulsions. J. Infect. Dis. 153:640–641. [DOI] [PubMed] [Google Scholar]
- 344.Powell, D. A., J. Hayes, D. E. Durrell, M. Miller, and M. J. Marcon. 1987. Malassezia furfur skin colonisation of infants hospitalised in intensive care units. J. Pediatr. 111:217–220. [DOI] [PubMed] [Google Scholar]
- 345.Powell, D. A., and M. J. Marcon. 1987. Failure to eradicate Malassezia furfur Broviac catheter infection with antifungal therapy. Pediatr. Infect. Dis. J. 6:579–580. [DOI] [PubMed] [Google Scholar]
- 346.Powell, D. A., M. J. Marcon, D. E. Durrell, and R. M. Pfister. 1987. Scanning electron microscopy of M. furfur attachment to Broviac catheters. Hum. Pathol. 18:740–745. [DOI] [PubMed] [Google Scholar]
- 347.Puhvel, S. M., and M. Sakamoto. 1977. A re-evaluation of fatty acids as inflammatory agents in acne. J. Investig. Dermatol. 68:93–97. [DOI] [PubMed] [Google Scholar]
- 348.Raad, I. 2000. Management of intravascular catheter-related infections. J. Antimicrob. Chemother. 45:267–270. [DOI] [PubMed] [Google Scholar]
- 349.Ran, Y., T. Yoshike, and H. Ogawa. 1993. Lipase of M. furfur: some properties and their relationship to cell growth. J. Med. Vet. Mycol. 31:77–85. [DOI] [PubMed] [Google Scholar]
- 350.Rapelanoro, R., P. Mortureux, B. Couprie, J. Maleville, and A. Taieb. 1996. Neonatal Malassezia furfur pustulosis. Arch. Dermatol. 132:190–193. [PubMed] [Google Scholar]
- 351.Rasool, O., A. Zargari, J. Almqvist, H. Eshaghi, P. Whitley, and A. Scheynius. 2000. Cloning, characterization and expression of complete coding sequences of three IgE binding Malassezia furfur allergens, Mal f 7, Mal f 8 and Mal f 9. Eur. J. Biochem. 267:4355–4361. [DOI] [PubMed] [Google Scholar]
- 352.Ratzer, M. 1969. The incidence of skin diseases in the west of Scotland. Br. J. Dermatol. 81:456–461. [DOI] [PubMed] [Google Scholar]
- 353.Ray, T. L., and K. D. Wuepper. 1976. Activation of the alternative (properdin) pathway of complement by Candida albicans and related species. J. Investig. Dermatol. 67:700–703. [DOI] [PubMed] [Google Scholar]
- 354.Redline, R. W., and B. B. Dahms. 1981. Malassezia pulmonary vasculitis in an infant on long-term intralipid therapy. N. Engl. J. Med. 305:1395–1398. [DOI] [PubMed] [Google Scholar]
- 355.Redline, R. W., S. S. Redline, B. Boxerbaum, and B. B. Dahms. 1985. Systemic M. furfur infections in patients receiving intralipid therapy. Hum. Pathol. 16:815–822. [DOI] [PubMed] [Google Scholar]
- 356.Reimer, L. G., M. L. Wilson, and M. P. Weinstein. 1997. Update on detection of bacteremia and fungemia. Clin. Microbiol. Rev. 10:444–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Rhie, S., R. Turcios, H. Buckley, and B. Suh. 2000. Clinical features and treatment of Malassezia folliculitis with fluconazole in orthotopic heart transplant recipients. J. Heart Lung Transplant. 19:215–219. [DOI] [PubMed] [Google Scholar]
- 358.Richardson, M. D., and G. S. Shankland. 1991. Enhanced phagocytosis and intracellular killing of Pityrosporum ovale by human neutrophils after expsoure to ketoconazole is correlated to changes of the yeast cell surface. Mycoses 34:29–33. [DOI] [PubMed] [Google Scholar]
- 359.Richet, H. M., M. M. McNeil, M. C. Edwards, and W. R. Jarvis. 1989. Cluster of Malassezia furfur pulmonary infections in infants in a neonatal intensive-care unit. J. Clin. Microbiol. 27:1197–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Riciputo, R. M., S. Oliveri, G. Micali, and A. Sapuppo. 1996. Phospholipase activity in Malassezia furfur pathogenic strains. Mycoses 39:233–235. [DOI] [PubMed] [Google Scholar]
- 361.Rider, E. D., R. D. Christensen, D. C. Hall, and G. Rothstein. 1988. Myeloperoxidase deficiency in neutrophils of neonates. J. Pediatr. 112:648–651. [DOI] [PubMed] [Google Scholar]
- 362.Rigopoulos, D., A. Katsambas, C. Antoiou, S. Theocharis, and J. Stratigos. 1994. Facial seborrhoeic dermatitis treated with fluconazole 2% shampoo Int. J. Dermatol. 33:136–137. [DOI] [PubMed] [Google Scholar]
- 363.Rivolta, S. 1873. Parassiti vegetali, p.469–471. di Giulio Speiranai F. Figli, Turin, Italy.
- 364.Roberts, S. O. B. 1969. Pityrosporum orbiculare: incidence and distribution on clinically normal skin. Br. J. Dermatol. 81:264–269. [DOI] [PubMed] [Google Scholar]
- 365.Roberts, S. O. B. 1969. Pityriasis versicolor: a clinical and mycological investigation. Br. J. Dermatol. 81:315–326. [DOI] [PubMed] [Google Scholar]
- 366.Roberts, S. O. B., and J. M. Lachapelle. 1969. Confluent and reticulate papillomatosis (Gougerot-Carteaud) and Pityrosporum orbiculare. Br. J. Dermatol. 81:841–845. [DOI] [PubMed] [Google Scholar]
- 367.Robin, C. 1853. Histoire naturelle des vegetaux parasites. J. B. Bailliere, Paris, France.
- 368.Robinson, R., and P. Ball. 1998. Does the Pall TNA-1E parenteral nutrition admixture filter retain Malassezia furfur? Nutrition 14:363–365. [DOI] [PubMed] [Google Scholar]
- 369.Rocha, G. L., C. Silva, O. Lima, and M. Goto. 1952. Experimental studies on Pityrosporum ovale: its pathogenicity and antigenic capacity. J. Investig. Dermatol. 19:289–292. [DOI] [PubMed] [Google Scholar]
- 370.Roilides, E., M. C. Dignani, E. J. Anaissie, and J. H. Rex. 1998. The role of immunoreconstitution in the management of refractory opportunistic fungal infections. Med. Mycol. 36(Suppl. 1):12–25. [PubMed] [Google Scholar]
- 371.Rokugo, M., H. Tagami, Y. Usuba, and Y. Tomita. 1990. Contact sensitivity to Pityrosporum ovale in patients with atopic dermatitis. Arch. Dermatol. 126:627–632. [PubMed] [Google Scholar]
- 372.Romano, A., E. Segal, and M. Blumenthal. 1978. Canaliculitis with isolation of Pityrosporum pachydermatis. Br. J. Ophthalmol. 62:732–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Rosenberg, E. W., and P. W. Belew. 1982. Microbial factors in psoriasis. Arch. Dermatol. 118:143–144. [PubMed] [Google Scholar]
- 374.Rosenberg, E. W., and P. W. Belew. 1982. Improvement of psoriasis of the scalp with ketoconazole. Arch. Dermatol. 118:370–371. [PubMed] [Google Scholar]
- 375.Rosenberg, E. W., P. Belew, and G. Bale. 1980. Effect of topical applications of heavy suspensions of killed Malassezia ovalis on rabbit skin. Mycopathologia 72:147–154. [DOI] [PubMed] [Google Scholar]
- 376.Rosenberg, E. W., P. W. Noah, R. B. Skinner, R. Vander Zwaag, S. K. West, and J. F. Browder. 1989. Microbial associations of 167 patients with psoriasis. Acta Dermato-Venereol. 146(Suppl):72–75. [PubMed] [Google Scholar]
- 377.Rulison, R. H., and W. J. Highman. 1924. Is dandruff seborrheal? Arch. Dermatol. Syphilol. 10:428–441. [Google Scholar]
- 378.Saadatzadeh, M. R., H. R. Ashbee, W. J. Cunliffe, and E. Ingham. 2001. Cell mediated immunity to the mycelial phase of Malassezia spp in patients with pityriasis versicolor and controls. Br. J. Dermatol. 144:77–84. [DOI] [PubMed] [Google Scholar]
- 379.Saadatzadeh, M. R., H. R. Ashbee, K. T. Holland, and E. Ingham. Production of the mycelial phase of Malassezia species in vitro. Med. Mycol., in press. [DOI] [PubMed]
- 380.Saadatzadeh, M. R. 1998. The immunology of the mycelial phase of Malassezia. Ph.D. thesis. University of Leeds, Leeds, United Kingdom.
- 381.Sabouraud, R. 1904. Maladies du cuir chevulu. Masson et cie, Paris, France.
- 382.Saint-Leger, D., A. M. Kligman, and J. J. Stoudemayer. 1989. The role of the resident microflora in the pathogenesis of dandruff. J. Soc. Cosmet. Chem. 40:109–119. [Google Scholar]
- 383.Salkin, F., and M. A. Gordon. 1977. Polymorphism of Malassezia furfur. Can. J. Microbiol. 23:471–475. [DOI] [PubMed] [Google Scholar]
- 384.Salzman, M. B., and L. G. Rubin. 1995. Intravenous catheter-related infections. Adv. Pediatr. Infect. Dis. 10:337–365. [PubMed] [Google Scholar]
- 385.Savolainen, J., and A. Broberg. 1992. Cross reacting IgE antibodies to Pityrosporum ovale and Candida albicans in atopic children. Clin. Exp. Allergy 22:469–474. [DOI] [PubMed] [Google Scholar]
- 386.Scheynius, A., J. Faergemann, and U. Forsum. 1984. Phenotypic characterisation in situ of inflammatory cells in pityriasis (tinea) versicolor. Acta Dermato-Venereol. 64:473–479. [PubMed] [Google Scholar]
- 387.Schleman, K. A., G. Tullis, and R. Blum. 2000. Intracardiac mass complicating Malassezia furfur fungemia. Chest 118:1828–1829. [DOI] [PubMed] [Google Scholar]
- 388.Schmidt, M., A. Zargari, P. Holt, L. Lindbom, U. Hellman, P. Whitley, I. van der Ploeg, B. Harfast, and A. Scheynius. 1997. The complete cDNA sequence and expression of the first major allergenic protein of Malassezia furfur, Mal f1. Euro. J. Biochem. 246:181–185. [DOI] [PubMed] [Google Scholar]
- 389.Schur, P. 1987. IgG subclasses— a review. Ann. Allergy 58:89–99. [PubMed] [Google Scholar]
- 390.Scott, D., W. J. Cunliffe, and G. Gowland. 1979. Activation of complement—a mechanism for inflammation in acne. Br. J. Dermatol. 101:315–32. [DOI] [PubMed] [Google Scholar]
- 391.Segal, R., D. Shmueli, A. Yussim, M. Sandbank, I. Alteras, and Z. Shapira. 1989. Renal transplant incidence of superficial fungal infection as related to immunosuppression therapy. Trans. Proc. 21:2114–2116. [PubMed] [Google Scholar]
- 392.Sei, Y., I. Takiuchi, S. Watanabe, M. Honda, F. Ito, T. Nishikawa, H. Ogawa, T. Harada, C. Nishiyama, and T. Katoh. 1997. Study on the usefulness of shampoo containing 0.75% miconazole nitrate for treatment of dandruff. A double blind comparative study. Jpn. J. Mycol. 38:87–97. [Google Scholar]
- 393.Seow, C. S. 1987. Double-blind, placebo-controlled trial on treatment of seborrhoeic dermatitis with oral ketoconazole. J. Med. Assoc. Thail. 70(Suppl. 3):45–50. [Google Scholar]
- 394.Shek, Y. H., M. C. Tucker, A. L. Viciana, H. J. Manz, and D. H. Connor. 1989. Malassezia furfur—disseminated infection in premature infants. Am. J. Clin. Pathol. 92:595–603. [DOI] [PubMed] [Google Scholar]
- 395.Shifrine, M., and A. G. Marr. 1963. The requirement of fatty acids by P. ovale. J. Gen. Microbiol. 32:263–270. [DOI] [PubMed] [Google Scholar]
- 396.Shimada, S., and S. I. Katz. 1988. The skin as an immunologic organ. Arch. Pathol. Lab. Med. 112:231–234. [PubMed] [Google Scholar]
- 397.Shparago, N. I., P. P. Bruno, and J. Bennett. 1995. Systemic Malassezia furfur infection in an adult receiving total parenteral nutrition. J. Am. Osteopath. Assoc. 95:375–377. [PubMed] [Google Scholar]
- 398.Shuster, S. 1984. The aetiology of dandruff and the mode of action of therapeutic agents. Br. J. Dermatol. 111:235–242. [DOI] [PubMed] [Google Scholar]
- 399.Silva, V., O. Fischman, and Z. P. de Camargo. 1997. Humoral immune response to Malassezia furfur in patients with pityriasis versicolor and seborrhoeic dermatitis. Mycopathologia 139:79–85. [DOI] [PubMed] [Google Scholar]
- 400.Silva, V., G. A. Moreno, L. Zaror, E. De Oliveora, and O. Fischman. 1997. Isolation of Malassezia furfur from patients with onychomycosis. J. Med. Vet. Mycol. 35:73–74. [PubMed] [Google Scholar]
- 401.Simmons, R. B., and D. G. Ahearn. 1987. Cell wall ultrastructure and diazonium blue B reactions of Speropachydermia quercuum, Bulleo tsugae and Malassezia spp. Mycologia 79:38–43. [Google Scholar]
- 402.Simmons, R. B., and E. Gueho. 1990. A new species of Malassezia. Mycol. Res. 94:1146–1149. [Google Scholar]
- 403.Sina, B., C. L. Kauffman, and C. S. Samorodin. 1995. Intrafollicular mucin deposits in Pityrosporum folliculitis. J. Am. Acad. Dermatol. 32:807–809. [DOI] [PubMed] [Google Scholar]
- 404.Sitges-Serra, A., and M. Girvent. 1999. Catheter-related bloodstream infections. World J. Surg. 23:589–595. [DOI] [PubMed] [Google Scholar]
- 405.Sizun, J., A. Karangwa, J. D. Giroux, O. Masure, A. M. Simitzis, D. Alix, and L. De Parscau. 1994. Malassezia furfur related colonization and infection of central venous catheters. Intensive Care Med. 20:496–499. [DOI] [PubMed] [Google Scholar]
- 406.Skinner R. B., P. W. Noah, M. D. Zanolli, and E. W. Rosenberg. 1986. The pathogenic role of microbes in seborrhoeic dermatitis. Arch. Dermatol. 122:16–17. [PubMed] [Google Scholar]
- 407.Slooff, W. C. 1970. Pityrosporum sabouraud, p.1167–1186. In J. Lodder (ed.), The yeasts—a taxonomic study, 2nd ed. North Holland Publishing Co., Amsterdam, The Netherlands.
- 408.Smith, K. J., H. G. Skelton, J. Yeager, R. Ledsky, W. McCarthy, D. Baxter, and K. F. Wagner. 1994. Cutaneous findings in HIV-1 positive patients: a 42 month prospective study. J. Am. Acad. Dermatol. 31:746–754. [DOI] [PubMed] [Google Scholar]
- 409.Sohnle, P. G., and C. Collins-Lech. 1978. Cell-mediated immunity to Pityrosporum orbiculare in tinea versicolor. J. Clin. Investig. 62:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Sohnle, P. G., and C. Collins-Lech. 1980. Relative antigenicity of P. orbiculare and C. albicans. J. Investig. Dermatol. 75:279–283. [DOI] [PubMed] [Google Scholar]
- 411.Sohnle, P. G., and C. Collins-Lech. 1982. Analysis of the lymphocyte transformation response to Pityrosporum orbiculare in patients with tinea versicolor. Clin. Exp. Immunol. 49:559–564. [PMC free article] [PubMed] [Google Scholar]
- 412.Sohnle, P. G., and C. Collins-Lech. 1983. Activation of complement by Pityrosporum orbiculare. J. Investig. Dermatol. 80:93–97. [DOI] [PubMed] [Google Scholar]
- 413.Sohnle, P. G., C. Collins-Lech, and K. E. Huhta. 1983. Class specific antibodies in young and aged humans against organisms producing superficial fungal infections. Br. J. Dermatol. 108:69–76. [DOI] [PubMed] [Google Scholar]
- 414.Solomkin, J. S., E. L. Mills, G. S. Giebink, R. D. Nelson, R. L. Simmons, and P. G. Quie. 1978. Phagocytosis of Candida albicans by human leukocytes—opsonic requirements. J. Infect. Dis. 137:30–37. [DOI] [PubMed] [Google Scholar]
- 415.Spoor, H. J., E. F. Traub, and M. Bell. 1954. P. ovale types cultured from normal and seborrhoeic subjects. Arch. Dermatol. Syphilol. 69:323–330. [DOI] [PubMed] [Google Scholar]
- 416.Spratt, M. G., and C. C. Kratzing. 1975. Oleic acid as a depressant of reticuloendothelial activity in rats and mice. J. Reticuloendothal. Soc. 17:135–140. [PubMed] [Google Scholar]
- 417.Squiquera, L., R. Galimberti, L. Morelli, L. Plotkin, R. Milicich, A. Kowalckzuk, and J. Leoni. 1994. Antibodies to proteins from Pityrosporum ovale in the sera from patients with psoriasis. Clin. Exp. Dermatol. 19:289–293. [DOI] [PubMed] [Google Scholar]
- 418.Sternberg, T. H., and F. M. Keddie. 1961. Immunofluorescence studies in tinea versicolor. Arch. Dermatol. 84:161–165. [DOI] [PubMed] [Google Scholar]
- 419.Stingl, G., E. Tschachler, V. Groh, K. Wolff, and C. Hauser. 1989. The immune functions of epidermal cells, p.3–72. In D. Norris (ed.), immune mechanisms in cutaneous disease. Marcel Dekker, Inc., New York, N.Y. [PubMed]
- 420.Streilein, J. W. 1989. Skin-associated lymphoid tissue, p.73–96. In D. Norris (ed.), Immune mechanisms in cutaneous disease. Marcel Dekker, Inc., New York, N.Y.
- 421.Surmont, I., A. Gavilanes, J. Vandepitte, H. Devlieger, and E. Eggermont. 1989. Malassezia furfur fungaemia in infants receiving intravenous lipid emulsions. A rarity or just underestimated ? Eur. J. Pediatr. 148:435–438. [DOI] [PubMed] [Google Scholar]
- 422.Suzuki, T., N. Ohno, and Y. Ohshima. 1998. Activation of complement system, alternative and classical pathways, by Malassezia furfur. Pharm. Pharmacol. Lett. 3:133–136. [Google Scholar]
- 423.Suzuki, T., N. Ohno, Y. Ohshima, and T. Yadomae. 1998. Soluble mannan and beta-glucan inhibit the uptake of Malassezia furfur by human monocytic cell line, THP-1. FEMS Immunol. Med. Microbiol. 21:223–230. [DOI] [PubMed] [Google Scholar]
- 424.Suzuki, T., A. Tsuzuki, N. Ohno, Y. Ohshima, and T. Yadomae. 2000. Enhancement of IL-8 production from human monocytic and granulocytic cell lines, THP-1 and HL-60, stimulated with Malassezia furfur. FEMS Immunol. Med. Microbiol. 28:157–162. [DOI] [PubMed] [Google Scholar]
- 425.Svejgaard, E., J. Faergemann, G. Jemec, M. Kieffer, and V. Ottevanger. 1989. Recent investigations on the relationship between fungal skin diseases and atopic dermatitis. Acta Dermato-Venereol. 144(Suppl.):140–142. [DOI] [PubMed] [Google Scholar]
- 426.Swift, J. A., and S. F. Dunbar. 1965. Ultrastructure of Pityrosporum ovale and P. canis. Nature 206:1174–1175. [DOI] [PubMed] [Google Scholar]
- 427.Tagami, H. 1992. The role of complement derived mediators in inflammatory skin diseases. Arch. Dermatol. Res. 284:S2–S9. [DOI] [PubMed] [Google Scholar]
- 428.Takahashi, M., T. Ushijima, and Y. Ozaki. 1981. Comparative studies of biological and serological characteristics of each species of Pityrosporum. Jpn. J. Med. Mycol. 22:314–321. [Google Scholar]
- 429.Takahashi, M., T. Ushijima, and Y. Ozaki. 1984. Biological activity of Pityrosporum. I. Enhancement of resistance in mice stimulated by Pityrosporum against S. typhimurium. Immunology 51:697–702. [PMC free article] [PubMed] [Google Scholar]
- 430.Takahashi, M., T. Ushijima, and Y. Ozaki. 1986. Biological activity of Pityrosporum. II. Antitumour and immune stimulating effect of Pityrosporum in mice. JNCI 77:1093–1097. [PubMed] [Google Scholar]
- 431.Takematsu, H., and H. Tagami. 1992. Preferential activation of the alternative pathway of complement in psoriatic lesional skin. Dermatology 184:159–160. [DOI] [PubMed] [Google Scholar]
- 432.Tanaka, M., and S. Imamura. 1979. Immunological studies on Pityrosporum genus and Malassezia furfur. J. Investig. Dermatol. 73:321–324. [DOI] [PubMed] [Google Scholar]
- 433.Taramelli, D., M. G. Malabarba, G. Sala, N. Basilico, and G. Cocuzza. 1996. Production of cytokines by alveolar and peritoneal macrophages stimulated by Aspergillus fumigatus conidia or hyphae. J. Med. Vet. Mycol. 34:49–56 [PubMed] [Google Scholar]
- 434.Tegner, E. 1983. Seborrhoeic dermatitis of the face induced by PUVA treatment. Acta Dermato-Venereol. 63:335–339. [PubMed] [Google Scholar]
- 435.Templeton, H. J. 1926. A study of dandruff and of the Pityrosporum of Malassez. Arch. Dermatol. Syphilol. 14:270–279. [Google Scholar]
- 436.Tengvall Linder, M., C. Johansson, A. Bengtsson, L. Holm, B. Harfast, and A. Scheynius. 1998. Pityrosporum orbiculare reactive T-cell lines in atopic dermatitis patients and healthy individuals. Scand. J. Immunol. 47:152–158. [DOI] [PubMed] [Google Scholar]
- 437.Tengvall Linder, M., C. Johansson, A. Scheynius, and C. F. Wahlgren. 2000. Positive atopy patch test reactions to Pityrosporum orbiculare in atopic dermatitis patients. Clin. Exp. Allergy 30:122–131. [DOI] [PubMed] [Google Scholar]
- 438.Tengvall Linder, M., C. Johansson, A. Zargari, A. Bengtsson, I. Van der Ploeg, I. Jones, B. Harfast, and A. Scheynius. 1996. Detection of Pityrosporum orbiculare reactive T cells from skin and blood in atopic dermatitis and characterization of their cytokine profiles. Clin. Exp. Allergy 26:1286–1297. [DOI] [PubMed] [Google Scholar]
- 439.Thompson, E., and J. R. Colvin. 1970. Composition of the cell wall of Pityrosporum ovale (Bizzozero) Castellani and Chalmers. Can. J. Microbiol. 16:263–265. [DOI] [PubMed] [Google Scholar]
- 440.Thygeson, P., and D. G. Vaughan. 1954. Seborrhoeic blepharitis. Trans. Am. Ophthalmol. Soc. 52:173–188. [PMC free article] [PubMed] [Google Scholar]
- 441.Tosti, A., S. Villardita, and M. L. Fazzini. 1972. The parasitic colonisation of the horny layer in tinea versicolor. J. Investig. Dermatol. 59:233–237. [DOI] [PubMed] [Google Scholar]
- 442.Tracey, K. J., and S. F. Lowry. 1990. The role of cytokine mediators in septic shock. Adv. Surg. 23:21–56. [PubMed] [Google Scholar]
- 443.Tupker, R. A., J. G. De Monchy, P. J. Coenraads, A. Homan, and J. B. van der Meer. 1996. Induction of atopic dermatitis by inhalation of house dust mite. J. Allergy Clin. Immunol. 97:1064–1070. [DOI] [PubMed] [Google Scholar]
- 444.Ushijima, T., M. Takahashi, and Y. Ozaki. 1981. Selective and differential media for isolation and tentative identification of each species of Pityrosporum residing on normal or diseased human skin Microbiol. Immunol. 25:1109–1118. [DOI] [PubMed] [Google Scholar]
- 445.Van Abbe, N. J. 1964. The investigation of dandruff. J. Soc. Cosmet. Chem. 15:609–630. [Google Scholar]
- 446.Van Der Wyk, R. W., and K. E. Hechemy. 1967. A comparison of the bacterial and yeast flora of the human scalp and their effect upon dandruff production. J. Soc. Cosmet. Chem. 18:629–639. [Google Scholar]
- 447.van Reijsen, F. C, C. A. F. M. Bruijnzeel-Koomen, F. S. Kalthoff, E. Maggi, S. Romagnani, J. K. T. Westland, and G. C. Mudde. 1992. Skin derived aeroallergen specific T cell clones of Th2 phenotype in patients with atopic dermatitis. J. Allergy Clin. Immunol. 90:184–193. [DOI] [PubMed] [Google Scholar]
- 448.van Reijsen, F. C., A. Felius, E. A. Wauters, C. A. Bruijnzeel-Koomen, and S. J. Koppelman. 1998. T cell reactivity for a peanut epitope in the skin of a young infant with atopic dermatitis. J. Allergy Clin. Immunol. 101:207–209. [DOI] [PubMed] [Google Scholar]
- 449.Vecchiarelli, A., C. Retini, D. Pietrella, C. Monari, C. Tascini, T. Beccari, and T. R. Kozel. 1995. Downregulation by cryptococcal polysaccharide of tumor necrosis alpha and interleukin 1 beta secretion from human monocytes. Infect. Immun. 63:2919–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Vicente Alves, E, J. E. Costa Martins, E. B. de Ribeiro, and M. N. Sotto. 2000. Pityrosporum folliculitis: renal transplantation. Case report. J. Dermatol. 27:49–51. [DOI] [PubMed] [Google Scholar]
- 451.Vidal, C., P. M. Girard, D. Dompmartin, J. L. Bosson, C. Met, P. Groslambert, J. P. Coulaud, and P. Amblard. 1990. Seborrhoeic dermatitis and HIV infection. J. Am. Acad. Dermatol. 23:1106–1110. [PubMed] [Google Scholar]
- 452.Vuillemin, P. 1931. Les champignon parasites et les mycoses de l’homme. Encyclopáe die Mycologique II, Paris, France.
- 453.Waersted, A., and N. Hjorth. 1985. Pityrosorum orbiculare—a pathogenic factor in atopic dermatitis of the face, scalp or neck? Acta Dermato-Venereol. 114:146–148. [DOI] [PubMed] [Google Scholar]
- 454.Wagner, D. K., and P. G. Sohnle. 1995. Cutaneous defences against dermatophytes and yeasts. Clin. Microbiol. Rev. 8:317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Wagner, D. K., and P. G. Sohnle. 1997. Cutaneous defence mechanisms against fungi. Basic Clin. Dermatol. 12:161–189. [Google Scholar]
- 456.Wakasugi, N., and J. L. Virelizier. 1985. Defective IFN-gamma production in the human neonate. I. Dysregulation rather than intrinsic abnormality. J. Immunol. 134:167–171. [PubMed] [Google Scholar]
- 457.Wallace, M., H. Bagnall, and D. Glen. 1979. Isolation of lipophilic yeast in “sterile” peritonitis. Lancet ii:956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Walters, C. E., E. Ingham, E. A. Eady, J. H. Cove, J. N. Kearney, and W. J. Cunliffe. 1995. In vitro modulation of keratinocyte-derived interleukin 1α (IL-1α) and peripheral blood mononuclear cell-derived IL-1β release in response to cutaneous commensal micro-organsisms. Infect. Immun. 63:1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Weary, P. E. 1970. Comedogenic potential of the lipid extract of P. ovale Arch. Dermatol. 102:84–91. [PubMed] [Google Scholar]
- 460.Weary, P. E., C. M. Russell, H. K. Butler, and Y. T. Hsu. 1969. Acneiform eruptions resulting from antibiotic administration. Arch. Dermatol. 100:179–183. [PubMed] [Google Scholar]
- 461.Webster, G. F., J. J. Leyden, and U. R. Nilsson. 1979. Complement activation in acne vulgaris: consumption of complement by comedones. Infect. Immun. 26:183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Webster, G. F., and W. P. McArthur. 1982. Activation of components of the alternative pathway of complement by Propionibacterium acnes cell wall carbohydrate. J. Investig. Dermatol. 79:137–140. [DOI] [PubMed] [Google Scholar]
- 463.Webster, G. F., and K. J. McGinley. 1980. Serologic analysis of the extractable carbohydrate antigens of Pityrosporum ovale. Microbios 28:41–45. [PubMed] [Google Scholar]
- 464.Weiss, S. J., P. E. Schoch, and B. A. Cunha. 1991. Malassezia furfur fungemia associated with central venous catheter lipid emulsion infusion. Heart Lung 20:87–90. [PubMed] [Google Scholar]
- 465.Welbel, S. F., M. M. McNeil, A. Pramanik, R. Silberman, A. D. Oberle, G. Midgley, S. Crow, and W. R. Jarvis. 1994. Nosocomial Malassezia pachydermatis bloodstream infections in a neonatal intensive care unit. Pediatr. Infect. Dis. J. 13:104–108. [DOI] [PubMed] [Google Scholar]
- 466.Werner, Y., and M. Lindberg. 1985. Transepidermal water loss in dry and clinically normal skin in patients with atopic dermatitis. Acta Dermato-Venereol. 65:102–105. [PubMed] [Google Scholar]
- 467.Wessels, M. W., G. Doekes, A. G. Van leperen-Van Dijk, W. J. Koers, and E. Young. 1991. IgE antibodies to Pityrosporum ovale in atopic dermatitis. Br. J. Dermatol. 125:227–232. [DOI] [PubMed] [Google Scholar]
- 468.Wikler, J. R., N. Janssen, D. P. Bruynzeel, and C. Nieboer. 1990. The effect of UV light on Pityrosporum yeasts: ultrastructural changes and inhibition of growth. Acta Dermato-Venereol. 70:69–72. [PubMed] [Google Scholar]
- 469.Wilde, P. F., and P. S. Stewart. 1968. A study of the fatty acid metabolism of the yeast Pityrosporum ovale. Biochem. J. 108:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Williamson, P., and A. M. Kligman. 1965. A new method for the quantitative investigation of cutaneous bacteria. J. Investig. Dermatol. 45:498–503. [DOI] [PubMed] [Google Scholar]
- 471.Wilson, C. L., and M. Walshe. 1988. Incidence of seborrhoeic dermatitis in spinal injury patients. Br. J. Dermatol. 119:s48. [Google Scholar]
- 472.Winiarczyk, S. 1992. The ultrastructure of Pityrosporum pachydermatis Arch. Vet. Pol. 32:4–13. [PubMed] [Google Scholar]
- 473.Wollenberg, A., and T. Bieber. 2000. Atopic dermatitis: from the genes to skin lesions. Allergy 55:205–213. [DOI] [PubMed] [Google Scholar]
- 474.Wolter, J. R. 1977. Pityrosporum species associated with dacryoliths in obstructive dacryocystitis. Am. J. Ophthalmol. 84:806–809. [DOI] [PubMed] [Google Scholar]
- 475.Wright, W. C., B. J. Ank, J. Herbert, and E. R. Steihm. 1975. Decreased bactericidal activity of leucocytes of stressed newborn infants. Pediatrics 56:579–584. [PubMed] [Google Scholar]
- 476.Wu, Y. C., and K. T. Chen. 1985. Humoral immunity in patients with tinea versicolor. J. Dermatol. 12:161–166. [DOI] [PubMed] [Google Scholar]
- 477.Wu, Y. C., and K. T. Chen. 1987. Lymphocyte proliferation to crude extract of Pityrosporum species and natural killer activity in tinea versicolor. J. Med. Assoc. Thail. 70(Suppl. 3):45. [Google Scholar]
- 478.Wurtz, R. M., and W. N. Knospe. 1988. Malassezia furfur fungernia in a patient without the usual risk factors. Ann. Intern. Med. 109:432–433. [DOI] [PubMed] [Google Scholar]
- 479.Wyre, H. W., Jr., and W. T. Johnson. 1981. Neonatal pityriasis versicolor. Arch. Dermatol. 117:752–753. [PubMed] [Google Scholar]
- 480.Yasueda, H., T. Hashida-Okado, A. Saito, K. Uchida, M. Kuroda, Y. Onishi, K. Takahashi, H. Yamaguchi, K. Takesako, and K. Akiyama. 1998. Identification and cloning of two novel allergens from the lipophilic yeast, Malassezia furfur. Biochem. Biophys. Res. Commun. 248:240–244. [DOI] [PubMed] [Google Scholar]
- 481.Yesudian, P. 1985. Confluent and reticulate papillomatosis. Arch. Dermatol. 121:1483. [PubMed] [Google Scholar]
- 482.Yesudian, P., S. Kamalam, and A. Razack. 1973. Confluent and reticulated papillomatosis (Gougerot-Carteaud). An abnormal host reaction to Malassezia furfur. Acta Dermato-Venerel. 53:381–384. [PubMed] [Google Scholar]
- 483.Yohn, J. J., J. Lucas, and C. Camisa. 1985. Malassezia folliculitis in immunocompromised patients. Cutis 35:536–538. [PubMed] [Google Scholar]
- 484.Yokoi, T., T. Miyawaki, A. Yachie, S. Ohzeki, and N. Taniguchi. 1982. Discrepancy in expression ability of Tac antigen and la determinants defined by monoclonal antibodies on activated and cultured cord blood T lymphocytes. J. Immunol. 129:1441–1445. [PubMed] [Google Scholar]
- 485.Young, E., W. J. Koers, and L. Berrens. 1989. Intracutaneous test with Pityrosporum extract in atopic dermatitis. Acta Dermato-Venereol. 144(Suppl.):122–125. [DOI] [PubMed] [Google Scholar]
- 486.Zargari, A., G. Doekes, A. G. van leperen-van Dijk, E. Landberg, B. Harfast, and A. Scheynius. 1995. Influence of culture period on the allergenic composition of Pityrosporum extracts. Clin. Exp. Allergy 25:1235–1245. [DOI] [PubMed] [Google Scholar]
- 487.Zargari, A., A. Emilson, G. Hallden, S. Johansson, and A. Scheynius. 1997. Cell surface expression of two major allergens in the Pityrosporum genus. Clin. Exp. Allergy 27:584–592. [PubMed] [Google Scholar]
- 488.Zargari, A., B. Harfast, S. G. O. Johansson, and A. Scheynius. 1994. Identification of allergen components of the opportunistic yeast Pityrosporum orbiculare by monoclonal antibodies. Allergy 49:50–56. [DOI] [PubMed] [Google Scholar]
- 489.Zargari, A., M. Schmidt, M. Lundberg, A. Scheynius, and P. Whitley. 1999. Immunologic characterization of natural and recombinant Mal f1 yeast allergen. J. Allergy Clin. Immunol. 103:877–884. [DOI] [PubMed] [Google Scholar]