To the Editor:
Ehlers-Danlos syndrome (EDS) is a heterogeneous group of heritable connective-tissue disorders, generally affecting skin, joints, and blood vessels. The most recent classification recognizes six subtypes (Beighton et al. 1998), of which the hypermobility type (HT-EDS [formerly EDS type III] [MIM 130020]) is the most common. This type of EDS is similar to benign joint hypermobility syndrome (BJHS), and both are often considered to represent the same hyperlaxity syndrome, since no clear clinical distinction can be made (Grahame 1999). Although various causative genes have been found in all other types of EDS, the genetic basis of HT-EDS or BJHS remains unexplained (Steinmann et al. 2002). One family has been described that has a missense mutation in COL3A1 (Narcisi et al. 1994), resulting in a phenotype that resembles HT-EDS, without obvious vascular complications. Mutations in COL3A1 generally result in the severe vascular type of EDS (MIM 130050). To our knowledge, no other cases of COL3A1 mutations in HT-EDS have been reported.
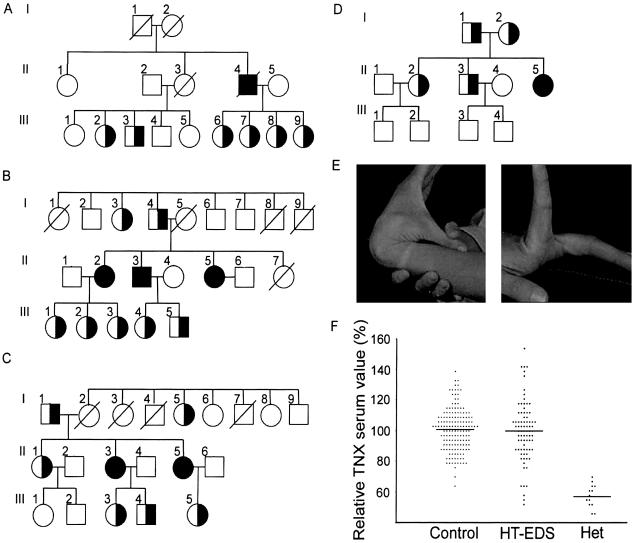
Recently, we showed that deficiency of the extracellular-matrix protein tenascin-X (TNX), encoded by the TNXB gene, causes a new type of recessively inherited EDS (Schalkwijk et al. 2001). Patients with complete deficiency of TNX showed marked joint hypermobility, skin hyperextensibility, and easy bruising. The absence of atrophic scars and recessive inheritance distinguishes TNX deficiency from the classical type of EDS. In our initial report (Schalkwijk et al. 2001), only a few heterozygous family members were available for examination. Here, we have examined all 20 heterozygous family members (individuals from families A–D in table 1) who were available for further study, regardless of clinical symptoms; in all of these individuals, we have found significantly reduced serum TNX levels (56% ± 6% vs. 100% ± 14% in the control population; P<.001, by Student's t test) (fig. 1f), and, in 17 of them, we have confirmed heterozygosity for a truncating TNXB mutation (table 1). Clinical examination revealed generalized joint hypermobility in nine family members (45%), using the Beighton score (Beighton et al. 1973), for HT-EDS, or the Brighton criteria (Grahame et al. 2000), for BJHS (table 1 and fig. 1e). Skin hyperextensibility and easy bruising, frequently seen in the individuals with complete TNX deficiency, were absent. A number of patients with haploinsufficiency had recurring joint dislocations and chronic joint pain, as are seen in HT-EDS and BJHS. Only four family members carrying two normal TNXB alleles were available for study, of whom none had hypermobility. The local medical ethics committee (CMO Regio Arnhem-Nijmegen) approved the study protocol, and informed consent was obtained from all patients.
Table 1.
Clinical and Molecular Findings in Individuals with TNXB Haploinsufficiency/Reduced Serum TNX Levels
| Individuala (Sex) | Yearof Birth | Mutation | BeightonScore | TNX Level(% of Control) | Clinical Feature(s) |
| AIII2 (F) | 1961 | [GT44906] ins | 5/9 | 52 | Velvety skin, piezogenic papules, back pain |
| AIII3 (M) | 1964 | [GT44906] ins | 4/9 | 57 | Loss of pliancy, Raynaud phenomenon |
| AIII6 (F) | 1974 | [GT44906] ins | 6/9 | 59 | Velvety skin, Raynaud phenomenon |
| AIII7 (F) | 1977 | [GT44906] ins | 5/9 | 52 | Striae |
| AIII8 (F) | 1981 | [GT44906] ins | 5/9 | 50 | Ankle sprains, knee pain |
| AIII9 (F) | 1984 | [GT44906] ins | 5/9 | 68 | Velvety skin |
| BI4 (M) | 1931 | Unknown | 0/9 | 56 | Multiple ankle sprains |
| BIII1 (F) | 1976 | Unknown | 5/9 | 65 | |
| BIII2 (F) | 1979 | Unknown | 5/9 | 62 | Wheelchair dependent, joint pain |
| BIII3 (F) | 1981 | 30-kb del | 5/9 | 62 | Joint pain |
| CI1 (M) | 1919 | [AA56063] del | 0/9 | 55 | |
| CI5 (F) | 1924 | [AA56063] del | ND | 54 | |
| CII1 (F) | 1944 | [AA56063] del | 1/9 | 46 | |
| CIII3 (F) | 1978 | [AA56063] del | 3/9 | 45 | Piezogenic papules, lymphedema |
| CIII4 (F) | 1980 | [AA56063] del | 6/9 | 53 | Velvety skin, multiple (sub)luxations |
| CIII5 (M) | 1981 | [AA56063] del | 0/9 | 57 | |
| DI1 (M) | 1919 | 30-kb del | 0/9 | 53 | Velvety skin |
| DI2 (F) | 1931 | 30-kb del | 0/9 | 54 | Multiple fractures |
| DII1 (F) | 1962 | 30-kb del | 2/9 | 59 | |
| DII2 (M) | 1964 | 30-kb del | 2/9 | 61 | Piezogenic papules |
| E (F) | 1961 | 30-kb del | 3/9 | 58 | Chronic joint pain, multiple (sub)luxations, wheelchair dependent, HT-EDS/BJHS according to Brighton criteria |
| F (F) | 1970 | [AA56063] del | 6/9 | 52 | |
| G1 (F) | 1972 | Unknown | 6/9 | 56 | Luxations, velvety skin, piezogenic papules |
| G2 (F) | 1977 | Unknown | 6/9 | 61 | Chronic pain, subluxations of multiple joints |
| H (F) | 1961 | Unknown | 2/9 | 64 | Wheelchair dependent, chronic musculoskeletal pain, shoulder luxations, HT-EDS/BJHS according to Brighton criteria |
| I (F) | 1954 | Unknown | ND | 65 | Diagnosis of HT-EDS |
| J (F) | 1974 | Unknown | ND | 54 | Diagnosis of HT-EDS |
Pedigrees for families A–D are depicted in figure 1. Patients E–J were identified in a cohort with HT-EDS. G2 is a sister of G1 and was identified independently of the screening of the 80 patients with HT-EDS/BJHS. We examined all patients available for study except individuals I and J.
ND = not determined.
Figure 1.
TNXB haploinsufficiency and generalized joint hypermobility. a–d, Pedigrees of families A–D (also see table 1). e, Joint hypermobility in individual III9 from family A. f, Distribution of serum TNX levels in control population, population with HT-EDS, and heterozygous (Het) population. Values are given as percentage of the control mean.
A striking finding is that 0 of the 6 males with haploinsufficiency fulfilled the clinical criteria for HT-EDS or BJHS, whereas 9 of 14 (64%) females were positive. This finding is in accordance with previous population-based studies that show a female preponderance in joint hypermobility syndromes (Larsson et al. 1987; Rikken-Bultman et al. 1997). In a control group of 30 unaffected females of the same age as the females with haploinsufficiency in the present study, we found no individuals with a Beighton score >4. This indicates that the prevalence of generalized joint hypermobility in a population of females with haploinsufficiency is significantly higher than in a control population (P<.001, by χ2 test). No sex differences in serum TNX levels in unaffected individuals and individuals with haploinsufficiency were found (not shown).
Because our observations in families carrying previously described TNXB mutations suggested an association between TNXB haploinsufficiency and joint hypermobility, we wondered about the prevalence of TNXB haploinsufficiency in patients with HT-EDS. We measured serum TNX levels (by ELISA) in an unselected cohort of 80 patients with HT-EDS who were recruited through the Dutch organization for patients with EDS. All patients were diagnosed with HT-EDS by a medical specialist, and ∼90% were female. Although the mean serum TNX level was not different in the cohort with HT-EDS overall (99.4%±19.7%) (fig. 1f), six of these patients (7.5% [all female]) had serum TNX levels >2.5 SDs (65%) below the mean for unaffected individuals. On the basis of the normal distribution of serum TNX levels, only 0.6% of individuals would be expected to have such low serum TNX levels, which is significantly less than the frequency found in the population with HT-EDS described in the present study (P<.001, by Fisher's exact test).
Clinically, patients with reduced TNX levels showed hypermobile joints, often associated with joint subluxations and chronic musculoskeletal pain (table 1). The clinical findings in these patients differ from those with complete TNX deficiency. Patients with haploinsufficiency do not have skin hyperextensibility and lack the easy bruising seen in patients with TNX deficiency. In addition, TNXB haploinsufficiency is expected to be an autosomal dominant trait, which is in accordance with the observed mode of inheritance of HT-EDS and BJHS.
On screening for the presence of a 30-kb deletion described previously (Burch et al. 1997; Schalkwijk et al. 2001), we found that this deletion was present in one of these six patients. The 30-kb deletion creates a fusion gene of TNXB and XA, a partial duplicate of TNXB. The XA gene has an internal deletion that truncates its ORF, rendering XA and the fusion gene nonfunctional (Gitelman et al. 1992). The deleted allele also lacks CYP21, so this individual is also a carrier for congenital adrenal hyperplasia. Subsequently, we PCR amplified and directly sequenced the coding regions and the intron-exon boundaries of TNXB in the other five patients presumed to have haploinsufficiency (for primers used, see Schalkwijk et al. 2001). One patient (individual F in table 1) was heterozygous for a 2-bp deletion, [AA56063] del, in exon 8, resulting in a premature stop codon at the position of amino acid 1231. In the other four patients, we were unable to identify mutations in TNXB. These patients may have mutations, in regulatory sequences or in exons of the TNXB gene, that have not yet been identified, or they may represent the extreme in normal variation of TNX expression.
In conclusion, in the present study, we have reported a genetic defect associated with HT-EDS or BJHS. On the basis of the observed phenotype in patients with complete TNX deficiency and the high prevalence of generalized joint hypermobility in heterozygous females, this is likely to be a causative relationship. Reduced TNX expression could disturb deposition of collagen (Mao et al. 2002) and the elastic fiber network (Burch et al. 1997), as has been shown for complete TNX deficiency, resulting in increased laxity of ligaments and tendons. TNXB haploinsufficiency is dominantly inherited and appears to produce clinical findings primarily in women, consistent with clinical descriptions of HT-EDS. Although we identified inactivating TNX mutations in only 2.5% of this cohort with HT-EDS, 7.5% had serum TNX levels low enough to affect collagen metabolism. The present study demonstrates that TNXB haploinsufficiency is associated with HT-EDS and suggests that locus heterogeneity exists for this disorder, as it does for other types of EDS (Byers 1994).
Acknowledgment
This work was supported, in part, by Public Health Services grant HL60875 (to J.B.).
Electronic-Database Information
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for HT-EDS and vascular type of EDS)
References
- Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ (1998) Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Am J Med Genet 77:31–37 [DOI] [PubMed] [Google Scholar]
- Beighton P, Solomon L, Soskolne CL (1973) Articular mobility in an African population. Ann Rheum Dis 32:413–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch GH, Gong Y, Liu W, Dettman RW, Curry CJ, Smith L, Miller WL, Bristow J (1997) Tenascin-X deficiency is associated with Ehlers-Danlos syndrome. Nat Genet 17:104–108 [DOI] [PubMed] [Google Scholar]
- Byers PH (1994) Ehlers-Danlos syndrome: recent advances and current understanding of the clinical and genetic heterogeneity. J Invest Dermatol Suppl 103:47S–52S [DOI] [PubMed] [Google Scholar]
- Gitelman SE, Bristow J, Miller WL (1992) Mechanism and consequences of the duplication of the human C4/P450c21/gene X locus. Mol Cell Biol 12:2124–2134 (erratum 12:3313–3314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame R (1999) Joint hypermobility and genetic collagen disorders: are they related? Arch Dis Child 80:188–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame R, Bird HA, Child A (2000) The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS). J Rheumatol 27:1777–1779 [PubMed] [Google Scholar]
- Larsson LG, Baum J, Mudholkar GS (1987) Hypermobility: features and differential incidence between the sexes. Arthritis Rheum 30:1426–1430 [DOI] [PubMed] [Google Scholar]
- Mao JR, Taylor G, Dean WB, Wagner DR, Afzal V, Lotz JC, Rubin EM, Bristow J (2002) Tenascin-X deficiency mimics Ehlers-Danlos syndrome in mice through alteration of collagen deposition. Nat Genet 30:421–425 [DOI] [PubMed] [Google Scholar]
- Narcisi P, Richards AJ, Ferguson SD, Pope FM (1994) A family with Ehlers-Danlos syndrome type III/articular hypermobility syndrome has a glycine 637 to serine substitution in type III collagen. Hum Mol Genet 3:1617–1620 [DOI] [PubMed] [Google Scholar]
- Rikken-Bultman DG, Wellink L, van Dongen PW (1997) Hypermobility in two Dutch school populations. Eur J Obstet Gynecol Reprod Biol 73:189–192 [DOI] [PubMed] [Google Scholar]
- Schalkwijk J, Zweers MC, Steijlen PM, Dean WB, Taylor G, Van Vlijmen IM, van Haren B, Miller WL, Bristow J (2001) A recessive form of the Ehlers-Danlos syndrome caused by tenascin-X deficiency. N Engl J Med 345:1167–1175 [DOI] [PubMed] [Google Scholar]
- Steinmann B, Royce PM, Superti-Furga A (2002) The Ehlers-Danlos syndrome. In: Royce PM, Steinmann B (eds) Connective tissue and its heritable disorders. Wiley-Liss, New York, pp 431–523 [Google Scholar]