Abstract
Voltage-gated sodium channels (NaVs) selectively permit diffusion of sodium ions across the cell membrane and, in excitable cells, are responsible for propagating action potentials. One of the nine human NaV isoforms, NaV1.8, is a promising target for analgesics, and selective inhibitors are of interest as therapeutics. One such inhibitor, the gating-modifier peptide Protoxin-I derived from tarantula venom, blocks channel opening by shifting the activation voltage threshold to more depolarized potentials, but the structural basis for this inhibition has not previously been determined. Using monolayer graphene grids, we report the cryogenic electron microscopy structures of full-length human apo-NaV1.8 and the Protoxin-I-bound complex at 3.1 Å and 2.8 Å resolution, respectively. The apo structure shows an unexpected movement of the Domain I S4-S5 helix, and VSDI was unresolvable. We find that Protoxin-I binds to and displaces the VSDII S3-S4 linker, hindering translocation of the S4II helix during activation.
Subject terms: Cryoelectron microscopy, Ion transport, Ion channels
Animal toxins can modulate action potentials and are important leads for therapeutics. Here, the authors use cryo-EM to show the interaction of the tarantula venom peptide Protoxin-I with a human voltage-gated sodium channel implicated in pain
Introduction
Voltage-gated sodium channels (NaVs) are integral membrane proteins responsible for the selective permeation of sodium ions into cells in response to membrane depolarization. The small differences in sequence that characterize the nine human NaV subtypes (hNaV1.1-1.9, Supplementary Fig. 1) nonetheless give rise to distinct electrophysiological properties that, together with varying expression levels in different tissues, give each hNaV isoform particular physiological roles.
NaV1.8, one of the three tetrodotoxin-resistant NaVs, is distinguished from other isoforms by the relatively depolarized voltage-dependency of activation and inactivation, slower inactivation kinetics, and an increased persistent current1–3; These attributes make NaV1.8 principally responsible for inward currents during the rising phase of the action potential4,5, and contribute to hyperexcitability and repetitive firing in the dorsal root ganglion (DRG) neurons where it is primarily localized6,7. Uniquely, it maintains its gating properties at cold temperatures8.
Multiple studies have linked NaV1.8 to nociception and chronic pain. Gain-of-function mutations in NaV1.8 causing increased excitability of DRG neurons have been identified in patients with peripheral neuropathy9,10, while a loss-of-function NaV1.8 mutation has been linked to reduced pain sensation11,12. NaV1.8 has also been linked to inflammatory pain13. Studies of Grasshopper mice (Onychomys torridus) showed that their insensitivity to pain induced by the venom of the Arizona bark scorpion (Centruroides exilicauda) derives from mutations in their NaV1.8 channels14; injection of the venom reduced the O. torridus pain response to the formalin test, demonstrating that inhibition of NaV1.8 is a viable analgesic strategy15.
Inhibitors of NaV1.8 are therefore of interest as pain treatments, and peptides derived from animal venom are renowned modulators of NaV activity. Unlike small-molecule inhibitors, which typically bind in the highly conserved pore domain (PD), peptide inhibitors frequently bind to the less-conserved extracellular regions above the voltage-sensing domains (VSDs) which provide greater scope for isoform selectivity in drug development. NaV1.8-selective peptides include scorpion venom peptide BmK I16, the μ-conotoxins MrVIA/MrVIB17,18 and TsIIIA19, and the tarantula venom peptide Protoxin-I (ProTx-I)20.
ProTx-I was isolated from the venom of the Peruvian green velvet tarantula (Thrixopelma pruriens) and is a gating-modifier peptide that shifts the voltage-dependence of activation to more depolarized potentials20. It shows slight selectivity for rat NaV1.8 (IC50 = 27 nM)21 over other human NaV isoforms (typically IC50 = 60–130 nM)22, as well as activity against T-type calcium channels23 and the TRPA1 channel24. ProTx-I is disulfide-rich and shares the inhibitor cystine knot (ICK) framework that is common among gating-modifier tarantula venom peptides25. Mutagenesis studies using hNaV1.7/KV2.1 chimeras localized the binding site of ProTx-I to NaV1.7 on the extracellular loops of VSDII and VSDIV26 but the exact binding mechanism remained undetermined.
Structural characterization of venom peptides in complex with NaVs is essential for understanding their pharmacological profiles and for realizing their potential as tool compounds and drugs. However, poor NaV expression yields and low local resolution for bound peptides have made these structures difficult to obtain. Full-length NaV-peptide complexes determined to date are limited to NaV1.2 in complex with the pore-blocking µ-conotoxin KIIIA27, NaV1.5 bound to the scorpion venom peptide LqhIII28, and the American cockroach channel NaVPaS bound to the spider venom peptide Dc1a29. Attempts to characterize complexes of human NaV1.7 with the spider venom peptides Protoxin-II and Huwentoxin-IV produced high-resolution reconstructions of the channel but could not sufficiently resolve the peptide for modeling30. Chimeric channels consisting of bacterial or invertebrate NaV scaffolds onto which human NaV domains have been grafted have also been developed to address this problem. A NaVAb/NaV1.7-VSDII chimera was used to determine the binding mechanism of Protoxin-II and Huwentoxin-IV31, which has additionally been characterized in complex with a NaChBac/NaV1.7-VSDII chimera32, while a NaV1.7-VSDIV chimera based on the NaVPaS scaffold aided in characterization of the scorpion venom peptide AaH2 as well as small molecule inhibitors33.
Mutagenesis screening and chimeric constructs can be effective in identifying channel variants with higher expression yield, but mutations can affect channel gating properties and chimeric constructs lack functional domains34. Experimental structures with full-length human channels are therefore preferred for rational structure-based drug design in order to minimize off-target interactions; maximizing yield and particle grid density through mammalian cell expression, biochemical and cryogenic electron microscopy (cryoEM) developments is therefore an attractive strategy.
Here, we used mammalian HEK293 cells to express full-length human NaV1.8 and determined the structure, with and without ProTx-I bound, by single-particle cryoEM. Optimization of the expression and purification, and the use of monolayer graphene grids, allowed the maximum number of useable particles for data collection from as low as 1.5 L of cell culture. The final reconstructions were determined at an overall resolution of 3.1 Å for apo-hNaV1.8 and 2.8 Å for the hNaV1.8-ProTx-I complex. Separate classifications revealed large movements of the S4-S5 linker leading to VSDI, and consequently this VSD was unresolvable. The resolution of the map in the ProTx-I region was sufficient for tracing of the peptide backbone and determination of its mechanism of binding. We anticipate that the developed protocols will be beneficial in the solution of future peptide-NaV complexes by cryoEM, and that these results will assist in the design of drugs targeting hNaV1.8.
Results
Apo-hNaV1.8
Human NaV1.8 α-subunit was co-expressed in HEK293 cells together with the β4-subunit and purified as described in Methods. NaV α-subunits are frequently co-expressed with β-subunits to stabilize the protein, increase expression levels, and maintain a more native environment. Evidence suggests that hNaV1.8 is capable of interacting with all four β-subunits, including β4, which affects hNaV1.8 activation and inactivation thresholds35. However, despite co-expression, only the α-subunit was observed after purification (Supplementary Fig. 2) and in the final map; loss of co-expressed β-subunits has been observed for other NaVs36–38and may reflect weak binding affinity between the proteins (see Discussion). To maximize the final hNaV1.8 yield, several parameters were screened (see Methods) and the overall time between solubilization and purification was minimized. Prior to cryoEM, particle purity and homogeneity were confirmed by negative stain (Supplementary Fig. 2d).
Screening freezing conditions using conventional holey carbon grids showed a preference for particles to accumulate over the carbon film, and low particle density in the holes (Supplementary Fig. 3a). Due to low yields, we attempted to increase the scale of the cell culture, but this introduced problems with solubilization and did not sufficiently increase the final usable protein concentration. The initial grid screening included a range of grid types, including one with a support film of monolayer graphene which showed improved particle distribution across the grid holes (Supplementary Fig. 3a). We therefore pursued the use of support film grids, including monolayer graphene, as an alternative to mutagenesis or large increases in the scale of cell culture. Using ultrathin (2–3 nm) carbon grids under similar conditions failed to produce a cryoEM dataset that could reach high resolution which we hypothesize was due to contrast loss resulting from particle packing. Graphene oxide grids indicated acceptable NaV particle density and contrast but were more susceptible to breakage from glow discharging (Supplementary Fig. 3b). All maps in this manuscript resulted from the use of monolayer graphene grids (0.4 nm thickness) which showed good particle distribution and contrast (Supplementary Fig. 3c); this allowed us to reconstruct the structures using just ~0.15 mg/mL of purified protein from as low as ~20 g wet cell pellet or 1.5 L of cell culture, significantly lower than typical NaV preparations37,39–41.
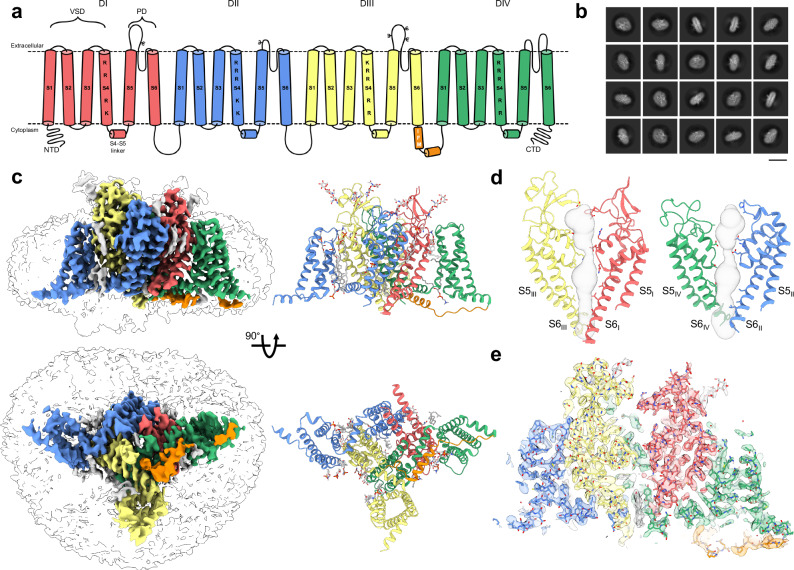
This approach allowed us to reconstruct apo-hNaV1.8 at an overall resolution of 3.1 Å (Fig. 1; Supplementary Figs. 4 and 5; Supplementary Table 1). Typical of other apo-NaV structures, the apo model shows features characteristic of an inactivated channel, with gating charge residues on all visible VSDs showing ‘up’ conformations (Supplementary Fig. 5) and the IFM fast inactivation motif on the VSDIII-VSDIV linker buried in its binding site between S6IV, S5IV and the VSDIII S4-S5 linker (Fig. 1a, c). Similar to a previous study36, the quality of the map allowed modeling of N-linked glycosylation at several positions (Asn residues 312, 819, 1312, 1328, and 1336) on the extracellular loops, as well as possible cholesterol, lipids, and detergent molecules bound to the transmembrane region; similar to other cryoEM structures of NaV channels we observe density for a bound molecule in the intracellular pore region, which we putatively assign as cholesterol (Fig. 1c, e)27,34,36,39,40.
Fig. 1. Overall architecture and reconstruction of apo-hNaV1.8.
a Topology of hNaV1.8 colored by domain: DI (red), DII (blue), DIII (yellow), IFM (orange) and DIV (green); VSD = voltage-sensing domain, PD = pore domain. The basic residues responsible for voltage-sensing in S4 of hNaV1.8 are labeled. hNaV1.8 glycosylation sites are labeled with the letter psi. b Example 2D class averages for apo-hNaV1.8; scale bar = 15 nm. c (left) Side and intracellular views of the final apo-hNaV1.8 map (colored according to the scheme in a) with transparent lower map threshold to indicate micelle and emerging NTD, and (right) the resulting final refined model. Glycosylation and small molecule ligands, including cholesterol in the pore are shown in gray. d Two views of the apo-hNaV1.8 pore domain showing the ion permeation path in gray. e Model-to-map fit for a central cross-section of apo-hNaV1.8.
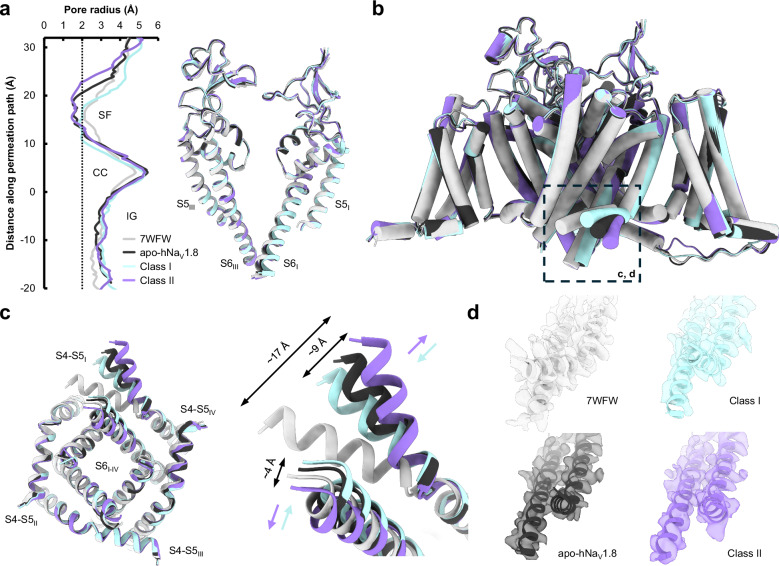
Density corresponding to VSDI is almost entirely absent in the 2D and 3D classifications, and in the final reconstruction (Fig. 1b, c), even as portions of the N-terminal domain (NTD) can be observed at lower map thresholds; this result is consistent with a prior report36. Attempts to improve the resolution in this region through 3D classifications steps, masking, and local refinements did not improve interpretability of VSDI but did reveal separate classes (denoted Class I and Class II, Supplementary Fig. 4) showing a distinct repositioning of the VSDI S4-S5 linker and a smaller movement of the lower portion of the VSDI S6 helix (Fig. 2, Supplementary Movie 1); The final apo map and structure was calculated from all particles making up Class I and Class II. In Class I the VSDI S4-S5 linker is positioned closer to the pore domain even as S6I moves outwards (Fig. 2c, cyan arrows), while in Class II the VSDI S4-S5 linker swings outwards (Fig. 2c, purple arrows); the S6I helix moves contrarily and tucks closer into the pore. In all our reconstructions, the VSDI S4-S5 linker is positioned significantly outward (by up to 17 Å) compared to the prior apo-NaV1.8 structure (PDB 7WFW)36. These movements necessarily affect the positioning, and likely contribute to the unresolvability, of VSDI; this is supported by 3D variability analysis, where, at low thresholds, density for the NTD is observed in slightly different positions (Supplementary Movie 2). Distinct positions for NTD and VSDI have also been observed in NaV1.7-M11, an engineered variant of hNaV1.7 containing 11 mutations that collectively induce a large depolarizing shift in the activation voltage42, underlining the connection between VSDI lability and activation thresholds.
Fig. 2. Structural comparisons of apo-hNaV1.8, highlighting dynamics of VSDI S4-S5 linker and S6 helices.
a (left) Pore radius for the overall apo-hNaV1.8 model (black) together with Class I (cyan), Class II (purple), and 7WFW (light gray)36, and (right) aligned pore domains showing minimal backbone movements. The selectivity filter (SF), central cavity (CC) and intracellular gate (IG) are indicated. b Comparison of the four apo-hNaV1.8 models contrasting the close overall structural agreement with the extensive outward movement of the VSDI S4-S5 linker (dashed black box). c (left) Intracellular view of the pore domain showing movements of the VSDI S4-S5 linker and lower S6I helix, with (right) close-up views highlighting the angular displacements (indicated by color-coded arrows) of the VSDI S4-S5 linker and S6 helix. Displacements between Class I and Class II measure ~9 Å for the VSDI S4-S5 linker and ~4 Å for the S6 helix d Model-to-map fits of the VSDI S4-S5 linker for all apo-hNaV1.8 structures.
Analysis of the ion permeation path shows the point of greatest restriction around the selectivity filter in the upper pore and is similar in all structures (Fig. 2a). The pore diameter through the intracellular gate is ~3 Å, consistent with the conformation of the S6 helices observed in hNaV1.734 and which allows space for the bound cholesterol molecule in our maps.
ProTx-I-bound complex
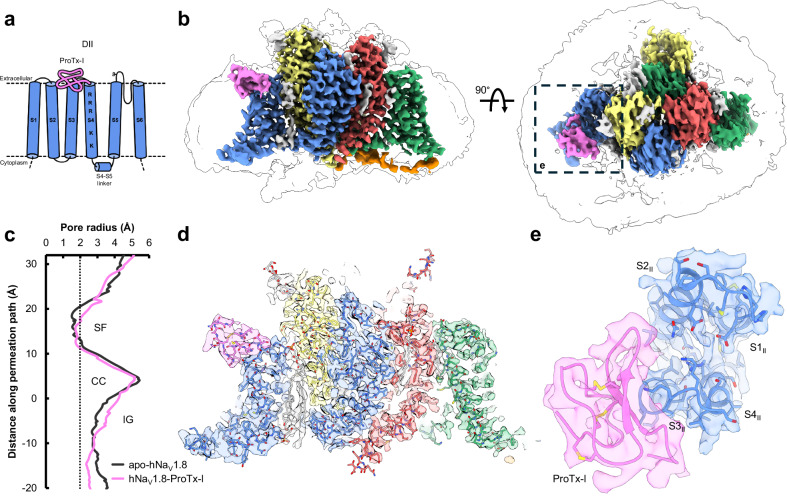
The hNaV1.8-ProTx-I complex was prepared by incubating ProTx-I solution with purified apo-hNaV1.8 prior to freezing with support film grids. We observed low particle contrast during initial screening, likely due to excess unbound ProTx-I (Supplementary Fig. 3b). We therefore introduced ProTx-I to hNaV1.8 prior to the final concentration step using a high molecular weight cutoff filter to remove excess unbound peptide (as described in Methods). Processing of the data produced a map with an overall resolution of 2.8 Å from 267,708 particles (Fig. 3; Supplementary Figs. 6 and 7). Outside the ProTx-I binding region, the refined hNaV1.8 structure in the complex is very similar to apo-hNaV1.8, with some slight rigid-body shifts in the VSDs and small movements in the extracellular loops. As with our apo-hNaV1.8 map, we observe density for multiple glycans in the map for the hNaV1.8-ProTx-I complex. The higher resolution of this map, likely enabled by the stabilizing effect of ProTx-I, allowed an additional extracellular loop (ECLI, D280-P295) to be traced in the hNaV1.8-ProTx-I map that was not possible for apo-hNaV1.8 (Supplementary Fig. 7). This loop has two further N-linked glycosylation sites (residues N284 and N288), but the glycans could not be resolved. The VSDI S4-S5 linker is again swung outward and consequently VSDI is not resolvable, despite additional processing, a larger dataset, and the higher overall resolution of the reconstruction.
Fig. 3. Overall architecture and reconstruction of hNaV1.8-ProTx-I complex.
a Schematic showing the positioning of ProTx-I (pink) on hNaV1.8 VSDII. b Side and extracellular views of the final hNaV1.8-ProTx-I complex map, colored as in Fig. 1c with ProTx-I in pink; the dashed box highlights the region binding ProTx-I. c Pore radius for the hNaV1.8-ProTx-I complex (pink) together with apo-hNaV1.8 (black). d Model-to-map fit for a central cross-section of hNaV1.8-ProTx-I, and e extracellular view of the fitted map in the ProTx-I-binding region.
Density for ProTx-I is clearly visible in the refined map; consistent with its electrophysiological effects as a gating modifier and previous structure-activity relationship studies26, ProTx-I binds to the S3-S4 linker on VSDII (Fig. 3b, d, e). The local resolution allowed the principal backbone of the peptide to be traced, which, with the assistance of the discernible β-loop near the peptide C-terminus, was sufficient to model ProTx-I into the map using an available NMR model (see Methods). The resolution of the ProTx-I portion of the map is highest immediately abutting the channel (likely due to stabilizing interactions) and is attenuated in the more distant regions (Supplementary Fig. 6c); this reduction in density in the more peripheral regions is a common feature of cryoEM studies of peptide-NaV complexes30,33. Evidence from mutagenesis experiments demonstrates that ProTx-I can also bind to hNaV1.7 VSDIV26, although electrophysiological recordings have not demonstrated that ProTx-I has any effect on channel inactivation thresholds that are typically governed by VSDIV. Despite high local resolution, we do not observe any density for ProTx-I above VSDIV in the hNaV1.8-ProTx-I structure, noting that the S1-S2 and S3-S4 linker regions on VSDIV are poorly conserved between hNaV1.7 and hNaV1.8 (Supplementary Fig. 1).
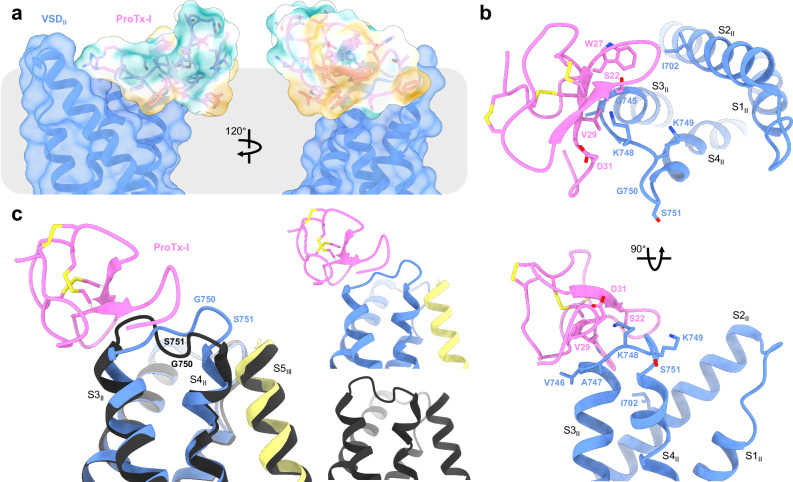
ProTx-I is partly buried in the detergent micelle (that mimics the membrane lipid bilayer). This interaction is mediated by a set of aromatic and aliphatic residues (W5, L6, W27, W30) that together form a ‘hydrophobic patch’, which is commonly observed in ICK peptides43 (Fig. 4a; Supplementary Movie 3) and explains prior observations that ProTx-I shows some affinity for model membranes, especially anionic membranes22. Tryptophan, in particular, is known to preferentially bind to the acyl carbonyl groups at the lipid-water interface44; these residues are proposed to anchor the peptide to the membrane and orientate it for interaction with the NaV VSDs45. ProTx-I shows only small conformational changes with respect to its unbound structure, as would be expected for an ICK peptide where the disulfide bonding network maintains rigidity in the peptide core (Supplementary Fig. 8a). The C-terminus is repositioned so as not to clash with the S3-S4 loop and permits the F34 sidechain access to the membrane.
Fig. 4. Interactions of ProTx-I with hNaV1.8 and the membrane.
a ProTx-I surface colored by hydrophobicity showing insertion of the hydrophobic patch into the membrane region (gray). Polar residues are indicated in teal, hydrophobic residues are indicated in gold. b Two views of the VSDII-ProTx-I binding interface showing W27 sidechain partially inserting into the S2/S3 cleft, V29 sitting atop S3II at V746 and A747, and the K748 sidechain on the VSDII S3-S4 linker in range to interact with the ProTx-I D31 and S22 sidechains. c (left) Overlay of the VSDII S3-S4 linker position in the hNaV1.8-ProTx-I complex (color scheme as in Fig. 3a) and apo-hNaV1.8 (black) showing inward movement towards the S4 helix, and (right) separated comparison of S3 and S4 helix positions.
The hNaV1.8-ProTx-I structure shows several points of interaction between the peptide and hNaV1.8 VSDII (Fig. 4). The membrane-embedded W27 sidechain partially inserts in the cleft formed by the S1/S2 and S3/S4 segments adjacent to I702 on S2 and G745 on S3, while the V29 sidechain is positioned directly on top of the S3 helix at V746/A747, likely hindering movement of this segment during activation (Fig. 4b). The structure places the S3II V746 sidechain directly below ProTx-I, and its replacement by the bulkier leucine in hNaV1.1-1.7 may hinder peptide binding and contribute to the slight increase in potency against NaV1.8.
The VSDII S3-S4 loop in the apo-hNaV1.8 map is of relatively lower resolution, which made tracing the loop backbone challenging; by contrast, the local resolution in this region of the hNaV1.8-ProTx-I complex map was improved (likely due to stabilization from the interaction with ProTx-I) and allowed straightforward tracing of the S3-S4 loop backbone at a higher threshold level (Supplementary Fig. 8b). Comparing the two structures shows that the binding of the toxin induces an inward movement of the top of the S3 helix together with a corresponding movement of the S3-S4 linker towards the pore domain (Fig. 4c and Supplementary Movie 4). This redirection of the S3-S4 linker due to ProTx-I binding propagates along its length such that G750 and S751 now sit directly atop S4 and adjacent to the pore domain, potentially hindering translocation of S4. This movement positions two adjacent lysine sidechains on the S3-S4 linker (K748 and K749, which are unique to hNaV1.8; see Discussion) upwards; the first of these lysine sidechains is positioned close to polar residues on ProTx-I (D31 and S22) where it may form hydrogen-bonding interactions. These observations are consistent with structure-activity studies of ProTx-I. A tethered-toxin alanine scan of ProTx-I against NaV1.7 identified multiple residues that significantly modified peptide activity (including L6, W27, V29, W30, and D31)26. The structure shows that many of these residues either form direct contacts with the channel or form part of the hydrophobic face that anchors the peptide to the membrane (Fig. 4a). Intriguingly, performing the same experiment with NaV1.2 shows an expanded pharmacophore compared with NaV1.7, with R3, W5, S22, R23, G32 also contributing to channel inhibition and suggesting that ProTx-I can adopt different binding modes depending on the NaV isoform that it is targeting24.
Mutagenesis experiments focusing on the channel have also explored the NaV-ProTx-I interaction. An alanine scan of S3-S4 in a NaV1.2-VSDII/KV1.2 chimera revealed several residues that modulated ProTx-I inhibition46. Mapping these residues to the hNaV1.8-ProTx-I structure provides a partial justification of these results. Significant reductions in potency were observed on mutation of the hydrophobic residues at the top of S3II; these correspond to V746 and A747 in NaV1.8, and which are directly involved in ProTx-I binding (Fig. 4b). Large changes were also observed for residues at the top of S4II, equivalent to S751 and S753 in hNaV1.8, which do not directly interact with ProTx-I in our structure but may instead relate to the inward push on the S3-S4 linker after binding.
Taken together, these structures justify prior structure-activity data as well as the observed pharmacological properties of ProTx-I on voltage-gated sodium channels. ProTx-I is observed to wrap around the top of the S3II helix, and inhibition is also likely mediated by the inward shift of the VSDII S3-S4 linker, which potentially restricts movement of the S4II helix. The positioning of residues which, in the hNaV1.8-ProTx-I structure, do not form direct interactions, also provides hints as to the relative promiscuity of ProTx-I towards hNaV isoforms (discussed below).
Discussion
This study reports the cryoEM structures of apo-hNaV1.8 and a hNaV1.8-ProTx-I complex and provides insights into the mechanism of channel inhibition by ProTx-I, as well as a useful point of comparison with other structures. ProTx-I was observed to bind to VSDII, with no density observed around VSDIV, despite evidence from mutagenesis experiments26. The addition of ProTx-I seemed to stabilize hNaV1.8 resulting in a better quality and higher resolution map, including in the peptide-binding region of the channel.
The decision to co-express with β-subunits was motivated by low expression yields of hNaV1.8 and poor particle distribution on the grid; we were also keen to minimize the volume of cell culture required to obtain sufficient particles for cryoEM reconstruction, which can reach 40 L in some cases47. The β4-subunit was selected based on evidence that it interacts with hNaV1.8 and affects activation and inactivation potentials35. However, no significant increase in the expression yield was seen and in subsequent purification and data collection steps only the hNaV1.8 α-subunit was identified. A cryoEM structure of hNaV1.1 together with β4 was able to resolve the β4 extracellular domain even as the co-expressed β3-subunit was not visible and demonstrated direct linking of β4 to the NaV1.1 α-subunit via a disulfide bond to the VSDII S5-S6 extracellular loop39. hNaV1.8 lacks the counterpart cysteine at this position required for disulfide bonding, which is likely to significantly weaken the interaction with β4; further investigation will be required to reveal the mechanism of gating modification of hNaV1.8 by β4.
Initial preparations produced homogeneous and good-quality particles, but in insufficient amounts to proceed with cryoEM. Since biochemical approaches did not significantly improve the yield of hNaV1.8, and we wished to avoid more drastic interventions (such as chimeras), we investigated different types of grids to optimize particle density and quality. The use of support films can drastically increase particle retention after blotting compared with conventional holey carbon grids48 and we found that grids with monolayer graphene support showed the most initial promise. Further optimization of the freezing conditions led to grids with a homogenous distribution of particles (Supplementary Fig. 3c) obtained from as low as 1.5 L cell culture and ultimately resulted in all the high-resolution reconstructions presented in this paper.
The structures of apo-hNaV1.8 revealed a large hinging movement of the VSDI S4-S5 linker that was resolvable in two separate classes (Fig. 2). This VSDI S4-S5 linker movement is not observed between the previously determined apo-hNaV1.8 and hNaV1.8-A-803467 complex structures36, despite the flexibility shown in VSDI S1-S4. By contrast the VSDI S4-S5 linker is consistently found tucked in closely to VSDII S5 and S6, as it is in other NaV structures, while the S6I helix is also positioned more closely into the pore. Notably, however, similar position shifts are observed between wild-type hNaV1.7 and hNaV1.7-M1142. Previous work ascribed the comparative flexibility of VSDI to unique mutations in hNaV1.8 VSDII S5 and identified two mutants (K806M and L809F) near the VSDII S5-VSDI interface that individually and collectively shift the voltage of activation to more polarized potentials36. Since we were unable to resolve VSDI in our structures we are unable to confirm the influence of these residues on VSDI flexibility, but we do observe an inward and upward shift of the VSDII S5 and S6 helices that justifies the connection between VSDI positioning and channel gating properties.
The unusual movements of the VSDI S4-S5 linker and S6 helix seen in our structures prompted closer examination of this region. Both regions are highly conserved across human NaV1.1-1.8, although hNaV1.9 shows lower sequence identity (Supplementary Fig. 1). Aside from hNaV1.9, only hNaV1.8 has non-conserved residues in the VSDI S4-S5 linker, with Val instead of Thr at position 234, and His replacing Glu at position 241. V234 points away from the rest of the channel and does not form any interactions except to solvent or detergent, but H241 is orientated towards the conserved E402 and Q403 residues on VSDI S6 (Supplementary Fig. 9). When the VSDI S4-S5 linker is in the conventional tucked position, the His/Glu sidechain is close enough to interact with these polar residues36. Both Glu and His are capable of simultaneously donating and accepting hydrogen bonds, but the imidazole ring on the His sidechain imposes additional geometric restraints; the Glu-His mutation observed at this position in hNaV1.8 may therefore affect the ability to form stabilizing interactions with VSDI S6 and may contribute to the lability of this region that was observed in our data.
The hNaV1.8-ProTx-I structure is obtained at higher resolution than apo-hNaV1.8, which allows an additional extracellular loop to be modeled into this map. The complex structure demonstrates binding of the peptide to the channel by wrapping around the top of the S3II helix. This interaction is mediated by anchoring of the peptide to the membrane via an external hydrophobic patch, together with acidic and polar residues that can potentially form hydrogen bonds with a lysine residue (unique to NaV1.8) on the VSDII S3-S4 linker. The binding of ProTx-I induces an inward shift of the VSDII S3-S4 linker such that it partially repositions on top of the S4II helix, which we hypothesize hinders the movement of S4II during activation and justifies the gating-modifier properties of ProTx-I.
Comparing the hNaV1.8-ProTx-I structure to other structures of gating-modifier peptides bound to NaVs shows some similarities and differences in their modes of action. A cryoEM study of the gating-modifier peptide Huwentoxin-IV in complex with a nanodisc-bound NaChBac-NaV1.7-VSDII chimera shows that the peptide is similarly orientated by its membrane-inserted hydrophobic patch to present polar residues towards the channel, particularly the K32 sidechain ‘stinger’ which is proposed to enter the VSDII cleft and come into proximity with negatively charged residues E822, D827, and E82949. This stinger interaction mechanism is maintained when Huwentoxin-IV binds to the channel in the resting conformation32. Notably, in hNaV1.8, D827 is modified to lysine while E829 is replaced by glycine; hNaV1.8 additionally has a second lysine at K748, replacing valine in hNaV1.7. The replacement of so many negatively charged residues in hNaV1.7 by positive or neutral residues in NaV1.8 would be sufficient to abolish these interactions and explain why hNaV1.8 is resistant to Huwentoxin-IV50. It also justifies the lack of a similar ‘stinger’ strategy by ProTx-I in its inhibition of hNaV1.8.
A similar chimeric strategy was used to obtain structures of Thrixopelma pruriens ICK peptide Protoxin-II in complex with NaV1.7-VSDII, which additionally revealed both activated and deactivated conformations31. While ProTx-I shows only mild selectivity towards NaV1.8 compared with other isoforms, Protoxin-II is notable for its potency (IC50 = 0.3 nM) and selectivity (>100-fold) in favor of hNaV1.7. As with the hNaV1.8-ProTx-I structure, a prominent tryptophan sidechain partitions into the VSDII S2-S3 cleft where it interacts with nearby hydrophobic residues (Fig. 4b). Similar to Huwentoxin-IV, Protoxin-II inserts a lysine residue sidechain to interact with E811, but additionally projects its R22 sidechain towards the acidic residues on the S3-S4 linker. The involvement of the arginine sidechain is of interest because ProTx-I also has an arginine at an equivalent position (R23), and which was identified as an important residue in targeting NaV1.224. The map density for this loop is not sufficient for confident placement of the arginine sidechain (suggestive of regional flexibility), but the hydrogen-bonding partner residues E694 and Q698 on S2II are within range for these interactions to occur and present an additional possible binding mode for NaV subtypes with acidic and polar residues in these positions. Notably, in hNaV1.7 the equivalent positions (E694 and Q698) are replaced by Lys and Ala, respectively, which would abolish any potential interactions with ProTx-I R23.
Here, the cryoEM reconstructions of apo- and ProTx-I-bound hNaV1.8 provide important insights into the versatile mechanisms that ProTx-I and other gating-modifier peptides have at their disposal to affect NaV gating. This was enabled by the use of monolayer graphene support that allowed 3D reconstructions from relatively low concentrations of protein stemming from small volumes of cell culture, which may be beneficial for studies of other hNaVs and similarly challenging proteins. These results will assist in the development of analgesic drugs targeting hNaV1.8, as well as aiding the structural characterization of peptide-NaV complexes yet to be determined.
Methods
Isoform and cloning of hNaV1.8 and β4
The hNaV1.8 sequence (Supplementary Fig. 1) used in this study was obtained from GenBank (NM_006514.3; the current canonical sequence NM_006514.4 has M1713 in place of Val, which does not significantly affect the electrophysiological properties of the channel)36. The hNaV1.8 sequence was N-terminally tagged with FLAG-tag, Twin-Strep-tag and a TEV protease site. The β4 sequence used in this study was obtained from GenBank (NM_174934.3) and C-terminally tagged with a TEV protease site and 6xHis-tag. The codon-optimized DNA was cloned into pcDNA3.1(+) and purchased from GenScript (genscript.com).
Transient expression of hNaV1.8 and β4
HEK293 cells (FreeStyle 293-F, Gibco) were seeded at ~0.3 × 106 cells/mL into 3 L of FreeStyle 293 Expression Medium (Gibco) in a baffled polycarbonate 5 L Erlenmeyer flask and incubated at 37 °C with 8% CO2 at 110 rpm. After 3 days, fresh media prewarmed to 37 °C was added to dilute the cells to 2 × 106 cells/mL. A total of 1.1 mg/L of DNA was used at a 2:1 ratio of hNaV1.8 to β4 and was mixed into 90 mL of Opti-MEM Reduced Serum Medium (Gibco). A total of 10 mL (1 mg/mL in PBS) of PEI Max 40 kDa (Polysciences Inc.) was added to the DNA and incubated for 20 min at room temperature. The cells were transiently co-transfected and harvested after 42 h at 800 × g for 30 min at 4 °C. The ~35–40 g wet cell pellet was flash frozen in liquid nitrogen and stored at −80 °C.
Protein purification of apo-hNaV1.8
A 35 g HEK293 cell pellet, equivalent to 3 L of cells, was homogenized in 60 mL of buffer A (165 mM NaCl, 27.5 mM HEPES pH 7.5, 2 mM MgCl2, 11% glycerol, 10 mM EDTA, and 3 x Pierce Protease Inhibitor Tablets (Thermo Scientific) supplemented with 5 units/mL of Benzonase (Millipore)) by plunging on ice in a glass Dounce tissue grinder with a large clearance pestle. The homogenate was diluted to 120 mL with buffer A and plunged on ice again with a small clearance pestle. The homogenate was diluted further with buffer A to a protein concentration of ~11 mg/mL (as determined by spectrophotometry using a Thermo Scientific NanoDrop). The membrane was solubilized for 2 h at 4 °C on a roller shaker at 30 rpm in 1% n-dodecyl-B-D-maltoside (DDM) (GoldBio), 0.2% CHS (Anatrace) by adding 10X solubilization buffer (10% DDM, 2% CHS) for a final protein concentration of ~10 mg/mL. The bulk of the cellular debris was pelleted at 4347 × g using an Eppendorf 5910 R centrifuge for 10 min at 4 °C. The supernatant was clarified further by ultracentrifugation with a Beckman Optima L ultracentrifuge equipped with a SW 32 Ti rotor at 25,000 rpm (rav 76,800 × g) for 30 min at 4 °C.
A 2 mL column volume (CV) of ANTI-FLAG M2 Affinity Gel (Millipore) was equilibrated in a gravity flow column with 2 CVs of buffer B (150 mM NaCl, 25 mM HEPES pH 7.5, 0.06% (w/v) glyco-diosgenin (GDN) (Anatrace)). The supernatant was mixed with the M2 affinity gel for 1 h at 4 °C on a roller shaker at 5 rpm. After collecting the flow through by gravity, the affinity gel was washed gradually into buffer B in the following 5 CVs buffer A and buffer B ratios: 50:50, 25:75, 12.5:87.5, 5:95 and 0:100. Protein was eluted by mixing the M2 affinity gel with 5 CVs of buffer B supplemented with 200 μg/mL of FLAG peptide for 30 min at 4 °C.
For size exclusion chromatography, two eluate fractions were loaded onto a Superose 6 Increase 10/300 GL column (Cytiva) connected to an ÄKTA pure system (Cytiva) in buffer C (150 mM NaCl, 25 mM HEPES pH 7.5, 0.006% (w/v) GDN) (Supplementary Fig. 2a). Eluate 1 consisted of the first 2 mL eluted from the FLAG column; Eluate 2 consisted of the remaining FLAG eluate, concentrated to 0.5 mL in a 4 mL 100 kDa MWCO Amicon Ultra centrifugal filter (3000 × g at 4 °C). The flow rate was 0.7 mL/min at 4 °C. Fractions 12-17 (11.8–14.8 mL) from both eluates were pooled and concentrated as before. The pooled fractions were again purified by size exclusion chromatography using the above method. Finally, fractions 13–15 (12.4–13.9 mL) were pooled and concentrated in a 0.5 mL 100 kDa MWCO Amicron Ultra centrifugal filter (3000 × g at 4 °C) to 60 µL at ~0.4 mg/mL.
Addition of ProTx-I to apo-hNaV1.8
Performed similarly to the forementioned protocol with the following changes. A 20 g HEK293 cell pellet, equivalent to 1.5 L of cell culture, was homogenized. Fractions 13–15 (12.4–13.9 mL) were pooled and concentrated to 250 µL at ~0.07 mg/mL. The concentrated apo-hNaV1.8 was mixed with 25 µL of 0.5 mM (2 mg/mL) ProTx-I (Smartox Biotechnology) in 1 M HEPES pH 7.4 for a final concentration of 45 µM and incubated on ice for 30 min. The mixture was concentrated in a 0.5 mL 100 kDa MWCO Amicon Ultra centrifugal filter (3000 × g at 4 °C) to 100 µL at ~0.15 mg/mL.
Negative staining
All samples were negatively stained following an established protocol51. Briefly; 3 µL of sample, ranging between 0.01 and 0.05 mg/mL, was pipetted onto glow-discharged carbon-coated 200-mesh Gilder Cu grids (Ted Pella). Excess sample was removed with filter paper, washed 5 times with 50 µL Milli-Q water drops, and finally stained with two 50 µL drops of 0.75% uranyl formate (Electron Microscopy Sciences) and excess stain was vacuum aspirated. Grids were carbon-coated using a Leica ACE200, negatively glow charged using a PELCO easiGlow (Ted Pella) prior to addition of sample and stain was freshly prepared. All grids were imaged with a JEOL JEM-2100F TEM equipped with a Gatan OneView 4k × 4k camera. Negative stain 2D class averages (Supplementary Fig. 2d) were calculated using RELION 3.152.
CryoEM grid freezing
Quantifoil R2/4 300 mesh Au grids with monolayer graphene support film (Graphenea) were negatively glow discharged using a PELCO easiGlow (Ted Pella) with the monolayer graphene (front) facing up. A Leica EM GP2 set to 10 °C and 96% humidity was used to freeze the grids. For apo-hNaV1.8, 0.4 mg/mL sample was diluted with buffer C to 0.25 mg/mL and 3 µL was applied to the front of the grid and incubated for 60 s before front blotting for 3 s. For the hNaV1.8-ProTx-I complex, 3 µL of 0.15 mg/mL sample was applied to the front of the grid and blotted as before. Grids were plunge frozen in liquid ethane and stored in liquid nitrogen.
CryoEM data collection
All movies were collected with a 300 kV FEI Titan Krios microscope equipped with a Gatan K3 direct electron detector. For apo-hNaV1.8, super-resolution movies were collected using SerialEM53 at a pixel size of 0.839 Å/pixel with a total dose of 60 e-/Å2 spread over 60 total frames, with a defocus range of −1 to −2.5 µm and a 100 µm objective aperture. Energy filter slit width was set to 20 eV. The hNaV1.8-ProTx-I acquisition was performed similarly with the following changes: data was collected at a pixel size of 0.827 Å/pixel with a defocus range of −1 to −2 µm. Full data collection parameters are highlighted in Supplementary Table 1.
CryoEM data processing of apo-hNaV1.8
The processing pipeline is described in Supplementary Fig. 4. Briefly; 13,124 movies were imported into CryoSPARC 4.254 for patch motion correction55 and patch contrast transfer function (CTF) estimation. 13,006 micrographs were selected for blob picking using circular and elliptical templates resulting in 4,878,994 particle coordinates. Selected 2D classes were utilized for template picking resulting in 11,792,840 particle coordinates. After multiple rounds of 2D classifications, a subset of particles showing different views of apo-hNaV1.8 were selected for ab initio initial 3D model building. Two 3D classes were selected and used as references to parse particles via heterogeneous refinement. Eventually 67,333 particles were used for non-uniform refinement56 to create a 3.5 Å map. This map was used for template picking resulting in 12,107,700 particle coordinates. Subsequent 2D classifications, 3D refinements, and 3D classifications resulted in a non-uniform refined and sharpened reconstruction at an overall resolution of 3.3 Å from 119,211 particles. This map was used for the final template picking resulting in 12,424,068 particle coordinates.
The 12,424,068 particles resulting from the final template pick were used in subsequent 2D classifications, 3D refinements, and 3D classifications which resulted in a non-uniform refined and sharpened reconstruction at an overall resolution of 3.2 Å from 120,821 particles. 3D variability analysis57 resulted in maps with varied conformations from distinct particles which were used for ab initio initial 3D model building. Two classes were subjected to non-uniform refinement and resulted in two distinct conformations of the VSDI S4-S5 linker as Class I with 84,466 particles and Class II with 82,542 particles at overall resolutions of 3.24 Å and 3.22 Å, respectively. The initial 3D model of Class II was used to refine a final sharpened map of apo-hNaV1.8 with all 120,821 particles at an overall resolution of 3.12 Å.
CryoEM data processing of hNaV1.8-ProTx-I
The processing pipeline is described in Supplementary Fig. 6. Processing of the hNaV1.8-ProTx-I dataset was performed similarly to the apo-hNaV1.8 dataset with the following changes. 15,400 movies were processed using CryoSPARC 4.4. Template picking using the final apo-hNaV1.8 map resulted in 10,509,847 particle coordinates for subsequent processing. After multiple rounds of 2D classifications, a subset of particles showing different views of hNaV1.8-ProTx-I were selected for ab initio initial 3D model building. A subset of 197,157 particles were used for non-uniform refinement into a 3D reconstruction at an overall resolution of 2.9 Å. Subsequent 2D classifications, 3D refinements, and 3D classifications resulted in a non-uniform refined and sharpened hNaV1.8-ProTx-I reconstruction at an overall resolution of 2.76 Å from 267,708 particles. Focus refinement of VSDI-II, as well as 3D classification did not aid in resolving VSDI or increasing the resolution of the ProTx-I binding region.
Model building, refinement and validation
hNaV1.8 from PDB 7WFW36 was rigid body fitted into the final apo-hNaV1.8 map using ChimeraX. No density for β4 was observed and therefore was not modeled. The apo-hNaV1.8 model was modified with M1713V, glycosylation sites were adjusted as necessary and the VSDI S4-S5 linker was positioned to best fit the map using Coot58. Additionally, 7WFW was found to contain a mutation (S894F) that differs from the canonical hNaV1.8 sequence (NM_006514.3 and NM_006514.4), which was updated in our model. Cholesterol and bound lipids from PDB 7WE4 were used for model building. The model was refined in Coot and subsequently refined against the corresponding map using Phenix real-space refinement59. Models for Class I and II were initially built using an earlier apo-hNaV1.8 model and similarly refined as described.
Modeling for hNaV1.8-ProTx-I used an earlier apo-hNaV1.8 model along with a single ProTx-I model from the NMR ensemble PDB 2M9L24 and both were rigid body fit into the hNaV1.8-ProTx-I map using ChimeraX60–62. Multiple orientations of ProTx-I were sampled to optimize the model-to-map fit. Steps for adjustments and refinements were performed similarly to apo-hNaV1.8.
Model validations were performed using Phenix and MolProbity63,64. Statistics are available in Supplementary Table 1. Pore path and radius were determined using MOLEonline65. All figures were prepared with UCSF ChimeraX, Fiji66, Adobe Photoshop and Microsoft PowerPoint. Supplementary movies were prepared with UCSF ChimeraX.
Antibody
The FLAG Tag antibody, HRP-conjugated, mouse Ab; GenScript cat #A01428-100; lot #21O5K001
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
The authors thank all members of the Gonen lab for helpful and critical discussions. The Gonen lab and this research was supported by the Defense Threat Reduction Agency HDTRA1-21−1-0004 and the National Institute of General Medical Sciences R35-GM142797.
Author contributions
B.N. performed the experimentation and cryoEM processing. B.N. and S.G. experimental design. B.N. with the guidance of S.M. and S.G. performed model building. All authors analyzed and interpreted the data. Project conception and work supervision by S.G. S.M. drafted an early version of the manuscript. All authors contributed to and approved the final manuscript.
Peer review
Peer review information
Nature Communications thanks Miao Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The cryoEM maps associated with this study have been deposited to the Electron Microscopy Data Bank (EMDB) under accession codes EMD-46718 (apo-hNaV1.8), EMD-46719 (Class I), EMD-46720 (Class II), and EMD-46721 (hNaV1.8-ProTx-I). The atomic coordinates associated with this study have been deposited to the Protein Data Bank (PDB) under the accession codes 9DBK (apo-hNaV1.8), 9DBL (Class I), 9DBM (Class II), and 9DBN (hNaV1.8-ProTx-I). Previously reported cryoEM maps and models referred to in this manuscript can be found under accession codes 2M9L (Solution structure of protoxin-1), EMD-32476 (Apo human Nav1.8), 7WFW (Apo human Nav1.8), and 7WE4 (Human Nav1.8 with A-803467, class I). Source data for Figs. 2a, 3c, and Supplementary Fig. 2a-c are provided with this paper. The data that support this study are available from the corresponding authors upon request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-55764-z.
References
- 1.Han, C. et al. Human Nav1.8: enhanced persistent and ramp currents contribute to distinct firing properties of human DRG neurons. J. Neurophysiol.113, 3172–3185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akopian, A. N., Sivilotti, L. & Wood, J. N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature379, 257–262 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Dib-Hajj, S. D. et al. Two tetrodotoxin-resistant sodium channels in human dorsal root ganglion neurons. FEBS Lett.462, 117–120 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Renganathan, M., Cummins, T. R. & Waxman, S. G. Contribution of Nav 1.8 sodium channels to action potential electrogenesis in DRG neurons. J. Neurophysiol.86, 629–640 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Blair, N. T. & Bean, B. P. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J. Neurosci.22, 10277–10290 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi, J. S. & Waxman, S. G. Physiological interactions between NA v1.7 and NA v1.8 sodium channels: a computer simulation study. J. Neurophysiol.106, 3173–3184 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Tan, Z. Y. et al. Tetrodotoxin-resistant sodium channels in sensory neurons generate slow resurgent currents that are enhanced by inflammatory mediators. J. Neurosci.34, 7190–7197 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann, K. et al. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature447, 855–858 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Faber, C. G. et al. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc. Natl Acad. Sci. USA109, 19444–19449 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang, J. et al. Small-fiber neuropathy Nav1.8 mutation shifts activation to hyperpolarized potentials and increases excitability of dorsal root ganglion neurons. J. Neurosci.33, 14087–14097 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan, G. et al. A SCN10A SNP biases human pain sensitivity. Mol. Pain.12, 1–16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Lopez, E. et al. Homozygosity for the SCN10A polymorphism rs6795970 is associated with hypoalgesic inflammatory bowel disease phenotype. Front. Med.5, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett, D. L., Clark, X. A. J., Huang, J., Waxman, S. G. & Dib-Hajj, S. D. The role of voltage-gated sodium channels in pain signaling. Physiol. Rev.99, 1079–1151 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Rowe, A. H. & Rowe, M. P. Physiological resistance of grasshopper mice (Onychomys spp.) to Arizona bark scorpion (Centruroides exilicauda) venom. Toxicon52, 597–605 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Rowe, A. H., Xiao, Y., Rowe, M. P., Cummins, T. R. & Zakon, H. H. Voltage-Gated Sodium Channel in Grasshopper Mice Defends Against Bark Scorpion Toxin. October441, 441–447 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye, P. et al. Scorpion toxin BmK I directly activates Nav1.8 in primary sensory neurons to induce neuronal hyperexcitability in rats. Protein Cell6, 443–452 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIntosh, J. M. et al. A new family of conotoxins that blocks voltage-gated sodium channels. J. Biol. Chem.270, 16796–16802 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Ekberg, J. et al. μO-conotoxin MrVIB selectively blocks NaV1.8 sensory neuron specific sodium channels and chronic pain behavior without motor deficits. Proc. Natl Acad. Sci. USA103, 17030–17035 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang, M. & Zhou, M. μ-conotoxin TsIIIA, a peptide inhibitor of human voltage-gated sodium channel hNav1.8. Toxicon186, 29–34 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Priest, B. T., Blumenthal, K. M., Smith, J. J., Warren, V. A. & Smith, M. M. ProTx-I and ProTx-II: gating modifiers of voltage-gated sodium channels. Toxicon49, 194–201 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Middleton, R. E. et al. Two tarantula peptides inhibit activation of multiple sodium channels. Biochemistry41, 14734–14747 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Agwa, A. J. et al. Gating modifier toxins isolated from spider venom: Modulation of voltage-gated sodium channels and the role of lipid membranes. J. Biol. Chem.293, 9041–9052 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohkubo, T., Yamazaki, J. & Kitamura, K. Tarantula toxin ProTx-I differentiates between human T-type voltage-gated Ca2+ channels Cav3.1 and Cav3.2. J. Pharm. Sci.112, 452–458 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Gui, J. et al. A tarantula-venom peptide antagonizes the TRPA1 nociceptor Ion channel by binding to the S1-S4 gating domain. Curr. Biol.24, 473–483 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laverne, V., Alewood, P. F., Mobli, M. & King, G. F. The structural universe of disulfide-Rich Venom Peptides. in Venoms to Drugs: Venom as a Source for the Development of Human Therapeutics (ed. King, G. F.) 37–70 (Royal Society of Chemistry, 2015).
- 26.Rupasinghe, D. B. et al. Mutational analysis of ProTx-I and the novel venom peptide Pe1b provide insight into residues responsible for selective inhibition of the analgesic drug target NaV1.7. Biochem. Pharm.181, 114080 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Pan, X. et al. Molecular basis for pore blockade of human Na+ channel Nav1.2 by the μ-conotoxin KIIIA. Science363, 1309–1313 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Jiang, D. et al. Structural basis for voltage-sensor trapping of the cardiac sodium channel by a deathstalker scorpion toxin. Nat. Commun.12, 128 (2021). [DOI] [PMC free article] [PubMed]
- 29.Shen, H. et al. Structural basis for the modulation of voltage-gated sodium channels by animal toxins. Science362, 1–8 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Shen, H., Liu, D., Wu, K., Lei, J. & Yan, N. Structures of human Nav1.7 channel in complex with auxiliary subunits and animal toxins. Science363, 1303–1308 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Xu, H. et al. Structural basis of Nav1.7 inhibition by a gating-modifier spider toxin. Cell176, 702–715 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Wisedchaisri, G. et al. Structural basis for high-affinity trapping of the NaV1.7 channel in its resting state by tarantula toxin. Mol. Cell81, 38–48.e4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clairfeuille, T. et al. Structural basis of a-scorpion toxin action on Nav channels. Science363, 80 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Huang, G. et al. High-resolution structures of human Nav1.7 reveal gating modulation through α-π helical transition of S6IV. Cell Rep.39, 110735 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Zhao, J., O’Leary, M. E. & Chahine, M. Regulation of Nav1.6 and Nav1.8 peripheral nerve Na+ channels by auxiliary β-subunits. J. Neurophysiol.106, 608–619 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang, X. et al. Structural basis for high-voltage activation and subtype-specific inhibition of human Nav1.8. Proc. Natl Acad. Sci.119, 1–9 (2022). [DOI] [PMC free article] [PubMed]
- 37.Li, Z. et al. Structural basis for pore blockade of the human cardiac sodium channel Nav1.5 by the Antiarrhythmic Drug Quinidine.Angew Chem. Int. Ed.60, 1174–11480 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Li, Z. et al. Structure of human Nav1.5 reveals the fast inactivation-related segments as a mutational hotspot for the long QT syndrome. Proc. Natl Acad. Sci. USA118, 1–7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan, X. et al. Comparative structural analysis of human Nav1.1 and Nav1.5 reveals mutational hotspots for sodium channelopathies. Proc. Natl Acad. Sci. USA118, 1–7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan, X. et al. Structure of the human voltage-gated sodium channel Nav1.4 in complex with β1. Science362, eaau2486 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Jiang, D. et al. Structure of the cardiac sodium channel. Cell180, 122–134.e10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang, G. et al. Unwinding and spiral sliding of S4 and domain rotation of VSD during the electromechanical coupling in Nav1.7. Proc. Natl Acad. Sci. USA119, 1–9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrat, G. & Darbon, H. An overview of the three dimensional structure of short spider toxins. Toxin Rev.24, 359–381 (2005). [Google Scholar]
- 44.Khemaissa, S., Sagan, S. & Walrant, A. Tryptophan, an amino-acid endowed with unique properties and its many roles in membrane proteins. Crystals11, 1032 (2021).
- 45.Henriques, S. T. et al. Interaction of tarantula venom peptide ProTx-II with lipid membranes is a prerequisite for its inhibition of human voltage-gated sodium channel NaV 1.7. J. Biol. Chem.291, 17049–17065 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bosmans, F., Martin-Eauclaire, M.-F. & Swartz, K. J. Deconstructing voltage sensor function and pharmacology in sodium channels. Nature456, 202–208 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan, X., Huang, J., Jin, X. & Yan, N. Cryo-EM structure of human voltage-gated sodium channel Nav1.6. Proc. Natl Acad. Sci. USA120, e2220578120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han, Y. et al. High-yield monolayer graphene grids for near-atomic resolution cryoelectron microscopy. Proc. Natl Acad. Sci. USA117, 1009–1014 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao, S. et al. Employing NaChBac for cryo-EM analysis of toxin action on voltage-gated Na+ channels in nanodisc. Proc. Natl Acad. Sci. USA117, 14187–14193 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao, Y. et al. Tarantula huwentoxin-IV inhibits neuronal sodium channels by binding to receptor site 4 and trapping the domain II voltage sensor in the closed configuration. J. Biol. Chem.283, 27300–27313 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonen, S. Progress Towards CryoEM: negative-Stain Procedures for Biological Samples. in cryoEM: Methods and Protocols (eds. Gonen, T. & Nannenga, B. L.) 145–160 (Humana Press, 2021). [DOI] [PubMed]
- 52.Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol.180, 519–530 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mastronarde, D. N. SerialEM: a program for automated tilt series acquisition on Tecnai microscopes using prediction of specimen position. Microsc. Microanal.9, 1182–1183 (2003). [Google Scholar]
- 54.Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. CryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods14, 290–296 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Rubinstein, J. L. & Brubaker, M. A. Alignment of cryo-EM movies of individual particles by optimization of image translations. J. Struct. Biol.192, 188–195 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods17, 1214–1221 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Punjani, A. & Fleet, D. J. 3D variability analysis: resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol.213, 107702 (2021). [DOI] [PubMed] [Google Scholar]
- 58.Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D. Biol. Crystallogr.66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. Sect. D. Struct. Biol.75, 861–877 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci.30, 70–82 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci.27, 14–25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meng, E. C. et al. UCSF ChimeraX: tools for structure building and analysis. Protein Sci.32, 1–13 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis, I. W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res.35, 375–383 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci.27, 293–315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pravda, L. et al. MOLEonline: a web-based tool for analyzing channels, tunnels and pores (2018 update). Nucleic Acids Res.46, W368–W373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schindelin, J. et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The cryoEM maps associated with this study have been deposited to the Electron Microscopy Data Bank (EMDB) under accession codes EMD-46718 (apo-hNaV1.8), EMD-46719 (Class I), EMD-46720 (Class II), and EMD-46721 (hNaV1.8-ProTx-I). The atomic coordinates associated with this study have been deposited to the Protein Data Bank (PDB) under the accession codes 9DBK (apo-hNaV1.8), 9DBL (Class I), 9DBM (Class II), and 9DBN (hNaV1.8-ProTx-I). Previously reported cryoEM maps and models referred to in this manuscript can be found under accession codes 2M9L (Solution structure of protoxin-1), EMD-32476 (Apo human Nav1.8), 7WFW (Apo human Nav1.8), and 7WE4 (Human Nav1.8 with A-803467, class I). Source data for Figs. 2a, 3c, and Supplementary Fig. 2a-c are provided with this paper. The data that support this study are available from the corresponding authors upon request. Source data are provided with this paper.