Abstract
Background and Aims
Abiotic and biotic components of the environment both limit plant reproduction, but how they interact with one another in combination is less understood. Understanding these interactions is especially relevant because abiotic and biotic environmental components respond differently to various drivers of global change. Here, we aim to understand whether the effects of pollination (biotic component) on plant reproduction depend on soil moisture (abiotic component), two factors known to affect plant reproduction and that are changing with global change.
Methods
We conducted pollen supplementation experiments for two plant species, Delphinium nuttallianum and Hydrophyllum fendleri, in subalpine meadows in the Western USA across 4 years that varied in soil moisture. In a separate 1-year field experiment, we crossed water addition with pollen supplementation factorially. We measured the proportion of fruit set, seeds per fruit and seeds per plant, in addition to stomatal conductance, to determine whether plant physiology responded to watering.
Key Results
In the 4-year study, only H. fendleri reproduction was pollen limited, and this occurred independently of soil moisture. Experimental water addition significantly increased soil moisture and stomatal conductance for both species. The effect of pollen addition on reproduction depended on the watering treatment only for H. fendleri fruit production. Reproduction in D. nuttallianum was not significantly affected by pollen addition or water addition, but it did respond to interannual variation in soil moisture.
Conclusions
Although we found some evidence for the effect of a biotic interaction depending on abiotic conditions, it was only for one aspect of reproduction in one species, and it was in an unexpected direction. Our work highlights interactions between the abiotic and biotic components of the environment as an area of further research for improving our understanding of how plant reproduction responds to global change.
Keywords: Delphinium nuttallianum (Pritz. Ex Walp.), Hydrophyllum fendleri (A. Gray) A. Heller, plant–pollinator interactions, pollen limitation, reproductive output, reproductive success, resource limitation, soil moisture, stomatal conductance, water stress
INTRODUCTION
Reproduction is an important part of the plant life cycle that is affected by a myriad of abiotic and biotic environmental factors. In terms of the abiotic environment, the effects of temperature and soil moisture on plant reproduction are fairly well understood (Saavedra et al., 2003; Caruso, 2006; Zinn et al., 2010; Scaven and Rafferty, 2013; Parmesan and Hanley, 2015). In terms of the biotic environment, various types of interactions among plants and between plants and other organisms, such as pathogens, mutualistic fungi, herbivores and pollinators, also affect plant reproduction (Goldberg and Barton, 1992; Jarosz and Davelos, 1995; Saikkonen et al., 1998; Singer and Parmesan, 2010; Thomson, 2010; Liu et al., 2011; Kudo and Ida, 2013; Koupilová et al., 2023). Although abiotic and biotic factors both limit plant reproduction independently, their potential interactive effects when combined are not as well understood. Interactions between the abiotic and biotic environment are important to understand because a biotic factor that seems critical for plant performance, such as pollination, might be important only in certain environmental conditions (Hulvey and Aigner, 2014; Eskelinen and Harrison, 2015; Faust and Iler, 2022). Conversely, the effect of the abiotic environment might be important only in certain biotic conditions (Gallagher and Campbell, 2017; Danguilan and Iler, 2023).
In terms of biotic factors that influence plant reproduction, fruit and seed production are often limited by pollen receipt in nature, such that addition of outcross pollen increases reproduction in comparison to freely pollinated plants (Burd, 1994; Knight et al., 2006; Bennett et al., 2020). Magnitudes of pollen limitation vary across space and time, but the reasons for this variation are not fully understood. One commonly posited hypothesis for why the magnitude of pollen limitation might vary across space and time is that pollinator abundance, hence pollination, also varies substantially across space and time (e.g. Petanidou et al., 2008; Carstensen et al., 2014; CaraDonna et al., 2017; Ogilvie and CaraDonna, 2022). A non-mutually exclusive, alternative hypothesis is that the abiotic environment also varies across space and time and might cause resource limitation, such that receipt of additional pollen has no effect on reproduction when plants are already resource limited (Campbell, 1987; Campbell and Halama, 1993; Totland, 2001).
The abiotic environment and pollination could have synergistic or subadditive effects on reproduction when combined. Both theory and empirical work predict synergistic interactions. Theory predicts simultaneous limitation of plant reproduction by pollination and resources, because of a balance between the demands of allocating resources to attract pollinators and allocating resources to provision seeds (Haig and Westoby, 1988). This is a particular type of synergistic interaction, whereby the addition of pollen and resources should not affect reproduction independently: only when added in combination should resources and pollen both limit plant reproduction (Brookes et al., 2008). Empirical work predicts that pollen addition will increase reproduction in favourable environmental conditions owing to release from resource limitation (Campbell and Halama, 1993; Brookes and Jesson, 2007; Andrzejak et al., 2022). In a slightly different type of synergistic interaction, reproduction is limited by abiotic factors only in harsh environments, such that addition of resources increases reproduction independently, and addition of pollen increases reproduction only in combination with resource addition. In terms of subadditive effects, the effect of combined pollen and resource addition would be masked or muted compared with when they are considered in isolation. Finally, indirect effects of resources are also possible, whereby resource addition alters allocation to floral rewards, therefore potentially affecting pollination (Campbell and Halama, 1993), but such indirect effects are not the focus of this research.
Given the large number of abiotic factors that could interact with pollen addition to affect plant reproduction, we are far from a synthetic understanding of how abiotic factors and pollination come together to affect plant reproduction, especially in the context of environmental change (Andrzejak et al., 2022). Understanding pollen and resource limitation and their interaction is especially relevant in light of climate change that is altering abiotic conditions (Andrzejak et al., 2022; IPCC, 2023) and pollinator declines that are altering pollination services to plants (Anderson et al., 2011; Burkle et al., 2013). Furthermore, to our knowledge, studies investigating pollen limitation, resource limitation and their interaction rely on experimental manipulations, making it unclear to what extent such interactions exist under natural variation (environmental heterogeneity across space or time).
We therefore combine a 4-year study of pollen limitation and interannual variation in soil moisture with a 1-year experiment in which both pollination and resources are manipulated. This allows us to test for the two types of interactions outlined above: whether pollen addition increases reproduction only in favourable abiotic conditions and/or whether pollen and resources simultaneously limit plant reproduction. We focus on soil moisture because it is known to affect plant reproduction, varies interannually across our study period, can be manipulated readily in the field and is changing as the climate changes (Descamps et al., 2021). At our study site in the Colorado Rocky Mountains of Western USA, spring snowmelt date is advancing (Anderson et al., 2012; Ogilvie et al., 2017; Cordes et al., 2020), leading to drier conditions, because of less water input into soils from snow, and a protracted dry period between spring snowmelt and mid-summer monsoon rains that might affect soil moisture for months after snow melts in the spring (Pederson et al., 2011; Blankinship et al., 2014; Sloat et al., 2015). If an interaction occurs as predicted by theory, plant reproduction would not increase in response to the addition of pollen or water independently, and reproduction would increase only when both pollen and water are added (or when soil moisture is higher in the case of the study relying on interannual variation in soil moisture). If an interaction occurs as predicted by empirical work, then we expect to see plant reproduction increase in response to the addition of water and the combined addition of pollen and water. We ask the following questions. First, do pollen and water limitation interact to affect plant reproduction? Second, if there is an interaction, does it support theoretical (simultaneous limitation by pollen and soil moisture) or empirical predictions (independent water limitation and pollen limitation only in favourable soil moisture conditions), or neither?
MATERIALS AND METHODS
Study species and site
We conducted this field experiment at the Rocky Mountain Biological Laboratory (RMBL) in Gothic, CO, USA (2900 m above sea level), in a subalpine plant community dominated by iteroparous perennials. Our study species were Nuttal’s larkspur, Delphinium nuttallianum (Ranunculaceae), and Fendler’s waterleaf, Hydrophyllum fendleri (Hydrophyllaceae). Both species are herbaceous, iteroparous perennial wildflowers native to Colorado, and both species depend on animal pollination for seed production (Waser, 1978; Beckmann, 1979). Delphinium nuttallianum plants produce a single inflorescence with 1–15 flowers that are pollinated primarily by bumble bee queens and hummingbirds (Waser and Price, 1981, 1990). There is no clonal reproduction or seed bank (Waser and Price, 1985). Delphinium nuttallianum has been shown to be pollen limited at the RMBL, flowers in mid-June and is self-compatible (Schiffer et al., 2023). Hydrophyllum fendleri plants produce one to six inflorescences per plant, each containing ~5–30 flowers. The flowers are pollinated primarily by bumble bees (A. M. Iler, unpublished data). Plants spread vegetatively via short rhizomes, and connections can be felt by gentle excavations of the soil surface (A. M. Iler, personal observation). Hydrophyllum fendleri plants have been shown to be pollen limited at the RMBL (A. M. Iler, unpublished data), flower in early July and are self-compatible (Beckmann, 1979).
Study 1 experimental design: interannual variation in soil moisture
Study areas were chosen based on accessibility and the population size of our two focal species (sufficient number of individuals). The D. nuttallianum population was located 600 m north-west of the RMBL, and the H. fendleri population was located 380 m south-east of the RMBL (in the RMBL ‘Research Meadow’).
We conducted a 4-year study of pollen limitation that consisted of two treatments: supplemental pollination, in which we added supplemental outcross pollen to all stigmas on the plants (details below), and a control, in which plants were not manipulated. The pollination treatments were applied in a randomized design in a series of 1 m × 10 m plots that were divided into eight treatment areas (0.5 m × 2.5 m; Fig. 1). Four pollination treatments were randomly assigned on each half of the plot; two of these pollination treatments were not used in the present study (two types of reduced pollination treatments). All plants of the study species were tagged with a unique identification number and mapped within each plot. There were six plots for D. nuttallianum and 15 plots for H. fendleri. On average across years, D. nuttallianum plots contained 47.67 flowering plants (range 16.2–103 plants per plot), and H. fendleri plots contained 11.8 flowering plants (range 2.0–34.8 plants per plot; Supplementary Data Table S1). For the supplemental pollination treatment, we added supplemental outcross pollen to all receptive stigmas on treatment plants three times per week throughout the flowering period of each species. Specifically, we removed anthers from donor plants outside of the plots using forceps and gently rubbed them across the stigmas of treatment plants until they were visibly covered in pollen, a standard technique in pollination biology for assessing pollen limitation (Kearns and Inouye, 1993; Ashman et al., 2004). Although it is possible that we occasionally transferred pollen from plants as near as 1 m from focal plants, pollen donors were usually further than 5 m away. Hand pollinations were performed during the morning when anthers contained visible pollen, typically 08:00–10:00 h.
Fig. 1.
Experimental design for the two studies in this paper aiming to test for interactions between pollen addition and soil moisture on plant reproduction in two subalpine plant species, Delphinium nuttallianum (top plant icon in study 1) and Hydrophyllum fendleri (bottom plant icon in study 1). Study 1 uses interannual variation in soil moisture and a 4-year pollen limitation experiment. Study 2 is a factorial manipulation of pollen and water addition in 1 year. Small pink dots indicate pollen supplementation, and blue droplets indicate water addition. Plant illustrations were created by J. Johnson.
Soil moisture was measured as the volumetric water content, once per week throughout the reproductive period of each species (from flowering to fruit maturation), using a FieldScout TDR 100 Meter at a probe depth of 7.62 cm (Spectrum Technologies, Aurora, IL, USA). We took five measurements per plot each week, at 0, 2.5, 5.0, 7.5 and 10 m along the western edge of all plots.
We measured plant size and reproduction from 2019 to 2022 for D. nuttallianum and from 2018 to 2021 for H. fendleri, using standard metrics of size from studies of plant demography. In the field, the size of D. nuttallianum was measured as the length and width of the longest leaf and later converted to leaf area using the formula for an ellipse: (leaf length × 0.5)(leaf width × 0.5) × π. The size of H. fendleri was measured as the number of leaves. We collected fruits as they matured, counted seeds and quantified the number of fruits per flower, seeds per fruit and seeds per plant as measures of reproductive success.
Analysis for study 1 (interannual variation in soil moisture)
We used interannual variation in volumetric water content of the soil to investigate the effects of soil moisture on reproduction, in addition to potential interactions with addition of pollen. We first calculated plot-level means of soil moisture for each year. We then used each reproductive response in either a linear mixed-effect model (LMM; seeds per fruit) or a generalized linear mixed-effect model (fruits per flower with betabinomial error distribution and seeds per plant with negative binomial error distribution). The betabinomial and negative binomial were used because binomial and Poisson models, respectively, were overdispersed. For D. nuttallianum, seeds per fruit was log-transformed to meet the assumptions for linear models. Mean soil moisture, size (leaf area for D. nuttallianum and number of leaves for H. fendleri) and plot-level plant density were continuous predictors, pollination treatment (supplement vs. control) and year were categorical predictors, and plot was a random intercept term. Initially, we included an interaction between treatment and soil moisture; if there was no evidence of an interaction using a type III ANOVA (P > 0.05), we used an additive model with no interaction term. We did not conduct model selection, because the goal of our analyses was inference (Tredennick et al., 2021).
Study 2 experimental design: watering experiment
The water addition experiment was conducted in 2022. Owing to different spatial arrangements of each species, we used a slightly different design to apply water addition and pollen supplementation treatments in a factorial combination for each species: a randomized block split-plot design for D. nuttallianum, in which plot pairs composed a block, and a randomized block design for H. fendleri, in which four individuals composed a block (Fig. 1).
For D. nuttallianum, we established six pairs of plots (12 in total) that were 1 m × 1 m in size, within the same population as study 1. Plot pairs were between 1.25 m and 1.5 m away from each other and had a similar slope and aspect. When possible, each pair was randomly assigned to either water addition or control. However, in cases where the watering treatment was upslope from the control, the treatments were switched to ensure that water did not run downslope into the control plot. Each half was randomly assigned a pollination treatment: supplement or control. We tagged 184 individuals, but many individuals contained fruits that were eaten, reducing our total sample to 106 plants. After accounting for frugivory, the control plots contained on average 8.5 plants (51 in total), and the watered plots contained on average 9.2 plants (55 in total; for number of plants per plot, see Supplementary Data Table S2).
For H. fendleri, we established ten blocks of four individuals each (40 individuals in total), also within the same population as study 1. These plants were located by identifying four plants that were near to each other, on a similar slope and aspect. The plants were each randomly assigned a pollination treatment (supplement or control) and a watering treatment (watering or control) in factorial combination.
Supplemented plants were hand pollinated every Monday, Wednesday and Friday, weather permitting, following a similar protocol to study 1. One exception is that visible pollen was removed and transferred to all supplemented flowers using a paintbrush, which was more efficient than removing anthers. Watering also took place every Monday, Wednesday and Friday, weather permitting. Delphinium nuttallianum watering occurred from 11 June to 1 July, and H. fendleri watering occurred from 15 June to 29 July. Watering occurred during the flowering period of each species, stopping after the last flower in each plot (for D. nuttallianum) or plant (for H. fendleri) was no longer open. We used a 15 L plastic backpack sprayer (SprayMate ‘Storm 2.5-Gallon 18V Battery Powered Sprayer’). Each watering application consisted of 4 L of water, following Faust and Iler (2022), who found that this amount of water increased soil moisture in plots of similar size. Water was sourced from the nearby East River with watering cans, attempting to exclude all macroorganisms via a filter, or from rainout shelter overflow water that was collected in plastic buckets in the same meadow as the H. fendleri experiment. Watering was done after 16:00 h each day to minimize evapotranspiration (Faust and Iler, 2022). Volumetric water content was measured twice per week in each plot corner for D. nuttallianum and at the base of each plant for H. fendleri.
To assess evidence of drought stress, we used a Meter SC-1 porometer to measure the stomatal conductance of leaves. Stomatal conductance is often, but not always, lower in plants experiencing drought stress, because stomatal closure conserves water (Tardieu et al., 2015). Therefore, when stomatal conductance is high, the plant is likely to be less water stressed. Owing to time constraints, we were unable to measure stomatal conductance on all plants. For D. nuttallianum, we measured stomatal conductance on 28 plants on 10 and 13 June in four plots on plants that were still flowering and beginning to mature fruits (two blocks; 17 control plants and 11 watered plants). We measured stomatal conductance on 32 H. fendleri plants on 27 July across eight blocks on plants in the fruiting phase (16 control and 16 watered plants).
Fruits were collected as they matured. Some H. fendleri inflorescences were mouldy once counting began in the laboratory. For these plants, it was too difficult to determine the number of flowers, hence we use fruits per inflorescence instead of fruits per flower in our analysis of H. fendleri. There were eight plants with at least one mouldy fruit from which it was not possible to count seeds; these plants were excluded from analysis of seeds per fruit and seeds per plant.
Analysis for study 2 (watering experiment)
To understand how the watering treatment affected soil moisture, we used LMMs with soil moisture as a response variable, watering treatment as a categorical predictor, and date as a random intercept term to account for repeated measures across multiple dates. The model for each species contained an additional random intercept term: plot nested within pairs for D. nuttallianum, and block for H. fendleri. Soil moisture was log-transformed to meet the assumptions of normality and heteroscedasticity. Log-transformed stomatal conductance was also analysed with an LMM following the same model structure, except that date was not a random intercept term (because it was not a repeated measure).
Plant reproduction responses were analysed separately and included fruits per flower (D. nuttallianum only), fruits per inflorescence (H. fendleri only), seeds per fruit and seeds per plant. We used generalized linear mixed-effect models with negative binomial error distributions for overdispersed count variables (seeds per plant), betabinomial error distributions for overdispersed proportion data (fruits per flower, with number of flowers as a weight) and LMMs for fruits per inflorescence and seeds per fruit. For D. nuttallianum, plot nested within pair was a random intercept term, and for H. fendleri block was a random intercept term. Plant size was included as a continuous predictor in all models to account for any effects of size on each measure of reproduction; however, we were unable to include size in the analysis of D. nuttallianum fruits per flower because models with size would not converge. Additionally, the number of plants per plot was included as a continuous predictor for D. nuttallianum. We first tested for an interaction between the pollination and watering treatments (using type III ANOVAs at P < 0.10). If there was no evidence for an interaction, we used an additive model (using type II ANOVAs). We used the package glmmTMB (Brooks et al., 2017) to fit our models in R v.4.4.0 (R Core Development Team, 2024) and the ggeffects package to make Fig. 2 (Lüdecke, 2018).
Fig. 2.
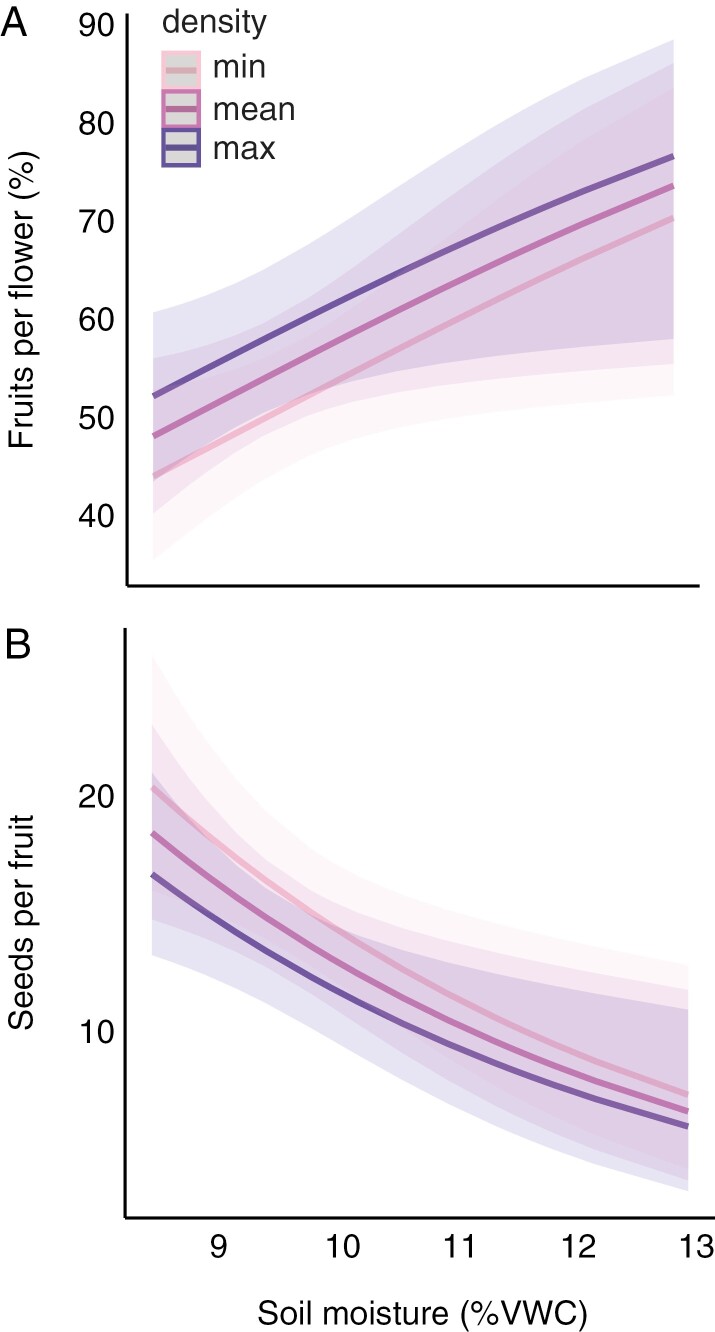
Relationships between interannual variation in soil moisture and fruits per flower (A) and seeds per fruit (B) in Delphinium nuttallianum, the only species in which soil moisture affected reproduction in study 1 (non-significant results are in Supplementary Data Fig. S1). Predicted lines are shown for low, medium and high (minimum, mean and maximum, respectively) densities of flowering plants, because density also affected reproduction. Error bars are 95 % confidence intervals. Abbreviation: VWC, volumetric water content.
RESULTS
Study 1: interannual variation in soil moisture
Volumetric soil water content ranged from 8.41 to 12.96 % across the 4-year study in D. nuttallianum plots (minimum and maximum of all weekly values across the 4 years). For D. nuttallianum, there were no significant interactions between soil moisture and pollination treatment for any measure of reproduction (interaction terms for each response: fruits per flower, χ21 = 0.33, P = 0.56; seeds per fruit, χ21 = 3.63, P = 0.059; seeds per plant, χ21 = 0.14, P = 0.71), hence we present results from additive models. There was no significant effect of pollination treatment on any measure of reproduction, suggesting that reproduction was not pollen limited (Table 1). Soil moisture had a significant effect on the number of fruits per flower and seeds per fruit, with more fruits per flower and fewer seeds per fruit as soil moisture increased (Table 1; Fig. 2). Likewise, plants matured a higher percentage of fruits per flower and fewer seeds per fruit as plant density increased (Fig. 2). Soil moisture did not significantly affect the number of seeds per plant (Table 1; Supplementary Data Fig. S1A). The number of seeds per plant was the only measure of reproduction that was size dependent, and all measures of reproduction varied significantly across years (Table 1).
Table 1.
Results from type II ANOVAs of linear mixed-effect models and generalized linear mixed-effect models of the effect of interannual variation in soil moisture and pollination treatment (control vs. pollen supplementation) on reproduction in two subalpine plant species, Delphinium nuttallianum and Hydrophyllum fendleri. Plant size and year were also included as predictors to account for size-dependent reproduction and interannual variation in reproduction unrelated to soil moisture, respectively. Plot was a random intercept term. Significant predictors are highlighted in bold (P < 0.05).
| Species | Response | Predictor | χ2 | d.f. | P-value |
|---|---|---|---|---|---|
| Delphinium nuttallianum | Fruits per flower | Mean soil moisture | 4.75 | 1 | 0.029 |
| Pollination treatment | 1.21 | 1 | 0.27 | ||
| Size | 2.31 | 1 | 0.13 | ||
| Year | 29.54 | 3 | <0.0001 | ||
| Density | 4.57 | 1 | 0.032 | ||
| Log(seeds per fruit) | Mean soil moisture | 7.83 | 1 | 0.0051 | |
| Pollination treatment | 0.26 | 1 | 0.61 | ||
| Size | 1.30 | 1 | 0.25 | ||
| Year | 67.23 | 3 | <0.0001 | ||
| Density | 5.82 | 1 | 0.016 | ||
| Seeds per plant | Mean soil moisture | 0.37 | 1 | 0.54 | |
| Pollination treatment | 0.04 | 1 | 0.89 | ||
| Size | 41.37 | 1 | <0.0001 | ||
| Year | 101.45 | 3 | <0.0001 | ||
| Density | 0.29 | 1 | 0.59 | ||
| Hydrophyllum fendleri | Fruits per flower | Mean soil moisture | 0.009 | 1 | 0.92 |
| Pollination treatment | 4.78 | 1 | 0.029 | ||
| Size | 0.25 | 1 | 0.62 | ||
| Year | 103.77 | 3 | <0.001 | ||
| Density | 0.32 | 1 | 0.57 | ||
| Seeds per fruit | Mean soil moisture | 0.33 | 1 | 0.56 | |
| Pollination treatment | 2.68 | 1 | 0.10 | ||
| Size | 1.41 | 1 | 0.24 | ||
| Year | 10.69 | 3 | 0.014 | ||
| Density | 0.25 | 1 | 0.62 | ||
| Seeds per plant | Mean soil moisture | 0.056 | 1 | 0.81 | |
| Pollination treatment | 3.65 | 1 | 0.056 | ||
| Size | 49.60 | 1 | <0.0001 | ||
| Year | 60.76 | 3 | <0.0001 | ||
| Density | 0.0014 | 1 | 0.97 |
Hydrophyllum fendleri volumetric soil water content ranged from 8.81 to 24.34 % across the 4-year study (minimum and maximum of all weekly values across the 4 years). Consistent with D. nuttallianum, there were no significant interactions between soil moisture and pollination treatment for any measure of reproduction in H. fendleri (interaction terms for each response: fruits per flower, χ21 = 0.22, P = 0.64; seeds per fruit, χ21 = 2.20, P = 0.14; seeds per plant, χ21 = 2.11, P = 0.15). In contrast to D. nuttallianum, the number of fruits per flower was significantly higher and the number of seeds per fruit and seeds per plant were marginally significantly higher in the pollen supplementation treatment compared with controls, indicating pollen limitation (Table 1; Fig. 3). Soil moisture did not have a significant effect on any measure of reproduction (Table 1; Supplementary Data Fig. S1B–D). All measures of reproduction varied significantly across years (Table 1).
Fig. 3.
Effects of pollination treatment on Hydrophyllum fendleri female reproductive success, shown as fruits per flower (A), seeds per fruit (B) and seeds per plant (C). Effects of pollination treatment occurred irrespective of soil moisture levels and are shown separately here. *P < 0.05, significant difference between pollination treatments. Open circles represent a marginally significant difference at 0.05 < P ≤ 0.10. Boxplots show the interquartile range (i.e. 25th–75th percentiles); the line is the median, and whiskers are 1.5 times the interquartile range.
Study 2: watering experiment
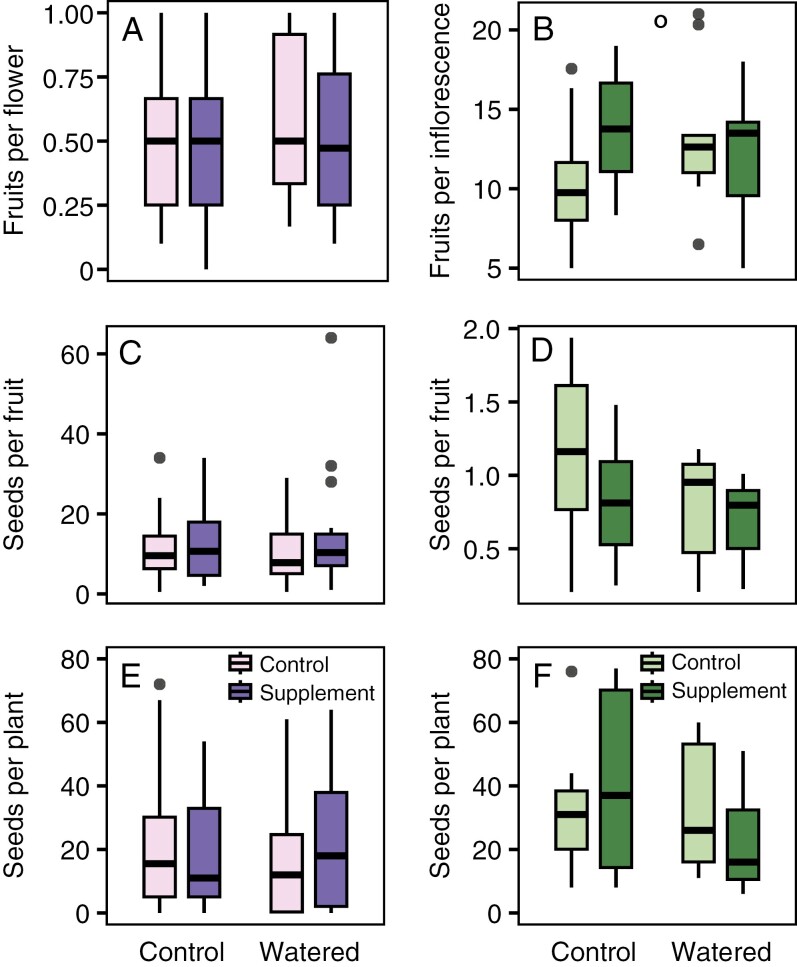
For D. nuttallianum, experimental water addition significantly increased volumetric soil moisture content by 1.1 % on average (χ21 = 5.45, P = 0.020; Fig. 4A). The experiment fell in the middle of the 4-year range of soil moisture, at a mean of 9.83 % for control plants and 10.90 % for watered plants. This increase in soil moisture was associated with significantly higher stomatal conductance in the watering treatment compared with the control (χ21 = 10.62, P = 0.0011; Fig. 4C). However, we did not find any evidence that watering or pollination affected any aspect of reproduction in D. nuttallianum. We present the results from additive models because interactions between the pollination and watering treatment were not significant (interaction terms for each response: fruits per flower, χ21 = 0.45, P = 0.50; seeds per fruit, χ21 = 0.0073, P = 0.93; seeds per plant, χ21 = 1.15, P = 0.28). The number of fruits per flower, seeds per fruit and seeds per plant were not significantly affected by water addition or pollen supplementation (Table 2; Fig. 5A, C, E).
Fig. 4.
The effects of experimental water addition on soil moisture (A, B), measured as percentage volumetric water content, and stomatal conductance (C, D) in two subalpine plant species, Delphinium nuttallianum and Hydrophyllum fendleri. Responses are shown on a logarithmic scale because they were log-transformed for analysis. Higher values of stomatal conductance are often indicative of lower levels of water stress. *P < 0.05, significant difference between watering treatments. Boxplots show the interquartile range (i.e. 25th–75th percentiles); the line is the median, and whiskers are 1.5 times the interquartile range.
Table 2.
Effects of water addition and pollen supplementation on reproduction in Delphinium nuttallianum and Hydrophyllum fendleri. Results are from type II ANOVAs of linear mixed-effect models and generalized linear mixed-effect models of the effect of pollen supplementation (pollination) and water addition (watering), except in cases where there was an interaction between pollination and watering treatments and a type III ANOVA was used. Degrees of freedom = 1 for each predictor in models shown below.
| Species | Response | Predictors | χ2 | P-value |
|---|---|---|---|---|
| Delphinium nuttallianum | Fruits per flower | Pollination | 0.064 | 0.80 |
| Watering | 0.71 | 0.40 | ||
| Density | 0.034 | 0.85 | ||
| Seeds per fruit | Pollination | 0.34 | 0.56 | |
| Watering | 0.042 | 0.84 | ||
| Size | 3.53 | 0.060 | ||
| Density | 0.54 | 0.46 | ||
| Seeds per plant | Pollination | 0.033 | 0.86 | |
| Watering | 0.10 | 0.75 | ||
| Size | 1.72 | 0.19 | ||
| Density | 0.53 | 0.47 | ||
| Hydrophyllum fendleri | Fruits per inflorescence |
Pollination | 3.25 | 0.071 |
| Watering | 2.80 | 0.094 | ||
| Size | 0.92 | 0.34 | ||
| Pollination × watering | 3.55 | 0.059 | ||
| Seeds per fruit | Pollination | 1.74 | 0.19 | |
| Watering | 2.50 | 0.11 | ||
| Size | 0.31 | 0.58 | ||
| Seeds per plant | Pollination | 0.002 | 0.99 | |
| Watering | 1.03 | 0.31 | ||
| Size | 5.28 | 0.02 |
Fig. 5.
The effects of experimental pollen and water addition on reproduction in two subalpine plant species, Delphinium nuttallianum (A, C, E) and Hydrophyllum fendleri (B, D, F). Boxplots show the interquartile range (i.e. 25th–75th percentiles); the line is the median, and whiskers are 1.5 times the interquartile range. Open circle represents a marginally significant interaction between watering and pollination treatment (P = 0.059).
For H. fendleri, experimental water addition significantly increased volumetric soil moisture content by 6.5 % on average (log-transformed soil moisture: χ21 = 228.5, P < 0.0001; Fig. 4B). The experiment fell in the upper part of the 4-year range, at a mean of 17.40 % for control plants and 23.90 % for watered plants. This increase in soil moisture was associated with significantly higher stomatal conductance in the watering treatment compared with the control (χ21 = 11.10, P = 0.0009; Fig. 4D). There was a marginally significant interaction between soil moisture and pollination treatment for fruits per inflorescence, but not for other aspects of reproduction (Table 2; Fig. 5B; interaction term for seeds per fruit: χ21 = 0.69, P = 0.41; seeds per plant: χ21 = 1.71, P = 0.19). The number of seeds per fruit and seeds per plant were not significantly affected by water addition or pollen supplementation (Table 2; Fig. 5D, F).
DISCUSSION
Our results support neither of our two hypotheses: that addition of pollen increases reproduction only in favourable soil moisture conditions or that reproduction is limited by pollen and resources simultaneously. Although reproduction varied across years in both species, we did not find evidence that this variation was explained by the interaction between soil moisture and pollination. Reproduction of H. fendleri was pollen limited in the 4-year study, fruit set of D. nuttallianum was water limited in the 4-year study, and there was an interaction between watering and pollen addition in the experiment, but in an unexpected direction. These experimental results contrast with two other studies that have investigated a factorial combination of water and pollen limitation, finding that water limited seed production, whereas pollen did not (Delph, 1986; de Jong and Klinkhamer, 1989), but our results are consistent with another study at the RMBL that found no effects of water addition on plant reproduction in a different species (Campbell and Halama, 1993). Plant-level seed production of both species appears to be resilient to changes in soil water availability, at least over the range of soil moisture values investigated here (volumetric water content range: 4.55 % for D. nuttallianum and 15.53 % for H. fendleri). Overall, our two study species largely maintained seed production, although soil moisture conditions varied enough to affect their stomatal conductance.
Although we found evidence of an interaction between pollination treatment and watering treatment in our water addition experiment, only for H. fendleri fruit set, it was in an unexpected, subadditive direction. This result was marginally significant (P = 0.059), but it is likely to be biologically meaningful, because there were only ten plants per treatment. Pollen limitation was detected only in unwatered plants, and water limitation was detected only in plants receiving ambient pollination, which, in turn, suggests that plants can make more fruits per inflorescence if they receive additional pollen (on average 3.1 more fruits per inflorescence) or additional water (on average 2.7 more fruits per inflorescence), but that increasing water and pollen together provides less than the additive benefits of each. In contrast to the watering experiment, in the 4-year study we found evidence that percentage fruit set and the number of seeds per plant were both pollen limited in H. fendleri, across a range of soil moisture values. Because variation in soil moisture is coming from variation across years in this experiment, it is likely that other environmental factors also varied across years. It is therefore possible that a resource other than soil moisture might affect reproduction, such as soil nutrients. Indeed, other studies at the RMBL have found evidence for nutrient limitation of plant reproduction (Campbell and Halama, 1993; Burkle and Irwin, 2009).
One explanation for the result that pollen limitation of H. fendleri reproduction occurred only in control soil moisture conditions is that we created a stressful environment by over-watering the plants. Indeed, the summer of 2022 was wetter than both the 10- and 30-year average (2022: 107.0 mm total precipitation in June and July vs. 30-year mean, 75.1 mm; 10-year mean, 81.3 mm; data from https://osf.io/bvzx9). However, several lines of reasoning do not support this hypothesis. First, the water-holding capacity of subalpine soils is generally low, making it difficult to over-water. Second, another study at the RMBL on a different plant species found increasing seed production with increasing soil moisture over a similar range to that in our study (up to ~21 %; Gallagher and Campbell, 2017), making it unlikely that we over-watered the plants. Third, neither the stomatal conductance nor the fruit set results support the over-watering hypothesis; fruit set was lowest in the unwatered plants that received ambient pollination.
We found evidence that soil moisture on its own, in addition to plant density, affected two aspects of reproduction in D. nuttallianum, fruits per flower and seeds per fruit, but only in the 4-year study (Fig. 2; Table 1). Plants make more fruits per flower as soil moisture and plant density increase (Fig. 2A; Table 1). Other studies at RMBL have found increased reproduction with addition of water in other polycarpic perennial species such as ours, suggesting water limitation (Gallagher and Campbell, 2017; Faust and Iler, 2022). An increasing proportion of fruits matured as plant density increases suggests intraspecific facilitation of pollination at higher plant densities (Heithaus et al., 1982; Moeller, 2004; Ghazoul, 2005). We find the opposite patterns for the number of seeds per fruit, which decreases as soil moisture and plant density increase (Fig. 2B; Table 1); this result suggests that intraspecific competition for resources might reduce the number of seeds per fruit at higher densities (Adler et al., 2018). However, it is unclear why the number of seeds per fruit would decrease with soil moisture (after accounting for effects of plant density). It is possible that the number of seeds per fruit is affected by another resource that covaries with soil moisture.
In contrast to the 4-year study, we did not find effects of soil moisture or density on D. nuttallianum reproduction in the watering experiment. It is likely that the amount of water stress in D. nuttallianum control plants was insufficient to affect reproduction in our 1-year experiment, suggesting that plants can adjust their physiology to maintain reproduction (i.e. isohydric plants that reduce stomatal conductance to maintain water potential; Onyemaobi et al., 2021). Generally speaking, less is known about the effects of reduced stomatal conductance on reproduction than on vegetative traits, but there is evidence that some crops also reduce stomatal conductance while maintaining yield (Onyemaobi et al., 2021; Liao et al., 2022). Because our study species are perennials, increased stomatal conductance in the watering treatment might have lagged effects owing to reallocation of resources across years, resulting in increased growth, survival or reproduction in the following year (sensuHorvitz and Schemske, 2002; Obeso, 2002; Iler et al., 2019). We did not find evidence of pollen limitation, either in the 4-year study or in the watering experiment, in contrast to other work showing that D. nuttallianum was pollen limited at the RMBL, at different locations from our study plots (Schiffer et al., 2023). This is unsurprising, because it is well known that pollen limitation can vary across space and time (Burd, 1994). Finally, even if plant reproduction is not limited by the quantity of pollen received, the abiotic environment could still interact with pollen addition to affect plant reproductive success if pollen quality affects reproduction (Faust and Iler, 2022). Although we did not assess pollen quality in this study, given that reproduction did not vary with soil moisture, it is unlikely to explain our results.
Interactions between pollination and resources appear to be relatively uncommon in nature. Of the 12 studies of which we are aware that have crossed pollen addition factorially with a manipulation of resources (usually soil nutrients) in the field, eight studies have found no evidence of an interaction between pollen and resource addition on plant reproduction (Galen, 1985; Delph, 1986; de Jong and Klinkhamer, 1989; Campbell and Halama, 1993; Ne’eman et al., 2006; Brookes and Jesson, 2007; Burkle and Irwin, 2009; Sletvold et al., 2010). Three studies detected an interaction but in unexpected directions (our study; Asikainen and Mutikainen, 2005; Andrzejak et al., 2022). One study found an interaction in the expected direction (pollen addition increased reproduction only in favourable environments), but this was not ubiquitous and occurred only in specific conditions (in one scent morph at one elevation; Galen, 1985). Finally, only one of these 12 studies found clear support for simultaneous limitation of pollen and resources (Brookes et al., 2008). Thus, experimental field studies generally do not support the theoretical prediction that pollination and resources simultaneously limit reproduction. However, this might be difficult to test in the field, especially because studies might focus on the ‘wrong’ limiting resource and because of spatially and temporally heterogeneous environments that would make it difficult to detect simultaneous limitation, even if this is a reasonable theoretical equilibrium. Because the studies that did find interactions tended to find them in unexpected directions, there are numerous ways that resources and pollination might interact to affect plant reproduction, beyond the two interactions we set out to study here. Therefore, to gain a better understanding of the consequences of global change on plant reproduction, we encourage future studies to consider how plant reproduction is affected by various interactions between abiotic and biotic environmental factors.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Table S1: number of flowering plants per plot in study 1 (interannual variation in soil moisture), averaged across years for each species and each plot. Table S2: number of flowering Delphinium nuttallianum plants per plot in study 2, the watering experiment. Figure S1: non-significant relationships between interannual variation in soil moisture and plant reproduction in two subalpine plant species, Delphinium nuttallianum (A) and Hydrophyllum fendleri (B–D).
ACKNOWLEDGEMENTS
We thank the Rocky Mountain Biological Laboratory for access to field sites and logistical support. We additionally thank Paul CaraDonna and three anonymous reviewers for helpful feedback on earlier versions of the paper.
Contributor Information
Isabella B Rodelius, Chicago Botanic Garden, Negaunee Institute of Plant Conservation Science and Action, Glencoe, IL 60022, USA; Rocky Mountain Biological Laboratory, Crested Butte, CO 81224, USA.
Amy M Iler, Chicago Botanic Garden, Negaunee Institute of Plant Conservation Science and Action, Glencoe, IL 60022, USA; Rocky Mountain Biological Laboratory, Crested Butte, CO 81224, USA; Northwestern University, Program in Plant Biology and Conservation, Evanston, IL 60208, USA.
FUNDING
This project was funded by a Research Experience for Postbaccalaureate Students (REPS) Supplemental Award to National Science Foundation grant DEB 1754518.
AUTHOR CONTRIBUTIONS
A.M.I. and I.B.R. designed the project. I.B.R. conducted fieldwork. A.M.I. and I.B.R. analysed data and wrote the manuscript.
Conflict of interest: None declared.
LITERATURE CITED
- Adler PB, Smull D, Beard KH, et al. 2018. Competition and coexistence in plant communities: intraspecific competition is stronger than interspecific competition. Ecology Letters 21: 1319–1329. [DOI] [PubMed] [Google Scholar]
- Anderson JT, Inouye DW, McKinney AM, Colautti RI, Mitchell-Olds T.. 2012. Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proceedings of the Royal Society B: Biological Sciences 279: 3843–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SH, Kelly D, Ladley JJ, Molloy S, Terry J.. 2011. Cascading effects of bird functional extinction reduce pollination and plant density. Science 331: 1068–1071. [DOI] [PubMed] [Google Scholar]
- Andrzejak M, Korell L, Auge H, Knight TM.. 2022. Effects of climate change and pollen supplementation on the reproductive success of two grassland plant species. Ecology and Evolution 12: e8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman T-L, Knight TM, Steets JA, et al. 2004. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85: 2408–2421. [Google Scholar]
- Asikainen E, Mutikainen P.. 2005. Pollen and resource limitation in a gynodioecious species. American Journal of Botany 92: 487–494. [DOI] [PubMed] [Google Scholar]
- Beckmann RL Jr. 1979. Biosystematics of the genus Hydrophyllum L. (Hydrophyllaceae). American Journal of Botany 66: 1053–1061. [Google Scholar]
- Bennett JM, Steets JA, Burns JH, et al. 2020. Land use and pollinator dependency drives global patterns of pollen limitation in the Anthropocene. Nature Communications 11: 3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankinship JC, Meadows MW, Lucas RG, Hart SC.. 2014. Snowmelt timing alters shallow but not deep soil moisture in the Sierra Nevada. Water Resources Research 50: 1448–1456. [Google Scholar]
- Brookes RH, Jesson LK.. 2007. No evidence for simultaneous pollen and resource limitation in Aciphylla squarrosa: a long-lived, masting herb. Austral Ecology 32: 370–377. [Google Scholar]
- Brookes RH, Jesson LK, Burd M.. 2008. A test of simultaneous resource and pollen limitation in Stylidium armeria. The New Phytologist 179: 557–565. [DOI] [PubMed] [Google Scholar]
- Burd M. 1994. Bateman’s principle and plant reproduction: the role of pollen limitation in fruit and seed set. The Botanical Review 60: 83–139. [Google Scholar]
- Burkle LA, Irwin RE.. 2009. The effects of nutrient addition on floral characters and pollination in two subalpine plants, Ipomopsis aggregata and Linum lewisii. Plant Ecology 203: 83–98. [Google Scholar]
- Burkle LA, Marlin JC, Knight TM.. 2013. Plant-pollinator interactions over 120 years: loss of species, co-occurrence and function. Science 339: 1611–1615. [DOI] [PubMed] [Google Scholar]
- Campbell DR. 1987. Interpopulational variation in fruit production: the role of pollination-limitation in the Olympic Mountains. American Journal of Botany 874: 269–273. [Google Scholar]
- Campbell DR, Halama KJ.. 1993. Resource and pollen limitations to lifetime seed production in a natural plant population. Ecology 74: 1043–1051. [Google Scholar]
- CaraDonna PJ, Petry WK, Brennan RM, et al. 2017. Interaction rewiring and the rapid turnover of plant–pollinator networks. Ecology Letters 20: 385–394. [DOI] [PubMed] [Google Scholar]
- Carstensen DW, Sabatino M, Trøjelsgaard K, Morellato LPC.. 2014. Beta diversity of plant-pollinator networks and the spatial turnover of pairwise interactions. PLoS One 9: e112903–e112907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso CM. 2006. Plasticity of inflorescence traits in Lobelia siphilitica (Lobeliaceae) in response to soil water availability. American Journal of Botany 93: 531–538. [DOI] [PubMed] [Google Scholar]
- Cordes LS, Blumstein DT, Armitage KB, et al. 2020. Contrasting effects of climate change on seasonal survival of a hibernating mammal. Proceedings of the National Academy of Sciences of the United States of America 117: 18119–18126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danguilan S, Iler AM.. 2023. Pollen supplementation alters how flowering phenology affects reproduction in a spring-flowering herb. International Journal of Plant Sciences 184: 586–600. [Google Scholar]
- de Jong TJ, Klinkhamer PGL.. 1989. Limiting factors for seed production in Cynoglossum officinale. Oecologia 80: 167–172. [DOI] [PubMed] [Google Scholar]
- Delph LF. 1986. Factors regulating fruit and seed production in the desert annual Lesquerella gordonii. Oecologia 69: 471–476. [DOI] [PubMed] [Google Scholar]
- Descamps C, Quinet M, Jacquemart A-L.. 2021. The effects of drought on plant–pollinator interactions: what to expect? Environmental and Experimental Botany 182: 104297. [Google Scholar]
- Eskelinen A, Harrison S.. 2015. Biotic context and soil properties modulate native plant responses to enhanced rainfall. Annals of Botany 116: 963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust MN, Iler AM.. 2022. Pollinator-mediated reproductive consequences of altered co-flowering under climate change conditions depend on abiotic context. Climate Change Ecology 3: 100043. [Google Scholar]
- Galen C. 1985. Regulation of seed-set in Polemonium viscosum: floral scents, pollination, and resources. Ecology 66: 792–797. [Google Scholar]
- Gallagher MK, Campbell DR.. 2017. Shifts in water availability mediate plant–pollinator interactions. The New Phytologist 215: 792–802. [DOI] [PubMed] [Google Scholar]
- Ghazoul J. 2005. Pollen and seed dispersal among dispersed plants. Biological Reviews of the Cambridge Philosophical Society 80: 413–443. [DOI] [PubMed] [Google Scholar]
- Goldberg DE, Barton AM.. 1992. Patterns and consequences of interspecific competition in natural communities: a review of field experiments with plants. The American Naturalist 139: 771–801. [Google Scholar]
- Haig D, Westoby M.. 1988. On limits to seed production. The American Naturalist 131: 757–759. [Google Scholar]
- Heithaus ER, Stashko E, Anderson PK.. 1982. Cumulative effects of plant-animal interactions on seed production by Bauhinia ungulata, a neotropical legume. Ecology 63: 1294–1302. [Google Scholar]
- Horvitz CC, Schemske DW.. 2002. Effects of plant size, leaf herbivory, local competition and fruit production on survival, growth and future reproduction of a neotropical herb. Journal of Ecology 90: 279–290. [Google Scholar]
- Hulvey KB, Aigner PA.. 2014. Using filter-based community assembly models to improve restoration outcomes. Journal of Applied Ecology 51: 997–1005. [Google Scholar]
- Iler AM, Compagnoni A, Inouye DW, Williams JL, CaraDonna PJ, Miller TEX.. 2019. Reproductive losses due to climate change-induced earlier flowering are not the primary threat to plant population viability in a perennial herb. Journal of Ecology 279: 384–313. [Google Scholar]
- IPCC, 2023. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Lee H and Romero J (eds.)]. IPCC, Geneva, Switzerland, pp. 35–115. doi: https://doi.org/ 10.59327/IPCC/AR6-9789291691647 [DOI] [Google Scholar]
- Jarosz AM, Davelos AL.. 1995. Effects of disease in wild plant populations and the evolution of pathogen aggressiveness. New Phytologist 129: 371–387. [Google Scholar]
- Kearns CA, Inouye DW.. 1993. Techniques for pollination biologists. Denver, CO, USA: University Press of Colorado. [Google Scholar]
- Knight TM, Steets JA, Ashman T-L.. 2006. A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. American Journal of Botany 93: 271–277. [DOI] [PubMed] [Google Scholar]
- Koupilová K, Koubek T, Kasner M, Janovský Z.. 2023. Anther smut pathogens as important drivers of population dynamics of long-lived perennial plants: a case study of Dianthus carthusianorum. Perspectives in Plant Ecology, Evolution and Systematics 59: 125729. [Google Scholar]
- Kudo G, Ida TY.. 2013. Early onset of spring increases the phenological mismatch between plants and pollinators. Ecology 94: 2311–2320. [DOI] [PubMed] [Google Scholar]
- Liao Q, Gu S, Kang S, et al. 2022. Mild water and salt stress improve water use efficiency by decreasing stomatal conductance via osmotic adjustment in field maize. The Science of the Total Environment 805: 150364. [DOI] [PubMed] [Google Scholar]
- Liu Y, Reich PB, Li G, Sun S.. 2011. Shifting phenology and abundance under experimental warming alters trophic relationships and plant reproductive capacity. Ecology 92: 1201–1207. [DOI] [PubMed] [Google Scholar]
- Lüdecke D. 2018. ggeffects: tidy data frames of marginal effects from regression models. Journal of Open Source Software 3: 772. [Google Scholar]
- Moeller DA. 2004. Facilitative interactions among plants via shared pollinators. Ecology 85: 3289–3301. [Google Scholar]
- Ne’eman G, Ne’eman R, Ellison AM.. 2006. Limits to reproductive success of Sarracenia purpurea (Sarraceniaceae). American Journal of Botany 93: 1660–1666. [DOI] [PubMed] [Google Scholar]
- Obeso JR. 2002. The costs of reproduction in plants. The New Phytologist 155: 321–348. [DOI] [PubMed] [Google Scholar]
- Ogilvie JE, CaraDonna PJ.. 2022. The shifting importance of abiotic and biotic factors across the life cycles of wild pollinators. The Journal of Animal Ecology 91: 2412–2423. [DOI] [PubMed] [Google Scholar]
- Ogilvie JE, Griffin SR, Gezon ZJ, et al. 2017. Interannual bumble bee abundance is driven by indirect climate effects on floral resource phenology. Ecology Letters 20: 1507–1515. [DOI] [PubMed] [Google Scholar]
- Onyemaobi O, Sangma H, Garg G, et al. 2021. Reproductive stage drought tolerance in wheat: importance of stomatal conductance and plant growth regulators. Genes 12: 1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan C, Hanley ME.. 2015. Plants and climate change: complexities and surprises. Annals of Botany 116: 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson GT, Gray ST, Ault T, et al. 2011. Climatic controls on the snowmelt hydrology of the Northern Rocky Mountains. Journal of Climate 24: 1666–1687. [Google Scholar]
- Petanidou T, Kallimanis AS, Tzanopoulos J, Sgardelis SP, Pantis JD.. 2008. Long-term observation of a pollination network: fluctuation in species and interactions, relative invariance of network structure and implications for estimates of specialization. Ecology Letters 11: 564–575. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2024. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Saavedra F, Inouye D, Price M, Harte J.. 2003. Changes in flowering and abundance of Delphinium nuttallianum (Ranunculaceae) in response to a subalpine climate warming experiment. Global Change Biology 9: 885–894. [Google Scholar]
- Saikkonen K, Faeth SH, Helander M, Sullivan TJ.. 1998. Fungal endophytes: a continuum of interactions with host plants. Annual Review of Ecology and Systematics 29: 319–343. [Google Scholar]
- Scaven VL, Rafferty NE.. 2013. Physiological effects of climate warming on flowering plants and insect pollinators and potential consequences for their interactions. Current Zoology 59: 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer A, Loy X, Morozumi C, Brosi BJ.. 2023. Differences in individual flowering time change pollen limitation and seed set in three montane wildflowers. American Journal of Botany 110: e16123. [DOI] [PubMed] [Google Scholar]
- Singer MC, Parmesan C.. 2010. Phenological asynchrony between herbivorous insects and their hosts: signal of climate change or pre-existing adaptive strategy? Philosophical Transactions of the Royal Society B: Biological Sciences 365: 3161–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletvold N, Grindeland JM, Ågren J.. 2010. Pollinator-mediated selection on floral display, spur length and flowering phenology in the deceptive orchid Dactylorhiza lapponica. The New Phytologist 188: 385–392. [DOI] [PubMed] [Google Scholar]
- Sloat LL, Henderson AN, Lamanna C, Enquist BJ.. 2015. The effect of the foresummer drought on carbon exchange in subalpine meadows. Ecosystems 18: 533–545. [Google Scholar]
- Tardieu F, Simonneau T, Parent B.. 2015. Modelling the coordination of the controls of stomatal aperture, transpiration, leaf growth, and abscisic acid: update and extension of the Tardieu–Davies model. Journal of Experimental Botany 66: 2227–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JD. 2010. Flowering phenology, fruiting success and progressive deterioration of pollination in an early-flowering geophyte. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 3187–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totland O. 2001. Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82: 2233–2244. [Google Scholar]
- Tredennick AT, Hooker G, Ellner SP, Adler PB.. 2021. A practical guide to selecting models for exploration, inference, and prediction in ecology. Ecology 102: e03336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waser NM. 1978. Competition for hummingbird pollination and sequential flowering in two Colorado wildflowers. Ecology 59: 934–944. [Google Scholar]
- Waser NM, Price MV.. 1981. Pollinator choice and stabilizing selection for flower color in Delphinium nelsonii. Evolution 35: 376–390. [DOI] [PubMed] [Google Scholar]
- Waser NM, Price MV.. 1985. Reciprocal transplant experiments with Delphinium nelsonii (Ranunculaceae): evidence for local adaptation. American Journal of Botany 72: 1726–1732. [Google Scholar]
- Waser NM, Price MV.. 1990. Pollination efficiency and effectiveness of bumble bees and hummingbirds visiting Delphinium nelsonii. Collectanea Botanica 19: 9–20. [Google Scholar]
- Zinn KE, Tunc-Ozdemir M, Harper JF.. 2010. Temperature stress and plant sexual reproduction: uncovering the weakest links. Journal of Experimental Botany 61: 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.