Abstract
Background and Aims
Stand-replacing crown fires are the most prevalent type of fire regime in boreal forests in North America. However, a substantial proportion of low-severity fires are found within fire perimeters. Here, we aimed to investigate the effects of low-severity fires on the reproductive potential and seedling recruitment in boreal forest stands in between stand-replacing fire events.
Methods
We recorded site and tree characteristics from 149 trees within 12 sites dominated by mature black spruce [Picea mariana (Mill.) B.S.P.] trees in the Northwest Territories, Canada. The presence of fire-scarred trees supported classification of sites as unburned or affected by low-severity fires in recent history. We used non-parametric tests to evaluate differences in site conditions between unburned and low-severity sites. We used linear and additive statistical models to evaluate differences in tree age, size and reproductive traits among unburned trees and trees from low-severity sites.
Key Results
The results showed a significantly higher density of dead black spruce trees in low-severity sites and marginally significant higher presence of permafrost. Trees from low-severity fire sites were significantly older, exhibited significantly lower tree growth and showed a tendency towards a higher probability of cone presence and percentage of open cones compared with trees from unburned sites. Surviving fire-scarred trees affected by more recent low-severity fires showed a tendency towards a higher probability of cone presence and cone production. The density of black spruce seedlings increased significantly with recent low-severity fires.
Conclusions
Trees in low-severity sites appeared to have escaped mortality from up to three fires, as indicated by fire-scar records and their older ages. Shallow permafrost at low-severity sites might cause lower flammability, allowing areas to act as fire refugia. Low-severity surface fires temporarily enhanced the reproductive capacity of surviving trees and the density of seedlings, probably as a stress response to fire events.
Keywords: Fire regime, fire scars, reproductive maturity, cone production, serotinous conifers
INTRODUCTION
The boreal biome composes one-third of the forested area of the world and stores 30–40 % of terrestrial carbon stocks (Bradshaw and Warkentin, 2015; Hayes et al., 2022). The boreal biome also contains extensive regions underlain by permafrost (Heginbottom et al., 1995; Gauthier et al., 2015) and is influenced by natural disturbances, such as repeated cycles of wildfires and vegetation recovery (Johnstone et al., 2010a). Fires in boreal North America are generally stand replacing, which leads to the mortality of most trees (Johnson, 1992; Walker et al., 2018). Specific adaptations of fire-adapted tree species include the retention of dead branches that are lichen covered and function as ladder fuels and the production of semi-serotinous cones (Van Cleve et al., 1983; Viereck, 1983; Pausas et al., 2017). Serotinous and semi-serotinous cones remain closed in the canopy for multiple years until opening is triggered by fire, releasing seeds in the initial years after a fire and facilitating self-replacement of serotinous conifers (Ilisson and Chen, 2009; Johnstone et al., 2009; Lamont et al., 2020). These evolved traits enhance post-fire recruitment, consistent with a hot-flammability strategy (Pausas et al., 2017). In this strategy, the offspring of fire-adapted species capitalize on the newly available space and favourable post-fire conditions. In boreal forests, fire creates optimal seedbeds by thinning organic soils, reducing competition from understorey vegetation including vascular and non-vascular plants, such as lichens and mosses, and warming the soil in these cold, high-latitude regions (Black and Bliss, 1980).
Fire severity (or burn severity) refers to the ecological impacts of fires on vegetation (above-ground) and soils (below-ground), including tree and vegetation mortality and changes in seedbed conditions that can impact the post-fire ecosystem response significantly (Keeley, 2009). High-intensity crown fires occur concurrently with burning of surface and ground layers (Viereck, 1983), resulting in a significant consumption of organic soils and below-ground carbon stocks (Walker et al., 2018). Despite the prevalence of high-intensity crown fires in Boreal North America, fire perimeters (especially large ones, e.g. >10 000 ha), contain heterogeneous burn patterns, i.e. different fire-severity classes, including a significant number of unburned islands (reviewed by Kolden et al., 2012). Unburned or minimally affected patches of vegetation within a fire perimeter can compose a substantial portion of the total enclosed area, accounting for ~20 % of burned area within fire perimeters in Alaska (Kasischke et al., 2002; Kolden et al., 2012; Veraverbeke et al., 2015). In Canada, fire-severity maps focused on surface organic layer consumption in the 2014 and 2015 fires in the Great Slave Lake area, Canada, found low severity to be the most abundant fire-severity class within fire perimeters (French et al., 2020).
Organic matter loss in low-severity surface fires is limited to scorching of tree stems and charring of surface litter -mosses and herbs-, leaving the surviving canopy trees with green needles and the soil organic layer (SOL) largely intact (Keeley, 2009). Because the canopy seed bank is less affected in unburned or low-severity fire areas, these provide an important source of seeds for post-fire regeneration (Turner et al., 1997; Arseneault, 2001).
Areas that experience localized low-severity fires are often at the edges of large stand-replacing fire perimeters (Johnson, 1992; Kolden et al., 2012), in open forest stands, where the potential for crown fire spread is limited, or near permanent features that limit fire spread, such as water bodies, topographic breaks or less flammable vegetation, such as peatlands (Héon et al., 2014). Additionally, forest stands underlain by permafrost maintain high surface soil moisture and cold soils, providing climatic buffering (temperature and moisture) against fire occurrence and severity (Stralberg et al., 2020). Surviving trees at the margin of unburned forest patches or within areas that experience localized surface fires often present fire scars (Héon et al., 2014). Fire scars are the product of heat damage to the cambium (Gutsell and Johnson, 1996) and provide high-resolution records to reconstruct the natural occurrence of low-severity fires (Swetnam et al., 1999; Falk et al., 2011).
Warming temperatures and changes in fire regime pose a risk to resilience of boreal forests to fire (Johnstone et al., 2016; Wang et al., 2020; Baltzer et al., 2021). In particular, short-interval and high-severity fires can prompt compositional shifts from dominant well-adapted conifers (e.g. black spruce [Picea mariana (Mill.) B.S.P.]) to deciduous dominance (e.g. Populus and Betula spp.) when excessive combustion of the SOL changes seedbed conditions to favour faster-growing tree species (Johnstone et al., 2010b; Baltzer et al., 2021). Additionally, severe canopy combustion can consume, kill or damage black spruce embryos within the aerial seedbank (Arseneault, 2001; Johnstone et al., 2009; Splawinski et al., 2019; Reid et al., 2023). Furthermore, increases in fire frequency can result in a higher proportion of younger stands burning, leading to greater immaturity risk (Keeley et al., 1999). When black spruce stands have insufficient time to reach reproductive maturity between fire cycles, i.e. to build up the aerial seedbank, it reduces post-fire recruitment potential and compromises the potential for self-replacement (Black and Bliss, 1980; Greene and Johnson, 1999; Viglas et al., 2013).
Although we know much about the impacts of high-intensity crown fires on the reproduction and self-replacement of dominant conifers, the impact of non-stand-replacing fires, such as low-severity fires, on boreal forest resilience has been understudied. Understanding the effects of low-severity fires on reproductive processes and the possible change in allocation patterns between reproduction and growth as a response to this stress (Lauder et al., 2019) is essential for anticipating the post-fire recovery trajectory of conifer-dominated boreal forests.
Here, we analysed the site conditions of naturally occurring, low-severity fires and their impact on the reproductive potential of surviving black spruce trees, one of the most dominant tree species in the boreal forests of western North America. Specifically: (1) we evaluated the site characteristics that were associated with low-severity burning; (2) we quantified natural black spruce regeneration (seedling recruitment); and (3) we compared differences in tree age, tree size [diameter at breast height (DBH) and annual radial growth] and reproductive attributes between trees at unburned sites and fire-scarred and non-scarred trees at low-severity fire sites. The results will offer an initial insight into where in the landscape these low-severity fires are more likely to occur, as well as their implications for the reproductive capacity of surviving black spruce trees. This information will improve our understanding of the impacts of low-severity fires on boreal forest resilience to subsequent disturbances.
MATERIALS AND METHODS
Sampled sites and data collection
The study area consists of unmanaged boreal forests in the discontinuous permafrost zone of the Taiga Plains ecoregion, spanning a latitudinal gradient of ~260 km along the Mackenzie Valley in the Northwest Territories, Canada. This region experiences large, stand-replacing fires that are not suppressed and is strongly dominated by even-aged stands of black spruce. White spruce [Picea glauca (Moench) Voss] and tamarack [Larix laricina (Du Roi) K. Koch] are also frequent in the region but rarely dominant. Reindeer lichens [Cladina spp. (Cladonia spp.)], feathermosses [e.g. Hylocomium splendens (Hedw.) Schimp. and Pleurozium shreberi (Willd. ex Brid.) Mitt.] and other mosses dominate the ground vegetation cover.
In July 2021 we established 20 monitoring sites across the ~260 km latitudinal gradient. In our sampling design, sites were selected based on their proximity to a series of boreholes instrumented with thermistor strings (Smith et al., 2009) and form part of a broader project linking ecosystem characteristics with permafrost thaw history. From the 20 sampled sites, we selected forest stands dominated by black spruce, with trees older than 30 years to avoid structurally immature trees (Viglas et al., 2013), and having <75 % of trees charred, corresponding to light or moderate burn severity classification according to Ruiz-Gallardo et al. (2004), i.e. non-stand-replacing fires. In total, we retained 12 sites that met our prerequisites.
At each site we established a 60 m2 belt transect (30 m × 2 m), running from south to north, in representative landscape areas and 30–50 m away from the boreholes to prevent disturbance in their measurements. Within the belt transects, we identified all stems taller than 1.3 m (breast height), measured their DBH and classified them as either alive or dead. Tree seedlings (individuals <1.3 m in height) were counted and the percentage ground cover was estimated in each of three 1 m2 quadrats established at 12 m intervals along the eastern side of the rectangular plot (at 0, 12 and 24 m). The SOL thickness was determined from three soil cores (60 cm in length) collected inside the belt transect at the same intervals as the quadrats. Site-level percentage cover by non-vascular plants and SOL thickness were calculated by taking the mean of the three quadrats. The dominant non-vascular functional group (i.e. reindeer lichens, feathermosses or other mosses) was determined using the mean non-vascular plant percentage cover. The presence of permafrost was determined at the time of sampling using a graduated steel rod (2 m in length) inserted into the soil at each site until it was met with resistance. Given that thaw was not at its maximal depth at the time of sampling, we used the presence or absence of permafrost rather than a measure of active layer thickness.
We sampled 149 living mature black spruce trees from these 12 sites, ranging from 6 to 18 living black spruce trees depending on the density of living trees at each site (Table 1). Trees were sampled outside the 60 m2 belt transects to avoid disturbing the monitoring sites in plots of variable size, ranging from 1.5 to 6.5 m2 (Table 1). Sampled trees represented the size distribution found within the belt transects. In each plot of variable size, we also counted all black spruce stems and seedlings. From each tree, we measured DBH and counted all the cones within the crown. Then, we cut the trees and collected a wood disc to study the presence or absence of fire scars, estimate tree ages and measure tree-ring widths. Trees were cut at the base to facilitate the study of fire scars and to estimate tree ages more accurately. All wood discs were sanded with different grits and scanned, using standard dendrochronological methods (Speer, 2010). In fire-scarred trees, we counted the number of fire scars per tree. Fire scars were dated by counting the number of rings from the last year of growth to the fire scar. Tree-ring widths were measured with CooRecorder (Cybis Elektronik & Data AB). Cross-dating of individual series was checked using CooRecorder and COFECHA (Holmes, 1983) programs.
Table 1.
Stand characteristics per site and means (in bold) for unburned and low-severity fire sampled sites. Abbreviations: BS, black spruce; SOL, soil organic layer. Shallow permafrost indicates permafrost detected within the top 2 m from the soil surface. Latitude and longitude coordinates correspond to the south-east corner of the 60 m2 belt transects.
| Site | Latitude | Longitude | Non-vascular functional group | Shallow permafrost | Variable plot radius (m2) | Sampled trees (n) | Fire-scarred trees (%) |
Mean fire scars (n) | Stand age (years) | Living BS trees (trees ha−1) | Dead BS trees (%) | Non-vascular plant cover (%) | BS regeneration (seedlings ha−1) | SOL thickness (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unburned | ||||||||||||||
| LS-02 | 64.429 | −124.733 | Feathermosses | No | 3.0 | 14 | 0 | 0 | 137 | 4,418 | 0 | 69.1 | 683 | 24.7 |
| BCN-01 | 65.403 | −127.318 | Feathermosses | No | 4.2 | 13 | 0 | 0 | 63 | 2,599 | 0.15 | 35.1 | 3,427 | 8.7 |
| OC-01 | 65.437 | −127.438 | Feathermosses | Yes | 3.8 | 15 | 0 | 0 | 67 | 4,176 | 0 | 78.4 | 503 | 23.7 |
| CL-01 | 65.896 | −128.240 | Reindeer lichen | No | 4.5 | 16 | 0 | 0 | 40 | 2,508 | 0 | 49.4 | 4,230 | 18.3 |
| JF-02 | 66.285 | −128.469 | Feathermosses | No | 3.2 | 12 | 0 | 0 | 63 | 3,071 | 0 | 45.8 | 12,123 | 8.7 |
| Mean | 0 | 0 | 74 | 3,354 | 0 | 55.6 | 4,193 | 16.2 | ||||||
| Low-severity fire | ||||||||||||||
| OFP-01 | 64.654 | −124.838 | Feathermosses | Yes | 3.5 | 18 | 55.6 | 1.3 | 140 | 3,656 | 0.15 | 61.8 | 507 | 26 |
| EC-01 | 65.520 | −127.621 | Reindeer lichen | Yes | 1.5 | 6 | 85.7 | 2.2 | 118 | 9,095 | 0.16 | 77.2 | 11,979 | 26.8 |
| HR-01 | 65.670 | −127.833 | Reindeer lichen | Yes | 6.5 | 14 | 92.9 | 1.0 | 87 | 726 | 15.16 | 70.5 | 2,293 | 38.0 |
| GL-01 | 65.747 | −127.888 | Reindeer lichen | Yes | 5.7 | 14 | 35.7 | 1.8 | 94 | 936 | 3.43 | 73.2 | 3,850 | 39.3 |
| FGHS-01 | 66.210 | −128.496 | Other mosses | No | 3.4 | 8 | 62.5 | 1.1 | 103 | 1,869 | 0.29 | 53.4 | 10,182 | 18.7 |
| PI-01 | 64.836 | −125.015 | Bare | Yes | 2.4 | 7 | 87.5 | 1.0 | 133 | 896 | 2.49 | 8.7 | 36,300 | 5.3 |
| PI-02 | 64.835 | −125.014 | Feathermosses | Yes | 2.6 | 12 | 41.7 | 1.6 | 132 | 3,447 | 1.60 | 56.5 | 9,293 | 30.7 |
| Mean | 64.0 | 1.4 | 115 | 1,575 | 4.59 | 57.3 | 10,629 | 26.4 | ||||||
For each site, the density of living and dead black spruce, in addition to the density of living black spruce seedlings, was calculated by compiling data from the belt transects or quadrats, respectively, with data from the plots of variable size.
Classification of trees and sites based on fire scars and fire history data
To determine whether our study sites were affected by low-severity fires, we studied the occurrence of fire-scarred trees at each site. We classified sites as unburned when there was no presence of fire-scarred trees, recognizing that these trees probably established after a stand-replacing fire in the past. We classified sites as affected by low-severity fires when there was the presence of at least two sampled trees that recorded fire scars during the same calendar year. Within the low-severity fire sites, trees with no signs of open (externally visible) or internal fire scars (Fig. 1) were classified as non-scarred trees, and trees with one or more fire scars were classified as fire-scarred trees (Supplementary Data Fig. S1). We used fire history records for the NWT (Government of the Northwest Territories and Canadian Forest Service) to confirm that the unburned sites had remained unaffected by forest fires since at least 1965, when historical fire data collection for the region began. We also checked the correspondence between fire-scar dates and recorded fire history in low-severity fire sites.
Fig. 1.
(A) Representative site (HR-01) affected by a fire in 1993, with a mix of dead and surviving black spruce trees (Table 1; Supplementary Data Fig. S1). (B–D) Examples of black spruce wood sections sampled with internal (B), semi-open (C) and open fire scars (D).
Statistical analyses at the site level
At the site level, we used the unpaired two-sample Wilcoxon test (non-parametric test for non-normally distributed data) to determine whether the density of living black spruce trees per hectare, proportion of dead black spruce trees, percentage of non-vascular plant cover, SOL thickness and density of black spruce seedlings per hectare (black spruce regeneration) differed significantly between the two-level factor site type, i.e. unburned or low-severity sites. We used the Fisher’s exact test to determine whether the presence of shallow permafrost differed between unburned and low-severity sites. Significant differences are reported for P < 0.05, but tendencies towards significance are also noted (0.05 < P < 0.1) because of low sample sizes.
To assess the effect of the time after the last low-severity fire on stand variables at low-severity fire sites, we ran Pearson correlation analyses of time after the last low-severity fire, defined as the most recent fire recorded in at least two trees per site (Supplementary Data Fig. S1), with the following stand variables: density of living black spruce trees per hectare, proportion of dead black spruce trees, density of black spruce seedlings per hectare (black spruce regeneration), percentage cover of non-vascular plants and SOL thickness.
Statistical models for tree age, DBH, annual tree growth and reproductive variables
We used linear mixed models (LMMs) to determine whether tree age and DBH differed significantly with a three-level factor fire impact (fixed effect), i.e. trees from unburned stands (unburned) and trees from low-severity fire sites with (fire-scarred) and without (non-scarred) fire scars. Site was included as a random effect to account for spatial variability.
We used a generalized additive mixed model (GAMM) to determine whether annual tree-ring width (TRW) differed significantly with the three-level factor fire impact, while controlling for size and age effects. Fixed effects for the tree growth GAMM included the three-level factor fire impact (unburned, non-scarred and fire-scarred trees) and the annual cumulative diameter and cambial age (ring number from the pith) using smooth terms, ‘s’ (Wood, 2017). We used the natural logarithm of TRW as the response variable because of the skewed distributions of their values. Tree identity was included as random effect to account for repeated measures within each tree. Site was not included as a random effect in the TRW GAMM owing to lower significance. To account for temporal autocorrelation within trees, akin to traditional detrending methods, we used the continuous autocorrelation function corCAR1 of the R package mgcv (Wood, 2017).
We used general linear models (GLMs) with a binomial response to model the probability of cone presence (reproductive maturity) and linear models (LMs) to model cone production and the percentage of open cones. To calculate reproductive maturity, we created a binary variable: one for trees with presence of cones in the canopy at the time of sampling and zero for trees with no cones at the time of sampling. Cones were categorized as closed or open, and estimates of cone production summed both categories per tree. To calculate the percentage of open cones per tree, we divided the number of open cones by the total cone production. Trees without cones were excluded from the analysis of cone production and the percentage of open cones. Fixed effects for the three models included tree age, DBH (log-transformed) and the three-level factor fire impact. Site was not included as a random effect in the reproductive models owing to lower significance and convergence issues when tested in GLMMs or LMMs using the same fixed effects as described above. Likewise, we avoided including other stand variables as predictors in the models owing to the limited sample size. An additional set of models used only fire-scarred trees to assess the effect of the time after the last low-severity fire on reproductive maturity (GLM with a binomial response) and the cone production and percentage of open cones (LMs), including as fixed effects tree age, DBH (log-transformed) and the date of the last fire recorded in each tree. To select the fixed variables resulting in the best-performing reproductive models, we used a stepwise procedure for model selection based on the Akaike information criterion (AIC) using the function stepAIC from the R package MASS (Venables and Ripley, 2002).
The LMMs, GLMs and LMs were fitted with the R package glmmTMB (Brooks et al., 2017). The TRW GAMM was fitted using function gamm of the R package mgcv (Wood, 2017). The model validation for LMMs, GLMs and LMs, including testing the assumptions of normally distributed model residuals, was conducted using the simulateResiduals function available in the R package DHARMa (Hartig, 2020), and homogeneity of variance across the three-level factor fire impact was assessed with the function leveneTest from the R package car (Fox and Weisberg, 2019). Statistical analyses were done using R statistical software v.4.1.2.
In models where the three-level factor fire impact variable resulted in a significant factor (P < 0.05), we examined significant differences among levels using post-hoc comparisons of pairwise differences with the R package emmeans (Lenth, 2022) for LMMS, GLMs and LMs, and using Wald tests with the wald_gam function from the itsadug package (van Rij et al., 2020) for the TRW GAMM.
RESULTS
Stand- and tree-level differences between low-severity fire and unburned sites
Seven sites comprised mature black spruce stands with presence of multiple fire-scarred trees that showed evidence of previous passages of natural low-severity fires. The remaining five sampled sites represented mature black spruce stands with no evidence of fire scarring that served as control or ‘unburned’ sites. In five of the seven low-severity fire sites, the forest fire dates obtained from the analysis of fire-scarred trees matched the information from fire history data. However, the correspondence between fire scar and fire history-based dates was not perfect, and in general, our fire-scarred records detected more fires per site than the fire history data. The occurrence of fire-scarred trees in low-severity sites ranged from 26 to 100 % of sampled trees. The mean number of fire scars in fire-scarred trees was 1.4, with some trees recording up to three fire scars (Table 1; Supplementary Data Fig. S1).
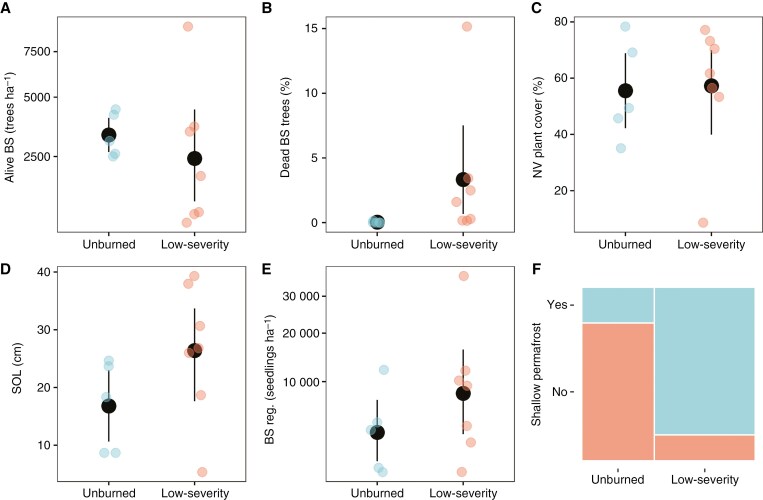
Unburned and low-severity fire sites exhibited similar densities of living black spruce trees and seedlings, a similar percentage of non-vascular plant cover and similar SOL thickness (Wilcoxon test, P > 0.1; Fig. 2A, C–E). In most of the unburned sites, feathermosses dominated the ground cover, whereas in low-severity fire sites there was no clear dominance, with sites dominated by reindeer lichen, feathermosses or bare soil (Table 1). However, low-severity fire sites exhibited a significantly higher proportion of dead black spruce trees (Wilcoxon test, P < 0.005) and a marginally significant higher presence of shallow permafrost (Fisher’s test, P < 0.1; Fig. 2B, F).
Fig. 2.
(A–E) Mean values and confidence intervals from living black spruce trees per hectare (A), percentage of dead black spruce (BS) trees (B), percentage of non-vascular (NV) plant cover (C), soil organic layer (SOL) thickness (D) and log-transformed black spruce regeneration (number of seedlings per hectare; E) for unburned and low-severity fire sites. (F) Proportion of sites with presence or absence of shallow permafrost for unburned and low-severity fire sites. In A and E, we have incorporated square root separations in the y-axis to enhance visualization.
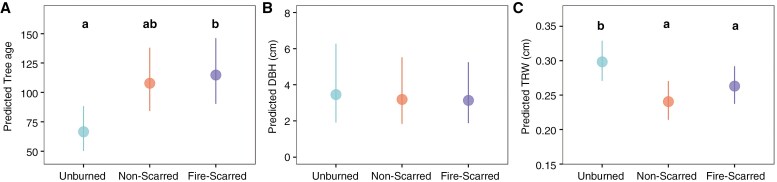
Tree age LMM and TRW GAMM results confirmed that low-severity fires had a significant effect (P < 0.05) on these two variables. Post-hoc tests indicated that fire-scarred trees were significantly (P < 0.05) older than unburned trees, whereas non-scarred trees showed no significant differences in tree age between fire-scarred and unburned trees (Fig. 3A; Supplementary Data Table S1A). Wald tests revealed significantly greater growth (P < 0.05) in unburned trees compared with non-scarred and fire-scarred trees. The DBH LMM results showed no significant differences in DBH among the three-level fire history factor (Fig. 3B; Supplementary Data Table S1B). Fig. 3C; Supplementary Data Table S1C
Fig. 3.
Predicted tree age (A), diameter at breast height (DBH; B) and tree-ring widths (TRW; C) (for output of models, see Supplementary Data Table S1). For TRW predictions, other fixed effects in the model are held constant. Lowercase letters indicate significant differences on post-hoc tests.
Drivers of reproductive maturity, cone production and cone opening in low-severity fire and unburned sites
We found that 63.5 % of the sampled trees were reproductive at the time of sampling. The probability of cone presence and cone production (number of cones per tree) increased with larger diameters and decreased with tree age, within our sample population of trees >30 years of age (the minimum tree age sampled) (Supplementary Data Table S2A, B; Fig. S2A–D). The percentage of open cones increased with DBH (Supplementary Data Table S2C; Fig. S2E).
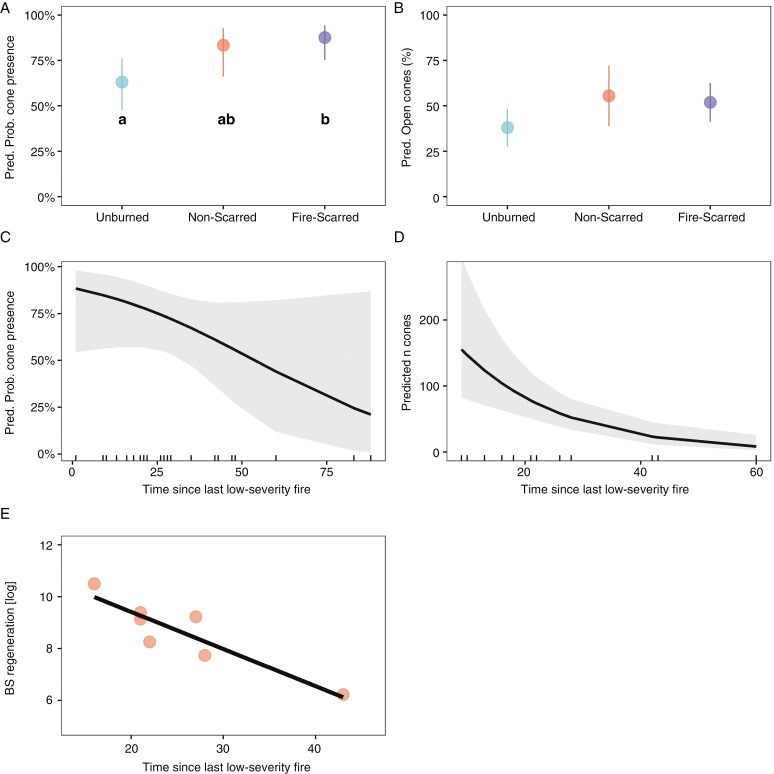
Stepwise model selection indicated a significant impact of fire history in the probability of cone presence GLM and in the percentage of open cones LM (Supplementary Data Table S2A, C). As such, we found that the probability of cone presence and the percentage of open cones increased in trees sampled from low-severity fire sites. Post-hoc tests revealed significantly higher probability of cone presence in fire-scarred trees than in trees from unburned sites, whereas non-scarred trees from low-severity fire sites did not differ from unburned and fire-scarred trees (Fig. 4A, B). Post-hoc tests showed no significant differences among the three-level fire impact factor in the percentage of open cones, despite the inclusion of this factor in the stepwise model selection.
Fig. 4.
(A, B) Predicted probability of cone presence (A) and predicted percentage of open cones (B) for trees from unburned stands (unburned) and trees with (fire-scarred) and without (non-scarred) fire scars from low-severity fire sites. (C, D) Predicted probability of cone presence (C) and predicted number of cones (cone production; D) as a function of time since last low-severity fire (for output of models, see Supplementary Data Tables S2 and S3). For each relationship shown, other fixed effects in the models were held constant; numerical terms were set to their means and factors to their reference level. (E) Log-transformed black spruce regeneration (number of tree seedlings per hectare) as a function of time since last low-severity fire.
Differences in reproductive attributes with time after the last low-severity fire
Time after the last low-severity fire was a selected variable in the probability of cone presence GLM and in the cone production LM stepwise selection processes (Supplementary Data Table S3A, B). As such, we found that the probability of cone presence and cone production in fire-scarred trees decreased with time after the last low-severity fire (significant for cone production, P < 0.001; Fig. 4C, D). For the percentage of open cones LM, the null model (model with no predictors) was the selected one in the stepwise model selection (Supplementary Data Table S3C).
The density of black spruce seedlings in low-severity fire sites increased significantly (r = 0.91, d.f. = 5, P < 0.05) with more recent fires (Fig. 4E). The density of living black spruce trees per hectare, proportion of dead black spruce trees, percentage of non-vascular plant cover and SOL thickness variables showed no significant correlations with time after the last low-severity fire.
DISCUSSION
Low-intensity fire represents a low-severity disturbance that, nevertheless, impacts the reproductive potential of black spruce stands, here represented by cone production and seedling recruitment. Fire-scarred trees were significantly older, displayed significantly lower radial growth and had a higher probability of cone presence and higher proportion of open cones than unburned trees. In addition, low-severity fire sites showed significantly higher seedling density than unburned sites. Thereby, low-severity fires seemed to stimulate increased reproductive capacity of surviving black spruce trees and advance regeneration of seedlings (forest infilling). It is noteworthy that the effects of fire history on reproductive maturity and tree-level cone production decreased significantly with time, indicating that low-severity fires create a pulsed change in reproductive potential. These results are likely to be important for the dynamics of boreal conifer post-fire recovery, owing to the large extent of burned area classified as low severity (French et al., 2020).
Stand-level differences between low-severity fire and unburned sites
Stand-replacing fire intervals can vary from 50 to 200 years in much of Alaska and western Canada (Viereck, 1983; Johnstone et al., 2010b). However, northern boreal wildfires tend to create a mosaic of different fire severities (Arseneault, 2001; Kolden et al., 2012; French et al., 2020; Guindon et al., 2021). In this study, we found evidence indicating the passage of low-severity fires since the last stand-replacing fire. Here, fire-scarred trees experienced between one and three stand-replacing fires, as recorded by the presence of up to three fire scars, some of them in the form of internal fire scars (Fig. 1; Supplementary Data Fig. S1). Multiple fire scars on individual trees are common because exposed wood wounds, produced after a tree compartmentalizes the fire injury, tend to be more prone to burning compared with the remaining bole. This is attributable to scar wounds having thinner bark, combined with the enhanced flammability of exposed wood and seeping resin within previously formed wounds (Swetnam et al., 1999; Falk et al., 2011).
Fire-scarred trees typically result from low-severity fires, frequently occurring at the edges of higher-severity patches. Here, within low-severity fire sites, we observed a pattern of more open stands with a higher percentage of dead trees, which might limit fuel availability. Additionally, we noted the presence of shallow permafrost, which is indicative of poor drainage (Bill et al., 2023) and low flammability (Pausas et al., 2017). Such characteristics contribute to the maintenance of cool, moist soils that could help to resist severe burning, except during the most severe fire-weather conditions (Johnstone et al., 2010b), and as such can be considered fire refugia (sensu Krawchuk et al., 2016: ‘places that are disturbed less frequently or less severely by wildfire’). Therefore, fire-scarred trees can also potentially be found in fire refugia within fire perimeters (Stralberg et al., 2020). This is a plausible explanation, because fire-scarred trees were significantly older than unburned trees.
Lower tree growth in low-severity fire sites is most probably linked to the challenges posed by the presence of permafrost on those sites, because cold and poorly drained soils can limit tree growth (e.g. González De Andrés et al., 2022). The lack of differences in tree growth between non-scarred and fire-scarred trees in low-severity fire sites aligns with previous research, in which natural or prescribed low-severity fires were found to have either short-term impacts or no impact at all on tree growth (Sala et al., 2005; Valor et al., 2015; Alfaro-Sánchez et al., 2016; Bottero et al., 2017; Espinosa et al., 2021).
Low-severity fires sites tend to favour black spruce regeneration (Johnston, 1977), mainly owing to the openings in the stands after the passage of low-intensity fires that burned part of the above-ground vegetation. Arseneault (2001) found that low-severity fires in boreal forests of eastern Canada resulted in a greater abundance of reindeer lichen within the lichen mat. Here, we found a predominance of reindeer lichen in three of the seven low-severity sites. However, we acknowledge the limited sample size of this study and recognize the need for further research to determine the specific site characteristics that promote or facilitate low-intensity fires, which lead to these low-severity disturbances and their ecological consequences. In contrast, unburned sites typically lacked permafrost and had a dominance in the understorey of feathermosses, which are indicators of later-successional stages that are more prone to active crown fires (Viereck, 1983). On these unburned sites, trees were significantly younger than fire-scarred trees but had similar DBH because they grew faster than trees from low-severity fire sites (fire-scarred or non-scarred trees).
Effects of low-severity fires on tree-level reproductive variables between stand-replacing fire cycles
All our sampled trees were ≥30 years old, and approximately two-thirds of them were reproductive. In northern boreal forests with relatively short fire-return intervals, tree species with an earlier onset in cone production, such as black spruce but especially jack pine (Pinus banksiana Lamb.), or resprouting strategies used by broadleaf species, have a successional advantage in the face of shorter fire-return intervals. Black spruce is semi-serotinous, which differs from fully serotinous species, such as jack pine, in that their cones open gradually over time even in the absence of fire. Here, we found a decline in the number of reproductive trees and in cone production with tree age. In contrast, when comparing trees with similar ages, larger trees exhibited earlier onset in reproduction, greater cone production and higher cone opening (Viglas et al., 2013; Alfaro-Sánchez et al., 2015, 2022; Andrus et al., 2020; Rodman et al., 2021).
Higher reproductive potential after recent low-severity fires (for certain variables studied and depending on tree fire damage) might be attributed to increased soil temperatures and/or a short pulse of increased soil resource availability after fire (Van Cleve et al., 1983; Certini, 2005). Low-severity fires also leave a legacy of more dead trees in a stand, which affects stand structure and habitat for organisms using dead wood. Fire-induced thinning by low-severity fires is likely to generate more open stands with higher light availability, resulting from partial stand mortality. This, in turn, also temporarily increases resource availability, potentially triggering an increased allocation to reproduction (Alfaro-Sánchez et al., 2015). However, fire scars do damage the tree (Arbellay et al., 2017), and it is more likely that this stress stimulates a response that increases allocation to reproduction (Roff, 1993; Santos-del-Blanco et al., 2013; Alfaro-Sánchez et al., 2022). Reproductive investment is often at the expense of other types of resource investment, such as tree growth (Obeso, 2002), which is consistent with our findings of significantly lower growth in low-severity fires. However, our study did not examine the short-term impacts of low-severity fires on tree growth and, as such, cannot discern whether the primary driver of increased cone production is the stress associated with fire damage, positive post-fire effects on competition or nutrients, or life-history stress associated with the presence of permafrost.
Conclusions
Black spruce forests are characterized by having a natural fire regime consisting of stand-replacing fires. However, unburned or lightly burned patches of forest are often found in the edge and within fire perimeters, owing to heterogeneous burn patterns. These patches play an important role in successional processes and ecosystem dynamics (Kolden et al., 2012; Stralberg et al., 2020). Current and future trajectories in boreal forest composition, structure and function are dictated by complex interactions and feedbacks among climate warming, wildfire, hydrology and permafrost thaw (Baltzer et al., 2014, 2021; Carpino et al., 2018). In recent decades, the accelerated rate of warming in the boreal biome has resulted in an increase in fire frequency and extent (Kasischke and Turetsky, 2006; Coops et al., 2018). An increase in the spatial extent of high-severity (stand-replacing) fires could reduce the area of unburned or lightly burned patches within the fire perimeter (Kolden et al., 2012), but might also lead to larger areas being affected by low-severity fires in the edges of fire perimeters. For fire-adapted species, such as black spruce, changes in the fire regime might lead to shifts in successional dynamics, potentially limiting reproductive success (Johnstone et al., 2010b). Understanding the prevalence of low-severity fires in northern boreal forests, in addition to their impact on the reproductive potential and regeneration dynamics of affected areas, will improve our projections of tree recruitment dynamics and shifts in species composition in response to climate warming and changing fire activity.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Table S1: estimated coefficients, standard errors (SE), P-values and R2 obtained in linear mixed models (LMMs) for tree age (A) and diameter at breast height (DBH; B). Table S2: estimated coefficients, standard errors (SE), P-values and R2 obtained in a binomial general linear model for the probability of cone presence (A) and in linear models for the number of cones (in reproductive trees; B) and the percentage of open cones (C). Table S3: estimated coefficients, standard errors (SE), P-values and R2 obtained in a binomial general linear model for the probability of cone presence (A) and in linear models for the number of cones (in reproductive trees; B) and for the percentage of open cones (C). Figure S1: vertical coloured solid lines indicate fire scars detected in black spruce trees in seven different sites. Figure S2: predicted probability of cone presence, as a function of DBH (A) and tree age (B).
ACKNOWLEDGEMENTS
We thank numerous students and technicians for field and laboratory assistance. We thank the GNWT Aurora Research Institute (NWT Scientific Research License #16816). The Wilfrid Laurier University–GNWT Partnership Agreement was instrumental in providing logistical support and laboratory space.
Contributor Information
Raquel Alfaro-Sánchez, Higher Technical School of Agricultural and Forestry Engineering and Biotechnology, University of Castilla-La Mancha, Albacete, Spain; Department of Biology, Wilfrid Laurier University, Waterloo, ON, Canada.
Jill F Johnstone, YukonU Research Centre, Yukon University, Whitehorse, YT, Canada; Institute of Arctic Biology, University of Alaska Fairbanks, Fairbanks, AK, USA.
Jennifer L Baltzer, Department of Biology, Wilfrid Laurier University, Waterloo, ON, Canada.
FUNDING
R.A.S. was supported by the María Zambrano postdoctoral research program MZ2021 and the British Ecological Society grant SR21/1291. J.L.B. was supported by the Canada Research Chairs Program. Field sampling was supported by the Government of the Northwest Territories Environmental Studies Research Fund, Natural Science and Engineering Research Council Discovery Grant and Northern Research Supplement Funding, Polar Continental Shelf Program and Northern Student Training Program.
LITERATURE CITED
- Alfaro-Sánchez R, Camarero JJ, López-Serrano FR, Sánchez-Salguero R, Moya D, Heras JDL. 2015. Positive coupling between growth and reproduction in young post-fire Aleppo pines depends on climate and site conditions. International Journal of Wildland Fire 24: 507–517. [Google Scholar]
- Alfaro-Sánchez R, Camarero JJ, Sánchez-Salguero R, Sangüesa-Barreda G, De Las Heras J. 2016. Post-fire Aleppo pine growth, C and N isotope composition depend on site dryness. Trees 30: 581–595. [Google Scholar]
- Alfaro-Sánchez R, Johnstone JF, Cumming SG, et al. 2022. What drives reproductive maturity and efficiency in serotinous boreal conifers? Frontiers in Ecology and Evolution 10: 869130. [Google Scholar]
- Andrus RA, Harvey BJ, Hoffman A, Veblen TT. 2020. Reproductive maturity and cone abundance vary with tree size and stand basal area for two widely distributed conifers. Ecosphere 11: e03092. [Google Scholar]
- Arbellay E, Daniels LD, Mansfield SD, Chang AS. 2017. Cambial injury in lodgepole pine (Pinus contorta): mountain pine beetle vs fire. Tree Physiology 37: 1611–1621. [DOI] [PubMed] [Google Scholar]
- Arseneault D. 2001. Impact of fire behavior on postfire forest development in a homogeneous boreal landscape. Canadian Journal of Forest Research 31: 1367–1374. [Google Scholar]
- Baltzer JL, Veness T, Chasmer LE, Sniderhan AE, Quinton WL. 2014. Forests on thawing permafrost: fragmentation, edge effects, and net forest loss. Global Change Biology 20: 824–834. [DOI] [PubMed] [Google Scholar]
- Baltzer JL, Day NJ, Walker XJ, et al. 2021. Increasing fire and the decline of fire adapted black spruce in the boreal forest. Proceedings of the National Academy of Sciences of the United States of America 118: e2024872118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill KE, Dieleman CM, Baltzer JL, et al. 2023. Post-fire recovery of soil organic layer carbon in Canadian boreal forests. Ecosystems 26: 1623–1639. [Google Scholar]
- Black RA, Bliss LC. 1980. Reproductive ecology of Picea mariana (Mill.) BSP., at tree line near Inuvik, Northwest Territories, Canada. Ecological Monographs 50: 331–354. [Google Scholar]
- Bottero A, D’Amato AW, Palik BJ, Kern CC, Bradford JB, Scherer SS. 2017. Influence of repeated prescribed fire on tree growth and mortality in Pinus resinosa forests, Northern Minnesota. Forest Science 63: 94–100. [Google Scholar]
- Bradshaw CJA, Warkentin IG. 2015. Global estimates of boreal forest carbon stocks and flux. Global and Planetary Change 128: 24–30. [Google Scholar]
- Brooks ME, Kristensen K, van Benthem KJ, et al. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9: 378–400. [Google Scholar]
- Carpino OA, Berg AA, Quinton WL, Adams JR. 2018. Climate change and permafrost thaw-induced boreal forest loss in Northwestern Canada. Environmental Research Letters 13: 084018. [Google Scholar]
- Certini G. 2005. Effects of fire on properties of forest soils: a review. Oecologia 143: 1–10. [DOI] [PubMed] [Google Scholar]
- Coops NC, Hermosilla T, Wulder MA, White JC, Bolton DK. 2018. A thirty year, fine-scale, characterization of area burned in Canadian forests shows evidence of regionally increasing trends in the last decade. PLoS One 13: e0197218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa J, Martin-Benito D, Rodríguez De Rivera O, Hernando C, Guijarro M, Madrigal J. 2021. Tree growth response to low-intensity prescribed burning in Pinus nigra stands: effects of burn season and fire severity. Applied Sciences 11: 7462. [Google Scholar]
- Falk DA, Heyerdahl EK, Brown PM, et al. 2011. Multi-scale controls of historical forest-fire regimes: new insights from fire-scar networks. Frontiers in Ecology and the Environment 9: 446–454. [Google Scholar]
- Fox J, Weisberg S. 2019. An R companion to applied regression. Thousand Oaks: Sage. [Google Scholar]
- French NHF, Graham J, Whitman E, Bourgeau-Chavez LL. 2020. Quantifying surface severity of the 2014 and 2015 fires in the Great Slave Lake area of Canada. International Journal of Wildland Fire 29: 892–906. [Google Scholar]
- Gauthier S, Bernier P, Kuuluvainen T, Shvidenko AZ, Schepaschenko DG. 2015. Boreal forest health and global change. Science 349: 819–822. [DOI] [PubMed] [Google Scholar]
- González De Andrés E, Shestakova TA, Scholten RC, Delcourt CJF, Gorina NV, Camarero JJ. 2022. Changes in tree growth synchrony and resilience in Siberian Pinus sylvestris forests are modulated by fire dynamics and ecohydrological conditions. Agricultural and Forest Meteorology 312: 108712. [Google Scholar]
- Greene DF, Johnson EA. 1999. Modelling recruitment of Populus tremuloides, Pinus banksiana, and Picea mariana following fire in the mixedwood boreal forest. Canadian Journal of Forest Research 29: 462–473. [Google Scholar]
- Guindon L, Gauthier S, Manka F, et al. 2021. Trends in wildfire burn severity across Canada, 1985 to 2015. Canadian Journal of Forest Research 51: 1230–1244. [Google Scholar]
- Gutsell SL, Johnson EA. 1996. How fire scars are formed: coupling a disturbance process to its ecological effect. Canadian Journal of Forest Research 26: 166–174. [Google Scholar]
- Hartig F. 2020. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. https://CRAN.R-project.org/package=DHARMa (11 March 2021, date last accessed). [Google Scholar]
- Hayes DJ, Butman DE, Domke GM, Fisher JB, Neigh CSR, Welp LR. 2022. Boreal forests. In: Balancing greenhouse gas budgets. Cambridge, MA: Elsevier, 203–236. [Google Scholar]
- Heginbottom JA, Dubreuil MA, Harker PA. 1995. Canada-permafrost. In: National Atlas of Canada, 5th edn. Ottawa, Canada: MCR 4177, Plate 2.1. doi: https://doi.org/ 10.4095/294672. [DOI] [Google Scholar]
- Héon J, Arseneault D, Parisien M-A. 2014. Resistance of the boreal forest to high burn rates. Proceedings of the National Academy of Sciences of the United States of America 111: 13888–13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes RL. 1983. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bulletin 43: 69–78. [Google Scholar]
- Ilisson T, Chen HYH. 2009. The direct regeneration hypothesis in northern forests. Journal of Vegetation Science 20: 735–744. [Google Scholar]
- Johnson EA. 1992. Fire and vegetation dynamics: studies from the North American boreal forest. Cambridge and New York: Cambridge University Press. [Google Scholar]
- Johnston WF. 1977. Manager’s handbook for black spruce in the north-central states. St. Paul: US Department of Agriculture, Forest Service, North Central Forest Experiment Station. [Google Scholar]
- Johnstone JF, Boby LA, Tissier E, Mack MC, Verbyla DL, Walker X. 2009. Postfire seed rain of black spruce, a semiserotinous conifer, in forests of interior Alaska. Canadian Journal of Forest Research 39: 1575–1588. [Google Scholar]
- Johnstone JF, Allen CD, Franklin JF, et al. 2016. Changing disturbance regimes, ecological memory, and forest resilience. Frontiers in Ecology and the Environment 14: 369–378. [Google Scholar]
- Johnstone JF, Chapin FS, Hollingsworth TN, Mack MC, Romanovsky V, Turetsky M. 2010a. Fire, climate change, and forest resilience in interior Alaska. Canadian Journal of Forest Research 40: 1302–1312. [Google Scholar]
- Johnstone JF, Hollingsworth TN, Chapin FS, Mack MC. 2010b. Changes in fire regime break the legacy lock on successional trajectories in Alaskan boreal forest. Global Change Biology 16: 1281–1295. [Google Scholar]
- Kasischke ES, Turetsky MR. 2006. Recent changes in the fire regime across the North American boreal region—spatial and temporal patterns of burning across Canada and Alaska. Geophysical Research Letters 33: L09703. [Google Scholar]
- Kasischke ES, Williams D, Barry D. 2002. Analysis of the patterns of large fires in the boreal forest region of Alaska. International Journal of Wildland Fire 11: 131–144. [Google Scholar]
- Keeley JE. 2009. Fire intensity, fire severity and burn severity: a brief review and suggested usage. International Journal of Wildland Fire 18: 116–126. [Google Scholar]
- Keeley JE, Ne’eman G, Fotheringham CJ. 1999. Immaturity risk in a fire-dependent pine. Journal of Mediterranean Ecology 1: 41–48. [Google Scholar]
- Kolden CA, Lutz JA, Key CH, Kane JT, Van Wagtendonk JW. 2012. Mapped versus actual burned area within wildfire perimeters: characterizing the unburned. Forest Ecology and Management 286: 38–47. [Google Scholar]
- Krawchuk MA, Haire SL, Coop J, et al. 2016. Topographic and fire weather controls of fire refugia in forested ecosystems of northwestern North America. Ecosphere 7: e01632. [Google Scholar]
- Lamont BB, Pausas JG, He T, Witkowski ETF, Hanley ME. 2020. Fire as a selective agent for both serotiny and nonserotiny over space and time. Critical Reviews in Plant Sciences 39: 140–172. [Google Scholar]
- Lauder JD, Moran EV, Hart SC. 2019. Fight or flight? Potential tradeoffs between drought defense and reproduction in conifers. Tree Physiology 39: 1071–1085. [DOI] [PubMed] [Google Scholar]
- Lenth RV. 2022. emmeans: estimated marginal means, aka least-squares means. [Google Scholar]
- Obeso JR. 2002. The costs of reproduction in plants. The New Phytologist 155: 321–348. [DOI] [PubMed] [Google Scholar]
- Pausas JG, Keeley JE, Schwilk DW. 2017. Flammability as an ecological and evolutionary driver. Journal of Ecology 105: 289–297. [Google Scholar]
- Reid KA, Day NJ, Alfaro-Sánchez R, et al. 2023. Black spruce (Picea mariana) seed availability and viability in boreal forests after large wildfires. Annals of Forest Science 80: 4. [Google Scholar]
- Rodman KC, Veblen TT, Andrus RA, et al. 2021. A trait-based approach to assessing resistance and resilience to wildfire in two iconic North American conifers. Journal of Ecology 109: 313–326. [Google Scholar]
- Roff D. 1993. Evolution of life histories: theory and analysis. New York, NY: Springer US. [Google Scholar]
- Ruiz-Gallardo JR, Castaño S, Calera A. 2004. Application of remote sensing and GIS to locate priority intervention areas after wildland fires in Mediterranean systems: a case study from south-eastern Spain. International Journal of Wildland Fire 13: 241–252. [Google Scholar]
- Sala A, Peters GD, McIntyre LR, Harrington MG. 2005. Physiological responses of ponderosa pine in western Montana to thinning, prescribed fire and burning season. Tree Physiology 25: 339–348. [DOI] [PubMed] [Google Scholar]
- Santos-del-Blanco L, Bonser SP, Valladares F, Chambel MR, Climent J. 2013. Plasticity in reproduction and growth among 52 range-wide populations of a Mediterranean conifer: adaptive responses to environmental stress. Journal of Evolutionary Biology 26: 1912–1924. [DOI] [PubMed] [Google Scholar]
- Smith SL, Chartrand J, Nguyen TN, Riseborough DW, Ednie M, Ye S. 2009. Geotechnical database and descriptions of permafrost monitoring sites established 2006–07 in the central and southern Mackenzie Corridor; Geological Survey of Canada, Open File 6041, 183p. [Google Scholar]
- Speer JH. 2010. Fundamentals of tree-ring research. Tucson, AZ: The University of Arizona Press, 521. [Google Scholar]
- Splawinski TB, Greene DF, Michaletz ST, Gauthier S, Houle D, Bergeron Y. 2019. Position of cones within cone clusters determines seed survival in black spruce during wildfire. Canadian Journal of Forest Research 49: 121–127. [Google Scholar]
- Stralberg D, Arseneault D, Baltzer JL, et al. 2020. Climate-change refugia in boreal North America: what, where, and for how long? Frontiers in Ecology and the Environment 18: 261–270. [Google Scholar]
- Swetnam TW, Allen CD, Betancourt JL. 1999. Applied historical ecology: using the past to manage for the future. Ecological Applications 9: 1189–1206. [Google Scholar]
- Turner MG, Romme WH, Gardner RH, Hargrove WW. 1997. Effects of fire size and pattern on early succession in Yellowstone National Park. Ecological Monographs 67: 411–433. [Google Scholar]
- Valor T, González-Olabarria JR, Piqué M. 2015. Assessing the impact of prescribed burning on the growth of European pines. Forest Ecology and Management 343: 101–109. [Google Scholar]
- Van Cleve K, Dyrness CT, Viereck LA, Fox J, Chapin FS, Oechel W. 1983. Taiga ecosystems in interior Alaska. BioScience 33: 39–44. [Google Scholar]
- van Rij J, Wieling M, Baayen RH, van Rijn H. 2020. itsadug: interpreting time series and autocorrelated data using GAMMs. https://CRAN.R-project.org/package=itsadug (13 October 2023, date last accessed). [Google Scholar]
- Venables WN, Ripley BD. 2002. Modern applied statistics with S. New York: Springer. [Google Scholar]
- Veraverbeke S, Rogers BM, Randerson JT. 2015. Daily burned area and carbon emissions from boreal fires in Alaska. Biogeosciences 12: 3579–3601. [Google Scholar]
- Viereck LA. 1983. The effects of fire in black spruce ecosystems of Alaska and Northern Canada. Pages 201-220. In: Wein RW, MacLean DA. eds. The role of fire in northern circumpolar ecosystems. New York: John Wiley and Sons. 322p. [Google Scholar]
- Viglas JN, Brown CD, Johnstone JF. 2013. Age and size effects on seed productivity of northern black spruce. Canadian Journal of Forest Research 43: 534–543. [Google Scholar]
- Walker XJ, Rogers BM, Baltzer JL, et al. 2018. Cross-scale controls on carbon emissions from boreal forest megafires. Global Change Biology 24: 4251–4265. [DOI] [PubMed] [Google Scholar]
- Wang JA, Sulla‐Menashe D, Woodcock CE, Sonnentag O, Keeling RF, Friedl MA. 2020. Extensive land cover change across Arctic–Boreal Northwestern North America from disturbance and climate forcing. Global Change Biology 26: 807–822. [DOI] [PubMed] [Google Scholar]
- Wood SN. 2017. Generalized additive models: an introduction with R, Second Edition (2nd ed.). Chapman and Hall/CRC. doi: https://doi.org/ 10.1201/9781315370279. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.