Abstract
This study investigates the thermodynamics, kinetics, and adsorption mechanisms of Lavender angustifolia extract (LAE) as a corrosion inhibitor for stainless steel (316SS) in desalination units. The primary aim is to evaluate the efficacy of LAE in mitigating corrosion in a 5.0 M HCl solution under dynamic conditions. High-Performance Liquid Chromatography (HPLC) analysis identified key components of the LAE extract that contribute to corrosion inhibition, including linalyl acetate (41.7%), linalool (13.6%), 1,8-cineole (8.3%), β-ocimene (6.2%), terpinen-4-ol (5.7%), lavandulyl acetate (7.5%), and camphor (4.7%). Results indicate that the inhibitory efficiency of LAE increases with concentration, peaking at 94.3% at 300 mg L⁻¹. The Freundlich adsorption isotherm model best describes the experimental adsorption data. Notably, the activation energy for corrosion increases from 7.17 kJ mol⁻¹ in the 5.0 M HCl solution to 21.65 kJ mol⁻¹ with the addition of LAE, reflecting enhanced protection. The enthalpy change (∆H*) in the presence of LAE (19.04 kJ mol⁻¹) is significantly greater than that of the extract-free solution (4.55 kJ mol⁻¹), indicating improved corrosion resistance of 316SS. Electrochemical techniques confirmed the mixed-type inhibition behavior of LAE, while UV and SEM-EDAX analyses demonstrated effective adsorption of the extract on the stainless steel surface.
Keywords: Stainless steel, Corrosion inhibitor, Lavender extract, Desalination, Hydrochloric acid
Subject terms: Chemistry, Electrochemistry
Introduction
Corrosion of stainless steel in hydrochloric acid (HCl) is an important consideration in various industries, including desalination, where HCl may be used for cleaning or acid treatment purposes1,2. Stainless steel is generally known for its corrosion resistance, but it can still be affected by certain conditions, including the concentration and temperature of the HCl solution3. Following are some things to think about when it comes to stainless steel corroding in HCl during the desalination process. Stainless steel, particularly grades containing chromium (e.g., 304, 316), forms a passive oxide layer on its surface, which provides corrosion resistance. The passive layer acts as a barrier between the metal and the corrosive environment, preventing direct contact and reducing the corrosion rate. However, the passive layer can be compromised or damaged under certain conditions, leading to localized corrosion4,5. The corrosion rate of stainless steel in HCl is influenced by the concentration of the acid. At low concentrations (e.g., dilute solutions), stainless steel is generally resistant to corrosion. However, at higher concentrations (e.g., concentrated HCl), the risk of corrosion increases, especially if the exposure is prolonged6. The temperature of the HCl solution can significantly affect the corrosion behavior of stainless steel. Higher temperatures can accelerate corrosion processes, making stainless steel more susceptible to attack, even at lower acid concentrations7,8. Elevated temperatures can also increase the risk of localized corrosion, such as pitting or crevice corrosion. Chloride ions present in HCl solutions can enhance the corrosion of stainless steel. Chloride ions can penetrate the passive oxide layer and initiate localized corrosion, leading to pitting or crevice corrosion. The combination of high chloride ion concentration, HCl concentration, and temperature can increase the corrosivity of the environment9.
The choice of stainless steel grade is crucial in minimizing corrosion in HCl environments. Grades with higher corrosion resistance, such as 316 stainless steel, are often preferred for applications involving HCl. Consideration should also be given to the presence of other corrosive species or impurities that may be present in the HCl solution.
Proper surface preparation and maintenance practices can help mitigate corrosion in stainless steel exposed to HCl.
To protect stainless steel from corrosion in hydrochloric acid (HCl) environments, corrosion inhibitors can be used10. In the context of desalination, where HCl may be used for cleaning or acid treatment purposes, here are some commonly used corrosion inhibitors for stainless steel in HCl: Organic inhibitors, such as amines, can be used to protect stainless steel from corrosion in HCl environments11. Ethanolamine is commonly used as an organic inhibitor in HCl solutions. It forms a protective film on the metal surface, preventing corrosion12. Imidazolium-based ionic liquids are other organic inhibitors that have been used to protect stainless steel in HCl10. It forms a protective film and reduces the corrosion rate. Mixed inhibitors are combinations of different compounds that work synergistically to provide corrosion protection13. It is important to note that the effectiveness of corrosion inhibitors can depend on various factors, including the concentration of HCl, temperature, exposure time, and the specific stainless steel grade.
While organic corrosion inhibitors offer several advantages, they also have some potential disadvantages.
Some organic corrosion inhibitors may have adverse effects on the environment14. Certain organic compounds used as inhibitors can be persistent and may not readily degrade, leading to potential pollution concerns if released into the environment. It is important to carefully consider the environmental impact and biodegradability of organic corrosion inhibitors.
To mitigate these disadvantages, it is crucial to carefully select and evaluate new corrosion inhibitors based on their compatibility with the specific application, environmental considerations, stability requirements, and cost-effectiveness. Natural compounds are generally considered more environmentally friendly compared to synthetic corrosion inhibitors, as they are derived from renewable sources and may have lower toxicity and biodegradability15,16.
Extracting natural compounds as corrosion inhibitors for stainless steel in hydrochloric acid (HCl) environments is an area of ongoing research. Natural extracts derived from plants, fruits, or other organic sources often contain compounds with potential corrosion inhibition properties. Polyphenolic and Caffeine compounds present in green tea have been investigated as corrosion inhibitors for stainless steel17. They form a protective film on the metal surface, reducing corrosion rates. Aloe vera contains various organic compounds, including polysaccharides and phenolic compounds, which have shown corrosion inhibition properties in acidic environments18. It’s important to note that the effectiveness of natural extracts as corrosion inhibitors can vary depending on factors such as extract concentration, exposure time, temperature, and the specific stainless steel grade. Additionally, compatibility and stability considerations should be taken into account when using natural extracts as corrosion inhibitors19.
Further research and testing are necessary to determine the optimal conditions and mechanisms of action for natural extract-based corrosion inhibitors in HCl environments.
To extent of what we know, there is no research available on the inhibitory impact of lavandula angustifolia extract (LAE) on 316SS corrosion in HCl conditions. It follows that the main goal of the current work is to ascertain the optimal concentration for safeguarding stainless steel (316SS) in 5.0 M HCl solution conditions during desalination, as well as to explore the possibilities of Lavandula angustifolia extract (LAE) as a novel corrosion inhibitor.
The use of 5.0 M HCl is representative of the concentrations typically employed in industrial acid cleaning processes, particularly in desalination units. Such high concentrations are effective for removing scale, rust, and other deposits that can accumulate on stainless steel surfaces.
LAE has gained attention as a potential corrosion inhibitor due to several advantages compared to traditional inhibitors. Lavender is widely cultivated and can be sourced at a relatively low cost, especially in regions where it is grown abundantly. The extraction process (e.g., steam distillation) is generally straightforward and can be performed at low costs compared to the synthesis of many industrial corrosion inhibitors. Lavender angustifolia is generally recognized as safe (GRAS) for various applications, including food and cosmetics. This low toxicity is a significant advantage over many synthetic corrosion inhibitors, which can be hazardous to health and the environment. Being a natural product, lavender extract is biodegradable, reducing environmental impact compared to persistent synthetic chemicals.
Weight loss, open circuit potential (OCP), electrochemical impedance spectroscopy (EIS), potentiodynamic polarization, and SEM-EDAX assessment have been used throughout the study.
Experimental part
Materials and solutions
316SS with a composition of 17.8% chromium, 13.6% nickel, 2.5% molybdenum, and the remaining Fe was assessed in the present study. 316SS sample was supplied from Desalination Company in Egypt. The 316SS sample was ground with emery paper grit size in the range of 600 to 1500 to achieve an extremely smooth and polished surface before inspection, and it was washed with distilled water and an ethyl alcohol solution.
For all studies, 5.0 M HCl concentrations have been obtained using de-ionizing water and Hydrochloric acid 37% (Merck). We purchased Lavender angustifolia extract (LAE) from Symrise. The main constituents of LAE are determined using HPLC (Agilent). The principal identified components of the LAE were linalyl acetate (41.7%), linalool (13.6%), 1,8-cineole (8.3%), β-ocimene (6.2%), terpinen-4-ol (5.7%), lavandulyl acetate (7.5%), and camphor (4.7%). The LAE extract was completely soluble in water and HCl solutions.
Electrochemical and chemical methods
The 316SS rectangular test specimens, sized 25 mm by 54 mm and having a hole and a thickness of 1.7 mm, are utilized for weight loss (WL) testing. WL tests were carried out in compliance with NACE TM0169/G31–21. A suitable-sized flask (often 500 cc) with a temperature-regulating device is standard testing equipment for WL tests. Each WL flask was placed in a programmed stirring hot plate model DAIHAN Scientific Co. to achieve a 500 rpm rotation speed during the 72-hour immersion period of the WL test. Upon the final cleaning of the surface of the specimen, the samples need to be placed in a desiccant till exposure.
This equation had been applied to calculate the 316SS corrosion rate (CR)20:
 |
1 |
(W: WL (mg), A: 316SS surface area (cm2), t: exposure time (h)).
The electrochemical tests were carried out in compliance with according to G3-89(2010). To identify the electrochemical testing, a potentiostat/3000-Gamry had been used. The tests were performed in a double-walled unit. The experiments comprised a 316SS having an effective surface of 0.349 cm2, a Platinum sheet to be a counter, and a saturated calomel electrode (SCE) to be a reference. The open circuit potential (OCP) was recorded during 60 min, and electrochemical impedance spectroscopy (EIS) tests were performed within the frequency starting at 100 kHz to 0.01 Hz by 10 mV amplitude AC voltage at OCP. Potentiodynamic polarization has been determined at ± 0.250 V/SCE vs. OCP, with a scanning rate of 0.125 mV s-1.
The WL and electrochemical studies were repeated three times, and their mean (M) and standard deviations (SD) were calculated.
Surface characterization
Using energy dispersive X-ray (EDAX) and scanning electron microscopy (SEM) (model: ZEISS-EVO), the surface morphological characteristics of the 316SS specimens immersed in 5.0 M HCl solution in the absence and presence of an acceptable quantity of the LAE extract as a corrosion inhibitor was investigated.
316SS samples were subjected to acid solution drops with and without LAE extract in order to measure the contact angle employing the Kruss instrument’s Drop Shape Analysis.
The UV-Visible spectrophotometer (PerkinElmer’s) was used to inspect the spectra of LAE extract in 5.0 M HCl. To illustrate the adsorption behavior of the LAE extract, UV examination was performed for the solutions before and after immersion (6.0 h) of the 316SS sample.
Results and discussion
Weight loss and corrosion rate studies
Weight loss and corrosion rate studies are commonly conducted to evaluate the corrosion performance of materials and assess the effectiveness of corrosion inhibitors or protective coatings. These studies involve measuring the weight loss of a material over a specific period of exposure to a corrosive environment and calculating the corresponding corrosion rate. The WL tests method was utilized to evaluate the corrosion rate and degree of inhibition for 316SS in 5.0 M HCl solution with LAE extract at 298 K under dynamic circumstances, as can be seen from the data displayed in Table 1.
Table 1.
WL parameters of 316SS in 5.0 M HCl solution with and without LAE extract at 298 K under dynamic conditions.
| LAE extract (mg L− 1) |
W (g) |
CR (µg cm− 2 h− 1) M ± SD |
ηW% |
|---|---|---|---|
|
Blank 50 100 150 200 250 300 |
0.595691 0.486089 0.427115 0.324648 0.183474 0.091138 0.050036 |
278.7 ± 3.8 227.42 ± 3.2 199.83 ± 2.6 151.89 ± 2.2 85.84 ± 1.8 42.64 ± 1.6 23.41 ± 1.2 |
- 18.4 28.3 45.5 69.2 84.7 91.6 |
The following formula has been employed for assessing the inhibitory power of LAE extract (ηW%)21:
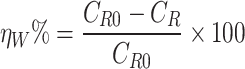 |
2 |
where CR and CR0 are the corrosion rate with and without of LAE extract, respectively.
The CR and ηW% at varying quantities of LAE extract are outlined in Table 1 shows that the average amount of CR decreases as LAE extract concentration increases. A trend regarding greater surface coverage of the 316SS with the help of LAE extract molecules is shown by the reduction in CR as LAE extract dosage increases. The adsorption of LAE extract molecules onto the 316SS surface is a key mechanism for corrosion inhibition22. Generally, an increase in the concentration of LAE extract molecules leads to a higher adsorption capacity. As the concentration rises, more LAE molecules are available for adsorption, resulting in a greater coverage of the 316SS surface and improved corrosion inhibition.
At 300 mg L− 1 of LAE extract, the greatest inhibitory effectiveness (ηW% = 91.6) was identified. No significant variations in inhibitory efficiency were seen above 300 mg L− 1 extract concentration. Prasad et al.22 discovered a similar trend, with inhibitory efficiency greater than 92% at 500 ppm concentration and enhanced efficiency via raising Mimosa pudica extract level from 100 to 500 ppm in the acid liquid.
Adsorption isotherm studies
Adsorption isotherm studies are conducted to understand and characterize the adsorption behavior of LAE extract molecules on the surface of 316SS surface. These studies provide valuable information about the relationship between the concentration of the LAE extract in 5.0 M HCl solution and the amount of LAE extract molecules adsorbed onto the 316SS surface.
Different types of adsorption isotherms can be constructed, such as Langmuir, Freundlich, or Temkin isotherms, depending on the nature of the adsorption process and assumptions made (see Eqs. 3, 4 and 5)23–25.
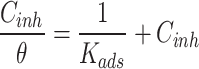 |
3 |
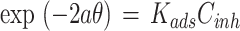 |
4 |
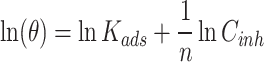 |
5 |
Where θ is surface coverage, Kads is adsorption constant, Cinh is LAE extract concentration, a is the lateral interaction, and n is a correction factor.
Fit the experimental data to various adsorption isotherm models to determine the best fit and extract relevant parameters. Several adsorption isotherm equations were fitted using the concentration of the LAE extract (Cinh) in 5.0 M HCl solution and the surface coverage (θ) derived from WL data (Fig. 1). The selection of the most ideally fitting adsorption isotherm is based on the regression coefficient (R2) value26. The R2 values for Langmuir, Temkin, and Freundlich isotherms are 0.123, 0.9151 and 0.9781, respectively. The model that best suited the results of the experiment was the Freundlich adsorption isotherm approach, as seen in Fig. 1c, which had R2 of 0.9781. The heterogeneous 316SS surface in 5.0 M HCl solution with LAE extract has been explored using the Freundlich isotherm model. The 316SS surface had been expected to be heterogeneous with varying adsorption energies, and this was demonstrated by their compliance to the Freundlich model. Similar results were found by Iloamaeke et al.27, who investigated the inhibition potential of Anthonotha macrophylla leaf extract on mild steel corrosion in 0.5 M H2SO4 medium and discovered that Freundlich adsorption isotherms fit the data more precisely than the Langmuir isotherm. The adsorption strength of the LAE extract molecules was determined by the value of the variable “1/n” in this model, which was stated in Eq. (5). It’s clear that the resulting value of 1/n = 0.9615 fell between 0 and 1. This demonstrates that the 316SS surface provides advantageous adsorption conditions for producing of cover layer of LAE extract molecules. According to Gusti and their group28, the heterogeneous multilayer of extract molecules that forms on the metal surface is explained by the Freundlich adsorption isotherm; each active group on the metal surface has a distinct adsorption capacity.
Fig. 1.

Adsorption isotherm plots: (a) Langmuir, (b) Temkin, (c) Freundlich for (LAE) extract adsorption on the surface of 316SS in 5.0 M HCl solution at 298 K under dynamic conditions.
Kads results reflect the degree of adsorption between the molecules of the LAE extract and the 316SS surface. It quantifies the equilibrium relationship between the concentration of the adsorbed species and the concentration of the species in the solution. A higher value of Kads (i.e. 255 L/mg) indicates a stronger affinity between the LAE extract molecules and the 316SS surface, suggesting a more favorable adsorption process. Raghvi et al.29 provided an explanation for this behaviour, pointing out that the higher Kads value for Dactyloctenium aegyptium extract + KI indicates a higher degree of adsorption and corrosion inhibition.
Thermodynamic studies
Thermodynamic studies are typically conducted by measuring the behavior of LAE extract at different temperatures (298–333 K) and analyzing the data using thermodynamic equations (Eqs. 6 and 7) and models (Arrhenius and transition-state). Table 2 displays the CR and ηW% for 316SS as variables that are influenced of temperature (298 to 333 K) in 5.0 M HCl solution; either without or with LAE extract (300 mg L− 1). Data indicates that the CR of 316SS in acid solution, either without or with LAE extract, continues to rise with increasing temperature.
Table 2.
WL parameters of 316SS in 5.0 M HCl solution with and without LAE extract (300 mg L− 1) at various temperatures under dynamic conditions.
| Temperature (K) |
LAE extract | CR (µg cm− 2 h− 1) M ± SD |
ηW (%) |
|---|---|---|---|
| 298 | 0 | 278.7 ± 3.8 | - |
| + | 23.41 ± 1.2 | 91.6 | |
| 313 | 0 | 299.2 ± 3.9 | - |
| + | 37.66 ± 1.7 | 87.4 | |
| 323 | 0 | 331.8 ± 3.3 | - |
| + | 44.23 ± 1.6 | 86.6 | |
| 333 | 0 | 380.4 ± 2.9 | - |
| + | 60.38 ± 1.8 | 84.1 |
Increasing the temperature generally leads to an increase in the corrosion rate of 316SS in acid solution. Higher temperatures accelerate the rates of chemical reactions, including oxidation and reduction reactions involved in corrosion processes. This increased reaction rate leads to a faster corrosion rate. In the same time, with elevated temperatures, the mobility of ions in the electrolyte solution increases. This increased mobility allows ions to more easily reach the 316SS surface and participate in electrochemical reactions, leading to higher corrosion rates30,31. Moreover, at elevated temperatures, the stability of these passive films of 316SS surface can decrease, leading to a higher susceptibility to corrosion32–34.
As the temperature goes up, the ηW% slightly drops from 91.6 to 84.1% (Table 2), This indicates that the adsorption may be weakened at elevated temperatures, reducing the inhibitor’s performance. LAE extract may be slightly susceptible to de-soprtion at higher temperatures, leading to a slightly decrease in their inhibitive properties. This also confirms their physisorption mechanism35.
The exact nature and mechanism of the inhibition of corrosion operation are able to evaluated by calculating thermodynamic variables, including activation energy (Ea), The enthalpy change (∆H*), and The entropy change (∆S*). Equations (6) and (7) on the Arrhenius and transition-state relationships provide thermodynamic variables36.
 |
6 |
 |
7 |
(h = 6.6261 10− 34 m2 kg s− 1, R = universal gas constant, N = Avogadro’s constant, and A = constant).
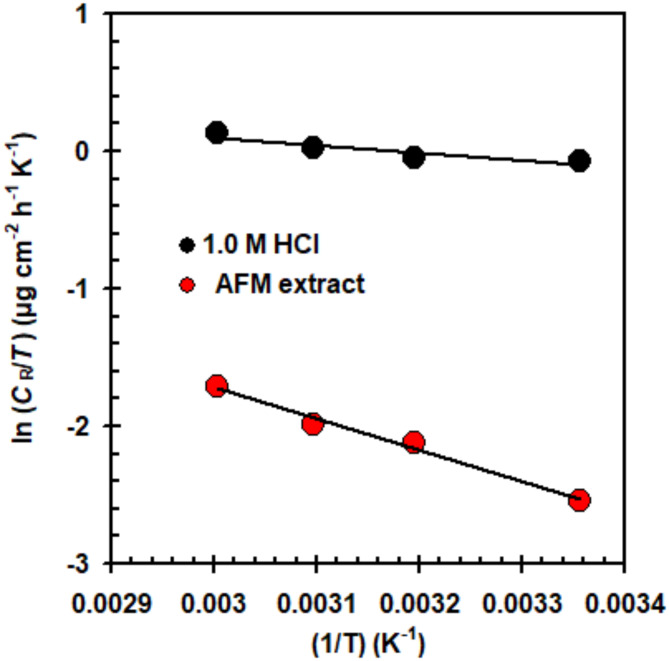
An Arrhenius curves (Fig. 2) has been employed to calculate the Ea, either with or without the 300 mg L− 1 LAE extract. The Ea rises to 21.65 kJ mol− 1 from 7.17 kJ mol− 1 (5.0 M HCl solution) upon the addition of LAE extract. The activation energy (Ea) is a measure of the energy barrier that needs to be overcome for the corrosion process to occur. When LAE extract is present, significantly greater activation energy slows down the corrosion of 316SS. Due to LAE extract’s adsorption on the 316SS surface, the double layer’s dimension grows, increasing the energy barrier needed to initiate the corrosion reaction. This phenomenon was associated with the molecules of the LAE extract possessing acceptable physical adsorption37. Kaur et al.38 observed comparable behavior. They found that the activation energy for a blank solution was estimated to be 9.09 kJ mol–1, but it was elevated to 50.31 kJ mol–1 at a concentration of 2000 mg L–1 of Pistacia Integerrima Gall Extract.
Fig. 2.
Arrhenius plots 316SS in 5.0 M HCl solution with and without LAE extract (300 mg L− 1) at various temperatures under dynamic conditions.
The transition figures (Fig. 3) have been employed to identify the ΔH* and ΔS* values. Adding LAE extract raised the ΔH* to 19.04 kJ mol− 1 versus 4.55 kJ mol− 1 (5.0 M HCl solution). The endothermic oxidation of 316SS in an acidic solution is illustrated by the positive amplitude of ΔH*39. There was a little change in the ΔS*, going from − 183 J mol− 1 K− 1 (5.0 M HCl solution) to -154 J mol− 1 K− 1 (in the presence of LAE extract).
Fig. 3.
Transition state plots 316SS in 5.0 M HCl solution with and without LAE extract (300 mg L− 1) at various temperatures under dynamic conditions.
Additionally, the decrease in negative values of ΔS* in the LAE extract solution can probably be attributed to an increase in H2O entropy that arises from H2O de-sorption over the 316SS surface when LAE extract is present40,41.
Electrochemical studies
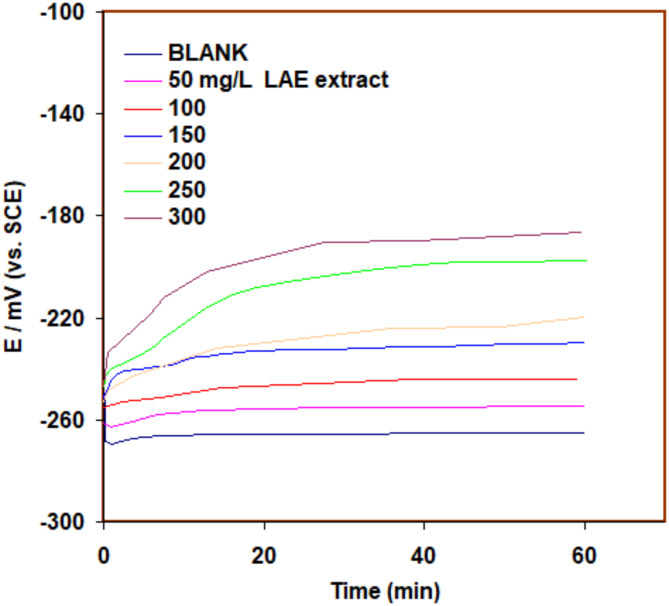
Assessing the open circuit potential (OCP) of 316SS in 5.0 M HCl with and without LAE extract can provide valuable information about the effectiveness of the LAE extract in mitigating corrosion. The OCP measurement helps to understand the electrochemical behavior and the tendency of the metal to corrode under specific conditions. The open circuit potential (OCP) assessments for 316SS in 5.0 M HCl have been established with and without the incorporation of LAE extract for duration of 60 min. The curves that are produced are shown in Fig. 4. In the absence of LAE extract, the OCP of 316SS in 5.0 M HCl will reflect the natural corrosion potential of the material in that particular environment. The OCP value will indicate the thermodynamic driving force for corrosion reactions to occur. When LAE extract is introduced, it can alter the electrochemical behavior of the system and affect the OCP of 316SS in 5.0 M HCl. The LAE extract may adsorb onto the 316SS surface, forming a protective layer or altering the electrochemical reactions occurring at the interface. This can cause the OCP to move to a more positive value, showing a reduction in corrosion propensity and implying that LAE extract inhibits anodic dissolution of the 316SS.
Fig. 4.
OCP curves of 316SS in 5.0 M HCl solution with and without Lavandula angustifolia (LAE) extract at 298 K under dynamic conditions.
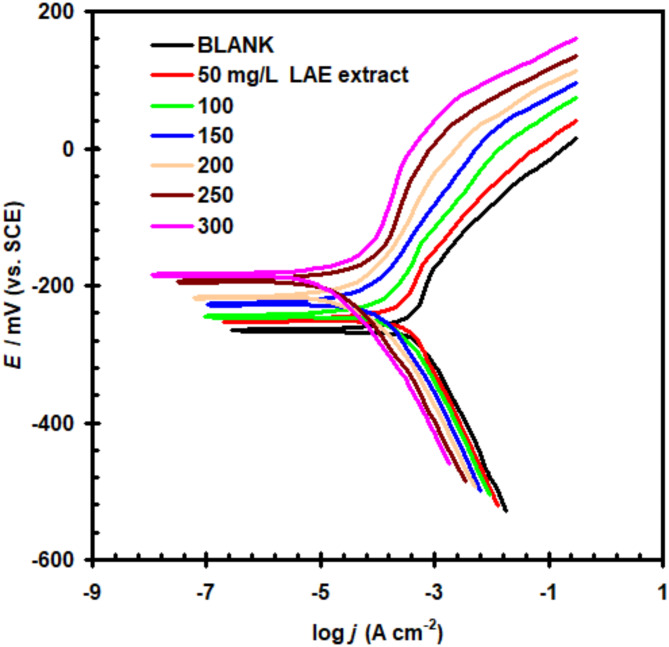
Figure 5 depicts the Tafel polarization behavior for 316SSin 5.0 M HCl using different LAE extract quantities at 298 K. Polarization investigation revealed that increasing the quantity of LAE extract alters both the cathodic and anodic curves. Table 3 gives the Tafel polarization results. The variation in Ecorr values in the absence and presence of LAE extract, which was below 85 mV, verified the mixed type behavior of the LAE extract. Similar outcomes were found when Tinospora cordifolia extract was used to suppress corrosion on low-carbon steel corrosion resistance in 0.5 M H2SO4 environment42. Sharma et al.43 further investigated this phenomenon and discovered that the presence of the Celastrus paniculatus seed extract lowers the anodic and cathodic current densities. This case shows how well the extract suppresses anodic metal oxidation and cathodic hydrogen evolution.
Fig. 5.
Polarization curves of 316SS in 5.0 M HCl solution with and without Lavandula angustifolia (LAE) extract at 298 K under dynamic conditions.
Table 3.
Polarization parameters of 316SS in 5.0 M HCl solution with and without LAE extract at 298 K under dynamic conditions.
| LAE extract (mg L− 1) |
-Ecorr mV (SCE) M ± SD |
jcorr. µA cm− 2 M ± SD |
βa (mV dec− 1) |
-βc (mV dec− 1) |
ηj% |
|---|---|---|---|---|---|
|
Blank 50 100 150 200 250 300 |
266 ± 2.2 253 ± 2.0 245 ± 2.1 228 ± 1.8 217 ± 1.3 193 ± 1.2 185 ± 1.4 |
545.00 ± 3.2 423.47 ± 3.0 356.43 ± 2.4 263.24 ± 2.6 122.08 ± 1.9 58.32 ± 1.2 30.97 ± 1.1 |
79 73 71 71 69 64 63 |
124 121 121 118 114 113 108 |
- 22.3 34.6 51.7 77.6 89.3 94.3 |
When the quantity of LAE extract was increased, the Ecorr value noticeably moved in a positive direction. Since neither the cathodic Tafel slop (βc) nor the anodic Tafel slop (βa) showed any appreciable changes, it is clear that the incorporation of LAE extract to the corrosion environment had no effect on the mechanisms of the anodic and cathodic activities44. The anodic and cathodic section current density declines in the presence of the LAE extract, based on the Tafel curves. This effect shows that the LAE extract can inhibit both anodic metal oxidation and the generation of cathodic hydrogen45. The addition of LAE extract resulted in a substantial reduction in the corrosion current density (jcorr).
This implies that LAE extract suppresses 316SS anode corrosion in 5.0 M HCl solutions. The inhibitory efficiency (ηj%) of the LAE extract has been determined via the formula below44:
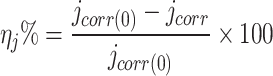 |
8 |
where jcorr and jcorr(0) is the corrosion current density with and without of LAE extract, respectively.
With increasing concentration, the inhibitory efficacy of LAE extract achieves its greatest value of 94.3% at 300 mg L− 1. These findings confirm that LAE extract significantly inhibits 316SS corrosion in 5.0 M HCl.
To investigate the effect of LAE extract on the corrosion behavior of 316SS in 5.0 M HCl in further detail, EIS experiments were carried out. The results that were reported (Nyquist diagram) are shown in Fig. 6. One moderately flattened semicircle and an inductive circuit beginning at low frequencies are detected in 5.0 M HCl, according to the Nyquist plots. The high adsorption of ions of chloride from solution onto the 316SS surface is most likely what causes the inductive circuit46. The Nyquist plot (see Fig. 6) shows that when the concentration of LAE extract increases, correspondingly increases the dimension of the semicircle. The corrosion rate is reduced as a consequence. According to Dutta et al.47, the semicircle’s size increased as the concentration of plant extract rose, indicating better extract adsorption.
Fig. 6.
Nyquist plot of 316SS in 5.0 M HCl solution with and without Lavandula angustifolia (LAE) extract at 298 K under dynamic conditions.
Figure 7 shows an estimated equivalent circuit (EC), which was utilized in order to interpret and reflect the Nyquist graphs. EC includes, polarization resistance (Rp) charge transfer resistance (Rct), solution resistance (Rs), film resistance (Rf) and the ideal capacitor is substituted by the constant phase element (Qdl), RL and L are resistance and inductivity of the chloride adsorption process. In the presence of LAE extract Rp equal to Rct + Rf. Table 4 presents the EIS variables that were identified by fitting, as revealed using the Nyquist curve. The relatively low goodness of fit (χ2) indicates accurate fitting processes48.
Fig. 7.
The equivalent electrical circuit for the impedance fitting for 316SS in 5.0 M HCl solution without (a) and with (b) LAE extract at 298 K under dynamic conditions.
Table 4.
EIS parameters for of 316SS in 5.0 M HCl solution with and without LAE extract at 298 K under dynamic conditions.
| LAE extract (mg L− 1) |
R
s
Ω cm2 M ± SD |
R
p
Ω cm2 M ± SD |
Qdl µF cm− 2 M ± SD |
χ2 | n | ηR% |
|---|---|---|---|---|---|---|
|
Blank 50 100 150 200 250 300 |
0.64 ± 0.02 0.65 ± 0.01 0.64 ± 0.01 0.56 ± 0.02 0.66 ± 0.02 0.74 ± 0.03 0.71 ± 0.02 |
22.00 ± 0.85 29.57 ± 1.10 35.60 ± 1.21 51.89 ± 2.32 111.11 ± 2.29 261.90 ± 2.20 305.56 ± 2.94 |
539 ± 3.4 447 ± 3.1 307 ± 2.3 143 ± 2.1 60 ± 1.5 52 ± 1.2 15 ± 0.9 |
1.2 × 10− 3 1.1 × 10− 3 1.7 × 10− 3 1.2 × 10− 3 1.0 × 10− 3 1.2 × 10− 3 1.6 × 10− 3 |
0.88 0.88 0.89 0.91 0.93 0.94 0.94 |
- 25.6 38.2 57.6 80.2 91.6 92.8 |
The Rct represents the resistance to electron transfer at the 316SS-HCl solution interface, typically associated with the corrosion reaction. The presence of LAE extract increases the Rp value. An increased Rp suggests that the LAE extract forms a protective layer on the 316SS surface, hindering the corrosion reaction and reducing the electron transfer rate49. The presence of LAE extract does not typically have a significant effect on Rs. This confirms that the LAE extract does not alter the conductivity of the electrolyte significantly. Table 4 shows that once LAE extract has been added, “n” values increase, suggesting an enhancement in surface homogeneity due to LAE extract adsorption50. Also, once the concentration of LAE extract became larger, the value of Qdl diminished. This is connected to the attachment of LAE extract molecules over the 316SS surface, which reduces the contact with the 316SS surface to HCl solution51.
The inhibitory efficiency (ηR%) of the LAE extract has been determined via the formula below52:
 |
2 |
where Rp and Rpo is the polarization resistance with and without of LAE extract, respectively.
Table 4 summarizes the obtained inhibition efficiencies ηR%. Upon rising concentrations, the ηR% of LAE extract reaches its greatest level of 92.8% at 300 mg L− 1. The polarization data (Table 3) are consistent with the calculated EIS values (Table 4). There is a little variation in the inhibition efficiency values between the EIS and polarization methods. This is because the polarization method captures the inhibition efficiency at a specific moment in time, reflecting the immediate impact of the LAE extract on the corrosion process. On the other hand, the EIS, being a time-dependent technique, offers insights into how inhibition efficiency may evolve over time or under varying conditions due to its frequency-dependent impedance measurements.
Surface morphology studies
Figure 8 (a) presents a SEM image of 316SS after 72 h of immersion in 5.0 M HCl at 298 K. The image of the 316SS surface in 5.0 M HCl (Fig. 8a) shows that the upper part of the 316SS was extremely damaged, leading in lots of cracks and holes across the surface. The associated EDAX consists of Fe (65.5%), Cr (16.3%), Ni (12.5%), Mo (2.3%), O (2.1%), and Cl (1.3%), showing the formation of Fe/Cr-oxides/hydroxides to be corrosion products that adhere to the 316SS surface. A high level of chloride ions on the 316SS surface transforms defensive oxides/hydroxides to soluble chlorides, resulting in pitting progress. Adding 300 mg L− 1 of LAE extract to the 5.0 M HCl solution (Fig. 8b) dramatically reduced its corrosive abilities and prevented corrosion of the 316SS surface, as demonstrated by a considerably clean 316SS surface that was virtually completely coated with LAE extract molecules. The related EDAX consists of Fe (62.6%), Cr (17.6%), Ni (13.4%), Mo (2.4%), O (1.4%), and C (2.6%), suggesting that the LAE extract molecules adhere onto the 316SS surface. The complete absence of Cl on the 316SS surface in the presence of LAE extract clearly demonstrates that LAE extract adsorbs on the 316SS surface, forming a barrier that limits corrosive harm.
Fig. 8.
SEM– EDAX surface images for (a) 316SS in 5.0 M HCl solution and (b) 316SS in 5.0 M HCl solution containing 300 mg L− 1 of LAE extract.
To determine whether the adsorbed LAE extract on the 316SS surface was hydrophilic or hydrophobic, contact angle analysis was utilized. On the 316SS surface immersed in 5.0 M HCl at 298 K, both in the absence and with 300 mg L− 1 of LAE extract, a contact angle measurement was carried out. Without LAE extract, the 316SS surface’s contact angle was measured to be 21.4°. The findings showed that the steel surface’s wettability imparts hydrophilicity, or a preference for water53. The addition of LAE extract causes the contact angle to rise to 57.8°, indicating that the adsorbed molecules of LAE extract have created a hydrophobic layer on the 316SS surface53.
The UV spectra of the LAE extract in 5.0 M HCl are displayed in Fig. 9 both before and after the 316SS sample was immersed. Following contact with the 316SS sheet and the corrosion experiment, the UV finger print significantly alters. This provides more convincing proof of the adsorption mechanism of LAE extract on the 316SS surface. Jaiswal et al.53 observed a change in the febuxostat band before and after 24 h of steel immersion, with a significant decrease in intensity. These two changes demonstrate the establishment of a corrosion inhibitor layer over the steel.
Fig. 9.
UV spectra of the LAE extract - HCl before and after immersion of the 316SS sample.
Proposed mechanism
LAE extract contains organic compounds (see Fig. 10), such as linalyl acetate, linalool, 1,8-cineole, β-ocimene, terpinen-4-o, lavandulyl acetate, and camphor, which have the ability to adsorb onto the 316SS surface. These organic compounds with functional groups such as hydroxyl groups that enable their adsorption onto metal surfaces. Additionally, the presence of aromatic rings enhances their π-π interactions with the 316SS surface53,54. The lone pair of electrons on the oxygen atom allows for the formation of coordinate bonds with metal ions, leading to their adsorption. These functional groups present in LAE extract enable interactions with the 316SS surface, such as hydrogen bonding, π-π interactions, coordination, and ionization55,56. These interactions facilitate the adsorption of the plant extract molecules onto the 316SS surface, forming a protective layer and inhibiting the corrosion process. On the 316SS surface, LAE extract may adsorb on both cathodic and anodic sites. However, due to electrical interaction with the partially-filled d-orbitals of Fe2+ and Cr3+, it is more likely to anodic adsorb.
Fig. 10.
Chemical structures of the main components of LAE extract.
The effectiveness of LAE extract and a few other published natural extracts for preventing stainless steel corrosion in HCl solutions are compared in Table 557–64. When compared to other extract components in acid solutions, Table 5 shows that LAE extract significantly suppresses 316SS corrosion in HCl solutions.
Table 5.
Comparative of the performance of LAE extract and some reported natural extract for stainless steel corrosion inhibition in HCl solutions.
| Extract type | Corrosion conditions | Extract concentration | Inhibition efficiency (%) | Ref. |
|---|---|---|---|---|
| Palm waste extract | 304 stainless steel in 1 M HCl solution | 1.5 g/L | 91.1% | 57 |
| Triticum aestivum extract | stainless steel (SS-410) in 15% M HCl solution | 4.0 g/L | 92.69% | 58 |
| Tea tree extract | 304 stainless steel in 1 M HCl | 0.75 g/L | 86.00% | 59 |
| Tectona grandis leaf extract | SS304 in 2 M HCl | 1.0 g/L | 71.70% | 60 |
| Tithonia diversifolia (Hemsl) A. Gray leaves extract | 304 SS in 1 M HCl | 2.0 g/L | 77.27% | 61 |
| Okra pectin | stainless steel (304 SS) in 1 M HCl | 2 g/L | 86.5 | 62 |
| Phyllanthus emblica seed extract | stainless steel (SS-410) in 15% HCl | 4 g/L | 92.43% | 63 |
| Citrus limetta pulp waste extract | stainless steel (SS)-410 in 15% HCl | 4 g/L | 90.90% | 64 |
| Lavandula angustifolia (LAE) extract | 316SS in 5.0 M HCl solution | 300 mg/L | 94.30% | This work |
It is important to note that the effectiveness of LAE extract molecules as corrosion inhibitors can vary depending on various factors, including the specific extract composition, concentration, exposure conditions, and the corrosive environment. Proper selection, optimization, and testing of the extract molecules are necessary to achieve the desired corrosion inhibition performance.
Conclusions
The main objective of this research is to explore into the ability of Lavandula angustifolia extract (LAE) to be a new corrosion inhibitor for stainless steel (316SS) in 5.0 M HCl solution. Weight loss, electrochemical, adsorption, thermodynamic, and surface morphology tests all validated this objective. The results revealed that at 300 mg L− 1 of LAE extract, the highest inhibitory efficiency (94.3%) had been observed. In 5.0 M HCl solution containing LAE extract, the heterogeneous 316SS surface was best fitted by the Freundlich isotherm model. At higher temperatures, there are likely to be a little de-sorption of LAE extract, which would support its physisorption mechanism and result in a slight diminution of its inhibitory effects. The presence of LAE extract significantly reduces the rate of 316SS corrosion by raising activation energy. The positive amplitude of ΔH* shows the endothermic oxidation of 316SS in an acidic solution. The mixed type behavior of the LAE extract was confirmed by the difference in Ecorr values between the presence and absence of the extract, which was less than 85 mV. In 5.0 M HCl, the Nyquist plots show one semicircle that has been substantially flattened and an inductive circuit that starts at low frequencies. The ability for contacts and bond formation between 316SS and LAE extract was shown by SEM–EDAX and UV studies. Plant extracts present a compelling alternative for corrosion inhibition in the future, which is expected to centre on creating environmentally friendly and sustainable solutions.
Acknowledgements
The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2025-20).
Author contributions
M. A. Deyab and Q. Mohsen , All authors reviewed the manuscript.
Funding
This research was funded by Taif University, Saudi Arabia, Project No. (TU-DSPP-2025-20).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhou Zou, Z. et al. Corrosion behavior of different building planes of selective laser melting 316L stainless steel in 0.1 M HCl solution. J. Mater. Res. Technol.28, 4738–4753 (2024). [Google Scholar]
- 2.Wang, D. Y., Li, Z. G., Liu, Y., Li, H. J. & Wu, Y. C. Investigation of efficient corrosion inhibitor during acid cleaning of reverse osmosis (RO) desalination plant. Corrosion Science. 208, 110609 (2022).
- 3.Hodgkiess, T., Al-Omari, K. H., Bontems, N. & Lesiak, B. Acid cleaning of thermal desalination plant: do we need to use corrosion inhibitors? Desalination, Volume 183, Issues 1–3, Pages 209–216. (2005).
- 4.Ningshen, S. & Kamachi Mudali, U. 2 - Uniform Corrosion of Austenitic Stainless Steels, Editor(s): H.S. Khatak, Baldev Raj, In Woodhead Publishing Series in Metals and Surface Engineering, Corrosion of Austenitic Stainless Steels, Woodhead Publishing, Pages 37–73, ISBN 9781855736139, (2002). 10.1533/9780857094018.74
- 5.Tsai, W. T. & Chen, M. S. Stress corrosion cracking behavior of 2205 duplex stainless steel in concentrated NaCl solution. Corros. Sci.42 (Issue 3), 545–559 (2000). [Google Scholar]
- 6.Hussain, C. M., Verma, C., Aslam, J. & Aslam, R. Saman Zehra, 13 - Metals and alloys susceptible for corrosion, Editor(s): Chaudhery Mustansar Hussain, Chandrabhan Verma, Jeenat Aslam, Ruby Aslam, Saman Zehra, Handbook of Corrosion Engineering, Elsevier, Pages 157–167, ISBN 9780323951852. (2023).
- 7.Pengcheng Wang, T. J. et al. High temperature corrosion of 321 stainless steel in purified NaCl-MgCl2 eutectic salt, Journal of Energy Storage, 84, 110845. (2024).
- 8.Li, H. et al. Effect of temperature on the Corrosion Behaviour of Super Austenitic Stainless Steel S32654 in Polluted Phosphoric Acid. Int. J. Electrochem. Sci.10 (Issue 6), 4832–4848 (2015). [Google Scholar]
- 9.Suresh Nuthalapati, K. E., Kee, S. R., Pedapati, K. & Jumbri A review of chloride induced stress corrosion cracking characterization in austenitic stainless steels using acoustic emission technique. Nuclear Eng. Technol.56 (Issue 2), 688–706 (2024). [Google Scholar]
- 10.Gadioli, A. O. et al. Imidazolium-based ionic liquids as corrosion inhibitors for stainless steel in different corrosive media: an overview. J. Mater. Res. Technol.29, 803–823 (2024). [Google Scholar]
- 11.Aslam, R., Mobin, M., Zehra, S. & Aslam, J. A comprehensive review of corrosion inhibitors employed to mitigate stainless steel corrosion in different environments. J. Mol. Liq.364, 119992 (2022). [Google Scholar]
- 12.De Vroey, S., Huynh, H., Lepaumier, H., Absil, P. & Thielens, M. L. Corrosion Investigations In 2-ethanolamine Based Post- Combustion CO2 Capture Pilot Plants, Energy Procedia, Volume 37, Pages 2047–2057. (2013).
- 13.Deyab, M. A. Sulfonium-based ionic liquid as an anticorrosive agent for thermal desalination units. J. Mol. Liq.296, 111742. 10.1016/j.molliq.2019.111742 (2019). [Google Scholar]
- 14.Zomorodian, A. & Behnood, A. Review of corrosion inhibitors in Reinforced concrete: conventional and green materials. Buildings13 (5), 1170. 10.3390/buildings13051170 (2023). [Google Scholar]
- 15.Deyab, M. A., Mohsen, Q. & Guo, L. Aesculus hippocastanum seeds extract as eco-friendly corrosion inhibitor for desalination plants: experimental and theoretical studies. J. Mol. Liq.361, 119594 (2022). [Google Scholar]
- 16.Deyab, M. A. & Mele, G. Polyaniline/Zn-phthalocyanines nanocomposite for protecting zinc electrode in Zn-air battery. J. Power Sources Volume. 44310.1016/j.jpowsour.2019.227264 (2019).
- 17.Devimeenakshi, S., Anandhi, M., Velkannan, V. & Balaji, G. Effect of green tea in artificial saliva on the corrosion resistance behaviour of stainless steel, Materials Today: Proceedings, Volume 52, Part 3, Pages 577–581. (2022).
- 18.Mehdipour, M., Ramezanzadeh, B. & Arman, S. Y. Electrochemical noise investigation of Aloe plant extract as green inhibitor on the corrosion of stainless steel in 1 M H2SO4. J. Ind. Eng. Chem.21, 318–327 (2015). [Google Scholar]
- 19.Salleh, S. Z., Yusoff, A. H., Zakaria, S. K., Taib, M. A. A. & Seman, A. A. Mohamad Najmi Masri, Mardawani Mohamad, Sarizam Mamat, Sharizal Ahmad Sobri, Arlina Ali, Pao Ter Teo, Plant extracts as green corrosion inhibitor for ferrous metal alloys: a review. J. Clean. Prod.304, 127030 (2021). [Google Scholar]
- 20.Deyab, M. A. Enhancement of corrosion resistance in MSF desalination plants during acid cleaning operation by cationic surfactant. Desalination. 456, 32–37 (2019).
- 21.Chuan Lai, B. et al. Adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by S-allyl-O,O′-dialkyldithiophosphates. Results in physics. 7, 3434–3443. (2017).
- 22.Dwarika Prasad, R., Maithani, B. E. & Ibrahimi Mimosa pudica extract corrosion inhibitive nature for stainless steel in 0.5 mol/L – 1 sulfuric acid media. J. Mol. Liq.389, 122940 (2023). [Google Scholar]
- 23.Su, H. et al. Enhancing the long-term anti-corrosion property of mg alloy by quaternary phosphonium salt: integrated experimental and theoretical approaches. Corros. Sci.178, Article109010 (2021). [Google Scholar]
- 24.Hosseini, M., Mertens, S. F. L., Ghorbani, M. & Arshadi, M. R. Asymmetrical schiff bases as inhibitors of mild steel corrosion in sulphuric acid media. Mater. Chem. Phys.78 (3), 800–808 (2003). [Google Scholar]
- 25.Deyab, M. A. & Mohsen, Q. Inhibitory influence of cationic Gemini surfactant on the dissolution rate of N80 carbon steel in 15% HCl solution. Sci. Rep.11, 10521. 10.1038/s41598-021-90031-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ituen, E., Akaranta, O. & James, A. Evaluation of performance of corrosion inhibitors using adsorption isotherm models: an overview. Chem. Sci. Int. J.18 (1), 1–34 (2017). [Google Scholar]
- 27.Ifeoma, M. et al. Biomass of Anthonotha macrophylla leaf extract as a mild steel acid corrosion inhibitor: experimental and theoretical study. Next Mater.7, 100344 (2025). [Google Scholar]
- 28.Gusti, D. R., Pardede, S. N., Tarigan, I. L., Damris, M. & Harizon Encapsulation of Acacia Bark Extract (Acacia mangium) by MaltodextrinCarrageenan for Corrosion Inhibition Steel in Sulfuric Acid Medium. Egypt. J. Chem. Vol. 67 (10), 1–9 (2024). [Google Scholar]
- 29.Raghvi, A., Saxena, J., Kaur, E., Berdimurodov, D. K. & Verma Synergistic mixture of the Dactyloctenium aegyptium extract and KI as an environment friendly and highly efficient corrosion inhibitor for steel in 0.5 M HCl. J. Indian Chem. Soc.101 (Issue 10), 101317 (2024). [Google Scholar]
- 30.Zhang, M. et al. Understanding microstructure evolution and corrosion behavior of wire arc cladding inconel 625 superalloy by thermodynamic approaches. J. Alloys Compd.947, 169530 (2023). [Google Scholar]
- 31.Konovalova, V. The effect of temperature on the corrosion rate of iron-carbon alloys, Materials Today: Proceedings, Volume 38, Part 4, Pages 1326–1329. (2021).
- 32.Zuocheng Wang, C. et al. Thermal stability of surface oxides on nickel alloys (NiCr and NiCrMo) investigated by XPS and ToF-SIMS, Applied Surface Science, Volume 576, Part B, 151836. (2022).
- 33.Li, X. et al. 1,4-Phenylenediamine-based Schiff bases as eco-friendly and efficient corrosion inhibitors for mild steel in HCl medium: experimental and theoretical approaches. J. Electroanal. Chem.955, 118052 (2024). [Google Scholar]
- 34.Deyab, M. A., Abd El-Rehim, S. S., Hassan, H. H. & Shaltot, A. M. Impact of rare earth compounds on corrosion of aluminum alloy (AA6061) in the marine water environment. J. Alloys Compd.820, 153428. 10.1016/j.jallcom.2019.153428 (2020). [Google Scholar]
- 35.Lai, C. et al. Corros. Porous Silicon Tetramethylammonium Hydroxide Solut. Corros. Sci., 85 471–476. (2014). [Google Scholar]
- 36.Azzaoui, K. et al. Eco friendly green inhibitor Gum Arabic (GA) for the corrosion control of mild steel in hydrochloric acid medium. Corros. Sci.120, 70–81 (2017). [Google Scholar]
- 37.Tang, C. et al. ACS Sustain. Chem. Eng.11 (1), 353–367 (2023). [Google Scholar]
- 38.Jasdeep Kaur, H. et al. Electrochemical and DFT studies of the Pistacia Integerrima Gall Extract: An Eco-friendly Approach towards the Corrosion of Steel in Acidic Medium. ACS Omega. 9, 7, 7643–7657 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gowraraju, N. D. et al. Adsorption characteristics of Iota-Carrageenan and Inulin biopolymers as potential corrosion inhibitors at mild steel/sulphuric acid interface. J. Mol. Liq. 232, 9–19. 10.1016/j.molliq.2017.02.054 (2017). [Google Scholar]
- 40.Ghareba, S. & Omanovic, S. 12-Amino dodecanoic acid as a corrosion inhibitor for carbon steel. Electrochim. Acta. 56, 3890–3898 (2011). [Google Scholar]
- 41.Dandia, A., Gupta, S. L., Singh, P. & Quraishi, M. A. Chem. Eng.1, 1303. (2013). [Google Scholar]
- 42.Saxena, A. et al. PDP, EIS, and Surface studies of the low-Carbon Steel by the Extract of Tinospora cordifolia: a Green Approach to the corrosion inhibition. Arab. J. Sci. Eng.46, 425–436. 10.1007/s13369-020-04894-9 (2021). [Google Scholar]
- 43.Ankita Sharma, J., Kaur, A. & Saxena Celastrus Paniculatus seeds as a green corrosion inhibitor for stainless steel in H2SO4 acidic solution. J. Indian Chem. Soc.101 (Issue 7), 101172 (2024). [Google Scholar]
- 44.Deyab, M. A., Słota, R., Bloise, E. & Mele, G. Exploring corrosion protection properties of alkyd@lanthanide bis-phthalocyanine nanocomposite coatings. RSC Adv.8, 1909–1916. 10.1039/C7RA09804A (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaur, J. et al. Euphorbia prostrata as an eco-friendly corrosion inhibitor for steel: electrochemical and DFT studies. Chem. Pap. 77, 957–976. 10.1007/s11696-022-02533-1 (2023). [Google Scholar]
- 46.Deyab, M. A. & Awadallah, A. E. Advanced anticorrosive coatings based on epoxy/functionalized multiwall carbon nanotubes composites. Progress in Organic Coatings. 139, 105423 (2020).
- 47.Anusuya Dutta, J., Kaur, A. & Saxena Boosting the Corrosion Inhibition Efficiency of the Clerodendrum Serratum Extract for Steel in the Presence of KCl, Chemical Data Collections, 52, 101148 (2024).
- 48.Zhao, L. et al. Jiangdong. A Comparative Study of Equivalent Circuit Models for Electro-Chemical Impedance Spectroscopy Analysis of Proton Exchange Membrane Fuel Cells. Energies. 15. 386. (2022). 10.3390/en15010386
- 49.Kaur, J. et al. Euphorbia prostrata as an eco-friendly corrosion inhibitor for steel: electrochemical and DFT studies. Chem. Pap. 77, 957–976. 10.1007/s11696-022-02533-0 (2023). [Google Scholar]
- 50.Hsu, C. H. & Mansfeld, F. Concerning the Conversion of the Constant Phase Element Parameter Y0 into a Capacitance, CORROSION 57 (9): 747–748. (2001).
- 51.Zhang, G. A., Liu, D., Li, Y. Z. & Guo, X. P. Corrosion behavior of N80 carbon steel in formation water under dynamic supercritical CO2 condition. Corros. Sci.120, 107–120 (2017).
- 52.Xu, B. et al. Experimental and theoretical evaluation of two pyridinecarboxaldehyde thiosemicarbazone compounds as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci.78, 260–268 (2014). [Google Scholar]
- 53.Mohit Jaiswal, A., Saxena, J. & Kaur Application of expired Febuxostat drug as an effective corrosion inhibitor for steel in acidic medium: experimental and theoretical studies. Chem. Data Collections. 52, 101149 (2024). [Google Scholar]
- 54.Li, X., Xie, X. & Deng, S. Du two phenylpyrimidine derivatives as new corrosion inhibitors for cold rolled steel in hydrochloric acid solution. Corros. Sci.87, 27–39 (2014). [Google Scholar]
- 55.Verma, C., Ebenso, E. E. & Quraishi, M. A. Corrosion inhibitors for ferrous and non-ferrous metals and alloys in ionic sodium chloride solutions: a review. J. Mol. Liq. 248, 927–942 (2017). [Google Scholar]
- 56.Walid Daoudi, A. et al. Paz Otero Fuertes, Abdelmalik El Aatiaoui, Carbon dots Nanoparticles: A Promising Breakthrough in Biosensing, Catalysis, Biomedical and Authers ApplicationsVolume 37101074 (Nano-Structures & Nano-Objects, 2024).
- 57.Sair, S. et al. Date Palm Waste Extract as Corrosion Inhibitor for 304 Stainless Steel in 1 M HCl solution. Int. J. Electrochem. Sci.13 (Issue 11), 10642–10653 (2018). [Google Scholar]
- 58.Bhardwaj, N., Sharma, P., & Kumar, V. Triticum aestivum extract as corrosion inhibitor for stainless steel (SS-410) in acidic media: experimental and theoretical study. Current Research in Green and Sustainable Chemistry. 4, 100189. (2021).
- 59.Kim, J. Y., Shin, I. & Byeon, J. W. Corrosion Inhibition of Mild Steel and 304 Stainless Steel in 1 M Hydrochloric Acid Solution by Tea Tree Extract and Its Main Constituents. Materials (Basel). 14(17), 5016 (2021). 10.3390/ma14175016. [DOI] [PMC free article] [PubMed]
- 60.Kadapparambil, S., Yadav, K., Ramachandran, M. & Victoria Selvam, N. Electrochemical investigation of the corrosion inhibition mechanism of Tectona grandis leaf extract for SS304 stainless steel in hydrochloric acid. Corros. Rev.35, 111–121 (2017). [Google Scholar]
- 61.Firdausi, S. & Kurniawan, F. Corrosion Inhibition by Tithonia diversifolia (Hemsl) A. Gray leaves extract for 304 SS in hydrochloric acid solution, Journal of Physics: Conference Series 710 012042. (2016).
- 62.Zhang, L. et al. Study of okra pectin prepared by sweeping frequency ultrasound/freeze-thaw pretreatment on corrosion inhibition of ANSI 304 stainless steel in acidic environment. Int. J. Biol. Macromol., 253, Part 1, 126587 (2023). [DOI] [PubMed]
- 63.Nishant Bhardwaj, P., Sharma, K., Singh, D., Rana, V. & Kumar Phyllanthus emblica seed Extract as Corrosion Inhibitor for Stainless Steel used in Petroleum Industry (SS-410) in Acidic MediumVolume 3100038 (Chemical Physics Impact, 2021).
- 64.Nishant Bhardwaj, P. et al. Monte Carlo simulation, molecular dynamic simulation, quantum chemical calculation and anti-corrosive behaviour of Citrus limetta pulp waste extract for stainless steel (SS-410) in acidic medium. Mater. Chem. Phys.284, 126052 (2022). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.